Abstract
Background:
Because immune responses are sensitive to environmental changes that drive selection of genetic variants, we hypothesized that polymorphisms of some xenobiotic response and immune response genes may be associated with specific types of immune-mediated diseases (IMD), while others may be associated with IMD as a larger category regardless of specific phenotype or ethnicity.
Objective:
To examine transethnic gene-IMD associations for single nucleotide polymorphism (SNP) frequencies of prototypic xenobiotic response genes—aryl hydrocarbon receptor (AHR), AHR nuclear translocator (ARNT), AHR repressor (AHRR) — and a prototypic immune response gene, protein tyrosine phosphatase, non-receptor type 22 (PTPN22), in subjects from the Environmental Polymorphism Registry (EPR).
Methods:
Subjects (n = 3,731) were genotyped for 14 SNPs associated with functional variants of the AHR, ARNT, AHRR, and PTPN22 genes, and their frequencies were compared among African Americans (n = 1,562), Caucasians (n = 1,838), and Hispanics (n = 331) with previously reported data. Of those genotyped, 2,015 EPR subjects completed a Health and Exposure survey. SNPs were assessed via PLINK for associations with IMD, which included those with autoimmune diseases, allergic disorders, asthma, or idiopathic pulmonary fibrosis. Transethnic meta-analyses were performed using METAL and MANTRA approaches.
Results:
ARNT SNP rs11204735 was significantly associated with autoimmune disease by transethnic meta-analyses using METAL (odds ratio, OR [95% confidence interval] = 1.29 [1.08-1.55]) and MANTRA (ORs ranged from 1.29 to 1.30), whereas ARNT SNP rs1889740 showed a significant association with autoimmune disease by METAL (OR = 1.25 [1.06-1.47]). For Caucasian females, PTPN22 SNP rs2476601 was significantly associated with autoimmune disease by allelic association tests (OR = 1.99, [1.30 – 3.04]). In Caucasians and Caucasian males, PTPN22 SNP rs3811021 was significantly associated with IMD (OR = 1.39 [1.12-1.72] and 1.50 [1.12-2.02], respectively) and allergic disease (OR = 1.39 [1.12-1.71], and 1.62 [1.19-2.20], respectively). In the transethnic meta-analysis, PTPN22 SNP rs3811021 was significantly implicated in IMD by METAL (OR = 1.31 [1.10-1.56]), and both METAL and MANTRA suggested that rs3811021 was associated with IMD and allergic disease in males across all three ethnic groups (IMD METAL OR = 1.50 [1.15-1.95]; IMD MANTRA ORs ranged from 1.47 to 1.50; allergic disease METAL OR = 1.58 [1.20-2.08]; allergic disease MANTRA ORs ranged from 1.55 to 1.59).
Conclusions:
Some xenobiotic and immune response gene polymorphisms were shown here, for the first time, to have associations across a broad spectrum of IMD and ethnicities. Our findings also suggest a role for ARNT in the development of autoimmune diseases, implicating environmental factors metabolized by this pathway in pathogenesis. Further studies are needed to confirm these data, assess the implications of these findings, define gene-environment interactions, and explore the mechanisms leading to these increasingly prevalent disorders.
Keywords: ARNT, PTPN22, autoimmune disease, immune-mediated disease, allergic disease, SNP
1. Introduction
Although clinically heterogeneous, immune-mediated diseases (IMD) appear to frequently co-occur and share genetic and environmental risk factors and biological pathways [1-8]. Thus, studying a broader array of disorders linked by shared pathology or mechanisms may allow for greater insight into immune-mediated disease pathogenesis. Genome-wide scans have revealed over 40 autoimmune disease regions that may be targets of natural selection [9]. Many of these targeted regions, including the human leukocyte antigen (HLA) region, ARHGAP31-CD80, PTPN22, TNFSF4, TNIP1, and TYK2, are shared among multiple diseases [9].
In response to environmental exposures, organisms have evolved sophisticated means of mitigating toxicity of potentially harmful xenobiotics, including a class of evolutionarily conserved xenobiotic response genes activated by environmental exposures to polycyclic aromatic hydrocarbons (PAH). Many of these PAH are chemically stable, persist in the environment, accumulate to toxic levels in living organisms, and may contribute to disease pathogenesis or progression [10]. Genes associated with the metabolism of PAH including the aryl hydrocarbon receptor (AHR), AHR nuclear translocator (ARNT), and AHR repressor (AHRR), which contain polymorphic variants that influence host responses to these chemicals [11]. In addition to regulating xenobiotic metabolism to compounds such as dioxin [12], transcriptional activation by AHR has been linked with various estrogenic effects and associated endocrine disorders, cell cycle control and tumor development, and autoinflammatory disorders [13-19].
Other than the HLA region, PTPN22 (protein tyrosine phosphatase, non-receptor type 22), called the archetypal non-HLA autoimmunity gene [20], is associated with the largest number of autoimmune diseases, including systemic lupus erythematosus, rheumatoid arthritis, Crohn’s disease, type 1 diabetes, vitiligo, myasthenia gravis, autoimmune thyroid disease, and ulcerative colitis [9]. PTPN22 encodes a cytosolic protein tyrosine phosphatase that influences multiple immune-regulatory pathways associated with T and B cell activation and Treg/Th17-mediated immune suppression [19, 21]. Polymorphisms of the PTPN22 gene—particularly the R620W missense allele encoded by an 1858 C>T single-nucleotide polymorphism (SNP; rs2476601)— are associated with susceptibility to several autoimmune disorders [22-26], suggesting that common mechanisms may be involved. PTPN22 is subject to pathogen-driven selection in at least one autoimmune disease, Crohn’s disease [9, 27].
We hypothesized that genetic polymorphisms of xenobiotic response and immune response genes influence susceptibility to environmental exposures and impact associated toxicities and disease development in many immune-mediated phenotypes in a transethnic fashion. While most association studies have been performed in populations of European descent, we took advantage of the diverse racial and ethnic population in a database and repository in the National Institute of Environmental Health Sciences (NIEHS) called the Environmental Polymorphisms Registry (EPR) [28-30] to perform a transethnic investigation of SNPs of the AHR, ARNT, AHRR, and PTPN22 to assess associations across a wide spectrum of autoimmune and allergic diseases, defined here as IMD, to determine whether these SNPs influence IMDs in a broad way and to determine whether there are any patterns in their SNP frequencies across race and gender.
2. Material and methods
2.1. Study participants
From 2005–2011, more than 16,000 subjects representing different ethnic, gender, and age groups were enrolled into the EPR. The EPR placed a special focus on enrolling members of minority populations in North Carolina [31]. The EPR was designed to enable studies of candidate genes and associated regulatory domains implicated in many common diseases (e.g. IMD and endocrine disorders) with known or suspected environmental risk factors [32, 33].
2.1.1. Study cohorts
Of the 3,731 participants genotyped for the SNPs of interest, 2,015 contributed data to the EPR Health and Exposure Survey administered between 2013 and 2014. The Health and Exposure survey was a questionnaire derived from validated sources that asked participants to provide information on health, disease, occupation, family health, lifestyle, home life, mood and fatigue. Subjects were divided into three groups based on their self-reported disease information, as follows:
IMD defined by presence of at least one of the following 17 conditions: allergic rhinitis, hay fever, or seasonal allergies; allergies or allergic reactions (other than seasonal); idiopathic pulmonary fibrosis; asthma; multiple sclerosis; celiac disease; Crohn’s disease; ulcerative colitis; scleroderma; lupus; Sjogren’s disease; myositis; rheumatoid arthritis; psoriasis; hypothyroidism; pernicious anemia; or hyperthyroidism.
Autoimmune disease defined by the presence of at least one of the following 13 conditions: multiple sclerosis, celiac disease, Crohn’s disease, ulcerative colitis, scleroderma, lupus, Sjogren’s disease, myositis, rheumatoid arthritis, psoriasis, hypothyroidism, pernicious anemia, or hyperthyroidism.
Allergic disease defined by the presence of at least one of the following conditions: Allergic rhinitis, hay fever, seasonal allergies or allergies or allergic reactions other than seasonal.
Participants with none of these 17 conditions served as controls for IMD (n = 870).
2.2. DNA extraction
Participant DNA was extracted from whole blood using the Gentra Autopure robot (Qiagen Inc., Valencia, CA) per the manufacturer’s protocol [34]. DNA concentrations were determined using a DTX 880 plate reader (Beckman Coulter, Jersey City, NJ) and normalized to 50 ng/μl A subset of EPR samples from the earliest recruiting efforts was isolated manually using a similar protocol and reagents. Purified DNA was stored at −80°C prior to use.
2.3. SNP selection
The custom program SNPselector was used to generate lists of SNPs spanning +/−5 kilobases of each target gene for possible inclusion in this study [35]. When feasible, SNPs were selected based on reported or predicted effects on gene function. At least one HapMap SNP (International HapMap Consortium 2005) was used to tag each haplotype bin. Fifteen SNPs were chosen; however, PTPN22 SNP rs34462362 was excluded from the analysis because it had a minor allele frequency (MAF) of 0 in the EPR population. Table S1 summarizes the fourteen SNP markers of the four genes (AHR, ARNT, AHRR, and PTPN22) studied, along with their corresponding major and minor allelic variants and reported phenotypic associations.
2.4. SNP genotyping and analysis
Genotyping was performed in triplicate as part of two custom 384-plex multiplex oligo pools for analysis of SNP assays using the Illumina BeadXpress system following the manufacturer’s instructions (Illumina, Inc., San Diego, CA). Illumina’s internal controls, including allele-specific extension, PCR uniformity, gender genotype, gap extension efficiency, annealing specificity, and hybridization, were used to assure data quality and fidelity.
Purified DNA was plated by the NIEHS Molecular Genetics Core Facility and processed through several activation and ligation steps followed by PCR with universal primers and subsequent hybridization to Illumina genotyping beads. After hybridization and washing, plates were scanned on the BeadXpress® reader. Raw data were normalized across samples using Illumina's BeadStudio software suite®, and SNP genotype calls were made using genotyping cluster files generated by BeadStudio. Seven samples with a call rate below 50% were excluded from the analysis. Genotyping duplicate reproducibility was 99.76%, with a call rate of 95.8%. Chi-square analysis was used to calculate p values (0.05 level) for comparisons of genotype frequencies between different ethnic groups in this exploratory study. MAFs were calculated by race and ethnicity and were compared to the Genome Aggregation Database (gnomAD) of the Broad Institute populations [36].
2.5. Statistical analyses
We analyzed 14 SNPs for association with the three main phenotypes derived from questions in the EPR Health and Exposure Survey: IMD, autoimmune disease, and allergic disease.
We performed preliminary descriptive allelic association analyses using PLINK software [37]. These analyses provided the input for meta-analyses to determine whether the initial findings from the three ethnic cohorts could be combined using METAL software [38]. We then utilized the METAL results as the basis for further analyses using MANTRA software (in a Bayesian approach [39]). METAL and MANTRA analyses were limited to the 7 SNPs with MAF ≥ 0.05. We also performed sex-specific analyses.
All p values (for PLINK and METAL analysis) were examined using the Holm-Bonferroni sequential procedure [40], which has more power than the conservative Bonferroni procedure. The Holm-Bonferroni adjusted p values allowed for either 14 SNPs (in the basic allelic association tests) or 7 SNPs (in METAL analyses).
2.6. Descriptive and association analyses using PLINK
Separate allelic association analyses (by race and the three phenotype groups [IMD, autoimmune disease, and allergic disease]) were run using PLINK software; SNP map data were downloaded from the UCSC Genome Browser [Human Feb. 2009 (GRCh37/hg19) Assembly].
2.7. Meta-analysis using METAL and MANTRA
Two methods of meta-analysis were used to combine association results for the samples from African American, Caucasian, and Hispanic subjects: METAL software [38, 41], and MANTRA [39]. Both methods incorporate testing for heterogeneous allele effects among different populations. For meta-analyses we included only the seven SNPs with MAF ≥ 0.05. MANTRA software is designed for transethnic meta-analysis of SNP association data [39]. It employs a Bayesian Partition Model to allow for genetic similarity between closely related populations when estimating allelic effects across multiple populations. This method produces more refined estimates of association effects while allowing for potential heterogeneity of effects between clusters.
MANTRA produces Bayesian estimates of association for each population as well as odds ratios and posterior standard deviations [42]. Stephens and Balding [43] provide a formula for estimating the upper limit of a Bayes factor given a p value satisfying the condition p < 1/e, where e ~ 2.72: BF < −1/[e p ln(p)]. Approximate equal-tailed 95% credible intervals for the ORs were calculated using the posterior standard deviations.
The upper limit log10 Bayes factor (log10BF) value of 1.018 corresponds to the first-test Holm-Bonferroni p value (0.0071) when testing the 7 SNPs appropriate for analysis.
2.8. Sex-specific Analyses
The analyses in sections 2.5-2.7 (basic PLINK allelic association testing followed by meta-analysis using METAL and MANTRA software) were repeated using single-sex samples (i.e., African American males, African American females, Caucasian males, Caucasian females, Hispanic males, and Hispanic females). The same thresholds (Holm-Bonferroni p values and log10BF value) were used to evaluate results.
3. Results
3.1. Selected population characteristics and associations
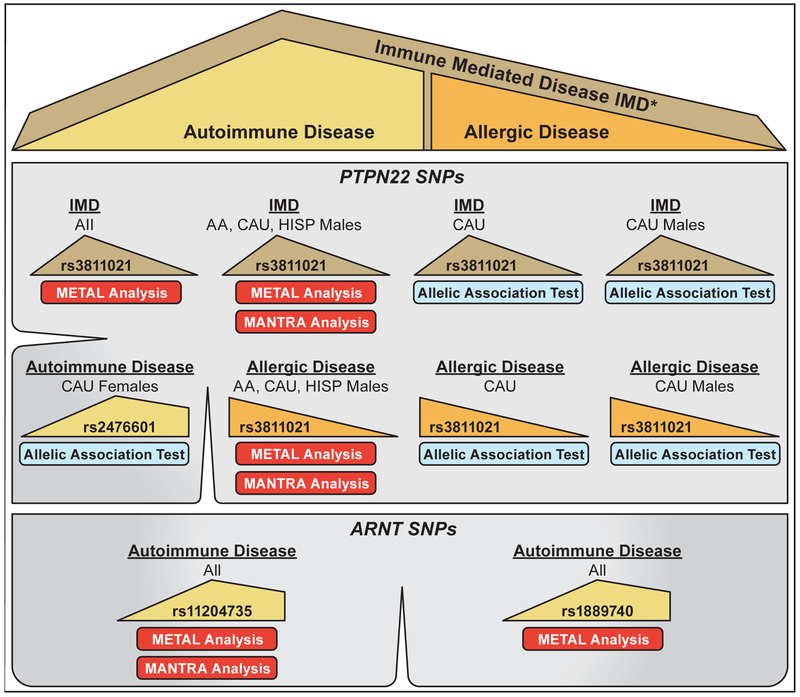
Of the 2,015 EPR participants with EPR Health and Exposure Survey data and SNP results, 749 (37%) were African American non-Hispanic, 1,119 (56%) were Caucasian non-Hispanic, and 147 (7%) were Hispanic; similarly, 1,158 (57%) were female and 857 (43%) were male (Table 1). Frequencies of the individual 17 diseases stratified by race/ethnicity are in Table S2. A summary of the key findings of SNP associations and meta-analyses for all phenotypes is shown in Figure 1.
Table 1.
Selected characteristics of the genotyped and phenotyped cohorts
| African American | Caucasian | Hispanic | Total | ||
|---|---|---|---|---|---|
| Genotyped Sample, n (%) | 1,562 (41.9) | 1,838 (49.3) | 331 (8.9) | 3,731 | |
| Phenotyped Sample, n (%) | 749 (37.2) | 1,119 (55.5) | 147 (7.3) | 2015 | |
| Female n (%) | 494 (66.0) | 577 (51.6) | 87 (59.2) | 1,158 (57.5) | |
| Immune-mediated disease | Case | 390 (52.1) | 680 (60.8) | 75 (51.0) | 1,145 (56.8) |
| Control | 359 (47.9) | 439 (39.2) | 72 (49.0) | 870 (43.2) | |
| Autoimmune disease a | Case | 112 (15.0) | 216 (19.3) | 28 (19.1) | 356 (17.7) |
| Control | 636 (85.0) | 902 (80.7) | 119 (80.9) | 1,657 (82.3) | |
| Allergic disease b | Case | 308 (41.1) | 580 (51.9) | 54 (36.7) | 942 (46.8) |
| Control | 441 (58.9) | 538 (48.1) | 93 (63.3) | 1,072 (53.2) | |
Two participants were missing information for the autoimmune phenotype.
One participant was missing information for the allergic disease phenotype.
Fig. 1.
Key genetic associations by meta-analyses (using METAL and MANTRA) and allelic association tests (using PLINK). Top figure panel is the key for populations where there is a significant finding. Large ‘tan’ triangles represent the entire Immune-Mediated Disease (IMD) population; ‘yellow’ shape represents the Autoimmune population; and the ‘orange’ triangle represents the Allergic Disease population. *The IMD population includes Autoimmune and Allergic Disease populations as well as asthma and idiopathic pulmonary fibrosis populations.
3.2. Allele frequencies
The distribution of the 14 SNPs of the four genes studied in the EPR sample (Table S1) was stratified based on self-reported ethnic origin (~42% African American, ~49% Caucasian, and ~9% Hispanic) as shown in Table S3. Of note, African American subjects were purposefully over-represented in the EPR study sample (~42% vs. ~22% in the U.S. 2010 North Carolina Census).
Comparisons of MAFs between EPR study subjects and published control populations from gnomAD of the Broad Institute [36] were generally consistent between corresponding ethnic groups (Table S4). These data demonstrate that SNP allele frequencies among these North Carolina EPR populations are generally consistent with and representative of published, ethnically-matched control populations. Seven SNPs had MAF < 0.05 in at least one ethnic group (Table S5).
3.3. Tests of Hardy-Weinberg equilibrium (HWE) and linkage disequilibrium (LD)
We used PLINK to examine SNPs for violations of HWE. Several SNPs were nominally significant in HWE testing among control participants (p values below 0.05) but not in the range of concern (e.g. p < 0.001), and these SNPs did not have associations with the phenotypes under study. The significant HWE p values were as follows: 1) among African Americans with IMD, AHR SNP rs4986826 (p = 0.041) and AHR SNP rs2237298 (p = 0.044); 2) among African Americans with allergic disease, AHR SNP rs4986826 (p = 0.032).
Results of linkage disequilibrium testing (Table S6) revealed relatively strong LD (r≥0.60) in African American and Caucasian groups for PTPN22 SNPs rs2476601 and rs2488457; in Caucasian and Hispanic groups for ARNT SNPs rs1889740 and rs11204735; and in Caucasians for AHR SNPs rs2237298 and rs2158041.
3.4. Association results (PLINK)
Three SNPs had at least one significant allelic association test result for the three disease groups, by race-ethnicity, and in the sub-analyses by sex (Table 2); all were from SNPs of PTPN22 in the Caucasian sample. SNP rs3811021 was associated with both IMD and allergic disease for Caucasians overall and for only male Caucasians. SNP rs2476601 was associated with autoimmune disease for only Caucasian females. Details of allelic association results of significant SNPs for this study are given in Table S7 for IMD, autoimmune, and allergic diseases. Sex-specific allelic association results are given in Table S8.
Table 2.
Allelic association tests reaching significance* in overall or sex-specific analyses, in immune-mediated, autoimmune, and allergic diseases
| African American | Caucasian | Hispanic | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gene/SNP | Sample | OR | L95 | U95 | OR | L95 | U95 | OR | L95 | U95 |
| Immune-mediated disease | ||||||||||
| PTPN22 / rs3811021 | Overall | 1.17 | 0.81 | 1.69 | 1.39a | 1.12 | 1.72 | 1.10 | 0.60 | 2.02 |
| Males | 1.32 | 0.68 | 2.53 | 1.50b | 1.12 | 2.02 | 2.14 | 0.64 | 7.17 | |
| Females | 1.11 | 0.71 | 1.75 | 1.21 | 0.88 | 1.67 | 0.96 | 0.46 | 2.01 | |
| Allergic disease | ||||||||||
| PTPN22 / rs3811021 | Overall | 0.86 | 0.59 | 1.24 | 1.39c | 1.12 | 1.71 | 1.20 | 0.63 | 2.27 |
| Males | 1.32 | 0.66 | 2.64 | 1.62d | 1.19 | 2.20 | 2.00 | 0.53 | 7.55 | |
| Females | 0.70 | 0.44 | 1.11 | 1.14 | 0.84 | 1.55 | 1.07 | 0.51 | 2.28 | |
| Autoimmune disease | ||||||||||
| PTPN22 / rs2476601 | Overall | 0.79 | 0.27 | 2.26 | 1.55 | 1.12 | 2.15 | 0.24 | 0.03 | 1.82 |
| Males | 0.83 | 0.11 | 6.53 | 1.17 | 0.68 | 2.00 | NE | NE | NE | |
| Females | 0.81 | 0.24 | 2.79 | 1.99e | 1.30 | 3.04 | 0.28 | 0.04 | 2.25 | |
SNP = single nucleotide polymorphism; OR = odds ratio; L95 = Lower boundary of 95% confidence interval; U95 = Upper boundary of 95% confidence interval; NE = not estimable.
Boldface indicates significant effect after Holm-Bonferroni correction.
p=0.002;
p=0.007;
p=0.003;
p=0.002;
p=0.001;
3.5. Results of meta-analysis using METAL and MANTRA software
Table 3 provides results for the 3 SNPs that showed significant (METAL) or credible (MANTRA) associations in the three disease groups. METAL delivers a single OR estimate when the racial/ethnic groups are combined, while MANTRA outputs an OR estimate for each ethnic group. METAL (frequentist approach) also produces confidence intervals, whereas MANTRA (Bayesian approach) produces credible intervals. The two approaches tend to agree (significance in one is often credible in the other). METAL indicated that PTPN22 SNP rs3811021 was significantly associated with IMD across the three ethnic groups (OR: 1.31 [1.10-1.56]); the separate MANTRA ORs for African Americans (1.28), Caucasians (1.31), and Hispanics (1.29) were almost identical, but did not reach the credibility threshold (Log10BF =1.02). However, METAL and MANTRA agreed on the association with males and IMD for SNP rs3811021 across the ethnic groups. Similarly, METAL and MANTRA agreed on the association with males for allergic disease and SNP rs3811021 across the ethnic groups. Thus, the meta-analyses extended the association between SNP rs3811021 and Caucasian males from Table 2 to all three ethnic groups.
Table 3.
Comparison of METAL and MANTRA results 1
| METAL | MANTRA | |||||
|---|---|---|---|---|---|---|
| SNP | ORall (95% CI2) | p value | ORAA (95% CrI3) | ORCA (95% CrI) | ORHI (95% CrI) | Log10BF |
| Immune-mediated disease | ||||||
| PTPN22 / rs3811021 | 1.31 (1.10-1.56) | 0.003 | 1.28 (1.03-1.59) | 1.31 (1.09-1.57) | 1.29 (1.06-1.58) | 0.520 |
| male | 1.50 (1.15-1.95) | 0.003 | 1.47 (1.04-2.09) | 1.50 (1.14-1.96) | 1.50 (1.07-2.09) | 1.245 |
| female | 1.15 (0.90-1.47) | 0.260 | 1.15 (0.88-1.51) | 1.16 (0.90-1.49) | 1.16 (0.88-1.52) | −0.176 |
| Autoimmune disease | ||||||
| ARNT / rs11204735 | 1.29 (1.08-1.55) | 0.005 | 1.30 (1.05-1.62) | 1.29 (1.08-1.55) | 1.29 (1.06-1.57) | 1.064 |
| male | 1.35 (0.99-1.85) | 0.062 | 1.38 (0.79-2.40) | 1.35 (0.97-1.87) | 1.12 (0.41-3.06) | 0.153 |
| female | 1.30 (1.04-1.62) | 0.022 | 1.30 (1.00-1.70) | 1.29 (1.02-1.63) | 1.31 (0.99-1.72) | 0.490 |
| ARNT / rs1889740 | 1.25 (1.06-1.47) | 0.009 | 1.23 (1.03-1.48) | 1.25 (1.05-1.48) | 1.25 (1.04-1.50) | 1.000 |
| male | 1.38 (1.02-1.86) | 0.036 | 1.45 (0.95-2.21) | 1.35 (0.99-1.86) | 1.20 (0.44-3.22) | 0.488 |
| female | 1.24 (1.01-1.52) | 0.038 | 1.22 (0.95-1.55) | 1.26 (1.01-1.57) | 1.27 (0.98-1.65) | 0.545 |
| Allergic disease | ||||||
| PTPN22 / rs3811021 | 1.23 (1.03-1.46) | 0.023 | 1.08 (0.73-1.59) | 1.28 (1.04-1.57) | 1.22 (0.90-1.64) | 0.815 |
| male | 1.58 (1.20-2.08) | 0.001 | 1.55 (1.11-2.18) | 1.59 (1.20-2.09) | 1.58 (1.12-2.23) | 1.847 |
| female | 0.99 (0.78-1.26) | 0.936 | 0.91 (0.61-1.35) | 1.02 (0.79-1.32) | 1.00 (0.74-1.36) | −0.338 |
Results are from 7-SNP analysis;
CI = Confidence Interval;
CrI = Credible Interval
SNP = single nucleotide polymorphism; L95 = Lower boundary of 95% confidence interval; U95 = Upper boundary of 95% confidence interval; ORall = odds ratio for all groups combined; ORAA = odds ratio for African American cohort, ORCA = odds ratio for Caucasian cohort; ORHI = odds ratio for Hispanic cohort; log10BF = log10 Bayes factor.
The bold entries show results that were significant after Holm-Bonferroni correction (METAL) or credible (Bayesian perspective) considering the upper limit of the log10BF (MANTRA).
The two ARNT SNPs showing association with autoimmune disease (Table 3) are not independent findings, since these two SNPs (rs11204735 and rs1889740) showed relatively high LD in Caucasian (r=0.84) and Hispanic (r=0.78) samples, and moderate LD (r=0.50) in African Americans (Table S6). METAL revealed a significant OR (1.29 [1.08-1.55]) across the 3 ethnic groups for rs11204735, and similarly (1.25 [1.06-1.47]) for rs1889740. MANTRA agreed for rs11204735, yielding three similar and credible ORs (Log10BF=1.064) for the three ethnic groups; the MANTRA ORs for rs1889740 were similar (log10BF=1.00, just shy of the 1.018 threshold).
Details of the METAL analyses, overall and by sex, of significant SNPs for this study are given in Table S9. Corresponding detailed results for MANTRA are in Table S10.
4. Discussion
IMD are a clinically heterogeneous group of disorders thought to result from interactions of genetic and environmental risk factors and to vary in prevalence, clinical presentation and responses to treatment in different racial and ethnic groups [44-49]. Despite their heterogeneity, increasing data supports the concept that IMD may share risk factors and pathogenetic mechanisms across ethnic groups and phenotypes, and that explorations of the IMD group as a whole may reveal novel associations (1-3). By combining individuals of different races and ethnicities with multiple diseases into the broad IMD phenotype, we searched for associations between SNPs of a gene known for its association with multiple autoimmune diseases (PTPN22) and SNPs of genes (AHR, ARNT, and AHRR) known for their associations with multiple environmental factors, in order to identify SNPs and possible pathways that might be shared within this broad range of immunologic disorders. By doing so, we identified for the first-time polymorphisms of PTPN22 transethnically associated with IMD, and those of ARNT transethnically associated with autoimmune disease.
We found that PTPN22 SNP rs2476601, which has been associated with multiple autoimmune diseases in GWAS studies [20, 50, 51], was significantly associated with autoimmune disease in Caucasian females (PLINK allelic association test; OR = 1.988), as expected given the reports linking this SNP to autoimmunity generally [20, 22, 24, 25]. The PTPN22-R620W variant protein (caused by the PTPN22-C1858T change of rs2476601) is likely involved in multiple stages of autoimmunity pathogenesis involving T cells, B cells, and myeloid cells. In T cells, for instance, the R-to-W substitution diminishes the interaction of PTPN22 and C-terminal Src kinase (Csk), a T cell receptor (TCR) signaling regulator, which leads to altered TCR signaling and autoimmune disease through many hypothesized though still unclear mechanisms [52]. In addition, in myeloid cells, experimental data have revealed that PTPN22-R620W could lead to defects in toll-like receptor (TLR) signaling through reduced K63-linked autopolyubiquination of tumor necrosis factor receptor-associated factor 3 (TRAF3) [53], which also interacts with Csk, resulting in upregulation of type 1 interferon (IFN) and in turn possible autoimmunity through defective myeloid cell capacity for type 1 IFN-dependent suppression of inflammation [52].
PTPN22 SNP rs3811021 has been associated with rheumatoid arthritis and systemic lupus erythematosus in Han Chinese [54] and urticaria in Caucasians [55] by focused arrays, but not by GWAS studies. Not taking sex into account, using METAL analysis, we found that risk allele A of PTPN22 SNP (rs3811021) was significantly implicated in the IMD group, but using MANTRA analysis, it did not reach the conclusive threshold. When analyzing sex-specific samples, both the METAL and MANTRA results indicated that the PTPN22 SNP rs3811021 associates with IMD in males across the three ethnic groups. We also found that the PTPN22 SNP rs3811021 was significantly implicated for males only in the allergy-related disease group, using both the METAL and MANTRA meta-analyses.
AHR pathway SNPs have not been extensively studied in IMD and no GWAS studies in IMD have been reported. We suspect the reason these ARNT SNPs have not been previously identified in GWAS partially relates to the unique IMD and combined autoimmune disease phenotypes we are focused on along with the transethnic analyses in this paper, which have not been previously evaluated in GWAS studies. Our results suggest that there may be a broad role for ARNT’S association with autoimmune disease because both of the ARNT SNPs examined showed an association with autoimmune disease. This may be because genes encoding proteins of the aryl hydrocarbon receptor pathway (e.g., AHR, ARNT, and AHRR) affect host responses to environmental toxins by inducing cytochrome P450 metabolic enzymes (e.g., CYP1A1) [56]. AHR also has a Per-Arnt-Sim (PAS) domain, which is broadly conserved among proteins across taxonomic kingdoms. PAS can bind and detect xenobiotic and endogenous small molecules, including polyaromatic hydrocarbons and cellular metabolites. Hence, they are found in pathways that regulate environmental change responses, which includes the dioxin response pathways in mammals [57]. Upon ligand binding, the cytosolic AHR migrates to the nucleus with the aid of ARNT and mediates transcriptional activation by xenobiotic response elements binding to target gene promoters. Activated AHR pathways, in addition to their established roles in regulating cell proliferation, development, and signaling in response to dioxin-type compounds, suppress innate and acquired immune responses presumptively by inducing regulatory T cells (Treg) and T helper type 17 cells (Th17) [18, 58]. In a murine model of multiple sclerosis (i.e., experimental autoimmune encephalomyelitis), an Ahr gene knock-out was associated with reduced Th17 immune suppressor activity, suggesting a link with fundamental pathways of innate and acquired immune activation [18]. Moreover, in murine models, Ahr affected regulatory T cells (Treg and Th17 pathways) in a ligand-specific manner, with the capacity to either attenuate or exacerbate autoimmune pathology [18]. Additionally, in the murine experimental autoimmune encephalomyelitis model, gallic acid promoted ARNT-AHR interactions and decreased inflammatory pathologic responses [59]. AHR-mediated immunosuppressive effects may be associated with PTPN22 activity, which also plays a regulatory role in immune activation and induction of Th17 regulatory pathways [18].
Environmental factors may have a role in inducing epigenetic changes that have been linked to cell types and tissues from individuals with a variety of autoimmune diseases including systemic lupus erythematosus, rheumatoid arthritis, and Sjögren’s syndrome [60]. DNA and histone modifications act as important signaling mediators between the environment and the genome and can regulate transcription, replication and a number of other intracellular processes [60]. Although little is known about the role of epigenetics for PTPN22 and ARNT, the methylation patterns of PTPN22 are altered in certain cancers [61], in other diseases such as antiphospholipid syndrome [62], and smoking [63]. Methylation patterns in ARNT, also known as HIF1β, are altered in certain cancers such as gastrointestinal stromal tumor [64]. DNA alterations, along with their specific environmental associates and effects, are important topics for further study in IMD.
The analysis of gene polymorphisms that modulate associations between phenotypes, such as IMD, and environmental exposures requires an understanding of the distributions of those polymorphisms in reference populations and the factors that impact them. Differences in MAFs of SNPs that we found among racial/ethnic groups were associated with a broad range of autoimmune/immune-related and allergic diseases, which suggests the presence of selective pressures of pathogenic and/or environmental factors. We, therefore, analyzed the EPR for MAFs that we thought might be related to IMD. Our examination of MAFs for the SNPs used in this study corroborated findings from the Broad Institute’s gnomAD cohort [36, 65]. We observed lower MAFs (in most cases 20% less) in African American populations compared to Caucasian and Hispanic cohorts for AHR SNP rs2158041, ARNT SNP rs11204735, and PTPN22 SNPs rs3811021 and rs2488457; and higher MAFs (~30% more) for the African American cohort than for Caucasian or Hispanic cohorts for AHR SNP rs2066853, AHRR SNP rs35008248, and PTPN22 SNP rs2040041. We observed that there are several minor alleles associated with functional variants of the AHR pathway and PTPN22 genes that were either under-represented or over-represented among African American subjects compared to Caucasian and Hispanic EPR populations. These findings, consistent with corresponding SNP MAFs reported for the gnomAD African American populations, suggest that selective pressures affecting autoimmune, immune-related, and allergic diseases differ among races and ethnicities in a consistent pattern.
Our findings should be interpreted in the context of the limitations of our study, which include limited power for some phenotypes, probable reduced accuracy of self-reported physician diagnoses compared to documented physician diagnoses, and that these association studies are descriptive and do not include mechanistic relationships.
5. Conclusions
For the first time, SNPs have been linked to a broad range of IMD in a transethnic fashion, supporting our hypothesis that IMD may share risk factors and pathogenic mechanisms. Despite limited statistical power for some phenotypes, we found an overall association in all three ethnic groups (African American, Caucasian, and Hispanic) for ARNT SNP rs11204735 and ARNT SNP rs1889740 with autoimmune diseases, suggesting a broad role for ARNT in processes underlying autoimmune diseases. For autoimmune disease, PTPN22 SNP rs2476601 was significantly associated with Caucasian females. PTPN22 SNP rs3811021 was associated, in males across all three cohorts, with IMD and allergic disease. These results suggest that both PTPN22 and ARNT are linked to immune-related processes across a broad range of diseases. Further studies need to be undertaken to confirm and expand on these studies and assess the related genetic-by-environmental risk factors, and the mechanisms that lead to these disorders.
Supplementary Material
Highlights.
Xenobiotic and immune response SNPs associate with immune-mediated diseases transethnically
ARNT polymorphisms associate with autoimmune diseases
PTPN22 SNP associations with immune-mediated diseases vary by ethnicity and gender
Acknowledgements
The authors thank Drs. Elaine Remmers and Paul Wade for their critical reading of the manuscript. The authors also thank members of the NIEHS Molecular Genetics Core Laboratory for their assistance in data collection and analysis, Nathaniel MacNell for his help with genetic association analyses, Paul Cacioppo for assistance with graphics, and Lisa Maroski and Wayne Pereanu for editorial assistance.
Funding Support statement
This research was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences under project Z01 ES101074 and contract HHSN273201600011C to Social and Scientific Systems, Inc.
Footnotes
Conflicts of interest
The authors have no known conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].David T, Ling SF, Barton A. Genetics of immune-mediated inflammatory diseases. Clin Exp Immunol, 2018;193:3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Robinson D Jr., Hackett M, Wong J, Kimball AB, Cohen R, Bala M et al. Co-occurrence and comorbidities in patients with immune-mediated inflammatory disorders: an exploration using US healthcare claims data, 2001-2002. Curr Med Res Opin, 2006;22:989–1000. [DOI] [PubMed] [Google Scholar]
- [3].Theofilopoulos AN, Kono DH, Baccala R. The multiple pathways to autoimmunity. Nature immunology, 2017;18:716–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Miller FW. Environmental agents and autoimmune diseases. Adv Exp Med Biol, 2011;711:61–81. [DOI] [PubMed] [Google Scholar]
- [5].Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. The New England journal of medicine, 2002;347:911–20. [DOI] [PubMed] [Google Scholar]
- [6].Parks CG, Miller FW, Pollard KM, Selmi C, Germolec D, Joyce K et al. Expert panel workshop consensus statement on the role of the environment in the development of autoimmune disease. Int J Mol Sci, 2014;15:14269–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bayry J, Radstake TR. Immune-mediated inflammatory diseases: progress in molecular pathogenesis and therapeutic strategies. Expert Rev Clin Immunol, 2013;9:297–9. [DOI] [PubMed] [Google Scholar]
- [8].Kuek A, Hazleman BL, Ostor AJ. Immune-mediated inflammatory diseases (IMIDs) and biologic therapy: a medical revolution. Postgrad Med J, 2007;83:251–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ramos PS, Shedlock AM, Langefeld CD. Genetics of autoimmune diseases: insights from population genetics. Journal of human genetics, 2015;60:657–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Alegbeleye OO, Opeolu BO, Jackson VA. Polycyclic Aromatic Hydrocarbons: A Critical Review of Environmental Occurrence and Bioremediation. Environ Manage, 2017;60:758–83. [DOI] [PubMed] [Google Scholar]
- [11].Abel J, Haarmann-Stemmann T. An introduction to the molecular basics of aryl hydrocarbon receptor biology. Biol Chem, 2010;391:1235–48. [DOI] [PubMed] [Google Scholar]
- [12].Furness SG, Lees MJ, Whitelaw ML. The dioxin (aryl hydrocarbon) receptor as a model for adaptive responses of bHLH/PAS transcription factors. FEBS Lett, 2007;581:3616–25. [DOI] [PubMed] [Google Scholar]
- [13].Das SK, Sharma NK, Chu WS, Wang H, Elbein SC. Aryl hydrocarbon receptor nuclear translocator (ARNT) gene as a positional and functional candidate for type 2 diabetes and prediabetic intermediate traits: Mutation detection, case-control studies, and gene expression analysis. BMC Med Genet, 2008;9:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sangrajrang S, Sato Y, Sakamoto H, Ohnami S, Laird NM, Khuhaprema T et al. Genetic polymorphisms of estrogen metabolizing enzyme and breast cancer risk in Thai women. Int J Cancer, 2009;125:837–43. [DOI] [PubMed] [Google Scholar]
- [15].Chen D, Tian T, Wang H, Liu H, Hu Z, Wang Y et al. Association of human aryl hydrocarbon receptor gene polymorphisms with risk of lung cancer among cigarette smokers in a Chinese population. Pharmacogenet Genomics, 2009;19:25–34. [DOI] [PubMed] [Google Scholar]
- [16].Figueroa JD, Malats N, García-Closas M, Real FX, Silverman D, Kogevinas M et al. Bladder cancer risk and genetic variation in AKR1C3 and other metabolizing genes. Carcinogenesis, 2008;29:1955–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Shin A, Shrubsole MJ, Rice JM, Cai Q, Doll MA, Long J et al. Meat intake, heterocyclic amine exposure, and metabolizing enzyme polymorphisms in relation to colorectal polyp risk. Cancer Epidemiol Biomarkers Prev, 2008;17:320–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E et al. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature, 2008;453:65–71. [DOI] [PubMed] [Google Scholar]
- [19].Veldhoen M, Duarte JH. The aryl hydrocarbon receptor: fine-tuning the immune-response. Curr Opin Immunol, 2010;22:747–52. [DOI] [PubMed] [Google Scholar]
- [20].Stanford SM, Bottini N. PTPN22: the archetypal non-HLA autoimmunity gene. Nature reviews Rheumatology, 2014;10:602–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].de St Groth BF. Regulatory T-cell abnormalities and the global epidemic of immuno-inflammatory disease. Immunol Cell Biol, 2012;90:256–9. [DOI] [PubMed] [Google Scholar]
- [22].Zoledziewska M, Perra C, Orru V, Moi L, Frongia P, Congia M et al. Further evidence of a primary, causal association of the PTPN22 620W variant with type 1 diabetes. Diabetes, 2008;57:229–34. [DOI] [PubMed] [Google Scholar]
- [23].Huang JJ, Qiu YR, Li HX, Sun DH, Yang J, Yang CL. A PTPN22 promoter polymorphism −1123G>C is associated with RA pathogenesis in Chinese. Rheumatology international, 2012;32:767–71. [DOI] [PubMed] [Google Scholar]
- [24].Carlton VE, Hu X, Chokkalingam AP, Schrodi SJ, Brandon R, Alexander HC et al. PTPN22 genetic variation: evidence for multiple variants associated with rheumatoid arthritis. American journal of human genetics, 2005;77:567–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Thompson SD, Sudman M, Ramos PS, Marion MC, Ryan M, Tsoras M et al. The susceptibility loci juvenile idiopathic arthritis shares with other autoimmune diseases extend to PTPN2, COG6, and ANGPT1. Arthritis and rheumatism, 2010;62:3265–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Provenzano C, Ricciardi R, Scuderi F, Maiuri MT, Maestri M, La Carpia F et al. PTPN22 and myasthenia gravis: replication in an Italian population and meta-analysis of literature data. Neuromuscular disorders : NMD, 2012;22:131–8. [DOI] [PubMed] [Google Scholar]
- [27].Cagliani R, Pozzoli U, Forni D, Cassinotti A, Fumagalli M, Giani M et al. Crohn's disease loci are common targets of protozoa-driven selection. Molecular biology and evolution, 2013;30:1077–87. [DOI] [PubMed] [Google Scholar]
- [28].Chulada PC, Vahdat HL, Sharp RR, DeLozier TC, Watkins PB, Pusek SN et al. The Environmental Polymorphisms Registry: a DNA resource to study genetic susceptibility loci. Human genetics, 2008;123:207–14. [DOI] [PubMed] [Google Scholar]
- [29].NIEHS Environmental Polymorphisms Registry - Main Site; http://dnaregistry.niehs.nih.gov/. 2019.
- [30].NIEHS Environmental Polymorphisms Registry - Clinical Study; https://www.niehs.nih.gov/research/clinical/studies/epr/index.cfm. 2019.
- [31].Chulada PC, Vainorius E, Garantziotis S, Burch LH, Blackshear PJ, Zeldin DC. The Environmental Polymorphism Registry: a unique resource that facilitates translational research of environmental disease. Environ Health Perspect, 2011;119:1523–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Gale SC, Gao L, Mikacenic C, Coyle SM, Rafaels N, Murray Dudenkov T et al. APOepsilon4 is associated with enhanced in vivo innate immune responses in human subjects. The Journal of allergy and clinical immunology, 2014;134:127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Jewell CM, Katen KS, Barber LM, Cannon C, Garantziotis S, Cidlowski JA. Healthy glucocorticoid receptor N363S carriers dysregulate gene expression associated with metabolic syndrome. American journal of physiology Endocrinology and metabolism, 2016;311:E741–E8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Automated purification of DNA from 8 or 16 samples of fresh or frozen whole blood on the Autopure LS®; https://www.qiagen.com/us/resources/download.aspx?id=689194bd-f646-4b8d-a0e9-193f8b6503b7&lang=en. 2009.
- [35].Xu H, Gregory SG, Hauser ER, Stenger JE, Pericak-Vance MA, Vance JM et al. SNPselector: a web tool for selecting SNPs for genetic association studies. Bioinformatics, 2005;21:4181–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature, 2016;536:285–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].PLINK software; http://pngu.mgh.harvard.edu/~purcell/plink/. 2019.
- [38].METAL software; http://csg.sph.umich.edu/abecasis/Metal/, 2019.
- [39].Morris AP Transethnic meta-analysis of genomewide association studies. Genetic epidemiology, 2011;35:809–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Holm S A Simple Sequentially Rejective Multiple Test Procedure. Scand J Stat, 1979;6:65–70. [Google Scholar]
- [41].Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics, 2010;26:2190–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Sawcer S Bayes factors in complex genetics. European journal of human genetics :EJHG, 2010;18:746–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Stephens M, Balding DJ. Bayesian statistical methods for genetic association studies. Nature reviews Genetics, 2009;10:681–90. [DOI] [PubMed] [Google Scholar]
- [44].Bae SC, Fraser P, Liang MH. The epidemiology of systemic lupus erythematosus in populations of African ancestry: a critical review of the "prevalence gradient hypothesis". Arthritis and rheumatism, 1998;41:2091–9. [DOI] [PubMed] [Google Scholar]
- [45].Goulielmos GN, Zervou MI, Vazgiourakis VM, Ghodke-Puranik Y, Garyfallos A, Niewold TB. The genetics and molecular pathogenesis of systemic lupus erythematosus (SLE) in populations of different ancestry. Gene, 2018;668:59–72. [DOI] [PubMed] [Google Scholar]
- [46].Drake KA, Galanter JM, Burchard EG. Race, ethnicity and social class and the complex etiologies of asthma. Pharmacogenomics, 2008;9:453–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ortega VE, Meyers DA. Implications of population structure and ancestry on asthma genetic studies. Curr Opin Allergy Clin Immunol, 2014;14:381–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wegienka G, Johnson CC, Zoratti E, Havstad S. Racial differences in allergic sensitization: recent findings and future directions. Curr Allergy Asthma Rep, 2013;13:255–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ortega VE, Meyers DA. Pharmacogenetics: implications of race and ethnicity on defining genetic profiles for personalized medicine. J Allergy Clin Immunol, 2014;133:16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Padyukov L, Seielstad M, Ong RT, Ding B, Ronnelid J, Seddighzadeh M et al. A genome-wide association study suggests contrasting associations in ACPA-positive versus ACPA-negative rheumatoid arthritis. Ann Rheum Dis, 2011;70:259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Bowes J, Loehr S, Budu-Aggrey A, Uebe S, Bruce IN, Feletar M et al. PTPN22 is associated with susceptibility to psoriatic arthritis but not psoriasis: evidence for a further PsA-specific risk locus. Ann Rheum Dis, 2015;74:1882–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Bottini N, Peterson EJ. Tyrosine phosphatase PTPN22: multifunctional regulator of immune signaling, development, and disease. Annu Rev Immunol, 2014;32:83–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Wang Y, Shaked I, Stanford SM, Zhou W, Curtsinger JM, Mikulski Z et al. The autoimmunity-associated gene PTPN22 potentiates toll-like receptor-driven, type 1 interferon-dependent immunity. Immunity, 2013;39:111–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Xue L, Pan C, Gu Z, Zhao S, Han B, Liu W et al. Genetic heterogeneity of susceptibility gene in different ethnic populations: refining association study of PTPN22 for Graves' disease in a Chinese Han population. PLoS One, 2013;8:e84514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Brzoza Z, Grzeszczak W, Rogala B, Trautsolt W, Moczulski D. PTPN22 polymorphism presumably plays a role in the genetic background of chronic spontaneous autoreactive urticaria. Dermatology, 2012;224:340–5. [DOI] [PubMed] [Google Scholar]
- [56].Chiaro CR, Patel RD, Marcus CB, Perdew GH. Evidence for an aryl hydrocarbon receptor-mediated cytochrome p450 autoregulatory pathway. Mol Pharmacol, 2007;72:1369–79. [DOI] [PubMed] [Google Scholar]
- [57].McIntosh BE, Hogenesch JB, Bradfield CA. Mammalian Per-Arnt-Sim proteins in environmental adaptation. Annu Rev Physiol, 2010;72:625–45. [DOI] [PubMed] [Google Scholar]
- [58].Marshall NB, Kerkvliet NI. Dioxin and immune regulation: emerging role of aryl hydrocarbon receptor in the generation of regulatory T cells. Ann N Y Acad Sci, 2010;1183:25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Abdullah A, Maged M, Hairul-Islam MI, Osama IA, Maha H, Manal A et al. Activation of aryl hydrocarbon receptor signaling by a novel agonist ameliorates autoimmune encephalomyelitis. PLoS One, 2019;14:e0215981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Karagianni P, Tzioufas AG. Epigenetic perspectives on systemic autoimmune disease. J Autoimmun, 2019:102315. [DOI] [PubMed] [Google Scholar]
- [61].Deng J, Zhang J, Wang C, Wei Q, Zhou D, Zhao K. Methylation and expression of PTPN22 in esophageal squamous cell carcinoma. Oncotarget, 2016;7:64043–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Weeding E, Coit P, Yalavarthi S, Kaplan MJ, Knight JS, Sawalha AH. Genome-wide DNA methylation analysis in primary antiphospholipid syndrome neutrophils. Clin Immunol, 2018;196:110–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Suter M, Ma J, Harris A, Patterson L, Brown KA, Shope C et al. Maternal tobacco use modestly alters correlated epigenome-wide placental DNA methylation and gene expression. Epigenetics, 2011;6:1284–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Hu F, Li H, Liu L, Xu F, Lai S, Luo X et al. Histone demethylase KDM4D promotes gastrointestinal stromal tumor progression through HIF1beta/VEGFA signalling. Mol Cancer, 2018;17:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Genome Aggregation Database (gnomAD); https://gnomad.broadinstitute.org/about. 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.