Abstract
It is estimated that up to 10% of proteins in eukaryotes require zinc for their function. While the majority of these proteins are located in the nucleus and cytosol, a small subset is secreted from cells or is located within an intracellular compartment. As many of these compartmentalized metalloproteins fold to their native state and bind their zinc cofactor inside an organelle, cells require mechanisms to maintain a supply of zinc to these compartments even under conditions of zinc deficiency. At the same time intracellular compartments can also be the site for storing zinc ions, which then can be mobilized when needed. In this review, we highlight insight that has been obtained from yeast models about how zinc homeostasis is maintained in the secretory pathway and vacuole.
Graphical Abstract
Introduction
In a zinc-replete environment up to 14% of zinc proteins are secreted from cells or are localized to organelles [1]. As some of these proteins fold to their native state and bind zinc within the lumen of an organelle, cells need to maintain an adequate supply of zinc ions within these organelles for the activation of these proteins. Organelles are also sites for the storage of zinc ions and where labile zinc ions accumulate for more specialized functions (Table 1). In some eukaryotes, zinc ions function as intracellular secondary messengers [2,3]. In these organisms, zinc ions are held in reserve in the endoplasmic reticulum or other intracellular vesicles until the appropriate signal triggers their release into the cytosol [4–6]. New studies in Arabidopsis have also revealed that zinc ions travel between cells to the xylem via a network of interconnected endoplasmic reticuli under conditions of zinc deficiency [7]. Thus, in some organisms, organelles may provide routes for the long distance transport of zinc ions.
Table 1.
Intracellular compartments that contain high concentrations of zinc ions
| Zinc storage | ||
|---|---|---|
| Intracellular compartment with high zinc accumulation | Organisms, cell, or tissue type | Reference |
| Vacuole |
Saccharomyces cerevisiae Cryptococcus neoformans Toxoplasma gondii Suillus luteus Arabidopsis halleri Thlaspi caerulescens Frey Thlaspi goesingense Cucumis sativus (cucumber) Oryza sativa (rice) |
[26,27,45–51] |
| Endoplasmic reticulum | Schizosaccharomyces pombe | [23] |
| Zincosomes* | Candida albicans | [52] |
| Acidocalcisome |
Leishmania amazonensis Phytomonas serpens |
[53] |
| Gut granules | Caenorhabditis elegans | [54] |
| Storage granules |
Drosophila melanogaster Drosophila hydei |
[55,56] |
| Granules | Crassostrea gigas (Pacific oyster) | [57] |
| Endosome/lysosome | Kidney cells | [58] |
| Other Functions | ||
| Synaptic vesicles | Neuronal cells | [59] |
| Secretory vesicles | Pancreatic beta cells Mast cells |
[60,61] |
As outlined in more detail in the following review [62], zincosomes are membrane bound vesicles of unknown origin that have been observed in a variety of cells types and organisms.
Zinc homeostasis in the secretory pathway: insight from yeast
While zinc ions have important functions inside of organelles, genetic or chemical approaches that lead to the buildup of zinc ions within the endoplasmic reticulum cause increased ER stress and reduced cell viability [8–10]. Multiple genetic diseases are also caused by mutations that affect the function of zinc transport proteins that supply or release zinc ions from compartments [11,12], whereas single nucleotide polymorphisms and gene dosage effects that alter the expression or function of intracellular zinc transporters frequently correlate with increased risks for multiple complex diseases [11,13,14]. Together these observations suggest that cells rely on mechanisms to tightly regulate zinc concentrations inside of organelles.
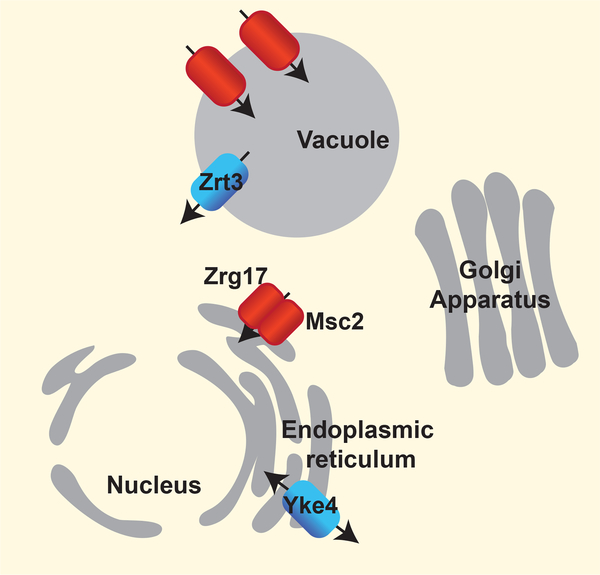
Yeast are genetically tractable systems that have been extensively used to study zinc homeostasis in the secretory pathway. In the budding yeast Saccharomyces cerevisiae, 4 proteins belonging to the Cation Diffusion Facilitator (CDF) family and 2 proteins belonging to the Zrt1- and Irt1-like protein (ZIP) family, transport zinc ions into and out of the secretory pathway and vacuole (Figure 1). Studies of these proteins have revealed that their activity can be regulated at a transcriptional and post-translational level in response to cellular zinc status, which in turn allows cells to balance the levels of zinc inside of the cytoplasm and organelles.
Figure 1.
Subcellular localization of zinc transporters that are localized to the secretory pathway in S. cerevisiae. Proteins belonging to the CDF and ZIP families are highlighted in red and blue respectively. Arrows indicate the direction of zinc transport.
The importance of transcriptional control
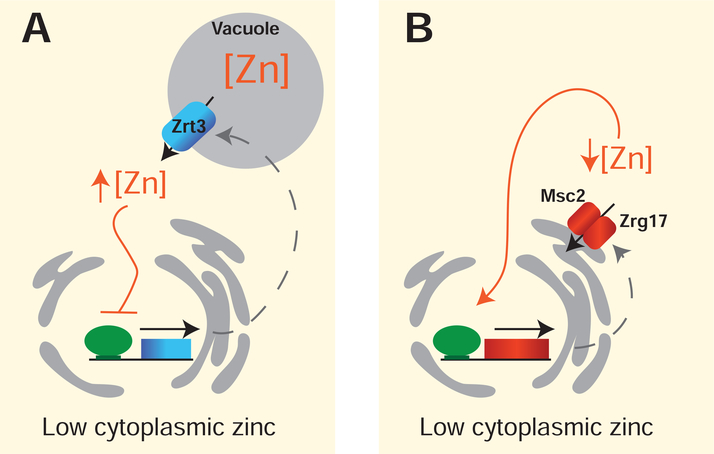
At a transcriptional level, genes encoding Zrt3, Zrg17, and Zrc1 are all expressed at higher levels under low zinc conditions in a manner that is dependent upon the zinc-responsive transcription factor Zap1 [15]. As discussed in past reviews, the Zap1-dependent regulation of ZRT3 creates a negative feedback circuit to help cells release zinc from stores when zinc ions in the cytosol are low (Figure 2A), whereas the Zap1-dependent regulation of ZRG17 creates a positive feedback circuit, enabling yeast to maintain a supply of zinc to organelles when zinc is limiting (Figure 2B) [15,16]. More recent studies suggest that related feedback mechanisms operate in other fungi with Zap1 homologs, as well as in other eukaryotes that use different zinc-responsive factors to control zinc homeostasis [17–20].
Figure 2.
The effects of Zap1-dependent regulation of ZRT3 and ZRG17 expression during zinc deficiency in S. cerevisiae. (A) Low levels of zinc in the cytoplasm increase Zap1 activity and the transcriptional activation of ZRT3. Increased expression of ZRT3 leads to increased levels of the Zrt3 protein and increased mobilization of zinc ions from the vacuolar stores. When cytosolic zinc ion concentrations are returned to an optimal level, Zap1 activity is inhibited creating a negative feedback circuit to prevent the accumulation of toxic levels of zinc in the cytosol. (B) Low cytoplasmic zinc levels also increase ZRG17 expression and Zrg17 protein levels. As Zrg17 works together with Msc2 to transport zinc ions out of the cytosol into the ER, a positive feedback circuit is generated, which maintains the supply of zinc to proteins in the ER, despite low zinc availability in the cytoplasm.
In most cases increased transcription of a zinc transporter gene correlates with a need for increased fluxes of zinc ions into or out of an organelle or the cytosol under a given growth condition. ZRC1 is an unusual Zap1 target gene as the Zap1-dependent increase in ZRC1 expression does not increase the transport of zinc ions into the vacuolar stores in low zinc, instead it is critical for protecting cells from a zinc shock - a growth condition where zinc-deficient cells are suddenly supplied with zinc ions [21].
The studies with Zrg17 and Zrc1 raises the question of why increased expression of some CDF proteins facilitates the transport of zinc ions out of a zinc-deficient cytosol, yet increased expression of others do not. One explanation is that there are other differences in the regulation or properties of the zinc transporters that affect their function. New studies in Schizosaccharomyces pombe provide additional evidence to support this hypothesis. In S. pombe, three members of the CDF family are localized to the secretory pathway. Cis4 and Zrg17 (the homologs of Msc2 and Zrg17) transport zinc ions into the cis-Golgi, and Zhf1 (the homolog of Zrc1 and Cot1) transports excess zinc ions into the endoplasmic reticulum [22,23]. By using genetically encoded high and low affinity zinc-responsive FRET reporters to examine changes in the labile pools of zinc in the cytosol, Choi et al. found that deletion of zhf1 had no effect on the concentrations of labile zinc in the cytosol under low zinc conditions, whereas deletion of zrg17 or cis4 resulted in higher levels of zinc accumulating in the cytosol [24]. On the other hand, when zinc-limited cells were exposed to zinc, Zhf1 is critical for the rapid removal of zinc ions from the cytosol, whereas Zrg17 and Cis4 have a more minor role in this process. As the expression of cis4, zrg17, and zhf1 is not affected by intracellular zinc status in fission yeast, these results suggest that there may be other regulatory mechanisms or differences in the intrinsic properties of the transporters that affect zinc transporter function.
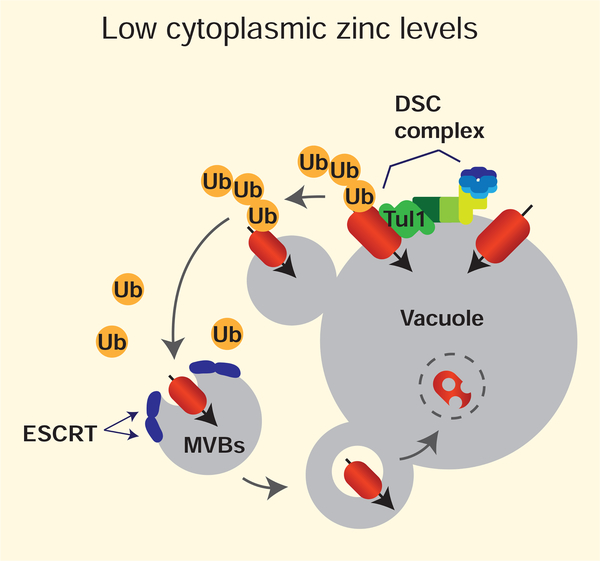
How else could the activity of zinc transporters be affected by cellular zinc status? While investigating mechanisms of membrane protein degradation in S. cerevisiae, other studies have revealed one possible answer. By testing whether the stability of the zinc transporters Zrt3 and Cot1 was dependent upon the levels of zinc, the Emr lab found that Zrt3 was ubiquitinated and targeted for degradation in high zinc, whereas Cot1 was ubiquitinated and targeted for degradation in low zinc [25]. In both cases polyubiquitination of each protein led to its degradation via the vacuole membrane recycling and degradation pathway (vReD) (Figure 3). Although the effects of these changes on zinc homeostasis remain to be tested, these analyses reveal a different mechanism by which cells can control the flux of zinc into and out of organelles in response to intracellular zinc status. Given that many zinc transporter genes are constitutively expressed, it is possible that similar mechanisms may be widespread in other eukaryotes, and that other mechanisms are present to control the activity of zinc transporters in response to cellular zinc status [26,27].
Figure 3.
Degradation of Cot1 in S. cerevisiae under low zinc conditions. When zinc levels are low, Cot1 is targeted for degradation in a manner that is dependent upon the Tul1 RING domain-containing E3 ligase and other members of the defective for SREBP cleavage (DSC) complex. While the signal that leads to the ubiquitination of Cot1 is unknown, polyubiquitination leads to its degradation via the vacuole membrane recycling and degradation pathway (vReD).
Coping with extreme zinc deficiency
While much is known about how cells alter gene expression to maintain zinc homeostasis, until now it has been unclear what happens to the entire zinc proteome under conditions of zinc deficiency. New studies from the Eide lab have used a combination of proteomic, in silico, and ionomic analyses to gain insight into what happens to the S. cerevisiae zinc proteome when intracellular zinc levels are low. Key findings from these analyses show that during conditions of zinc deficiency there are ~3-fold more zinc binding sites than there are zinc ions [1]. These results suggest that when zinc is limiting, the majority of zinc-binding sites in proteins are not occupied by zinc or are potentially mis-metalated with the wrong metal.
If there are insufficient zinc ions to metalate all proteins under conditions of zinc deficiency, how do cells prioritize the delivery of zinc ions to essential compartmentalized metalloproteins under this condition? As discussed above, one strategy is to control the levels or activity of zinc transporters that facilitate the flux of zinc into or out of organelles. Another strategy is to reduce the expression of non-essential zinc binding proteins to conserve zinc for more important functions. Such ‘zinc-sparing’ mechanisms typically result in reduced expression of abundant cytosolic zinc binding proteins [28,29]. However, in S. cerevisiae the vacuolar zinc-binding Pho8 alkaline phosphatase is degraded when cells are starved of zinc [30]. The targeted removal of non-essential compartmentalized metalloenzymes could therefore be a mechanism to facilitate the reallocation of zinc to more essential metalloproteins under conditions of extreme zinc deficiency.
In fission yeast Pho8 activity is also dependent upon zinc. In this yeast, pho8 is expressed at a higher level under conditions of zinc deficiency, which leads to the accumulation of the inactive apo-Pho8 protein that can be rapidly activated as soon as zinc is available [31]. As a number of the enzymes that potentially bind zinc in the endoplasmic reticulum are essential for life, the proactive accumulation of apo-Pho8 under conditions of zinc deficiency suggests that there are mechanisms present to prioritize the delivery of zinc to the other essential zinc-requiring proteins when zinc levels are low. While this hypothesis remains to be tested, in humans the activation of some secreted zinc proteins requires specific CDF family members [32–34]. New studies of the chaperone ERp44 have shown that it only forms zinc-bridged homodimers in the slightly acidic environment of the cis-Golgi, resulting in the exposure of amino acid residues critical for the retrieval of its client proteins back to the ER [35]. Thus, the environment of a subcellular compartment as well as other factors may affect metalation and protein activity of zinc proteins within the secretory pathway.
Coping with too much zinc
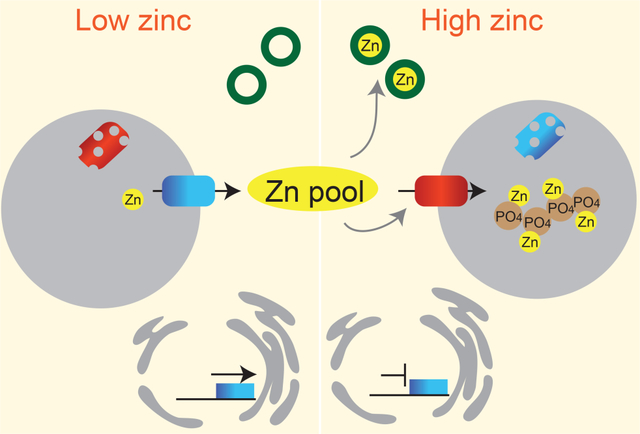
In the cytoplasm excessive concentrations of zinc ions are potentially toxic because they can displace other redox-active metals from their cognate sites. An important unanswered question is therefore how can high concentrations of metal ions be stored inside intracellular compartments without causing toxicity? In some organisms, specific molecules have been identified that help to buffer zinc ions inside of cells [36]. However, for the most part, detailed knowledge is lacking about the ligands that buffer zinc ions inside of the cytosol and organelles. Gaining knowledge of the intracellular buffering environment for zinc ions in vivo is complicated by the fact that there are many low molecular weight (LMW) small molecules that could potentially buffer zinc ions [37–42]. These ligands also include the N, O, and S donors from the side chains of the amino acids histidine, glutamate/aspartate and cysteine, raising the possibility that these side chains on exposed surfaces of proteins and peptides also contribute to the zinc buffer. The extent to which each of these molecules contribute to the zinc buffering environment is also likely to be influenced by the pH of the compartment, the concentration of ligand within the compartment, and competition effects from other metal ions. The zinc buffering environment could therefore be specific to individual compartments, as well as the number and types of buffering molecules produced under a different growth condition.
Despite this complexity, in yeast the labile zinc pools within the vacuole are affected by the concentrations of glutathione and its derivatives [43]. New studies in S. cerevisiae have also revealed that multiple zinc-containing LMW complexes accumulate in the vacuole, most of which were removed by phosphatase treatment [44]. While these studies provide some insight into the speciation of zinc within compartments, to date, enzymes involved in polyphosphate synthesis and degradation have only been found to be important for resistance to high zinc in some fungal species [45]. As multiple other zinc buffering molecules accumulate within compartments, future studies are needed to determine the extent to which these molecules contribute to zinc buffering inside organelles.
Conclusions
A growing amount of evidence suggests that the zinc ion concentrations within intracellular compartments are dynamic in nature and are highly regulated. Studies in yeast have also revealed that transcriptional and post-transcriptional mechanisms contribute to organelle zinc homeostasis and that these mechanisms include altering the zinc proteome and expression of zinc transporters that supply and release zinc ions from intracellular compartments.
Highlights.
Labile pools of zinc ions are present in many different organelles
Cells tightly regulate the levels of zinc ions inside of organelles
Cells have multiple mechanisms to control zinc transport into and out of organelles
When zinc is limiting, most zinc-binding sites in proteins are not occupied by zinc.
Acknowledgement
The authors would like to acknowledge funding from the National Institutes of Health grant R01 GM105695. We also thank Dr. R. Michael Townsend and members of the Bird lab for critical reading of the manuscript.
Footnotes
Conflict of interest
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Wang Y, Weisenhorn E, MacDiarmid CW, Andreini C, Bucci M, Taggart J, Banci L, Russell J, Coon JJ, Eide DJ: The cellular economy of the Saccharomyces cerevisiae zinc proteome. Metallomics 2018, 10:1755–1776.••The authors use a combination of in silico, iononic, and proteomic approaches to show that under conditions of zinc deficiency the number of zinc binding sites is in excess of the number of zinc ions inside of cells. Biochemical analyses of aldolase and methionine synthetase confirm that these metalloenzymes do not contain their required zinc cofactor under these conditions.
- 2.Chabosseau P, Woodier J, Cheung R, Rutter GA: Sensors for measuring subcellular zinc pools. Metallomics 2018, 10:229–239. [DOI] [PubMed] [Google Scholar]
- 3.Carter KP, Carpenter MC, Fiedler B, Jimenez R, Palmer AE: Critical Comparison of FRET-Sensor Functionality in the Cytosol and Endoplasmic Reticulum and Implications for Quantification of Ions. Anal Chem 2017, 89:9601–9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao Y, Tan CH, Krauchunas A, Scharf A, Dietrich N, Warnhoff K, Yuan Z, Druzhinina M, Gu SG, Miao L, et al. : The zinc transporter ZIPT-7.1 regulates sperm activation in nematodes. PLoS Biol 2018, 16:e2005069.•ZIPT-7.1 was identified in screens to identify genes required for sperm activation. Additional analyses show that the ZIPT-7.1-dependent release of zinc from intracellular stores into the cytoplasm triggers a signal tranduction pathway required for sperm activation.
- 5.Kjellerup L, Winther AL, Wilson D, Fuglsang AT: Cyclic AMP Pathway Activation and Extracellular Zinc Induce Rapid Intracellular Zinc Mobilization in Candida albicans. Front Microbiol 2018, 9:502.•The authors find that Candida albicans contains labile pools of zinc in the endoplasmic reticulum that are perturbed by changes in cellular zinc status.
- 6.Maret W: Zinc in Cellular Regulation: The Nature and Significance of “Zinc Signals”. Int J Mol Sci 2017, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sinclair SA, Senger T, Talke IN, Cobbett CS, Haydon MJ, Kramer U: Systemic Upregulation of MTP2- and HMA2-Mediated Zn Partitioning to the Shoot Supplements Local Zn Deficiency Responses. Plant Cell 2018, 30:2463–2479.••The authors find that zinc deficiency in the shoots of Arabidopsis triggers increased transcription of CDF family member MTP2 and enhanced root-to-shoot transport. In plants, the ER lumen is contigious between neighboring cells via plasmodesmata, suggesting that in low zinc the ER faciliates the ‘intra-ER’ symplastic movement of zinc ions between cells.
- 8.Woodruff G, Bouwkamp CG, de Vrij FM, Lovenberg T, Bonaventure P, Kushner SA, Harrington AW: The Zinc Transporter SLC39A7 (ZIP7) Is Essential for Regulation of Cytosolic Zinc Levels. Mol Pharmacol 2018, 94:1092–1100. [DOI] [PubMed] [Google Scholar]
- 9.Ohashi W, Kimura S, Iwanaga T, Furusawa Y, Irie T, Izumi H, Watanabe T, Hijikata A, Hara T, Ohara O, et al. : Zinc Transporter SLC39A7/ZIP7 Promotes Intestinal Epithelial Self-Renewal by Resolving ER Stress. PLoS Genet 2016, 12:e1006349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nolin E, Gans S, Llamas L, Bandyopadhyay S, Brittain SM, Bernasconi-Elias P, Carter KP, Loureiro JJ, Thomas JR, Schirle M, et al. : Discovery of a ZIP7 inhibitor from a Notch pathway screen. Nat Chem Biol 2019, 15:179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anzilotti C, Swan DJ, Boisson B, Deobagkar-Lele M, Oliveira C, Chabosseau P, Engelhardt KR, Xu X, Chen R, Alvarez L, et al. : An essential role for the Zn(2+) transporter ZIP7 in B cell development. Nat Immunol 2019, 20:350–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar L, Michalczyk A, McKay J, Ford D, Kambe T, Hudek L, Varigios G, Taylor PE, Ackland ML: Altered expression of two zinc transporters, SLC30A5 and SLC30A6, underlies a mammary gland disorder of reduced zinc secretion into milk. Genes Nutr 2015, 10:487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barresi V, Valenti G, Spampinato G, Musso N, Castorina S, Rizzarelli E, Condorelli DF: Transcriptome analysis reveals an altered expression profile of zinc transporters in colorectal cancer. J Cell Biochem 2018, 119:9707–9719. [DOI] [PubMed] [Google Scholar]
- 14.Kambe T, Tsuji T, Hashimoto A, Itsumura N: The Physiological, Biochemical, and Molecular Roles of Zinc Transporters in Zinc Homeostasis and Metabolism. Physiol Rev 2015, 95:749–784. [DOI] [PubMed] [Google Scholar]
- 15.Eide DJ: Homeostatic and adaptive responses to zinc deficiency in Saccharomyces cerevisiae. J Biol Chem 2009, 284:18565–18569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi S, Bird AJ: Zinc’ing sensibly: controlling zinc homeostasis at the transcriptional level. Metallomics 2014, 6:1198–1215. [DOI] [PubMed] [Google Scholar]
- 17.Tiwari A, Ngiilmei SD, Tamuli R: The NcZrg-17 gene of Neurospora crassa encodes a cation diffusion facilitator transporter required for vegetative development, tolerance to endoplasmic reticulum stress and cellulose degradation under low zinc conditions. Curr Genet 2018, 64:811–819. [DOI] [PubMed] [Google Scholar]
- 18.Roh HC, Collier S, Deshmukh K, Guthrie J, Robertson JD, Kornfeld K: ttm-1 encodes CDF transporters that excrete zinc from intestinal cells of C. elegans and act in a parallel negative feedback circuit that promotes homeostasis. PLoS Genet 2013, 9:e1003522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanford L, Carpenter MC, Palmer AE: Intracellular Zn(2+) transients modulate global gene expression in dissociated rat hippocampal neurons. Sci Rep 2019, 9:9411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bottcher B, Palige K, Jacobsen ID, Hube B, Brunke S: Csr1/Zap1 Maintains Zinc Homeostasis and Influences Virulence in Candida dubliniensis but Is Not Coupled to Morphogenesis. Eukaryot Cell 2015, 14:661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacDiarmid CW, Milanick MA, Eide DJ: Induction of the ZRC1 metal tolerance gene in zinc-limited yeast confers resistance to zinc shock. J Biol Chem 2003, 278:15065–15072. [DOI] [PubMed] [Google Scholar]
- 22.Fang Y, Sugiura R, Ma Y, Yada-Matsushima T, Umeno H, Kuno T: Cation diffusion facilitator Cis4 is implicated in Golgi membrane trafficking via regulating zinc homeostasis in fission yeast. Mol Biol Cell 2008, 19:1295–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clemens S, Bloss T, Vess C, Neumann D, Nies DH, Zur Nieden U: A transporter in the endoplasmic reticulum of Schizosaccharomyces pombe cells mediates zinc storage and differentially affects transition metal tolerance. J Biol Chem 2002, 277:18215–18221. [DOI] [PubMed] [Google Scholar]
- 24.Choi S, Hu YM, Corkins ME, Palmer AE, Bird AJ: Zinc transporters belonging to the Cation Diffusion Facilitator (CDF) family have complementary roles in transporting zinc out of the cytosol. PLoS Genet 2018, 14:e1007262.••A genetic study that uses low and high affinity zinc-responsive FRET sensors to determine if different CDF proteins have an equivalent role in transporting zinc ions out of the cytosol. The authors find that different CDF proteins play a primary role in removing zinc from the cytosol under low and high zinc conditions.
- 25.Li M, Koshi T, Emr SD: Membrane-anchored ubiquitin ligase complex is required for the turnover of lysosomal membrane proteins. J Cell Biol 2015, 211:639–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Migocka M, Malas K, Maciaszczyk-Dziubinska E, Posyniak E, Migdal I, Szczech P: Cucumber Golgi protein CsMTP5 forms a Zn-transporting heterodimer with high molecular mass protein CsMTP12. Plant Sci 2018, 277:196–206. [DOI] [PubMed] [Google Scholar]
- 27.Ruytinx J, Coninx L, Nguyen H, Smisdom N, Morin E, Kohler A, Cuypers A, Colpaert JV: Identification, evolution and functional characterization of two Zn CDF-family transporters of the ectomycorrhizal fungus Suillus luteus. Environ Microbiol Rep 2017, 9:419–427. [DOI] [PubMed] [Google Scholar]
- 28.Ehrensberger KM, Mason C, Corkins ME, Anderson C, Dutrow N, Cairns BR, Dalley B, Milash B, Bird AJ: Zinc-dependent regulation of the Adh1 antisense transcript in fission yeast. J Biol Chem 2013, 288:759–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bird AJ, Gordon M, Eide DJ, Winge DR: Repression of ADH1 and ADH3 during zinc deficiency by Zap1-induced intergenic RNA transcripts. EMBO J 2006, 25:5726–5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qiao W, Ellis C, Steffen J, Wu CY, Eide DJ: Zinc status and vacuolar zinc transporters control alkaline phosphatase accumulation and activity in Saccharomyces cerevisiae. Mol Microbiol 2009, 72:320–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu YM, Boehm DM, Chung H, Wilson S, Bird AJ: Zinc-dependent activation of the Pho8 alkaline phosphatase in Schizosaccharomyces pombe. J Biol Chem 2019, 294:12392–12404.•Biochemical and genetic analyses show that fission yeast grown under zinc-limiting conditions accumulates apo-Pho8 within the secretory pathway as a proactive mechanism to allow rapid restoration of Pho8 activity when zinc is available.
- 32.Fujimoto S, Tsuji T, Fujiwara T, Takeda TA, Merriman C, Fukunaka A, Nishito Y, Fu D, Hoch E, Sekler I, et al. : The PP-motif in luminal loop 2 of ZnT transporters plays a pivotal role in TNAP activation. Biochem J 2016, 473:2611–2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kambe T, Matsunaga M, Takeda TA: Understanding the Contribution of Zinc Transporters in the Function of the Early Secretory Pathway. Int J Mol Sci 2017, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsuji T, Kurokawa Y, Chiche J, Pouyssegur J, Sato H, Fukuzawa H, Nagao M, Kambe T: Dissecting the Process of Activation of Cancer-promoting Zinc-requiring Ectoenzymes by Zinc Metalation Mediated by ZNT Transporters. J Biol Chem 2017, 292:2159–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watanabe S, Amagai Y, Sannino S, Tempio T, Anelli T, Harayama M, Masui S, Sorrentino I, Yamada M, Sitia R, et al. : Zinc regulates ERp44-dependent protein quality control in the early secretory pathway. Nat Commun 2019, 10:603.••Structural and biochemical analyses of ERp44 show that it forms zinc-bridged homodimers in the cis-Golgi. These homodimers expose residues critical for the capture and return of client proteins back to the endoplasmic reticulum.
- 36.Ma Z, Chandrangsu P, Helmann TC, Romsang A, Gaballa A, Helmann JD: Bacillithiol is a major buffer of the labile zinc pool in Bacillus subtilis. Mol Microbiol 2014, 94:756–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abiria SA, Krapivinsky G, Sah R, Santa-Cruz AG, Chaudhuri D, Zhang J, Adstamongkonkul P, DeCaen PG, Clapham DE: TRPM7 senses oxidative stress to release Zn(2+) from unique intracellular vesicles. Proc Natl Acad Sci U S A 2017, 114:E6079–E6088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang G, Ulrich PN, Storey M, Johnson D, Tischer J, Tovar JA, Moreno SN, Orlando R, Docampo R: Proteomic analysis of the acidocalcisome, an organelle conserved from bacteria to human cells. PLoS Pathog 2014, 10:e1004555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kawano-Kawada M, Kakinuma Y, Sekito T: Transport of Amino Acids across the Vacuolar Membrane of Yeast: Its Mechanism and Physiological Role. Biol Pharm Bull 2018, 41:1496–1501. [DOI] [PubMed] [Google Scholar]
- 40.Wellenreuther G, Cianci M, Tucoulou R, Meyer-Klaucke W, Haase H: The ligand environment of zinc stored in vesicles. Biochem Biophys Res Commun 2009, 380:198–203. [DOI] [PubMed] [Google Scholar]
- 41.Krezel A, Maret W: The biological inorganic chemistry of zinc ions. Arch Biochem Biophys 2016, 611:3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eide DJ: Bacillithiol, a new role in buffering intracellular zinc. Mol Microbiol 2014, 94:743–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steiger MG, Patzschke A, Holz C, Lang C, Causon T, Hann S, Mattanovich D, Sauer M: Impact of glutathione metabolism on zinc homeostasis in Saccharomyces cerevisiae. FEMS Yeast Res 2017, 17. [DOI] [PubMed] [Google Scholar]
- 44.Nguyen TQ, Dziuba N, Lindahl PA: Isolated Saccharomyces cerevisiae vacuoles contain low-molecular-mass transition-metal polyphosphate complexes. Metallomics 2019.••A combination of liquid chromatography followed by ICP-MS reveals that low-molecular-mass zinc polyphosphate complexes are present in the vacuoles of yeast.
- 45.Kretschmer M, Reiner E, Hu G, Tam N, Oliveira DL, Caza M, Yeon JH, Kim J, Kastrup CJ, Jung WH, et al. : Defects in phosphate acquisition and storage influence virulence of Cryptococcus neoformans. Infect Immun 2014, 82:2697–2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simm C, Lahner B, Salt D, LeFurgey A, Ingram P, Yandell B, Eide DJ: Saccharomyces cerevisiae vacuole in zinc storage and intracellular zinc distribution. Eukaryot Cell 2007, 6:1166–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chasen NM, Stasic AJ, Asady B, Coppens I, Moreno SNJ: The Vacuolar Zinc Transporter TgZnT Protects Toxoplasma gondii from Zinc Toxicity. mSphere 2019, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gustin JL, Loureiro ME, Kim D, Na G, Tikhonova M, Salt DE: MTP1-dependent Zn sequestration into shoot vacuoles suggests dual roles in Zn tolerance and accumulation in Zn-hyperaccumulating plants. Plant J 2009, 57:1116–1127. [DOI] [PubMed] [Google Scholar]
- 49.Kupper H, Zhao FJ, McGrath SP: Cellular compartmentation of zinc in leaves of the hyperaccumulator Thlaspi caerulescens. Plant Physiology 1999, 119:305–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Neumann D, zur Nieden U: Silicon and heavy metal tolerance of higher plants. Phytochemistry 2001, 56:685–692. [DOI] [PubMed] [Google Scholar]
- 51.Menguer PK, Farthing E, Peaston KA, Ricachenevsky FK, Fett JP, Williams LE: Functional analysis of the rice vacuolar zinc transporter OsMTP1. J Exp Bot 2013, 64:2871–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Crawford AC, Lehtovirta-Morley LE, Alamir O, Niemiec MJ, Alawfi B, Alsarraf M, Skrahina V, Costa A, Anderson A, Yellagunda S, et al. : Biphasic zinc compartmentalisation in a human fungal pathogen. PLoS Pathog 2018, 14:e1007013.•Reveals that zinc stores are critical for the virulence of the human pathogen Candida albicans.
- 53.Miranda K, Docampo R, Grillo O, de Souza W: Acidocalcisomes of trypanosomatids have species-specific elemental composition. Protist 2004, 155:395–405. [DOI] [PubMed] [Google Scholar]
- 54.Roh HC, Collier S, Guthrie J, Robertson JD, Kornfeld K: Lysosome-related organelles in intestinal cells are a zinc storage site in C. elegans. Cell Metab 2012, 15:88–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zierold K, Wessing A: Mass dense vacuoles in Drosophila Malpighian tubules contain zinc, not sodium. A reinvestigation by X-ray microanalysis of cryosections. Eur J Cell Biol 1990, 53:222–226. [PubMed] [Google Scholar]
- 56.Tejeda-Guzman C, Rosas-Arellano A, Kroll T, Webb SM, Barajas-Aceves M, Osorio B, Missirlis F: Biogenesis of zinc storage granules in Drosophila melanogaster. J Exp Biol 2018, 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thomson JD, Pirie BJS, George SG: Cellular Metal Distribution in the Pacific Oyster, Crassostrea-Gigas (Thun) Determined by Quantitative X-Ray Microprobe Analysis. Journal of Experimental Marine Biology and Ecology 1985, 85:37–45. [Google Scholar]
- 58.Palmiter RD, Cole TB, Findley SD: ZnT-2, a mammalian protein that confers resistance to zinc by facilitating vesicular sequestration. EMBO J 1996, 15:1784–1791. [PMC free article] [PubMed] [Google Scholar]
- 59.Palmiter RD, Cole TB, Quaife CJ, Findley SD: ZnT-3, a putative transporter of zinc into synaptic vesicles. Proc Natl Acad Sci U S A 1996, 93:14934–14939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chimienti F, Favier A, Seve M: ZnT-8, a pancreatic beta-cell-specific zinc transporter. Biometals 2005, 18:313–317. [DOI] [PubMed] [Google Scholar]
- 61.Ho LH, Ruffin RE, Murgia C, Li L, Krilis SA, Zalewski PD: Labile zinc and zinc transporter ZnT4 in mast cell granules: role in regulation of caspase activation and NF-kappaB translocation. J Immunol 2004, 172:7750–7760. [DOI] [PubMed] [Google Scholar]
- 62.Eide DJ: Zinc transporters and the cellular trafficking of zinc. Biochim Biophys Acta 2006, 1763:711–722. [DOI] [PubMed] [Google Scholar]