Abstract
Sickle cell disease (SCD) is a monogenic disorder, estimated to affect over three million people worldwide1,2. Acute systemic painful vaso-occlusive episode (VOE) is the primary reason for emergency medical care among SCD patients3. VOE may also progress to acute chest syndrome (ACS), a type of acute lung injury and one of the primary reasons for mortality among SCD patients3. Recently, P-selectin monoclonal antibodies were shown to attenuate VOE in SCD patients and lung vaso-occlusion in transgenic humanized SCD mice, highlighting the therapeutic benefit of P-selectin inhibition in SCD4,5. Here, we use quantitative fluorescence intravital lung microscopy (qFILM) to show that tandem-P-selectin-glycoprotein-ligand-immunoglobulin (TSGL-Ig) fusion molecule containing four P-selectin binding sites, significantly attenuated intravenous (IV) oxy-hemoglobin (oxy-Hb) triggered lung vaso-occlusion in SCD mice. These findings highlight the therapeutic potential of TSGL-Ig to prevent VOE and ACS in SCD.
Sickle cell anemia, the most common form of sickle cell disease (SCD) is caused by the homozygous mutation in the β-globin gene, which leads to erythrocyte sickling, impaired rheology, vaso-occlusion and premature hemolysis6. Vaso-occlusion and hemolysis are the two predominant pathophysiological events in SCD that contribute to chronic organ damage and acute systemic painful vaso-occlusive episode (VOE)3,6. Earlier, we have shown that VOE involves entrapment of large neutrophil-platelet aggregates in lung arterioles of SCD mice, which is inhibited following IV administration of P-selectin function blocking antibody5. These findings were supported by the SUSTAIN clinical trial that reported significant reduction in the frequency of VOE in SCD patients receiving IV administration of P-selectin monoclonal antibody crizanlizumab4. P-selectin-glycoprotein-ligand-1 (PSGL-1) constitutively expressed on neutrophils, binds to P-selectin on activated platelets and both P- and E-selectin on activated endothelial cells to promote neutrophil-platelet aggregation and neutrophil adhesive-rolling along vascular endothelium, respectively5,7,8. Previously, a soluble form of recombinant PSGL-1 was shown to prevent ischemia-reperfusion injury of liver allografts in both mice and humans by competitively inhibiting E-/P-selectin-PSGL-1 dependent neutrophil-endothelium adhesion without increasing any risk of bleeding9–11. Taken together, these findings suggest that biomolecules mimicking the binding domain of PSGL-1 can be therapeutically beneficial in preventing vaso-occlusion in SCD. TSGL-Ig is a recombinant fusion protein that carries two P-selectin sulfated-glycopeptide-binding domains in a tandem configuration on a single polypeptide chain12. Such two polypeptide chains are fused to an inactivated Fc domain of human IgG1, resulting in a dimer with four P-selectin binding sites per molecule of TSGL-Ig (Figure S1)12. Recently, TSGL-Ig was shown to prevent ischemia-reperfusion injury of orthotopic liver transplants in mice by attenuating leukocyte sequestration in the liver microcirculation12. The current study assesses the therapeutic efficacy of TSGL-Ig in preventing the lung vaso-occlusion in SCD mice.
Oxy-hemoglobin (oxy-Hb) released during hemolysis is an erythrocyte-derived-damage-associated-molecular-pattern (eDAMP) molecule, which promotes vaso-occlusion by causing endothelial dysfunction, nitric oxide depletion, oxidative stress and sterile inflammation, leading to activation of leukocytes, platelets and vascular endothelium6. Therefore, a non-lethal dose of IV oxy-Hb (10 μmol/kg) was established after multiple titrations, and IV administered in SCD mice to trigger VOE. Based on previous studies13, IV vet-starch was used as a negative control (vehicle) to account for the changes in volume and viscosity caused by IV oxy-Hb administration. As shown in the experimental scheme (Figure 1A), Townes-SS (SCD) mice were IV administered with 10 μmol/kg oxy-Hb or 80 μl Vet-starch or 10 μmol/kg oxy-Hb with 100 μg/mouse of TSGL-Ig or 10 μmol/kg oxy-Hb with 100 μg/mouse of recombinant human IgG1-Fc (rh IgG1-Fc). Two hours later, mice were IV administered FITC-dextran, AlexaFluor546 (AF546) conjugated anti-mouse Ly6G mAb and Violet450 (V450) conjugated anti-mouse CD49b mAb for visualization of blood vessels, and in vivo staining of neutrophils and platelets, respectively. Lung microcirculation in live mice was assessed using the qFILM imaging approach described previously in detail5,14. Refer to Methods for experimental details. TSGL-Ig did not result in excessive bleeding during the qFILM imaging in SCD mice. IV oxy-Hb triggered lung vaso-occlusion in SCD mice, which involved occlusion of pulmonary arteriole bottlenecks (junction of pulmonary arterioles and capillaries) by neutrophil-platelet aggregates (representative field of view (FOV) shown in Figure 1B and Movie S1). IV oxy-Hb did not promote vaso-occlusion in control mice (data not shown). Unlike IV oxy-Hb, IV administration of vet-starch did not promote lung vaso-occlusion in SCD mice (representative FOV shown in Figure 1C and Movie S2). Majority of FOVs in the lung of SCD mice IV administered both oxy-Hb and TSGL-Ig were free of vaso-occlusion (representative FOV shown in Figure 1D and Movie S3), while IV administration of control rh IgG1-Fc had no effect on IV oxy-Hb induced lung vaso-occlusion in SCD mice (representative FOV shown in Figure 1E and Movie S4). Figure 1E and Movie S4 show a neutrophil-platelet aggregate occluding the pulmonary arteriole bottle-neck in SCD mice IV administered both oxy-Hb and rh IgG1-Fc.
Figure 1. TSGL-Ig prevents lung vaso-occlusion in SCD mice in vivo.
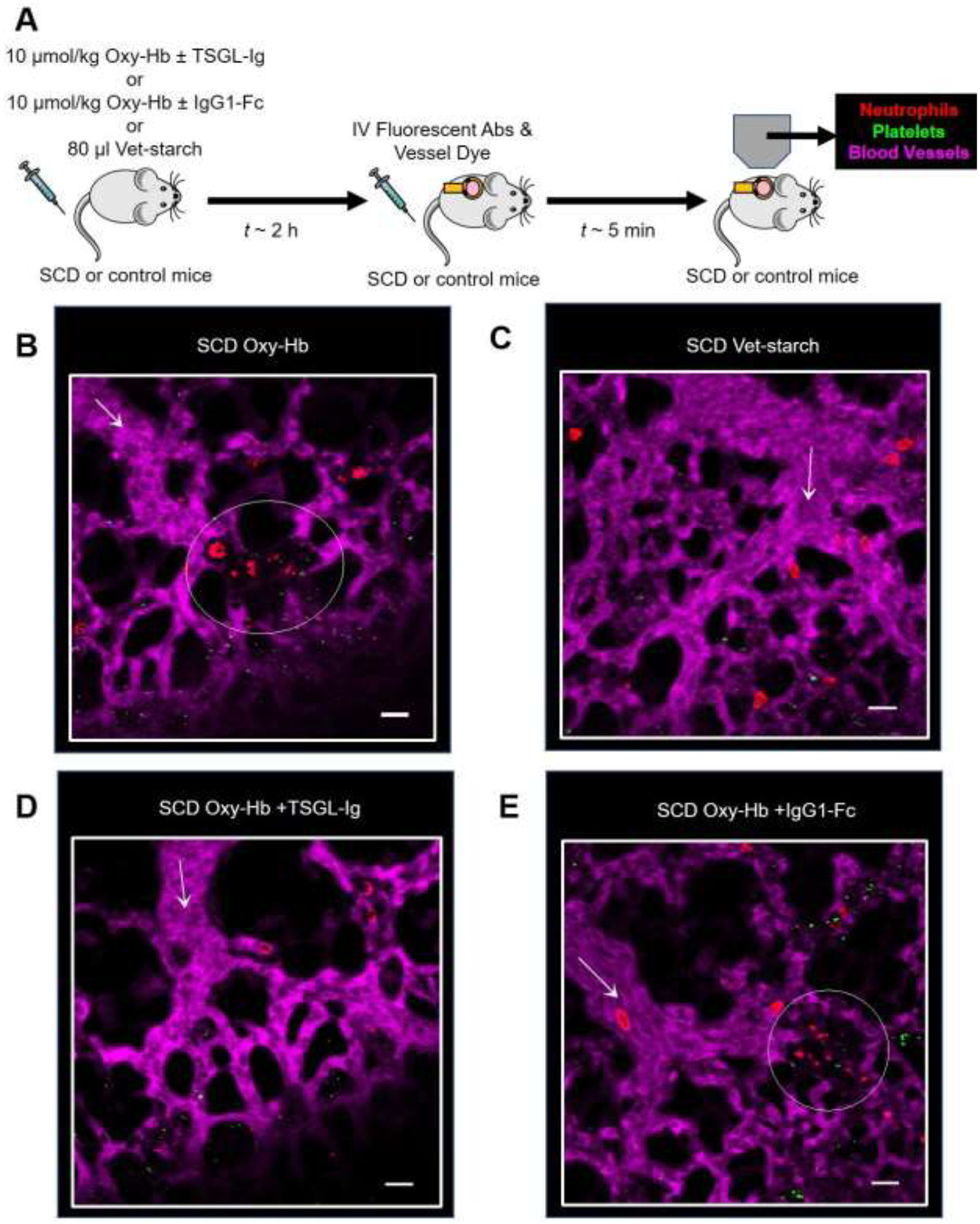
(A) Experimental scheme- SCD mice were IV administered with 10 μmol/kg oxy-Hb ± 100 μg/mouse TSGL-Ig or 10 μmol/kg oxy-Hb ± 100 μg/mouse rh IgG1-Fc or 80 μl vet-starch and quantitative fluorescence intravital lung microscopy (qFILM) was used to assess the absence or presence of lung vaso-occlusion. (B) Representative field of view (FOV) showing large platelet-neutrophil aggregate (dotted white circle) occluding the arteriolar bottle-neck in the lung of SCD mice IV administered oxy-Hb. (C) Representative FOV showing absence of neutrophil-platelet aggregates in pulmonary arterioles of SCD mice administered IV vet-starch. (D) Representative FOV showing absence of neutrophil-platelet aggregates in pulmonary arterioles of SCD mice administered IV oxy-Hb + TSGL-Ig. (E) Representative FOV showing large platelet-neutrophil aggregate (dotted white circle) occluding the arteriolar bottle-neck in the lung of SCD mice IV administered oxy-Hb + rh IgG1-Fc. The complete time series of FOVs in B, C, D and E are shown in Supplementary Movies S1, S2, S3 and S4, respectively. Pulmonary microcirculation (pseudo-colored purple), neutrophils (red) and platelets (pseudo-colored green) were labeled in vivo by IV administration of FITC dextran, AF546-anti-Ly6G Ab and V450-anti-CD49b Ab, respectively. White arrows denote the direction of blood flow within the arterioles. FOV~65,536 μm2. Size of arterioles in B, C, D and E are 28 μm, 34 μm, 32 μm and 33 μm respectively. Scale bars 20 μm.
Next, pulmonary vaso-occlusions were analyzed in 12 to15 FOVs (FOV size ~ 65,536 μm2) per mouse with 3 to 4 mice in each treatment group and quantified based on following three parameters: 1) average number of pulmonary vaso-occlusions per FOV; 2) percent of total FOVs with pulmonary vaso-occlusions; and 3) average number of large pulmonary vaso-occlusions (size > 1000 μm2) per FOV. This analysis approach has been described previously5,15,16 and the same details are also included in Methods. Based on our previous studies5,15,16, vaso-occlusions covering an area > 1000 μm2 in the 2D qFILM images were referred as large pulmonary vaso-occlusions. Large vaso-occlusions are known to be present in the pulmonary arterioles of ACS patients17,18. Following treatment with IV oxy-Hb, SCD mice manifested an average of at least one pulmonary vaso-occlusion per FOV (black bar in Figure 2A), at least one pulmonary vaso-occlusion was present in 80% of all the FOVs examined (black bar in Figure 2B), and an average of 0.4 large pulmonary vaso-occlusions per FOV (black bar in Figure 2C; i.e. every third FOV in SCD mice had a large pulmonary vaso-occlusion). Although TSGL-Ig treatment led to significant reduction in all the three parameters (Figure 2A–C), the largest reduction was observed in number of large vaso-occlusions (Figure 2C). Average number of pulmonary vaso-occlusions per FOV was reduced by 40% (grey bar in Figure 2A), percent FOVs with pulmonary vaso-occlusion was reduced by 33% (grey bar in Figure 2B), and average number of large pulmonary vaso-occlusions per FOV was reduced by 50% (grey bar in Figure 2C) in SCD mice administered IV oxy-Hb + TSGL-Ig compared to SCD mice administered IV oxy-Hb only. In contrast, the average number of pulmonary vaso-occlusions per FOV was unchanged in SCD mice administered IV oxy-Hb + rh IgG1-Fc compared to SCD mice administered IV oxy-Hb only (Figure 2D).
Figure 2. TSGL-Ig attenuates pulmonary vaso-occlusion in SCD mice.
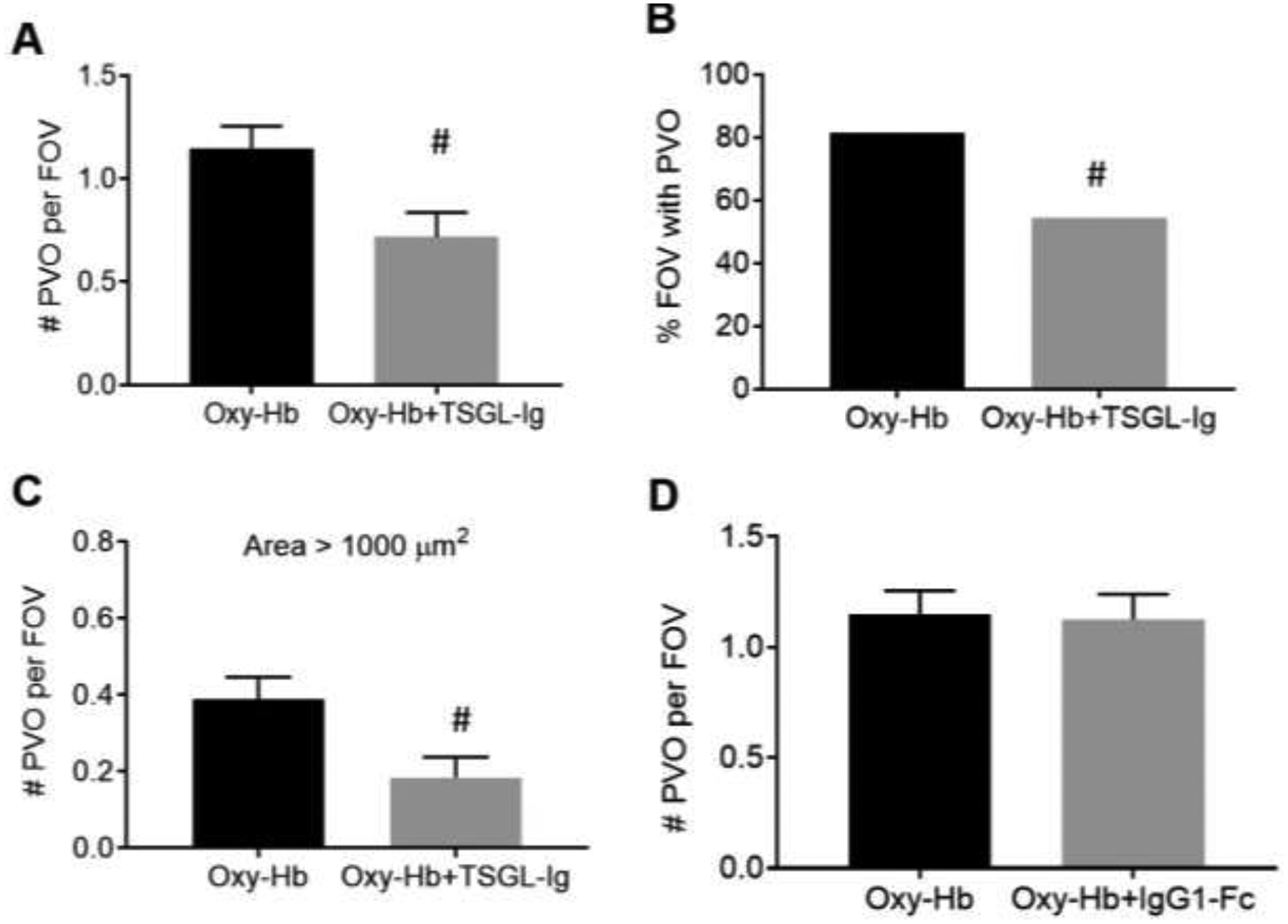
Time series of qFILM images were analyzed in 10–12 field of views (FOV~65,536 μm2) per mouse and 3 to 4 mice per treatment group to determine average number of pulmonary vaso-occlusions per FOV, percent FOVs with pulmonary vaso-occlusions, and average number of large pulmonary vaso-occlusions (area > 1000 μm2) per FOV. Means were compared using unpaired Student’s t test and percents were compared using four-fold table analysis with χ2 statistics to assess the effect of TSGL-Ig or rh IgG1-Fc on IV oxy-Hb triggered lung vaso-occlusion in SCD mice. Treatment with TSGL-Ig significantly reduced (A) average number of pulmonary vaso-occlusions per FOV, (B) percent FOVs with pulmonary vaso-occlusions, and (C) average number of large pulmonary vaso-occlusions per FOV in SCD mice. (D) Average number of pulmonary vaso-occlusions per FOV was not affected by IV administration of control rh IgG1 Fc. Error bars SE. # p < 0.05. IV Oxy-Hb SCD (n = 4 mice); IV Oxy-Hb + TSGL-Ig SCD (n = 3 mice); IV Oxy-Hb + rh IgG1-Fc SCD (n = 3 mice). PVO, pulmonary vaso-occlusion,
Taken together, our data suggest that IV oxy-Hb triggered lung vaso-occlusion in SCD mice by promoting the occlusion of pulmonary arterioles by neutrophil-platelet aggregates. TSGL-Ig attenuated lung vaso-occlusion in SCD mice by reducing both the number and size of neutrophil-platelet aggregates in the pulmonary arterioles. These findings highlight the need for clinical studies to evaluate the safety and efficacy of TSGL-Ig in preventing VOE and/or ACS in SCD patients.
METHODS
Reagents
Violet 450 (V450) conjugated rat anti-mouse CD49b mAb (clone DX5) was purchased from BD Biosciences (San Jose, CA). Alexa Fluor 546 (AF546) conjugated rat anti-mouse Ly-6G mAb (clone 1A8) was purchased from BioLegend (San Diego, CA). Fluorescein isothiocyanate (FITC) dextran (MW 70,000) was purchased from Molecular Probes Inc. (Eugene, OR). Vet-starch was purchased from Henry Schein Animal Health (Dublin, Ohio). Recombinant human lgG1 Fc (rh IgG1 Fc) was purchased from BioCell (New Hampshire, USA). Ketamine HCl was purchased from (Henry Shein Animal Health; Dublin, OH) and xylazine was purchased from (LLOYD Laboratories; Shenandoah, IA).
Tandem-P-selectin-glycoprotein-ligand-immunoglobulin (TSGL-Ig)
Recombinant TSGL-Ig fusion molecule was provided by Quell Pharma Inc. (Greater Boston Area, USA). TSGL-Ig was produced in HEK293 cells after transfection with plasmids encoding human FucT-VII cDNA and TSGL-Ig, essentially as described elsewhere19. Secreted TSGL-Ig was purified from conditioned media using protein A-sepharose and P-selectin binding kinetics were subsequently confirmed via multiple Octet binding assays.
Oxy-hemoglobin preparation
Oxy-hemoglobin (Oxy-Hb) was prepared from expired, leukocyte-reduced red blood cell units from healthy human donors. These red blood cell units were provided by the Institute for Transfusion Medicine (IT×M, Pittsburgh, PA). Oxy-Hb purification was performed as described previously20–22 with minor modifications. Red blood cells were washed with PBS in a centrifuge at 5000g for 10 min to obtain a packed cell pellet. Cells were lysed hypotonically by resuspending in distilled water (3:1; v/v) and mixing for 30 min at room temperature. Cell debris were removed by centrifuging at 25000g for 30 min. The supernatant was dialyzed against PBS and the final product stored at −80 °C.
Mice
Male and female (age ~12–16 weeks) Townes SCD mice (SS, homozygous for Hbatm1(HBA)Tow, homozygous for Hbbtm2(HBG1,HBB*)Tow) were used in this study23. In Townes SCD mice, human α and β-sickle (βS) genes are knocked into the locus where mouse α and β genes were knocked out23. Townes SS mice were bred and genotyped in-house. All animal experiments were approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh.
Quantitative Fluorescence Intravital Lung Microscopy (qFILM)
Quantitative Fluorescence Intravital Lung Microscopy (qFILM) was developed by our group to study pulmonary vaso-occlusion in SCD mice14. In this study, qFILM was used to visualize the lung microcirculation of SCD mice. Detail experimental set up of qFILM has been described elsewhere5. qFILM was performed using a Nikon A1R multi-photon excitation upright motorized microscope (Nikon Instruments; Tokyo, Japan). The two dimensional time series of qFILM images were acquired with NIS-Elements software using a prechirped Chameleon Laser Vision (Coherent; Santa Clara, CA) emitting an excitation wavelength of 850 nm, an APO LWD 25x water immersion objective with 1.1 NA, a high speed resonant scanning mode capable of acquisition at 512 × 512 resolution with 2x line averaging and bi-directional scanning (~15 frames per second) and four GaAsP NDD detectors. The four detectors collected fluorescent light transmitted through 450/20 nm (detector 1; blue channel), 525/50 nm (detector 2; green channel), 576/26 nm (detector 3; red channel) and 685/70 nm (detector 4; far red channel) band pass filters. In this study, we used detector 1 for V450, detector 2 for FITC and detector 3 for AF546. The position of the microscope stage was selected in the z and x–y planes through a Nano-Drive (Mad City Labs Inc.; Madison, WI) and a control pad (Prior Scientific Inc.; Rockland, MA), respectively.
Experimental design of qFILM studies
Approximately 2–2.5 hours prior to qFILM, SCD mice were intravenously (IV) administered (via tail vein) with 80 μl of vet-starch, or 10 μmole/kg of oxy-Hb, or 10 μmole/kg of oxy-Hb with 100 μg/mouse of TSGL-Ig, or 10 μmole/kg of oxy-Hb with 100 μg/mouse of rh IgG1-Fc. Mice were anesthetized with an intraperitoneal (IP) injection of 100 mg/kg ketamine HCl and 20 mg/kg xylazine. Just prior to imaging, IV fluorescent dyes and mAbs against blood cell lineage markers were intravascularly administered through a carotid artery catheter to enable the visualization of the lung microcirculation and in vivo staining of neutrophils and platelets. IV fluorescent dyes included following: 75 μg/mouse FITC-dextran, 12 μg/mouse of AF546-conjugated anti-mouse Ly6G mAb to stain neutrophils, and 7 μg/mouse of V450-conjugated anti-mouse CD49b mAb to stain platelets. qFILM was performed on a mouse for a total period of 30 minutes and the presence or absence of pulmonary vaso-occlusion was assessed for 30 seconds in each FOV by using a Nikon’s NIS elements 4.20 software. Pulmonary vaso-occlusions were assessed in ~12–15 FOVs in each mouse and across multiple mice per test group (n = 3 to 4) as previously described5,15,16.
qFILM Image processing and analysis
The time series of qFILM images were processed using an image subtraction, a median filter, a noise-reduction algorithm and adjustment of intensity histograms as described previously using the Nikon NIS elements 4.20 software5,6,14,22. The lung microcirculation was pseudo colored as purple, neutrophils and platelets were red and green, respectively. Pulmonary vaso-occlusion were compared between treatment groups using the following parameters as described previously5,15,16: average number of pulmonary vaso-occlusions per FOV, percent FOVs with pulmonary vaso-occlusions, and the average number of pulmonary vaso-occlusions (with area > 1000 μm2) per FOV.
Statistical Analysis
Statistical analyses were conducted using the approach described in our previous studies5,15,16. The average number of pulmonary vaso-occlusions per FOV and the average number of large pulmonary vaso-occlusions (size > 1000 μm2) per FOV were compared using the 2-tailed unpaired Student’s t-test. The percent FOVs with pulmonary vaso-occlusions was compared using the four-fold table analyses with χ2 statistics. Data presented as mean ± SEM. A p-value of less than 0.05 was used to determine the statistical significance.
Supplementary Material
Highlights.
Intravenous administration of hemoglobin triggers lung vaso-occlusion in SCD mice.
Hemoglobin triggers sequestration of neutrophil-platelet aggregates in lung arterioles.
TSGL-Ig prevents lung vaso-occlusion in SCD mice.
Safety and efficacy of TSGL-Ig should be evaluated in SCD patients.
ACKNOWLEDGEMENT
This study was supported by NIH-NHLBI 1R01HL128297-01 (PS) and 1R01HL141080-01A1 (PS), 2RO1-HL125886 (JT), American Heart Association 18TPA34170588 (PS) and funds from the Hemophilia Center of Western Pennsylvania and Vitalant (PS). RV was supported by American Heart Association 19PRE34430188. The Nikon multiphoton excitation microscope was funded by NIH grant 1S10RR028478-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE OF CONFLICT OF INTEREST
GDS is an inventor of US Patent 8,889,628 relevant to TSGL molecules and is a founder of Quell Pharma Inc. Remaining authors have declared that no conflict of interest exists.
REFERENCES
- 1.Piel FB, Patil AP, Howes RE, et al. Global epidemiology of sickle haemoglobin in neonates: a contemporary geostatistical model-based map and population estimates. Lancet. 2013;381(9861):142–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kato GJ, Piel FB, Reid CD, et al. Sickle cell disease. Nat Rev Dis Primers. 2018;4:18010. [DOI] [PubMed] [Google Scholar]
- 3.Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet. 2010;376(9757):2018–2031. [DOI] [PubMed] [Google Scholar]
- 4.Ataga KI, Kutlar A, Kanter J, et al. Crizanlizumab for the Prevention of Pain Crises in Sickle Cell Disease. N Engl J Med. 2017;376(5):429–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennewitz MF, Jimenez MA, Vats R, et al. Lung vaso-occlusion in sickle cell disease mediated by arteriolar neutrophil-platelet microemboli. JCI Insight. 2017;2(1):e89761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sundd P, Gladwin MT, Novelli EM. Pathophysiology of Sickle Cell Disease. Annu Rev Pathol. 2019;14:263–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sundd P, Gutierrez E, Koltsova EK, et al. ‘Slings’ enable neutrophil rolling at high shear. Nature. 2012;488(7411):399–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koltsova EK, Sundd P, Zarpellon A, et al. Genetic deletion of platelet glycoprotein Ib alpha but not its extracellular domain protects from atherosclerosis. Thromb Haemost. 2014;112(6):1252–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dulkanchainun TS, Goss JA, Imagawa DK, et al. Reduction of hepatic ischemia/reperfusion injury by a soluble P-selectin glycoprotein ligand-1. Ann Surg. 1998;227(6):832–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amersi F, Farmer DG, Shaw GD, et al. P-selectin glycoprotein ligand-1 (rPSGL-Ig)-mediated blockade of CD62 selectin molecules protects rat steatotic liver grafts from ischemia/reperfusion injury. Am J Transplant. 2002;2(7):600–608. [DOI] [PubMed] [Google Scholar]
- 11.Busuttil RW, Lipshutz GS, Kupiec-Weglinski JW, et al. rPSGL-Ig for improvement of early liver allograft function: a double-blind, placebo-controlled, single-center phase II study. Am J Transplant. 2011;11(4):786–797. [DOI] [PubMed] [Google Scholar]
- 12.Zhang C, Zhang Y, Liu Y, et al. A Soluble Form of P Selectin Glycoprotein Ligand 1 Requires Signaling by Nuclear Factor Erythroid 2-Related Factor 2 to Protect Liver Transplant Endothelial Cells Against Ischemia-Reperfusion Injury. Am J Transplant. 2017;17(6):1462–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raat NJ, Tabima DM, Specht PA, et al. Direct sGC activation bypasses NO scavenging reactions of intravascular free oxy-hemoglobin and limits vasoconstriction. Antioxid Redox Signal. 2013;19(18):2232–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bennewitz MF, Watkins SC, Sundd P. Quantitative intravital two-photon excitation microscopy reveals absence of pulmonary vaso-occlusion in unchallenged Sickle Cell Disease mice. Intravital. 2014;3(2):e29748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bennewitz MF, Tutuncuoglu E, Gudapati S, et al. P-selectin-deficient mice to study pathophysiology of sickle cell disease. Blood Adv. 2020;4(2):266–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vats R, Brzoska T, Bennewitz MF, et al. Platelet Extracellular Vesicles Drive Inflammasome-IL-1beta-Dependent Lung Injury in Sickle Cell Disease. Am J Respir Crit Care Med. 2020;201(1):33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anea CB, Lyon M, Lee IA, et al. Pulmonary platelet thrombi and vascular pathology in acute chest syndrome in patients with sickle cell disease. Am J Hematol. 2016;91(2):173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mekontso Dessap A, Deux JF, Abidi N, et al. Pulmonary artery thrombosis during acute chest syndrome in sickle cell disease. Am J Respir Crit Care Med. 2011;184(9):1022–1029. [DOI] [PubMed] [Google Scholar]
- 19.Barbaux S, Poirier O, Pincet F, Hermand P, Tiret L, Deterre P. The adhesion mediated by the P-selectin P-selectin glycoprotein ligand-1 (PSGL-1) couple is stronger for shorter PSGL-1 variants. J Leukoc Biol. 2010;87(4):727–734. [DOI] [PubMed] [Google Scholar]
- 20.Geraci G, Parkhurst LJ, Gibson QH. Preparation and properties of alpha- and beta-chains from human hemoglobin. J Biol Chem. 1969;244(17):4664–4667. [PubMed] [Google Scholar]
- 21.Huang Z, Shiva S, Kim-Shapiro DB, et al. Enzymatic function of hemoglobin as a nitrite reductase that produces NO under allosteric control. J Clin Invest. 2005;115(8):2099–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tejero J, Basu S, Helms C, et al. Low NO concentration dependence of reductive nitrosylation reaction of hemoglobin. J Biol Chem. 2012;287(22):18262–18274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu LC, Sun CW, Ryan TM, Pawlik KM, Ren J, Townes TM. Correction of sickle cell disease by homologous recombination in embryonic stem cells. Blood. 2006;108(4):1183–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


