Abstract
Mitochondria are essential for neuronal function because they serve not only to sustain energy and redox homeostasis but also are harbingers of death. A dysregulated mitochondrial network can cascade until function is irreparably lost, dooming cells. TBI is most prevalent in the young and comes at significant personal and societal costs. Traumatic brain injury (TBI) triggers a biphasic and mechanistically heterogenous response and this mechanistic heterogeneity has made the development of standardized treatments challenging. The secondary phase of TBI injury evolves over hours and days after the initial insult, providing a window of opportunity for intervention. However, no FDA approved treatment for neuroprotection after TBI currently exists. With recent advances in detection techniques, there has been increasing recognition of the significance and roles of mitochondrial redox lipid signaling in both acute and chronic central nervous system (CNS) pathologies. Oxidized lipids and their downstream products result from and contribute to TBI pathogenesis. Therapies targeting the mitochondrial lipid composition and redox state show promise in experimental TBI and warrant further exploration. In this review, we provide 1) an overview for mitochondrial redox homeostasis with emphasis on glutathione metabolism, 2) the key mechanisms of TBI mitochondrial injury, 3) the pathways of mitochondria specific phospholipid cardiolipin oxidation, and 4) review the mechanisms of mitochondria quality control in TBI with consideration of the roles lipids play in this process.
Keywords: Traumatic brain injury, mitochondria, lipid peroxidation, redox lipidomics, mitochondria quality control, mitophagy
1.0: Introduction
Brain bioenergetics rely on mitochondrial oxidative phosphorylation for energy production. In addition to ATP production, mitochondria are known for their essential contribution to metabolic pathways (e.g, for purines and pyrimidines) as well as for their semi-autonomous role in survival and death signaling, oxidative stress, and intracellular calcium buffering. Aberrant mitochondrial dynamics and metabolism have a central role in the pathogenesis of numerous acute and chronic neurologic diseases. These changes interfere with normal function and stimulate downstream signaling events. Increased electron leakage due to electron transport chain dysregulation and/or aberrant catalytic mechanisms of TCA dehydrogenases such as α-ketoglutarate dehydrogenase (α-KGDH), α-glycerophosphate dehydrogenase (α-GPDH) (Adam-Vizi and Tretter, 2013) results in increased free radical generation, which can overcome endogenous compensatory mechanisms and detrimentally alter DNA, proteins, and lipids. Similarly, a diverse array of pathogenetic mechanisms converge to prompt release of mitochondrially sequestered regulated death and inflammatory factors. Mitochondrial function and dynamic regulation in response to both acute and chronic brain injury are integral to neuronal survival and homeostatic function.
Traumatic brain injury (TBI) is remarkable because of the heterogeneity of neuronal survival, death, and inflammatory signaling mechanisms involved. TBI pathogenesis is biphasic; it entails both a primary mechanical injury and secondary delayed injury mechanisms (Prins et al., 2013). The primary injury is initiated by the acute mechanical disruption of brain tissue and cellular architecture. The mechanical injury damages axons and synapses leading to excitotoxic injury, while tearing of blood vessels results in both hemorrhagic and ischemic injury. Secondary TBI injury is delayed, developing hours-to-weeks later. Both injury phases prompt maladaptive mitochondria-specific lipid oxidation, inflammation, and initiation of regulated death pathways. Each of these processes bears close mechanistic similarities with other acute brain injury pathologies (e.g. ischemia-reperfusion, spinal cord injury, subarachnoid hemorrhage).
Both clinical observations and experimental studies recognize the central role of structural and functional mitochondrial injury in TBI pathogenesis. Mitochondrial dysregulation is a primary driver of neuronal loss and worse clinical outcomes following TBI (Cheng et al., 2012). Therefore, understanding how the heterogenous injury mechanisms of TBI converge on mitochondria and induce a shift from normal to pathological activity can guide rational therapy development. In particular, we illustrate how mitochondria-derived (oxidized) lipid signaling has emerged as an opportunity-rich and exciting area of ongoing TBI research. This review provides: 1) an overview for mitochondrial redox homeostasis with emphasis on glutathione metabolism, 2) the key mechanisms of TBI mitochondrial injury, 3) the pathways of mitochondrial lipid oxidation, focusing on cardiolipin oxidation, and 4) an overview of mitochondria quality control mechanisms in TBI with consideration of the role lipids play in these processes.
1.1: Mitochondrial Structure and Redox Function
Mitochondria are complex semi-autonomous organelles. They are unique because they have their own plasmid-like circular genome (mitochondrial DNA, mtDNA) that expresses a small but vital subset of redox proteins (Lagouge and Larsson, 2013). The remaining suite of mitochondrial proteins are nuclearly encoded and must be imported then renatured at their specific submitochondrial destination. The dual mitochondrial membranes are protein rich. The inner (IMM) and outer (OMM) mitochondrial membranes phospholipid (PL)-to-protein ratio are 0.20 and 0.45 (w/w), respectively. In comparison, the plasma membrane has a higher 0.67 PL-to-protein ratio (Horvath and Daum, 2013). Most important, however, is the precise lipid composition and high abundance of oxidizable polyunsaturated acyl chains means mitochondria are a unique and important site for generation of lipid mediators and downstream products.
The electron transport chain (ETC) is the chief source of ATP, but is also a potentially dangerous site of intracellular oxidant production (Willems et al., 2015). The ETC is composed of five protein complexes and the mobile electron carriers, coenzyme Q10 and cytochrome c (cyt c). ETC Complex I and II receive high energy electrons derived from NADH/FADH2. Electrons are then shuttled through a series of redox centers in Complexes I, III, and IV – a process that culminates in the translocation of protons from the matrix to intermembrane space (IMS). This proton pumping action produces a negative IMM transmembrane potential (Δψm, −150 to −180 mV) (Kamo et al., 1979). Complex V (F1/F0 ATP Synthase) is powered by this electrochemical gradient (i.e. protonmotive force). Proton traversing back across the IMM via Complex V powers a stepwise rotation in the synthase’s F0 c-ring motor, which in turn drives ATP synthesis by the F1 motor. The establishment and maintenance of Δψm are absolutely required for healthy mitochondrial function. Beyond energy production, mitochondrial Ca2+ buffering activity and mitochondrial protein import depends on and influences Δψm (Hansford and Zorov, 1998; Kulawiak et al., 2013).
Mitochondrial power comes with risk. The ETC is a significant source of intracellular reactive oxygen species (ROS) – such as superoxide anion radicals (O2•-) and hydrogen peroxide (H2O2) – and secondary reactive nitrogen species (RNS) (Loschen et al., 1974). Dysregulation of the ETC following TBI or other acute/chronic CNS pathologies can prompt high energy electrons to “jump the track” and react with molecular oxygen (O2) to form O2•- (Jastroch et al., 2010). Aside from the respiratory complexes, several dehydrogenases essential for the physiology of neurons can generate O2•- / H2O2 in mitochondria. Chief among them is the TCA cycle’s α-ketoglutarate dehydrogenase (α-KGDH), whose E3 subunit generates O2•- / H2O2 at a rate dependent on the NADH/NAD+ ratio (Adam-Vizi and Tretter, 2013). α-KGDH-associated ROS production is enhanced in several naturally occurring E3 subunit mutants (e.g. G194C common in Ashkenazi Jewish and some Arab Muslim populations) (Hong et al., 2003; Shaag et al., 1999). In generating acetyl-CoA for the TCA cycle, pyruvate dehydrogenase complex (PDHC) activity similarly yields significant amounts of H2O2 (Mailloux et al., 2018; O’Brien et al., 2017). Finally, mitochondrial α-glycerophosphate dehydrogenase (α-GPDH, GPD2) is an IMM protein that, together with cytosolic α-GPDH (GPD1), helps shuttle NADH-derived reducing equivalents across the IMM. As mitochondrial Ca2+ levels increase, GPD2-associated ROS production increases and normal shuttling function is subsequently inhibited. Regardless of origin, nascent O2•- is rapidly dismutated to H2O2 by nearby mitochondrial manganese superoxide dismutase (MnSOD, SOD2). H2O2 is then reduced in mitochondria by the glutathione peroxidase system or diffuse out into cytosol and be eliminated by catalase. Alternatively, H2O2 can be utilized by a number of metallo-enzymes, particularly heme-peroxidases, as a source of oxidizing equivalents to feed a variety of oxidation reactions yielding oxidized products, including oxidized lipids with signaling functions (Kanner et al., 1987). Unfortunately, these systems can be overwhelmed under pathological conditions, leading to exacerbation of mitochondrial injury and eventual Δψm depletion. Even with brain’s reliance on mitochondrial respiration, it is also comparatively sensitive to oxidative insult, due in part to its: 1) unusually high concentration of molecular oxygen (using ~20% of body O2 supply), 2) relatively low catalase expression (10% of the liver), 3) a high iron/ascorbate ratio that has been shown to be pro-oxidant, and 4) enrichment of oxidizable polyunsaturated fatty acids (Marklund et al., 1982; Zaleska et al., 1989). Therefore, redundancies exist both in and out of the mitochondria to help keep tight control on cells’ redox balance.
1.2: Mitochondria & Glutathione Regulation
Members of the glutathione peroxidase (GPX) family are among the most prominent and abundant alternatives to the SOD/catalase system. They function to preserve neuronal mitochondrial function and redox balance. Eight families of human glutathione peroxidases have been identified (Brigelius-Flohé and Maiorino, 2013). GPX1–4 and 6 rely on selenocysteine for their catalytic cycle, while GPX5, 7, and 8 contain standard cysteine. H2O2 is the primary oxidizing equivalent for GPX1, 3, 7, and 8. However, GPX1 and 3 can additionally reduce small organic hydroperoxides, including soluble peroxidated free fatty acids. GPX4 is unique in its ability to reduce membrane-associated, PL-esterified peroxidated cholesterols, and polyunsaturated fatty acids (Brigelius-Flohé and Maiorino, 2013). GPX4 is localized throughout the cell, including in mitochondria (m-GPX4), where there is a high preponderance of ROS and oxidizable polyunsaturated PLs.
Unlike catalase, GPX relies on glutathione (γ-glutamylcysteinylglycine, GSH) tripeptide to reduce H2O2 or organic peroxides. Mechanistically, GPX4 and other selenocysteine GPXs utilize a two-stage ping-pong mechanism. The first step reduces the target lipid peroxide (L-OOH → L-OH) and oxidizes GPX’s selenocysteine residue. In step two, GSH is oxidized to regenerate reduced selenocysteine and active enzyme (Brigelius-Flohé and Maiorino, 2013). Glutathione reductase then converts oxidized GSH (GSSG dimers) back into free GSH using reducing potential derived from NADPH. Alternatively, GSH-mediated ROS detoxification may also proceed non-enzymatically. In this case, GSH reacts with hydroxyl radical, nitric oxide, or superoxide radical (Dringen, 2000) producing the strongly oxidizing thiyl radical (GS•). The reactive thiyl radical is a liability for cells. Therefore, this non-enzymatic mechanism is likely not the primary pathway of GSH-mediated protection, but thiyl radical abundance, measured by electron paramagnetic resonance spectrometry (EPR), can reflect the cellular redox state (Sagristá et al., 2002; Sturgeon et al., 1998).
Synthesis of GSH occurs only in the cytosol and is then imported into most organelles (Griffith and Meister, 1985). GSH is required for many vital functions: 1) modulating protein s-glutathionylation (Dalle-Donne et al., 2009); 2) supplying cysteine for protein synthesis (Meister and Anderson, 1983); 3) DNA synthesis (Suthanthiran et al., 1990); 4) immune response (Furukawa et al., 1987); 5) metal ion (iron) storage and homeostasis (Jozefczak et al., 2012); 6) maintaining protein redox states (e.g. glutaredoxins); and 7) providing an antioxidant defense (Lu, 2009). As mitochondria are the primary ROS sources within cells, a correspondingly significant proportion of GSH is partitioned to mitochondria (10–15% total cellular GSH) (Griffith and Meister, 1985). Import is accomplished by two IMM membrane anion transporters, the dicarboxylate carrier (DIC, SLC25A10) and the 2-oxoglutarate carrier (OGC, SLC25A11) (Lash, 2006; Meijer et al., 1972). Further, while final GSH synthesis takes place in the cytosol, import of the GSH precursor, cystine (Cys), by the cystine/glutamate antiporter (system Xc−, SLC7A11) is regulated by cytosolic glutamate (Glu) concentration. A high cytosolic-to-extracellular Glu gradient promotes cystine uptake and downstream GSH synthesis. Cytosolic Glu levels are, in turn, controlled by mitochondrial metabolism and glutaminolysis. Within the mitochondrial matrix, glutamine (Gln) is converted to Glu by glutaminase (GLS). Glu maybe then transaminated into TCA cycle intermediates (e.g. α-ketoglutarate) by glutamate dehydrogenase (GDH) or by the alanine/aspartate transaminases (TAs) (Watford, 2000). Therefore, the levels of cytosolic Glu and Cys are ultimately influenced by the activity of the mitochondrial TCA cycle and specifically, GDH and TA. GSH insufficiency leads to inactivation of GPX4/GSH system and accumulation of peroxidated PLs (PL-OOH). Accumulation of PL-OOHs – particularly peroxidized phosphatidylethanolamines (PEox) – in the setting of GPX4 inhibition (or GSH depletion) results in ferroptotic cell death (Kagan et al., 2017). This was observed following experimental TBI (Kenny et al., 2019). Therapeutically administered glutathione precursors such as n-acetylcysteine (NAC) and a more CNS bioavailable version, n-acetylcysteine amide (NACA), have demonstrated promise in experimental TBI and spinal cord injury (SCI) (Pandya et al., 2014; Xiong et al., 1999). A small double-blind, placebo controlled clinical study showed that early NAC administration (<24 h) following blast-induced mild TBI was linked to reduced neuropsychological deficit and greater rate of symptom resolution by 7-days post-TBI (Hoffer et al., 2013). Safety and efficacy studies of NAC and NACA in treatment of TBI are ongoing (Clark et al., 2017). In summary, mitochondrial metabolic activity influences both cytosolic Glu and Cys levels and, in turn, regulates GSH production, corresponding GPX activity, and related regulated cell death signaling in TBI.
1.3: TBI Mitochondrial Pathology Overview
Beginning in hours and continuing for weeks, the secondary phase of TBI is marked by excitotoxic, pro-death, and inflammatory responses (Prins et al., 2013). Diffuse depolarization of neurons occurs in the hyperacute stage of injury (first 24 hours after inciting incident). This event results in an unregulated release of excitatory neurotransmitters, primarily glutamate and aspartate (Fig. 1) (Bullock et al., 1998; Faden et al., 1989). Increased levels of extracellular glutamate can last as long as 4 days with initial glutamate levels (first 24 hours, >20 μmol/L) and concentration positively correlating with worse outcomes (Chamoun et al., 2010). Excitotoxic injury results from overactivation of N-methyl-D-aspartate (NMDA) receptors, α-amino-3-hydroxy-5-methyl-4-isoxazoleproprionic acid (AMPA) receptors, and various-gated Na+ and Ca2+ channels, which leads to a swift rise in neuronal cytosolic Ca2+ concentration (Arundine and Tymianski, 2004). Increases in cytosolic Ca2+ activate and are potentiated by Ca2+-dependent phospholipases and proteases. Examples of the latter include calpains and downstream caspases that induce either necrotic and apoptotic death (Jastroch et al., 2010). If death is not outright, Ca2+ is buffered by mitochondrial-associated endoplasmic reticulum and mitochondria using various transporters, including: mitochondrial calcium uniporter (mCU), uncoupling proteins (UCP), H+/Ca2+, and Na+/Ca2+ exchangers (Williams et al., 2013). Calcium influx is associated with an early transient Δψm hyperpolarization that drives increased ROS and RNS production. RNS levels are particularly increased due to the upregulation of inducible nitric oxide synthase early after TBI (Bayır et al., 2005). ROS/RNS modification of nuclear and mitochondrial DNA, lipids, and proteins further exacerbates the injury and interferes with recovery mechanisms (Opii et al., 2007). Therefore, the initial hyperpolarization exacerbates injury and gives way into depolarization. Finally, Δψm-reliant processes fail and assembly of the mitochondrial permeability transition pore (mPTP) begins.
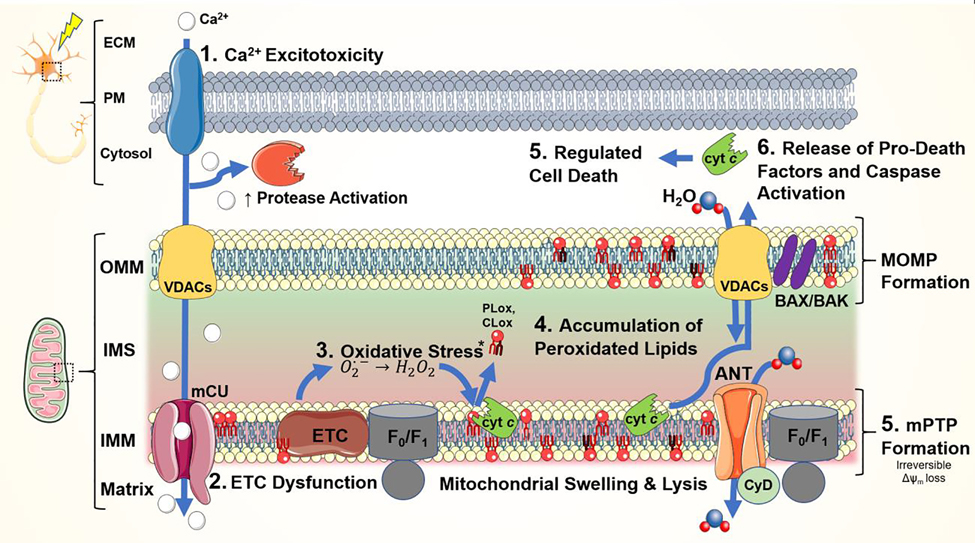
Figure 1. Mitochondria-focused mechanisms of neuronal injury following TBI.
1) Widespread neuronal depolarization results in the unregulated release of excitatory neurotransmitters and the activation of their corresponding ion channels. Increased cytosolic Ca2+ is buffered, in part, by the mitochondria leading to disruption of the mitochondrial membrane potential (Δψm). Ensuing 2) electron transport chain (ETC) dysfunction causes protracted increases in 3) ROS & RNS production. Injury becomes self-perpetuating as endogenous oxidant stress response systems are overwhelmed. 4) Mitochondrial lipids, especially cardiolipin (CL), are important sources of oxidized free fatty acid inflammatory mediators. Both non-enzymatic and cyt c/CL peroxidase-mediated enzymatic CL peroxidation occurs in the setting of high ROS levels. 5) Permeabilization of the mitochondrial membranes and oxidation of CL prompts the release of pro-death factors – ultimately leading to neuronal death. (OMM, outer mitochondrial membrane; IMS, intermembrane space; IMM, inner mitochondrial membrane; ANT, adenine nucleotide translocator; BAK/BAX, Bcl-2-associated X protein; CyD, cyclophilin D; F0/F1, ATP synthase; mCU, mitochondrial calcium uniporter, VDAC, voltage-dependent anion channel)
mPTP formation is the point of no return and when Δψm loss becomes irreversible (Szabó and Zoratti, 1991). The exact constituents of the mPTP are still debatable, but three primary suspects have been described (Fig. 1) (Halestrap, 2009). The first component is adenosine nucleotide transporter (ANT), a thiol-containing IMM protein. In its oxidized state, ANT binds cyclophilin D (CyD) and possibly other mitochondrial proteins. It is believed that ANT, CyD, and OMM-localized VDACs form the mPTP complex (Tsujimoto and Shimizu, 2007). ATP synthase subunit c may also participate in the formation of the mPTP conduit (Giorgio et al., 2013; Neginskaya et al., 2019). However, its precise role in the mPTP complex remains controversial; recent studies indicate mPTP formation persist despite mutations in ATP synthase subunits (He et al., 2017). The final mPTP complex creates continuous connection bridging the mitochondrial matrix, IMM and cytosolic space. This permits the release of mitochondrial proteins, such as cyt c, SMAC/diablo and apoptosis-inducing factor (AIF), that trigger pathways of caspase-dependent and -independent death (Galluzzi et al., 2009). Further OMM permeabilization occurs secondary to VDAC and other OMM proteins conformational changes induced by BCL-2-associated X protein (BAX) and BCL-2 antagonist or killer (BAK) that lead to the formation of the mitochondrial outer membrane permeabilization (MOMP) channel (Tait and Green, 2010).
Together, the formation of MOMP and mPTP conclusively deplete Δψm and eventually lead to osmotic swelling then lysis of mitochondria (Kalkavan and Green, 2018; Kitsis and Molkentin, 2010). Consequently, IMS proteins are released into the cytosol. Interactions between the released cyt c, apoptotic protease activating factor (Apaf-1) and ATP yields apoptosomes that active caspase 3 and downstream caspases (Ola et al., 2011). In summary, the combined inhibition of mitochondrial bioenergetic function leads to GSH depletion and ferroptotic cell death while also contributing to the irreversible release of pro-apoptotic proteins that ensures the neuronal death (Kitsis and Molkentin, 2010).
2.0: Mitochondrial Lipid Redox Signaling
2.1: CNS Pathology & Lipids Overview
Abnormalities in lipid abundance and metabolism are observed in numerous acute and chronic neurological diseases. Brain characteristically is enriched with oxidizable polyunsaturated lipids and overall possesses a high lipid-to-protein ratio (O’Brien and Sampson, 1965). The advent of high fidelity liquid chromatography tandem mass spectrometry (LC-MS/MS) lipidomics methods has aided the recognition of signaling roles lipids play in the CNS (Anthonymuthu et al., 2018). With these advances, the extent and consequences of PL oxidation in CNS insults have begun to emerge. For example, analysis of CSF PLs from ten TBI patients showed a distinct difference in the speciation, time course, and abundance of PL between those that survived vs succumbed to their injuries (Pasvogel et al., 2010). Similarly, levels of polyunsaturated free fatty acids, namely arachidonic, docosahexaenoic, and linoleic acids, were found to be increased in CSF of TBI patients compared to healthy controls (Pilitsis et al., 2003). In acute cerebral ischemia, membrane PL alterations are among the earliest pathological events observed (Bazan and Rodriguez de Turco, 1980). Plasma levels of the global lipid peroxidation marker, F2-isoprostane (F2-isoP), correlates with the infarct growth in stroke (Lorenzano et al., 2018). Even chronic conditions such as Alzheimer’s disease (AD) display altered levels of PL, ceramides, and sulfatides within the brain, CSF, and blood (Kosicek and Hecimovic, 2013). The following sections will focus on the contributions of mitochondrial PL signaling and injury in neurological diseases, including TBI.
2.2: Mitochondrial Lipid Composition
Every organelle has its own unique lipid signature that is essential for its structure and function (van Meer et al., 2008). Like other organelles, mitochondrial membranes are largely composed of phospholipids (PL) with the following unique characteristics: 1) mitochondria are almost devoid of sphingolipids, phosphatidylserine (PS), and sterols; 2) they have a high protein-to-phospholipid ratio compared to other organelles; 3) the mitochondria-specific PL, cardiolipin (CL), represents 15–20% of all mitochondrial lipids; 4) mitochondrial PLs are enriched with unsaturated and oxidizable fatty acyl chains (Daum, 1985). Mitochondrial membrane PLs are asymmetrically distributed across the OMM and IMM (Daum and Vance, 1997). In particular, CL and phosphatidylinositol (PI) are significantly enriched within the inner leaflet (matrix side) of the IMM, whereas phosphatidylethanolamine (PE) and phosphatidylcholine (PC) are randomly distributed across the IMM and OMM (Horvath and Daum, 2013). Lipid exchange between mitochondria and smooth endoplasmic reticulum (sER) is critical for import and overall maintenance of mitochondrial lipid composition (Tatsuta et al., 2014). For example, PS transferred from sER to mitochondria is rapidly decarboxylated to PE in mitochondria. Mitochondria contain synthetic machinery to produce four major classes of glycerophospholipids, namely: CL, PE, phosphatidic acid (PA), and phosphatidylglycerol (PG) (Flis and Daum, 2013; Vance and Vance, 2004).
CL is the one true mitochondria-specific lipid class. Canonical mitochondrial CL synthesis involves two key phases: 1) de novo CL synthesis via cardiolipin synthase that produces hetero-acylated, nascent-CL; and 2) secondary remodeling steps carried out by phospholipid-lysophospholipid transacylases, such as Tafazzin, that convert hetero-acylated CLs to homo-acylated, mature CL (Schlame, 2008). The identity of CL’s new acyl chain depends on both the transacylase specificity and PUFA abundance. Apart from the cerebellum, the brain expresses relatively little Tafazzin and has a correspondingly heterogenous CL population vs most other tissues (e.g. heart). Complete loss-of-function mutations in the gene which encodes Tafazzin (TAZ) results in a rare X-linked disorder called Barth Syndrome (BTHS). Barth syndrome is best known as a cardiac disease but has been acknowledged as a multi-syndrome disorder, including the CNS causing various cognitive deficits. Mice that contain mutated/knockdown TAZ have decreased CL – most prevalent in long-chain polyunsaturated fatty acids (PUFA) (22:6, 22:5 and 22:4). Further, loss of intact CL was matched by accumulation of monolyso-CL (mCL) (19-fold increase observed in whole brains from TAZ knockdown vs wild-type mice), which were enriched in short-chain saturated 16:0/16:1 and 18:0/18:1 fatty acids (Cole et al., 2018). The result was CNS mitochondria from these mice showed an increased state I respiration, attenuated spare capacity, and increased ROS generation. While motor function is retained, defects in memory retention in line with hippocampal degeneration are observed (Cole et al., 2018). These findings demonstrate how proper CL speciation and remodeling is critical for normal brain mitochondrial function and memory.
Though the results from many studies linked neurological diseases with altered mitochondrial lipid regulation, the precise roles of mitochondrial lipids and their oxidation products long remained unclear (Nakahara et al., 1991). The recent advent of high-fidelity liquid chromatography-tandem mass spectrometry (LC-MS/MS) lipidomics approaches has allowed for study of low abundance but important mitochondrial signaling lipids, like cardiolipin. These new approaches have provided a wealth of information on mitochondria-specific lipid injury during neurological diseases.
2.3: Linking CNS Injury & Mitochondrial Lipid Perturbations
Both acute and chronic CNS disorders commonly lead to increased ROS/RNS levels in mitochondria (Salim, 2017). This, combined with abundance of highly oxidizable polyunsaturated acyl chain-containing PLs, creates an ideal environment for lipid peroxidation in mitochondria (Vladimirov et al., 1980). Throughout the cell and in mitochondria, polyunsaturated PL peroxidation proceeds either non-enzymatically through a transition metal ion (iron/copper)-initiated free radical reaction or enzymatically catalyzed by cytochrome c, cyclooxygenases (COX) and lipoxygenases (LOX). Compared to the free radical mechanisms, enzymatic oxidation generates specific, structurally-defined PL peroxidation products that serve as specific downstream signals (Niki, 2009). If not rapidly reduced by the GPX4/GSH system, PL-OOH species break down non-enzymatically to yield a suite of small reactive electrophiles. These reactive electrophiles are a liability, and unabated, detrimentally alkylate DNA (nuclear & mitochondrial) and key nucleophilic protein residues.
One breakdown product of PL oxidation, 4-hydroxynonenal (4-HNE), is particularly well documented as increased in both acute and chronic CNS pathologies (Liu et al., 2011; Zhong and Yin, 2015). 4-HNE is a potent protein-modifying electrophile that inhibits mitochondrial respiration (Vaishnav et al., 2010). Studies in controlled cortical impact (CCI) and contusion spinal cord injury (SCI) showed increased mitochondrial 4-HNE beginning within 3 hours and lasting at least 72h after injury (Carrico et al., 2009; Singh et al., 2013; Sullivan et al., 2007). Likewise, modification of the ETC complex V, ATP synthase α subunit by 4-HNE in the entorhinal cortex of Braak stages I/II Alzheimer’s (AD) patients suggests the involvement of mitochondrial lipid peroxidation in AD neurofibril formation (Siegel et al., 2007; Terni et al., 2010). In Parkinson disease (PD), 4-HNE has been detected in Lewy bodies. Increased accumulation of 4-HNE alkylation products is linked to development of mitochondrial dysfunction in parkin-deficient mice (Palacino et al., 2004). Similar observations have been made in the Huntington disease models as well (Lee et al., 2011). Although 4-HNE is a good marker of global lipid peroxidation, it is nonspecific and cannot provide conclusive information about the identity of its upstream parent oxidized lipid. This is an important consideration because understanding the identity of the intact parent lipid can reveal the localization, drug targeting, and potential signaling roles of oxidized lipids in both pathologic and reparative processes.
2.4: Enzymatic Cardiolipin Oxidation in TBI
The specific signaling roles of oxidized mitochondrial lipids in CNS pathology have been well demonstrated for CL. Alterations in CL metabolism show the best link between neuronal injury and druggable enzymatic mitochondrial lipid oxidation (Fig. 2). Selective oxidation of brain CL was observed in TBI models starting from 3h post-injury (Bayir et al., 2007; Chao et al., 2018). Upon injury, the asymmetric distribution of CL collapses and cytochrome c (cyt c) can interact and bind CL (Chao et al., 2019). The initial interaction is via the electrostatic interface of the positively charged lysines in cyt c and two negatively charged phosphates of CL (Kagan et al., 2009; Kooijman et al., 2017). This interaction leads to a considerable disruption of the native hexa-coordinated compact structure of the protein. The notable conformational effects are: 1) opening of the heme crevice; 2) decreased hydrophobic core volume; 3) disruption of the heme iron (Fe) to Met80 bond; 4) distal movement of Trp59 from the heme leading to change in the protein tertiary structure; 5) generation of a mix of penta-coordinated and His33/His26 hexa-coordinated heme (Droghetti et al., 2006); 6) decreased Fe(II)/Fe(III) couple redox potential that is ~400 mV more negative than native cyt c, meaning it can no longer shuttle electrons for the ETC (Barker et al., 1996; Di Marino et al., 1987); and 7) acquisition of peroxidase activity. Breaking of the Met80-Fe bond further permits access of substrate into the newly formed peroxidase active site. The newfound peroxidase activity relies on several residues, including His26, His33, Arg38, and Arg91 that aid in the H-bonding and proton shuttling (McClelland et al., 2014), as well as four tyrosine and a tryptophan residue that serve as radial intermediates in the peroxidase catalytic cycle (Tsong, 1976). Heme-containing peroxidases can oxidize substrates using different inorganic (e.g. H2O2) or, more rarely, organic peroxides (e.g. lipid hydroperoxides). The Cyt c/CL complex has superior lipid hydroperoxidase activity compared to its H2O2 peroxidase activity. It has an affinity for anionic lipid substrates, including oxidized CL (CL-OOH, CLox). In turn, CLox is a good source of oxidizing equivalents for membrane-associated cyt c/CL (Kagan et al., 2009). CL is most enriched near ETC Complex I, III, and IV – potent sources of pathology-induced H2O2 production. Therefore, the proximity, abundance, and electrostatic affinity of CL to the newly formed cyt c/CL peroxidase enzyme makes CL a preferred lipid peroxidation substrate (Fig. 2) (Ardail et al., 1990). CL peroxidation by cyt c in the presence of mitochondrially-generated H2O2 prompts pro-apoptotic mitochondrial permeabilization and cytosolic cyt c release (Kagan et al., 2005). Alternatively, the liberation of CL’s oxidized acyl chains through the action of phospholipases (e.g. iPLA2γ) yields a suite of pro- and anti-inflammatory signaling molecules.
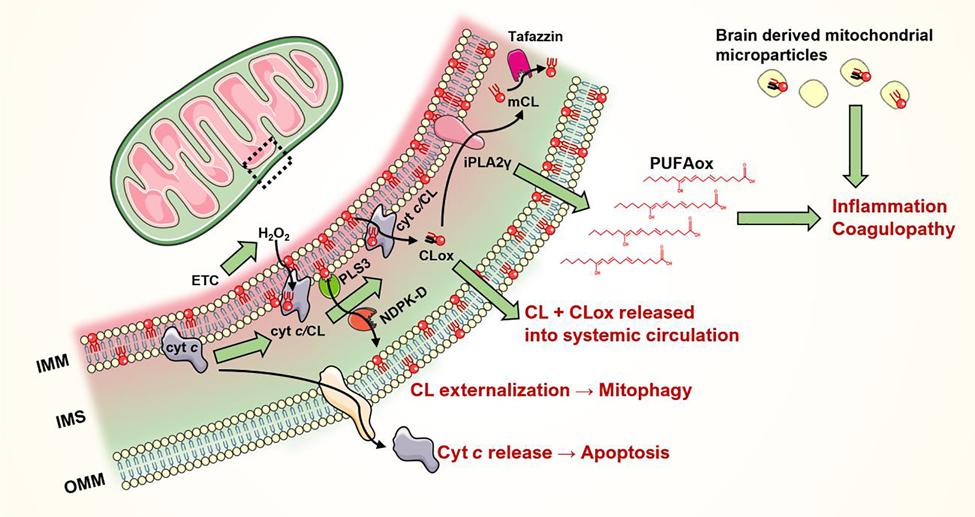
Figure 2. Mitochondrial CL signaling in brain injury.
The inner leaflet of mitochondrial inner membrane (IMM) is enriched in cardiolipins (CL). Upon injury, CLs are translocated to the outer leaflet of IMM, where it forms a complex with cytochrome c. The cytochrome c/cardiolipin (cyt c/CL) complex is a potent peroxidase that oxidizes the polyunsaturated acyl chains of CL (or other anionic lipids, to a lesser extent). Oxidation of CL (CLox) leads to the translocation of cyt c to the cytoplasm and subsequent apoptosis. IMM-localized non-oxidized CL are translocated to the outer membrane by the phospholipid scramblase 3 (PLS3) and mitochondrial nucleoside diphosphate kinase (NDPK-D). Externalized CL serve as a “eat me” signal for mitophagy. CLs previously oxidized by the cyt c/CL complex undergo hydrolysis by calcium-independent phospholipase A2 - gamma (iPLA2γ) to release oxidized polyunsaturated fatty acids (PUFAox). These PUFAox serve as lipid mediators that regulate inflammation and can lead to coagulopathy. Intact tetraacylated CLs can be regenerated from monolyso-CL (mCL) by phospholipid-lysophospholipid transacylases (e.g. tafazzin). CLs present on the brain-derived mitochondrial microparticles also regulates the injury-related inflammation and coagulation.
2.5: Inflammatory & Pro-coagulant Roles of Cardiolipin in TBI
Apart from signaling apoptosis, CLox is also involved in the generation of various lipid mediators (Fig. 2). Hundreds of unique oxidized cardiolipins species are produced by the cyt c/CL complex after TBI (Ji et al., 2012). They are then hydrolyzed by mitochondrial calcium-independent phospholipase A2 (iPLA2γ) (Tyurina et al., 2014). Hydrolysis of CLox following experimental TBI produces a plethora of well-characterized inflammatory mediators such as hydroxyoctadecadienoic acids (HODEs), hydroperoxyoctadecadienoic acids (HpODEs), and hydroxyeicasotetraenoic acids (ETEs) (Fig. 2) (Liu et al., 2017). These mediators are known for regulating pro- and anti-inflammatory responses (Dennis and Norris, 2015). For example, 9-HODE, 12-HETE are involved in the pro-inflammatory response such as the recruitment of leukocytes (Vangaveti et al., 2010). In contrast, 13-HODE, 15-HETE, 14,15-epoxyeicasatrienoic acid (EpETrE) and 11,12-EpETrE are known to promote anti-inflammatory responses (Roman, 2002). Levels of these pro and anti-inflammatory signals increase by 4h after CCI, fitting well with the timeline of CL oxidation (Anthonymuthu et al., 2017). Treatments targeting CL oxidation or CL hydrolysis attenuate neurodegeneration and improve neurocognitive outcome after CCI (Chao et al., 2018; Ji et al., 2012). These data suggest that both oxidation and hydrolysis of CL play important roles in neuronal injury response to TBI.
Brain-derived CLs are released into systemic circulation acutely following TBI (Anthonymuthu et al., 2019a). Some of the released brain CLs are likely present in the form of brain-derived microparticles (Zhao et al., 2016). However, the specific identities and oxidation states of various CLs in these microparticles has not been analytically verified (Fig. 2). Surface exposure of anionic phospholipids, (oxidized) PS and CL, promotes phagocytosis of cells and particles by macrophages and microglia (Balasubramanian et al., 2015). Additionally, CLs and PS present in microparticles are potent anti-coagulation factors contributing to trauma-associated coagulopathy (Midura et al., 2015; Tian et al., 2015). Coagulopathy is common in TBI patients (Stein and Smith, 2004), and it is associated with poor outcome (Sun et al., 2011). Thus, prevention of coagulopathy may be a potential therapeutic target in TBI. In line with this, induction of phagocytotic clearance of brain-derived microparticles by apoptotic cell-scavenge factor lactadherin was shown to prevent TBI-Induced coagulopathy and improve outcome in rodents (Zhou et al., 2018b). In most cases, this will lead to an anti-inflammatory phenotype, but it has also been suggested that clearance of brain-derived microparticles by microglia produces a secondary pro-inflammatory response following intracerebral injury (Kumar et al., 2017). With regards to extracellular CL, it causes immune-paralysis of innate immune cells by preventing the formation of TLr4-Md2 hetero-oligomeric complex required for activation of macrophages/microglia to produce and release cytokines (Balasubramanian et al., 2015).
2.6: Release of Cardiolipin in Systemic Circulation
TBI is accompanied by a reduction of CL content in the contusional and peri-contusional regions (Chao et al., 2018; Sparvero et al., 2016). Despite mounting evidence suggesting the release of CL into the systemic circulation, identification of CL in plasma was not possible until recently. Technical limitations prevented accurate identification and speciation of low abundance CL products circulating in plasma following CNS injury. Exploiting the power of mass spectrometry-based lipidomics approach; we recently developed a sensitive LC-MS/MS method for the identification of circulating CL. The brain expresses a uniquely diverse CL profile, rich in long-chain PUFA, due to its relatively low CL remodeling activity. In comparison, other tissues express a relatively homogenous and lower molecular weight CL population, dominantly composed of tetralinoleic CL (TLCL). Therefore, the appearance of brain-type CL in plasma early after TBI was hypothesized to correlate with brain injury severity. Moreover, the approach may provide benefit in cases of poly-trauma where brain-type CL (and associated CNS injury) can be distinguished from CL released from non-CNS tissue. We found increased plasma brain-type CL content after clinical or experimental cardiac arrest-induced ischemic brain injury and CCI in rats (Anthonymuthu et al., 2019a). In animal models of global cerebral ischemia and trauma, the release of brain-specific CL into systemic circulation correlated with the loss of brain CL. Plasma concentration of brain-type CL sampled within 6 hours post-cardiac arrest correlated with the brain injury severity and long-term outcome in a cohort of 41 patients (Anthonymuthu et al., 2019b). Therefore, detection and quantification of plasma CL levels have potential utility as an early marker of traumatic (or ischemic) brain injury severity and therapeutic response.
2.7: Therapeutic Targeting of Mitochondrial Phospholipid Oxidation
The various pathologic effects of mitochondrial PL oxidation make therapeutic targeting of these pathways an exciting avenue for TBI research. By virtue of their lipophilic nature, many of the antioxidants are effective in preventing mitochondrial PL peroxidation without specific mitochondrial targeting (Maiti et al., 2018). However, previous TBI studies showed non-targeted global antioxidants administration (e.g. vitamin E, CoQ10, or nitroxyl radicals) provided little to no benefit on survival or neurocognitive function. This failure was likely, in part, due to lack of outcome specificity (i.e. understanding the source/localization/identity of oxidized PL), poor localization of therapeutic compound to the pathogenic target site, and/or deleterious extramitochondrial off-target and side-effects (Lamade et al., 2019). These deficiencies have been addressed by 1) LC-MS/MS based lipidomics have allowed deeper understanding of TBI’s effects on mitochondrial lipids and their redox states, and 2) by the use of specific mitochondria-targeted antioxidants and enzyme inhibitors that overcome localization concerns. Mitochondrially-targeted therapeutics have shown preclinical success in both ischemic and traumatic brain injury. They are composed of two key components: 1) a mitochondrial targeting/binding moiety; and 2) an antioxidant, electron scavenger, or enzyme inhibitor moiety. Lipophilic but positively charged molecules, such as triphenyphosphonium ion (TPP+), are drawn to mitochondria’s highly negative Δψm matrix. Based on the Nernst equation, passively transported cations accumulate 10x per 61 mV trans-membrane potential difference at 37°C. For neurons with a membrane resting potential of −70 mV, cation-conjugated drug accumulates 5 to 10-fold in cytosol vs extracellular fluid and 100 to 500-fold more in mitochondria vs cytosol at equilibrium. Two key examples of Δψm-driven mitochondria-localizing drugs that showed promise in TBI are Mito-Q (TPP-CoQ10) and SS-31 (Zhu et al., 2018). Treatment of mice subjected to a weight drop model of TBI with Mito-Q was shown to improve neurological deficits, alleviate brain edema and inhibit cortical neuronal apoptosis. This improvement was associated with the reduction in levels of nonspecific lipid peroxidation end-product (e.g. malondialdehyde) and greater mitochondrial membrane integrity. These together point towards likely inhibition of mitochondrial lipid peroxidation by Mito-Q (Zhou et al., 2018a). The results of the Mito-Q clinical trials in Parkinson’s Disease have shown Mito-Q to be nontoxic, even at high concentrations (Snow et al., 2010). The drawback of Δψm-reliant strategies is that pathology (i.e. TBI) can significantly dissipate Δψm, causing a significant reduction in drug localization efficiency. Moreover, movement of TPP+ and other cations into the matrix necessarily dissipates Δψm, conceivably exacerbating injury at high concentrations. In contrast, development of Δψm-independent mitochondrial localization strategies can minimize these concerns while improving chances for consistent, efficacious therapeutic effects. This alternate strategy takes advantage of a targeting moiety’s affinity towards mitochondria-specific/enriched proteins or lipids. For example, the short peptide, hemigramicidin (HS, a derivative of gramicidin S), enriches in mitochondria >100-fold. This effect is likely propelled by HS’s affinity to IMM-localized CL rather than Δψm.
One particularly promising example of this alternative strategy is the mitochondria-targeted nitroxide is XJB-5–131. It is comprised of a hemigramicidin (HS) peptide conjugated to TEMPO. The HS-TEMPO conjugate can cross the intact blood-brain barrier while TEMPO by itself cannot (Ji et al., 2012). XJB-5–131 has been shown to attenuate lesion volume and improve behavioral deficits following experimental TBI. Mechanistically, XJB-5–131 and the structurally similar JP4–039 act as electron scavengers to prevent the formation of O2•- and H2O2. In the context of mitochondrial lipid peroxidation, reduced mitochondrial ROS limits substrate for the cyt c/CL peroxidase complex resulting attenuation of CLox generation and CL loss. Alternative strategies that decrease CL oxidation include mitochondrial-targeted imidazole-conjugated fatty acids that inhibit the peroxidase activity of the cyt c/CL complex (Kagan et al., 2015); or mitochondria-targeted oleic or stearic acids that promote remodeling of the CL lipidome into a less oxidizable form rich in mono-unsaturated fatty acids. The latter remodeling approach has shown potential in various disease states where mitochondrial generation of ROS is a key in the pathophysiology of the disease (Atkinson et al., 2011; Jiang et al., 2014; Tyurina et al., 2012). Further development and clinical translation of mitochondria-targeted therapies will require detailed studies of unique pharmacodynamic and pharmacokinetic properties of these compounds.
3.0: Mitochondrial Quality Control
3.1: Mitochondrial Fate Decisions
Mitochondrial injury following TBI produces a litany of pro-survival and death effects, discussed previously. In reaction, the mitochondrial network is dynamically regulated to preserve functional mitochondrion units while removing potential liabilities. To maintain homeostasis, the mitochondrial quality control system (MQC) governs the opposing pathways of mitochondrial fission and fusion. Broadly, fusion is a consolidating force. It enables salvage of intact, functional mitochondrial components to increase cristae density and maximize bioenergetic function necessary for post-injury survival and eventual recovery. Conversely, fission has two divergent roles: removal of damaged, dangerous mitochondrion or proliferation and expansion of the mitochondrial network (Youle and van der Bliek, 2012). Mitophagy is responsible for the ultimate recycling of fissioned, dysfunctional mitochondria. An organelle-specific example of autophagy, mitophagy orchestrates the bulk turnover of mitochondria through an autophagosomal-lysosomal mechanism. Mitophagy provides potential benefits to injured neurons by reducing the mitochondrial contribution to vicious redox cycles, including pathways governed by oxidative lipid signaling (Chao et al., 2019; Diskin et al., 2005). However, there is a delicate balance between mitophagy’s beneficial effects vs harm – the latter harm stems from potentially overzealous elimination of pro-survival mitochondrial functional capacity. MQC system dysfunction contributes to multiple acute and chronic brain pathologies, including in traumatic and ischemic brain injury (Lipinski et al., 2015; Sarkar et al., 2014).
3.2: Mitochondrial Fusion
Mitochondrial fusion is a coordinated process involving the discriminative union of inner and outer mitochondrial membranes, as well as the contents of the intermembrane space and matrix. Once initiated, mitochondrial merging can proceed rapidly over several minutes (Jakobs et al., 2003; Twig et al., 2008), but the process maintains sufficient fidelity to ensure compartment-specific transfer of mitochondrial DNA, proteins, lipids, and metabolites. This autonomous mechanism can proceed in the absence of active nuclear instructions or de novo translation (Pernas and Scorrano, 2016). Fusion pathways are fundamentally conserved in eukaryotes (Okamoto and Shaw, 2005). Loss of fusion function is incompatible with life and leads to embryonic lethality in mice (Chen et al., 2003). Fusion is also a selective process with most mitochondrial contacts not resulting in union (Twig et al., 2008). Suitability of fusion candidates is dictated by Δψm with depolarized mitochondria being unlikely to fuse compared to their more functional counterparts. Several studies have demonstrated that uncoupling drugs not only increase mitochondrial fragmentation (fission) but also arrest fusion (Ishihara et al., 2003; Malka et al., 2005). This selective mechanism may help ensure that only healthy mitochondrial fragments are reconsolidated after TBI (Wu et al., 2016) and other acute/chronic neurodegenerative conditions (Knott et al., 2008).
At the center of both fusion and fission pathways are several highly conserved GTPases. These ‘large GTPases’ are distinguished from “small regulatory” GTPase (e.g. Ras-like regulatory GTPases or α-subunits of heterotrimeric G-proteins) not only by their size, but also, by their 1) oligomerization-dependent GTPase activation, 2) low GTP-binding affinities, 3) self-oligomerization capacity, and 4) affinity for lipid membranes (Praefcke and McMahon, 2004). The three critical GTPases of mitochondrial fusion are the Mitofusins (MFN1 and MFN2) and dominant optic atrophy 1 (OPA1). MFN1 and 2 regulate the first key steps of OMM fusion. As fusion-destined mitochondria approach, OMM-anchored MFNs dimerize and bridge the (~20 nm) gap between membranes. Subsequent GTP binding and hydrolysis induces a conformation change in the MFNs, bringing the outer membranes into closer proximity (Fig. 3A) (Cohen and Tareste, 2018). The lipid bilayers must then be integrated. This is again facilitated by MFNs, specifically the HR1 (alternatively, HB1) and HR2 domains. These domains were shown to facilitate the fusion of OMM-like PC liposomes in vitro and were required for mitochondrial fusion in cells (Daste et al., 2018). Once brought in proximity by MFNs’ GTPase activity, HR1 helps disrupt the OMM stability to allow integration of the two mitochondria. OMM regions containing lipid packing defects are most susceptible to HR1. These regions contain increased content of cone-shaped lipids, such as PE, which aid HR1 disrupt the bilayer and induce joining. Other bulky mitochondrial lipids may also play a role in fusion, such as PA and CL. Interestingly, while most CL is IMM-localized, TBI induces phospholipid scramblase 3 (PLS3)-mediated CL translocation to the OMM where it has been implicated in mitophagy, discussed below. Moreover, CL content is enriched at OMM-IMM contact sites (Paradies et al., 2014), a proposed nidus for final membrane fusion (Fritz et al., 2001). Changes in OMM lipid composition due to TBI-induced PL loss or oxidation (which changes lipid geometry and packing efficiency) likely serve as an additional regulatory checkpoint in fusion.
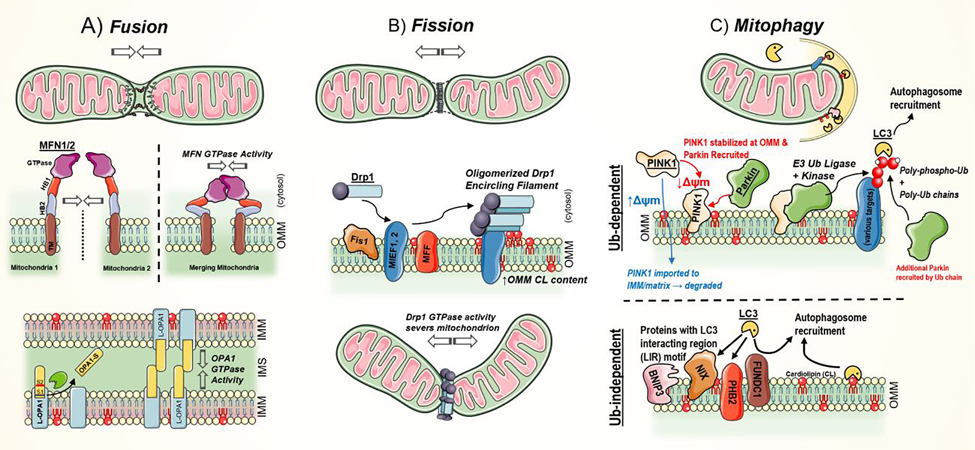
Figure 3. Mitochondrial quality control system and mitophagy.
A) Mitochondrial fusion leads to the joining of two mitochondrion. As fusion-destined mitochondria approach, outer mitochondrial membrane (OMM)-anchored mitofusins 1 and 2 (MFN1/2) dimerize and bridge the (~20 nm) gap between membranes. Subsequent MFN GTPase activity pulls the two organelles into proximity. The HB domains then participate in membrane destabilization and integration. This process is most common at sites of lipid packing detects – due to presence of bulky or conical lipids, like cardiolipin. B) Mitochondrial fusion leads to increased network fragmentation. The large GTPase, dynamin-related protein 1 (Drp1), drives fission by mechanically constricting and severing both the OMM and inner mitochondrial membrane (IMM). Soluble Drp1 is initially recruited to the OMM with assistance by OMM-bound adaptors: mitochondrial fission 1 (Fis1), mitochondrial fission factor (MFF), and mitochondrial elongation factors 1 & 2 (MIEF1, MIEF2). Increased OMM cardiolipin and phosphatidic acid content are linked to efficient fission activity. C) Mitophagy is a selective autophagy of mitochondria. Broadly, it can proceed in a ubiquitin (Ub)-dependent and -independent manner. In the Ub-dependent pathway, phosphatase and tensin homologue (PTEN)-induced punitive kinase 1 (PINK1) accumulates in the OMM when mitochondrial membrane potential (Δψm) is depleted. PINK1 recruits Parkin, an E3 Ub ligase. The PINK1/Parkin complex then proceeds to poly-phospho-ubiquitinate a range of mitochondrial proteins (e.g. translocase of the OMM, Miro, MFNs) and itself in feed-forward mechanism. By direct engagement or assistance of several adaptor proteins (OPTN, NDP52, p62, TAX1Bp1, and NBR1), LC3 (LC3-II) binds these poly-Ub and poly-phospho-ubiquitinated proteins, leading to autophagosomal engulfment. Ub-independent mechanisms similarly proceed in an LC3-dependent manner. However, in this case, LC3 directly engages various OMM protein (contain an LC3 interacting motif, e.g. BNIP3, NIX, PHB2, and FUNDC1) or select lipids, like cardiolipin (CL). This leads to autophagosome recruitment.
Relatively less information is known about the coordination of IMM fusion beyond the role of OPA1, which is present in eight isoforms (Del Dotto et al., 2018). Deletion of OPA1 1) decreases mitochondrial network connectivity (increased fragmentation), 2) leads to disorganization of cristae membranes (Frezza et al., 2006), and 3) overall decreases mitochondrial function (Δψm) (Di Pietro et al., 2017). Conversely, overexpression confers protection in neurodegenerative disease models (Ramonet et al., 2013). OPA1 is localized to the IMM, directed by a conventional Δψm-dependent N-terminal mitochondrial targeting sequence and affinity for IMM-localized CL (Liu and Chan, 2017). At the IMM, it is renatured into its membrane-bound long isoform (L-OPA1). Further proteolytic processing may occur, which produces the soluble short OPA1 isoform (S-OPA1). Only the L-OPA1 isoform is required for stress-induced mitochondrial fusion (Song et al., 2007). In contrast, both the S- and L-OPA1 appear to contribute to cristae organization in unstressed conditions at a 1:1 ratio (Ishihara et al., 2006). Stress-induced (decreased Δψm and reduced ATP levels) activation of OPA1 proteases (i.e. OMA1, YME1), enhances S-OPA1 production and depletes L-OPA1 (Alavi and Fuhrmann, 2013). Upregulation and multimerization of S-OPA1 further inhibits mitochondrial fusion and promotes OMM permeabilization. Therefore, the fusion activity is reduced in unhealthy mitochondria – including acutely after TBI (Fischer et al., 2016). Indeed, persistent depolarization and energetic failure simulate the core fission GTPase, dynamin-related protein-1 (DRP1), and eventual mitophagy.
3.3: Fission and Mitophagy
Fission pathways cause mitochondrion to fragment. In unstressed cells, fission supports mitochondrial proliferation and regionalization. This enables both the proper and persistent distribution of healthy mitochondria across the cell, including after injury. For example, following diffuse axonal injury, newly created mitochondria are segregated and then transported through repaired axons to sites of need (boutons, terminal synapses). Conversely, under active stress conditions, fission enables the mitophagy-mediated elimination of dangerous, dysfunctional mitochondrial components. It may also promote cytosolic release of pro-apoptotic factors (Estaquier and Arnoult, 2007). The large GTPase, DRP1, drives fission by mechanically constricting and severing both the OMM and IMM (Fig. 3B). Fission is regulated at both the level of DRP1 activity (Taguchi et al., 2007) and its docking with mitochondria. DRP1 lacks a membrane-binding domain and is recruited to the mitochondrial membrane with assistance by several adaptor proteins: mitochondrial fission 1 (Fis1), mitochondrial fission factor (MFF), and mitochondrial elongation factors 1 & 2 (MIEF1, MIEF2). Their precise mechanisms and relative contributions to DRP1-mediated fission remains unsettled (Otera et al., 2010; Samangouei et al., 2018; Zhao et al., 2011). More recent studies suggest the critical role of OMM lipid composition in anchoring and activation of both DRP1 and its adaptors (Macdonald et al., 2014). Notably, increased OMM CL content has been linked to increased fission activity (Bustillo-Zabalbeitia et al., 2014; Francy et al., 2017). As discussed previously, CL is externalized from the IMM to OMM with TBI (Chao et al., 2019; Chu et al., 2013; Ji et al., 2012). Conversely, increased PA abundance suppresses DRP1-driven fission (Adachi et al., 2016; Kameoka et al., 2018). Once membrane-bound and active, electron microscopy studies of yeast demonstrated that DRP1 forms large spiral structures (average 60 nm radius) (Mears et al., 2011). These DRP1monomers oligomerize into constricting filaments, encircling the mitochondria and breaking it apart (Bui and Shaw, 2013).
Newly generated mitochondrial fragments are then distributed or eliminated. Elimination takes place via mitophagy – the selective autophagic removal of mitochondria. Several different autophagic mechanisms have been described (Glick et al., 2010). The common denominator of these mechanisms is microtube-associated A1/1B-light chain 3 (LC3). More specifically, LC3 can be modified into its active LC3-II form following association with various lipids, including PE. LC3 binding to the OMM mediates the final recognition and targeting of mitochondria to the autophagosome. Mitophagy can proceed via ubiquitin (Ub)-dependent or -independent pathways (Fig. 3C).
First, the prototypical Ub-dependent pathway relies on the binding and activities of phosphatase and tensin homologue (PTEN)-induced punitive kinase 1 (PINK1) and Parkin. In functional mitochondria with normal Δψm, PINK1 is transported to the IMM where it is proteolytically degraded (Sekine and Youle, 2018). However, under stress conditions (including TBI), membrane potential dissipation inhibits PINK1 import and promotes PINK1 stabilization on the OMM. Subsequent OMM-localized PINK1 and other factors (e.g. BECN1) (Choubey et al., 2014) promote Parkin recruitment and activation (Aguirre et al., 2017). The E3 ubiquitin (Ub)-ligase, Parkin, and PINK1 together phospho-poly-ubiquitinate a range of OMM proteins (Harper et al., 2018; Shiba-Fukushima et al., 2014) in a feed-forward fashion (Ordureau et al., 2014). While not required for mitophagy, fission and DRP1 may serve to help restrict Parkin activity to dysfunctional mitochondrial subdomains (Burman et al., 2017). Conversely, Parkin-mediated mitofusin ubiquitination prevents the reintegration of segregated mitochondria while ubiquitination of Miro (Rho-GTPase, serving as an OMM-cytoskeleton anchor) inhibits mitochondrial trafficking (Shlevkov et al., 2016). Other E3 ubiquitin ligases also contribute to Parkin-independent pro-mitophagic OMM ubiquitination in a cell- and/or tissue-dependent manner (Palikaras et al., 2018; Szargel et al., 2016). Ultimately, phospho-poly-Ub OMM proteins are recognized by the central autophagic protein, LC3, with assistance by several adaptors (OPTN, NDP52, p62, TAX1Bp1, and NBR1) (Wong and Holzbaur, 2014). Finally, LC3 mediates autophagosome formation and final execution of mitophagy (Tanida et al., 2008).
Second, ubiquitin-independent mitophagy involves direct interaction of LC3 (LC3-II) and OMM proteins or CL. Several OMM proteins possess an LC3-interacting region (LIR) motif. These may directly mediate LC3 mitophagy (Hanna et al., 2012; Liu et al., 2012) while also promoting Parkin-dependent mitophagy (Gao et al., 2015; Yoo and Jung, 2018). Several have been characterized, including in their contributions to TBI-relevant hypoxic injury. They are BNIP3, NIX (Ma et al., 2019), PHB2 (Wei et al., 2017), and FUNDC1 (Chen et al., 2014; Zhou et al., 2017). However, the precise regulation of LC3’s interaction with these targets is not fully understood. Like its roles in both fusion and fission, CL also serves to mediate LC3-mediated mitophagy (Chu et al., 2014). Externalization of the CL from the inner leaflet of the IMM to the surface of the OMM functions as a mitophageal signal recognized by the LC3. Two translocations occur during externalization of CL from inner leaflet of IMM to the outer leaflet of the OMM. The first one is from the inner leaflet to the outer leaflet of IMM catalyzed by PLS3. The second one is from the outer leaflet of IMM to the inner leaflet of the OMM. The hexameric intermembrane space protein, NDPK-D (or NM23-H4), is responsible from translocation of CL from IMM to the OMM (Fig. 2) (Kagan et al., 2016). A R90D NDPK-D mutant that does not bind CL is inactive in promoting mitophagy. Furthermore, mitophagy-inducing CL-transfer activity of NDPK-D is closely associated with the dynamin-like GTPase OPA1, implicating fission-fusion dynamics in mitophagy regulation. Mitochondrial depolarization secondary to TBI prompts phospholipid scramblase 3 (PLS3) activation and externalization of CL from the IMM inner leaflet to the outer leaflet of the OMM. Externalized CL interacts with the terminal helices of LC3 and aids recruitment of the autophagosome (Chao et al., 2019). Knockdown of PLS3 or inhibition of CL synthesis inhibits TBI-induced mitophagy (Chao et al., 2019). The diverse suite and interactions of receptor and adaptor molecules participating in mitochondrial quality control and mitophagy highlights the fine control necessary to maintain cellular homeostasis.
3.4: Pharmacologic Targeting Mitophagy in TBI
The mitochondrial quality control system and mitophagy are indispensable for homeostasis and restoration of function following TBI. Over- or underutilization of these systems may worsen outcomes. A delicate balance is required, making development of clinical therapies targeting mitophagy, and more generally, autophagy, challenging. Further confounding clinical translation, the protective or harmful effects of mitophagy-targeted therapies may be highly dependent on injury severity and time course. Specific and non-toxic mitophagy-inducers have not been well described. Melatonin-mediated upregulation of mitophagy was associated with protection following subarachnoid hemorrhage (Cao et al., 2017; Wang et al., 2018), commonly seen with TBI. Conversely, inhibition of fission and subsequent mitophagy via the small molecule DRP1 inhibitor, mitochondrial division inhibitor 1 (Mdivi1), worsened outcomes and increased neuronal injury following CCI (Niu et al., 2019). Other studies showed contradictory results, suggesting Mdivi1-mediated mitophagy inhibition preserved Δψm and blood-brain barrier function after TBI (Wu et al., 2018). More generally, autophagy inducers and inhibitors have had mixed results following TBI (Zhang and Wang, 2018). Development of specific small molecule inhibitors of the key players in fusion, fission, and mitophagy is required to better understand the extent, timing, and therapeutic potential of mitophagy modulation after TBI.
4.1: Conclusions
The secondary injury of TBI persists chronically even after the amelioration of the initial acute phase. The consequences of secondary TBI poses a heavy individual and societal burden. Despite the great need, no effective therapies exist. Restoration of mitochondrial function after TBI is necessary to preserve cellular energy production and redox homeostasis. However, preserving mitochondrial function following TBI is challenging due to interaction between the multiple converging pathways that each cause and feed-forward into dysfunction. A combination of several agents that comprehensively cover the complex mechanism may be the optimal but pharmacologically complicated route. The role of mitochondrial redox lipid signaling in development of TBI secondary injury has begun to emerge in recent years. Early accumulation of CLox after CCI is followed by hydrolytic reactions, which produce monolyso-CLs and PUFAox signaling intermediates (Chao et al., 2018). Mitochondrial CLox is directly implicated in triggering and execution of apoptosis (Kagan et al., 2009). It is likely that mitochondria control other programs of regulated neuronal death via indirect but essential metabolic mechanisms. For example, excessive production of H2O2 by mitochondria ETC and dehydrogenases creates excessively high peroxidase tone by turning on a variety of GSH-dependent peroxidases, which ends up depleting intracellular GSH. The GSH insufficiency causes inactivation of the GPX4/GSH system inducing alternative cell death mechanisms beyond apoptosis, like ferroptotsis. The direct role of mitochondrial-localized ferroptotic PE oxidation, as well as opposing anti-ferroptotic mechanisms (i.e. ferroptosis suppressor protein 1, FSP1), in TBI-induced neuronal death are emerging and need systematic exploration. Drugs that specifically target lipid localization or redox state have shown optimistic outcomes in experimental models, but translation to human subjects is necessary to demonstrate therapeutic efficacy. Similarly, therapies targeting MQC systems – fusion, fission, mitophagy – have shown promise but lack of clarity around injury time course and severity has produced contradictory results. Further exploration of the molecular and cellular mechanism of mitochondria lipid signaling and complimentary MCQ pathways in secondary injury is warranted.
Acknowledgments
This work was supported by NIH (F30HL142130, U19AI068021, HL114453, NS076511, NS061817, NS117000, GM113908, and CA165065).
Footnotes
Declaration of Interests
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adachi Y, Itoh K, Yamada T, Cerveny KL, Suzuki TL, Macdonald P, Frohman MA, Ramachandran R, Iijima M, Sesaki H, 2016. Coincident Phosphatidic Acid Interaction Restrains Drp1 in Mitochondrial Division. Mol Cell 63, 1034–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam-Vizi V, Tretter L, 2013. The role of mitochondrial dehydrogenases in the generation of oxidative stress. Neurochemistryinternational 62, 757–763. [DOI] [PubMed] [Google Scholar]
- Aguirre JD, Dunkerley KM, Mercier P, Shaw GS, 2017. Structure of phosphorylated UBL domain and insights into PINK1-orchestrated parkin activation. Proc Natl Acad Sci U S A 114, 298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alavi MV, Fuhrmann N, 2013. Dominant optic atrophy, OPA1, and mitochondrial quality control: understanding mitochondrial network dynamics. Mol Neurodegener 8, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthonymuthu TS, Kenny EM, Amoscato AA, Lewis J, Kochanek PM, Kagan VE, Bayır H, 2017. Global assessment of oxidized free fatty acids in brain reveals an enzymatic predominance to oxidative signaling after trauma. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 1863, 2601–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthonymuthu TS, Kenny EM, Hier ZE, Clark RS, Kochanek PM, Kagan VE, Bayır H, 2019a. Detection of brain specific cardiolipins in plasma after experimental pediatric head injury. Experimental neurology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthonymuthu TS, Kenny EM, Lamade AM, Gidwani H, Krehel NM, Misse A, Gao X, Amoscato AA, Straub AC, Kagan VE, 2019b. Lipidomics Detection of Brain Cardiolipins in Plasma Is Associated With Outcome After Cardiac Arrest. Critical care medicine 47, e292–e300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthonymuthu TS, Kenny EM, Lamade AM, Kagan VE, Bayir H, 2018. Oxidized phospholipid signaling in traumatic brain injury. Free radical biology & medicine 124, 493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardail D, Privat JP, Egret-Charlier M, Levrat C, Lerme F, Louisot P, 1990. Mitochondrial contact sites. Lipid composition and dynamics. The Journal of biological chemistry 265, 18797–18802. [PubMed] [Google Scholar]
- Arundine M, Tymianski M, 2004. Molecular mechanisms of glutamate-dependent neurodegeneration in ischemia and traumatic brain injury. Cellular and Molecular Life Sciences CMLS 61, 657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson J, Kapralov AA, Yanamala N, Tyurina YY, Amoscato AA, Pearce L, Peterson J, Huang Z, Jiang J, Samhan-Arias AK, 2011. A mitochondria-targeted inhibitor of cytochrome c peroxidase mitigates radiation-induced death. Nature communications 2, 497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian K, Maeda A, Lee JS, Mohammadyani D, Dar HH, Jiang JF, St Croix CM, Watkins S, Tyurin VA, Tyurina YY, Kloditz K, Polimova A, Kapralova VI, Xiong Z, Ray P, Klein-Seetharaman J, Mallampalli RK, Bayir H, Fadeel B, Kagan VE, 2015. Dichotomous roles for externalized cardiolipin in extracellular signaling: Promotion of phagocytosis and attenuation of innate immunity. Science signaling 8, ra95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker PD, Nerou EP, Cheesman MR, Thomson AJ, de Oliveira P, Hill HA, 1996. Bis-methionine ligation to heme iron in mutants of cytochrome b562. 1. Spectroscopic and electrochemical characterization of the electronic properties. Biochemistry 35, 13618–13626. [DOI] [PubMed] [Google Scholar]
- Bayır H, Kagan VE, Borisenko GG, Tyurina YY, Janesko KL, Vagni VA, Billiar TR, Williams DL, Kochanek PM, 2005. Enhanced oxidative stress in iNOS-deficient mice after traumatic brain injury: support for a neuroprotective role of iNOS. Journal of Cerebral Blood Flow & Metabolism 25, 673–684. [DOI] [PubMed] [Google Scholar]
- Bayir H, Tyurin VA, Tyurina YY, Viner R, Ritov V, Amoscato AA, Zhao Q, Zhang XJ, Janesko Feldman, K.L., Alexander H, 2007. Selective early cardiolipin peroxidation after traumatic brain injury: an oxidative lipidomics analysis. Annals of neurology 62, 154–169. [DOI] [PubMed] [Google Scholar]
- Bazan NG, Rodriguez de Turco EB, 1980. Membrane lipids in the pathogenesis of brain edema: phospholipids and arachidonic acid, the earliest membrane components changed at the onset of ischemia. Advances in neurology 28, 197–205. [PubMed] [Google Scholar]
- Brigelius-Flohé R, Maiorino M, 2013. Glutathione peroxidases. Biochimica et Biophysica Acta (BBA) - General Subjects 1830, 3289–3303. [DOI] [PubMed] [Google Scholar]
- Bui HT, Shaw JM, 2013. Dynamin assembly strategies and adaptor proteins in mitochondrial fission. Curr Biol 23, R891–R899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock R, Zauner A, Woodward JJ, Myseros J, Choi SC, Ward JD, Marmarou A, Young HFJJo.n., 1998. Factors affecting excitatory amino acid release following severe human head injury. 89, 507–518. [DOI] [PubMed] [Google Scholar]
- Burman JL, Pickles S, Wang C, Sekine S, Vargas JNS, Zhang Z, Youle AM, Nezich CL, Wu X, Hammer JA, Youle RJ, 2017. Mitochondrial fission facilitates the selective mitophagy of protein aggregates. The Journal of Cell Biology 216, 3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustillo-Zabalbeitia I, Montessuit S, Raemy E, Basañez G, Terrones O, Martinou J-C, 2014. Specific Interaction with Cardiolipin Triggers Functional Activation of Dynamin-Related Protein 1. PloS one 9, e102738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao S, Shrestha S, Li J, Yu X, Chen J, Yan F, Ying G, Gu C, Wang L, Chen G, 2017. Melatonin-mediated mitophagy protects against early brain injury after subarachnoid hemorrhage through inhibition of NLRP3 inflammasome activation. Scientific Reports 7, 2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico KM, Vaishnav R, Hall ED, 2009. Temporal and spatial dynamics of peroxynitrite-induced oxidative damage after spinal cord contusion injury. Journal of neurotrauma 26, 1369–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamoun R, Suki D, Gopinath SP, Goodman JC, Robertson C, 2010. Role of extracellular glutamate measured by cerebral microdialysis in severe traumatic brain injury. Journal of neurosurgery 113, 564–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao H, Anthonymuthu TS, Kenny EM, Amoscato AA, Cole LK, Hatch GM, Ji J, Kagan VE, Bayır H, 2018. Disentangling oxidation/hydrolysis reactions of brain mitochondrial cardiolipins in pathogenesis of traumatic injury. JCI insight 3, e97677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao H, Lin C, Zuo Q, Liu Y, Xiao M, Xu X, Li Z, Bao Z, Chen H, You Y, Kochanek PM, Yin H, Liu N, Kagan VE, Bayır H, Ji J, 2019. Cardiolipin-Dependent Mitophagy Guides Outcome after Traumatic Brain Injury. The Journal of Neuroscience 39, 1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Han Z, Feng D, Chen Y, Chen L, Wu H, Huang L, Zhou C, Cai X, Fu C, Duan L, Wang X, Liu L, Liu X, Shen Y, Zhu Y, Chen Q, 2014. A regulatory signaling loop comprising the PGAM5 phosphatase and CK2 controls receptor-mediated mitophagy. Mol Cell 54, 362–377. [DOI] [PubMed] [Google Scholar]
- Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC, 2003. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol 160, 189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G, Kong R. h., Zhang L. m., Zhang J. n., 2012. Mitochondria in traumatic brain injury and mitochondrial-targeted multipotential therapeutic strategies. British Journal of Pharmacology 167, 699–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choubey V, Cagalinec M, Liiv J, Safiulina D, Hickey MA, Kuum M, Liiv M, Anwar T, Eskelinen EL, Kaasik A, 2014. BECN1 is involved in the initiation of mitophagy: it facilitates PARK2 translocation to mitochondria. Autophagy 10, 1105–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu CT, Bayir H, Kagan VE, 2014. LC3 binds externalized cardiolipin on injured mitochondria to signal mitophagy in neurons: implications for Parkinson disease. Autophagy 10, 376–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu CT, Ji J, Dagda RK, Jiang JF, Tyurina YY, Kapralov AA, Tyurin VA, Yanamala N, Shrivastava IH, Mohammadyani D, 2013. Cardiolipin externalization to the outer mitochondrial membrane acts as an elimination signal for mitophagy in neuronal cells. Nature cell biology 15, 1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RSB, Empey PE, Bayır H, Rosario BL, Poloyac SM, Kochanek PM, Nolin TD, Au AK, Horvat CM, Wisniewski SR, Bell MJ, 2017. Phase I randomized clinical trial of N-acetylcysteine in combination with an adjuvant probenecid for treatment of severe traumatic brain injury in children. PloS one 12, e0180280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MM, Tareste D, 2018. Recent insights into the structure and function of Mitofusins in mitochondrial fusion. F1000Res 7, F1000 Faculty Rev-1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole LK, Kim JH, Amoscato AA, Tyurina YY, Bay RH, Karimi B, Siddiqui TJ, Kagan VE, Hatch GM, Kauppinen TM, 2018. Aberrant cardiolipin metabolism is associated with cognitive deficiency and hippocampal alteration in tafazzin knockdown mice. Biochim Biophys Acta Mol Basis Dis 1864, 3353–3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalle-Donne I, Rossi R, Colombo G, Giustarini D, Milzani A, 2009. Protein S-glutathionylation: a regulatory device from bacteria to humans. Trends in biochemical sciences 34, 85–96. [DOI] [PubMed] [Google Scholar]
- Daste F, Sauvanet C, Bavdek A, Baye J, Pierre F, Le Borgne R, David C, Rojo M, Fuchs P, Tareste D, 2018. The heptad repeat domain 1 of Mitofusin has membrane destabilization function in mitochondrial fusion. EMBO Rep 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daum G, 1985. Lipids of mitochondria. Biochimica et Biophysica Acta (BBA) - Reviews on Biomembranes 822, 1–42. [DOI] [PubMed] [Google Scholar]
- Daum G, Vance JE, 1997. Import of lipids into mitochondria. Progress in lipid research 36, 103–130. [DOI] [PubMed] [Google Scholar]
- Del Dotto V, Fogazza M, Carelli V, Rugolo M, Zanna C, 2018. Eight human OPA1 isoforms, long and short: What are they for? Biochimica et Biophysica Acta (BBA) - Bioenergetics 1859, 263–269. [DOI] [PubMed] [Google Scholar]
- Dennis EA, Norris PC, 2015. Eicosanoid storm in infection and inflammation. Nature Reviews Immunology 15, 511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marino M, Marassi R, Santucci R, Brunori M, Ascoli F, 1987. A spectroelectrochemical study of carboxymethylated cytochrome-c. Bioelectrochemistry and Bioenergetics 17, 27–34. [Google Scholar]
- Di Pietro V, Lazzarino G, Amorini AM, Signoretti S, Hill LJ, Porto E, Tavazzi B, Lazzarino G, Belli A, 2017. Fusion or Fission: The Destiny of Mitochondria In Traumatic Brain Injury of Different Severities. Scientific Reports 7, 9189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diskin T, Tal-Or P, Erlich S, Mizrachy L, Alexandrovich A, Shohami E, Pinkas-Kramarski R, 2005. Closed head injury induces upregulation of Beclin 1 at the cortical site of injury. Journal of neurotrauma 22, 750–762. [DOI] [PubMed] [Google Scholar]
- Dringen R, 2000. Metabolism and functions of glutathione in brain. Progress in neurobiology 62, 649–671. [DOI] [PubMed] [Google Scholar]
- Droghetti E, Oellerich S, Hildebrandt P, Smulevich G, 2006. Heme coordination states of unfolded ferrous cytochrome C. Biophys J 91, 3022–3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estaquier J, Arnoult D, 2007. Inhibiting Drp1-mediated mitochondrial fission selectively prevents the release of cytochrome c during apoptosis. Cell death and differentiation 14, 1086–1094. [DOI] [PubMed] [Google Scholar]
- Faden AI, Demediuk P, Panter SS, Vink RJS, 1989. The role of excitatory amino acids and NMDA receptors in traumatic brain injury. 244, 798–800. [DOI] [PubMed] [Google Scholar]
- Fischer TD, Hylin MJ, Zhao J, Moore AN, Waxham MN, Dash PK, 2016. Altered Mitochondrial Dynamics and TBI Pathophysiology. Front Syst Neurosci 10, 29–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flis VV, Daum G, 2013. Lipid transport between the endoplasmic reticulum and mitochondria. Cold Spring Harbor perspectives in biology 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francy CA, Clinton RW, Fröhlich C, Murphy C, Mears JA, 2017. Cryo-EM Studies of Drp1 Reveal Cardiolipin Interactions that Activate the Helical Oligomer. Scientific reports 7, 10744–10744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frezza C, Cipolat S, Martins de Brito O, Micaroni M, Beznoussenko GV, Rudka T, Bartoli D, Polishuck RS, Danial NN, De Strooper B, Scorrano L, 2006. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell 126, 177–189. [DOI] [PubMed] [Google Scholar]
- Fritz S, Rapaport D, Klanner E, Neupert W, Westermann B, 2001. Connection of the mitochondrial outer and inner membranes by Fzo1 is critical for organellar fusion. The Journal of cell biology 152, 683–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa T, Meydani S, Blumberg J, 1987. Reversal of age-associated decline in immune responsiveness by dietary glutathione supplementation in mice. Mechanisms of ageing and development 38, 107–117. [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Blomgren K, Kroemer G, 2009. Mitochondrial membrane permeabilization in neuronal injury. Nature Reviews Neuroscience 10, 481–494. [DOI] [PubMed] [Google Scholar]
- Gao F, Chen D, Si J, Hu Q, Qin Z, Fang M, Wang G, 2015. The mitochondrial protein BNIP3L is the substrate of PARK2 and mediates mitophagy in PINK1/PARK2 pathway. Hum Mol Genet 24, 2528–2538. [DOI] [PubMed] [Google Scholar]
- Giorgio V, von Stockum S, Antoniel M, Fabbro A, Fogolari F, Forte M, Glick GD, Petronilli V, Zoratti M, Szabó I, Lippe G, Bernardi P, 2013. Dimers of mitochondrial ATP synthase form the permeability transition pore. Proceedings of the National Academy of Sciences 110, 5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick D, Barth S, Macleod KF, 2010. Autophagy: cellular and molecular mechanisms. J Pathol 221, 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith OW, Meister A, 1985. Origin and turnover of mitochondrial glutathione. Proceedings of the National Academy of Sciences 82, 4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap AP, 2009. What is the mitochondrial permeability transition pore? Journal of molecular and cellular cardiology 46, 821–831. [DOI] [PubMed] [Google Scholar]
- Hanna RA, Quinsay MN, Orogo AM, Giang K, Rikka S, Gustafsson AB, 2012. Microtubule-associated protein 1 light chain 3 (LC3) interacts with Bnip3 protein to selectively remove endoplasmic reticulum and mitochondria via autophagy. The Journal of biological chemistry 287, 19094–19104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansford RG, Zorov D, 1998. Role of mitochondrial calcium transport in the control of substrate oxidation. Molecular and cellular biochemistry 184, 359–369. [PubMed] [Google Scholar]
- Harper JW, Ordureau A, Heo J-M, 2018. Building and decoding ubiquitin chains for mitophagy. Nature Reviews Molecular Cell Biology 19, 93–108. [DOI] [PubMed] [Google Scholar]
- He J, Ford HC, Carroll J, Ding S, Fearnley IM, Walker JE, 2017. Persistence of the mitochondrial permeability transition in the absence of subunit c of human ATP synthase. Proceedings of the National Academy of Sciences 114, 3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffer ME, Balaban C, Slade MD, Tsao JW, Hoffer B, 2013. Amelioration of acute sequelae of blast induced mild traumatic brain injury by N-acetyl cysteine: a double-blind, placebo controlled study. PloS one 8, e54163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong YS, Korman SH, Lee J, Ghoshal P, Wu Q, Barash V, Kang S, Oh S, Kwon M, Gutman A, Rachmel A, Patel MS, 2003. Identification of a common mutation (Gly194Cys) in both Arab Moslem and Ashkenazi Jewish patients with dihydrolipoamide dehydrogenase (E3) deficiency: possible beneficial effect of vitamin therapy. J Inherit Metab Dis 26, 816–818. [DOI] [PubMed] [Google Scholar]
- Horvath SE, Daum G, 2013. Lipids of mitochondria. Progress in lipid research 52, 590–614. [DOI] [PubMed] [Google Scholar]
- Ishihara N, Fujita Y, Oka T, Mihara K, 2006. Regulation of mitochondrial morphology through proteolytic cleavage of OPA1. The EMBO journal 25, 2966–2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara N, Jofuku A, Eura Y, Mihara K, 2003. Regulation of mitochondrial morphology by membrane potential, and DRP1-dependent division and FZO1-dependent fusion reaction in mammalian cells. Biochemical and biophysical research communications 301, 891–898. [DOI] [PubMed] [Google Scholar]
- Jakobs S, Martini N, Schauss AC, Egner A, Westermann B, Hell SW, 2003. Spatial and temporal dynamics of budding yeast mitochondria lacking the division component Fis1p. Journal of Cell Science 116, 2005–2014. [DOI] [PubMed] [Google Scholar]
- Jastroch M, Divakaruni AS, Mookerjee S, Treberg JR, Brand MD, 2010. Mitochondrial proton and electron leaks. Essays In Biochemistry 47, 53–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J, Kline AE, Amoscato A, Arias AS, Sparvero LJ, Tyurin VA, Tyurina YY, Fink B, Manole MD, Puccio AM, Okonkwo DO, Cheng JP, Alexander H, Clark RSB, Kochanek PM, Wipf P, Kagan VE, Bayýr H, 2012. Global lipidomics identifies cardiolipin oxidation as a mitochondrial target for redox therapy of acute brain injury. Nature neuroscience 15, 1407–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Bakan A, Kapralov AA, Silva KI, Huang Z, Amoscato AA, Peterson J, Garapati VK, Saxena S, Bayir H, 2014. Designing inhibitors of cytochrome c/cardiolipin peroxidase complexes: mitochondria-targeted imidazole-substituted fatty acids. Free Radical Biology and Medicine 71, 221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jozefczak M, Remans T, Vangronsveld J, Cuypers A, 2012. Glutathione is a key player in metal-induced oxidative stress defenses. Int J Mol Sci 13, 3145–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan VE, Bayır HA, Belikova NA, Kapralov O, Tyurina YY, Tyurin VA, Jiang J, Stoyanovsky DA, Wipf P, Kochanek PM, 2009. Cytochrome c/cardiolipin relations in mitochondria: a kiss of death. Free Radical Biology and Medicine 46, 1439–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan VE, Jiang J, Huang Z, Tyurina YY, Desbourdes C, Cottet-Rousselle C, Dar HH, Verma M, Tyurin VA, Kapralov AA, Cheikhi A, Mao G, Stolz D, St Croix CM, Watkins S, Shen Z, Li Y, Greenberg ML, Tokarska-Schlattner M, Boissan M, Lacombe ML, Epand RM, Chu CT, Mallampalli RK, Bayir H, Schlattner U, 2016. NDPK-D (NM23-H4)-mediated externalization of cardiolipin enables elimination of depolarized mitochondria by mitophagy. Cell death and differentiation 23, 1140–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan VE, Mao G, Qu F, Angeli JP, Doll S, Croix CS, Dar HH, Liu B, Tyurin VA, Ritov VB, Kapralov AA, Amoscato AA, Jiang J, Anthonymuthu T, Mohammadyani D, Yang Q, Proneth B, Klein-Seetharaman J, Watkins S, Bahar I, Greenberger J, Mallampalli RK, Stockwell BR, Tyurina YY, Conrad M, Bayir H, 2017. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nature chemical biology 13, 81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan VE, Tyurin VA, Jiang J, Tyurina YY, Ritov VB, Amoscato AA, Osipov AN, Belikova NA, Kapralov AA, Kini V, 2005. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nature chemical biology 1, 223. [DOI] [PubMed] [Google Scholar]
- Kagan VE, Tyurina YY, Tyurin VA, Mohammadyani D, Angeli JP, Baranov SV, Klein-Seetharaman J, Friedlander RM, Mallampalli RK, Conrad M, Bayir H, 2015. Cardiolipin signaling mechanisms: collapse of asymmetry and oxidation. Antioxidants & redox signaling 22, 1667–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkavan H, Green DR, 2018. MOMP, cell suicide as a BCL-2 family business. Cell death and differentiation 25, 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameoka S, Adachi Y, Okamoto K, Iijima M, Sesaki H, 2018. Phosphatidic Acid and Cardiolipin Coordinate Mitochondrial Dynamics. Trends in cell biology 28, 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamo N, Muratsugu M, Hongoh R, Kobatake Y, 1979. Membrane potential of mitochondria measured with an electrode sensitive to tetraphenyl phosphonium and relationship between proton electrochemical potential and phosphorylation potential in steady state. The Journal of membrane biology 49, 105–121. [DOI] [PubMed] [Google Scholar]
- Kanner J, German JB, Kinsella JE, 1987. Initiation of lipid peroxidation in biological systems. Crit Rev Food Sci Nutr 25, 317–364. [DOI] [PubMed] [Google Scholar]
- Kenny EM, Fidan E, Yang Q, Anthonymuthu TS, New LA, Meyer EA, Wang H, Kochanek PM, Dixon CE, Kagan VE, Bayir H, 2019. Ferroptosis Contributes to Neuronal Death and Functional Outcome After Traumatic Brain Injury. Critical care medicine 47, 410–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitsis RN, Molkentin JD, 2010. Apoptotic cell death “Nixed” by an ER–mitochondrial necrotic pathway. Proceedings of the National Academy of Sciences 107, 9031–9032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott AB, Perkins G, Schwarzenbacher R, Bossy-Wetzel E, 2008. Mitochondrial fragmentation in neurodegeneration. Nat Rev Neurosci 9, 505–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooijman EE, Swim LA, Graber ZT, Tyurina YY, Bayır H, Kagan VE, 2017. Magic angle spinning (31)P NMR spectroscopy reveals two essentially identical ionization states for the cardiolipin phosphates in phospholipid liposomes. Biochim Biophys Acta Biomembr 1859, 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosicek M, Hecimovic S, 2013. Phospholipids and Alzheimer’s disease: alterations, mechanisms and potential biomarkers. Int J Mol Sci 14, 1310–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulawiak B, Hopker J, Gebert M, Guiard B, Wiedemann N, Gebert N, 2013. The mitochondrial protein import machinery has multiple connections to the respiratory chain. Biochimica et biophysica acta 1827, 612–626. [DOI] [PubMed] [Google Scholar]
- Kumar A, Stoica BA, Loane DJ, Yang M, Abulwerdi G, Khan N, Kumar A, Thom SR, Faden AI, 2017. Microglial-derived microparticles mediate neuroinflammation after traumatic brain injury. Journal of neuroinflammation 14, 47–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagouge M, Larsson NG, 2013. The role of mitochondrial DNA mutations and free radicals in disease and ageing. J Intern Med 273, 529–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamade AM, Kenny EM, Anthonymuthu TS, Soysal E, Clark RS, Kagan VE, Bayır H, 2019. Aiming for the target: Mitochondrial drug delivery in traumatic brain injury. Neuropharmacology 145, 209–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lash LH, 2006. Mitochondrial glutathione transport: physiological, pathological and toxicological implications. Chemico-biological interactions 163, 54–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Kosaras B, Del Signore SJ, Cormier K, McKee A, Ratan RR, Kowall NW, Ryu H, 2011. Modulation of lipid peroxidation and mitochondrial function improves neuropathology in Huntington’s disease mice. Acta Neuropathol 121, 487–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinski MM, Wu J, Faden AI, Sarkar C, 2015. Function and Mechanisms of Autophagy in Brain and Spinal Cord Trauma. Antioxidants & redox signaling 23, 565–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G-Y, Moon SH, Jenkins CM, Li M, Sims HF, Guan S, Gross RW, 2017. The phospholipase iPLA2γ is a major mediator releasing oxidized aliphatic chains from cardiolipin, integrating mitochondrial bioenergetics and signaling. Journal of Biological Chemistry 292, 10672–10684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Feng D, Chen G, Chen M, Zheng Q, Song P, Ma Q, Zhu C, Wang R, Qi W, Huang L, Xue P, Li B, Wang X, Jin H, Wang J, Yang F, Liu P, Zhu Y, Sui S, Chen Q, 2012. Mitochondrial outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in mammalian cells. Nature cell biology 14, 177–185. [DOI] [PubMed] [Google Scholar]
- Liu R, Chan DC, 2017. OPA1 and cardiolipin team up for mitochondrial fusion. Nature cell biology 19, 760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Porter NA, Schneider C, Brash AR, Yin H, 2011. Formation of 4-hydroxynonenal from cardiolipin oxidation: Intramolecular peroxyl radical addition and decomposition. Free radical biology & medicine 50, 166–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzano S, Rost NS, Khan M, Li H, Lima FO, Maas MB, Green RE, Thankachan TK, Dipietro AJ, Arai K, Som AT, Pham LD, Wu O, Harris GJ, Lo EH, Blumberg JB, Milbury PE, Feske SK, Furie KL, 2018. Oxidative Stress Biomarkers of Brain Damage: Hyperacute Plasma F2-Isoprostane Predicts Infarct Growth in Stroke. Stroke 49, 630–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loschen G, Azzi A, Richter C, Flohé L, 1974. Superoxide radicals as precursors of mitochondrial hydrogen peroxide. FEBS letters 42, 68–72. [DOI] [PubMed] [Google Scholar]
- Lu SC, 2009. Regulation of glutathione synthesis. Molecular Aspects of Medicine 30, 42–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Ni H, Rui Q, Liu H, Jiang F, Gao R, Gao Y, Li D, Chen G, 2019. Potential Roles of NIX/BNIP3 L Pathway in Rat Traumatic Brain Injury. Cell Transplant 28, 585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald PJ, Stepanyants N, Mehrotra N, Mears JA, Qi X, Sesaki H, Ramachandran R, 2014. A dimeric equilibrium intermediate nucleates Drp1 reassembly on mitochondrial membranes for fission. Molecular biology of the cell 25, 1905–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailloux RJ, Young A, O’Brien M, Gill RM, 2018. Simultaneous Measurement of Superoxide/Hydrogen Peroxide and NADH Production by Flavin-containing Mitochondrial Dehydrogenases. J Vis Exp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiti AK, Spoorthi B, Saha NC, Panigrahi AK, 2018. Mitigating peroxynitrite mediated mitochondrial dysfunction in aged rat brain by mitochondria-targeted antioxidant MitoQ. Biogerontology 19, 271–286. [DOI] [PubMed] [Google Scholar]
- Malka F, Guillery O, Cifuentes-Diaz C, Guillou E, Belenguer P, Lombes A, Rojo M, 2005. Separate fusion of outer and inner mitochondrial membranes. EMBO Rep 6, 853–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund SL, Westman NG, Lundgren E, Roos G, 1982. Copper- and zinc-containing superoxide dismutase, manganese-containing superoxide dismutase, catalase, and glutathione peroxidase in normal and neoplastic human cell lines and normal human tissues. Cancer Res 42, 1955–1961. [PubMed] [Google Scholar]
- McClelland LJ, Mou TC, Jeakins-Cooley ME, Sprang SR, Bowler BE, 2014. Structure of a mitochondrial cytochrome c conformer competent for peroxidase activity. Proc Natl Acad Sci U S A 111, 6648–6653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mears JA, Lackner LL, Fang S, Ingerman E, Nunnari J, Hinshaw JE, 2011. Conformational changes in Dnm1 support a contractile mechanism for mitochondrial fission. Nature Structural & Molecular Biology 18, 20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer A, Brouwer A, Reijngoud D, Hoek J, Tager J, 1972. Transport of glutamate in rat-liver mitochondria. Biochimica et Biophysica Acta (BBA)-Bioenergetics 283, 421–429. [DOI] [PubMed] [Google Scholar]
- Meister A, Anderson ME, 1983. Glutathione. Annual review of biochemistry 52, 711–760. [DOI] [PubMed] [Google Scholar]
- Midura EF, Jernigan PL, Kuethe JW, Friend LA, Veile R, Makley AT, Caldwell CC, Goodman MD, 2015. Microparticles impact coagulation after traumatic brain injury. The Journal of surgical research 197, 25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahara I, Kikuchi H, Taki W, Nishi S, Kito M, Yonekawa Y, Goto Y, Ogata N, 1991. Degradation of mitochondrial phospholipids during experimental cerebral ischemia in rats. Journal of neurochemistry 57, 839–844. [DOI] [PubMed] [Google Scholar]
- Neginskaya MA, Solesio ME, Berezhnaya EV, Amodeo GF, Mnatsakanyan N, Jonas EA, Pavlov EV, 2019. ATP Synthase C-Subunit-Deficient Mitochondria Have a Small Cyclosporine A-Sensitive Channel, but Lack the Permeability Transition Pore. Cell reports 26, 11–17.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niki E, 2009. Lipid peroxidation: physiological levels and dual biological effects. Free Radical Biology and Medicine 47, 469–484. [DOI] [PubMed] [Google Scholar]
- Niu F, Dong J, Xu X, Zhang B, Liu B, 2019. Mitochondrial Division Inhibitor 1 Prevents Early-Stage Induction of Mitophagy and Accelerated Cell Death in a Rat Model of Moderate Controlled Cortical Impact Brain Injury. World Neurosurg 122, e1090–e1101. [DOI] [PubMed] [Google Scholar]
- O’Brien JS, Sampson EL, 1965. Lipid composition of the normal human brain: gray matter, white matter, and myelin. Journal of lipid research 6, 537–544. [PubMed] [Google Scholar]
- O’Brien M, Chalker J, Slade L, Gardiner D, Mailloux RJ, 2017. Protein S-glutathionylation alters superoxide/hydrogen peroxide emission from pyruvate dehydrogenase complex. Free radical biology & medicine 106, 302–314. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Shaw JM, 2005. Mitochondrial morphology and dynamics in yeast and multicellular eukaryotes. Annu Rev Genet 39, 503–536. [DOI] [PubMed] [Google Scholar]
- Ola MS, Nawaz M, Ahsan H, 2011. Role of Bcl-2 family proteins and caspases in the regulation of apoptosis. Molecular and cellular biochemistry 351, 41–58. [DOI] [PubMed] [Google Scholar]
- Opii WO, Nukala VN, Sultana R, Pandya JD, Day KM, Merchant ML, Klein JB, Sullivan PG, Butterfield DA, 2007. Proteomic identification of oxidized mitochondrial proteins following experimental traumatic brain injury. Journal of neurotrauma 24, 772–789. [DOI] [PubMed] [Google Scholar]
- Ordureau A, Sarraf SA, Duda DM, Heo JM, Jedrychowski MP, Sviderskiy VO, Olszewski JL, Koerber JT, Xie T, Beausoleil SA, Wells JA, Gygi SP, Schulman BA, Harper JW, 2014. Quantitative proteomics reveal a feedforward mechanism for mitochondrial PARKIN translocation and ubiquitin chain synthesis. Mol Cell 56, 360–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otera H, Wang C, Cleland MM, Setoguchi K, Yokota S, Youle RJ, Mihara K, 2010. Mff is an essential factor for mitochondrial recruitment of Drp1 during mitochondrial fission in mammalian cells. The Journal of cell biology 191, 1141–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacino JJ, Sagi D, Goldberg MS, Krauss S, Motz C, Wacker M, Klose J, Shen J, 2004. Mitochondrial dysfunction and oxidative damage in parkin-deficient mice. The Journal of biological chemistry 279, 18614–18622. [DOI] [PubMed] [Google Scholar]
- Palikaras K, Lionaki E, Tavernarakis N, 2018. Mechanisms of mitophagy in cellular homeostasis, physiology and pathology. Nature cell biology 20, 1013–1022. [DOI] [PubMed] [Google Scholar]
- Pandya JD, Readnower RD, Patel SP, Yonutas HM, Pauly JR, Goldstein GA, Rabchevsky AG, Sullivan PG, 2014. N-acetylcysteineamide confers neuroprotection, improves bioenergetics and behavioral outcome following TBI. Experimental neurology 257, 106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradies G, Paradies V, De Benedictis V, Ruggiero FM, Petrosillo G, 2014. Functional role of cardiolipin in mitochondrial bioenergetics. Biochimica et Biophysica Acta (BBA) - Bioenergetics 1837, 408–417. [DOI] [PubMed] [Google Scholar]
- Pasvogel AE, Miketova P, Moore IM, 2010. Differences in CSF Phospholipid Concentration by Traumatic Brain Injury Outcome. Biological Research For Nursing 11, 325–331. [DOI] [PubMed] [Google Scholar]
- Pernas L, Scorrano L, 2016. Mito-Morphosis: Mitochondrial Fusion, Fission, and Cristae Remodeling as Key Mediators of Cellular Function. Annual Review of Physiology 78, 505–531. [DOI] [PubMed] [Google Scholar]
- Pilitsis JG, Coplin WM, O’Regan MH, Wellwood JM, Diaz FG, Fairfax MR, Michael DB, Phillis JW, 2003. Free fatty acids in cerebrospinal fluids from patients with traumatic brain injury. Neuroscience Letters 349, 136138. [DOI] [PubMed] [Google Scholar]
- Praefcke GJK, McMahon HT, 2004. The dynamin superfamily: universal membrane tubulation and fission molecules? Nature Reviews Molecular Cell Biology 5, 133–147. [DOI] [PubMed] [Google Scholar]
- Prins M, Greco T, Alexander D, Giza CC, 2013. The pathophysiology of traumatic brain injury at a glance. Disease Models & Mechanisms 6, 1307–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramonet D, Perier C, Recasens A, Dehay B, Bove J, Costa V, Scorrano L, Vila M, 2013. Optic atrophy 1 mediates mitochondria remodeling and dopaminergic neurodegeneration linked to complex I deficiency. Cell death and differentiation 20, 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman RJ, 2002. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiological reviews 82, 131–185. [DOI] [PubMed] [Google Scholar]
- Sagristá ML, GarcÍa AF, De Madariaga MA, Mora M, 2002. Antioxidant and pro-oxidant effect of the thiolic compounds N-acetyl-L-cysteine and glutathione against free radical-induced lipid peroxidation. Free radical research 36, 329–340. [DOI] [PubMed] [Google Scholar]
- Salim S, 2017. Oxidative Stress and the Central Nervous System. The Journal of pharmacology and experimental therapeutics 360, 201–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samangouei P, Crespo-Avilan GE, Cabrera-Fuentes H, Hernández-Reséndiz S, Ismail NI, Katwadi KB, Boisvert WA, Hausenloy DJ, 2018. MiD49 and MiD51: New mediators of mitochondrial fission and novel targets for cardioprotection. Cond Med 1, 239–246. [PMC free article] [PubMed] [Google Scholar]
- Sarkar C, Zhao Z, Aungst S, Sabirzhanov B, Faden AI, Lipinski MM, 2014. Impaired autophagy flux is associated with neuronal cell death after traumatic brain injury. Autophagy 10, 2208–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlame M, 2008. Cardiolipin synthesis for the assembly of bacterial and mitochondrial membranes. Journal of lipid research 49, 1607–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine S, Youle RJ, 2018. PINK1 import regulation; a fine system to convey mitochondrial stress to the cytosol. BMC Biol 16, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaag A, Saada A, Berger I, Mandel H, Joseph A, Feigenbaum A, Elpeleg ON, 1999. Molecular basis of lipoamide dehydrogenase deficiency in Ashkenazi Jews. Am J Med Genet 82, 177–182. [PubMed] [Google Scholar]
- Shiba-Fukushima K, Arano T, Matsumoto G, Inoshita T, Yoshida S, Ishihama Y, Ryu KY, Nukina N, Hattori N, Imai Y, 2014. Phosphorylation of mitochondrial polyubiquitin by PINK1 promotes Parkin mitochondrial tethering. PLoS Genet 10, e1004861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlevkov E, Kramer T, Schapansky J, LaVoie MJ, Schwarz TL, 2016. Miro phosphorylation sites regulate Parkin recruitment and mitochondrial motility. Proceedings of the National Academy of Sciences 113, E6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel SJ, Bieschke J, Powers ET, Kelly JW, 2007. The oxidative stress metabolite 4-hydroxynonenal promotes Alzheimer protofibril formation. Biochemistry 46, 1503–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh IN, Gilmer LK, Miller DM, Cebak JE, Wang JA, Hall ED, 2013. Phenelzine mitochondrial functional preservation and neuroprotection after traumatic brain injury related to scavenging of the lipid peroxidation -derived aldehyde 4-hydroxy-2-nonenal. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 33, 593–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow BJ, Rolfe FL, Lockhart MM, Frampton CM, O’Sullivan JD, Fung V, Smith RA, Murphy MP, Taylor KM, Protect Study G, 2010. A double-blind, placebo-controlled study to assess the mitochondria-targeted antioxidant MitoQ as a disease-modifying therapy in Parkinson’s disease. Mov Disord 25, 1670–1674. [DOI] [PubMed] [Google Scholar]
- Song Z, Chen H, Fiket M, Alexander C, Chan DC, 2007. OPA1 processing controls mitochondrial fusion and is regulated by mRNA splicing, membrane potential, and Yme1L. The Journal of cell biology 178, 749–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparvero LJ, Amoscato AA, Fink AB, Anthonymuthu T, New LA, Kochanek PM, Watkins S, Kagan VE, Bayır H, 2016. Imaging mass spectrometry reveals loss of polyunsaturated cardiolipins in the cortical contusion, hippocampus, and thalamus after traumatic brain injury. Journal of neurochemistry 139, 659–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein SC, Smith DH, 2004. Coagulopathy in traumatic brain injury. Neurocritical care 1, 479–488. [DOI] [PubMed] [Google Scholar]
- Sturgeon BE, Sipe HJ, Barr DP, Corbett JT, Martinez JG, Mason RP, 1998. The fate of the oxidizing tyrosyl radical in the presence of glutathione and ascorbate implications for the radical sink hypothesis. Journal of Biological Chemistry 273, 30116–30121. [DOI] [PubMed] [Google Scholar]
- Sullivan PG, Krishnamurthy S, Patel SP, Pandya JD, Rabchevsky AG, 2007. Temporal characterization of mitochondrial bioenergetics after spinal cord injury. Journal of neurotrauma 24, 991–999. [DOI] [PubMed] [Google Scholar]
- Sun Y, Wang J, Wu X, Xi C, Gai Y, Liu H, Yuan Q, Wang E, Gao L, Hu J, Zhou L, 2011. Validating the incidence of coagulopathy and disseminated intravascular coagulation in patients with traumatic brain injury--analysis of 242 cases. British journal of neurosurgery 25, 363–368. [DOI] [PubMed] [Google Scholar]
- Suthanthiran M, Anderson ME, Sharma VK, Meister A, 1990. Glutathione regulates activation-dependent DNA synthesis in highly purified normal human T lymphocytes stimulated via the CD2 and CD3 antigens. Proceedings of the National Academy of Sciences 87, 3343–3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabó I, Zoratti M.J.J.o.B.C., 1991. The giant channel of the inner mitochondrial membrane is inhibited by cyclosporin A. 266, 3376–3379. [PubMed] [Google Scholar]
- Szargel R, Shani V, Abd Elghani F, Mekies LN, Liani E, Rott R, Engelender S, 2016. The PINK1, synphilin-1 and SIAH-1 complex constitutes a novel mitophagy pathway. Hum Mol Genet 25, 3476–3490. [DOI] [PubMed] [Google Scholar]
- Taguchi N, Ishihara N, Jofuku A, Oka T, Mihara K, 2007. Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. The Journal of biological chemistry 282, 11521–11529. [DOI] [PubMed] [Google Scholar]
- Tait SW, Green DR, 2010. Mitochondria and cell death: outer membrane permeabilization and beyond. Nature reviews Molecular cell biology 11, 621. [DOI] [PubMed] [Google Scholar]
- Tanida I, Ueno T, Kominami E, 2008. LC3 and Autophagy. Methods Mol Biol 445, 77–88. [DOI] [PubMed] [Google Scholar]
- Tatsuta T, Scharwey M, Langer T, 2014. Mitochondrial lipid trafficking. Trends in cell biology 24, 44–52. [DOI] [PubMed] [Google Scholar]
- Terni B, Boada J, Portero-Otin M, Pamplona R, Ferrer I, 2010. Mitochondrial ATP-Synthase in the Entorhinal Cortex Is a Target of Oxidative Stress at Stages I/II of Alzheimer’s Disease Pathology. Brain Pathology 20, 222–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y, Salsbery B, Wang M, Yuan H, Yang J, Zhao Z, Wu X, Zhang Y, Konkle BA, Thiagarajan P, Li M, Zhang J, Dong JF, 2015. Brain-derived microparticles induce systemic coagulation in a murine model of traumatic brain injury. Blood 125, 2151–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsong TY, 1976. Ferricytochrome c chain folding measured by the energy transfer of tryptophan 59 to the heme group. Biochemistry 15, 5467–5473. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y, Shimizu S, 2007. Role of the mitochondrial membrane permeability transition in cell death. Apoptosis 12, 835–840. [DOI] [PubMed] [Google Scholar]
- Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, Alroy J, Wu M, Py BF, Yuan J, Deeney JT, Corkey BE, Shirihai OS, 2008. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. The EMBO journal 27, 433–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyurina YY, Poloyac SM, Tyurin VA, Kapralov AA, Jiang J, Anthonymuthu TS, Kapralova VI, Vikulina AS, Jung MY, Epperly MW, Mohammadyani D, Klein-Seetharaman J, Jackson TC, Kochanek PM, Pitt BR, Greenberger JS, Vladimirov YA, Bayir H, Kagan VE, 2014. A mitochondrial pathway for biosynthesis of lipid mediators. Nature chemistry 6, 542–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyurina YY, Tungekar MA, Jung M-Y, Tyurin VA, Greenberger JS, Stoyanovsky DA, Kagan VE, 2012. Mitochondria targeting of non peroxidizable triphenylphosphonium conjugated oleic acid protects mouse embryonic cells against apoptosis: Role of cardiolipin remodeling. FEBS letters 586, 235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaishnav RA, Singh IN, Miller DM, Hall ED, 2010. Lipid Peroxidation-Derived Reactive Aldehydes Directly and Differentially Impair Spinal Cord and Brain Mitochondrial Function. Journal of neurotrauma 27, 1311–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer G, Voelker DR, Feigenson GW, 2008. Membrane lipids: where they are and how they behave. Nature Reviews Molecular Cell Biology 9, 112–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance JE, Vance DE, 2004. Phospholipid biosynthesis in mammalian cells. Biochemistry and cell biology 82, 113–128. [DOI] [PubMed] [Google Scholar]
- Vangaveti V, Baune BT, Kennedy RL, 2010. Hydroxyoctadecadienoic acids: novel regulators of macrophage differentiation and atherogenesis. Therapeutic advances in endocrinology and metabolism 1, 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vladimirov YA, Olenev VI, Suslova TB, Cheremisina ZP, 1980. Lipid Peroxidation in Mitochondrial Membrane, in: Paoletti R, Kritchevsky D (Eds.), Advances in Lipid Research. Elsevier, pp. 173–249. [DOI] [PubMed] [Google Scholar]
- Wang S, Zhao Z, Feng X, Cheng Z, Xiong Z, Wang T, Lin J, Zhang M, Hu J, Fan Y, Reiter RJ, Wang H, Sun D, 2018. Melatonin activates Parkin translocation and rescues the impaired mitophagy activity of diabetic cardiomyopathy through Mst1 inhibition. Journal of Cellular and Molecular Medicine 22, 5132–5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watford M, 2000. Glutamine and Glutamate Metabolism across the Liver Sinusoid. The Journal of Nutrition 130, 983S–987S. [DOI] [PubMed] [Google Scholar]
- Wei Y, Chiang WC, Sumpter R Jr., Mishra P, Levine B, 2017. Prohibitin 2 Is an Inner Mitochondrial Membrane Mitophagy Receptor. Cell 168, 224–238.e210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems, Peter HGM, Rossignol R, Dieteren, Cindy EJ, Murphy, Michael P, Koopman, Werner JH, 2015. Redox Homeostasis and Mitochondrial Dynamics. Cell Metab 22, 207–218. [DOI] [PubMed] [Google Scholar]
- Williams GSB, Boyman L, Chikando AC, Khairallah RJ, Lederer WJ, 2013. Mitochondrial calcium uptake. Proceedings of the National Academy of Sciences 110, 10479–10486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong YC, Holzbaur ELF, 2014. Optineurin is an autophagy receptor for damaged mitochondria in parkinmediated mitophagy that is disrupted by an ALS-linked mutation. Proceedings of the National Academy of Sciences 111, E4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Gao C, Wang H, Zhang X, Li Q, Gu Z, Shi X, Cui Y, Wang T, Chen X, Wang X, Luo C, Tao L, 2018. Mdivi-1 alleviates blood-brain barrier disruption and cell death in experimental traumatic brain injury by mitigating autophagy dysfunction and mitophagy activation. The International Journal of Biochemistry & Cell Biology 94, 44–55. [DOI] [PubMed] [Google Scholar]
- Wu Q, Xia S-X, Li Q-Q, Gao Y, Shen X, Ma L, Zhang M-Y, Wang T, Li Y-S, Wang Z-F, Luo C-L, Tao L-Y, 2016. Mitochondrial division inhibitor 1 (Mdivi-1) offers neuroprotection through diminishing cell death and improving functional outcome in a mouse model of traumatic brain injury. Brain research 1630, 134–143. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Peterson PL, Lee CP, 1999. Effect of N-acetylcysteine on mitochondrial function following traumatic brain injury in rats. Journal of neurotrauma 16, 1067–1082. [DOI] [PubMed] [Google Scholar]
- Yoo S-M, Jung Y-K, 2018. A Molecular Approach to Mitophagy and Mitochondrial Dynamics. Mol Cells 41, 18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youle RJ, van der Bliek AM, 2012. Mitochondrial fission, fusion, and stress. Science (New York, N.Y.) 337, 10621065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaleska MM, Nagy K, Floyd RA, 1989. Iron-induced lipid peroxidation and inhibition of dopamine synthesis in striatum synaptosomes. Neurochemical research 14, 597–605. [DOI] [PubMed] [Google Scholar]
- Zhang L, Wang H, 2018. Autophagy in Traumatic Brain Injury: A New Target for Therapeutic Intervention. Front Mol Neurosci 11, 190–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Liu T, Jin S, Wang X, Qu M, Uhlen P, Tomilin N, Shupliakov O, Lendahl U, Nister M, 2011. Human MIEF1 recruits Drp1 to mitochondrial outer membranes and promotes mitochondrial fusion rather than fission. The EMBO journal 30, 2762–2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Wang M, Tian Y, Hilton T, Salsbery B, Zhou EZ, Wu X, Thiagarajan P, Boilard E, Li M, Zhang J, Dong J. f., 2016. Cardiolipin-mediated procoagulant activity of mitochondria contributes to traumatic brain injury-associated coagulopathy in mice. Blood. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H, Yin H, 2015. Role of lipid peroxidation derived 4-hydroxynonenal (4-HNE) in cancer: focusing on mitochondria. Redox Biol 4, 193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Zhu P, Guo J, Hu N, Wang S, Li D, Hu S, Ren J, Cao F, Chen Y, 2017. Ripk3 induces mitochondrial apoptosis via inhibition of FUNDC1 mitophagy in cardiac IR injury. Redox Biol 13, 498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Wang H, Shen R, Fang J, Yang Y, Dai W, Zhu Y, Zhou M, 2018a. Mitochondrial-targeted antioxidant MitoQ provides neuroprotection and reduces neuronal apoptosis in experimental traumatic brain injury possibly via the Nrf2-ARE pathway. Am J Transl Res 10, 1887–1899. [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Cai W, Zhao Z, Hilton T, Wang M, Yeon J, Liu W, Zhang F, Shi F-D, Wu X, 2018b. Lactadherin promotes microvesicle clearance to prevent coagulopathy and improves survival of severe TBI mice. Blood 131, 563–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Wang H, Fang J, Dai W, Zhou J, Wang X, Zhou M, 2018. SS-31 Provides Neuroprotection by Reversing Mitochondrial Dysfunction after Traumatic Brain Injury. Oxid Med Cell Longev 2018, 4783602. [DOI] [PMC free article] [PubMed] [Google Scholar]