Abstract
In the era of effective antiretroviral therapy (ART), HIV has become a manageable disease marked by an elevated risk of non-AIDS-related comorbidities, including stroke. Rates of stroke are higher in people living with HIV (PLWH) compared with the general population. Elevated stroke risk may be attributable to traditional risk factors, HIV-associated chronic inflammation and immune dysregulation, and possible adverse effects of long-standing ART use. Tailoring stroke prevention strategies for PLWH requires knowledge of how stroke pathogenesis may differ from non-HIV-associated stroke, knowledge of long-term stroke outcomes in HIV, and accurate stroke risk assessment tools. As a result, the approach to primary and secondary stroke prevention in PLWH relies heavily on guidelines developed for the general population, with an emphasis on optimization of traditional vascular risk factors and early initiation of ART. This review summarizes existing evidence on HIV-associated stroke mechanisms and considerations for stroke prevention for PLWH.
Keywords: HIV, stroke, cerebrovascular disease, prevention, atherosclerosis, antiretroviral therapy
INTRODUCTION
People living and aging with HIV are experiencing a rising burden of stroke in the contemporary era of combination antiretroviral therapy (ART). In the US, the number of patients with HIV admitted for stroke rose by 43% between 1997 and 2006.1 In regions of sub-Saharan Africa where HIV is endemic, HIV is the leading risk factor for stroke in young populations with a population-attributable fraction of 46%.2
In addition to high rates of traditional cardiovascular disease (CVD) risk factors, people living with HIV (PLWH) are at risk of physiologic changes that occur from ART and HIV infection itself. Both immunosuppression with lower CD4 count and uncontrolled HIV viremia appear to be associated with greater stroke risk. Little is known about stroke prevention specifically in HIV, and it is unclear whether the more general methods of modifying stroke risk provide a similar benefit in PLWH compared with HIV-uninfected patients. Due to the lack of data for optimal prevention of stroke in PLWH, current clinical practice tends to follow guidelines for stroke prevention developed for the population at large.
This review summarizes the potential mechanisms underlying the pathogenesis of stroke in PLWH and strategies for stroke prevention in this high-risk population.
EPIDEMIOLOGY
Early in the HIV epidemic, stroke typically occurred in the setting of advanced HIV infection, often with attendant opportunistic infections, coagulopathies, or AIDS-related malignancies.3 In the ART era, the association between HIV and stroke remains compelling, especially as PLWH age and the prevalence of traditional vascular risk factors increases, both in high- and in low- and middle-income countries (LMIC), and as ART regimens evolve.4 The number of PLWH who are aged 50 years and older has tripled since 2000, and5 CVD, including stroke, is a leading cause of death among PLWH in high-income countries.6–8
Recent studies from the modern ART treatment era have demonstrated increased stroke risk in PLWH independent of age and traditional vascular risk factors.1,9–12 A large, retrospective Danish study showed that the incidence of a cerebrovascular event in PLWH was 1.6 times higher than for HIV-uninfected patients, even after controlling for intravenous drug use and other traditional vascular risk factors.10 In a large American healthcare claims database, PLWH had an elevated risk of stroke, with rates nearly triple that of uninfected controls after adjustment for sex and age.11 Immunosuppression may also modulate the risk of stroke. Several studies have demonstrated that low CD4 count and higher viral load are associated with a higher incidence of stroke in PLWH.9,10,12 While ischemic stroke is the most frequent type of stroke in PLWH, similar to the general population, the risk of hemorrhagic stroke may also be increased in HIV populations, especially among those with uncontrolled viremia, who are at higher risk of aneurysmal arteriopathy and opportunistic infections.13,14 Knowledge gaps remain regarding the extent to which socioeconomic factors (e.g. education, income) play a role in explaining increased stroke risk in PLWH.
Low- and middle-income countries
In LMIC, the incidence of stroke has more than doubled in the last 40 years.15,16 The burden of HIV is high in sub-Saharan Africa, where a substantial proportion of PLWH have a CD4 count less than 200 cells/mm3.17,18 In these regions, stroke in PLWH affects a much younger patient population with advanced, untreated disease, and stroke may be the initial presentation of HIV infection.2 One study in Malawi found that HIV was the second most common risk factor associated with stroke.18 PLWH in these regions may be particularly vulnerable to stroke given the high burden of advanced infection combined with increasing prevalence of traditional CVD risk factors in those with controlled infection.19,20
ISCHEMIC STROKE SUBTYPES
The distribution of ischemic stroke subtypes that occur with HIV may provide insight into the mechanisms underlying increased stroke risk and aid in predicting recurrent stroke risk and prognosis. Ischemic stroke has classically been divided into 3 main subtypes: cardioembolic, large artery atherosclerosis, and small vessel disease. Cardioembolic strokes in HIV may be secondary to atrial fibrillation and HIV-associated dilated cardiomyopathy. Strokes attributed to large artery atherosclerosis are defined by infarction distal to a large vessel stenosis, such as proximal intracranial arteries or the extracranial carotid artery. Small vessel strokes, or lacunar strokes, are typically defined by anatomical location (e.g. small subcortical infarct) and specific lacunar syndrome. In HIV-infected stroke populations, a higher proportion of strokes may be attributed to “undetermined” or “other determined” etiologies. A few small studies suggest that large artery atherosclerosis, small vessel disease, and stroke of undetermined etiology are the most common stroke subtypes among treated PLWH.21–23 A recent retrospective cohort showed that PLWH may be at a greater proportional risk of stroke of undetermined etiology compared with uninfected individuals.23 The high proportion of cryptogenic strokes in this patient population may be due to practice variation in the work-up of stroke etiologies in PLWH or novel mechanisms for stroke in the context of HIV infection.
MECHANISMS OF STROKE IN HIV INFECTION
In addition to the standard approach of classifying ischemic stroke mechanisms, immune status must be considered in the evaluation of stroke in PLWH. The pathophysiology of HIV-associated stroke is likely multifactorial and includes an interplay of traditional CVD risk factors, exposure to ART, virologic suppression, and chronic inflammation (Figure).
Figure 1: Mechanisms of Ischemic Stroke in HIV.
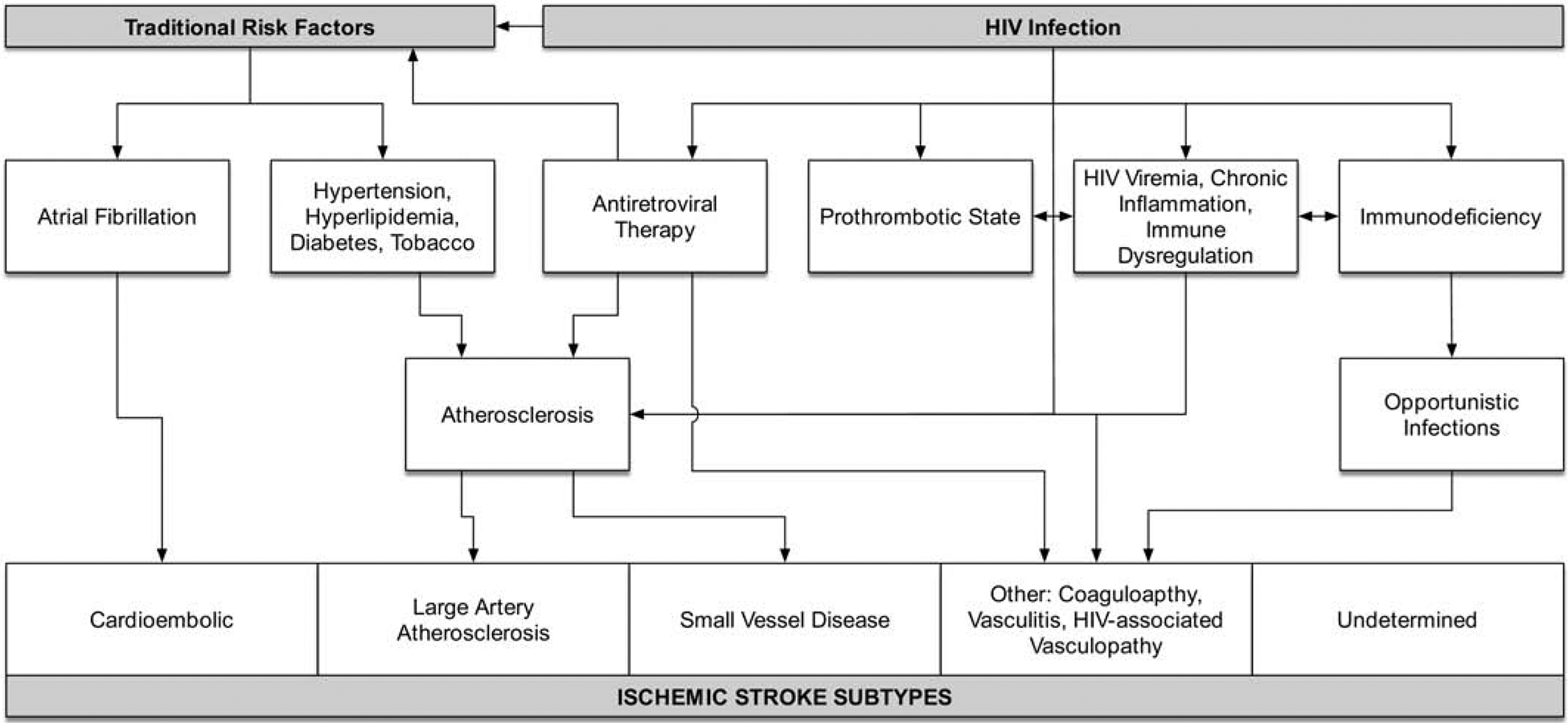
Traditional CVD Risk Factors
PLWH have a higher prevalence of traditional CVD risk factors, including hypertension, dyslipidemia, diabetes, and smoking.24–26 Smoking, for example, is highly prevalent among PLWH. A nationally representative US sample of PLWH found that 42% were current smokers and 20% were former smokers.27 As PLWH age, traditional CVD risk factors may play a more significant role in stroke events than HIV-related risk factors such as viral load and CD4 count.28,29
Infection-Associated Strokes
Cerebral infections are an important cause of secondary vasculitis and may cause both ischemic and hemorrhagic strokes. Immunosuppression related to HIV increases susceptibility to acquisition or reactivation of opportunistic infections associated with strokes such as tuberculosis, neurosyphilis, varicella-zoster virus, and cryptococcus. These infections may induce extensive cerebrovascular inflammation leading to endarteritis and a prothrombotic state that predisposes patients to ischemic strokes. The recent surge of syphilis cases in Canada and the US may increase the incidence of syphilis-associated strokes in PLWH.30–32 It is important to distinguish strokes in PLWH from stroke mimics due to primary central nervous system (CNS) lymphoma, CNS toxoplasmosis, CNS tuberculomas, brain abscesses and other infections.
Prothrombotic States
Several studies have identified prothrombotic states such as protein C and S deficiency, and less commonly, antiphospholipid antibodies in PLWH with uncontrolled infection who have had a stroke.33–35 While biomarkers of coagulation and inflammation in HIV can decrease on ART, they may remain elevated compared with HIV-uninfected individuals.36 In a case-control study of 82 PLWH with strokes, hypercoagulability was deemed responsible for 9% (7/82) of strokes, with protein S deficiency found in 45% (10/22) and anticardiolipin antibodies found in 29% (9/31) of tested patients.37 However, a South African study found no statistically significant difference in the prevalence of Protein S deficiency in PLWH with and without stroke.38 A causal relationship between these procoagulant factors and stroke in PLWH is not clearly established and may instead represent an epiphenomenon of uncontrolled HIV infection.39
Accelerated Atherosclerosis
Traditional CVD risk factors, HIV-specific novel risk factors, including chronic inflammation, and side effects of long-term use of some ART appear to contribute to accelerated atherosclerosis and higher risk of CVD in PLWH. Endothelial dysfunction and arterial inflammation are thought to be critical to the mechanism of accelerated atherosclerosis in PLWH. Carotid intima-media thickness, a subclinical marker of atherosclerosis and CVD risk, has been shown to be increased in PLWH compared with uninfected individuals in cross-sectional and longitudinal studies.40,41 HIV infection itself, immunodeficiency, and ART influence the progression of atherosclerosis.41,42 In a study of treated, virologically suppressed PLWH, atherosclerotic CVD risk was associated with carotid wall thickness on MRI, a known marker of CVD, whereas HIV status was not.43 Therefore, traditional CVD risk factors remain the strongest determinants of carotid atherosclerotic disease progression in PLWH.44
Nonatherosclerotic Vasculopathy
Non-atherosclerotic vasculopathy is an evolving term used to describe arterial changes in PLWH in the absence of atherosclerosis, infective vasculitis, or neoplastic involvement of the vessels. This form of vasculopathy has been primarily described in young, immunocompromised individuals.18 Vessel abnormalities include extra- or intracranial stenotic lesions or cerebral aneurysmal lesions that are hypothesized to result directly or indirectly from HIV infection. Clinical presentation is variable and may include transient ischemic attack (TIA), cerebral infarcts, or intracranial hemorrhage due to aneurysmal rupture. In one study of PLWH with predominantly unsuppressed infection, PLWH were more likely to develop adventitial inflammation, suggesting a different phenotype of vascular disease than the intimal inflammation associated with atherosclerosis seen in an aging population.45
Effects of HIV Treatment
ART may also contribute to the risk of stroke, both directly by accelerating atherosclerosis and indirectly by increasing life expectancy and shifting competing risks. Certain antiretroviral drugs and drug classes have been associated with elevated risk of CVD events, though less data are available on the association between ART and stroke. Other studies, however, have found an association between ART use and lower stroke risk,12,21,46 perhaps because ART use may function as a proxy for control of HIV infection. Protease inhibitors (PIs) have been associated with CVD because of their association with dyslipidemia, lipodystrophy, and metabolic syndrome. One study investigating newer PIs found an association between cumulative ritonavir-boosted darunavir use and increased CVD, including stroke, even after adjustment for CVD risk factors and viral load.47 Carotid atheromatosis progression has been shown to be associated with nonnucleoside reverse transcriptase inhibitor (NNRTI) exposure.48 Newer generations of nucleoside reverse transcriptase inhibitors (NRTIs) have fewer metabolic side effects, but there is conflicting evidence on abacavir’s association with elevated CVD risk. While a meta-analysis of randomized controlled trials did not support an increased risk of CVD events with abacavir,49 a meta-analysis of several observational studies demonstrated an association between abacavir and increased risk of CVD, including ischemic stroke.50 These older observational studies may have been subject to confounding by indication, since abacavir may have been used more frequently in patients with chronic kidney disease with higher baseline CVD risk,51 although in current practice abacavir is often avoided in patients at high CVD risk.
VASCULAR DISEASE AND HIV-ASSOCIATED COGNITIVE IMPAIRMENT
The relationship between CVD risk factors, atherosclerosis, and cognitive impairment has received increasing attention in the general population. A parallel association has been explored in HIV-associated cognitive impairment, which remains prevalent in the era of ART.52–54 In a substudy of the Strategies for Management of Antiretroviral Therapy (SMART) trial, a history of CVD resulted in a six-fold higher odds of baseline cognitive impairment in individuals with well-controlled HIV infection, whereas markers of control of HIV infection were not associated with test performance.55 CV risk factors and markers of CVD, ranging from diabetes mellitus to abdominal obesity, have also been linked to cognitive impairment in PLWH.56–61 Age-related chronic small vessel ischemic changes, which are strongly tied to stroke and dementia in the general population,62 have been shown to be more prevalent in PLWH28 and linked to HIV-associated cognitive impairment.63,64 Confirmation of a major role that CVD plays in the pathophysiology of HIV-associated cognitive impairment raises the exciting possibility of targeting CVD risk factors to preserve cognitive health. However, much work needs to be done on how these data can be translated to interventions that effectively improve cognition or delay cognitive decline in PLWH.
STROKE PREVENTION IN HIV
Due to the lack of specific data guiding primary and secondary stroke prevention for HIV infection, the approach to prevention and management of stroke for PLWH should follow, at a minimum, current guidelines for the general public. This approach to stroke prevention in PLWH requires early, effective treatment of HIV infection as well as assessment and treatment of traditional CVD risk factors (Table).
Table: Key stroke prevention strategies in HIV.
Primary prevention should focus on early initiation of ART and accurate assessment and optimization of vascular risk factors. Secondary prevention for non-cardioembolic strokes includes aspirin, high-intensity statin, and aggressive management of vascular risk factors. Carotid revascularization should be considered for moderate/severe stenosis. Cardioembolic strokes should be treated with anticoagulation.
| Primary Prevention | Strategy | ||
| Antiretroviral Therapy * | |||
| Assess and optimize vascular risk factors (e.g., hypertension, hyperlipidemia, diabetes mellitus) | |||
| Lifestyle modification (e.g., tobacco cessation) | |||
| Secondary Prevention | Ischemic Stroke Subtype | Strategy | Comments |
| Small vessel disease | Aspirin | Consider short-term dual antiplatelet therapy
|
|
| Intracranial stenosis | |||
| Carotid Atherosclerosis | Carotid revascularization for moderate/severe stenosis | CEA is preferred revascularization procedure for most patients | |
| Atrial Fibrillation | Anticoagulation** | Integrase inhibitors do not have any significant DDI with DOACs | |
Careful selection of cART to avoid DDI and minimize vascular risk factors (consider avoiding abacavir if high baseline CVD risk)
Caution with DDI
CEA, carotid endarterectomy; TIA, transient ischemic attack; DDI, drug-drug interactions; DOACs, direct oral anticoagulants
Predicting Stroke Risk
Current CVD risk prediction models were developed for use in the general population and were neither derived from nor validated in HIV-infected cohorts. These risk estimation tools may underestimate cardiovascular risk in HIV.65 PLWH have unique factors, such as the state of infection and inflammation, whose impacts on stroke risk are difficult to measure. There are mixed data regarding which existing risk assessment tool most accurately predicts CVD risk among PLWH.66,67 An HIV-specific CVD risk assessment tool derived from the D:A:D cohort which included traditional CVD risk factors as well as markers of immune function and exposure to two PIs and abacavir demonstrated an improved ability to accurately predict CVD events compared with the Framingham Risk Score (FRS).67 Since it is unclear how much ART contributes to CVD risk, an updated reduced D:A:D model omitted ART and still outperformed the FRS.68 However, generalizability to other HIV-infected populations is unknown since the tool has only been validated in a subset of the D:A:D cohort and not in an external cohort.
The Framingham Stroke Risk Score (FSR-S) combines stroke risk factors (age, sex, systolic blood pressure, use of antihypertensive, presence/absence of left ventricular hypertrophy on ECG, prevalent CVD, current smoking status, current/previous atrial fibrillation, and diabetes) to predict the 10-year probability of stroke. However, the FRS-S may underestimate the long-term risk of stroke in PLWH. In the Multicenter AIDS Cohort (MACS), despite higher incidence of stroke in HIV-infected men (3.3 vs 1.7 per 1000 person-years), mean 10-year FRS-S was lower in HIV–infected participants (4.5% vs 6.6%, p<0.04).69 Developing a stroke risk prediction tool that integrates traditional stroke risk factors along with HIV-related variables associated with stroke may improve our ability to identify PLWH at increased cerebrovascular risk. However, addition of HIV-related factors to the atherosclerotic cardiovascular disease (ASCVD) risk score in one study of the Centers for AIDS Research Network of Integrated Clinical Systems (CNICS) did not improve model performance.70
Role of HIV Therapy
While there are contradictory results regarding the negative effect of ART on CVD and cerebrovascular risk, ART is essential for stroke prevention in PLWH for several reasons. Early initiation of ART reduces serious AIDS-related complications including opportunistic infections that cause cerebral vasculitis and secondary strokes. In addition, uncontrolled viral load and low CD4 count have been consistently associated with higher rates of CVD, including stroke. Suppression of HIV viremia reduces immune activation and inflammation, thereby diminishing end-organ damage, including of the heart and brain.71 The SMART trial demonstrated that ART treatment interruption is associated with a higher risk of CVD events.72 While ART might lower the stroke risk associated with uncontrolled HIV infection, in the long term, there is some mixed evidence that it may contribute to the progression of atherosclerosis. Therefore, although the data are not clear cut, avoidance of ART that may be associated with higher CVD risk (e.g., select PIs, abacavir) and those that may have adverse cardiometabolic effects is reasonable when selecting an ART regimen. Limited data have suggested that stroke risk may be elevated in the period after initiation of ART in immunosuppressed patients, possibly due to an immune reconstitution inflammatory phenomenon. However, this finding should be interpreted with caution as it is based on a small number of people who started ART in a case-control study.29
Managing Traditional CVD Risk Factors
Traditional stroke risk factors such as hypertension, dyslipidemia, and smoking should be managed aggressively in PLWH. As in the general population, adherence to a healthy lifestyle is an essential first step for primary and secondary stroke prevention in PLWH. Smoking cessation is particularly important given the high prevalence of smoking among PLWH and the clear role of smoking in atherosclerosis and stroke. Regular physical activity is also an essential aspect of lifestyle optimization in HIV given the association of improved inflammation and cardiometabolic health with increasing physical activity in HIV.73–75 Absent HIV-specific data on optimal diets to prevent stroke, adherence to ACC/AHA dietary guidelines is reasonable.76 Similarly, diabetes, hypertension, and dyslipidemia should be managed per guidelines for the general population given insufficient data to recommend a divergent approach in HIV.
Statin Therapy
In the general population, the evidence supporting statin therapy in primary prevention of strokes is derived mainly from studies designed to evaluate the efficacy of statins for prevention of coronary heart disease in patients with high CVD risk, including hypertension and diabetes. These trials found a relative risk reduction of stroke ranging from 18–51%.77 In a meta-analysis of 47 trials, high-dose statin was associated with reduced risk of stroke by 17% compared with low-dose statin therapy.78 Current 2014 AHA/ASA guidelines for primary prevention of ischemic stroke recommend a statin in patients estimated to have a high 10-year risk for CVD events79. Given the aforementioned limitations in reliably incorporating HIV infection into CVD risk models, whether further intensification or earlier initiation of statin therapy among people with HIV would be warranted is unclear.
For patients who have had a TIA or ischemic stroke of atherosclerotic origin, high-intensity statin therapy is recommended for secondary stroke prevention, independent of the baseline low density lipoprotein (LDL) cholesterol.76,80 The Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial was the first to show the benefits of atorvastatin 80mg for patients with a history of recent non-disabling stroke or TIA and no coronary heart disease or hypercholesterolemia, with a 16% relative risk reduction in recurrent stroke.81 An LDL cholesterol level of < 70mg/dL was related to a 28% reduction in risk of stroke. Therefore, LDL cholesterol remains the primary lipid treatment target for reduction of stroke risk. PLWH who have a history of ischemic stroke of atherosclerotic origin should have a goal LDL of <70 mg/dL.
There is a paucity of large, randomized clinical trial data to inform management of dyslipidemia and stroke prevention in HIV-infected populations. Statins may be useful in PLWH as they have the advantage of lowering LDL cholesterol but may also reduce immune activation and inflammation. Given the increased prevalence of dyslipidemia and earlier incidence and progression of atherosclerosis in PLWH, statin therapy might be particularly beneficial for PLWH. However, there is a dearth of quality trial data evaluating clinical outcomes in PLWH on simultaneous ART and statin therapy. The risk estimation tools used to guide management of dyslipidemia in primary stroke prevention were not derived from nor validated in HIV-infected cohorts.82 Furthermore, statin therapy may be underutilized in PLWH. Despite the prevalence of dyslipidemia in up to 80% of PLWH, as few as 5.9% of PLWH on ART are on statins.83 In a cohort study of 258 PLWH, 14% (36/258) were at a high risk of stroke based on the 10-year stroke FRS and although 97.2% (35/36) of those patients required statin therapy, only 37.1% (13/35) were receiving a statin.84 The low rates of statin prescription may be due to clinicians’ concerns regarding statin metabolism and potential drug-drug interactions between ART and several statins.
Statin therapy may confer additional protection against CVD beyond lipid-lowering by decreasing biomarkers of arterial inflammation and thrombosis. Immune activation, even in individuals with well-controlled infection, may mediate increased CVD risk in PLWH, resulting in increased CVD events. A randomized control trial in PLWH (SATURN-HIV) suggested that rosuvastatin 10mg was associated with a reduction in some markers of inflammation, monocyte activation, and vascular inflammation compared with placebo.85,86 However, other studies have not demonstrated similar effects of statins on inflammatory indices (e.g., IL-6 hsCRP, and D-dimer).87,88 Rosuvastatin 10mg was associated with 0.019mm (95% CI 0.002–0.037mm) lower carotid IMT change over 96 weeks in SATURN-HIV.89 It remains to be established if the pleiotropic effects of statin on plaque indices and inflammatory markers translates to a reduction of CVD events.87
Statin use in HIV is complicated by potential drug interactions, although newer statins and ART may have fewer drug-drug interactions. Statins such as simvastatin and lovastatin and, to a lesser extent, atorvastatin are metabolized by cytochrome P (CYP) 450 3A4, which is inhibited by PIs and pharmacokinetic boosters such as cobicistat, resulting in increased statin systemic concentration. In an open-label study, the addition of an older PI nelfinavir to simvastatin was associated with a 505% and 517% increase in steady-state and maximum concentrations of simvastatin.90 Therefore, simvastatin is contraindicated in PLWH on PIs. Pravastatin, rosuvastatin, and pitavastatin appear to have the most benign safety profiles among statins when co-administered with ART and may not require dose adjustment. Atorvastatin, for which there is the most evidence for secondary stroke prevention, is used most often clinically, though usually at a lower dose in patients on PIs. Although some NNRTIs may induce CYP enzymes (resulting in lower statin concentrations in blood and tissues), dose adjustments when administered with statins may not be required. Careful monitoring is advised, however, particularly for PLWH on high-dose statins.91
At this time, there are insufficient large-scale, randomized data to form evidence-based recommendations regarding statin therapy in primary prevention of stroke in the HIV-infected population. Given the risk of accelerated atherosclerosis and CVD associated with HIV, it is reasonable to treat PLWH on ART at least as aggressively as the general population, though providers should be mindful of potential drug-drug interactions between statins and ART. Results from the National Institutes of Health-funded Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE), a randomized double-blind trial currently underway of pitavastatin 4mg versus placebo for primary CVD prevention in >7,500 PLWH with low or moderate risk of CVD, are eagerly awaited.92 The primary outcome of REPRIEVE is major adverse CVD events, including CVD-related mortality, myocardial infarction, stroke, unstable angina, peripheral artery disease, and cardiac revascularization. REPRIEVE will also include a substudy of 800 participants investigating the mechanism of action of pitavastatin, particularly in reducing immune activation and inflammation.93
Antiplatelet Therapy
For decades, aspirin has been recommended for cardiac but not stroke prevention in the general population. Recent primary prevention trials have shown less overall benefit of prophylactic aspirin for CVD94,95. As a result, the new 2019 ACC/AHA guidelines for primary prevention of ASCVD suggest that aspirin for ASCVD prophylaxis (including but not specific to stroke) may be considered among select adults 40 to 70 years of age who are at higher ASCVD risk but not at increased bleeding risk.82 A meta-analysis of 9 clinical trials including 50,868 participants found no overall benefit of aspirin for primary prevention of stroke.79,96 Any potential benefit of aspirin in preventing ischemic stroke appears to be offset by an increase in hemorrhagic stroke.97 However, there are certain populations in whom aspirin may be more beneficial for stroke primary prevention. Aspirin may be useful for the prevention of a first stroke among women, including those with diabetes, whose risk is sufficiently high for the benefits to outweigh the risks associated with treatment.79,98
Antiplatelet therapy is the mainstay of secondary prevention of ischemic stroke in patients with a history of non-cardioembolic stroke or TIA. In a meta-regression analysis of placebo-controlled trials of aspirin therapy for secondary stroke prevention, the relative risk reduction for any type of stroke was 15%.99 Aspirin monotherapy or a combination of aspirin is indicated as initial therapy after TIA or ischemic stroke for secondary prevention, although clopidogrel monotherapy is also a reasonable option.80 Short-term dual antiplatelet therapy (aspirin and clopidogrel for 21 days) is recommended in patients with a recent minor ischemic stroke or high-risk TIA100,101 and for 90 days in patients with symptomatic large artery intracranial atherosclerosis.102 However, there is currently no indication for long-term dual antiplatelet therapy for secondary stroke prevention.
There are limited data evaluating whether the benefit of antiplatelet therapy for stroke prevention in PLWH differs from the general population. Underutilization of aspirin for prevention of CVD in PLWH has been reported.84,103–105 An Italian multicenter prospective cohort study found that only about 50% of PLWH requiring aspirin for primary and secondary CVD prevention were prescribed aspirin.106 No studies are available regarding the utilization of aspirin specifically for secondary stroke prevention in PLWH. Of the antiplatelet agents, aspirin has fewer drug-drug interactions with ART than clopidogrel or dipyridamole. Aspirin reduces abacavir-induced in vivo platelet activation and platelet hyperactivity in PLWH, and may reduce the risk of recurrent ASCVD events for PLWH who remain on abacavir after an initial event,107,108 These studies raise the question of whether aspirin should be prescribed for all PLWH on abacavir-containing ART. In addition, the utility of aspirin may extend beyond its antiplatelet properties to include immunomodulatory benefits. In a pilot study, aspirin was shown to reduce platelet aggregation and markers of T-cell and monocyte activation in virologically suppressed PLWH.109 However, in a larger randomized controlled trial, aspirin had no significant effect on markers of inflammation, T-cell or monocyte activation, or endothelial function compared with placebo among PLWH on suppressive ART.110
Extracranial Carotid Atherosclerosis
Given the risk of accelerated atherosclerosis in HIV,40–42 management of extracranial carotid atherosclerosis is a critical component of stroke prevention for PLWH. The prevalence of HIV among those undergoing carotid intervention increased between 2004 and 2014, and PLWH who undergo carotid intervention tend to be younger than individuals without HIV infection.111 It is unclear if the benefit of revascularization in carotid stenosis differs between PLWH and non-HIV infected individuals. In the absence of specific data guiding carotid stenosis management in HIV infection, the approach to prevention of stroke in PLWH with carotid stenosis should follow current guidelines for the general public. Revascularization with carotid endarterectomy (CEA) is recommended for individuals with recent TIA or ischemic stroke due to ipsilateral severe (70%−99%) carotid artery stenosis and in select patients with moderate (50%−69%) carotid stenosis.80 Carotid stenting may be an alternative to CEA for symptomatic patients with severe carotid artery stenosis whom are younger or at low risk of complications associated with endovascular intervention.80 Patients with asymptomatic carotid stenosis should receive medical management including aspirin, statin and optimization of CVD risk factors. It is reasonable to consider CEA in individuals with asymptomatic severe (>70%) stenosis of the internal carotid artery, though its effectiveness compared with contemporary best medical management alone is not well established.79,80 Results from the ongoing CREST-2 trial, a large randomized controlled trial comparing carotid revascularization versus contemporary medical management alone for preventing stroke in patients with asymptomatic high-grade carotid stenosis, will help to address this uncertainty.
Atrial Fibrillation
HIV-related immunosuppression and traditional CVD risk factors have been shown to be associated with increased risk of atrial fibrillation/atrial flutter among PLWH.112 Cardioembolic stroke may account for up to 20% of ischemic stroke among PLWH.23,112 Risk stratification tools such as CHA2DS2-VASc and HAS-BLED scores estimate cardioembolic stroke and hemorrhagic complications of anticoagulation therapy, respectively, and are used to guide stroke prevention in the general population with atrial fibrillation. The reliability of these scores in PLWH is unclear,113 as is the safety of anticoagulants and their interactions with ART.114,115 European and American guidelines for the management of atrial fibrillation recommend direct oral anticoagulants (DOACs) and warfarin as equivalent options in the general population; however, there are limited data on the use of anticoagulants in PLWH taking ART. Warfarin was previously the mainstay of anticoagulation in people with HIV due to providers’ familiarity with warfarin and the ability to monitor with INRs; however, it has significant interactions with antiretrovirals that are metabolized via CYP450 pathways. DOACs are an appealing alternative to warfarin and there is some evidence for the safety and efficacy of concomitant dabigatran and ART.116,117 For PLWH on PIs or NNRTIs, dabigatran has no significant interactions, while the strong interaction with rivaroxaban precludes coadministration, and the interaction with apixaban may require dose reduction (to 2.5mg twice daily). Integrase inhibitors, which are commonly recommended as first-line antiretrovirals worldwide, do not have any significant interactions with DOACs.114,118
Managing Novel Risk Factors
Alternative and adjunctive approaches are needed to reduce excess CVD and stroke risk in HIV. Studies evaluating the efficacy of strategies that address risk factors unique to PLWH, such as persistent inflammation and immune activation, will be essential to develop more targeted stroke prevention strategies in the future. The aforementioned REPRIEVE trial is underway to assess the effect of statin use for primary prevention of CVD events in PLWH without known CVD.119 In non-HIV populations, large studies have demonstrated that aggressive LDL cholesterol lowering in populations at high CVD risk reduces CVD events.120–122 Proprotein convertase subtilsin-kexin type 9(PCSK9), which binds and degrades LDL receptors and results in an increase in LDL cholesterol, is increased in PLWH, especially in those with HCV co-infection.123 PCSK9 is also increased in parallel with inflammatory markers, such as interleukin (IL)-6,123 especially in those who are ART naive.124 PCSK9 inhibitors are monoclonal antibodies with minimal significant drug-drug interactions that reduce LDL cholesterol by ~60% even in the setting of high-intensity statin therapy.121 The EPIC-HIV study is currently underway to investigate the impact of PCSK9 inhibitor therapy on lipids, inflammatory markers, and subclinical ASCVD in PLWH.125(p9)
While early initiation of ART lowers inflammation in HIV, inflammatory markers remain high compared with HIV-uninfected individuals126,127 and are associated with increased CVD risk.128 The IL-1 pathway, which has been implicated in both atherogenesis and HIV disease pathogenesis, is an attractive target to reduce inflammation and potentially prevent CVD, including stroke, in PLWH. CANTOS (Canakinumab Anti-Inflammatory Thrombosis Outcomes Study), a randomized, placebo-controlled trial of over 10,000 participants which specifically excluded PLWH, demonstrated that canakinumab, a monoclonal antibody targeting IL-1β, significantly reduced IL-6 and high-sensitivity C-reactive protein (hsCRP) and led to lower rates of recurrent CVD events in individuals with known CVD and hsCRP > 2mg/L.98 Although there was no significant difference in all-cause mortality between the canakinumab and placebo groups, a higher rate of fatal infections was observed in those assigned to canakinumab. A placebo-controlled trial of canakinumab to reduce atherosclerotic inflammation in PLWH is in progress; preliminary results demonstrated a significant reduction in inflammatory markers and atherosclerotic inflammation in the aorta and carotids measured by FDG-PET.129 A recent phase II trial of low-dose methotrexate to improve endothelial function and inflammation in people with treated, virologically suppressed HIV demonstrated more adverse events in those receiving methotrexate compared with placebo.130 Although the prespecified noninferiority margin of 15% was not exceeded in the trial, the results underscore the critical balance between potential benefits and risks of anti-inflammatory interventions in HIV-infected populations, even in PLWH with treated, suppressed infection.
CONCLUSION AND FUTURE DIRECTIONS
The risk of stroke is elevated in PLWH compared with the general population, and the prevalence of stroke will continue to increase as PLWH age. Optimal stroke risk reduction strategies in HIV are not well defined given the lack of large-scale data on stroke risk stratification, prevention, and clinical outcomes in PLWH. At a minimum, PLWH should be treated as aggressively as the general population for stroke prevention and management as per current guidelines. Further investigation into the pathogenesis of stroke and the mechanisms underlying increased stroke risk will be essential to developing targeted interventions to lower cerebrovascular risk in PLWH. Future studies should also address knowledge gaps in successful implementation and uptake of stroke risk reduction strategies for PLWH. Aggressive public health measures and a primary care-focused approach to combat the high prevalence of traditional vascular risk factors in PLWH will be crucial for effective primary and secondary stroke prevention in HIV infection.
Disclosures/COI:
Ivy Nguyen - none.
Anthony Kim - SanBio; research grants unrelated to the current work.
Felicia Chow - research grants from NIH
Abbreviations:
- ART
antiretroviral therapy
- ASCVD
atherosclerotic cardiovascular disease
- CEA
carotid endarterectomy
- CNS
central nervous system
- CVD
cardiovascular disease
- CYP
cytochrome P
- DOAC
direct oral anticoagulant
- FRS
Framingham Risk Score
- hsCRP
high sensitivity C-reactive protein
- IL
interleukin
- LDL
low density lipoprotein
- LMIC
low- and middle-income countries
- NNRTI
nonnucleoside reverse transcriptase inhibitor
- NRTI
nucleoside reverse transcriptase inhibitor
- PI
protease inhibitors
- PLWH
people living with HIV
- PCSK9
proprotein convertase subtilsin-kexin type 9
- TIA
transient ischemic attack
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ovbiagele B, Nath A. Increasing incidence of ischemic stroke in patients with HIV infection. Neurology. 2011;76(5):444–450. doi: 10.1212/WNL.0b013e31820a0cfc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdallah A, Chang JL, O’Carroll CB, et al. Stroke in HIV-infected individuals in sub-Saharan Africa (SSA): A systematic review. J Stroke Cerebrovasc Dis. 2018;27(7):1828–1836. doi: 10.1016/j.jstrokecerebrovasdis.2018.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singer EJ, Valdes-Sueiras M, Commins DL, Yong W, Carlson M. HIV stroke risk: evidence and implications. Therapeutic Advances in Chronic Disease. 2013;4(2):61–70. doi: 10.1177/2040622312471840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Autenrieth CS, Beck EJ, Stelzle D, Mallouris C, Mahy M, Ghys P. Global and regional trends of people living with HIV aged 50 and over: Estimates and projections for 2000–2020. PLoS One. 2018;13(11). doi: 10.1371/journal.pone.0207005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bogorodskaya M, Chow FC, Triant VA. Stroke in HIV. Canadian Journal of Cardiology. 2019;35(3):280–287. doi: 10.1016/j.cjca.2018.11.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shahbaz S, Manicardi M, Guaraldi G, Raggi P. Cardiovascular disease in human immunodeficiency virus infected patients: A true or perceived risk? World J Cardiol. 2015;7(10):633–644. doi: 10.4330/wjc.v7.i10.633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanna DB, Ramaswamy C, Kaplan RC, et al. Trends in Cardiovascular Disease Mortality Among Persons With HIV in New York City, 2001–2012. Clin Infect Dis. 2016;63(8):1122–1129. doi: 10.1093/cid/ciw470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mocroft A, Reiss P, Gasiorowski J, et al. Serious fatal and nonfatal non-AIDS-defining illnesses in Europe. J Acquir Immune Defic Syndr. 2010;55(2):262–270. doi: 10.1097/QAI.0b013e3181e9be6b [DOI] [PubMed] [Google Scholar]
- 9.Marcus JL, Leyden WA, Chao CR, et al. HIV infection and incidence of ischemic stroke: AIDS. 2014;28(13):1911–1919. doi: 10.1097/QAD.0000000000000352 [DOI] [PubMed] [Google Scholar]
- 10.Rasmussen LD, Engsig FN, Christensen H, et al. Risk of cerebrovascular events in persons with and without HIV: a Danish nationwide population-based cohort study. AIDS. 2011;25(13):1637–1646. doi: 10.1097/QAD.0b013e3283493fb0 [DOI] [PubMed] [Google Scholar]
- 11.Alonso A, Barnes AE, Guest JL, Shah A, Shao IY, Marconi V. HIV Infection and Incidence of Cardiovascular Diseases: An Analysis of a Large Healthcare Database. JAHA. 2019;8(14). doi: 10.1161/JAHA.119.012241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chow FC, Regan S, Feske S, Meigs JB, Grinspoon SK, Triant VA. Comparison of Ischemic Stroke Incidence in HIV-Infected and Non–HIV-Infected Patients in a US Health Care System: JAIDS Journal of Acquired Immune Deficiency Syndromes. 2012;60(4):351–358. doi: 10.1097/QAI.0b013e31825c7f24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Behrouz R, Topel CH, Seifi A, et al. Risk of intracerebral hemorrhage in HIV/AIDS: a systematic review and meta-analysis. J Neurovirol. 2016;22(5):634–640. doi: 10.1007/s13365-016-0439-2 [DOI] [PubMed] [Google Scholar]
- 14.Chow FC, He W, Bacchetti P, et al. Elevated rates of intracerebral hemorrhage in individuals from a US clinical care HIV cohort. Neurology. 2014;83(19):1705–1711. doi: 10.1212/WNL.0000000000000958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.GBD 2016 Stroke Collaborators. Global, regional, and national burden of stroke, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(5):439–458. doi: 10.1016/S1474-4422(19)30034-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim AS, Cahill E, Cheng NT. Global Stroke Belt: Geographic Variation in Stroke Burden Worldwide. Stroke. 2015;46(12):3564–3570. doi: 10.1161/STROKEAHA.115.008226 [DOI] [PubMed] [Google Scholar]
- 17.Mlay M, Bakari M. The prevalence of HIV among patients admitted with stroke at the Muhimbili National Hospital, Dar es Salaam, Tanzania. Tanzania Journal of Health Research. 2010;12(2):105–113–113. doi: 10.4314/thrb.v12i2.56397 [DOI] [Google Scholar]
- 18.Benjamin LA, Allain TJ, Mzinganjira H, et al. The Role of Human Immunodeficiency Virus-Associated Vasculopathy in the Etiology of Stroke. J Infect Dis. 2017;216(5):545–553. doi: 10.1093/infdis/jix340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feinstein MJ, Bogorodskaya M, Bloomfield GS, et al. Cardiovascular Complications of HIV in Endemic Countries. Curr Cardiol Rep. 2016;18(11):113. doi: 10.1007/s11886-016-0794-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel P, Rose CE, Collins PY, et al. Noncommunicable diseases among HIV-infected persons in low-income and middle-income countries: a systematic review and meta-analysis. AIDS. 2018;32(Suppl 1):S5–S20. doi: 10.1097/QAD.0000000000001888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corral I, Quereda C, Moreno A, et al. Cerebrovascular ischemic events in HIV-1-infected patients receiving highly active antiretroviral therapy: incidence and risk factors. Cerebrovasc Dis. 2009;27(6):559–563. doi: 10.1159/000214219 [DOI] [PubMed] [Google Scholar]
- 22.Vinikoor MJ, Napravnik S, Floris-Moore M, Wilson S, Huang DY, Eron JJ. Incidence and clinical features of cerebrovascular disease among HIV-infected adults in the Southeastern United States. AIDS Res Hum Retroviruses. 2013;29(7):1068–1074. doi: 10.1089/aid.2012.0334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chow FC, Price RW, Hsue PY, Kim AS. Greater Risk of Stroke of Undetermined Etiology in a Contemporary HIV-Infected Cohort Compared with Uninfected Individuals. Journal of Stroke and Cerebrovascular Diseases. 2017;26(5):1154–1160. doi: 10.1016/j.jstrokecerebrovasdis.2017.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92(7):2506–2512. doi: 10.1210/jc.2006-2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gutierrez J, Albuquerque ALA, Falzon L. HIV infection as vascular risk: A systematic review of the literature and meta-analysis. PLoS ONE. 2017;12(5):e0176686. doi: 10.1371/journal.pone.0176686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sico JJ, Chang C-CH, So-Armah K, et al. HIV status and the risk of ischemic stroke among men. Neurology. 2015;84(19):1933–1940. doi: 10.1212/WNL.0000000000001560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mdodo R, Frazier EL, Dube SR, et al. Cigarette smoking prevalence among adults with HIV compared with the general adult population in the United States: cross-sectional surveys. Ann Intern Med. 2015;162(5):335–344. doi: 10.7326/M14-0954 [DOI] [PubMed] [Google Scholar]
- 28.Moulignier A, Savatovsky J, Assoumou L, et al. Silent Cerebral Small-Vessel Disease Is Twice as Prevalent in Middle-Aged Individuals With Well-Controlled, Combination Antiretroviral Therapy-Treated Human Immunodeficiency Virus (HIV) Than in HIV-Uninfected Individuals. Clin Infect Dis. 2018;66(11):1762–1769. doi: 10.1093/cid/cix1075 [DOI] [PubMed] [Google Scholar]
- 29.Benjamin LA, Corbett EL, Connor MD, et al. HIV, antiretroviral treatment, hypertension, and stroke in Malawian adults: A case-control study. Neurology. 2016;86(4):324–333. doi: 10.1212/WNL.0000000000002278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ling DI, Janjua NZ, Wong S, et al. Sexually transmitted infection trends among gay or bisexual men from a clinic-based sentinel surveillance system in British Columbia, Canada. Sex Transm Dis. 2015;42(3):153–159. doi: 10.1097/OLQ.0000000000000250 [DOI] [PubMed] [Google Scholar]
- 31.Nguyen TQ, Kohn RP, Ng RC, Philip SS, Cohen SE. Historical and Current Trends in the Epidemiology of Early Syphilis in San Francisco, 1955 to 2016. Sex Transm Dis. 2018;45(9S Suppl 1):S55–S62. doi: 10.1097/OLQ.0000000000000870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Novak RM, Ghanem A, Hart R, et al. Risk Factors and Incidence of Syphilis in Human Immunodeficiency Virus (HIV)-Infected Persons: The HIV Outpatient Study, 1999–2015. Clin Infect Dis. 2018;67(11):1750–1759. doi: 10.1093/cid/ciy348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mochan A, Modi M, Modi G. Stroke in black South African HIV-positive patients: a prospective analysis. Stroke. 2003;34(1):10–15. doi: 10.1161/01.str.0000043821.35051.fa [DOI] [PubMed] [Google Scholar]
- 34.Casado Naranjo I, Toledo Santos JA, Antolin Rodriguez MA. Ischemic stroke as the sole manifestation of human immunodeficiency virus infection. Stroke. 1992;23(1):117–118. [PubMed] [Google Scholar]
- 35.Vishnu P, Aboulafia DM. Haematological manifestations of human immune deficiency virus infection. Br J Haematol. 2015;171(5):695–709. doi: 10.1111/bjh.13783 [DOI] [PubMed] [Google Scholar]
- 36.Funderburg NT. Markers of coagulation and inflammation often remain elevated in ART-treated HIV-infected patients. Curr Opin HIV AIDS. 2014;9(1):80–86. doi: 10.1097/COH.0000000000000019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ortiz G, Koch S, Romano JG, Forteza AM, Rabinstein AA. Mechanisms of ischemic stroke in HIV-infected patients. Neurology. 2007;68(16):1257–1261. doi: 10.1212/01.wnl.0000259515.45579.1e [DOI] [PubMed] [Google Scholar]
- 38.Mochan A, Modi M, Modi G. Protein S deficiency in HIV associated ischaemic stroke: an epiphenomenon of HIV infection. J Neurol Neurosurg Psychiatry. 2005;76(10):1455–1456. doi: 10.1136/jnnp.2004.059733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qureshi AI. HIV infection and stroke: if not protein S deficiency then what explains the relationship? Journal of Neurology, Neurosurgery & Psychiatry. 2005;76(10):1331–1331. doi: 10.1136/jnnp.2005.072017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hanna DB, Post WS, Deal JA, et al. HIV Infection Is Associated With Progression of Subclinical Carotid Atherosclerosis. Clin Infect Dis. 2015;61(4):640–650. doi: 10.1093/cid/civ325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hsue PY, Lo JC, Franklin A, et al. Progression of atherosclerosis as assessed by carotid intima-media thickness in patients with HIV infection. Circulation. 2004;109(13):1603–1608. doi: 10.1161/01.CIR.0000124480.32233.8A [DOI] [PubMed] [Google Scholar]
- 42.Msoka TF, Van Guilder GP, van Furth M, et al. The effect of HIV infection, antiretroviral therapy on carotid intima-media thickness: A systematic review and meta-analysis. Life Sci. 2019;235:116851. doi: 10.1016/j.lfs.2019.116851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mee TC, Aepfelbacher J, Krakora R, et al. Carotid magnetic resonance imaging in persons living with HIV and 10-year atherosclerotic cardiovascular disease risk score. Antivir Ther (Lond). 2018;23(8):695–698. doi: 10.3851/IMP3258 [DOI] [PubMed] [Google Scholar]
- 44.Mangili A, Polak JF, Skinner SC, et al. HIV infection and progression of carotid and coronary atherosclerosis: the CARE study. J Acquir Immune Defic Syndr. 2011;58(2):148–153. doi: 10.1097/QAI.0b013e31822d4993 [DOI] [PubMed] [Google Scholar]
- 45.Gutierrez J, Menshawy K, Gonzalez M, et al. Brain large artery inflammation associated with HIV and large artery remodeling. AIDS. 2016;30(3):415–423. doi: 10.1097/QAD.0000000000000927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bozzette SA, Ake CF, Tam HK, Chang SW, Louis TA. Cardiovascular and cerebrovascular events in patients treated for human immunodeficiency virus infection. N Engl J Med. 2003;348(8):702–710. doi: 10.1056/NEJMoa022048 [DOI] [PubMed] [Google Scholar]
- 47.Ryom L, Lundgren JD, El-Sadr W, et al. Cardiovascular disease and use of contemporary protease inhibitors: the D:A:D international prospective multicohort study. Lancet HIV. 2018;5(6):e291–e300. doi: 10.1016/S2352-3018(18)30043-2 [DOI] [PubMed] [Google Scholar]
- 48.Psichogiou M, Kapelios CJ, Konstantonis G, et al. Prevalence, Incidence, and Contributors of Subclinical Atheromatosis, Arteriosclerosis, and Arterial Hypertrophy in HIV-Infected Individuals: A Single-Center, 3-Year Prospective Study. Angiology. 2019;70(5):448–457. doi: 10.1177/0003319718801093 [DOI] [PubMed] [Google Scholar]
- 49.Ding X, Andraca-Carrera E, Cooper C, et al. No association of abacavir use with myocardial infarction: findings of an FDA meta-analysis. J Acquir Immune Defic Syndr. 2012;61(4):441–447. doi: 10.1097/QAI.0b013e31826f993c [DOI] [PubMed] [Google Scholar]
- 50.Bavinger C, Bendavid E, Niehaus K, et al. Risk of Cardiovascular Disease from Antiretroviral Therapy for HIV: A Systematic Review. Landay A, ed. PLoS ONE. 2013;8(3):e59551. doi: 10.1371/journal.pone.0059551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bedimo RJ, Westfall AO, Drechsler H, Vidiella G, Tebas P. Abacavir use and risk of acute myocardial infarction and cerebrovascular events in the highly active antiretroviral therapy era. Clin Infect Dis. 2011;53(1):84–91. doi: 10.1093/cid/cir269 [DOI] [PubMed] [Google Scholar]
- 52.Heaton RK, Franklin DR, Ellis RJ, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17(1):3–16. doi: 10.1007/s13365-010-0006-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heaton RK, Clifford DB, Franklin DR, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75(23):2087–2096. doi: 10.1212/WNL.0b013e318200d727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cysique LA, Maruff P, Brew BJ. Prevalence and pattern of neuropsychological impairment in human immunodeficiency virus-infected/acquired immunodeficiency syndrome (HIV/AIDS) patients across pre- and post-highly active antiretroviral therapy eras: a combined study of two cohorts. J Neurovirol. 2004;10(6):350–357. doi: 10.1080/13550280490521078 [DOI] [PubMed] [Google Scholar]
- 55.Wright EJ, Grund B, Robertson K, et al. Cardiovascular risk factors associated with lower baseline cognitive performance in HIV-positive persons. Neurology. 2010;75(10):864–873. doi: 10.1212/WNL.0b013e3181f11bd8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Becker JT, Kingsley L, Mullen J, et al. Vascular risk factors, HIV serostatus, and cognitive dysfunction in gay and bisexual men. Neurology. 2009;73(16):1292–1299. doi: 10.1212/WNL.0b013e3181bd10e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Valcour VG, Sacktor NC, Paul RH, et al. Insulin resistance is associated with cognition among HIV-1-infected patients: the Hawaii Aging With HIV cohort. J Acquir Immune Defic Syndr. 2006;43(4):405–410. doi: 10.1097/01.qai.0000243119.67529.f5 [DOI] [PubMed] [Google Scholar]
- 58.SATTLER F, HE J, LETENDRE S, et al. Abdominal Obesity Contributes to Neurocognitive Impairment in HIV Infected Patients with Increased Inflammation and Immune Activation. J Acquir Immune Defic Syndr. 2015;68(3):281–288. doi: 10.1097/QAI.0000000000000458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schouten J, Su T, Wit FW, et al. Determinants of reduced cognitive performance in HIV-1-infected middle-aged men on combination antiretroviral therapy. AIDS. 2016;30(7):1027–1038. doi: 10.1097/QAD.0000000000001017 [DOI] [PubMed] [Google Scholar]
- 60.Valcour V, Rubin LH, Tien P, et al. Human immunodeficiency virus (HIV) modulates the associations between insulin resistance and cognition in the current combination antiretroviral therapy (cART) era: a study of the Women’s Interagency HIV Study (WIHS). J Neurovirol. 2015;21(4):415–421. doi: 10.1007/s13365-015-0330-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McCutchan JA, Marquie-Beck JA, Fitzsimons CA, et al. Role of obesity, metabolic variables, and diabetes in HIV-associated neurocognitive disorder. Neurology. 2012;78(7):485–492. doi: 10.1212/WNL.0b013e3182478d64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wallin A, Roman GC, Esiri M, et al. Update on Vascular Cognitive Impairment Associated with Subcortical Small-Vessel Disease2. J Alzheimers Dis. 62(3):1417–1441. doi: 10.3233/JAD-170803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Watson C, Busovaca E, Foley JM, et al. White matter hyperintensities correlate to cognition and fiber tract integrity in older adults with HIV. J Neurovirol. 2017;23(3):422–429. doi: 10.1007/s13365-016-0509-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Su T, Wit FWNM, Caan MWA, et al. White matter hyperintensities in relation to cognition in HIV-infected men with sustained suppressed viral load on combination antiretroviral therapy. AIDS. 2016;30(15):2329–2339. doi: 10.1097/QAD.0000000000001133 [DOI] [PubMed] [Google Scholar]
- 65.Triant VA, Perez J, Regan S, et al. Cardiovascular Risk Prediction Functions Underestimate Risk in HIV Infection. Circulation. 2018;137(21):2203–2214. doi: 10.1161/CIRCULATIONAHA.117.028975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Comparing Cardiovascular Disease Risk Scores for Use in HIV-Infected Individuals | CROI Conference. http://www.croiconference.org/sessions/comparing-cardiovascular-disease-risk-scores-use-hiv-infected-individuals. Accessed October 7, 2019.
- 67.Friis-Møller N, Thiébaut R, Reiss P, et al. Predicting the risk of cardiovascular disease in HIV-infected patients: the data collection on adverse effects of anti-HIV drugs study. Eur J Cardiovasc Prev Rehabil. 2010;17(5):491–501. doi: 10.1097/HJR.0b013e328336a150 [DOI] [PubMed] [Google Scholar]
- 68.Friis-Møller N, Ryom L, Smith C, et al. An updated prediction model of the global risk of cardiovascular disease in HIV-positive persons: The Data-collection on Adverse Effects of Anti-HIV Drugs (D:A:D) study. Eur J Prev Cardiol. 2016;23(2):214–223. doi: 10.1177/2047487315579291 [DOI] [PubMed] [Google Scholar]
- 69.Mateen FJ, Post WS, Sacktor N, et al. Long-term predictive value of the Framingham Risk Score for Stroke in HIV-positive vs HIV-negative men. Neurology. 2013;81(24):2094–2102. doi: 10.1212/01.wnl.0000437296.97946.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Feinstein MJ, Nance RM, Drozd DR, et al. Assessing and Refining Myocardial Infarction Risk Estimation Among Patients With Human Immunodeficiency Virus: A Study by the Centers for AIDS Research Network of Integrated Clinical Systems. JAMA Cardiol. 2017;2(2):155–162. doi: 10.1001/jamacardio.2016.4494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Giorgi JV, Hultin LE, McKeating JA, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis. 1999;179(4):859–870. doi: 10.1086/314660 [DOI] [PubMed] [Google Scholar]
- 72.Strategies for Management of Antiretroviral Therapy (SMART) Study Group, El-Sadr WM, Lundgren JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355(22):2283–2296. doi: 10.1056/NEJMoa062360 [DOI] [PubMed] [Google Scholar]
- 73.d’Ettorre G, Ceccarelli G, Giustini N, Mastroianni CM, Silvestri G, Vullo V. Taming HIV-Related Inflammation with Physical Activity: A Matter of Timing. AIDS Res Hum Retroviruses. 2014;30(10):936–944. doi: 10.1089/aid.2014.0069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jaggers JR, Prasad VK, Dudgeon WD, et al. Associations between physical activity and sedentary time on components of metabolic syndrome among adults with HIV. AIDS Care. 2014;26(11):1387–1392. doi: 10.1080/09540121.2014.920075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Blashill AJ, Mayer KH, Crane H, et al. Physical activity and health outcomes among HIV-infected men who have sex with men: a longitudinal mediational analysis. Ann Behav Med. 2013;46(2):149–156. doi: 10.1007/s12160-013-9489-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Grundy Scott M, Stone Neil J, Bailey Alison L, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1082–e1143. doi: 10.1161/CIR.0000000000000625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Castilla-Guerra L, Del Carmen Fernandez-Moreno M, Colmenero-Camacho MA. Statins in Stroke Prevention: Present and Future. Curr Pharm Des. 2016;22(30):4638–4644. doi: 10.2174/1381612822666160510125229 [DOI] [PubMed] [Google Scholar]
- 78.Ribeiro RA, Ziegelmann PK, Duncan BB, et al. Impact of statin dose on major cardiovascular events: a mixed treatment comparison meta-analysis involving more than 175,000 patients. Int J Cardiol. 2013;166(2):431–439. doi: 10.1016/j.ijcard.2011.10.128 [DOI] [PubMed] [Google Scholar]
- 79.Meschia James F, Bushnell Cheryl, Boden-Albala Bernadette, et al. Guidelines for the Primary Prevention of Stroke. Stroke. 2014;45(12):3754–3832. doi: 10.1161/STR.0000000000000046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the Prevention of Stroke in Patients With Stroke and Transient Ischemic Attack: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2014;45(7):2160–2236. doi: 10.1161/STR.0000000000000024 [DOI] [PubMed] [Google Scholar]
- 81.High-Dose Atorvastatin after Stroke or Transient Ischemic Attack. New England Journal of Medicine. 2006;355(6):549–559. doi: 10.1056/NEJMoa061894 [DOI] [PubMed] [Google Scholar]
- 82.2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. 2019:51. [Google Scholar]
- 83.Feinstein MJ, Achenbach CJ, Stone NJ, Lloyd-Jones DM. A Systematic Review of the Usefulness of Statin Therapy in HIV-Infected Patients. Am J Cardiol. 2015;115(12):1760–1766. doi: 10.1016/j.amjcard.2015.03.025 [DOI] [PubMed] [Google Scholar]
- 84.Park TE, Yusuff J, Sharma R. Use of aspirin and statins for the primary prevention of myocardial infarction and stroke in patients with human immunodeficiency virus infection. Int J STD AIDS. 2016;27(6):447–452. doi: 10.1177/0956462415585448 [DOI] [PubMed] [Google Scholar]
- 85.Funderburg NT, Jiang Y, Debanne SM, et al. Rosuvastatin reduces vascular inflammation and T-cell and monocyte activation in HIV-infected subjects on antiretroviral therapy. J Acquir Immune Defic Syndr. 2015;68(4):396–404. doi: 10.1097/QAI.0000000000000478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Funderburg NT, Jiang Y, Debanne SM, et al. Rosuvastatin treatment reduces markers of monocyte activation in HIV-infected subjects on antiretroviral therapy. Clin Infect Dis. 2014;58(4):588–595. doi: 10.1093/cid/cit748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lo J, Lu MT, Ihenachor EJ, et al. Effects of statin therapy on coronary artery plaque volume and high-risk plaque morphology in HIV-infected patients with subclinical atherosclerosis: a randomised, double-blind, placebo-controlled trial. Lancet HIV. 2015;2(2):e52–63. doi: 10.1016/S2352-3018(14)00032-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Eckard AR, Jiang Y, Debanne SM, Funderburg NT, McComsey GA. Effect of 24 weeks of statin therapy on systemic and vascular inflammation in HIV-infected subjects receiving antiretroviral therapy. J Infect Dis. 2014;209(8):1156–1164. doi: 10.1093/infdis/jiu012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Longenecker CT, Sattar A, Gilkeson R, McComsey GA. Rosuvastatin slows progression of subclinical atherosclerosis in patients with treated HIV infection. AIDS. 2016;30(14):2195–2203. doi: 10.1097/QAD.0000000000001167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hsyu P-H, Schultz-Smith MD, Lillibridge JH, Lewis RH, Kerr BM. Pharmacokinetic Interactions between Nelfinavir and 3-Hydroxy-3-Methylglutaryl Coenzyme A Reductase Inhibitors Atorvastatin and Simvastatin. Antimicrob Agents Chemother. 2001;45(12):3445–3450. doi: 10.1128/AAC.45.12.3445-3450.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wiggins BS, Lamprecht DG, Page RL, Saseen JJ. Recommendations for Managing Drug-Drug Interactions with Statins and HIV Medications. Am J Cardiovasc Drugs. 2017;17(5):375–389. doi: 10.1007/s40256-017-0222-7 [DOI] [PubMed] [Google Scholar]
- 92.Grinspoon SK, Fitch KV, Overton ET, et al. Rationale and design of the Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE). Am Heart J. 2019;212:23–35. doi: 10.1016/j.ahj.2018.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hoffmann U, Lu MT, Olalere D, et al. Rationale and design of the Mechanistic Substudy of the Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE): Effects of pitavastatin on coronary artery disease and inflammatory biomarkers. Am Heart J. 2019;212:1–12. doi: 10.1016/j.ahj.2019.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.ASCEND Study Collaborative Group, Bowman L, Mafham M, et al. Effects of Aspirin for Primary Prevention in Persons with Diabetes Mellitus. N Engl J Med. 2018;379(16):1529–1539. doi: 10.1056/NEJMoa1804988 [DOI] [PubMed] [Google Scholar]
- 95.McNeil JJ, Nelson MR, Woods RL, et al. Effect of Aspirin on All-Cause Mortality in the Healthy Elderly. New England Journal of Medicine. 2018;379(16):1519–1528. doi: 10.1056/NEJMoa1803955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bartolucci AA, Tendera M, Howard G. Meta-analysis of multiple primary prevention trials of cardiovascular events using aspirin. Am J Cardiol. 2011;107(12):1796–1801. doi: 10.1016/j.amjcard.2011.02.325 [DOI] [PubMed] [Google Scholar]
- 97.Raju N, Sobieraj-Teague M, Hirsh J, O’Donnell M, Eikelboom J. Effect of aspirin on mortality in the primary prevention of cardiovascular disease. Am J Med. 2011;124(7):621–629. doi: 10.1016/j.amjmed.2011.01.018 [DOI] [PubMed] [Google Scholar]
- 98.Ridker PM, Cook NR, Lee I-M, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352(13):1293–1304. doi: 10.1056/NEJMoa050613 [DOI] [PubMed] [Google Scholar]
- 99.Johnson ES, Lanes SF, Wentworth CE, Satterfield MH, Abebe BL, Dicker LW. A metaregression analysis of the dose-response effect of aspirin on stroke. Arch Intern Med. 1999;159(11):1248–1253. doi: 10.1001/archinte.159.11.1248 [DOI] [PubMed] [Google Scholar]
- 100.Wang Y, Wang Y, Zhao X, et al. Clopidogrel with Aspirin in Acute Minor Stroke or Transient Ischemic Attack. New England Journal of Medicine. 2013;369(1):11–19. doi: 10.1056/NEJMoa1215340 [DOI] [PubMed] [Google Scholar]
- 101.Johnston SC, Easton JD, Farrant M, et al. Clopidogrel and Aspirin in Acute Ischemic Stroke and High-Risk TIA. New England Journal of Medicine. 2018;379(3):215–225. doi: 10.1056/NEJMoa1800410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus Aggressive Medical Therapy for Intracranial Arterial Stenosis. New England Journal of Medicine. 2011;365(11):993–1003. doi: 10.1056/NEJMoa1105335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Burkholder GA, Tamhane AR, Salinas JL, et al. Underutilization of Aspirin for Primary Prevention of Cardiovascular Disease Among HIV-Infected Patients. Clin Infect Dis. 2012;55(11):1550–1557. doi: 10.1093/cid/cis752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tornero C, Ventura A, Mafe M. Aspirin is indicated for primary prevention of cardiovascular events in HIV-infected patients. J Acquir Immune Defic Syndr. 2010;54(5):560. doi: 10.1097/QAI.0b013e3181d913fd [DOI] [PubMed] [Google Scholar]
- 105.Suchindran S, Regan S, Meigs JB, Grinspoon SK, Triant VA. Aspirin Use for Primary and Secondary Prevention in Human Immunodeficiency Virus (HIV)-Infected and HIV-Uninfected Patients. Open Forum Infectious Diseases. 2014;1(3):ofu076–ofu076. doi: 10.1093/ofid/ofu076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.De Socio GV, Ricci E, Parruti G, et al. Statins and Aspirin use in HIV-infected people: gap between European AIDS Clinical Society guidelines and clinical practice: the results from HIV-HY study. Infection. 2016;44(5):589–597. doi: 10.1007/s15010-016-0893-z [DOI] [PubMed] [Google Scholar]
- 107.Falcinelli E, Francisci D, Schiaroli E, et al. Effect of aspirin treatment on abacavir-associated platelet hyperreactivity in HIV-infected patients. Int J Cardiol. 2018;263:118–124. doi: 10.1016/j.ijcard.2018.04.052 [DOI] [PubMed] [Google Scholar]
- 108.Sabin CA, Ryom L, d’Arminio Monforte A, et al. Abacavir use and risk of recurrent myocardial infarction. AIDS. 2018;32(1):79–88. doi: 10.1097/QAD.0000000000001666 [DOI] [PubMed] [Google Scholar]
- 109.O’Brien M, Montenont E, Hu L, et al. Aspirin attenuates platelet activation and immune activation in HIV-1-infected subjects on antiretroviral therapy: a pilot study. J Acquir Immune Defic Syndr. 2013;63(3):280–288. doi: 10.1097/QAI.0b013e31828a292c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.O’Brien MP, Hunt PW, Kitch DW, et al. A Randomized Placebo Controlled Trial of Aspirin Effects on Immune Activation in Chronically Human Immunodeficiency Virus-Infected Adults on Virologically Suppressive Antiretroviral Therapy. Open Forum Infect Dis. 2017;4(1):ofw278. doi: 10.1093/ofid/ofw278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lin TC, Burton BN, Barleben A, Hoenigl M, Gabriel RA. Association of HIV infection with age and symptomatic carotid atherosclerotic disease at the time of carotid intervention in the United States. Vasc Med. 2018;23(5):467–475. doi: 10.1177/1358863X18789783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sanders JM, Steverson AB, Pawlowski AE, et al. Atrial arrhythmia prevalence and characteristics for human immunodeficiency virus-infected persons and matched uninfected controls. PLoS ONE. 2018;13(3):e0194754. doi: 10.1371/journal.pone.0194754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chau KH-Y, Scherzer R, Grunfeld C, Hsue PY, Shlipak MG. CHA2DS2-VASc Score, Warfarin Use, and Risk for Thromboembolic Events Among HIV-Infected Persons With Atrial Fibrillation. J Acquir Immune Defic Syndr. 2017;76(1):90–97. doi: 10.1097/QAI.0000000000001470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.West TA, Perram J, Holloway CJ. Use of direct oral anticoagulants for treatment of atrial fibrillation in patients with HIV: a review. Curr Opin HIV AIDS. 2017;12(6):554–560. doi: 10.1097/COH.0000000000000412 [DOI] [PubMed] [Google Scholar]
- 115.Benjamin L, Khoo S. HIV infection and stroke In: Handbook of Clinical Neurology. Vol 152 Elsevier; 2018:187–200. doi: 10.1016/B978-0-444-63849-6.00015-3 [DOI] [PubMed] [Google Scholar]
- 116.Perram J, Joseph J, Holloway C. Novel oral anticoagulants and HIV: dabigatran use with antiretrovirals. BMJ Case Rep. 2015;2015. doi: 10.1136/bcr-2015-211651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Barco S, Coppens M, van den Dool E-J, van de Kerkhof D, Stroobants AK, Middeldorp S. Successful co-administration of dabigatran etexilate and protease inhibitors ritonavir/lopinavir in a patient with atrial fibrillation. Thromb Haemost. 2014;112(4):836–838. doi: 10.1160/TH14-03-0214 [DOI] [PubMed] [Google Scholar]
- 118.Egan G, Hughes CA, Ackman ML. Drug interactions between antiplatelet or novel oral anticoagulant medications and antiretroviral medications. Ann Pharmacother. 2014;48(6):734–740. doi: 10.1177/1060028014523115 [DOI] [PubMed] [Google Scholar]
- 119.Gilbert JM, Fitch KV, Grinspoon SK. HIV-Related Cardiovascular Disease, Statins, and the REPRIEVE Trial. Top Antivir Med. 2015;23(4):146–149. [PMC free article] [PubMed] [Google Scholar]
- 120.Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. New England Journal of Medicine. 2015;372(25):2387–2397. doi: 10.1056/NEJMoa1410489 [DOI] [PubMed] [Google Scholar]
- 121.Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. New England Journal of Medicine. 2017;376(18):1713–1722. doi: 10.1056/NEJMoa1615664 [DOI] [PubMed] [Google Scholar]
- 122.Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366(9493):1267–1278. doi: 10.1016/S0140-6736(05)67394-1 [DOI] [PubMed] [Google Scholar]
- 123.Kohli P, Ganz P, Ma Y, et al. HIV and Hepatitis C-Coinfected Patients Have Lower Low-Density Lipoprotein Cholesterol Despite Higher Proprotein Convertase Subtilisin Kexin 9 (PCSK9): An Apparent “PCSK9-Lipid Paradox.” J Am Heart Assoc. 2016;5(5). doi: 10.1161/JAHA.115.002683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Boccara F, Ghislain M, Meyer L, et al. Impact of protease inhibitors on circulating PCSK9 levels in HIV-infected antiretroviral-naive patients from an ongoing prospective cohort. AIDS. 2017;31(17):2367–2376. doi: 10.1097/QAD.0000000000001633 [DOI] [PubMed] [Google Scholar]
- 125.Effect of PCSK9 Inhibition on Cardiovascular Risk in Treated HIV Infection (EPIC-HIV Study) - Full Text View - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03207945. Accessed October 25, 2019.
- 126.Neuhaus J, Jacobs DR, Baker JV, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010;201(12):1788–1795. doi: 10.1086/652749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Baker JV, Sharma S, Grund B, et al. Systemic Inflammation, Coagulation, and Clinical Risk in the START Trial. Open Forum Infect Dis. 2017;4(4):ofx262. doi: 10.1093/ofid/ofx262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Duprez DA, Neuhaus J, Kuller LH, et al. Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLoS ONE. 2012;7(9):e44454. doi: 10.1371/journal.pone.0044454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.IL-1β INHIBITION SIGNIFICANTLY REDUCES ATHEROSCLEROTIC INFLAMMATION IN TREATED HIV | CROI Conference. http://www.croiconference.org/sessions/il-1%CE%B2-inhibition-significantly-reduces-atherosclerotic-inflammation-treated-hiv. Accessed November 25, 2019.
- 130.Hsue PY, Ribaudo HJ, Deeks SG, et al. Safety and Impact of Low-dose Methotrexate on Endothelial Function and Inflammation in Individuals With Treated Human Immunodeficiency Virus: AIDS Clinical Trials Group Study A5314. Clin Infect Dis. 2019;68(11):1877–1886. doi: 10.1093/cid/ciy781 [DOI] [PMC free article] [PubMed] [Google Scholar]


