Abstract
The recently solved crystal structures of the human cysteine desulfurase NFS1, in complex with the LYR protein ISD11, the acyl carrier protein ACP and the main scaffold ISCU, have shed light on the molecular interactions that govern initial cluster assembly on ISCU. Here, we aim to highlight recent insights into iron-sulfur (Fe-S) cluster biogenesis in mammalian cells that have arisen from the crystal structures of the core iron sulfur cluster (ISC) assembly complex. We will also discuss how iron sulfur clusters are delivered to recipient proteins, and the challenges that remain in dissecting the pathways that deliver clusters to numerous Fe-S recipient proteins in both the mitochondrial matrix and cytosolic compartments of mammalian cells.
1.1. Fe-S clusters are versatile cofactors involved in multiple, essential cellular processes
Iron sulfur (Fe-S) clusters are co-factors composed of iron and inorganic sulfur that are generally ligated to three or more sulfhydryl groups of cysteine residues in proteins [1]. Recent work has revealed that these cofactors are much more commonly associated with mammalian proteins than was recognized as recently as a decade ago [2, 3]. A combination of different approaches has revealed that Fe-S proteins are essential for ribosomal function and translation, as well as many aspects of DNA metabolism (reviewed in [2–4]). Bioinformatics tools applied to the radical S-adenosylmethionine (SAM) superfamily have dramatically expanded the repertoire of Fe-S proteins involved in an astonishing array of reactions that impact numerous cellular processes [5, 6]. Iron sulfur clusters are versatile cofactors that are able to serve in electron relay chains, as in the mitochondrial respiratory chain, store reducing power, donate inorganic sulfur for the biogenesis of essential cofactors (e.g. lipoate), and serve as the substrate ligand for enzymes like aconitase [3]. Not surprisingly, the mechanisms by which Fe-S clusters are synthesized in mammalian cells are highly complex. The basic building block, a rhombic cluster containing two iron and two sulfur atoms, is built upon a scaffold, and then transferred to specific recipient proteins that transiently interact with the ISC biogenesis machinery, as outlined in the following sections of this review.
1.2. Initial formation of nascent clusters on ISCU
ISC biogenesis is a processive, protein-mediated process that involves a critical initial step catalyzed by a pyridoxal-5’-phosphate (PLP)-dependent transaminase, the cysteine desulfurase NFS1 (Figure 1). NFS1 converts cysteine to alanine, while generating a persulfide species on a mobile cysteine-containing loop (Cys381 in human NFS1) that delivers sulfane sulfur to the scaffold protein ISCU. The accessory protein ISD11 (also known as LYRM4; Figure 1), a member of the LYRM family, characterized by the presence of the highly conserved Leu-Tyr-Arg motif [7], is uniquely present in eukaryotes, and was shown to stabilize NFS1 and to interact with the acyl-carrier protein ACP (NDUFAB1 in human; Figure 1) [8]. Interestingly, the human NFS1/ISD11 proteins recombinantly expressed in E. coli yielded a complex that contained stoichiometrically relevant amounts of the endogenous bacterial Acp (Acpec) in a 1:1:1 NFS1/ISD11/Acpec ratio [9]. Moreover, three recently solved structures of the initial ISC core complex, obtained by co-expressing in E. coli mammalian NFS1, ISD11 and/or ISCU and FXN, revealed that bacterial Acp co-purified with the ISC biogenesis complex [10–12]. The mitochondrial ACP is a bacterial-type acyl-carrier protein involved in mitochondrial fatty acid synthesis (mFAS), a pathway that provides lipoic acid (see later in this review), through binding of nascent acyl chains on the 4’-phosphopantetheine (4’-PP) cofactor bound to serine 112 of ACP [13]. Combined structural and biochemical analyses showed that a critical aspect of the ISD11-ACP interaction involved the insertion of the entire acyl chains (of at least 12 carbons) and the 4’-PP group of ACP into the hydrophobic barrel created by the three-helical structure of ISD11, a mechanism known as chain-flipping (Figures 2A–C) [10–12, 14, 15]. The role of ACP in ISC biogenesis remains unclear. It has been speculated that ACP may link fatty acid metabolism, through its role in mitochondrial fatty acid biosynthesis, to Fe-S cluster biogenesis [8]. Interestingly, an observation that strengthens the relationship between the integrity of the Fe-S biogenesis pathway and lipid homeostasis was the discovery that acute disruption of Fe-S biogenesis by the inducible expression of dominant negative mutants of the main scaffold ISCU markedly increased fatty acid biosynthesis relative to cellular proliferation rates, leading to a more than 12-fold increase in citrate levels and to cytosolic lipid droplet accumulation [16].
Figure 1. A model of the highly conserved Fe-S biogenesis pathway in mitochondria (based on the recently solved structures of the ISC core complex[11, 12]).
Nascent Fe-S clusters are initially assembled on the main scaffold protein ISCU. A cysteine desulfurase, NFS1, forms a dimer to which monomers of the primary scaffold ISCU bind at either end. ISD11 (also known as LYRM4) and ACP with its bound acyl chain are structural components of the core complex in eukaryotes. Frataxin (FXN) transiently binds in a pocket-like region between NFS1 and ISCU and promotes sulfur transfer from NFS1 to ISCU. The cluster assembles upon ISCU when iron is provided together with the reducing equivalents needed to generate the final electronic configuration of the cluster. (A) Direct cluster transfer from ISCU to recipient apoproteins is assisted by a dedicated chaperone/co-chaperone (HSPA9/HSC20) system. Accessory factors, like the LYR proteins SDHAF1 and LYRM7, which bind to HSC20, can assist the transfer step of Fe-S clusters to specific recipients, such as SDHB (Fe-S subunit of complex II) or UQCRFS1 (Rieske protein of complex III). (B) Cluster transfer mediated by secondary carriers: the HSPA9/HSC20 chaperone/co-chaperone system can facilitate cluster transfer from the main scaffold ISCU to secondary carriers (e. g. NFU1, GlRX5, ISCA1, ISCA2, BOLA3) that target a subset of Fe-S recipient proteins (e.g. lipoic acid synthase, LIAS).
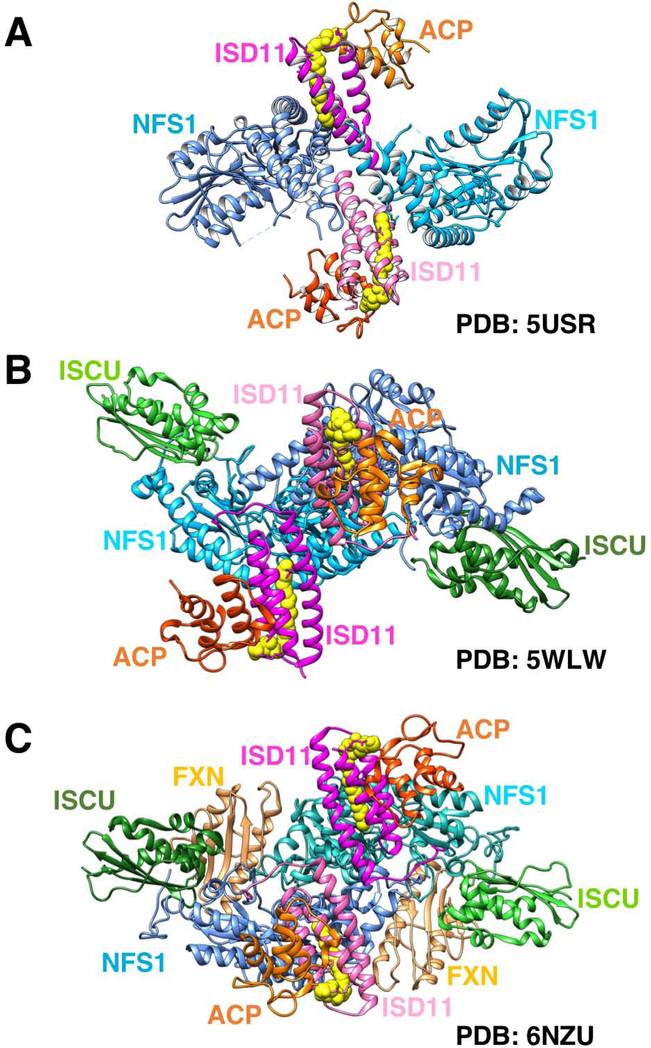
Figure 2. Recently solved crystal structures of the human ISC core complex.
(A) Structure of the (NFS1/ISD11/ACP)2 homodimeric complex by Cory et al.,[10] (PDB ID: 5USR). NFS1 protomers are in shades of blue, ISD11 in magenta, ACP in orange. The acyl chain covalently attached to the 4’-PP group of ACP is shown in yellow and fits within the three-helical structure of ISD11. (B) Structure of the homodimeric (NFS1/ISD11/ACP/ISCU-Zn2+)2 complex by Boniecki et al.,[11] (PDB ID: 5WLW). The two ISCU protomers are shown in shades in green. (C) Structure of the homodimeric (NFS1/ISD11/ACP/ISCU-Zn2+-FXN)2 complex by Fox et al.,[12] (PDB ID: 6NZU). FXN binds in a pocket like region between ISCU and NFS1 protomers.
1.2.1. Does ACP have a special affinity for proteins of the LYRM family?
In addition to its binding to ISD11, mitochondrial ACP was found to interact with the LYR proteins Sdh6, Sdh7 and Mzm1 in yeast [7, 17]. Mammalian ACP was crystallized as part of mitochondrial complex I, through binding to the LYR subunits NDUFA6 (also known as LYRM6) and NDUFB9 (also known as LYRM3) [18], and as a component of an assembly intermediate of the mitochondrial ribosomes bound to a previously unannotated LYR motif-containing protein L0R8F8, also known as MIEF1 [19]. So, does ACP have a special affinity for proteins of the LYRM family? It should be noted that the ACP-LYR protein interactions are mainly stabilized through the insertion of the acyl chain of ACP into the hydrophobic core of the three-helical structures of ISD11, NDUFA6 and NDUFB9 [10, 11, 18], whereas the distinctive Leu-Tyr-Arg motif of the LYR proteins is not directly involved in interactions with ACP [11, 12, 14]. Moreover, mutational analyses showed that substitution of Leu12 or Arg14 of the LYR of ISD11 into alanines didn’t affect binding to ACP [14], whereas mutagenesis of Tyr13 of the motif into alanine profoundly affected the stability of ISD11, precluding proper assessment of the effect of the mutation on the stability of the ACP-ISD11 interaction [14]. Supporting the notion that the ACP-LYR protein interactions were mainly mediated by the acyl chain of ACP was the fact that when the deacylated ACPS112A, which lacked the 4’-PP group bound to the invariant serine residue (S112 in human), was used as a bait in co-immunoprecipitation experiments performed in mammalian cells, the majority of the interactions detected with wild type ACP were lost, including those with ISD11, the complex I subunits and other LYR proteins [14]. Moreover, in prokaryotes that lack the LYR family of proteins [7], Acp is known to interact with a vast array of proteins [20, 21], including the cysteine desulfurase IscS [22, 23], which is the ortholog of human NFS1. The Acp-IscS interaction is particularly intriguing because it takes place without involving the small LYR protein ISD11, which is absent in prokaryotes [7]. The bacterial Acp-IscS complex has not been crystallized and the interface of interaction between the two proteins therefore remains uncharacterized. As a further argument against the selectivity of ACP for LYR proteins, the landscape of human ACP interactions expands well beyond the 11 annotated members of the LYRM family and includes hundreds of non-LYR proteins [14].
1.2.2. Insights into the frataxin-mediated activation of the core ISC complex revealed by a novel cryo-electron microscopy structure
The NFS1/ISD11/ACP/ISCU core complex is a symmetric hetero-octamer, comprising two copies of each of the four constituent proteins [11, 12] (Figures 1, 2B and 2C). It forms a (NFS1/ISD11/Acpec)2 homodimeric core, with ISCU bound to the end of each monomeric complex. Frataxin (FXN), which has been proposed to act as an allosteric regulator of ISC biogenesis by driving efficient sulfur transfer from the cysteine loop of NFS1 (Cys381) to ISCU (Cys138) [24], has recently been co-crystallized with the NFS1/ISD11/Acpec/ISCU complex [12]. FXN occupied a cavity at the interface between NFS1 and ISCU [12] (schematically depicted in Figure 1 and shown as part of the solved cryo-EM structure in Figure 2C), and did not make direct physical contacts with ISD11, whereas it simultaneously interacted with both NFS1 protomers of the complex, consistent with the structures solved by Boniecki et al.[11], and Fox et al.[12] (Figures 2B and 2C), but not compatible with the NFS1/ISD11/ACP structure from Cory et al.[10] (Figure 2A), in which the two NFS1 protomers minimally contacted each other. The crystal structure of the core complex containing FXN also revealed two key contact regions with ISCU. One ISCU-FXN interface was through the conserved Ala-loop of ISCU (residues Ala66-Asp71 of the human protein), containing the Fe-S ligating cysteine (Cys69). The second and more extensive ISCU-FXN interface involved the conserved L131PPVKLHC138SM140 sequence motif of ISCU, which contains the LPPVK motif recognized by the chaperone HSPA9, the Cys138, which is the proposed sulfur acceptor from NFS1 [24], and Met140, a residue reported to confer FXN-dependence of ISC biogenesis in S. cerevisiae [25], or FXN-independence in prokaryotes [26]. The NFS1/ISD11/ACP/ISCU complex recombinantly expressed in E. coli was also purified with variable amounts of Zn2+ bound to ISCU [11, 12, 27]. In the structure reported by Boniecki and colleagues [11], Cys381 of the cysteine loop of NFS1 was involved in ligating zinc, along with Asp71, Cys95 and His137 of ISCU (Figure 3A), a conformation that explains why zinc inhibits NFS1 activity in vitro, as zinc-binding constrains Cys381 to a position distant from the catalytic cleft [12] (Figure 3A). In the cryo-electron microscopy structure of the NFS1/ISD11/ACP/ISCU-Zn2+ complex, addition of FXN displaced the zinc ligand, freeing His137 of ISCU to interact with FXN, and Cys381 of NFS1 to engage in sulfur transfer [12] (Figure 3B). The structure of the ISC core complex including FXN revealed how FXN unlocked the zinc inhibition of the NFS1/ISD11/ACP/ISCU-Zn2+ complex in vitro to activate NFS1 by mobilizing the cysteine loop of NFS1to generate and transfer sulfide to ISCU (Figure 3B). Although zinc often mimicked the Fe-S cluster in crystal structures solved in aerobic conditions, it remains to be determined whether Zn2+ plays any role in vivo.
Figure 3. Proposed mechanism of the activation of NFS1 by frataxin.
(A) Conformation of the cysteine loop of NFS1, containing the catalytic Cys381, in the absence of FXN (modeled upon the structure solved by Boniecki et al.,[11] PDB ID: 5WLW). In the absence of FXN, Cys381 of NFS1, upon which the mobilized sulfur is initially bound as a persulfide group, is isolated far from the catalytic cleft by ligating zinc, along with Cys95 and Asp71 of ISCU. (B) Binding of FXN to the ISC core complex disengages His137 of ISCU, which becomes available to interact with Trp155 of FXN, and Cys381 of NFS1, enabling sulfur transfer onto Cys138 of ISCU [24]. The conformation of the cysteine loop of NFS1 in the presence of FXN was modeled upon the structure from Fox et al.,[12] (PDB ID: 6NZU). NFS1 protomers are in shades of blue, ISD11 in magenta, ACP in orange and its acyl chain in yellow, ISCU in green and frataxin in tan. PLP (in yellow) is pyridoxal 5’-phosphate, a cofactor of NFS1.
The recently solved crystal structures of the ISC core complex have elucidated critical aspects of the initial steps of Fe-S cluster biogenesis. However, several questions still remain unanswered, such as the source of iron for the cluster. A glutathione-iron complex has been identified as a key component of the cytoplasmic labile iron pool [28] that supplies iron to cytoplasmic iron(II)-dependent enzymes, to mitochondria and likely to the cytoplasmic Fe-S biogenesis pathway. It remains to be seen whether the iron-glutathione complex can provide iron to the mitochondrial Fe-S biogenesis machinery.
1.3. Transfer of nascent Fe-S clusters to recipient proteins requires the interaction of holo-ISCU with a chaperone/co-chaperone complex
Advances in our understanding of mammalian Fe-S protein biogenesis have resulted from the biochemical characterization of the multimolecular complexes devoted to initial Fe-S cluster assembly in bacteria and yeast and their counterparts in mammalian cells. Likewise, transfer of a newly assembled cluster downstream of the main scaffold protein ISCU in mammalian cells relies on the activity of a chaperone/co-chaperone system analogous to the bacterial HscA/HscB and yeast Ssq1/Jac1 complexes [29, 30]. The multifunctional member of the HSP70 chaperone family, HSPA9 in mammalian cells (also known as mortalin/PBP74/GRP75), works with the specialized DnaJ type III protein, HSC20, also referred to as DNAJC20 or HSCB, to facilitate Fe-S cluster transfer downstream of ISCU (Figure 1) [31, 32]. The N-terminal J-domain of HSC20 contains the invariant histidine, proline, aspartate (HPD) motif that is responsible for stimulating the ATPase activity of HSPA9, whereas the C-terminus forms a three-helical bundle that is directly involved in binding the scaffold protein ISCU with four highly conserved non-contiguous hydrophobic residues (Leu162, Met166, Tyr220, Phe221) [32].
1.3.1. The co-chaperone HSC20 facilitates Fe-S cluster transfer by selectively recognizing and binding LYR motifs present in Fe-S recipient proteins or in accessory factors
Recent studies have revealed that the human co-chaperone HSC20 is the major component of the Fe-S transfer machinery that guides the multifunctional HSP70 chaperone HSPA9 to deliver Fe-S clusters from ISCU to recipient proteins [32]. Since there is only one highly conserved HSC20 homologue in the human genome [31], HSC20 binding was used to identify potential target Fe-S proteins. HSC20 served as a bait in a stringent yeast two-hybrid (Y2H) screen, a method used to reveal direct molecular interactions between pairs of proteins [32]. Multiple HSC20 binding partners were identified among the library of proteins. Notably, multiple individual HSC20 interacting clones encoded succinate dehydrogenase subunit B (SDHB) [32], which is the Fe-S cluster containing subunit of complex II. The interaction between HSC20 and SDHB was further validated in vivo in mammalian cells [32]. SDHB contains three deeply buried clusters of different nuclearities; [Fe2-S2], [Fe4-S4] and [Fe3-S4] [33], which are likely inserted during initial folding.
The Y2H screen was also conducted in order to identify potential amino acid motifs present in recipient Fe-S proteins, which may act as a binding site for the HSC20/HSPA9 complex, functioning as molecular signatures that guide specific recruitment of the Fe-S transfer complex through direct binding to HSC20. SDHB, which contains three Fe-S clusters, appeared to be an ideal candidate for identifying the potential motifs that mediate direct binding to the cochaperone HSC20. Upon subdivision of the primary sequence of SDHB into multiple prey constructs, three independent binding sites for HSC20 were identified on SDHB, and two of these were iterations of a tripeptide motif, leucine, tyrosine and arginine (the LYR motif)[32]. Notably, the two LYR consensus sequences in SDHB are evolutionarily highly conserved and located in unstructured loops of the crystal structure proximal to the clusters of cysteine residues that ligate the Fe-S cofactors [3]. Importantly, substitution of Arg46 into Gln of the first IYR motif in SDHB was identified as a cause of one type of renal cancer [34], and substitutions of the IYR, LYR and cysteine residues important for ligating Fe-S clusters were identified as frequent causal mutations in rare tumors such as paragangliomas and gastrointestinal stromal tumors [35].
1.3.2. LYR motifs present in assembly factors of respiratory complexes II and III mediate an interaction with the Fe-S transfer complex through binding to HSC20
SDHAF1 (also known as LYRM8) was previously known to be important for SDH activity and for assembly of the holo-complex in fibroblasts [36]. Homozygous mutations in SDHAF1 cause a distinctive early-onset leukoencephalopathy in which accumulations of lactate and succinate in the white matter are associated with selective loss of SDH activity [36]. In recent studies on cell lines derived from patients with SDHAF1 mutations [37], SDHAF1 was shown to recruit the Fe-S transfer complex to the C-terminus of SDHB through direct and transient binding of its N-terminal LYR motif to the co-chaperone HSC20. The region L53-R65 of SDHAF1, enriched in arginine residues, was found to interact with SDHB at several binding sites rich in aromatic amino acids, a recognized type of binding known as cation-pi interaction [38]. Binding of SDHAF1 to SDHB and recruitment of the HSC20-HSPA9-holo-ISCU complex by its first LYR motif was shown to be required for Fe-S cluster incorporation into SDHB and assembly of functional succinate dehydrogenase complexes [37].
LYRM7, the Rieske Fe-S protein chaperone of complex III [39] and an annotated member of the LYR family, was also identified in the Y2H screening and shown to be a direct high-affinity interacting partner of HSC20 (Figure 1; steps 2 to 4) [32]. Further investigations in vivo demonstrated that binding of HSC20 to the LYR motif of LYRM7 was required for Fe-S cluster incorporation into UQCRFS1, the Rieske protein of complex III [40]. The HSC20 transfer complex was also shown to be critical for Fe-S acquisition by complex I [40], which contains eight Fe-S clusters (Figure 1).
These findings supported the notion that the general underlying principles of Fe-S cluster acquisition by recipient proteins were shared, and that each unique recipient protein possessed the necessary information that allowed the Fe-S transfer apparatus to bind and transfer its Fe-S cargo. The HSC20 transfer complex may also convey the cluster to secondary carriers that target a subset of specific recipient proteins to which they bind through as yet unidentified mechanisms (Figure 1. See section 1.4).
Interestingly, there are two other examples in which the function of small peptide motifs is critical in the ISC pathway. The first is the well-known role of the tripeptide His, Pro, Asp (HPD) of HSC20, which activates the ATPase activity of its partner chaperone [41]. A second example is provided by the tripeptide Pro, Val, Lys (PVK) of ISCU, which binds the chaperone HSPA9 as part of the activation mechanism of the Fe-S cluster transfer cycle.
1.4. Secondary carriers involved in Fe-S cluster delivery
The HSC20/HSPA9 transfer complex in mammalian cells is responsible for direct transfer of Fe-S clusters from ISCU to a subset of recipient proteins (Figure 1; step B) [32, 37, 40]. A number of ISC biogenesis factors have been proposed to act as secondary carriers that deliver clusters downstream of ISCU to specific Fe-S proteins. These include NFU1, BOLA3, IBA57, GLRX5, ISCA1 and ISCA2. Interestingly, clinical and biochemical investigations of several recently described human diseases caused by mutations in NFU1, BOLA3, IBA57, ISCA1 or ISCA2 suggest that transfer of Fe-S clusters to secondary carriers that function downstream of the holo-ISCU/chaperone/co-chaperone represent specific delivery pathways to recipients that interact with distinct subsets of carriers [42, 43]. The distinctive phenotypes associated with the various disease gene mutations reveal our lack of knowledge about how many individual Fe-S proteins are targeted for Fe-S delivery.
The multiple mitochondrial dysfunctions syndromes (MMDSs) have recently emerged as a group of mitochondrial disorders that are inherited in an autosomal recessive manner. Six MMDSs have been described so far. These include MMDS1, which is caused by mutations in NFU1 (OMIM #605711)[44, 45]; MMDS2, caused by BOLA3 gene defects (OMIM #614299)[44, 46, 47]; MMDS3 due to IBA57 mutations (OMIM #615330)[48]; MMDS4 due to ISCA2 mutations (OMIM #616370)[49]; MMDS5 due to ISCA1 mutations (OMIM #617613)[50]; and MMDS6 due to the PMPCB gene defect (OMIM #617954) [51], which encodes the catalytic subunit of the essential mitochondrial processing protease (MPP), required for maturation of the majority of mitochondrial precursor proteins. MMDSs 1–5 are caused by mutations in components of the ISC biogenesis pathway and manifest with different patterns of defects in the levels of lipoylated mitochondrial pyruvate dehydrogenase, α-ketoglutarate dehydrogenase, the branched-chain ketoacid dehydrogenase and glycine cleavage system and concomitant loss of respiratory chain function (recently reviewed in [42, 43]). The specific targets of the secondary carriers have not been assigned, although it appears that NFU1 and BOLA3 play an essential and non-redundant role in the biogenesis of the two [Fe4-S4] clusters of lipoic acid synthase [44, 45], whereas acquisition of the [Fe4-S4] cluster of mitochondrial aconitase does not depend on NFU1 and BOLA3 in humans [44, 45]. Defects in the respiratory complexes I and II have also been reported in association with mutations in NFU1 and BOLA3 [44, 45]. However, the interpretation of the phenotypes associated with mutations in these late-acting ISC components is particularly complex due to indirect metabolic and respiratory alterations arising from impairment of the lipoic-acid dependent complexes. Moreover, defects in lipoic acid biosynthesis negatively affect tRNA processing by the RNase P leading to attenuation of mitochondrial translation [52, 53], which ultimately affects the assembly of respiratory complexes I and III-V, which all contain subunits encoded by the mitochondrial DNA.
1.4.1. NFU and delivery of [4Fe-4S] clusters to recipient proteins
NFU1, one of the late-acting carrier proteins, homodimerizes to ligate a [4Fe-4S] cluster [54, 55]. ISCU and A-type proteins have been implicated as the cluster donors for NFU1, but more work is required to delineate the cluster acquisition pathway for NFU1. In human cells, NFU1 localizes to both cytosol and the mitochondria [55]. Recent studies have shed light on the mechanism of Fe-S cluster acquisition by the bacterial lipoyl synthase (LipA) by NfuA, the bacterial ortholog of human NFU1 [56, 57], NfuA was found to physically interact with LipA [56, 57]. LipA ligates two [Fe4-S4] clusters and donates two sulfur atoms from its auxiliary cluster to octanoic acid to form lipoic acid. NfuA was shown to catalytically repair and regenerate the auxiliary cluster of LipA, allowing multiple turnovers [56]. The source of the second Fe-S cluster of LipA that binds S-adenosylmethionine remains unknown. Interestingly, other carrier proteins including BOLA3 and A-type proteins also function in lipoylation, perhaps by contributing this second [Fe4-S4] cluster of LipA that interacts with S-adenosylmethionine.
1.5. The existence of a parallel system for Fe-S biogenesis in the cytosolic/nuclear compartment of mammalian cells
In mammalian cells, a full complement of Fe-S biogenesis proteins is synthesized and targeted to the mitochondrial matrix, but almost all of these proteins have also been detected in the cytosolic/ nuclear compartments of cells, where they localize as a result of alternative splicing of the transcript that also encodes the mitochondrial isoform [58], a weak mitochondrial targeting signal [59], and alternative utilization of initiator AUGs that retained or skipped the mitochondrial targeting encoding sequence [60]. These proteins have not been found in the cytosol of the model system S. cerevisiae, although the cysteine desulfurase is clearly present and functional [61, 62]. Despite a high degree of sequence homology of the ISC components from bacteria to yeast to human, and common basic molecular pathways for Fe-S biogenesis, the increased biological complexity in multicellular organisms, which evolved with a corresponding demand for more elaborate mechanisms of transcriptional/translational control, may explain the alleged differential subcellular distribution of some ISC biogenesis factors between yeast and human cells [2, 43].
A recent study in mammalian cells revealed that a de novo cytosolic Fe-S assembly pathway that contained the full complement of initial biosynthetic enzymes as well as many involved in later Fe-S transfer was present and functional [59] (Figure 4). Fe-S clusters have emerged as essential cofactors in enzymes involved in all aspects of DNA processing [63, 64]. By using a combination of proteomic and biochemical approaches, cytosolic HSC20 (C-HSC20) was found to facilitate Fe-S cluster transfer to cytoplasmic and nuclear recipients (Figure 4; subsets 4a, 4b, 5a and 5b). A subset of cytosolic Fe-S proteins, including NUBP1, NUBP2, and GLRX3 appeared to receive their clusters directly from the cytosolic HSC20/HSPA9/ISCU transfer complex (Figure 4, step 4a). Alternatively, a subset of Fe-S proteins involved in DNA metabolism acquired their clusters through the intermediary activities of a large multisubunit ISC/CIA complex (Figure 4, step 4b). While most nuclear Fe-S protein acquired their clusters by the CIAO1/FAM96B/MMS19 complex, the Fe-S cluster helicase XPD appears to be an exception in that it was found to be able to interact with MMS19 independently of FAM96B and CIAO1 [65]. In the CIA-mediated Fe-S transfer mechanism, cytosolic HSC20 bridged components of the initial biogenesis (ISC) pathway, ISCU1, NFS1, HSPA9, to the CIA targeting complex, consisting of CIAO1, FAM96B and MMS19 through its dimerization (Figure 4; step 4b) [59]. Functional assays demonstrated that in the absence of cytosolic HSC20, several Fe-S proteins, including POLD1, DPYD, NUBP2, ERCC2, ELP3, and PPAT failed to acquire their clusters [59]. Notably, these findings directly contrast with a commonly proposed model that cytosolic Fe-S proteins derive their Fe-S co-factors solely from a precursor (named X-S) in the mitochondrial matrix, a model based on studies performed mainly in S. cerevisiae [43].
Figure 4. Proposed model for the biogenesis of Fe-S clusters in the cytosol of mammalian cells.
1. De novo assembly of Fe-S clusters occurs in the cytosol of mammalian cells upon the main scaffold protein ISCU by the concerted action of a multimeric complex, which consists of the cytosolic cysteine desulfurase NFS1 and the accessory protein ISD11. 2. The HSC20/HSPA9 cochaperone/chaperone system interacts with ISCU to facilitate cluster transfer to recipient proteins. 3. The functional unit of HSC20 is a dimer [59]. 4a. A subset of recipient Fe-S proteins acquire their clusters directly from the HSC20/HSPA9/ISCU1 complex [59]. 4b. Binding of HSC20 to the LYR motif of CIAO1 recruits the CIA targeting complex, which is known to form a platform to which Fe-S recipients involved in DNA metabolism dock to acquire their clusters (5a and 5b). The Fe-S proteins shown in the model were all identified as C-HSC20 interacting partners [59] (i.e. NUBP2, GLRX3, CIAPIN1, ABCE1, ERCC2, POLD1, PRIM2, PPAT, ELP3, CPSF30, DDX11, etc.).
Nuclear genome instability has been linked to defects in Fe-S cluster biogenesis since 2009 [66], when nuclear genome instability was proposed to occur because of a defect in mitochondrial Fe-S cluster biogenesis. The dependence of the cytosolic Fe-S biogenesis pathway on mitochondria was based on genetic studies conducted in yeast, which proposed that assembly of Fe-S clusters for nuclear proteins, while primarily taking place in the cytoplasm, depends on the mitochondrial Fe-S biogenesis machinery for the synthesis of a sulfur-containing compound that is exported to the cytosol by the ABC transporter Atm1 (ABCB7 in human) and utilized by the CIA machinery for Fe-S cluster assembly [43]. However, the indirect evidence that suggested that Atm1 exports Fe–S clusters from mitochondria should be analyzed with caution (for a detailed explanation see [59, 67]). The effect of mitochondrial dysfunction on genome instability can reasonably be explained by the fact that several enzymes of the metabolic pathways responsible for the biosynthesis of cytosolic ribo- and deoxyribonucleotides are located in mitochondria and the rate limiting steps of these processes are known to be affected by mitochondrial function [68]. Importantly, cytosolic NFS1 is a functional sulfur mobilizing enzyme [69, 70], an observation that obviates the need for export of a sulfur containing compound from the mitochondrial matrix. Recently NFS1 has also been reported to localize at the tips of the centrosome, further confirming the cytosolic localization of the cysteine desulfurase in mammalian cells [62].
Conclusions and future perspectives
In comparison to the bacterial ISC biogenesis, the mammalian Fe-S biogenesis pathway delivers Fe-S cofactors to hundreds of proteins, many of which are likely not yet known to be Fe-S proteins, in multiple subcellular compartments. Fe-S delivery relies on protein-protein interactions that enshroud the labile nascent Fe-S clusters, enabling them to be delivered intact to the correct specific recipients from among hundreds of proteins that have enough cysteines to potentially ligate an Fe-S cofactor. Zinc-finger proteins contain repeats of four cysteine and/or histidine residues within their primary amino acid sequence, which coordinate zinc. Therefore, zinc-finger proteins possess the ligand set to coordinate Fe-S clusters [71]. Several Fe-S proteins have been misannotated as bona fide zinc proteins and subsequently experimentally characterized as Fe-S proteins, such as the human cleavage and polyadenylation specificity factor 30 (CPSF30)[72], human CISD2 (also known as MINER1 or endoplasmic reticulum intermembrane small protein (ERIS))[73], and DNA polymerases[74]. In vivo the ISC biogenesis machinery is able to distinguish zinc finger proteins from Fe-S proteins presumably using a highly selective path that depends on specific motif recognition and consumption of ATP to drive folding of recipient proteins to accommodate the Fe-S cofactor. We envision that as yet uncharacterized chaperones and cochaperones will be important for Fe-S transfer at steps beyond synthesis of the initial nascent cluster on ISCU. As acquisition of Fe-S clusters by recipient proteins is associated with final three-dimensional conformational changes needed to accommodate the Fe-S cluster, chaperone/cochaperone complexes may serve the dual function of shielding transfer of the cluster with remodeling the conformation of the recipient Fe-S protein. Multiple cochaperones other than HSC20 have been identified in mass spectrometry analyses of interacting partners of components of the CIA machinery, CIAO1, FAM96B and MMS19 [63, 75], and we suggest that these cochaperones will prove to be key to later steps of Fe-S biogenesis. Also, we predict that other motifs analogous to the LYR motif will be involved in guiding Fe-S cofactors to appropriate recipient proteins, preventing wasteful side interactions of this highly complex and specific pathway.
Other remaining questions involve the recently discovered role of ACP1; future structural and functional studies will be needed to define how it contributes to initial Fe-S biogenesis. Insights into the role of frataxin in Fe-S biogenesis have arisen from recent structural and biochemical studies, an important future direction will be to search for drugs that can mimic frataxin activity to treat patients with Friedreich’s ataxia, a disease caused by low frataxin expression [76]. The source of iron for Fe-S biogenesis remains unsettled and future work will likely focus on how iron is delivered to the Fe-S biogenesis machinery. Much progress has been made, but many basic questions remain unanswered in the complex process of mammalian Fe-S biogenesis.
Highlights.
Fe-S clusters are metal cofactors involved in multiple, essential cellular processes
Fe-S clusters can accept or donate single electrons over a wide range of potentials
Defined pathways assemble Fe-S clusters de novo in mammalian mitochondria and cytosol
The acyl carrier protein is a component of the core ISC machinery
Frataxin is an allosteric regulator that accelerates sulfur transfer from NFS 1 to ISCU
A chaperone/cochaperone system facilitates cluster transfer downstream of ISCU
Acknowledgements:
the authors would like to acknowledge support from the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Funding: this work was supported by the Intramural research program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement: the authors declare no conflict of interest.
REFERENCES
- 1.Beinert H, Holm RH, and Munck E (1997). Iron-sulfur clusters: nature’s modular, multipurpose structures. Science 277, 653–659. [DOI] [PubMed] [Google Scholar]
- 2.Rouault TA (2019). The indispensable role of mammalian iron sulfur proteins in function and regulation of multiple diverse metabolic pathways. Biometals 32, 343–353. [DOI] [PMC free article] [PubMed] [Google Scholar]; (*This manuscript provides insights into the crucial role of Fe-S proteins in multiple metabolic pathways).
- 3.Rouault TA, and Maio N (2017). Biogenesis and functions of mammalian iron-sulfur proteins in the regulation of iron homeostasis and pivotal metabolic pathways. J Biol Chem 292, 12744–12753. [DOI] [PMC free article] [PubMed] [Google Scholar]; (*This manuscript discusses Fe-S cluster biogenesis in mammalian cells and focuses on the cross talk between Fe-S biogenesis and iron homeostasis).
- 4.Melber A, and Winge DR (2018). Steps Toward Understanding Mitochondrial Fe/S Cluster Biogenesis. Methods Enzymol 599, 265–292. [DOI] [PubMed] [Google Scholar]
- 5.Landgraf BJ, McCarthy EL, and Booker SJ (2016). Radical S-Adenosylmethionine Enzymes in Human Health and Disease. Annu Rev Biochem 85, 485–514. [DOI] [PubMed] [Google Scholar]
- 6.Broderick JB, Duffus BR, Duschene KS, and Shepard EM (2014). Radical S-adenosylmethionine enzymes. Chem Rev 114, 4229–4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Angerer H (2015). Eukaryotic LYR Proteins Interact with Mitochondrial Protein Complexes. Biology (Basel) 4, 133–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Vranken JG, Jeong MY, Wei P, Chen YC, Gygi SP, Winge DR, and Rutter J (2016). The mitochondrial acyl carrier protein (ACP) coordinates mitochondrial fatty acid synthesis with iron sulfur cluster biogenesis. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]; (*This manuscript provided the first experimental evidence that the acyl carrier protein is a component of the ISC core machinery).
- 9.Cai K, Frederick RO, Tonelli M, and Markley JL (2017). Mitochondrial Cysteine Desulfurase and ISD11 Coexpressed in Escherichia coli Yield Complex Containing Acyl Carrier Protein. ACS Chem Biol 12, 918–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cory SA, Van Vranken JG, Brignole EJ, Patra S, Winge DR, Drennan CL, Rutter J, and Barondeau DP (2017). Structure of human Fe-S assembly subcomplex reveals unexpected cysteine desulfurase architecture and acyl-ACP-ISD11 interactions. Proc Natl Acad Sci U S A 114, E5325–E5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boniecki MT, Freibert SA, Muhlenhoff U, Lill R, and Cygler M (2017). Structure and functional dynamics of the mitochondrial Fe/S cluster synthesis complex. Nat Commun 8, 1287. [DOI] [PMC free article] [PubMed] [Google Scholar]; (*This manuscript provides structural insights into the arrangement of the ISC core components).
- 12.Fox NG, Yu X, Feng X, Bailey HJ, Martelli A, Nabhan JF, Strain-Damerell C, Bulawa C, Yue WW, and Han S (2019). Structure of the human frataxin-bound iron-sulfur cluster assembly complex provides insight into its activation mechanism. Nat Commun 10, 2210. [DOI] [PMC free article] [PubMed] [Google Scholar]; (**Besides providing insights into the structure of the ISC core complex in the presence of frataxin, the manuscript reveals the mechanism of action of frataxin as an activator of NFS1).
- 13.Chan DI, and Vogel HJ (2010). Current understanding of fatty acid biosynthesis and the acyl carrier protein. Biochem J 430, 1–19. [DOI] [PubMed] [Google Scholar]
- 14.Majmudar JD, Feng X, Fox NG, Nabhan JF, Towle T, Ma T, Gooch R, Bulawa C, Yue WW, and Martelli A (2019). 4’-Phosphopantetheine and long acyl chain-dependent interactions are integral to human mitochondrial acyl carrier protein function. Medchemcomm 10, 209–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cronan JE (2014). The chain-flipping mechanism of ACP (acyl carrier protein)-dependent enzymes appears universal. Biochem J 460, 157–163. [DOI] [PubMed] [Google Scholar]
- 16.Crooks DR, Maio N, Lane AN, Jarnik M, Higashi RM, Haller RG, Yang Y, Fan TW, Linehan WM, and Rouault TA (2018). Acute loss of iron-sulfur clusters results in metabolic reprogramming and generation of lipid droplets in mammalian cells. J Biol Chem 293, 8297–8311. [DOI] [PMC free article] [PubMed] [Google Scholar]; (*The manuscript contains interesting findings on the early metabolic changes associated with loss of Fe-S proteins in multiple subcellular compartments).
- 17.Van Vranken JG, Nowinski SM, Clowers KJ, Jeong MY, Ouyang Y, Berg JA, Gygi JP, Gygi SP, Winge DR, and Rutter J (2018). ACP Acylation Is an Acetyl-CoA-Dependent Modification Required for Electron Transport Chain Assembly. Mol Cell 71, 567–580 e564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fiedorczuk K, Letts JA, Degliesposti G, Kaszuba K, Skehel M, and Sazanov LA (2016). Atomic structure of the entire mammalian mitochondrial complex I. Nature 538, 406–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown A, Rathore S, Kimanius D, Aibara S, Bai XC, Rorbach J, Amunts A, and Ramakrishnan V (2017). Structures of the human mitochondrial ribosome in native states of assembly. Nat Struct Mol Biol 24, 866–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gully D, and Bouveret E (2006). A protein network for phospholipid synthesis uncovered by a variant of the tandem affinity purification method in Escherichia coli. Proteomics 6, 282–293. [DOI] [PubMed] [Google Scholar]
- 21.Butland G, Peregrin-Alvarez JM, Li J, Yang W, Yang X, Canadien V, Starostine A, Richards D, Beattie B, Krogan N, et al. (2005). Interaction network containing conserved and essential protein complexes in Escherichia coli. Nature 433, 531–537. [DOI] [PubMed] [Google Scholar]
- 22.Gully D, Moinier D, Loiseau L, and Bouveret E (2003). New partners of acyl carrier protein detected in Escherichia coli by tandem affinity purification. FEBS Lett 548, 90–96. [DOI] [PubMed] [Google Scholar]
- 23.Flint DH (1996). Escherichia coli contains a protein that is homologous in function and N-terminal sequence to the protein encoded by the nifS gene of Azotobacter vinelandii and that can participate in the synthesis of the Fe-S cluster of dihydroxy-acid dehydratase. J Biol Chem 271, 16068–16074. [PubMed] [Google Scholar]
- 24.Bridwell-Rabb J, Fox NG, Tsai CL, Winn AM, and Barondeau DP (2014). Human frataxin activates Fe-S cluster biosynthesis by facilitating sulfur transfer chemistry. Biochemistry 53, 4904–4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoon H, Knight SA, Pandey A, Pain J, Zhang Y, Pain D, and Dancis A (2014). Frataxin-bypassing Isu1: characterization of the bypass activity in cells and mitochondria. Biochem J 459, 71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roche B, Agrebi R, Huguenot A, Ollagnier de Choudens S, Barras F, and Py B (2015). Turning Escherichia coli into a Frataxin-Dependent Organism. PLoS Genet 11, e1005134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fox NG, Martelli A, Nabhan JF, Janz J, Borkowska O, Bulawa C, and Yue WW (2018). Zinc(II) binding on human wild-type ISCU and Met140 variants modulates NFS1 desulfurase activity. Biochimie 152, 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hider RC, and Kong XL (2011). Glutathione: a key component of the cytoplasmic labile iron pool. Biometals 24, 1179–1187. [DOI] [PubMed] [Google Scholar]
- 29.Vickery LE, and Cupp-Vickery JR (2007). Molecular chaperones HscA/Ssq1 and HscB/Jac1 and their roles in iron-sulfur protein maturation. Critical reviews in biochemistry and molecular biology 42, 95–111. [DOI] [PubMed] [Google Scholar]
- 30.Craig EA, and Marszalek J (2002). A specialized mitochondrial molecular chaperone system: a role in formation of Fe/S centers. Cellular and molecular life sciences : CMLS 59, 1658–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uhrigshardt H, Singh A, Kovtunovych G, Ghosh M, and Rouault TA (2010). Characterization of the human HSC20, an unusual DnaJ type III protein, involved in iron-sulfur cluster biogenesis. Hum Mol Genet 19, 3816–3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maio N, Singh A, Uhrigshardt H, Saxena N, Tong WH, and Rouault TA (2014). Cochaperone binding to LYR motifs confers specificity of iron sulfur cluster delivery. Cell Metab 19, 445–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singer TP, and Johnson MK (1985). The prosthetic groups of succinate dehydrogenase: 30 years from discovery to identification. FEBS Lett 190, 189–198. [DOI] [PubMed] [Google Scholar]
- 34.Ricketts C, Woodward ER, Killick P, Morris MR, Astuti D, Latif F, and Maher ER (2008). Germline SDHB mutations and familial renal cell carcinoma. J Natl Cancer Inst 100, 1260–1262. [DOI] [PubMed] [Google Scholar]
- 35.Saxena N, Maio N, Crooks DR, Ricketts CJ, Yang Y, Wei MH, Fan TW, Lane AN, Sourbier C, Singh A, et al. (2016). SDHB-Deficient Cancers: The Role of Mutations That Impair Iron Sulfur Cluster Delivery. J Natl Cancer Inst 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghezzi D, Goffrini P, Uziel G, Horvath R, Klopstock T, Lochmuller H, D’Adamo P, Gasparini P, Strom TM, Prokisch H, et al. (2009). SDHAF1, encoding a LYR complex-II specific assembly factor, is mutated in SDH-defective infantile leukoencephalopathy. Nat Genet 41, 654–656. [DOI] [PubMed] [Google Scholar]
- 37.Maio N, Ghezzi D, Verrigni D, Rizza T, Bertini E, Martinelli D, Zeviani M, Singh A, Carrozzo R, and Rouault TA (2016). Disease-Causing SDHAF1 Mutations Impair Transfer of Fe-S Clusters to SDHB. Cell Metab 23, 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma JC, and Dougherty DA (1997). The Cation-pi Interaction. Chem Rev 97, 1303–1324. [DOI] [PubMed] [Google Scholar]
- 39.Sanchez E, Lobo T, Fox JL, Zeviani M, Winge DR, and Fernandez-Vizarra E (2013). LYRM7/MZM1L is a UQCRFS1 chaperone involved in the last steps of mitochondrial Complex III assembly in human cells. Biochimica et biophysica acta 1827, 285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maio N, Kim KS, Singh A, and Rouault TA (2017). A Single Adaptable Cochaperone-Scaffold Complex Delivers Nascent Iron-Sulfur Clusters to Mammalian Respiratory Chain Complexes I-III. Cell Metab 25, 945–953 e946. [DOI] [PMC free article] [PubMed] [Google Scholar]; (*This manuscript provides insights into the mechanism of Fe-S cluster delivery to the respiratory complexes during their assembly).
- 41.Voisine C, Cheng YC, Ohlson M, Schilke B, Hoff K, Beinert H, Marszalek J, and Craig EA (2001). Jac1, a mitochondrial J-type chaperone, is involved in the biogenesis of Fe/S clusters in Saccharomyces cerevisiae. Proceedings of the National Academy of Sciences of the United States of America 98, 1483–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maio N, and Rouault TA (2015). Iron-sulfur cluster biogenesis in mammalian cells: New insights into the molecular mechanisms of cluster delivery. Biochim Biophys Acta 1853, 1493–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Braymer JJ, and Lill R (2017). Iron-sulfur cluster biogenesis and trafficking in mitochondria. J Biol Chem 292, 12754–12763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cameron JM, Janer A, Levandovskiy V, Mackay N, Rouault TA, Tong WH, Ogilvie I, Shoubridge EA, and Robinson BH (2011). Mutations in iron-sulfur cluster scaffold genes NFU1 and BOLA3 cause a fatal deficiency of multiple respiratory chain and 2-oxoacid dehydrogenase enzymes. Am J Hum Genet 89, 486–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Navarro-Sastre A, Tort F, Stehling O, Uzarska MA, Arranz JA, Del Toro M, Labayru MT, Landa J, Font A, Garcia-Villoria J, et al. (2011). A fatal mitochondrial disease is associated with defective NFU1 function in the maturation of a subset of mitochondrial Fe-S proteins. Am J Hum Genet 89, 656–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nishioka M, Inaba Y, Motobayashi M, Hara Y, Numata R, Amano Y, Shingu K, Yamamoto Y, Murayama K, Ohtake A, et al. (2018). An infant case of diffuse cerebrospinal lesions and cardiomyopathy caused by a BOLA3 mutation. Brain Dev 40, 484–488. [DOI] [PubMed] [Google Scholar]
- 47.Yu Q, Tai YY, Tang Y, Zhao J, Negi V, Culley MK, Pilli J, Sun W, Brugger K, Mayr J, et al. (2019). BOLA (BolA Family Member 3) Deficiency Controls Endothelial Metabolism and Glycine Homeostasis in Pulmonary Hypertension. Circulation 139, 2238–2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ajit Bolar N, Vanlander AV, Wilbrecht C, Van der Aa N, Smet J, De Paepe B, Vandeweyer G, Kooy F, Eyskens F, De Latter E, et al. (2013). Mutation of the iron-sulfur cluster assembly gene IBA57 causes severe myopathy and encephalopathy. Hum Mol Genet 22, 2590–2602. [DOI] [PubMed] [Google Scholar]
- 49.Al-Hassnan ZN, Al-Dosary M, Alfadhel M, Faqeih EA, Alsagob M, Kenana R, Almass R, Al-Harazi OS, Al-Hindi H, Malibari OI, et al. (2015). ISCA2 mutation causes infantile neurodegenerative mitochondrial disorder. J Med Genet 52, 186–194. [DOI] [PubMed] [Google Scholar]
- 50.Shukla A, Hebbar M, Srivastava A, Kadavigere R, Upadhyai P, Kanthi A, Brandau O, Bielas S, and Girisha KM (2017). Homozygous p.(Glu87Lys) variant in ISCA1 is associated with a multiple mitochondrial dysfunctions syndrome. J Hum Genet 62, 723–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vogtle FN, Brandl B, Larson A, Pendziwiat M, Friederich MW, White SM, Basinger A, Kucukkose C, Muhle H, Jahn JA, et al. (2018). Mutations in PMPCB Encoding the Catalytic Subunit of the Mitochondrial Presequence Protease Cause Neurodegeneration in Early Childhood. Am J Hum Genet 102, 557–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hiltunen JK, Schonauer MS, Autio KJ, Mittelmeier TM, Kastaniotis AJ, and Dieckmann CL (2009). Mitochondrial fatty acid synthesis type II: more than just fatty acids. J Biol Chem 284, 9011–9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schonauer MS, Kastaniotis AJ, Hiltunen JK, and Dieckmann CL (2008). Intersection of RNA processing and the type II fatty acid synthesis pathway in yeast mitochondria. Mol Cell Biol 28, 6646–6657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cai K, Liu G, Frederick RO, Xiao R, Montelione GT, and Markley JL (2016). Structural/Functional Properties of Human NFU1, an Intermediate [4Fe-4S] Carrier in Human Mitochondrial Iron-Sulfur Cluster Biogenesis. Structure 24, 2080–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tong W-H, Jameson GNL, Huynh BH, and Rouault TA (2003). Subcellular compartmentalization of human Nfu, an iron–sulfur cluster scaffold protein, and its ability to assemble a [4Fe–4S] cluster. Proceedings of the National Academy of Sciences 100, 9762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McCarthy EL, and Booker SJ (2017). Destruction and reformation of an iron-sulfur cluster during catalysis by lipoyl synthase. Science 358, 373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]; (**The manuscript enlightens the mechanism of catalysis of lipoyl synthase, which requires disruption and reformation of the auxiliary cluster to provide sulfur to octanoic acid for lipoic acid synthesis).
- 57.McCarthy EL, Rankin AN, Dill ZR, and Booker SJ (2019). The A-type domain in Escherichia coli NfuA is required for regenerating the auxiliary [4Fe-4S] cluster in Escherichia coli lipoyl synthase. J Biol Chem 294, 1609–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tong WH, and Rouault TA (2006). Functions of mitochondrial ISCU and cytosolic ISCU in mammalian iron-sulfur cluster biogenesis and iron homeostasis. Cell Metab 3, 199–210. [DOI] [PubMed] [Google Scholar]
- 59.Kim KS, Maio N, Singh A, and Rouault TA (2018). Cytosolic HSC20 integrates de novo iron-sulfur cluster biogenesis with the CIAO1-mediated transfer to recipients. Hum Mol Genet 27, 837–852. [DOI] [PMC free article] [PubMed] [Google Scholar]; (*This manuscript outlines a mammalian cytoplasmic de novo Fe-S biogenesis pathway).
- 60.Land T, and Rouault TA (1998). Targeting of a human iron-sulfur cluster assembly enzyme, nifs, to different subcellular compartments is regulated through alternative AUG utilization. Mol Cell 2, 807–815. [DOI] [PubMed] [Google Scholar]
- 61.Marelja Z, Stocklein W, Nimtz M, and Leimkuhler S (2008). A novel role for human Nfs1 in the cytoplasm: Nfs1 acts as a sulfur donor for MOCS3, a protein involved in molybdenum cofactor biosynthesis. J Biol Chem 283, 25178–25185. [DOI] [PubMed] [Google Scholar]
- 62.Neukranz Y, Kotter A, Beilschmidt L, Marelja Z, Helm M, Graf R, and Leimkuhler S (2019). Analysis of the Cellular Roles of MOCS3 Identifies a MOCS3-Independent Localization of NFS1 at the Tips of the Centrosome. Biochemistry 58, 1786–1798. [DOI] [PubMed] [Google Scholar]
- 63.Stehling O, Vashisht AA, Mascarenhas J, Jonsson ZO, Sharma T, Netz DJ, Pierik AJ, Wohlschlegel JA, and Lill R (2012). MMS19 assembles iron-sulfur proteins required for DNA metabolism and genomic integrity. Science 337, 195–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gari K, Leon Ortiz AM, Borel V, Flynn H, Skehel JM, and Boulton SJ (2012). MMS19 links cytoplasmic iron-sulfur cluster assembly to DNA metabolism. Science 337, 243–245. [DOI] [PubMed] [Google Scholar]
- 65.Odermatt DC, and Gari K (2017). The CIA Targeting Complex Is Highly Regulated and Provides Two Distinct Binding Sites for Client Iron-Sulfur Proteins. Cell Rep 18, 1434–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Veatch JR, McMurray MA, Nelson ZW, and Gottschling DE (2009). Mitochondrial dysfunction leads to nuclear genome instability via an iron-sulfur cluster defect. Cell 137, 1247–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maio N, Kim KS, Holmes-Hampton G, Singh A, and Rouault TA (2019). Dimeric ferrochelatase bridges ABCB7 and ABCB10 homodimers in an architecturally defined molecular complex required for heme biosynthesis. Haematologica. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Desler C, Lykke A, and Rasmussen LJ (2010). The effect of mitochondrial dysfunction on cytosolic nucleotide metabolism. J. Nucleic Acids 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Marelja Z, Mullick Chowdhury M, Dosche C, Hille C, Baumann O, Lohmannsroben HG, and Leimkuhler S (2013). The L-cysteine desulfurase NFS1 is localized in the cytosol where it provides the sulfur for molybdenum cofactor biosynthesis in humans. PLoS One 8, e60869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marelja Z, Stocklein W, Nimtz M, and Leimkuhler S (2008). A novel role for human Nfs1 in the cytoplasm: Nfs1 acts as a sulfur donor for MOCS3, a protein involved in molybdenum cofactor biosynthesis. J. Biol. Chem 283, 25178–25185. [DOI] [PubMed] [Google Scholar]
- 71.Shimberg GD, Pritts JD, and Michel SLJ (2018). Iron-Sulfur Clusters in Zinc Finger Proteins. Methods Enzymol 599, 101–137. [DOI] [PubMed] [Google Scholar]
- 72.Shimberg GD, Michalek JL, Oluyadi AA, Rodrigues AV, Zucconi BE, Neu HM, Ghosh S, Sureschandra K, Wilson GM, Stemmler TL, et al. (2016). Cleavage and polyadenylation specificity factor 30: An RNA-binding zinc-finger protein with an unexpected 2Fe-2S cluster. Proc Natl Acad Sci U S A 113, 4700–4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Conlan AR, Axelrod HL, Cohen AE, Abresch EC, Zuris J, Yee D, Nechushtai R, Jennings PA, and Paddock ML (2009). Crystal structure of Miner1: The redox-active 2Fe-2S protein causative in Wolfram Syndrome 2. J Mol Biol 392, 143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Netz DJ, Stith CM, Stumpfig M, Kopf G, Vogel D, Genau HM, Stodola JL, Lill R, Burgers PM, and Pierik AJ (2011). Eukaryotic DNA polymerases require an iron-sulfur cluster for the formation of active complexes. Nat Chem Biol 8, 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stehling O, Mascarenhas J, Vashisht AA, Sheftel AD, Niggemeyer B, Rosser R, Pierik AJ, Wohlschlegel JA, and Lill R (2013). Human CIA2A-FAM96A and CIA2B-FAM96B integrate iron homeostasis and maturation of different subsets of cytosolic-nuclear iron-sulfur proteins. Cell Metab 18, 187–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rouault TA (2012). Biogenesis of iron-sulfur clusters in mammalian cells: new insights and relevance to human disease. Dis Model Mech 5, 155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]