Abstract
Human herpesvirus-6 (HHV-6) is a ubiquitous pathogen associated with nervous and endocrine autoimmune disorders. The aim of this study was to investigate the presence of HHV-6 in pancreatic tissue sections from non-diabetic, auto-antibody positive (AAB+), and donors with type 1 diabetes (T1D) and explore whether there is any association between HHV-6 and MHC class I hyperexpression and CD8 T cell infiltration.
HHV-6 DNA was detected by PCR and its protein was examined by indirect immunofluorescence assay followed by imaging using high-resolution confocal microscopy. Viral DNA (U67) was found in most pancreata of non-diabetic (3 out of 4), AAB+ (3 out of 5) and T1D donors (6 out of 7). Interestingly, HHV-6 glycoprotein B (gB) was more expressed in islets and exocrine pancreas of donors with T1D. However, gB expression was not directly associated with other pathologies. Out of 20 islets with high gB expression, only 3 islets (15%) showed MHC class I hyperexpression. Furthermore, no correlation was found between gB expression and CD8 T cell infiltration on a per-islet basis in any of the groups.
Our observations indicate that HHV-6 DNA and protein are present in the pancreas of non-diabetic subjects but gB expression is higher in the pancreas of donors with T1D. The possible role of HHV-6 as a contributory factor for T1D should therefore be further investigated.
Keywords: Human herpesvirus-6, Type 1 diabetes, Environmental factors
1. Introduction
Type 1 diabetes (T1D) is characterized by the immune-mediated destruction of insulin-producing beta cells (β cells) in the pancreas. It is thought that an interaction between predisposing genes and environmental factors such as viral infections may trigger the disease. Several viruses, mainly from the Enterovirus genus have been considered as potential causal agents for human T1D [1]. However, little is known about the involvement of other viruses such as herpesviruses.
Following primary infection, herpesviruses remain in a latent state in various tissues of the human host and become reactivated later in life [2], particularly in patients with severe immunosuppression. Human Herpesvirus-6 (HHV-6), a Beta-herpesvirus subfamily member, is a ubiquitous virus associated with roseola or exanthema subitum [3]. Some reports have implicated HHV-6 in several autoimmune diseases such as multiple sclerosis, autoimmune connective tissue diseases, and Hashimoto’s thyroiditis [2]. HHV-6 preferentially replicates in activated T cells but can also infect natural killer cells and several non-immune cells, and it is known to cause immunosuppression [2]. It is also commonly present in salivary glands, thyroid, liver, kidney, and therefore potentially present in pancreas.
HHV-6 utilizes envelope glycoproteins such as glycoprotein B (gB), an abundantly expressed glycoprotein, for membrane fusion and viral entry [4]. Previous reports have suggested the involvement of HHV-6 in fulminant T1D [5,6]. However, the contribution of viral triggers in T1D development has been controversial and there is no documented association between roseola infection and T1D. Thus, here we attempted to explore the possible role of HHV-6 on disease pathogenesis and its presence in the pancreas. In line with this idea, the presence of HHV-6 in the pancreas has been examined recently by Ericsson et al., [7], where the authors detected HHV6-B DNA in pancreatic islets from brain-dead diabetic and non-diabetic donors by PCR assay [7]. In our study, in addition to PCR, we have used high-resolution confocal microscopy on a per-islet basis to investigate the presence or absence of the HHV-6 gB protein in pancreatic tissues. We also examined whether HHV-6 infection is associated with MHC class I expression and CD8 T cell infiltration, which are key hallmarks of T1D. Our results indicate that HHV-6 is not associated with MHC class I expression or CD8 infiltration. However, the virus is more frequently present in diabetic pancreata than in subjects without diabetes, suggesting that diabetic pancreata might be more susceptible to acute and persistent viral infections.
2. Materials and Methods
2.1. Subjects:
Human pancreata were collected from cadaveric organ donors via nPOD through accredited organ procurement organizations that are authorized to serve all regions across the United States. Six μm sections from formalin-fixed paraffin-embedded (FFPE) tissue samples from the head (PH), body (PB) and tail region (PT) were obtained from non-diabetic (n = 4) donors, non-diabetic autoantibody positive donors (n = 5), and donors with T1D (n = 7). Donor information is summarized in Supplementary Table 1.
The experimental procedures of this study were performed in accordance with relevant guidelines and regulations. La Jolla Institute for Immunology Institutional Review Board approved all experimental procedures (protocol number DI3-054-1112). Informed consent from donor families was obtained by Organ Recovery Partners at the nPOD (https://www.jdrfnpod.org/for-partners/organ-recovery-partners/).
2.2. Indirect immunofluorescence (IF):
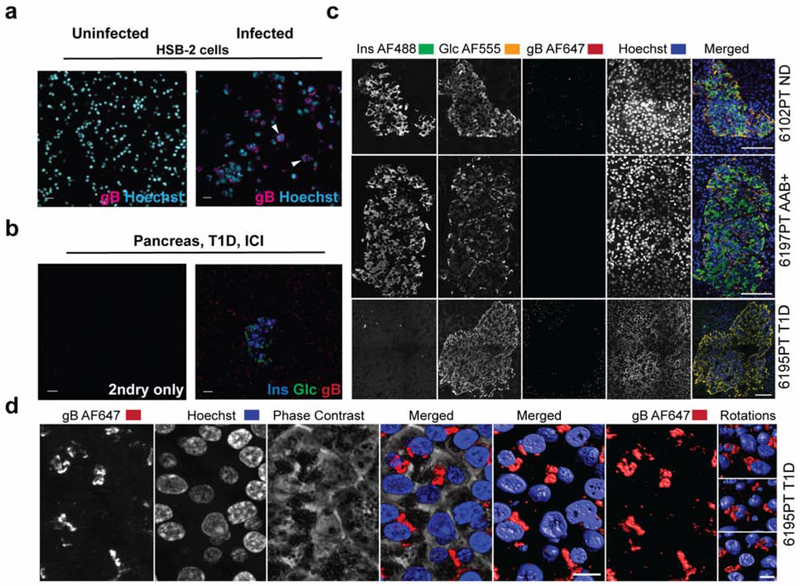
To determine the expression of HHV-6 gB, MHC class I, and CD8 T cells, pancreas sections were subjected to a standard triple IF staining protocol. After deparaffinization and rehydration in descending ethanol concentrations, sections were exposed to heat-based citrate antigen retrieval for 20 minutes. Sections were blocked with 10% goat serum for 1 hour. HHV-6 protein was detected using a monoclonal mouse antibody H-AR-2 (10 mg/ml) directed against gp110 glycoprotein of both HHV-6A and HHV-6B (kindly provided by Janos Luka, Bioworld Consulting Laboratories; 1:2000) incubated overnight at 4°C followed by an Alexa Fluor 647 conjugated goat anti-mouse IgG antibody (Life Technologies, Grand Island NY; 1:1000) incubated at room temperature for 30 minutes. Staining for insulin and glucagon was performed subsequently at room temperature for 1 hour using the following antibodies: polyclonal guinea pig anti-insulin (Dako, Carpinteria CA; 1:600) and polyclonal rabbit anti-glucagon (Dako, Carpinteria CA; 1:2000). Detection was performed at room temperature for 30 minutes using polyclonal goat anti-guinea pig IgG Alexa Fluor 488 (Life Technologies; 1:1000) and polyclonal goat anti-rabbit IgG Alexa Fluor 555 (Life Technologies; 1:1000), respectively. The specificity of the HHV-6 gB antibody was confirmed by using HSB-2 cell lines (NIH AIDS Reagent Program) [8] infected or uninfected with HHV-6 as a positive and negative control, respectively (Fig. 1a).
Figure 1. Validation of the anti-gB antibody using HHV-6-infected cells and pancreas sections.
(a) HSB-2 cells uninfected (left panel) or HHV-6-infected (right panel) were stained with anti-HHV-6 gB (H-AR-2) antibody. Goat anti-mouse AF647 was used as a secondary antibody and Hoechst was used for nuclear staining. Arrowheads show gB positive staining (magenta). (b) Representative triple staining of insulin (blue), glucagon (green), and gB (red) in the absence or presence of the primary antibodies (pancreatic tail region, case 6212). (c) Representative phenotypes of gB expression in a non-diabetic donor, AAB+ donor and a donor with T1D. Sections were stained for insulin (AF488), glucagon (AF555), gB (AF647), and Hoechst for the nuclear staining. Last panel (right) shows the merged channels. Case number and the corresponding group are indicated on the right side of each panel. (d) Representative image of gB expression in high magnification (AF647) in a donor with T1D, case 6195, is shown. Fluorescently labeled samples were optically sectioned and reconstructed in 3 dimensions using a high-resolution microscope (Zeiss 780 LSCM) and Imaris software. T1D: Type 1 diabetes; ICI: Insulin-Containing Islets. Scale bars for (a) and (b): 20 μm, (c): 100 μm, (d): 10 μm.
For CD8 and MHC class I staining, a combination of insulin, CD8, and MHC class I antibodies was used in a consecutive section to the one stained for gB. Deparaffinization, rehydration, heat-induced antigen retrieval, and blocking steps were similar to the gB staining as mentioned above. CD8 staining was performed by using a polyclonal rabbit antibody (ab4055, Abcam, Cambridge MA; 1:400) incubated overnight at 4°C followed by an Alexa Fluor 555 conjugated goat anti-rabbit IgG antibody (Life Technologies, Grand Island NY; 1:1000) incubated at room temperature for 30 minutes. Staining for insulin and MHC class I was performed subsequently at room temperature for 1 hour using the polyclonal guinea pig anti-insulin (Dako, Carpinteria CA; 1:600) and monoclonal mouse anti-human anti-HLA class I ABC (Abcam, ab70328; 1:200). Detection was performed at room temperature for 30 minutes using polyclonal goat anti-guinea pig IgG Alexa Fluor 488 (Life Technologies; 1:1000) for insulin and polyclonal goat anti-mouse IgG Alexa Fluor 647 (Life Technologies; 1:1000) for MHC class I. For both sets of the staining (insulin/glucagon/gB or insulin/CD8/MHC class I), after applying the secondary antibodies, sections were washed with distilled water, and counterstained with Hoechst 33342 (Life Technologies; 1:200) for 20 minutes. Sections were then mounted with Prolong Gold anti-fade medium (Life technologies).
2.3. Image acquisition:
Z stacks of images for insulin/glucagon/gB staining and consecutive sections for insulin/CD8/MHC class I staining were acquired using a laser scanning Olympus FV10i confocal microscope and a 60x/1.3NA objective. Images were acquired at Nyquist resolution (using a step size of 0.5μm and optimal image frame size at 1024x1024), obtained as multi-paneled stacks that overlapped at 10%, and were automatically stitched using the FV10-ASW 4.2 Olympus software. These multi-stitched images were flattened using Fiji software (LOCI, Madison, WI) at maximum intensity and then imported into Image-Pro Premier software (Media Cybernetics, Rockville, MD) for further processing. All 8-bit images were acquired using the full dynamic intensity range (0-256) that was set based on the population of cells, which displayed the highest signal. The same instrument settings (based on laser power and gain at the detector) were also used to acquire all islets from the various donors. Similarly, they were used to obtain secondary antibody alone images and these were in turn used to define thresholds of real signal above background.
2.4. Exocrine tissue imaging:
To determine the presence or absence of gB in the exocrine tissue, the slides were scanned by a Zeiss Axio Scan Z. 1 slide scanner (Carl Zeiss microscopy) using an Orca Flash 4.0 v2 fluorescent camera with 20x objective.
2.5. gB quantification Analysis:
Endocrine analysis:
In Image-Pro Premier, up to 10 islets per case were randomly analyzed for levels of fluorescent signal above background. All images received the same thresholding in order to define the real signal above background signal. However, donor 6081 displayed a different background and the threshold was selected manually for this case. Periphery was defined as the exocrine area around the islet in the captured image.
Exocrine analysis:
In order to make sure that the analysis excluded the islets, both images labeled for insulin and glucagon were used to make masks of the islets and to subtract those islet regions from the images labeled with gB only, therefore enabling us to analyze exocrine gB only in Image-Pro Premier (See image preparation in supplementary methods). This was accomplished using macros created in image J (NIH-FIJI-J). The images were imported into Image-Pro Premier and then, the smart segmentation feature was used to highlight, detect and auto outline all the gB positive zones in all the images. The analyzed data was sent into a data collector and then to excel in an automated fashion through custom-made macros using automate and batch processing modules within Image-Pro Premier project explorer. All techniques, image acquisition and quantification were approved by histology and microscopy specialists at the La Jolla Institute for Immunology in order to guarantee the appropriate experimental setup and analysis for this study. Information regarding the number of cases and islets that were analyzed for gB analysis is summarized in Supplementary Table. 2a.
2.6. MHC class I Analysis:
For MHC class I analysis, consecutive sections were used to find the same islets as those stained for gB (up to 10 per donor). For MHC class I quantification, similar image acquisition and quantification method as gB analysis was used. Information regarding the number of cases and islets analyzed for MHC class I is summarized in Supplementary Table. 2b.
2.7. CD8 T cell Analysis:
In order to assess the number of CD8 T cells inside the islets, consecutive sections to the one stained for gB were used to find the same islets (up to 10 islets per case) (Supplementary Table. 2c). CD8 positive T cells were manually counted on the sections that were scanned by the Zeiss Axio Scan Z.1 scanner using an Orca Flash 4.0 v2 fluorescent camera with 20x objective. Due to the different background levels between sections, contrast stretching was applied to the tissue sections in order to maximize the signal. To calculate the CD8 positive T cell density inside each islet, the number of CD8 positive T cells in each islet was divided by the islet size.
2.8. DNA extraction from pancreatic FFPE tissue sections
DNA was extracted from the consecutive FFPE sections to those stained by IF. Depending on the tissue size, 3 to 5 sections were used for DNA extraction, which was performed using the QIAmp DNA FFPE Tissue protocol (Qiagen). Briefly, pancreatic tissue sections were scraped using a razor blade. Paraffin was dissolved and removed using xylene followed by two times ethanol wash. Consequently, samples were lysed under denaturing conditions with Proteinase K. Formalin crosslinking was reversed by heat incubation. Finally, DNA was washed and purified.
2.9. Polymerase Chain Reaction
The extracted DNA was used to perform a PCR to amplify the U67 gene of both HHV-6A and HHV-6B. External primers [7] forward (GCGTTTTCAGTGTGTAGTTCGGCAG) and reverse (TGGCCGCATTCGTACAGATACGGAGG) generated a 520 bp fragment.
PCR products were used to perform a nested PCR using internal primers. Forward (GCTAGAACGTATTTGCTGCAGAACG) and reverse (ATCCGAAACAACTGTCTGACTGGCA) primers generated a 258 bp fragment.
The amplified nested PCR products were analyzed by electrophoresis on a 2% agarose gel containing GelRed™ (Biotium). A 100 bp DNA ladder (Qiagen) was used as a molecular mass marker.
2.10. Statistical analysis
Data are presented as mean ± SD and analyzed using one-way ANOVA followed by Tukey’s multiple comparison test. Analyses were performed using GraphPad Prism version 7 (GraphPad Software, San Diego, CA). A value of P < 0.05 was considered significant. The P values were corrected for multiple comparisons using the Benjamini and Hochberg method.
3. Results
3.1. gB protein of HHV-6 is detected more frequently in the pancreas of donors with type 1 diabetes than auto-antibody positive donors or donors without diabetes
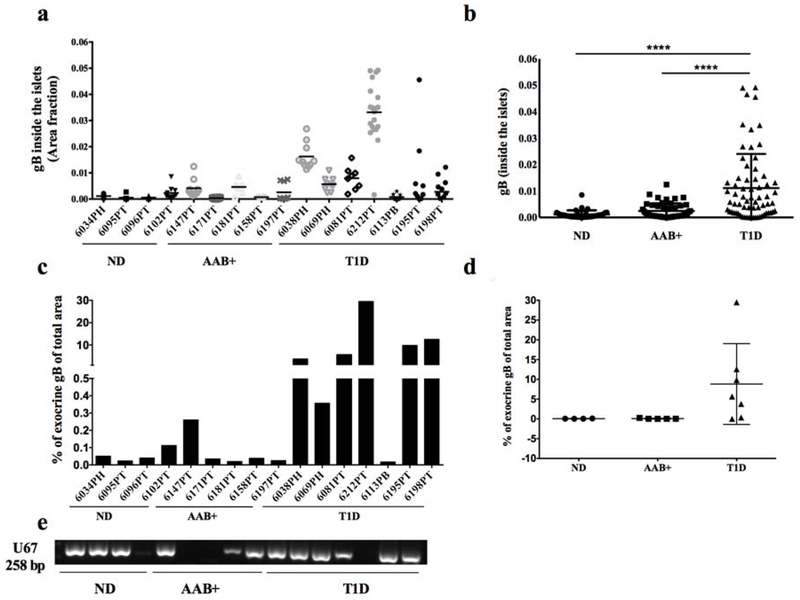
HHV-6 gB antibody has been validated on infected and uninfected HSB-2 cells (materials and methods) (Fig. 1a) as well as on a pancreas section (Fig. 1b). Pancreas sections from 16 donors were assessed for the presence of gB protein by IF. Representative images of the triple staining of insulin (blue), glucagon (green) and gB (red) are presented in Fig. 1c and a higher magnification of gB expression is presented in Fig. 1d. A significantly higher level of gB expression was found in the islets of donors with T1D compared with AAB+ donors and donors without diabetes (Fig. 2a, b). gB was detected in the islets of 6 out of 7 T1D donors compared to 1 out of 4 non-diabetic cases and 2 out of 5 AAB+ donors (Fig. 2a). Consistent with high intra-islet expression of gB in the islets in donors with T1D, a higher expression level of gB protein in the periphery of the islets was also observed (Supplementary Fig. 1). In order to find out if gB expression was restricted to the islets and their surroundings, the presence of gB in the exocrine tissue was investigated. We observed that gB was more expressed in the exocrine tissue in donors with diabetes compared to non-diabetic donors (Fig. 2c–d). Indeed, gB was detectable in the exocrine compartment of all donors with T1D except case 6113. Also, gB was expressed at low levels in case 6034 and 6102 in the non-diabetic group. In AAB+ donors, gB was only detected in the exocrine tissue of case 6147 (Fig. 2c–d).
Figure 2. gB is more frequently detected in the pancreas of T1D donors.
Quantification of gB expression inside the islets (a, b) or in the exocrine (c, d) from non-diabetic donors (n=4), AAB+ donors (n=5) and donors with T1D (n=7). Each dot represents an islet and is presented as the positive fraction of the total islet area (a, b) or percentage of gB positive of total area (c). For each case, up to 10 islets were quantified. In (d), each dot represents a donor. (e) DNA was extracted from ND, AAB+ and donors with T1D, and U67 viral gene (258bp) was detected by nested PCR. ND: Non diabetic; AAB+: Auto-antibody positive; T1D: Type 1 diabetes.
3.2. HHV-6 DNA is detected in the pancreas of both donors with and without diabetes
Genomic DNA was extracted from the pancreas sections of non-diabetic donors, AAB+ donors, and donors with diabetes. In order to detect HHV-6 genomic DNA, the samples were amplified by a nested PCR using specific primers. U67 gene, a major capsid protein of both HHV6-A and HHV6-B was present in 3 out of 4 non-diabetic donors, 3 out of 5 AAB+ donors, and 6 out of 7 donors with T1D (Fig. 2e).
3.3. Intra-islet gB does not correlate with MHC class I expression or CD8 T cell infiltration in donors with type 1 diabetes
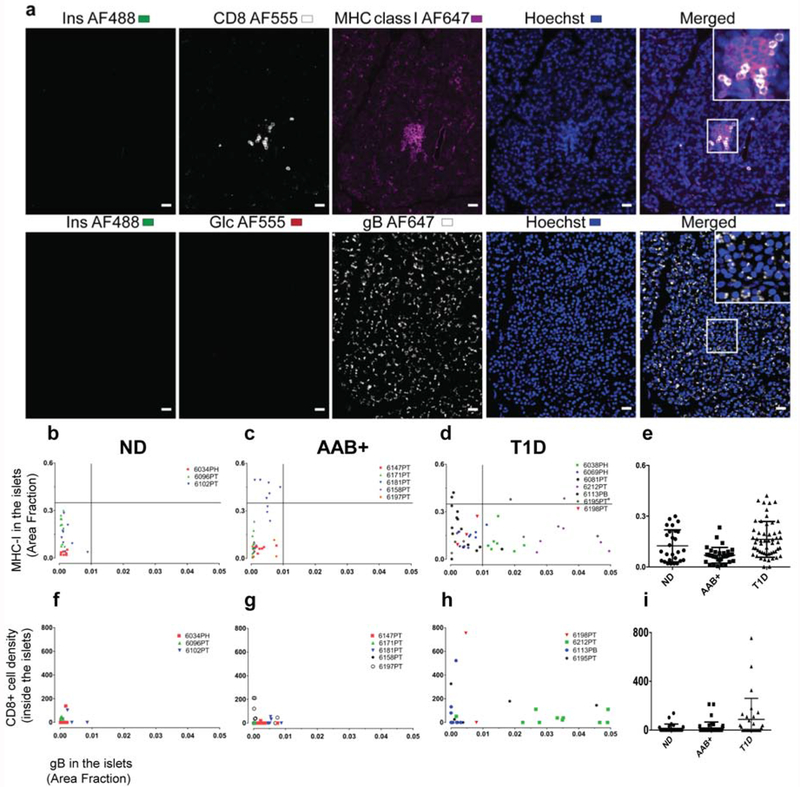
We analyzed MHC class I expression on consecutive sections to those stained for gB (Supplementary Table. 2b). A representative image of MHC class I staining and gB staining on the consecutive section is shown in Fig. 3a, top and bottom panel, respectively. MHC class I protein level in the pancreatic islets was quantified similarly to the gB quantification as described above. In order to find out if there is a correlation between the expression of gB and MHC class I in the islets, data based on the area fraction was used for both gB and MHC class I. However, no direct association between the presence of gB and MHC class I expression was found regardless of the diabetic status (Fig. 3b–d).
Figure 3. Islet MHC class I expression and CD8 T cells infiltration is not associated with gB expression.
Representative images from case 6195 (T1D) positive for CD8, MHC class I, and gB staining (a). Top panel: section stained with insulin (green), CD8 (white), MHC class I (magenta), and Hoechst (blue). Bottom panel: the consecutive section was stained with insulin (green), glucagon (red), gB (white), and Hoechst (blue). Last column in each panel (right) shows the merged channels and inserts show magnification of the area inside the box.
Correlation between gB protein level and MHC class I expression inside the islets is presented based on the area fraction in (b) non-diabetic donors (n=3), (c) AAB+ donors (n=5), (d) donors with T1D (n=7) and (e) in groups. For each case up to 10 islets were quantified. Each dot represents an islet and each color represents an individual donor. Correlation between gB protein level and CD8 T cells density inside the islets in each group are presented (f-i). Each dot represents an islet and each color represents an individual donor. CD8 counting inside the islets was manually performed from scanned images and the CD8 density was presented as total CD8 number divided by the islet size. For ease of presentation, this value was multiplied by 10x6.
We performed a CD8 staining in combination with MHC class I staining on consecutive slides to that of gB staining, which enabled us to locate the same islets in both sections and perform a CD8 T cell correlation analysis (see Methods for more details). A representative image for CD8 staining and gB staining on the consecutive section is presented in Fig. 3a, top and bottom panel, respectively. Although we observed an increase in the number of CD8+ T cells in donors with T1D (Fig. 3f–i), we could not find any association between the gB expression level and CD8 T cell numbers inside the islets on a per-islet basis (Fig. 3f–h and Supplementary Table. 2c).
4. Discussion
Epidemiological and serological studies have suggested that several viruses including rotaviruses, retroviruses and other picornaviruses may have a role as triggers for the development of T1D [1]. However, due to the complexity of the human pancreas and the lack of available samples, there is limited information about the presence of other viruses such as HHV-6 in the pancreas. This is the first study in which the association of a viral infection with single islet pathology and not merely disease status is investigated.
The current study is different in many aspects from those of other investigators who reported the presence of HHV6 in pancreata of healthy and T1D donors. In the present study, HHV-6 gB protein has been quantified and its possible association with MHC class I or CD8 T cell infiltration has been investigated.
Several considerations in the present report are noteworthy. Using high-resolution confocal microscopy, we observe a higher level of gB protein in the pancreatic islets from donors with T1D compared to non-diabetic donors. Similar results were obtained in the periphery of the islets as well as in the exocrine tissue of both diabetic and non-diabetic subjects. Furthermore, using nested PCR, the U67 viral gene has been detected in 75% of ND donors, 60% in AAB+ donors and 85.7% in T1D donors. We have focused on U67, which is a late gene and expresses a major capsid protein of both HHV6-A and HHV6-B. The detection of U67 with HHV-6 gB protein suggests an active viral infection. Our data are further supported by the detection of the viral receptor OX40 in the pancreas (data not shown). In accordance with our results, the presence of HHV-6 DNA in the pancreas from brain-dead organ donors with or without T1D has been recently reported [7]. However, in the study by Skog et al. there was a lack of concordance between PCR and IF.
According to the fertile field hypothesis, certain viruses may infect the pancreas, thus creating a fertile field for preexisting islet autoimmunity, resulting in its augmentation, increased β cell destruction, and ultimately leading to T1D development [12]. Our results indicate that HHV-6 infection might play such an indirect role in fostering the autoimmune process and may still act as a general pathogenic factor enhancing the development of the disease.
Hyper-expression of MHC class I has been reported in mouse models of T1D [13] as well as in the islets of patients with recent-onset T1D [14,15]. In agreement with previous studies [9], we observed a higher level of MHC class I expression in donors with T1D than in those without diabetes. However, there was no correlation between gB and MHC class I expression on a per-islet basis. The absence of correlation could be explained by the fact that HHV-6 downregulates the expression of MHC class I on the surface and within infected cells [16].
HHV-6 infects both CD4 and CD8 T cells in vivo [2]. Although we observed a higher number of CD8+ T cells in islets of donors with T1D, we were unable to find any correlation between gB protein level and CD8 T cell infiltration on a per-islet basis (Fig. 3f–h).
These results indicate that, while there might be a link between gB expression and T1D, HHV-6 is not strongly associated with pancreas pathology during T1D (or in pre-diabetes) on a per-islet basis. Diabetic pancreata might be more susceptible to viral infections in general, indirectly contributing to disease pathogenesis. It is also possible that the higher expression level of gB seen in the pancreata from donors with T1D could be associated with exocrine subclinical pancreatitis [17] which often accompanies the disease, and not directly with the islet pathology.
Our findings are novel and indicate that although it is unlikely that HHV-6 is a primary cause for T1D and is not associated with MHC class I expression or CD8 infiltration, yet the virus is more frequently present in diabetic pancreata, which is similar to what has been reported for enterovirus infections [1,18].
The conclusion that one can observe increased frequency of infection by a virus like HHV-6 in type 1 diabetes pancreata without a direct association with islet pathology is novel and points towards a more indirect mechanism of potential causality. Maybe, pancreata of type 1 patients are in general more susceptible to infections, possibly due to alterations in the interferon pathway [19], which could result in more general deleterious effects of many different viruses during type 1 pathogenesis.
Our findings should prompt further studies to understand why certain viral infections occur more frequently in the diabetic pancreas and how they might trigger and/or aid in the development of the disease.
Supplementary Material
Highlights:
HHV-6 gB protein was more frequently detected in the islets and exocrine tissue of donors with T1D.
HHV-6 genomic sequence (U67) was found in the pancreatic tissues of both non-diabetic and diabetic donors.
No correlation was found between HHV-6 gB protein expression and CD8 T cell infiltration
3 out of 20 islets with gB high expression showed MHC class I expression on a per-islet basis.
Acknowledgements
The authors would like to thank Zbigniew Mikulski and Yasaman Lajevardiat from the La Jolla Institute for Immunology for help with the image acquisition and analysis and technical assistance, respectively. The following reagent was obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH: HSB2 HHV6A (GS) from Dr. Dharam Ablashi. We would like to acknowledge Dr. Kristin Loomis of the HHV-6 Foundation for her helpful comments.
Funding: This research was performed with the support of nPOD, a collaborative type 1 diabetes research project sponsored by the Juvenile Diabetes Research Foundation International. Organ Procurement Organizations, partnering with nPOD to provide research resources, are listed at www.jdrfnpod.org/our-partners.php. This study was also supported by the National Institutes of Health/National Institute of Allergy and Infectious Diseases grant #U01AI102370-08.
S.S. was supported by the NIDDK of the NIH under Award number T32DK007494. M.A.B. was supported by a SPARK award from La Jolla Institute for Immunology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing financial interests: The authors declare no competing financial interests.
References
- [1].Rodriguez-Calvo T, Sabouri S, Anquetil F, von Herrath MG, The viral paradigm in type 1 diabetes: Who are the main suspects?, Autoimmun Rev. 15 (2016) 964–969. doi: 10.1016/j.autrev.2016.07.019. [DOI] [PubMed] [Google Scholar]
- [2].Agut H, Bonnafous P, Gautheret-Dejean A, Update on infections with human herpesviruses 6A, 6B, and 7, Med Mal Infect. 47 (2017) 83–91. doi: 10.1016/j.medmal.2016.09.004. [DOI] [PubMed] [Google Scholar]
- [3].Yamanishi K, Okuno T, Shiraki K, Takahashi M, Kondo T, Asano Y, Kurata T, Identification of human herpesvirus-6 as a causal agent for exanthem subitum, Lancet. 1 (1988) 1065–1067. doi: 10.1016/s0140-6736(88)91893-4. [DOI] [PubMed] [Google Scholar]
- [4].Tanaka Y, Suenaga T, Matsumoto M, Seya T, Arase H, Herpesvirus 6 glycoproteins B (gB), gH, gL, and gQ are necessary and sufficient for cell-to-cell fusion, J. Virol 87 (2013) 10900–10903. doi: 10.1128/JVI.01427-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Imagawa A, Hanafusa T, Fulminant type 1 diabetes--an important subtype in East Asia, Diabetes Metab. Res. Rev 27 (2011) 959–964. doi: 10.1002/dmrr.1236. [DOI] [PubMed] [Google Scholar]
- [6].Yoneda S, Imagawa A, Fukui K, Uno S, Kozawa J, Sakai M, Yumioka T, Iwahashi H, Shimomura I, A Histological Study of Fulminant Type 1 Diabetes Mellitus Related to Human Cytomegalovirus Reactivation, J. Clin. Endocrinol. Metab 102 (2017) 2394–2400. doi: 10.1210/jc.2016-4029. [DOI] [PubMed] [Google Scholar]
- [7].Ericsson M, Skog O, Presence of Human Herpesvirus 6B in the Pancreas of Subjects With and Without Type 1 Diabetes, Pancreas. 46 (2017) 1341–1346. doi: 10.1097/MPA.0000000000000927. [DOI] [PubMed] [Google Scholar]
- [8].Ablashi DV, Balachandran N, Josephs SF, Hung CL, Krueger GR, Kramarsky B, Salahuddin SZ, Gallo RC, Genomic polymorphism, growth properties, and immunologic variations in human herpesvirus-6 isolates, Virology. 184 (1991) 545–552. [DOI] [PubMed] [Google Scholar]
- [9].Richardson SJ, Rodriguez-Calvo T, Gerling IC, Mathews CE, Kaddis JS, Russell MA, Zeissler M, Leete P, Krogvold L, Dahl-Jørgensen K, von Herrath M, Pugliese A, Atkinson MA, Morgan NG, Islet cell hyperexpression of HLA class I antigens: a defining feature in type 1 diabetes, Diabetologia. 59 (2016) 2448–2458. doi: 10.1007/s00125-016-4067-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Willcox A, Richardson SJ, Bone AJ, Foulis AK, Morgan NG, Analysis of islet inflammation in human type 1 diabetes, Clin. Exp. Immunol 155 (2009) 173–181. doi: 10.1111/j.1365-2249.2008.03860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rodriguez-Calvo T, Suwandi JS, Amirian N, Zapardiel-Gonzalo J, Anquetil F, Sabouri S, von Herrath MG, Heterogeneity and Lobularity of Pancreatic Pathology in Type 1 Diabetes during the Prediabetic Phase, J. Histochem. Cytochem 63 (2015) 626–636. doi: 10.1369/0022155415576543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].von Herrath MG, Fujinami RS, Whitton JL, Microorganisms and autoimmunity: making the barren field fertile?, Nat. Rev. Microbiol 1 (2003) 151–157. doi: 10.1038/nrmicro754. [DOI] [PubMed] [Google Scholar]
- [13].Thomas HE, Parker JL, Schreiber RD, Kay TW, IFN-gamma action on pancreatic beta cells causes class I MHC upregulation but not diabetes, J. Clin. Invest 102 (1998) 1249–1257. doi: 10.1172/JCI2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Foulis AK, Farquharson MA, Hardman R, Aberrant expression of class II major histocompatibility complex molecules by B cells and hyperexpression of class I major histocompatibility complex molecules by insulin containing islets in type 1 (insulin-dependent) diabetes mellitus, Diabetologia. 30 (1987) 333–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Coppieters KT, Dotta F, Amirian N, Campbell PD, Kay TWH, Atkinson MA, Roep BO, von Herrath MG, Demonstration of islet-autoreactive CD8 T cells in insulitic lesions from recent onset and long-term type 1 diabetes patients, J. Exp. Med 209 (2012) 51–60. doi: 10.1084/jem.20111187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ota M, Serada S, Naka T, Mori Y, MHC class I molecules are incorporated into human herpesvirus-6 viral particles and released into the extracellular environment, Microbiology and Immunology. 58 (2014) 119–125. doi: 10.1111/1348-0421.12121. [DOI] [PubMed] [Google Scholar]
- [17].Capua I, Mercalli A, Pizzuto MS, Romero-Tejeda A, Kasloff S, De Battisti C, Bonfante F, Patrono LV, Vicenzi E, Zappulli V, Lampasona V, Stefani A, Doglioni C, Terregino C, Cattoli G, Piemonti L, Influenza A viruses grow in human pancreatic cells and cause pancreatitis and diabetes in an animal model, J. Virol 87 (2013) 597–610. doi: 10.1128/JVI.00714-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hyöty H, Taylor KW, The role of viruses in human diabetes, Diabetologia. 45 (2002) 1353–1361. doi: 10.1007/s00125-002-0852-3. [DOI] [PubMed] [Google Scholar]
- [19].Richardson SJ, Morgan NG, Enteroviral infections in the pathogenesis of type 1 diabetes: new insights for therapeutic intervention, Curr Opin Pharmacol. 43 (2018) 11–19. 10.1016/j.coph.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.