Abstract
Although overall mortality rates in dialysis patients have improved during the last decade or so, infections remain a leading cause of death, second only to cardiovascular disease. In addition, infections account for a major share of hospitalizations in this patient population. Receiving hemodialysis treatments in an outpatient dialysis facility significantly contributes to patients’ risks for infection. In dialysis units, patient-to-patient transmission of viral pathogens such as hepatitis B virus, hepatitis C virus, and human immunodeficiency virus can occur; proper screening and vaccination of patients can decrease the risk for transmission. Strict adherence to hand hygiene, use of appropriate personal protective equipment, transmission-based precautions, and maintaining aseptic technique while connecting the access to the hemodialysis machine can substantially decrease the likelihood of bacterial infections. With an effective infection control program in place, infection prevention becomes part of the dialysis facility’s culture and results in improved patient safety. In this installment of the Core Curriculum series, we highlight best practices that should be followed by health care workers in the dialysis unit and discuss the role of the medical director in promoting initiatives to reduce infection rates.
Index Words: Outpatient care, isolation & infection control, viral infection, hand disinfection, PPE, review
FEATURE EDITOR:
Asghar Rastegar
ADVISORY BOARD:
Ursula C. Brewster
Michael Choi
Ann O’Hare
Manoocher Soleimani
The Core Curriculum aims to give trainees in nephrology a strong knowledge base in core topics in the specialty by providing an overview of the topic and citing key references, including the foundational literature that led to current clinical approaches.
Introduction
There are approximately 750,000 patients with kidney failure in the United States, with more than 450,000 of them undergoing outpatient hemodialysis. These patients are at an increased risk for infections, which are second only to cardiovascular disease as a cause of death in this patient population. There are various factors that are responsible for high rates of infection in patients undergoing hemodialysis in the outpatient setting, including immunocompromised status of the patient, need to access the bloodstream through catheters/fistulas, exposure of blood to an extracorporeal circuit, proximity of patients to each other, and care provided by the same health care workers to multiple patients at the same time. Therefore, the potential of harm in this patient care setting is high if the recommended infection control practices are not followed. One of the many challenges faced in implementing proper infection control in dialysis units is the lack of understanding and training of health care workers.
The opportunities to decrease infections start with proper screening and vaccinations in patients who initiate outpatient dialysis. Because viral pathogens such as hepatitis B virus (HBV), hepatitis C virus (HCV), and human immunodeficiency virus (HIV) can undergo patient-to-patient transmission, all incident hemodialysis patients should be screened for these infections and vaccinated when appropriate. After initiation of dialysis, contaminated water, equipment, and environmental surfaces can be a source of infection. Therefore, maintenance of water quality, use of standard precautions, appropriate use of personal protective equipment (PPE), hand hygiene, and proper disinfection of the vascular access site are paramount in reducing the risk for infection. Given the scope of this problem, various international organizations have generated guidelines for infection prevention in hemodialysis units. Because an in-depth discussion of all infectious organisms and recommended infection control measures cannot be covered in a single article, in this article we focus on the common infection-related issues faced by dialysis unit physicians and staff.
Screening Recommendations for Hemodialysis Patients
Case: Mr J. S. is a 65-year-old man with a 15-year history of diabetes mellitus and hypertension. During the last 3 years, his serum creatinine level has stayed stable between 2.5 and 3.0 mg/dL. However, 3 months ago, he was admitted with osteomyelitis of his right foot, which required a right transmetatarsal amputation. During the hospitalization, his glomerular filtration rate worsened and he reached kidney failure. He was started on hemodialysis through a tunneled right internal jugular dialysis catheter. After a prolonged stay at a rehabilitation facility, he is now ready for discharge. As medical director of an outpatient hemodialysis facility, you have received a referral to admit him to your unit.
Question 1: In reviewing his records, it seems that he has not been vaccinated against HBV. Before admission to the outpatient hemodialysis unit, which of the following tests should be completed in the hospital?
-
a)
Hepatitis B surface antigen (HBsAg) and antibody to hepatitis B core antigen (anti-HBc)
-
b)
HBsAg and antibody to HBsAg (anti-HBs)
-
c)
HBsAg, anti-HBs, and anti-HBc
-
d)
HBsAg, anti-HBs, and hepatitis B e-antigen
For the answer to the question, see the following text.
HBV infections are a significant problem in the dialysis unit, with a prevalence of ~1%. HBV can be transmitted through exposure to infectious blood or body fluids of an infected person, direct contact with mucous membranes, or through contaminated environmental surfaces in the dialysis unit. The US Centers for Disease Control and Prevention (CDC) recommends screening for HBV infection in all patients starting hemodialysis and these have been fully adopted by the Centers for Medicare & Medicaid Services (CMS) as a part of Medicare’s Conditions for Coverage for End-Stage Renal Disease Facilities. Based on CDC and American Association for the Study of Liver Diseases recommendations, screening should include testing for HBsAg, anti-HBs, and anti-HBc before admission in an outpatient dialysis facility. The interpretation of these tests is summarized in Table 1 .
Table 1.
Interpretation of HBV Serologic Test Results in Patients
| HBsAg | Anti-HBs | Anti-HBc |
Interpretation | Notes | |
|---|---|---|---|---|---|
| IgM | Total | ||||
| Negative | Negative | Negative | Negative | No exposure | Susceptible to infection: needs vaccination |
| Negative | Positive | Negative | Negative | Successful vaccination | Patient has acquired immunity |
| Negative | Positive | Negative | Positive | Previous infection (now cleared) | Patient has natural immunity |
| Positivea | Negative | Negative | Negative | Early infection (first 2-4 wk) or false positive | Patient is infective |
| Positive | Negative | Positive | Positive | Acute infection | Patient is infective |
| Positive | Negative | Negative | Positive | Chronic infection | Patient is infective |
| Negative | Negative | Negative or positive | Positive | 4 possibilities:
|
Patient needs further testing |
Abbreviations: anti-HBc, antibody to hepatitis B core antigen; anti-HBs, antibody to hepatitis B surface antigen; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; IgM, immunoglobulin M.
The authors originally published this table in Nephrology Self-Assessment Program, Vol 18, No 3, July 2019.
A caveat is that recent exposure to vaccine may give a positive test result in which HBsAg can be detected for up to 4 weeks.
Patients who are susceptible to HBV infection despite vaccination (negative results for HBsAg and anti-HBs) should be screened monthly for HBsAg so that seroconversion can be identified early and appropriate isolation procedures can be implemented. If patients are immune to HBV as evidenced by a positive anti-HBs test result, they should have anti-HBs levels screened annually and be revaccinated if levels decrease to <10 IU/L. Table 2 summarizes the HBV screening recommendations from the CDC for patients based on their HBV serologic status.
Table 2.
HBV Screening Guidelines for Patients
| Patient Characteristic | Serologic Results at Baseline | Screening Recommendation |
|---|---|---|
| Susceptible to HBV infection | Negative HBsAg and negative anti-HBs | Monthly screening with HBsAg only |
| Immune from a prior vaccination | Anti-HBs > 10 IU/mL | Annual screening with anti-HBs |
| Immune from a prior infection | Anti-HBs > 10 IU/mL and anti-HBc positive | No further testing required |
Abbreviations: anti-HBc, antibody to hepatitis B core antigen; anti-HBs, antibody to hepatitis B surface antigen; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus.
The authors originally published this table in Nephrology Self-Assessment Program, Vol 18, No 3, July 2019.
As a result of these vaccination, isolation, and infection-control practices in the United States regarding HBV, transmission of HBV in dialysis clinics has been very rare.
In addition to HBV testing, at the start of dialysis patients should also be screened for HCV infection by detection of antibodies to HCV. This is a grade 1A recommendation in the 2018 KDIGO (Kidney Disease: Improving Global Outcomes) hepatitis C guideline. The guideline also recommends that patients should then be tested regularly at 6-month intervals (graded 1B). If patients test positive for HBV or HCV, they should be considered for appropriate treatment.
In addition, CDC recommends HIV screening for all patients between the ages of 13 and 64 years, using an HIV antibody test that is consistent with US Preventive Services Task Force guidelines. HIV testing of patients must be voluntary and performed after informed consent. HIV infection and the meaning of a positive and negative test result should be explained carefully to patients and they should be given an opportunity to ask questions and decline testing. In the event of a positive test result, maintaining confidentiality is imperative because HIV diagnosis still carries a stigma that concerns many patients. Given the lack of privacy in an outpatient hemodialysis unit due to the proximity of other patients, positive test results should be communicated through personal contact by a physician, nurse, midlevel practitioner, or a qualified staff member in a private setting and patients should also promptly be referred for consideration of antiretroviral therapy.
Patients with kidney failure often have impaired cellular immunity, predisposing them to develop tuberculosis. Tuberculin skin test is generally used to screen patients with kidney failure for tuberculosis; however, dialysis patients can have high rates of anergy. Given that some studies have shown higher sensitivity and specificity with interferon gamma release assays (IGRAs) such as T-SPOT.TB (Oxford Immunotec Limited) or QuantiFERON-TB Gold (QIAGEN), there has been increased interest in using these as an alternative screening test but there is no consensus at this time. CDC currently recommends screening dialysis patients with either a tuberculin skin test or IGRA blood test.
Returning to the patient presented in case 1, he should be tested for HBsAg, anti-HBs, and anti-HBc; thus the correct answer is option (c). HBsAg is positive in patients who are HBV carriers or either have an acute or chronic HBV infection. A positive anti-HBs test indicates immunity to HBV through either vaccination or a prior infection that has now cleared. A positive total anti-HBc result can be seen in acute, chronic, or a previously cleared infection.
Checking for HBsAg and anti-HBc (option (a)) is not sufficient because that will not give us information regarding the susceptibility of this patient to HBV infection and the need for vaccination. If only HBsAg and anti-HBs is measured (option (b)), an acute infection in the window period (when HBsAg has been cleared but anti-HBs has not yet developed) can be misdiagnosed. Checking HBeAg (option (d)) gives information regarding the infectivity of a patient but without checking anti-HBc, there is still the potential pitfall of missing an acute infection.
Vaccination Recommendations for Hemodialysis Patients
Hepatitis B Virus
HBV vaccination of susceptible patients and staff is one of the key initiatives to decrease the risk for HBV infection. Patients are more likely to respond to vaccination if they receive it before starting dialysis, but if they are vaccinated after initiation of dialysis, a higher dose is recommended [40 μg of Engerix-B (GlaxoSmithKline) at 0, 1, 2, and 6 months or 40 μg of Recombivax HB (Merck & Co.) at 0, 1, and 6 months]. After 1 to 2 months of completion of the vaccination series, patients should be tested for anti-HBs and if they are not immune, they should get another series of HBV vaccination. For patients who do not respond to the second series, no additional doses of vaccines have proved to be beneficial.
Influenza
To prevent morbidity from seasonal influenza, CDC includes dialysis patients as a priority group for receiving annual influenza vaccine. There is no preference between inactivated influenza vaccines, including standard quadrivalent and high-dose trivalent, but the live attenuated influenza vaccine is contraindicated in patients treated by maintenance hemodialysis.
All dialysis patients should be offered the influenza vaccine and counseled that although influenza vaccine is not 100% protective, it is the best tool that is currently available. In case of patient refusal, health care providers should try and understand underlying specific concerns and address them accordingly.
All physicians and staff working in the dialysis unit should be also be vaccinated annually based on American College of Physicians recommendations.
Shingles
CDC recommends vaccination against herpes zoster in immunocompetent adults 50 years or older, including patients who are dialysis dependent. The CDC recommends 2 doses of recombinant zoster vaccine (RZV, Shingrix) 2 to 6 months apart as the preferred vaccine over zoster vaccine live (ZVL, Zostavax), which is a live vaccine.
Pneumonia
Although the overall efficacy of pneumococcal vaccine is lower in the dialysis population compared with the general population, immunization is still recommended to decrease the morbidity and mortality associated with invasive pneumococcal disease. Dialysis patients should receive both 23 valent pneumococcal capsular polysaccharide vaccine [Pneumovax or PPSV 23 (Merck & Co.)] and 13 valent pneumococcal conjugate vaccine [PCV 13 (Wyeth Pharmaceuticals Inc.)]. If PCV 13 is given first, PPSV 23 should be given after 8 weeks or longer, and if PPSV 23 is given first, PCV 13 should be given a year later. All patients should be given a second dose of PPSV 23 at 5 years after the prior dose.
Additional Readings
-
➢
Branson BM, Handsfield HH, Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1-17.
-
➢
Centers for Disease Control and Prevention. Guidelines for vaccinating kidney dialysis patients and patients with chronic kidney disease. 2012. https://www.cdc.gov/vaccines/pubs/downloads/dialysis-guide-2012.pdf. Accessed January 24, 2020.
-
➢
Centers for Disease Control and Prevention. Consultant meeting to update recommendations for the prevention and control of bloodborne and other infections among chronic hemodialysis patients 1999. https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5005a1.htm#tab3. Accessed January 25, 2020. ★ Essential Reading
-
➢
Hess S, Bren V. Essential components of an infection prevention program for outpatient hemodialysis centers. Semin Dial. 2013;26(4):384-398.
-
➢
Grohskopf LA, Sokolow LZ, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices – United States, 2018-19 influenza season. MMWR Recomm Rep. 2018;67(3):1-20.
-
➢
KDIGO 2018 clinical practice guideline for the prevention, diagnosis, evaluation, and treatment of hepatitis C in chronic kidney disease. Kidney Int Suppl. 2018;8(3):91-165.
-
➢
Segall L, Covic A. Diagnosis of tuberculosis in dialysis patients: current strategy. Clin J Am Soc Nephrol. 2010;5(6):1114-1122.
-
➢
Terrault N, Lok A, McMahon B, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. https://www.aasld.org/sites/default/files/2019-06/HBVGuidance_Terrault_et_al-2018-Hepatology.pdf. Accessed January 24, 2020.
Standard Precautions: Hand Hygiene and PPE
The CDC recommends that standard precautions be followed in all patient care settings. Hand hygiene and PPE use are considered important components in preventing the spread of infection from patient to patient and protecting health care providers.
Hand Hygiene
Case, cont: Mr J. S. is tolerating in-center hemodialysis well and he is referred to a vascular surgeon for creation of an arteriovenous (AV) fistula. As part of your dialysis facility–wide effort to achieve a bloodstream infection (BSI)-free days target of 100 days or longer, you elect to perform an audit on hand hygiene practices at your facility by observing a team of dialysis staff caring for patients on an afternoon shift.
Question 2: Which of the following situations necessitates appropriate hand hygiene?
-
a)
Before collecting a box of clean gloves from storage
-
b)
After reviewing a patient chart at the nursing station
-
c)
Before placing chest pads during cardiac resuscitation of a patient
-
d)
After using chairside computers for charting
Question 3: Which of the following scenarios is appropriate for use of alcohol-based hand rubs (ABHRs)?
-
a)
After touching a patient with a recent diagnosis of Clostridium difficile colitis
-
b)
When a few drops of thin fluid are visible over your hands
-
c)
Before visiting the next patient to answer a machine alarm
-
d)
Immediately after wiping a blood spill on the floor
For the answer s to the questions, see the following text.
Hand hygiene is deemed a critical step in the prevention of infection spread in health care settings. The term “hand hygiene” refers to both handwashing with either plain (ie, nonantimicrobial) soap and water or antiseptic-containing soap and water and use of ABHRs. ABHRs are effective in reducing microorganisms on the hands of health care personnel, are readily accessible and easier to use, and have been demonstrated to cause less skin irritation than handwashing with soap and water.
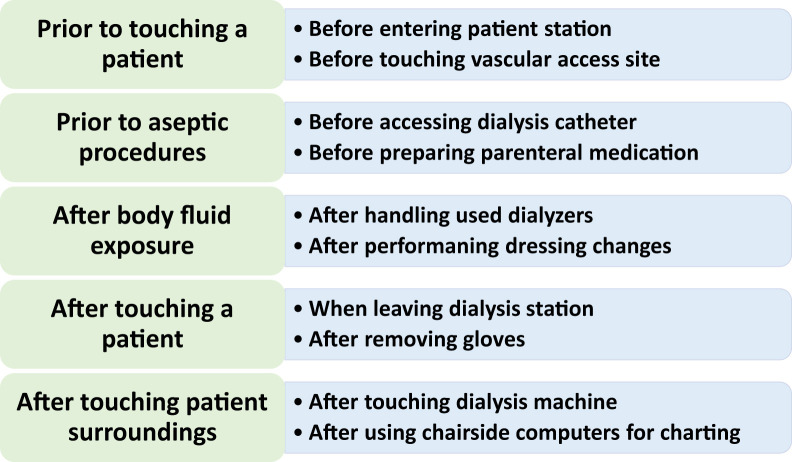
There are several important opportunities for hand hygiene at hemodialysis facilities. The World Health Organization (WHO) recommends 5 such opportunities as follows (see Fig 1 and additional readings for specific examples):
-
1.
Before touching a patient
-
2.
Before clean/aseptic procedure
-
3.
After body fluid exposure
-
4.
After touching a patient
-
5.
After touching patient surroundings
Figure 1.
Recommended situations for hand hygiene at dialysis. Adapted from the World Health Organization and Centers for Disease Control and Prevention documents; additional examples specific to dialysis facilities are available from these sources.
In regard to question 2, the correct answer is option (d). Hand hygiene is required after using chairside computers for charting because a chairside computer is considered part of the patient surroundings. Although hand hygiene is always encouraged, particularly in health care settings, it is not necessary for the other scenarios described. Dialysis staff use chairside computers multiple times during any single hemodialysis treatment to review dialysis orders, enter notes, and otherwise update documentation. More importantly, dialysis staff often care for multiple patients during a dialysis shift. Therefore, it is important they adhere to hand hygiene procedures in between patients to minimize the risk for infection spread.
According to the CDC and WHO recommendations, the preferred hand hygiene method is use of ABHRs with the following exceptions when hand washing with soap and water is required: (1) when hands are visibly dirty or soiled with blood or other body fluids, and (2) if potential spore-forming pathogens exposure (eg, C difficile, which is highly resistant to being killed by ABHRs) is strongly suspected or proven. Therefore, for question 3, the correct answer is option (c). For the other scenarios, hand washing with soap and water are required based on the mentioned CDC recommendations.
Hand hygiene is indicated multiple times during hemodialysis treatments at any dialysis facility. It is critical that each dialysis facility is equipped with a sufficient number of easily accessible ABHR dispensers and clearly identified hand washing sinks along with soap, water, and appropriate drying methods.
As part of overall infection control measures, regular monitoring of appropriate hand hygiene practices is central to successful outcomes at any dialysis facility. All dialysis staff, including physicians, should be regularly observed and personnel provided with timely and helpful feedback regarding their performance. It is also important to educate dialysis patients on proper hand hygiene procedures. There is also opportunity for dialysis facilities to engage their patients in observation of hand hygiene practices and create a culture in which everyone, including patients, is encouraged to speak up and contribute to the upkeeping of dialysis safety.
A number of helpful Audit Tools, including a hand hygiene tool for use at hemodialysis facilities, are available at the links provided in the additional readings.
Additional Readings
-
➢
Centers for Disease Control and Prevention. Audit tool: hemodialysis hand hygiene observations. https://www.cdc.gov/dialysis/PDFs/collaborative/Hemodialysis-Hand-Hygiene-Observations.pdf. Accessed July 25, 2018.
-
➢
Centers for Disease Control and Prevention. FAQs for clinicians about C. diff. https://www.cdc.gov/cdiff/clinicians/faq.html. Accessed January 17, 2020.
-
➢
NHSN. Hand Hygiene. NHSN prevention process measures in outpatient dialysis facilities. https://www.cdc.gov/nhsn/pdfs/training/dialysis/ppm-op-dialysis-facilities.pdf. Accessed January 16, 2020.
-
➢
The Joint Commission. Hand hygiene. https://www.jointcommission.org/resources/patient-safety-topics/infection-prevention-and-control/hand-hygiene/. Accessed January 16, 2020.
-
➢
WHO Guidelines on hand hygiene in health care. https://www.who.int/gpsc/5may/background/5moments/en/. Accessed January 16, 2020. Note: this guide is available as a download via a link near the bottom of the webpage.
Personal Protective Equipment
Case, cont: At the start of Mr J. S.’s third outpatient dialysis treatment, his patient care technician (PCT) notices a light brown stain over the lower portion of his disposable gown. Soon the PCT realizes that Mr J. S.’s left leg dressing is wet with brown drainage.
Question 4: Which of the following is the most appropriate next step the PCT should follow?
-
a)
The gloves are clean, therefore proceed with accessing the catheter without delay
-
b)
Cover the left leg region with a large sterile gauze, then access catheter
-
c)
Replace gloves, then proceed with catheter access
-
d)
Replace gloves and gowns immediately
For the answer to the question, see the following text.
During hemodialysis procedures, potential exposure to blood and contaminated articles can be routinely anticipated. PPE refers to specialized apparel or equipment worn for protection against contamination of infectious materials. When choosing PPE, it is important to address the type of potential exposure from the patient interaction, the suitability of the PPE for the task, and likely modes of pathogen transmission. The main PPE components include gloves, gown, and face protection articles (masks, goggles, and face shields). It is important that dialysis staff replace PPE immediately if it becomes soiled with blood, body fluids, secretions, or excretions. Thus, for question 4, the correct answer is option (d). Because the PCT’s gown is visibly soiled, the gown needs to be replaced along with a new pair of clean gloves. Importantly, appropriate hand hygiene must be performed between replacing gloves and gown. Dialysis facilities should make sure that PPE in multiple sizes are readily available for use by staff, patients, and visitors, when indicated.
At the dialysis facilities, dedicated PPE use is required when dialysis staff come in contact with patients who pose higher risk for spreading infectious organisms to other patients and staff. For example, HBsAg-positive patients, those with open skin wounds with drainage that is not contained by dressings, and patients with uncontrolled diarrhea with or without incontinence and suspected C difficile infection.
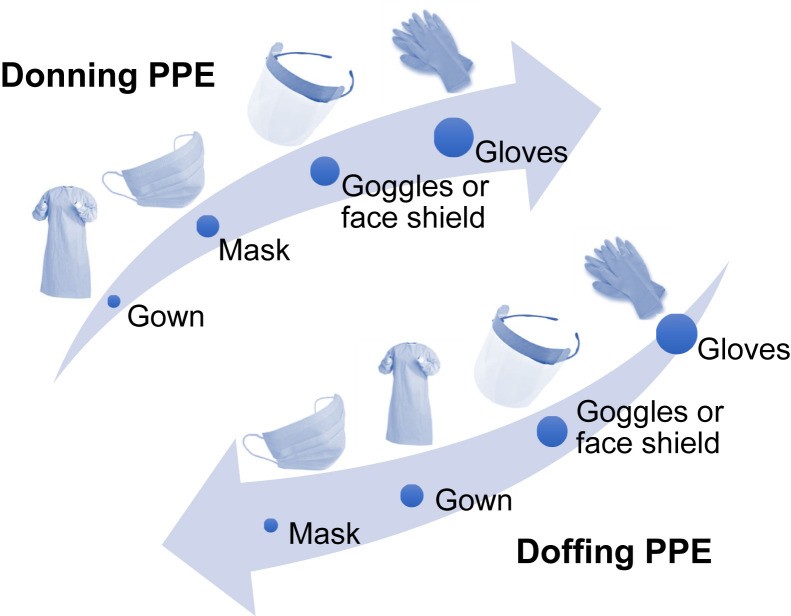
Adherence to correct sequencing of donning (putting on) and doffing (taking off) of PPE is important for successful PPE use at dialysis facilities (Fig 2 ).
Figure 2.
Sequences for donning and doffing personal protective equipment (PPE). Adapted from a Centers for Disease Control and Prevention document: https://www.cdc.gov/HAI/pdfs/ppe/ppeposter148.pdf.
Currently, dialysis facilities in the United States have discretion in selecting appropriate resources to help develop and implement their hand hygiene infection control and prevention programs. It is important for individual facilities to establish practice standards based on their unique circumstances. An example of hand hygiene and PPE use practices implemented at a dialysis facility is provided in Box 1 .
Box 1. Sample Hand Hygiene and PPE Use Practices for a Dialysis Clinic.
-
1.Before entering patient areas
-
•Stop and perform hand hygiene per the dialysis facility policy
-
•Wear appropriate PPE
-
•Wipe down stethoscope with alcohol pad/wipes
-
•
-
2.If touching patient, dialysis machine, chairside keyboard, or patient chair, undertake the following steps:
-
•Before touching a patient or any item
-
⋄Wear gloves, mask, and/or face protection, if appropriate
-
⋄
-
•After touching a patient or any item
-
⋄Remove gloves and discard appropriately
-
⋄Wash hands or use hand sanitizer to clean hands
-
⋄Wipe down stethoscope, if used, with alcohol pad/wipes
-
⋄
-
•
-
3.Before leaving the dialysis facility
-
•Remove and discard all PPE
-
•Wipe down stethoscope with alcohol pad/wipes
-
•Wash hands per policy
-
•
Abbreviation: PPE, personal protective equipment.
Dialysis staff, including physicians, are encouraged to avoid carrying any personal items to patient care areas. If the cell phone or pager needs to be answered in patient care areas, these articles need to be promptly cleaned using appropriate facility procedures.
Additional Readings
-
➢
Centers for Disease Control and Prevention. Infection prevention in dialysis settings. https://www.cdc.gov/dialysis/clinician/ce/infection-prevent-outpatient-hemo.html. Accessed October 10, 2018.
-
➢
Centers for Medicare & Medicaid Services, Department of Health and Human Services. Conditions for Coverage for End-Stage Renal Disease Facilities; Final Rule. Fed Regist. 2008;73:20369-20484.
-
➢
Patel PR, Brinsley-Rainisch K. The Making Dialysis Safer for Patients Coalition: a new partnership to prevent hemodialysis-related infections. Clin J Am Soc Nephrol. 2018;13(1):175-181.
-
➢
Pyrek KM. Mobile technology disinfection: contaminated devices pose threat to patients. Infection Control Today. February 17, 2017. https://www.infectioncontroltoday.com/transmission-prevention/mobile-technology-disinfection-contaminated-devices-pose-threat-patients. Accessed January 16, 2020.
-
➢
Shimokura G, Weber DJ, Miller WC. Factors associated with personal protection equipment use and hand hygiene among hemodialysis staff. Am J Infect Control. 2006;34(3):100-107.
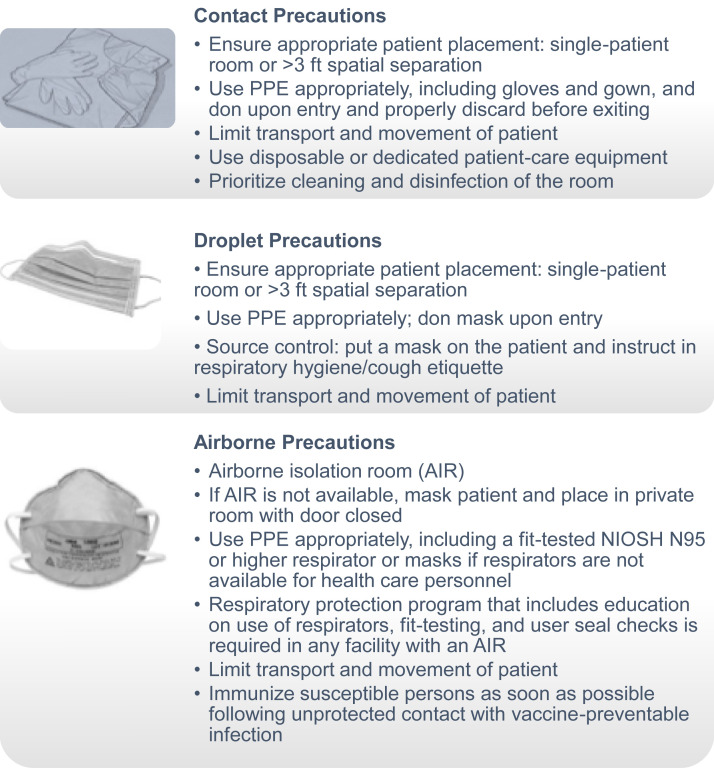
Transmission-Based Precautions: Contact, Droplet, and Airborne Precautions
In addition to standard precautions, additional measures, referred to as transmission-based precautions, should be undertaken to prevent transmission of infections when interacting with patients who may be infected or colonized with certain infectious agents. In these patients, standard precautions alone are considered ineffective in completely interrupting routes of transmission of infectious agents. The 3 categories of transmission-based precautions include contact precautions, droplet precautions, and airborne precautions (Fig 3 ). It is important to point out that these transmission-based precautions should not be used alone but rather in addition to standard precautions.
Figure 3.
Transmission-based precautions: contact, droplet, and airborne precautions. Abbreviations: NIOSH, National Institute for Occupational Safety and Health; PPE, personal protective equipment. Adapted from https://www.cdc.gov/infectioncontrol/basics/transmission-based-precautions.html.
Contact precautions are recommended when there is a high likelihood of spread of infectious agents either by direct or indirect contact with the patient or the patient’s environment. The following situations would require contact precautions: a patient infected or colonized with a multidrug-resistant organism, an open wound with excessive drainage, and fecal incontinence or other body discharges that could potentially contaminate the environment and cause transmission.
Droplet precautions are recommended for prevention of microorganism spread by closed respiratory or mucous membrane contact with respiratory secretions. Additional ventilation and air handling precautions are generally not required for these organisms (eg, influenza virus, adenovirus, rhinovirus, Neisseria meningitides, and Bordetella pertussis) because they do not remain infectious over long distances. Masks are worn at all times in these conditions.
Airborne precautions are advocated for the prevention of microorganism spread when they are presumed to remain infectious over long distances when suspended in the air (eg, varicella virus, rubeola virus, Mycobacterium tuberculosis, and SARS [severe acute respiratory syndrome]). When airborne precautions are indicated, patients should be placed in a room, referred to as an airborne isolation room (AIR), which is furnished with special air handling and ventilation capabilities. Typically, outpatient dialysis facilities in the United States rarely include AIRs. Therefore, if a maintenance dialysis patient is suspected to have an active tuberculosis infection or any of the other listed infections, the alternate precautions should be undertaken until the patient can be safely transferred to a facility with an AIR (eg, hospital). These alternate precautions include placing a mask on the patient, moving them to a private room with the door kept closed, and providing health care personnel with respirators or masks rated as N95 (filtering at least 95% of airborne particles) or greater.
Additional Readings
-
➢
Centers for Disease Control and Prevention. Transmission-Based Precautions. https://www.cdc.gov/infectioncontrol/basics/transmission-based-precautions.html. Accessed July 27, 2018.
-
➢
Siegel JD, Rhinehart E, Jackson M, Chiarello L, and the Healthcare Infection Control Practices Advisory Committee. 2007 Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Healthcare Settings. https://www.cdc.gov/infectioncontrol/guidelines/isolation/index.html. Accessed July 28, 2019.
Environmental Cleaning and Disinfection
Dialysis facilities are already at heightened risk for transmission of pathogenic microorganism(s) due to the intensive nature of hemodialysis treatments, in which patient blood is circulated in an extracorporeal circuit for up to 4 hours per treatment. Additional factors might pose further risks for pathogen transmission at dialysis facilities, including but not limited to the following: close proximity of several hemodialysis patients in an open area without separation by walls or other boundaries, typical 1:4 dialysis technician to patient ratio, and swift changing of patients between dialysis shifts that does not allow adequate time for cleaning and disinfection. Any items (dialysis machine, chair, television, adjacent tables or work surfaces, intravenous pole, clip boards, clamps, jugs, etc) that are in close proximity to the patient or in the designated treatment areas warrant disinfection. In the interval between the completion of a dialysis treatment and completion of the set up for the next patient, it is critical to ensure that no patients occupy that station. Strict adherence to proper cleaning and disinfection of environmental surfaces is important and sufficient time should be allocated for these procedures.
According to CDC guidelines, there are 2 levels of disinfection needed at outpatient dialysis facilities, low- and intermediate-level disinfections. Low-level disinfection with use of an Environmental Protection Agency (EPA)-registered disinfectant (a list of products is available from the EPA website and periodically updated to reflect revisions such as label changes, withdrawals, and transfers of product registrations) would suffice for the following: common and noncritical environmental surfaces, including dialysis chair, exterior surfaces of hemodialysis machines; water treatment and distribution systems of dialysis fluid concentrates and hemostats, clamps, scissors, blood pressure cuffs, and stethoscopes, etc without visible blood.
In contrast, when visible blood is present in the treatment area and other areas including the bathroom and waiting room, intermediate-level disinfection, which is a 2-step process, is recommended. First, visible blood should be removed immediately and effectively with use of a cloth soaked in tuberculocidal disinfectant or 1:100 dilution of a hypochlorite solution (500–600 ppm free chlorine). Next, a second application of disinfectant with use of a new towel or cloth is indicated. In addition, the intermediate-level disinfection is also required for the following: interior pathways of hemodialysis machine, hemodialyzer port caps, and the presence of significant biofilm within water treatment and distribution systems.
Disinfection of a hemodialysis machine’s internal hydraulic pathways is often incorporated into the design of dialysis machines and must be performed at the end of each day. Typically, there are 2 options; heat disinfection and chemical disinfection. Heat disinfection is preferred for machines amenable to this disinfection technique because it is less labor intensive and residual chemical disinfectant hazard will not be a concern. More frequent (ie, in between patients’ dialysis treatments) disinfection of hemodialysis machine internal pathways is necessary if dialyzer blood leaks occur.
Additional Readings
-
➢
Centers for Disease Control and Prevention. Environmental surface disinfection in dialysis facilities: notes for clinical managers. https://www.cdc.gov/dialysis/PDFs/collaborative/Env_notes_508.pdf. Accessed August 8, 2018.
-
➢
WA Rutala, DJ Weber, and the Healthcare Infection Control Practices Advisory Committee. Guideline for disinfection and sterilization in healthcare facilities 2008. https://www.cdc.gov/infectioncontrol/guidelines/disinfection/index.html. Accessed August 9, 2018.
-
➢
Centers for Disease Control and Prevention. Guidelines for environmental infection control in health-care facilities. MMWR Recomm Rep. 2003;52(RR10):1-42.
-
➢
Environmental Protection Agency. Selected EPA-registered disinfectants. (October 22, 2012). http://www.epa.gov/oppad001/chemregindex.htm. Accessed August 9, 2018.
Infection Control Measures for Common Viral Infections
Influenza
Influenza leads to hospital admissions and deaths across the world and dialysis patients are more susceptible to contracting influenza and experiencing complications. Influenza virus is spread by droplets and in some cases by fomites, so patient-to-patient transmission in the dialysis unit is possible. If a dialysis patient has influenza, a surgical mask should be worn by the patient to prevent droplet transmission. In addition, it is important to adhere to hand hygiene and to cleaning of environmental surfaces with a virucidal agent. Physical separation of at least 6 feet of the infected patient from other patients is also recommended. Patients with influenza symptoms should be treated with oseltamivir and the recommended dosing is a 30 mg initial dose followed by 30 mg at every dialysis session for 5 days.
If there is concern for an influenza outbreak in the dialysis unit, all patients and staff should wear surgical masks and there should be enhanced emphasis on hand hygiene and environmental cleaning. Prophylactic oseltamivir is also recommended for exposed hemodialysis patients with an initial dose of 30 mg, followed by 30 mg during each dialysis session for a total of 7 days.
Herpes Zoster Infection
Herpes zoster virus infection, commonly known as “shingles,” is particularly common among adults older than 50 years and is highly contagious. Standard infection-control precautions should be followed in all cases of herpes zoster infection. In addition, airborne (often in a hospital) and contact precautions are needed when disseminated zoster (defined as “appearance of lesions in >3 dermatomes”) is suspected or the patient is immunocompromised. Antiviral agents have been shown to decrease viral shedding and lower the duration of herpes zoster lesions.
Additional Readings
-
➢
Centers for Disease Control and Prevention. Influenza antiviral medications: summary for clinicians 2018. https://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm. Accessed January 21, 2020.
-
➢
Centers for Disease Control and Prevention. Influenza vaccination information for health care workers. https://www.cdc.gov/flu/professionals/healthcareworkers.htm. Accessed January 21, 2020.
-
➢
Centers for Disease Control and Prevention. Preventing varicella-zoster virus (VZV) transmission from zoster in healthcare settings. https://www.cdc.gov/shingles/hcp/hc-settings.html. Accessed January 17, 2020.
-
➢
Centers for Disease Control and Prevention. Shingles (Herpes Zoster). Vaccination. https://www.cdc.gov/shingles/vaccination.html. Accessed January 17, 2020.
Water Treatment in the Outpatient Hemodialysis Unit
Question 5: What is the frequency of microbial testing of dialysis water?
-
a)
Daily
-
b)
Weekly
-
c)
Monthly
-
d)
Quarterly
For the answer to the question, see the following text.
Because dialysis patients are exposed to a large volume of water during treatment, water quality assurance in a dialysis unit is of utmost importance. In the short term, exposure to high levels of bacteria and endotoxin is associated with complications ranging from pyrogenic reactions such as chills and fever to septicemia with severe hypotension and shock.
The 2008 End-Stage Renal Disease Conditions for Coverage reference standards from the Association of the Advancement of Medical Instrumentation (AAMI) regarding the safety of hemodialysis water define action levels and maximum allowable levels for contamination. These standards are summarized in Table 3 .
Table 3.
Testing Thresholds for Microbial Contaminants
| Contaminant | Maximum Allowable Level | Typical Action Level |
|---|---|---|
| Bacteria water and dialysate | <200 CFU/mL | 50 CFU/mL |
| Endotoxin water and dialysate | <2 EU/mL | 1 EU/mL |
Abbreviations: EU, endotoxin unit; CFU, colony-forming unit
Information based on American National Standards Institute/Association of the Advancement of Medical Instrumentation RD52:2004 and current Centers for Medicare & Medicaid Services standards.
If the bacterial or endotoxin levels are in excess of the typical action level, the medical director should be notified so that immediate steps can be taken, including deciding whether it is safe to continue dialyzing patients. Subsequently, the medical director must determine additional disinfection measures to bring the water quality within AAMI standards.
Cultures and endotoxin analysis of water and dialysate should be performed monthly as a proactive strategy; thus, the correct answer to question 5 is option (c). The results of these tests should be reviewed by the medical director and water engineer to ensure that the disinfection schedule and process are successfully controlling bacteria and endotoxin.
Additional Readings
-
➢
ANSI/AAMI RD52:2004. Dialysate For Hemodialysis . Arlington, VA: Association for the Advancement of Medical Instrumentation; 2004.
-
➢
Centers for Medicare & Medicaid Services, Department of Health and Human Services. Medicare and Medicaid programs: conditions for coverage for end-stage renal disease facilities; Final Rule. Fed Regist. 2008;73:20369-20484.
-
➢
Kasparek T, Rodriguez OE. What medical directors need to know about dialysis facility water management. Clin J Am Soc Nephrol. 2015;10(6):1061-1071. ★ ESSENTIAL READING
Reducing Vascular Access–Related BSIs in Hemodialysis Patients
Case, cont: You are seeing Mr J. S. during your monthly hemodialysis rounds. He has been referred to vascular surgery for an AV fistula creation but has not been evaluated yet. He still has a right internal jugular tunneled hemodialysis catheter for access. He has read that hemodialysis catheters are associated with a high risk for infection and asks you the following question.
Question 6: What of the following measures in the dialysis unit can reduce chances of a patient acquiring a bloodstream infection?
-
a)
Hand hygiene observation of clinical staff
-
b)
Vascular access care observation of clinical staff
-
c)
Using alcohol-based chlorhexidine at the exit site as antiseptic during dressing changes
-
d)
Catheter hub disinfection with an antiseptic when the catheter is accessed or disconnected
-
e)
All of the above
For the answer to the question, see the following text.
BSIs remain a major cause of morbidity and mortality in hemodialysis patients and the use of a tunneled catheter as the vascular access has an 8.5 times higher risk for BSI compared to an AV fistula. Therefore, it is alarming that in the United States, 80% of patients start with a catheter and even 90 days after initiation of hemodialysis, 68.5% are still using the catheter as their access despite national initiatives to decrease the rate of catheter use.
In addition to decreasing the use of catheters, a number of other measures can reduce the risk for BSI. After dialysis staff perform hand hygiene, the exit site should be examined for any signs of infection. Proper exit-site care includes disinfection of the exit site with alcohol-based chlorhexidine (>0.5% solution) for at least 60 seconds. Acceptable alternatives are 10% povidone iodine solution used for 2 to 3 minutes or 70% alcohol. Moreover, if a gauze bandage is applied over the exit site, povidone iodine or a triple antibiotic ointment should be used. Alternatively, a chlorhexidine disk [Biopatch (Johnson & Johnson)] with a Tegaderm (3M) dressing can be used and must be changed weekly.
Before connecting the patient’s catheter to the machine, the catheter should be placed on a nonsterile pad and the catheter cap should be removed after clamping the tubing. The dialysis staff should perform hand hygiene and wear gloves and a mask while handling the catheter. Before the connection of the catheter, the hub should be scrubbed with either chlorhexidine or a 70% alcohol pad for at least 15 seconds. This process of “scrub the hub” should be repeated at the time of disconnection.
In patients with a permanent AV access like an AV fistula or graft, the AV access should always be washed with soap and water and examined for signs of infection. After performing hand hygiene and wearing gloves, the dialysis staff should disinfect the access in one of the following ways (Fig 1):
-
1.
Alcohol-based chlorhexidine (>0.5%)
-
2.
10% povidone iodine solution
-
3.
70% alcohol
After disinfection, the AV access can be cannulated using standard precautions. Before decannulation of the AV access, dialysis staff should perform hand hygiene and then place a nonsterile gauze pad over the needle and pressure should be applied at the insertion site after the needle has been removed.
Thus, the correct answer to question 6 is option (e) because hand hygiene, vascular access observation, disinfection of the exit site, and catheter hub disinfection have all been shown to decrease the risk for BSIs.
The CDC Dialysis BSI Prevention Collaborative has audit tools regarding the steps for appropriate exit-site care and connection and disconnection of catheters and AV access cannulations, which are included in additional reading suggestions.
Additional Readings
-
➢
CDC Dialysis Collaborative. Audit Tool: Arteriovenous fistula/graft cannulation observations. https://www.cdc.gov/dialysis/PDFs/collaborative/AV-Fistula-Graft-Can-Decannulation-Observations-AT.pdf. Accessed July 15, 2019.
-
➢
CDC Dialysis Collaborative. Audit Tool: Catheter connection and disconnection observations. https://www.cdc.gov/dialysis/PDFs/collaborative/Catheter-Connection-Disconnection-Observations.pdf. Accessed July 15, 2019.
-
➢
CDC Dialysis Collaborative. Audit Tool: Catheter exit site care observations. https://www.cdc.gov/dialysis/PDFs/collaborative/Catheter-Exit-Site-Care-Observations.pdf. Accessed July 15, 2019.
-
➢
Centers for Disease Control and Prevention. Preventing Bloodstream Infections in Outpatient Hemodialysis Patients. http://youtu.be/_0zhY0JMGCA. Accessed July 15, 2019. ★ ESSENTIAL READING
-
➢
Centers for Disease Control and Prevention. Guidelines for the Prevention of Intravascular Catheter Related-Infections, 2011. https://www.cdc.gov/infectioncontrol/guidelines/bsi/index.html. Accessed July 15, 2019.
-
➢
Patel P, Kallen AJ. Bloodstream infection prevention in ESRD: forging a pathway for success. Am J Kidney Dis. 2014;63(2):180-182. ★ ESSENTIAL READING
-
➢
Patel PR, Yi SH, Booth S, et al. Bloodstream infection rates in outpatient hemodialysis facilities participating in a collaborative prevention effort: a quality improvement report. Am J Kidney Dis. 2013;62(2):322-330.
-
➢
Taylor D, Gravel D, Johnston L, et al. Incidence of bloodstream infection in multicenter inception cohorts of hemodialysis patients. Am J Infect Control. 2004;32(3):155-160.
-
➢
Yi SH, Kallen AJ, Hess S, et al. Sustained infection reduction in outpatient hemodialysis centers participating in a collaborative bloodstream infection prevention effort. Infect Control Hosp Epidemiol. 2016;37(7):863-866.
Quality Assessment and Program Improvement
Case, cont: Shortly after your conversation with Mr J. S. about his concerns regarding catheter use, you are reviewing data from the dialysis unit in your capacity as Medical Director. You notice that the rate of catheter use in your dialysis unit has increased to 30% from 25% last year.
Question 7: What would be the next best step to address this problem?
-
a)
Refer all patients with a catheter for AV access evaluation
-
b)
Start cannulating fistulas early at 4 weeks after fistula creation
-
c)
Perform a root cause analysis for why the catheter rates are high
-
d)
Refer all patients with catheters for a peritoneal dialysis evaluation
For the answer to the question, see the following text.
An important aspect of nurturing a culture of quality and safety at dialysis facilities is to develop and maintain an effective Quality Assessment and Performance Improvement (QAPI) process. An interdisciplinary team approach involving social workers, dieticians, PCTs, nurses, attending physicians, and the regional quality manager should be undertaken. The Medical Director’s active leadership role is crucial for the success of an effective QAPI process.
The QAPI process is extremely helpful in developing and evaluating successful infection control programs at dialysis facilities. A systems-thinking approach using a methodology such as Plan-Do-Check-Act has been shown to be highly effective in promoting quality and safety improvement activities toward infection control efforts. First, at the “plan” stage, it is important to recognize the problem, brainstorm ideas, determine root cause(s), develop a simple a plan for change, designate personnel and resources, and decide on a timeline. Second, at the “do” stage, collect relevant data and document findings. At the next “check” stage, examine the findings, reassess the problem, determine whether the process is working, and document progress. At the final “act” step, if the goals are met, adopt the process and consider expanding to other areas of deficiencies. If results are suboptimal, then repeat Plan-Do-Check-Act and consider a new plan.
To illustrate the QAPI process, in the rest of this section we describe a project led by a nephrology fellow under the supervision of an attending physician. The QAPI team from an outpatient dialysis facility noted a slow increase in the number of BSIs over a 3-month period. Therefore, at a QAPI program meeting, the team decided to develop a project to reduce the number of infections with the overall goal of achieving “zero infection rate” at their facility.
First, the QAPI team identified a lower adherence rate to appropriate hand hygiene practices at their facility. An initial root cause analysis performed by the team revealed a possible lack of understanding by dialysis patients regarding the importance of hand hygiene and the minimum duration recommended for hand washing. Then a simple plan to improve hand hygiene was developed and appropriate personnel to lead the project were selected: 1 second-year nephrology fellow during his or her outpatient dialysis rotation, 1 attending physician, and 2 nurses.
Twenty hemodialysis patients agreed to take part. To simulate germ exposure, they were asked to apply Glo Germ (Glo Germ Company) powder, a product that glows under UV light, evenly to both hands. Then they were observed during their routine hand washing; the time spent per patient during each hand washing session was recorded. After hand washing, the nephrology fellow then scored on a scale of 0 to 4 the extent of residual illumination (under UV light) left on the patients’ hands. The patients then received a 5-minute educational session on proper hand washing techniques, with particular emphasis on appropriate duration of hand washing, deep hand rubbing, and coverage of finger wedges.
One week later, leading a team, the nephrology fellow scored a second hand washing session. There was a statistically significant improvement in both hand stain score (ie, cleaner hands) and time spent with hand washing. Many of the patients enjoyed using the Glo Germ product and were eager to show what they had learned. These findings were reported back to the entire QAPI team and the findings were also shared at the ASN Kidney News Fellows Corner. Based on these findings, the educational procedure was expanded to the entire facility involving all dialysis staff.
As illustrated in the example, the first step in a QAPI process should be to determine the root cause of the problem. Thus, the correct answer to question 7 is option (c).
Additional Reading
-
➢
Chu C. How to make handwashing a fun experience for patients in the dialysis unit. ASN Kidney News. 2017;9(5):11-12.
Conclusion
In-center hemodialysis patients are at an increased risk for infections and one of the major contributing factors is spread of infections in the hemodialysis units from other patients, as well as health care providers. Stringent infection-control measures including screening for infections, vaccinations, and hand hygiene, and appropriate isolation of patients should be implemented in dialysis units. Although the ultimate responsibility lies with the medical director, every member of the dialysis team should strive to make the dialysis unit a safer place for the patients. A QAPI program ensures that there is a process to identify gaps in the care of our patients so that these issues can be addressed in a timely fashion.
Article Information
Authors’ Full Names and Academic Degrees
Sana Waheed, MD, and Marie Philipneri, MD, PhD.
Acknowledgements
The authors acknowledge the members of the Nephrologist Transforming Dialysis Safety Project for their contribution to the development of this curriculum.
Support
None.
Financial Disclosure
Dr Philipneri serves on the medical advisory board for Baxter. Dr Waheed declares that she has no relevant financial interests.
Peer Review
Received September 2, 2019, in response to an invitation from the journal. Evaluated by 2 external peer reviewers and a member of the Feature Advisory Board, with direct editorial input from the Feature Editor and a Deputy Editor. Accepted in revised form February 25, 2020.
Footnotes
Complete author and article information provided at end of article.