There is tremendous concern in the liver transplant (LT) community about the coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Limited data raise questions regarding risk and severity, management of immunosuppression, and hepatic injury related to COVID-19. The state of New York, specifically New York City, was previously the first US epicenter of the pandemic. The Mount Sinai Hospital is a tertiary care academic medical center that supports the Recanati/Miller Transplantation Institute. Here, we describe our initial experience with COVID-19 in LT recipients.
Methods
Additional details are provided in the Supplementary Methods. A retrospective analysis of electronic medical records was performed of LT recipients diagnosed with COVID-19 (confirmed by positive SARS-CoV-2 testing result) from March 18, 2020, to April 13, 2020. The severity of COVID-19 for hospitalized patients was categorized as mild (oxygen saturation ≥94% on room air and no radiographic evidence of pneumonia), moderate (oxygen saturation <94% or radiographic evidence of pneumonia), or severe (advanced oxygen delivery device use); severity was determined by the worst experienced during hospitalization.
Results
Of 38 LT recipients with COVID-19, the first case was diagnosed on March 18. Demographic characteristics, including presenting symptoms, are reported in Supplementary Table 1. Gastrointestinal symptoms (diarrhea, abdominal pain, or nausea/vomiting) were reported in 42%, and 71% were hospitalized, with a median time to admission from symptom onset of 7 days (range, 0–30 d). Three patients were diagnosed while already hospitalized, with a median stay of 33 days (range, 19–49 d) before diagnosis. Hospitalized patients were older (65 vs 39 y; P = .02) and had at least 1 comorbid condition (66% vs 18%; P = .047) compared to nonhospitalized patients. Of the 38, most were taking a tacrolimus-based regimen (3% cyclosporine); 50% took concomitant mycophenolic acid therapy. Fifteen patients (39%) were taking corticosteroid therapy; 13 patients were taking low-dose therapy, and 2 were taking higher doses for treatment of recent allograft rejection and immune thrombocytopenic purpura, respectively.
The severity of COVID-19 was assessed in 24 of 27 hospitalized patients (3 hospitalized at outside medical centers were excluded). Of the 24, 8% had mild disease, 46% had moderate disease, and 46% had severe disease. Most hospitalized patients had medical comorbidities (92%), and 54% presented with acute kidney injury (AKI). Serum cytokine profiles were elevated but without differences across severity, and 92% had radiographic evidence of pneumonia (Table 1 ).
Table 1.
Clinical Characteristics of COVID-19 in Hospitalized LT Recipientsa
| Variables | Severity of COVID-19 for hospitalized patients |
|||
|---|---|---|---|---|
| Overall (N = 24a) | Mild to moderate (n = 13, 54%) | Severe (n = 11, 46%) | P value | |
| Age, y | 66 (30–80) | 65 (30–80) | 66 (39–79) | .62 |
| Age ≥65 y, n (%) | 14 (58) | 8 (62) | 6 (55) | 1 |
| Body mass index, kg/m2, median (range) | 28.4 (18.7–46.6) | 26.8 (18.7–46.6) | 31.9 (24.3–35.7) | .25 |
| Obesity, BMI ≥ 30 kg/m2, n (%) | 10 (42) | 4 (31) | 6 (55) | .41 |
| Comorbidities, at least 1, n (%) | 22 (92) | 12 (92) | 10 (91) | 1 |
| Hypertension | 17 (71) | 7 (54) | 10 (91) | .08 |
| Diabetes mellitus | 12 (50) | 6 (46) | 6 (55) | 1 |
| Cardiovascular disease | 10 (42) | 5 (38) | 5 (45) | 1 |
| Chronic kidney disease | 17 (71) | 11 (85) | 6 (55) | .18 |
| Time from LT, y, median (range) | 4.7 (0.02–28.2) | 10.6 (0.1–27.2) | 3.6 (0.02–28.2) | .23 |
| Days since symptom onset to hospital, median (range) | 7 (1–30) | 7 (1–21) | 7 (1–30) | .74 |
| Imaging findings of COVID-19, n (%) | 22 (92) | 11 (85) | 11 (100) | .48 |
| Laboratory assessment on admission | ||||
| Alkaline phosphatase, U/L, median (range) | 131 (48–1302) | 159 (49–915) | 79 (48–1302) | .35 |
| Total bilirubin, mg/dL, median (range) | 0.7 (0.2–4.5) | 0.6 (0.2–3.9) | 1.0 (0.4–4.5) | .20 |
| AST, U/L, median (range) | 31 (10–1691) | 32 (10–1691) | 29 (12–255) | .91 |
| ALT, U/L, median (range) | 22 (5–1578) | 24 (5–1578) | 19 (5–199) | .58 |
| Albumin, g/dL, median (range) | 3.2 (1.7–4.3) | 3.1 (1.7–4.3) | 3.3 (2.2–4.3) | .77 |
| INR, median (range) | 1.1 (1.0–1.9) | 1.1 (1.0–1.2) | 1.2 (1.0–1.9) | .06 |
| Lymphocyte count, ×103/μL, median (range) | 0.6 (0.2–5.6) | 0.6 (0.2–5.6) | 0.6 (0.2–1.5) | .75 |
| Acute kidney injury, n (%) | 13 (54) | 6 (46) | 8 (73) | .44 |
| Inflammatory variables on admission, median (range) | ||||
| Ferritin, ng/mL | 986 (36–4677) | 871 (71–4677) | 1148 (36–2909) | .69 |
| C-reactive protein, mg/L | 65.9 (6.2–430.3) | 56.4 (6.2–314.0) | 80.7 (26.9–430.3) | .46 |
| Procalcitonin, ng/mL | 0.33 (0.08–36.46) | 0.36 (0.08–11.44) | 0.30 (0.09–36.46) | .73 |
| Lactate dehydrogenase, U/L | 314 (160–889) | 287 (160–702) | 315 (253–889) | .25 |
| D-dimer, μg/mL | 1.67 (0.27–8.62) | 1.63 (0.27–8.53) | 2.50 (0.27–8.62) | .75 |
| Serum cytokine profile, median (range)b | ||||
| Interleukin 6, pg/mL | 66.3 (12.5–218.0) | 45.7 (12.5–162.0) | 71.6 (19.5–218.0) | .25 |
| Interleukin 8, pg/mL | 44 (13.2–100.0) | 42.3 (16.7–88.1) | 47.1 (13.2–100.0) | 1 |
| Interleukin 1β, pg/mL | 0.5 (0.3–1.8) | 0.6 (0.3–1.8) | 0.5 (0.3–0.8) | .32 |
| Tumor necrosis factor α, pg/mL | 33.7 (15.6–111.0) | 41.1 (21.3–74.5) | 29.1 (15.6–111.0) | .28 |
| Therapy provided, n (%) | ||||
| Supplemental oxygenation | 18 (75) | 7 (54) | 11 (100) | .02 |
| Mechanical ventilation | 8 (33) | 0 | 8 (73) | <.01 |
| Hydroxychloroquine ± azithromycin therapy | 18 (75) | 8 (62) | 10 (91) | .17 |
| Intravenous glucocorticoid therapy | 5 (21) | 0 | 5 (45) | .01 |
| Anticoagulation therapy | 8 (33) | 1 (8) | 7 (64) | .01 |
| Type of immunosuppression before admission, n (%) | ||||
| Tacrolimus | 23 (96) | 13 (100) | 10 (91) | .46 |
| Cyclosporine | 1 (4) | 0 | 1 (9) | .46 |
| Mycophenolic acid | 13 (54) | 6 (46) | 7 (64) | .44 |
| Corticosteroid | 12 (50) | 6 (46) | 6 (55) | 1 |
| Decrease in immunosuppression, n (%) | ||||
| Overall regimen | 19 (79) | 9 (69) | 10 (91) | .33 |
| Calcineurin inhibitorc | 15 (63) | 7 (54) | 8 (73) | .42 |
| Mycophenolic acidc | 13 (100) | 6 (100) | 7 (100) | — |
| Corticosteroidc | 2 (17) | 1 (17) | 1 (17) | 1 |
| Intensive care, n (%) | 8 (33) | 0 | 8 (73) | <.01 |
| Discharged, n (%) | 14 (58) | 12 (92) | 2 (18) | <.01 |
| Length of stay,bd, median (range) | 9 (4–22) | 8 (4–19) | 18 (13–22) | .07 |
| Death, n (%) | 7 (29) | 0 | 7 (64) | <.01 |
| Remain hospitalized, n (%) | 3 (13) | 1 (8) | 2 (18) | .58 |
NOTE. Wilcoxon signed-rank test and Fisher’s test were used to compare samples and proportions as appropriate. All statistical analyses were performed using R 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria). Bolded values indicate P-values less than .05 (for visual purposes).
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; INR, internationalized ratio.
n = 24 (27 patients were hospitalized, 3 at outside medical centers with unavailable data).
n = 19.
Total number derived from the type of immunosuppression taken.
Immunosuppression was decreased in 79% of hospitalized patients (Table 1). Three patients experienced elevations in liver enzymes after immunosuppression reduction; the pattern was hepatocellular, with a range of 2 to 20 times the upper limit of normal. No patients underwent biopsy.
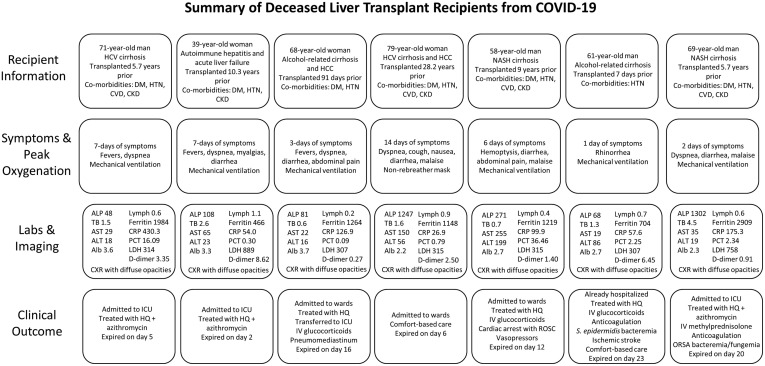
Seven LT recipients died (18% overall, 29% hospitalized) (Supplementary Figure 1). The median time to death from symptom onset was 19 days (range, 9–24 d). All 7 patients had 1 or more comorbidities, and 57% had AKI on admission. The preliminary autopsy results of patient 2 showed dense lung parenchyma, focal left ventricular subendocardial hemorrhage, and pancreatic congestion with hemorrhage.
Supplementary Figure 1.
Summary of the 7 LT recipients with severe COVID-19 resulting in death. Alb, albumin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CKD, chronic kidney disease; CRP, C-reactive protein; CVD, cardiovascular disease; CXR, chest radiograph; DM, diabetes mellitus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HQ, hydroxychloroquine; HTN, hypertension; ICU, intensive care unit; IV, intravenous; Labs, laboratory tests; LDH, lactate dehydrogenase; NASH, nonalcoholic steatohepatitis; ORSA, oxacillin-resistant Staphylococcus aureus; PCT, procalcitonin; ROSC, return of spontaneous circulation; TB, total bilirubin.
Eight recipients (21%) were infected within 1 year after LT, and the earliest was 7 days after LT. This patient underwent LT before mandatory testing of donors and recipients; the donor subsequently tested negative by stored serum. Three of the 8 recipients had severe COVID-19, of whom 2 died; 1 remains hospitalized in critical condition.
Admission liver tests were relatively normal across severity of COVID-19. Six patients had pre-existing elevated alkaline phosphatase, and only 3 patients presented with aminotransferase elevations >3 times the upper limit of normal.
Discussion
We describe our initial single-center experience of COVID-19 in 38 LT recipients. Gastrointestinal symptoms were common, similar to a report of 90 infected solid organ transplant recipients (including 13 LT, 1 liver-kidney) (42% vs 31%).1 Most LT recipients required hospitalization, and associated factors included older age and presence of comorbidities. The typical hepatocellular pattern of liver test elevations in severe COVID-192 was not common. Most patients had radiographic evidence of pneumonia (92% hospitalized, 100% in severe cases), similar to kidney transplant recipients (96%)3 but greater than rates from the general population in China (59% overall, 77% in severe cases).4 Approximately 33% of hospitalized patients required mechanical ventilation; only 25% survived.
A recent study reported AKI as a possible risk factor for worse outcomes in COVID-19.5 We describe a high proportion of LT recipients presenting with AKI; recipients are at risk of renal failure given the presence of sepsis in the background of calcineurin inhibitor use. Comorbidities after transplant have also been associated with poor outcomes in COVID-19, particularly hypertension.1 We similarly report a high rate of comorbidities, which was associated with hospitalization.
Early experience of COVID-19 in LT recipients from Italy showed mild disease with a 3% mortality rate in long-term LT survivors.6 , 7 In contrast, our study describes 3 severe cases (2 dead, 1 in critical condition) in patients receiving transplants within a year. Additionally, a report of solid organ transplant recipients described a 27.8% overall mortality rate (including 2 of 6 LT).8 Pereira et al1 reported mortality rates similar to our study (18% vs 18% overall and 24% vs 26% hospitalized). These high rates of mortality related to COVID-19 are concerning, suggesting greater risk in allograft recipients.
Although immunosuppression may attenuate the initial inflammatory response, it may increase virologic injury, resulting in higher rates of severe COVID-19 and mortality. Most of our hospitalized patients had immunosuppression decreased similar to the practice of a neighboring center.1 In severe cases, providers can consider decreasing immunosuppression, given risks of bacterial or fungal superinfection.
Limitations of this study include the small sample size and single-center experience. The strengths of our study are in the uniformity of data collected and definitions applied, which may be limited in larger registry studies. Not all LT recipients at our center received testing, therefore, incidence is unknown.
Based on these findings, we recommend a low threshold to test for SARS-CoV-2 in LT recipients. We report high mortality in LT recipients across both early and long-term survivors. AKI and comorbidities were common. The long-term impact of COVID-19 is not well understood but will be monitored to better understand its effect on graft and patient outcomes.
Acknowledgments
Members of the COBE Study Group are as follows: Ben L. Da, Recanati/Miller Transplantation Institute, New York, NY; Robert Mitchell, Recanati/Miller Transplantation Institute, New York, NY; and Saikiran Kilaru, Recanati/Miller Transplantation Institute, New York, NY.
The authors would like to thank Morgan Resta-Flarer for his contribution to data collection.
CRediT Authorship Contributions
Brian T. Lee, MD (Conceptualization: Equal; Data curation: Lead; Formal analysis: Lead; Investigation: Lead; Methodology: Lead; Writing – original draft: Lead; Writing – review & editing: Equal); Ponni V. Perumalswami, MD (Formal analysis: Equal; Writing – review & editing: Lead); Gene Y. Im, MD (Conceptualization: Lead; Formal analysis: Supporting; Investigation: Supporting; Writing – review & editing: Equal); Sander Florman, MD (Writing – review & editing: Supporting); Thomas D. Schiano, MD (Conceptualization: Supporting; Formal analysis: Supporting; Investigation: Supporting; Methodology: Supporting; Supervision: Lead; Writing – review & editing: Supporting).
Footnotes
Conflicts of interest The authors disclose no conflicts.
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at https://doi.org/10.1053/j.gastro.2020.05.050.
Contributor Information
COBE Study Group:
Supplementary Methods
Testing for SARS-CoV-2 at our center used a real-time polymerase chain reaction assay by the Roche (Basel, Switzerland) Cobas 6800 system; all specimens were obtained by a nasopharyngeal swab. All patients tested positive on the initial swab.
Categorization of COVID-19 severity for hospitalized patients was defined by our colleagues in the Division of Infectious Diseases at Mount Sinai. Conventional therapy included supportive care, supplemental oxygen, hydroxychloroquine, and/or azithromycin therapy, unless contraindicated. Advanced oxygen delivery devices included high-flow nasal cannula, non-rebreather, bilevel positive airway pressure, or mechanical ventilation. In severe cases, intravenous glucocorticoid therapy was considered. Toward the peak of the pandemic, therapeutic anticoagulation was started in qualifying patients who did not have obvious contraindications. One patient was enrolled into a clinical trial.
Laboratory values were collected at initial presentation during hospitalization. Elevations in liver test values were defined by an increase in serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels greater than 3 times the upper limit of normal of our laboratory parameters (AST, 35 U/L; ALT, 45 U/L). AKI was defined by the Kidney Disease: Improving Global Outcomes (KDIGO) criteria. No patients had hepatitis C viremia at the time of COVID-19 diagnosis.
At our center, induction therapy after LT consists of intravenous glucocorticoid therapy, even after simultaneous liver-kidney transplantation. Tacrolimus-based immunosuppression is typically used. Data regarding immunosuppression were collected based on the regimen taken immediately before hospitalization. Low-dose corticosteroid use was defined as the use of prednisone at 5–10 mg daily.
Baseline characteristics and laboratory values are described as median (range) or frequency (percentage). Wilcoxon’s signed-rank test and Fisher’s test were used to compare samples and proportions as appropriate. All statistical analyses were performed with R 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria).
Supplementary Table 1.
Demographics and Presenting Symptoms of LT Recipients With COVID-19 (n = 38)
| Characterisitics | Values |
|---|---|
| Age, y, median (range) | 63 (27–81) |
| Male sex, n (%) | 26 (68) |
| BMI, kg/m2, median (range) | 28.3 (18.7–50.0) |
| Ethnicity, n (%) | |
| White | 15 (39) |
| African American | 5 (13) |
| Hispanic | 14 (37) |
| Asian | 2 (5) |
| Other | 2 (5) |
| ABO blood type, n (%) | |
| A | 12 (32) |
| B | 9 (24) |
| AB | 5 (14) |
| O | 11 (30) |
| Residence, n (%) | |
| Manhattan | 3 (9) |
| Brooklyn | 7 (20) |
| Bronx | 4 (11) |
| Queens | 7 (20) |
| Staten Island | 3 (9) |
| Outside New York City | 5 (15) |
| Other state | 6 (17) |
| Indication for liver transplant, n (%) | |
| Alcohol-associated liver disease | 2 (5) |
| Hepatitis C | 16 (42) |
| Hepatitis B | 2 (5) |
| Autoimmune hepatitis | 2 (5) |
| Primary sclerosing cholangitis | 5 (13) |
| Primary biliary cholangitis | 1 (3) |
| Nonalcoholic fatty liver disease | 6 (16) |
| Polycystic liver disease | 2 (5) |
| Hemochromatosis | 1 (3) |
| Drug-induced liver injury | 1 (3) |
| Hepatocellular carcinoma, n (%) | 8 (21) |
| Type of LT, n (%) | |
| Liver alone | 32 (84) |
| Simultaneous liver-kidney | 6 (16) |
| Repeat transplantation | 2 (5) |
| Time from recent transplant, y, median (range) | 3.8 (0.02–28.2) |
| Symptoms, n (%) | |
| Asymptomatica | 2 (5) |
| Fever | 23 (61) |
| Cough | 21 (55) |
| Dyspnea | 13 (34) |
| Myalgias | 9 (24) |
| Malaise | 11 (29) |
| Rhinorrhea | 3 (8) |
| Gastrointestinal | 16 (42) |
| Anosmia | 1 (3) |
| Comorbidities, n (%) | |
| Hypertension | 24 (63) |
| Diabetes mellitus | 18 (47) |
| Cardiovascular disease | 11 (29) |
| Chronic kidney disease | 24 (63) |
| Malignancy | 2 (5) |
| Type of immunosuppression before admission, n (%) | |
| Tacrolimus | 37 (97) |
| Cyclosporine | 1 (3) |
| Everolimus | 1 (3) |
| Mycophenolic acid | 19 (50) |
| Corticosteroid | 15 (39) |
One patient was tested before endoscopy, and the other was tested to determine hospital cohorting.
References
- 1.Pereira M.R. Am J Transplant. 2020;20:1800–1808. doi: 10.1111/ajt.15941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cai Q. J Hepatol. 2020 Sep;73(3):566–574. doi: 10.1016/j.jhep.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akalin E. N Engl J Med. 2020;382:2475–2477. doi: 10.1056/NEJMc2011117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan W.J. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richardson S. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhoori S. Lancet Gastroenterol Hepatol. 2020;5:532–533. doi: 10.1016/S2468-1253(20)30116-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D’Antiga L. Liver Transpl. 2020;26:1064–1065. [Google Scholar]
- 8.Fernández-Ruiz M. Am J Transplant. 2020;20:1849–1858. doi: 10.1111/ajt.15929. [DOI] [PMC free article] [PubMed] [Google Scholar]