Abstract
The lack of vaccines and antiviral treatment, along with the increasing number of cases of Zika virus (ZIKV) and Chikungunya virus (CHIKV) infections, emphasize the need for searching for new therapeutic strategies. In this context, the marine brown seaweed Canistrocarpus cervicornis has been proved to hold great antiviral potential. Hence, the aim of this work was to evaluate the anti-ZIKV and anti-CHIKV activity of a marine dolastane isolated from brown seaweed C. cervicornis and its crude extract. Vero cells were used in antiviral assays, submitted to ZIKV and CHIKV, and treated with different concentrations of C. cervicornis extract or dolastane. The crude extract of C. cervicornis showed inhibitory activities for both ZIKV and CHIKV, with EC50 values of 3.3 μg/mL and 3.1 μg/mL, respectively. However, the isolated dolastane showed a more significant and promising inhibitory effect (EC50 = 0.95 µM for ZIKV and 1.3 µM for CHIKV) when compared to both the crude extract and ribavirin, which was used as control. Also, the dolastane showed a very potent virucidal activity against CHIKV and was able to inhibit around 90% of the virus infectivity at 10 μM. For the ZIKV, the effects were somewhat lower, although interesting, at approximately 64% in this same concentration. Further, we observed that both the extract and the dolastane were able to inhibit the replication of ZIKV and CHIKV at different times of addition post-infection, remaining efficient even if added after 8 hours post-infection, but declining soon after. A synergistic effect using sub-doses of the extract and isolates was associated with ribavirin, inhibiting above 80% replication even at the lowest concentrations. Therefore, this work has unveiled the anti-ZIKV and CHIKV potential of C. cervicornis crude extract and an isolated dolastane, which, in turn, can be used as a preventive or therapeutic strategy in the future.
Subject terms: Biologics, Drug screening, Viral infection
Introduction
Arthropod-borne viruses, mainly described as arboviruses, pose a significant threat to human and animal health in many parts of the world. Clearly, it is now known that such pathogens are transmitted by arthropod vectors, such as mosquitoes and ticks, to susceptible vertebrates. In addition, they are targets of studies and precautions due to the possibility of emerging and reemerging virus infections1. Humans are mostly incidental hosts because they do not produce significant viremia and do not contribute to the transmission cycle. Thus, it is observed that they acquire the viral infection during the blood-feeding process of an infected vector and that mosquitoes are the main vector for most arboviruses2,3.
The infection mechanisms of arboviruses are vastly classified. They can lead to central nervous system impairment with compromises ranging from mild to paralytic and death processes, as well as hemorrhagic lesions, fever, capillary leakage, shock, jaundice, liver damage, and culminating in death. In addition, polyarthritis and rash may be common. However, a large number of these infections presents asymptomatically or may result in nonspecific symptoms4,5.
The Zika virus (ZIKV), so named in reference to the Ugandan forest, was first isolated in 1947 and is a flavivirus closely related to dengue. It was initially recognized as causing an asymptomatic infection or producing a febrile disease in humans and, for decades, did not express major public health concerns6. However, several cases of microcephaly were initially linked to the virus. Physicians working in the Northeast region of Brazil have detected a significant increase in the incidence of children being born with microcephaly following the identification of virus entry in the country. The discovery of the possible transmission of the virus through sexual contact or secretions (saliva, urine), and the absence of vaccines or specific treatments have been determinant for a high level of worldwide concern. However, diagnostic confirmation has been difficult due to the lack of markers or tests that have real diagnostic capacity, leading to constant false-positive cases7,8. In addition, during the outbreak in French Polynesia, it was observed that 42 patients with symptoms of ZIKV infection presented Guillain-Barré syndrome, which represented a considerable increase in the number of cases. These observations had already been related to flavivirus at another time, but not to ZIKV infection. Studies have associated ZIKV infection with fetal microcephaly, and it was declared by the WHO in 2016 as the “public health emergency of international concern”9. However, the relationship between intrauterine infection by ZIKV and recent cases of microcephaly has been panicky for many women at any gestational age.
The Chikungunya virus (CHIKV) is a virus transmitted by arthropods to humans mainly by the mosquito Aedes aegypti. The virus was primarily isolated in Tanzania in 1952 and for years has been reported in sporadic cases and several outbreaks. To date, reports of CHIKV infection were obtained in several African countries, in the Indian subcontinent, and Southeast Asia. In recent years, many outbreak cases have been reported in a large geographical area that includes African islands in the Indian Ocean and Indian subcontinent. Studies have shown that in August 2015, the autochthonous transmission was detected in 33 countries and territories of the Americas, and Latin America registered almost one million cases10. Severe forms of CHIKV infection appear to be associated with multiple organ failure, hepatitis, meningitis, nephritis, encephalitis, bullous dermatitis, myocarditis, and cardiac arrhythmias. However, studies show that although the most severe or atypical manifestations of CHIKV infection are uncommon, except for severe arthralgia, the overall mortality rate of these complications has remained quite high.
Previous studies have shown the antiviral potential of C. cervicornis against HSV-1 in vitro assays11 and also its therapeutic efficacy in BALB/c mice infected with the virus and treated with an extract ointment12. We also demonstrated the potential of two dolastanes and a secodolastane isolated from C. cervicornis against HIV-113.
Experimental Section
Seaweed material
Specimens of brown seaweed C. cervicornis (Kützing) De Paula and De Clerck were collected at Praia do Velho in Angra dos Reis, in the south of Rio de Janeiro State, Brazil (lat. 23°01′S, long. 44° 00′W). Voucher specimens (HRJ 10754) were deposited in the Herbarium of the Universidade do Estado do Rio de Janeiro (UERJ). The seaweeds were washed with local seawater and separated from sediments, epiphytes, and other associated organisms.
Extraction
In order to prepare the crude extract, air-dried seaweeds were exhaustively extracted with CH2Cl2 at room temperature. In previous studies, the use of CH2Cl2 extracts most of the diterpenes from the algae. The extract was evaporated under reduced pressure, yielding a brownish residue. The crude extract was acetylated to increase the amount and stability of the most abundant diterpenes by replacing the secondary hydroxyl groups by acetyl groups as described previously by our group14. The acetylated extract was subjected to silica gel chromatography (3 × 40 cm) eluted with 100% n-hexane to 100% EtOAc, in steps of 10% and 100 mL, from which 44 fractions were obtained. The fractionation was monitored by thin-layer chromatography, and spots of diterpenes were detected as pink-colored spots after heating the plated specimens at 100 °C for 3 minutes. The fractions 26, 27, and 28 yielded the dolastane diterpene (4R,9S,14S)-4α-acetoxy-9β,14α-dihidroxydolast-1(15),7-diene14. The structure of the diterpene was assigned by comparison of physical and spectroscopic data with reported values15 (Fig. 1).
Figure 1.
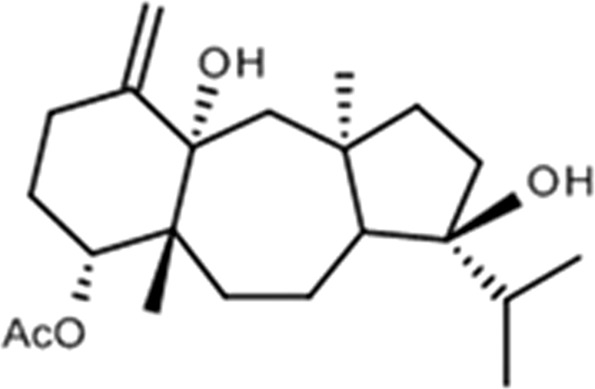
Chemical structure of compound 1 from Canistrocarpus cervicornis.
Cells and virus
Vero cells (African green monkey kidney) were grown in Dulbecco’s Modified Eagle Medium (DMEM; Invitrogen, cat. no 11960) supplemented with 5% Fetal Bovine Serum (FBS; Invitrogen), 2 mmol L−1 L-glutamine (Invitrogen, cat. no. 25030). Antibiotics were added at a final concentration of 50 units/mL penicillin and streptomycin (Invitrogen, cat. no. 15070). ZIKV (ATCC® VR-1839™) was amplified in C6/36 mosquito cells line from Aedes albopictus, adapted to grow at 28 °C, was cultured in L-15 Medium (Leibovitz) supplemented with 0.5% tryptose phosphate broth, 0.03% glutamine, 1% MEM non-essential amino acids solution and 5% FBS. CHIKV experiments were performed with an isolated virus of infected patients, and the sequence was deposited in GenBank under accession number: MK910738 (BRA/RJ/1 F). The manipulation was carried out in a Safety Laboratory, level 3 risk classification, as determined by the Ministry of Health, Ordinance No. 2,349, of September 142017.
Effect of the compounds on the cellular viability
Cytotoxicity of the seaweed crude extract and dolastane in Vero cells was tested in vitro using the MTT method16. Briefly, 2 × 104 Vero cells were seeded into each well of a 96 well plate and incubated for 24 hours (h) at 37oC with different increasing concentrations ranging from 50 to 1000 μg/mL and 50 to 1000 µM of extract and dolastane, respectively, diluted in DMEM. After this period, the cells were submitted to the MTT method and evaluated on a microplate reader for the determination of cell viability as described in the method. Cells treated with DMSO (1.0%) in DMEM were used as control. Relative cell viability was calculated as a percentage comparing treated cells with the untreated cells and the cells in DMSO. Concentrations that maintained more than 90% of cell viability after treatment were considered non-toxic.
TCID50 assay for infectious virus
To assess whether the compounds affect replication of ZIKV and CHIKV, 2 × 105 Vero cells were seeded into each well of a 24-well plate the day before infection. The virus inoculum was diluted to a multiplicity of infection (MOI) of 0.1 in serum-free DMEM. After 1 h of adsorption at 37 °C, the viral inoculum was removed by aspiration, the cells were washed with PBS to remove the non-adsorbed viral residual, and fresh medium was added at the indicated concentration of the compounds. Infected cells were also treated with culture medium containing 1% DMSO to determine the residual antiviral effect, and ribavirin was used as a positive control for antiviral activity. After 24 h of incubation at 37 oC, the culture medium was collected, and the yield of the infectious virus in the cell supernatant was determined by titration using the TCID50 assay in VERO cells and was also evaluated by assay for the production of viral plaques.
Time-of-Drug-addition assay
To initially understand the mechanism of action of the C. cervicornis extract and dolastante, a time-of-drug-addition assay was carried out. Monolayers of Vero cells grown in 24-well plates were infected with ZIKV and CHIKV at an MOI of 3 at time zero. Cells were treated with the extract or dolastane at a final concentration of 10 μg/mL and 10 µM, respectively, at the following times: 0, 1, 2, 4, 6, and 8 h after virus infection. After a 12 h incubation, the supernatants were collected and added to naive cells. Viruses adsorption was allowed for 2 h; then cells were washed and covered with 2.5% carboxymethylcellulose in culture medium. After 72 h of incubation at 37 oC, the cells were fixed with 20% formaldehyde for 2 h and stained with crystal violet for 5 minutes. Plates formed after each treatment were counted and titer calculated.
Determination of virucidal activity
To assess whether dolastane and C. cervicornis extract have a direct effect on the infectivity of ZIKV and CHIKV, approximately 200 plaque-forming units of the viruses were incubated at 37oC in DMEM containing both compounds at three indicated concentrations (5, 10 and 20 μg/mL for extract and 5, 10 and 20 µM for dolastane). In order to the virus adsorption to occur, cells were incubated for 1 h and then washed for removal of the residual virus. After the incubation time, the mixture of virus and compounds was diluted 10 times; that way, the concentration was outside the inhibition range. DMSO 1% and ribavirin were used as controls. Virus titer was determined by plaque assays, as abovementioned.
Evaluation of the combinatorial effect of the compounds
To assess whether the combined compounds show increased antiviral effects, more directly described as a synergistic effect, VERO cells were infected and treated with the ZIKV and CHIKV viruses with MOI of 0.1 and incubated for 2 h, then the compounds were added separately at different concentrations and 0.5 µM for dolastane and Ribavirin and 0.5 μg/mLfor the extract (doses with a low inhibitory effect <20%). In addition, they were also added at higher doses like 10 µM for dolastane and ribavirin and 10 μg/mL for the extract (doses with high inhibitory effect > 80%). In other wells, the compounds were added combined (dolastane plus Ribavirin and Extract plus Ribavirin) in low and high doses, as indicated above. After the plates were washed and carboxymethyl cellulose (CMC) was added for evaluation of viral plaque formation. Cells were fixed and stained after 48–72 h, and viral plaques were counted. Additionally, the results were analyzed by the Loewe and Bliss synergy and antagonism methods using Combenefit software, for the determination of synergism17.
Statistical analysis
The data were analyzed by one-way analysis of variance (ANOVA) followed by Tukey post-test using the GraphPad Instat version 3 program. A p-value of <0.05 was considered statistically significant. In the case of the Synergism results, the T-test was used for the best determination of significance.
Results
Effect of Dolastane and extract of C. cervicornis on cell viability
Cell viability in the presence of C. cervicornis extract and dolastane was evaluated using the MTT method. As a comparison, we used broad-spectrum antiviral ribavirin, which has also been shown to have antiviral activity against both ZIKV and CHIKV. The compounds added at increasing concentrations showed a low dose toxicity profile, and besides, the compounds showed less toxicity than ribavirin. The compounds maintained cell viability above 80% at concentrations up to 200 μg/mL for the extract and 200 μM for dolastane, declining at higher concentrations (data not shown). The CC50 values presented by the compounds were high for both C. cervicornis extract (438 μg/mL) and dolastane (935 μM), and ribavirin showed higher cytotoxicity than the compounds (297 µg.mL−1). Therefore, lower concentrations than those reported above for each drug were selected for the virus production assay (Table 1).
Table 1.
Cytotoxicity (CC50), anti-ZIKV, or anti-CHIKV profile (EC50) and selectivity index (SI) of dolastane diterpenes.
| Compounds | ZIKV | CHIKV | |||
|---|---|---|---|---|---|
| CC50a(μg or μM) | EC50b(μg or μM) | SIc | EC50b(μg or μM) SIc | ||
| Extract | 438 μg/mL ± 5.18 | 2.15 ± 0.22 | 203 | 2.45 ± 1.02 | 178 |
| Dolastane | 935 µM/mL ± 11.0 | 0.75 ± 0.18 | 1246 | 1.28 ± 0.47 | 730 |
| Ribavirin | 297 μM/mL ± 4.25 | 3.95 ± 0.95 | 75.2 | 2.42 ± 0.49 | 122 |
The mean values ± standard deviations are representative of three independent experiments.
aConcentration required to reduce 50% cell viability when compared to untreated controls.
bConcentration that reduced 50% of ZIKV or CHIKV replication when compared to infected and untreated controls.
cSelectivity index was defined as the ratio between CC50 and EC50.
Virus yield assay
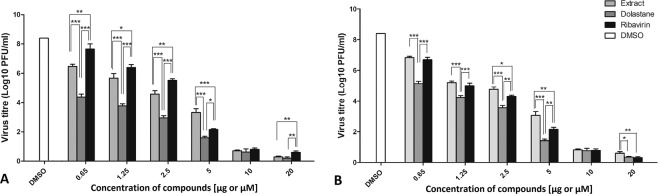
The evaluation of the antiviral effect was performed on Vero cells. Vero cells were infected with ZIKV and CHIKV using a multiplicity of infection of 0.1 and treated for 24 h with increasing concentrations of the substances. The TCID50 method was used to titrate the virus yield, and the concentration capable of reducing virus yield by 50% (EC50) was obtained from a dose-response plot and is shown in Fig. 2A,B. Ribavirin was used as control. The results showed that concentrations of the compounds ranging from 0.65 to 20 μM led to a significant reduction in infectious viral yields when compared to the treatment. The data demonstrate a dose-response curve, reaching about 90% inhibition or more than 5-fold reduced virus production for both viruses. The relationship between the cytotoxicity and the antiviral effect of the compounds was evaluated by means of the Selectivity Index - SI (ratio CC50/EC50) and determined for each molecule, obtaining unitless values. Our results show that dolastane presented an expressive SI for both ZIKV equal to 1246 and CHIKV equal to 730, proving to be much higher than the extract of C. cervicornis and Ribavirin as can be observed in Table 1.
Figure 2.
Inhibition of ZIKV or CHIKV replication by C. cervicornis extract, dolastane, and ribavirin. (A) Inhibition of ZIKV replication. (B) Inhibition of CHIKV replication. Vero cells were infected with ZIKV or CHIKV (MOI 0.1) and treated at concentrations of 0.65, 1.25, 2.5, 5, 10, or 20 μg/mL or µM. The results were evaluated from three independent experiments in triplicate. Data are presented as the percentage of virus titer when compared to control cells and are expressed as the mean of three experiments ± standard error. Statistical analysis was performed using the Tukey test in comparison to C. cervicornis extract, dolastane, and ribavirin in each concentration: *p < 0.05; **p < 0.01; ***p < 0.001.
Time-of-drug-addition assay
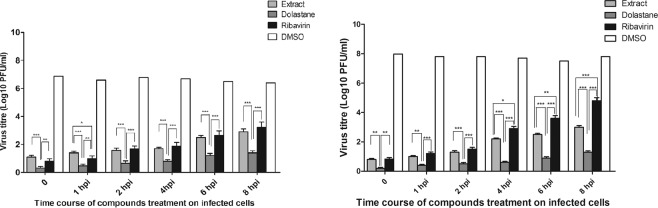
In the time-of-addition assay, we observed that the extract and the dolastane had a very potent inhibitory effect with a 4 or 6-fold decreased virus production for both viruses when added at time zero (simultaneous treatment) (Fig. 3A,B). The effects were observed in post-treatment by inhibition of plaque assay for CHIKV and ZIKV in addition to the determination of viral inhibition by TCID50. As can be seen, the inhibitory potential for the compounds was quite significant with virus inhibition of more than 3 Log10. The dolastane effects on ZIKV presented better results when compared to ribavirin, demonstrating to be a potent inhibitor up to the eighth h of post-treatment (Fig. 3A). On the CHIKV inhibiting front, dolastane presents a potent inhibition of viral replication for up to 8 h, while ribavirin significantly reduces its inhibitory potential within 4 h of post-treatment, and the extract behaves similarly to ribavirin (Fig. 3B).
Figure 3.
Effect of the addition of C. cervicornis extract, dolastane, and ribavirin on ZIKV or CHIKV replication over time. (A) Inhibition of ZIKV replication. (B) Inhibition of CHIKV replication. Monolayers of Vero cells were infected with ZIKV or CHIKV at an MOI of 3 at time zero. At times indicated, extract, dolastane or ribavirin was added to a final concentration of 10 μg/mL for extract or 10 μM/mL, for dolastane or ribavirin. The data were presented by the virus titers in PFU/mL when compared to control cells and are expressed as the mean of three experiments ± standard error. Statistical analysis was performed using the Tukey test in comparison to C. cervicornis extract, dolastane, and ribavirin in each concentration: *p < 0.05; **p < 0.01; ***p < 0.001.
Determination of the virucidal activity of the compounds
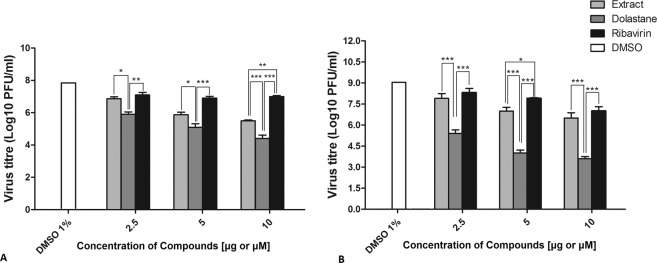
The virucidal activity assay was performed using Vero cells to explore whether the inactivation of the virus particle is a mechanism of action of the studied products. Virus titer was determined by plaque assay and TCID50. The C. cervicornis extract inhibited the replication of ZIKV and CHIKV in a dose-dependent manner. However, we observed an inhibition of ZIKV replication up to 40% or (1 Log10 inhibition) in contrast to the inhibition of CHIKV that was able to inhibit up to 60% or (1 Log10 inhibition) at the concentration of 10 µM used (Fig. 4a,b). On the other hand, the dolastane exhibited more potent effects against both viruses, inhibiting nearly 2 to 3 Logs to the production of ZIKV and CHIKV, respectively, at 10 µM. Ribavirin showed weak virucidal activity, decreasing up to 1 log10 of ZIKV and CHIKV titers.
Figure 4.
Virucidal effect. (A) Effect on the ZIKV. (B) Effect on the CHIKV. The viral suspension (ZIKV or CHIKV) was incubated with C. cervicornis extract, dolastane, and ribavirin at concentrations of 2.5, 5, and 10 µg/mL for extract or 10 µM for dolastane or ribavirin for 2 h and then added to Vero cells. Viral cytopathic effect was assessed after 72 h of incubation. Data are presented as the percentage of virus titer when compared to control cells and are expressed as the mean of three experiments ± standard error. Statistical analysis was performed using the Tukey test in comparison to C. cervicornis extract, dolastane, and ribavirin in each concentration: *p < 0.05; **p < 0.01; ***p < 0.001.
Evaluation of the synergistic effect of compounds with Ribavirin
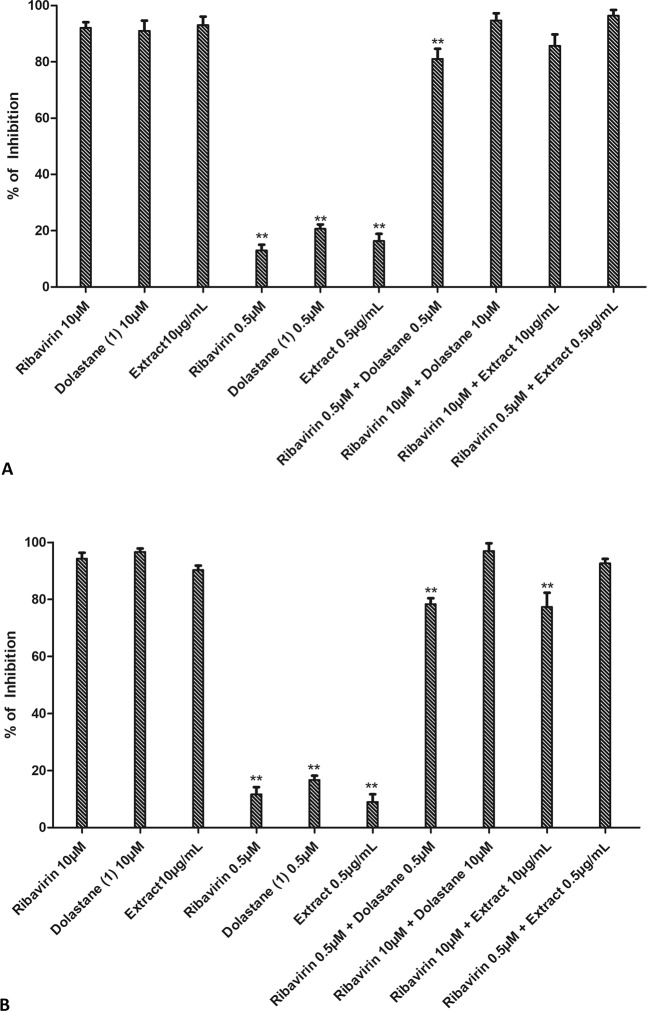
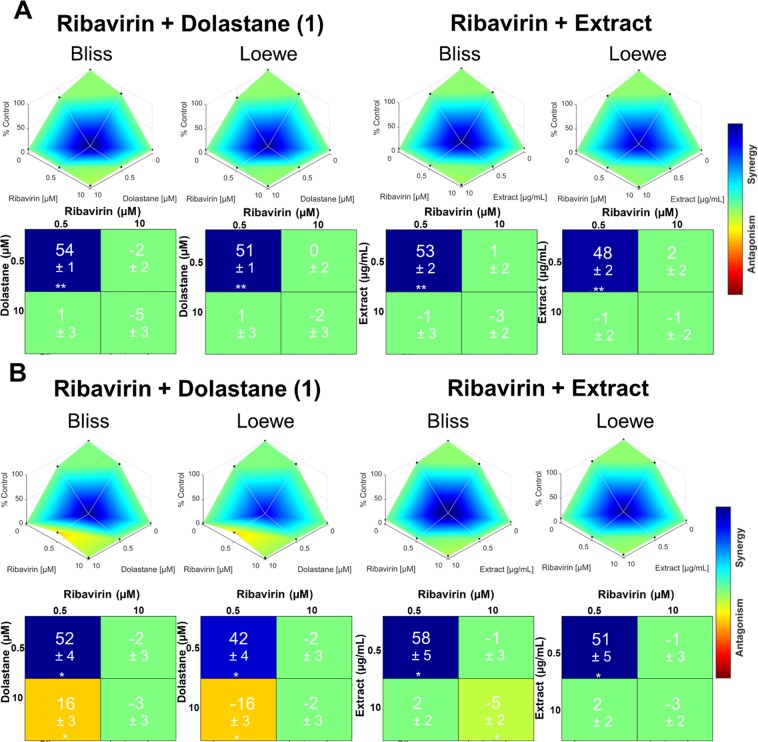
The determination of the synergistic effect may be an essential strategy for the use of drugs. For this, the antiviral effects were initially determined at concentrations previously studied with low inhibitory potential (0.5 μg/mL extract or 0.5 μM dolastane and ribavirin), which showed replication inhibition rates lower than 20% at these concentrations. Also, they were used separately at higher inhibition concentrations (10 μg/mL extract or 10 μM dolastane and ribavirin), which were able to inhibit above 90% of viral replication. The compounds were then combined at the lower concentrations (extract 0.5 μg/mL plus 0.5 μM ribavirin) and at higher concentrations (10 µg.mL−1 extract plus 10 μM Ribavirin). In Fig. 5A,B, we can observe that the association of the lower concentrations of both C. cervicornis extract with Ribavirin and Dolastane with Ribavirin was able to inhibit up to 80% to 90% of viral replication for both ZIKV and or CHIKV. These effects were similar to the ones observed of the combination of the compounds with Ribavirin at the highest concentrations that were between 80 and 90%. In light of the limitations of individual synergy models18, we employed a consensus strategy by combining results from both Loewe and Bliss synergy and antagonism methods. Interestingly, the combination of ribavirin (0.5 µM) with the extract (0.5 μg/mL) or the isolated dolastane (0.5 µM) resulted in a substantial synergy effect for both ZIKV and CHIKV, with an increased effect of 52–58% and 42–51% according to Bliss and Loewe models, respectively. In contrast, other combinations showed mostly additive effects (Figure Fig. 6A,B). Most surprisingly, we noticed an antagonism effect for the combination of ribavirin (0.5 µM) and dolastane (10 µM) against CHIKV.
Figure 5.
Evaluation of the synergistic effect in combination of C. cervicornis extract or dolastane with ribavirin on ZIKV or CHIKV replication. (A) Inhibition of ZIKV replication. (B) Inhibition of CHIKV replication. Monolayers of Vero cells were infected with ZIKV at an MOI of 0.1 and subsequently treated with extract and Ribavirin sub-doses (0.5 µg/mL and 0.5 µM, respectively) and concentrations of 10 µg/mL and 10 µM. In addition, the dose combination was also performed at both concentrations for synergism assessment. Evaluation of the synergistic antiviral effect was determined by inhibition of cytopathic effect by plaque assay. Data are presented as the percentage of virus titer when compared to control cells and are expressed as the mean of three experiments ± standard error. Statistical significance following a one-sample t-test is indicated (*p < 0.05; **p < 0.001).
Figure 6.
Synergistic effects of ribavirin and extract or dolastane against ZIKV and CHIKV. Synergy distribution plots and score matrix tables calculated by Bliss and Loewe methods for combination effects of ribavirin and extract or dolastane against (A) ZIKV, and (B) CHIKV are shown. Synergy scores are represented as mean ± standard deviation (n = 3). Statistical significance following a one-sample t-test is indicated (*p < 0.05; **p < 0.001).
Discussion
In the last years, the rapid widespread of ZIKV and CHIKV infections worldwide7,19 associated with severe manifestations and high impact on public health care and quality of patients’ life have led to the search for new antiviral compounds to tackle these diseases20,21. Marine organisms, such as seaweeds, have great potential as sources of drug candidates with low toxicity and high antiviral activity against several viruses22,23. Herein, we isolated a dolastane from the seaweed C. cervicornis and evaluated the compounds and the crude seaweed extract against ZIKV and CHIKV.
Both products had an interesting bioactivity profile. For instance, they exhibited potent antiviral activity with EC50 values of 2.15 μg/mL and 0.75 µM for the extract and the isolated compound, respectively, against ZIKV replication. Likewise, both products were able to inhibit CHIKV replication with EC50 values of 2.45 µg.mL−1 for the extract and 1.28 μM for the dolastane. Interestingly, low cytotoxicity was observed for the dolastane (CC50 = 935 μM) and the crude extract (CC50 = 438 μg/mL), resulting in promising SI for the extract (203 and 178 for ZIKV and CHIKV, respectively) and the dolastane (1246 and 730 for ZIKV and CHIKV, respectively). These data are representative when we consider that the dolastane, which showed the best antiviral potential, is a compound isolated from the C. cervicornis seaweed that displays an interesting anti-ZIKV and anti-CHIKV activity, similar to that observed for the other compounds tested, but with high inhibitory potential24–26.
Investigating the mechanism of action of compounds is an essential step in drug development and may help in the evaluation of their promising and specificity profiles. In this scenario, finding compounds with low toxicity and that inhibit different steps of viral replication may be important to determine whether their clinical use is feasible in the future27. Thereby, we evaluated the inhibitory effect of the products added at different times after viral infection. Interestingly, the dolastane maintained its inhibitory potential above 80% against CHIKV even when added at 24 h post-infection while the similar effects were observed against ZIKV when treated after up to 16 h post-infection. Our studies demonstrate that it may be feasible to search for new compounds capable of acting on viral replication at different stages and increasing the prospect of discovering new drugs for clinical use similar to that occurring for the treatment of HIV infection28, herpes29, hepatitis30 or even to more than one viral treatment31,32. Previously, we performed time-of-drug-addition studies against ZIKV with the viral polymerase inhibitor 7DMA, considering only post-treatment at different time points up to 24 h post-infection. They showed that the addition of the compound to infected cells could be delayed up to ~10 h post-infection without much loss of antiviral efficacy, which suggests these compounds may act at both pre-entry and post-entry stages of infection.
Another approach widely explored for tackling viral diseases is the development of preventive strategies such as products that inactivate the viral particle before entering into host cells33. We observed that dolastane showed a dose-dependent virucidal activity, inhibiting up to 4 Log10 the CHIKV production (Fig. 4A,B). However, although the compounds exhibit some virucidal activity, this moderate expression can inhibit up to 3 Log10 of viral particle infectivity in the highest concentration tested. In the preparation of preventive measures on the activity of some viral infections, many different working groups are aiming to act directly on the viral particle preventing the appearance of symptoms or even reducing the morbidity and mortality caused by the infection34. Thus, these compounds have therapeutic potential, as it acts directly on the virus particle. This effect is significant due to the possible sexual transmission of ZIKV35. In this sense, this extract has potential as a preventive microbicide for preventing the transmission of the virus through sexual intercourse36.
Combining different drugs has proved to be an interesting strategy to increase the desired biological activity considerably with a decreased toxicity, as already proposed by some research groups37,38. In our studies, we evaluated the combination of the tested compounds, both the C. cervicornis extract and the isolated dolastane combined with ribavirin, both at low concentrations, i.e., concentrations that were not able to expressively inhibit the replication of ZIKV or CHIKV, inhibiting only up to 20% of viral replication if added separately. Our results demonstrated that the combination determined a strong synergism in both inhibition of ZIKV (Fig. 5) and inhibition of CHIKV (Fig. 6), reaching over 90% inhibition of replication when combined, which may suggest that the combined use can be an important treatment strategy, bringing lower risk to people’s health.
Conclusions
Our findings showed that the crude extract of seaweed C. cervicornis tested showed activity against ZIKV and CHIKV. Also, the dolastane extracted from these algae has shown an even more expressive result, demonstrating that marine algae are an interesting source for drug discovery and the development of novel anti-ZIKV and anti-CHIKV agents. Furthermore, upcoming studies focused on the specific mechanism of action aspects and in vivo experiments may corroborate the importance of this compound and its potential for reducing morbidity and mortality of infections caused by these viruses.
Acknowledgements
The authors are grateful to CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) for financial support and for Productivity Fellowships to ICNPP and VLT (443930/2014-7 and 304070/2014-9). ICNPP and VLT (E-26/201.442/2014) also thank the FAPERJ (Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro) for the Cientista do Nosso Estado Fellowship. CCCS thanks to CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) for the Postdoc fellowship and CSB thanks FAPERJ for the Postdoc fellowship (E-26/201.344/2016). The postgraduate program in sciences and biotechnology of UFF (PPBI-UFF). This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001.
Author contributions
C.C.C.S., C.S.B., M.C.O., R.C.A., V.W.-H.R., D.F.F., and I.C.N.P.P. conducted biological (antiviral and virucidal) tests. C.S.B. and V.L.T. conducted algae identification, extraction, isolation, and elucidation of dolastane. C.C.C.S., C.S.B., V.L.T., and I.C.N.P.P. drafted the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Claudio Cesar Cirne-Santos, Email: claudiocirne@gmail.com.
Izabel Christina Nunes de Palmer Paixão, Email: izabeluff@gmail.com.
References
- 1.Weaver SC, Reisen WK. Present and future arboviral threats. Antiviral research. 2010;85:328–345. doi: 10.1016/j.antiviral.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weaver SC, Barrett ADT. Transmission cycles, host range, evolution and emergence of arboviral disease. Nature Reviews Microbiology. 2004;2:789. doi: 10.1038/nrmicro1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arora N, Banerjee AK, Narasu ML. Zika virus: an emerging arboviral disease. Future Virology. 2016;11:395–399. doi: 10.2217/fvl-2016-0048. [DOI] [Google Scholar]
- 4.Levi J. Arbovirus epidemics and blood safety in Brazil. ISBT Science Series. 2017;12:233–238. doi: 10.1111/voxs.12334. [DOI] [Google Scholar]
- 5.Vasilakis N. Arboviruses: Molecular Biology, Evolution and Control. American Journal of Tropical Medicine and Hygiene. 2016;95:488–489. doi: 10.4269/ajtmh.16-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fauci AS, Morens DM. Zika virus in the Americas—yet another arbovirus threat. New England journal of medicine. 2016;374:601–604. doi: 10.1056/NEJMp1600297. [DOI] [PubMed] [Google Scholar]
- 7.Musso D, Nilles E, Cao-Lormeau VM. Rapid spread of emerging Z ika virus in the P acific area. Clinical Microbiology and Infection. 2014;20:O595–O596. doi: 10.1111/1469-0691.12707. [DOI] [PubMed] [Google Scholar]
- 8.Mead PS, Hills SL, Brooks JT. Zika virus as a sexually transmitted pathogen. Current opinion in infectious diseases. 2018;31:39–44. doi: 10.1097/qco.0000000000000414. [DOI] [PubMed] [Google Scholar]
- 9.Gulland, A. Zika virus is a global public health emergency, declares WHO. British Medical Journal Publishing Group (2016). [DOI] [PubMed]
- 10.Cunha RVD, Trinta KS. Chikungunya virus: clinical aspects and treatment - A Review. Memorias do Instituto Oswaldo Cruz. 2017;112:523–531. doi: 10.1590/0074-02760170044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vallim MA, et al. In vitro antiviral activity of diterpenes isolated from the Brazilian brown alga Canistrocarpus cervicornis. Journal of Medicinal Plants Research. 2010;4:2379–2382. [Google Scholar]
- 12.de Souza Barros C, et al. Therapeutic efficacy in BALB/C mice of extract from marine alga Canistrocarpus cervicornis (Phaeophyceae) against herpes simplex virus type 1. Journal of applied phycology. 2017;29:769–773. doi: 10.1007/s10811-016-0865-9. [DOI] [Google Scholar]
- 13.de Souza Barros C, et al. Anti-HIV-1 activity of compounds derived from marine alga Canistrocarpus cervicornis. Journal of applied phycology. 2016;28:2523–2527. doi: 10.1007/s10811-015-0776-1. [DOI] [Google Scholar]
- 14.Barros, C. d. S. et al. Anti-HIV-1 activity of compounds derived from marine alga Canistrocarpus cervicornis. Journal of Applied Phycology, 1–5 (2015).
- 15.Teixeira VL, Tomassini T, Fleury BG, Kelecom A. Dolastane and Secodolastane Diterpenes from the Marine Brown Alga, Dictyota cericornis. Journal of natural products. 1986;49:570–575. doi: 10.1021/np50046a002. [DOI] [Google Scholar]
- 16.Tricarico PM, Caracciolo I, Crovella S, D’Agaro P. Zika virus induces inflammasome activation in the glial cell line U87-MG. Biochemical and biophysical research communications. 2017;492:597–602. doi: 10.1016/j.bbrc.2017.01.158. [DOI] [PubMed] [Google Scholar]
- 17.Di Veroli GY, et al. Combenefit: an interactive platform for the analysis and visualization of drug combinations. Bioinformatics. 2016;32:2866–2868. doi: 10.1093/bioinformatics/btw230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pemovska T, Bigenzahn JW, Superti-Furga G. Recent advances in combinatorial drug screening and synergy scoring. Current opinion in pharmacology. 2018;42:102–110. doi: 10.1016/j.coph.2018.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cavalcanti LPdG, Freitas ARR, Brasil P, Cunha RV. d. Surveillance of deaths caused by arboviruses in Brazil: from dengue to chikungunya. Memórias do Instituto Oswaldo Cruz. 2017;112:583–585. doi: 10.1590/0074-02760160537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masmejan, S., Baud, D., Musso, D. & Panchaud, A. Zika virus, vaccines and antiviral strategies. Expert review of anti-infective therapy (2018). [DOI] [PubMed]
- 21.Rausch K, et al. Screening bioactives reveals nanchangmycin as a broad spectrum antiviral active against Zika virus. Cell reports. 2017;18:804–815. doi: 10.1016/j.celrep.2016.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atta-ur-Rahman, F. Studies in natural products chemistry. (Elsevier, 2016).
- 23.Newman DJ, Cragg GM. Natural products as sources of new drugs from 1981 to 2014. Journal of natural products. 2016;79:629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- 24.Eyer L, et al. Nucleoside inhibitors of Zika virus. The. Journal of infectious diseases. 2016;214:707–711. doi: 10.1093/infdis/jiw226. [DOI] [PubMed] [Google Scholar]
- 25.Barrows NJ, et al. A screen of FDA-approved drugs for inhibitors of Zika virus infection. Cell host & microbe. 2016;20:259–270. doi: 10.1016/j.chom.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weber C, Sliva K, von Rhein C, Kümmerer BM, Schnierle BS. The green tea catechin, epigallocatechin gallate inhibits chikungunya virus infection. Antiviral research. 2015;113:1–3. doi: 10.1016/j.antiviral.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Abdelnabi, R., Neyts, J. & Delang, L. Antiviral Strategies Against Chikungunya Virus. Methods in Molecular Biology, vol 1426. Humana Press, New York, NY, Springer 243–253 (2016). [DOI] [PMC free article] [PubMed]
- 28.Rebensburg S, et al. Potent in vitro antiviral activity of Cistus incanus extract against HIV and Filoviruses targets viral envelope proteins. Scientific reports. 2016;6:20394. doi: 10.1038/srep20394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Oliveira A, Prince D, Lo C-Y, Lee LH, Chu T-C. Antiviral activity of theaflavin digallate against herpes simplex virus type 1. Antiviral research. 2015;118:56–67. doi: 10.1016/j.antiviral.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ottosen S, et al. In vitro antiviral activity and preclinical and clinical resistance profile of miravirsen, a novel anti-hepatitis C virus therapeutic targeting the human factor miR-122. Antimicrobial agents and chemotherapy. 2015;59:599–608. doi: 10.1128/AAC.04220-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nothias-Scaglia L-F, et al. Antiviral activity of diterpene esters on chikungunya virus and HIV replication. Journal of natural products. 2015;78:1277–1283. doi: 10.1021/acs.jnatprod.5b00073. [DOI] [PubMed] [Google Scholar]
- 32.Zmurko J, et al. The Viral Polymerase Inhibitor 7-Deaza-2’-C-Methyladenosine Is a Potent Inhibitor of In Vitro Zika Virus Replication and Delays Disease Progression in a Robust Mouse Infection Model. PLOS Negl Trop Dis. 2016;10:e0004695. doi: 10.1371/journal.pntd.0004695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siddharta A, et al. Virucidal Activity of World Health Organization–Recommended Formulations Against Enveloped Viruses, Including Zika, Ebola, and Emerging Coronaviruses. The. Journal of Infectious Diseases. 2017;215:902–906. doi: 10.1093/infdis/jix046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carneiro BM, Batista MN, Braga ACS, Nogueira ML, Rahal P. The green tea molecule EGCG inhibits Zika virus entry. Virology. 2016;496:215–218. doi: 10.1016/j.virol.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 35.Towers S, et al. Estimate of the reproduction number of the 2015 Zika virus outbreak in Barranquilla, Colombia, and estimation of the relative role of sexual transmission. Epidemics. 2016;17:50–55. doi: 10.1016/j.epidem.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 36.Buckheit RW, Watson KM, Morrow KM, Ham AS. Development of topical microbicides to prevent the sexual transmission of HIV. Antiviral research. 2010;85:142–158. doi: 10.1016/j.antiviral.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu J-W, et al. Synergistic effects of combination treatment using EGCG and suramin against the chikungunya virus. Biochemical and biophysical research communications. 2017;491:595–602. doi: 10.1016/j.bbrc.2017.07.157. [DOI] [PubMed] [Google Scholar]
- 38.Snyder B, Goebel S, Koide F, Ptak R, Kalkeri R. Synergistic antiviral activity of Sofosbuvir and type-I interferons (α and β) against Zika virus. Journal of medical virology. 2018;90:8–12. doi: 10.1002/jmv.24932. [DOI] [PubMed] [Google Scholar]