Abstract
The P2X receptor family of ATP-gated cation channels are attractive drug targets for pain and inflammatory disease, but no subtype-selective agonists, and few partially selective agonists have been described to date. As proof-of-concept for the discovery of novel P2X receptor agonists, here we demonstrate the use of Drosophila taste neurons heterologously expressing rat P2X2 receptors as a screening platform. We demonstrate that wild-type rat P2X2 expressed in Drosophila is fully functional (ATP EC50 8.7 µM), and that screening of small (2 µl) volumes of a library of 80 adenosine nucleotide analogues is rapid and straightforward. We have determined agonist potency and specificity profiles for rat P2X2 receptors; triphosphate-bearing analogues display broad activity, tolerating a number of substitutions, and diphosphate and monophosphate analogues display very little activity. While several ATP analogues gave responses of similar magnitude to ATP, including the previously identified agonists ATPγS and ATPαS, we were also able to identify a novel agonist, the synthetic analogue 2-fluoro-ATP, and to confirm its agonist activity on rat P2X2 receptors expressed in human cells. These data validate our Drosophila platform as a useful tool for the analysis of agonist structure-activity relationships, and for the screening and discovery of novel P2X receptor agonists.
Subject terms: Ligand-gated ion channels, Ion channels in the nervous system, Extracellular recording, Receptor pharmacology
Introduction
P2X receptors are ATP-gated ion channels which play diverse roles in physiology and thereby affect a variety of diseases and conditions, including, although not exhaustively: pain sensation1, cancer2, arthritis3,4, osteoporosis5, and hypertension6. Together with the metabotropic P2Y receptors, P2X receptors mediate cellular responses to extracellular ATP7. ATP functions as an intercellular signaling molecule through release as a (co)transmitter in many synapses, during muscle contraction in skeletal and heart muscle, and by receptor-mediated release in many non-excitable tissues. ATP is also released from cells following damage or stress to the cell membrane by a variety of conductive mechanisms8, and is released constitutively at low flux rates9. Seven P2X genes in mammals (P2RX1-P2RX7), some of which may be alternatively spliced, encode channel subunits that trimerize to form a complex repertoire of homo- and heterotrimeric P2X receptors. Each subunit has two transmembrane domains connected by a large extracellular loop; N- and C-termini are intracellular. P2X genes have also been identified in many other classes of vertebrates and invertebrates, as well as in lower organisms, such as slime mold, where the P2X receptor has an intracellular function in vesicle fusion10,11.
The P2X2 subunit in mammals functions as a homotrimer, or in association with other subunits, in signaling in both the peripheral nervous system (PNS) and central nervous system (CNS). In the PNS, P2X2 receptors of the carotid sinus nerve endings are crucial to carotid body oxygen signal transduction6. Functions of P2X2 receptors have also been reported in trigeminal and myenteric nerves12. In the CNS, P2X2 receptor expression is widespread7 with functional receptors identified in cultured Purkinje cells13, neurons of the dorsal horn14 and neuron terminals in the posterior pituitary15,16. In association with P2X3, P2X2 functions in taste sensation, pain and inflammation17.
Substantial early research in P2X receptors on ATP binding has been carried out using rat P2X218, and parallel work focused on human P2X119–22. Studies on P2X2 and P2X4 suggested that the mode of binding ATP is common among P2X receptors23. The determination of the crystal structures of zebra fish P2X4 in the closed apo-24,25 and in the ATP-bound open state25, and human P2X3 with bound ATP26, has corroborated most of the interactions between the P2X receptor and ATP that were proposed in these earlier studies, in addition to providing detailed insight into the overall architecture and structural rearrangements between the open and closed conformers (reviewed by27–29).
The knowledge of the structure of the P2X binding pocket and its interactions with the primary ligand offers a unique opportunity to understand the mechanistic basis of ligand specificity of the receptor. The ligand specificity of P2X receptors has been explored in previous studies (reviewed in30–32), and most recently in a systematic study of agonist recognition and specificity in rat P2X2 receptors33, where a series of ATP analogues with modifications to the base and ribose were examined. In our study we have further expanded the range of molecules tested on rat P2X2 by using a library of 80 adenosine nucleotides. In order to test this library we developed a rapid and sensitive assay, which uses very small quantities of ligand, based on overexpressing P2X receptors in Drosophila taste neurons. Taste neurons in the distal mouthparts of Drosophila (Fig. 1A) are housed in hair-like cuticular structures called sensilla34,35, which have an apical pore that allows chemicals to enter the sensillum and interact with receptors expressed in the neurons’ dendrites. The architecture of the sensilla, together with their accessible position on the labellae, means that the action potential activity of taste neurons in response to panels of chemicals can be readily recorded. Several adenosine nucleotides gave responses as large as ATP, including the previously identified agonists ATPγS and ATPαS. We also identified a novel agonist, 2-fluoro-ATP (2F-ATP), from our screen in Drosophila, and confirmed its activity on rat P2X2 by measuring currents using whole-cell voltage-clamp on HEK cells heterologously expressing the receptor. Rat P2X2 exhibited a relatively broad activity profile to adenosine nucleotides with three phosphoryl groups, but did not respond robustly either to ADP or AMP and their derivatives. This suggested that the binding pocket can accommodate a range of modifications of the primary ligand. A T184I P2X2 receptor mutant showed reduced activity in Drosophila taste neurons, which could be explained by loss of hydrogen bonding to ATP as deduced from the crystal structures of other P2X receptors.
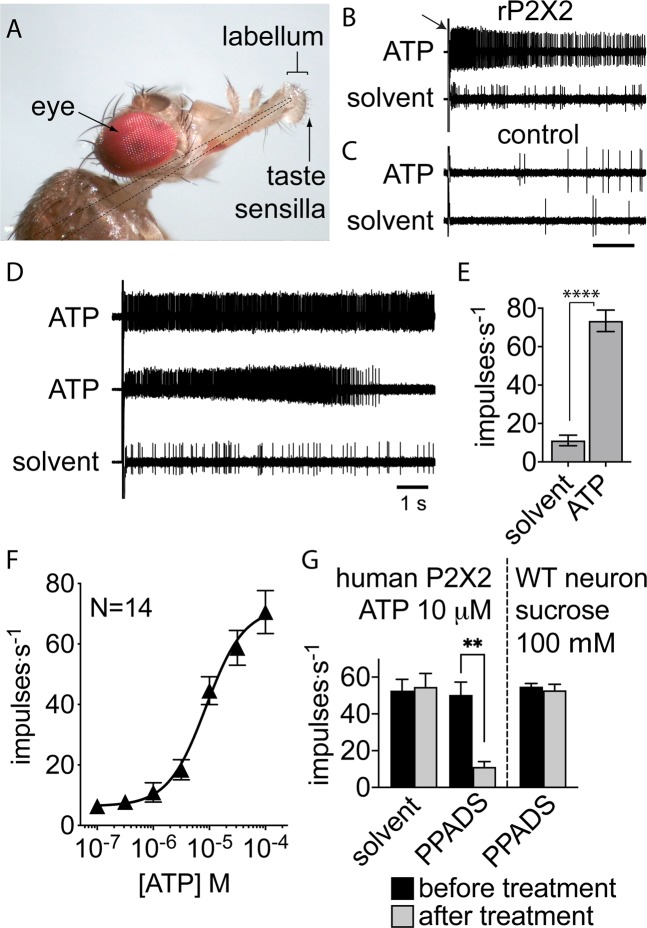
Figure 1.
P2X2 mediates responses to ATP in Drosophila sugar taste neurons. (A) Photo of fly preparation for recording. Dashed lines indicate outline of reference electrode, which is placed in the fly with its tip reaching inside the distal end of the proboscis near the labella. The hair-like taste sensilla are accessible on the exterior with a pipette containing the stimulus. The pipette also serves as recording electrode. (B) Recording of action potential response to 100 μM ATP (top) and solvent (bottom) from rat P2X2 expressing flies. Arrow indicates contact artifact when recording pipette makes initial contact with the sensillum. Flies carry a single copy each of UAS-rat P2X2::mCherry, where:: denotes a construct encoding a fusion protein, and Gr5a-GAL4. (C) As in (B) but from control flies carrying the UAS transgene but no driver to express it. (D) Variability in temporal kinetics of responses to ATP in rP2X2 expressing flies: phasic-tonic as in (B), tonic (top trace) or with slow onset and rapid off kinetics (middle trace). (E) Responses to solvent and 100 μM ATP (mean and SEM, N = 12, P < 0.0001 paired t-test). (F) Response to ATP in flies expressing rat P2X2 to increasing concentrations of ATP (mean and SEM, N = 14, EC50 = 8.7 μM with 95%CI 5.1 to 14.7 μM). (G) Effect of 2 minute treatment of 30 μM PPADS on the subsequent response of human P2X2 to 10 μM ATP (middle pair of bars); responses before and after treatment with PPADS differed significantly (mean and SEM, N = 6, P = 0.004 paired t-test). Solvent treatment had no significant effect on the ATP response in flies expressing P2X2 (shown at left: mean and SEM, N = 6, P > 0.05 paired t-test), and PPADS treatment had no significant effect on the sucrose response in wild-type flies (shown at right: mean and SEM, N = 6, P > 0.05 paired t-test).
In summary, we have developed a sensitive functional assay in Drosophila taste neurons for mammalian ligand-gated ion channels, which permits the rapid screening of compound libraries in small (2 µl per application) quantities. We have used this assay to profile the activity of 80 compounds from an adenosine nucleotide library against rat P2X2, discovering a novel agonist and expanding our understanding of the structure-activity relationship of agonist action.
Results
Rat P2X2 is functional in taste neurons of Drosophila
To determine whether rat P2X2 formed a functional channel in Drosophila taste neurons, we tested the responses to ATP in taste neurons of flies expressing rat P2X2 via the GAL4-UAS system under control of the Gr5a promoter. ATP at 100 μM elicited strong excitatory responses from taste neurons in sensilla on the mouthparts of these flies, while the solvent by itself did not (Fig. 1B), suggesting rat P2X2 is functional in the sugar taste neuron. Moreover, Drosophila taste neurons do not show endogenous responses to ATP; control flies carrying a transgene of UAS-ratP2X2 but without a GAL4 driver did not respond to ATP (Fig. 1C). We noted that in solvent-only recordings, flies expressing rat P2X2 exhibited higher action potential activity than control flies (Fig. 1B and C). The temporal kinetics of the responses to ATP in flies expressing rat P2X2 varied from phasic-tonic (Fig. 1B), to tonic (Fig. 1D, top trace) while many neurons showed a delayed onset of response (Fig. 1D, middle trace). The response to ATP differed significantly from the solvent response (Fig. 1E). The response to ATP mediated by rat P2X2 was concentration-dependent (Fig. 1F), with an EC50 of 8.7 μM (95%CI: 5.1 to 14.7 μM). The human P2X2 ortholog was also tested in Drosophila taste neurons, as we intend to study the human receptor after beginning with the rat receptor as a proof-of-concept, and it responded to ATP in a similar fashion. Among several antagonists of P2X receptors that have been identified, PPADS acts strongly on homomeric P2X1, P2X2, P2X3 and P2X5 receptors (reviewed in30). To test if P2X2 is similarly inhibited in the context of the Drosophila taste system, we applied it for two minutes at 30 μM and observed strong inhibition of the ATP response (Fig. 1G). A two-minute solvent treatment did not affect the ATP response. In addition, the sucrose response in wild-type flies was not significantly affected by PPADS treatment, which suggests that the inhibitor indeed acted directly on the expressed P2X receptor.
Screening adenosine nucleotides validates known ligands and identifies additional agonists
Having determined that rat P2X2 forms a functional channel in taste neurons of Drosophila, we proceeded to screen a library of 80 adenosine nucleotides for their agonist activity. A small number of chemicals in the library have been previously tested on P2X receptors, as described below, and were useful in validation of the Drosophila assay platform.
In ATP the adenine base is linked to C1′ of the sugar ribose to which three phosphoryl groups are connected at C5′ (Fig. 2A). The library comprised chemicals with changes in the adenine base, the ribose sugar, and the phosphate moieties of ATP and combinations of changes in these three moieties, as well as a number of dinucleotides. Responses to the 80 nucleotides ranged in a smooth progression from full agonist activity, with responses similar to ATP, to no response (Fig. 2B). We did not find nucleotides that exceeded the activity of ATP on the receptor by >20%. For all three moieties, there were some changes that had little effect on agonist activity: in ATPγS the gamma phosphoryl group is replaced with a thiophosphoryl group, 2F-ATP has a fluoro substitution on the adenine base, and 2′F-ATP has a fluoro substitution on the ribose ring. ATPγS and ATPαS have previously been shown to act as agonists on P2X2 expressed in Xenopus oocytes36 and both were also effective agonists in the Drosophila system. 2F-ATP, ATPγS and ATPαS did not elicit responses greater than that of solvent alone in control flies not expressing rat P2X2 receptors (data not shown).
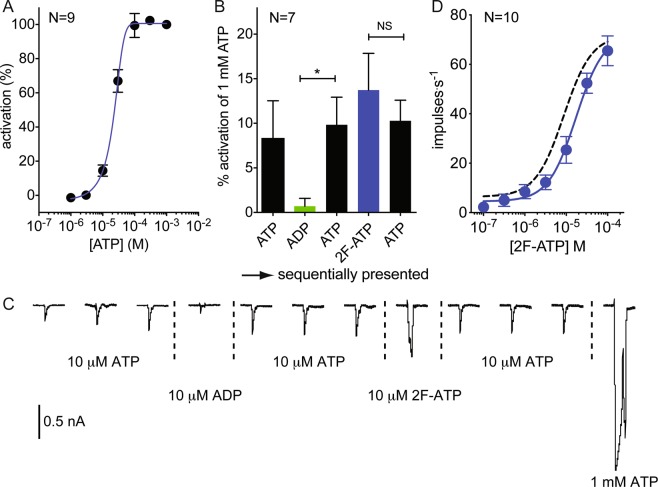
Figure 2.
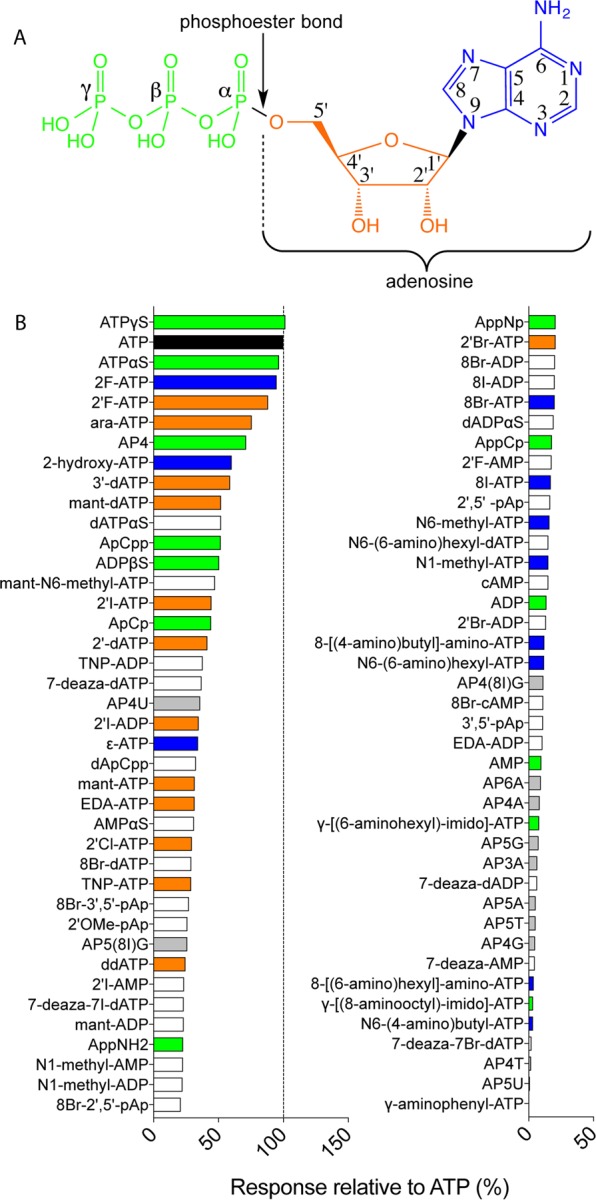
Responses in taste neurons expressing rat P2X2 to a library of adenosine nucleotides. (A) Structure of ATP. The adenine base (in blue) is linked to C1′ of the sugar ribose (in orange) to which three phosphoryl groups (in green) are connected at C5′. (B) Mean response to 80 adenosine nucleotides tested at 100 μM, N = 3–6. Nucleotides are arranged in order of activity relative to the response to ATP (in black). Derivatives of ATP include modifications in the phosphoryl groups (green), the ribose moiety (orange), the adenine base (blue), a combination of two or more of these (white) or are dinucleotides (grey).
The presence of three phosphoryl groups is a necessary condition for strong agonist activity, as none of the ADP and AMP derivatives elicited >50% of the response to ATP. However, it is not a sufficient condition, as most modifications of chemicals with three phosphoryl groups still led to a reduction or complete loss of activity at 100 μM. Additions to, or substitutions in, positions 1, N6, 7 and 8 of the adenine ring were all detrimental, while position 2 allowed some modification (2F-ATP and 2OH-ATP). In the ribose ring, changes were detrimental to agonist activity both at C2′ (but 2′F-ATP showed some activity) and C3′.
We noted that diadenosine tetraphosphate (AP4A) did not elicit responses from rat P2X2 in the Drosophila taste system, although it has been reported to be a full agonist when expressed in Xenopus oocytes37.
Whole-cell voltage clamp of rat P2X2 in HEK cells validates a novel agonist
Screening the library on rat P2X2 expressed in Drosophila taste neurons led to the identification of 2-fluoro-ATP (2F-ATP) as a novel full agonist. To confirm its activity in an established expression platform, 2F-ATP was presented to HEK 293 cells stably expressing rat P2X2 receptors. We first verified the responsiveness to ATP of these cells by measuring whole-cell transmembrane currents in voltage-clamp to concentrations from 10–6 to 10–3 M; ATP elicited concentration-dependent cationic currents in this range (Fig. 3A). Subsequently, responses were recorded to ATP, ADP and 2F-ATP at 10 μM. 2F-ATP elicited currents that did not differ significantly from currents elicited by ATP (Fig. 3B,C), which provided an independent experimental validation of the finding from the Drosophila assay. Due to the high cost of 2F-ATP, we were unable to purchase sufficient volumes to generate a concentration response curve using voltage-clamp in HEK 293 cells. However, this was possible in the Drosophila system due to the small application volumes required (2 µl). We found that the potency of 2F-ATP was similar to that of ATP, with EC50 values of 17.2 μM (Figs. 3D) and 8.7 µM (Fig. 1F), respectively.
Figure 3.
Responses mediated by rat P2X2 confirm 2-fluoro-ATP (2F-ATP) is a full agonist. (A) Recordings under whole-cell voltage clamp from HEK cells expressing rat P2X2 show the response to ATP increases with concentration. (B) Responses to ATP, ADP, ATP (repeat stimulus), 2F-ATP and ATP (repeat stimulus) presented sequentially each at 10 μM, relative to ATP at 1 mM (mean and SEM, *P < 0.05 ANOVA with Bonferroni post-test, NS non-significant). (C) Current traces in one of the experiments underlying data in (B). Applications of ADP, 2F-ATP and 1 mM ATP each followed three applications of ATP. (D) Response to 2F-ATP in flies expressing rat P2X2 to increasing concentrations (mean and SEM, N = 10, EC50 = 17.2 μM with 95%CI 9.5 to 31.3 μM). Black dashed curve is fitted to ATP data from Fig. 1F for comparison.
An amino acid substitution in the ATP binding pocket alters ligand activity in P2X2
We serendipitously generated a Drosophila line of rat P2X2 encoding the channel with a T184I substitution. Thr-184 is a conserved residue that corresponds to T189 in zebra fish P2X4 and to T172 in human P2X3. This residue has been shown in structural studies to interact through hydrogen bonding with the adenine moiety of ATP in the P2X binding pocket25,26, and substitution of this residue has previously been demonstrated to reduce ATP potency and activity18. The sugar taste neurons expressing the T184I mutant still responded to ATP at 100 μM, albeit with slow onset kinetics and low impulse rates (Fig. 4A,B). To broaden and enhance expression in the other taste neurons of the sensilla, flies carrying either WT or T184I UAS-rat P2X2 were crossed to a strong pan-neural driver. Preliminary observation of flies with pan-neural expression of WT rat P2X2 suggested that the ion channel interferes with neuronal physiology. The flies exhibited a tremor or shaking phenotype at rest and their locomotor activity appeared to be inhibited. These phenotypes appeared three days post-eclosion in flies expressing the WT rat P2X2 but were absent in flies expressing the T184I variant. Testing concentrations from 10–6 to 10–3 M showed that ATP was less potent at the T184I receptor (Fig. 4C), with an EC50 of 145 μM (95%CI: 92 to 370 μM), than at the WT receptor (Fig. 1F). Microscopy showed strong expression of the T184I-GFP fusion protein under control of the pan-neural driver in neurons throughout the animal (Fig. 4D), suggesting low responses to ATP were not due to low levels of expression or poor dendritic localization. Also, while it is possible that the low responses to ATP were caused by interference of the C-terminal GFP fusion tag with the function of the receptor, previous studies on rat P2X2 expressed in Xenopus oocytes showed that responses mediated by a C-terminal GFP fusion tagged receptor and a wild-type receptor are similar in EC50, peak current and desensitization kinetics38,39. Rat P2X3 constructs carrying other C-terminal fluorescent tags (ECFP or DsRed2) also did not differ from wild-type receptors in their affinity for the ligand or in their ion conductance properties40, suggesting that members of the P2X family may generally be tolerant of C-terminal fluorescent protein tags.
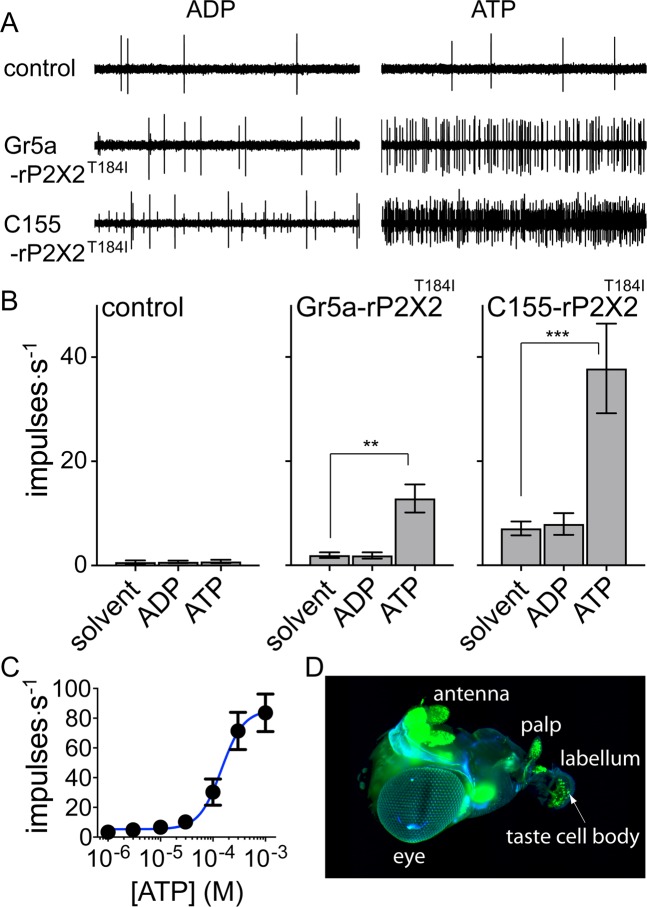
Figure 4.
Responses mediated by T184I rP2X2::GFP in taste neurons. (A) 100 μM ATP elicits responses from mutated rP2X2 while 100 μM ADP does not. Top traces from control flies carrying a UAS transgene but no driver, middle traces from flies expressing T184I rP2X2 in sugar taste neurons under control of the Gr5a promoter, bottom traces from flies with pan-neural expression of T184I rP2X2 under control of the C155 driver. (B) Mean responses (with SEM) to solvent, ADP and ATP in control flies and in flies expressing T184I rP2X2 in sugar taste neurons and in all neurons (ns p ≥ 0.05 **p < 0.01 ***p < 0.001 paired Anova and post-hoc Dunnett’s test). Solvent elicited impulse rates among genotypes differ significantly between Gr5a-rP2X2T184I and C155-rP2X2T184I, p = 0.0001 (unpaired Anova and post-hoc Dunnett’s test). (C) Response to ATP in flies expressing rat P2X2 to increasing concentrations of ATP (mean and SEM, N = 14, EC50 = 145 μM with 95%CI 92 to 370 μM). (D) Light-sheet fluorescence micrograph of neurons with pan-neural expression of T184I rP2X2::GFP (in green).
We subsequently tested the library of 80 adenosine nucleotides on flies expressing rat P2X2T184I and found that ATPγS, ATPαS and 2F-ATP elicited responses similar to ATP (Fig. 5); mirroring the situation in the WT receptor. However, the ribose modified 2′F-ATP, ara-ATP and the nucleotide with four phosphoryl groups AP4 as well as 2OH-ATP elicited no significant responses even though the WT rat P2X2 mediated an intermediate response to these ligands (see Fig. 2).
Figure 5.
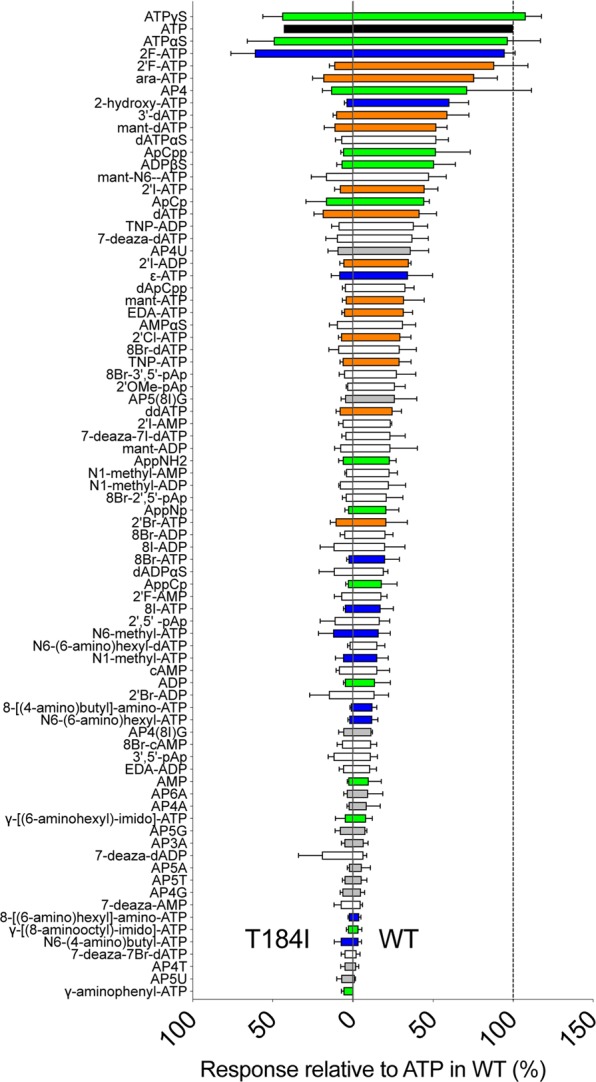
Comparative response to 80 adenosine nucleotides tested at 100 μM at wild-type and T184I rat P2X2 (mean and SEM, N = 3–6) expressed in Drosophila taste neurons. Wild type (WT) rat P2X2 (data from Fig. 2) at right and T184I mutant at left. Nucleotides are arranged in order of activity relative to the response to ATP in WT. ATP (black) and its derivatives include modifications in the phosphoryl groups (green), the ribose moiety (orange), the adenine base (blue), a combination of two or more of these (white) or are dinucleotides (grey).
In order to rationalize our experimental data, we constructed a homology model of rat P2X2 based on the zebrafish P2X4 ATP-bound crystal structure25. In the modeling procedure, the conformation of ATP was constrained, and so matched that observed in the original crystal structure. The ATP binding site, indicating the key conserved amino acid residues which contribute polar contacts, is shown in Fig. 6A. The side-chain hydroxyl group of Thr-184 forms a hydrogen bond with the N1 position of the adenine ring, which would be disrupted in the T184I mutant, explaining the lower potency and activity of ATP at this mutant receptor. In order to assess the binding of other nucleotide analogues to our model, we first performed molecular docking simulations with ATP (Fig. 6B). The docked ATP displayed very similar conformation to that of the ATP derived from the crystal structure (RMSD = 1.4799), with one notable difference: instead of forming an intramolecular hydrogen bond with the γ-phosphate oxygen, the 2′ hydroxyl group of the ribose formed an intermolecular hydrogen bond with the main chain of Leu-211. Docking with the novel full agonist 2F-ATP (Fig. 6C) predicted a docking conformation more similar to that of ATP (RMSD = 1.1970), preserving the intramolecular hydrogen bond and demonstrating that the fluorine substitution is well accommodated in the binding pocket. It has recently been demonstrated that 2′-dATP is not an effective agonist at P2X1 receptors. This has been proposed to arise from the loss of an intramolecular hydrogen bond between the 2′ hydroxyl and a γ-phosphate oxygen which destabilizes the U-shaped conformation of ATP required for efficient agonist action41. In our molecular docking simulations using 2′-dATP (Fig. 6D,E), we observed two distinct conformations of 2′-dATP; one where the U-shaped conformation was lost, leading to a significant translation of the adenine ring and complete rearrangement of the critical lysine-phosphate interactions (Fig. 6D, RMSD = 4.0540), and one where the U-shaped conformation was maintained by formation of an intramolecular hydrogen bond between the γ-phosphate oxygen and the 3′-hydroxyl of the ribose (Fig. 6E) leading to a significant translation of the adenine ring relative to that observed in our molecular model derived from the ATP-bound crystal structure.
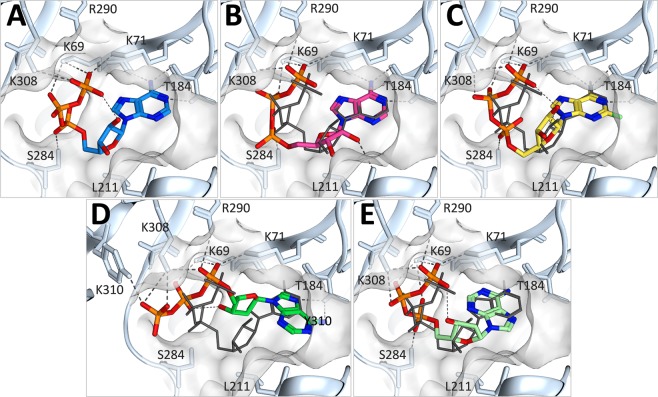
Figure 6.
Docking of ATP, 2F-ATP and 2′-dATP into the ATP binding site of rat P2X2. (A) Molecular model of rat P2X2 derived from the ATP-bound crystal structure of zebrafish P2X4 (PDB ID 4DW1) showing the key polar contacts (dashed lines) involved in ATP binding. (B) Representative pose from molecular docking of ATP into the rat P2X2 model using Glide XP (pink and orange sticks), compared to ATP from the molecular model (dark grey lines; RMSD = 1.4799). The position of the adenine ring is near-identical and the overall conformation, positioning and interactions of the phosphates are very similar. The ribose moiety is rotated, and the 2′ hydroxyl forms a hydrogen bond with the main chain of Leu-211. (C) Docking of 2F-ATP (yellow and orange sticks) compared to ATP from the molecular model (RMSD = 1.1970). In the 2F-ATP dock, the hydrogen bond between the 2′ hydroxyl and the γ-phosphate oxygen is maintained. (D,E) are representative poses obtained for 2′-dATP; either the U-shaped conformation of ATP is not maintained (D); RMSD = 4.0540), or the adenine ring is in a very different orientation (E), probably induced by a change in conformation of the ribose to accommodate a hydrogen bond between the 3′ hydroxyl and the γ-phosphate oxygen. N288 is not labeled for clarity.
Discussion
Heterologously expressing a mammalian P2X receptor in Drosophila taste neurons allowed us to rapidly screen 80 adenosine nucleotides for agonist activity, suggesting that P2X receptors can function alongside insect taste receptors in mediating action potential responses to ligands in taste neurons. Sugar taste in Drosophila is mediated by receptors encoded by members of the Gr gene family42,43, but the mechanism by which sugar reception is transduced to an action potential response is not yet clear. The sugar receptor BmGr-9 from the silkworm Bombyx mori mediates responses to D-fructose when expressed in Xenopus oocytes or HEK 293 cells44. Pharmacological experiments suggest that Ca2+ influx in response to fructose does not depend on G-protein coupled cascades, and further electrophysiological analyses of macroscopic and unitary currents supported the hypothesis that BmGr-9 forms a non-selective cation channel. P2X receptors are ligand-gated ion channels permeable to cations including K+, Na+ and Ca2+, although P2X5 also carries Cl−45. In rat P2X2, the Ca2+ current contributes 5.7% of the whole cell current, as measured by patch-clamp photometry46. Therefore, activation of P2X2 receptors can lead to membrane depolarization and may also affect cellular physiology through Ca2+ influx. The analogies in function between mammalian P2X receptors and an insect sugar Gr suggest sugar taste neurons may provide a useful and responsive expression system to assay P2X ligand specificities. The EC50 of 8.7 μM for rat P2X2-mCherry expressed in sugar taste neurons is indeed similar to the potency of ATP for rat P2X2 and rat P2X2-GFP expressed in Xenopus oocytes (EC50 = 6.5 µM and 13.5 µM, respectively38), and HEK 293 cells47. Rat P2X2 receptors have been previously expressed in Drosophila neurons and were shown to be functional in the CNS48 using both bath application of ATP on cholinergic neurons expressing the receptor and photo-release of caged ATP for the receptor expressed in the Giant Fiber system. Our experiments show that rat P2X2 is also functional in the peripheral nervous system.
As an assay platform, the Drosophila taste system is a neuronal expression system with endogenous receptors whose mechanisms of transduction show some analogy to P2X receptors, as discussed above. It may therefore be particularly suitable for functional studies of neuronally expressed P2X2/P2X3 heteromeric- or P2X3 homomeric receptors that are thought to act in nociceptive, inflammatory and neuropathic pain49,50. Some further practical considerations support the value of the Drosophila taste system as an assay platform for P2X receptors: (1) stimuli can be prepared in small quantities as a presentation can be achieved using only 2 μl of solution; (2) once transgenic flies are obtained, maintenance of the flies is straightforward and economical; (3) taste recording from insect sensilla is a well-established technique51. However, the observation that flies with pan-neural expression of wild-type rat P2X2 showed behavioral phenotypes three days after eclosion suggests that the receptor interferes to some extent with neuronal physiology in adults. Strong pan-neural expression of rat P2X2 under control of the elav-GAL4 driver line was previously reported to cause subtle incoordination in Drosophila while lower expression levels under control of a weaker driver line did not48. Larvae of Drosophila with pan-neural expression of rat P2X2 did not show conspicuous phenotypes. While members of the P2X receptor family are present in diverse clades of the Metazoa, they appear to be absent in insects52 and our observations suggest adult Drosophila may be affected by expression of ligand-gated channels with high sensitivity for extracellular ATP. We do not know whether the extracellular space around Drosophila neurons contains enough ATP to activate P2X channels, but taste neurons expressing rat P2X2 did show a higher baseline action potential activity than neurons in control flies even in the absence of experimentally applied ATP. A larger frequency of miniature excitatory junction potentials in recorded muscles was also noted when rat P2X2 was expressed in cholinergic neurons innervating abdominal muscles via the glutamatergic motor neurons48. Single channel recordings on P2X2 have shown no spontaneous channel opening53,54, so the increased baseline of activity that we observed is unlikely to be caused by stochastic opening of non-stimulated channels. Since the expression of the T184I mutant did not appear to affect the behavior of the flies, poor tolerance for high levels of mammalian membrane protein expression per se do not seem to underlie the behavioral phenotypes observed in flies expressing WT rat P2X2 receptors.
Here, we have demonstrated that 2F-ATP is a novel full agonist for rat P2X2, and have shown computationally that it adopts a very similar conformation to ATP in the P2X2 receptor binding pocket. Together with partial activity of 2-hydroxy-ATP tested here, and full activity of 2-chloro-ATP reported previously55, we suggest that some substitutions on the 2-position of the adenine base are well-tolerated. Diadenosine tetraphosphate (AP4A) did not elicit responses in the taste neurons although it was reported to act as a full agonist at 100 μM in Xenopus oocytes37. In this regard, it might be informative to examine the activity of AP4A on P2X2 receptors expressed in other systems, such as HEK 293 cells. However, the taste neuron responses to ATPγS and ATPαS, the conserved low responses to αβ-meATP (ApCpp) and βγ-meATP (AppCp) reviewed in30,32, together with our direct demonstration of reduced ATP potency and activity of the T184I mutation18, would suggest very strongly that rat P2X2 in the Drosophila system does function similarly as it does in other heterologous systems. This means the variance we have demonstrated in activity of AP4A at the rat P2X2 receptor are likely to be due to differences in the expression systems used.
The compound library comprises a variety of adenosine nucleotides and most show less activity than ATP, indicating that several different moieties of ATP can be recognized in the ATP binding pocket. Indeed, earlier findings from systematic alanine and cysteine scanning mutational analyses have pointed to an extensive number of residues that co-ordinate binding of ATP (reviewed by56) and this is corroborated by the solved P2X structures. Lysine residues interact with the phosphoryl groups (in rat P2X2 numbering: K69, K71, and K308 through hydrogen bonds and K188 via solvent molecules) as do N288 and R290. The ribose ring is recognized through hydrophobic interactions with L211 and the adenine moiety is co-ordinated by hydrogen bonding with K69 and T184 as well as through hydrophobic interactions with L186 and I226. T184 interacts with the N atoms in adenine at N1 and C6 through two hydrogen bonds. Indeed, substitution of this residue greatly reduced the potency and activity of ATP in our study, as has been shown previously18. Adenine position C2 does not appear to be co-ordinated by the binding pocket, and several modifications at this position show full (2F-ATP) or partial (2-hydroxy-ATP) agonist activity. In contrast, several chemicals, with modifications of ATP at positions that do not interact with residues in the binding pocket in the solved P2X structure, such as in 2′-dATP or modifications at adenine C8, did display reduced activity. Fryatt et al. reported that while 3′-dATP was a full agonist at P2X1 receptors, 2′-dATP displayed no agonist activity41. They used molecular dynamics simulations to demonstrate that the loss of an intramolecular hydrogen bond between the 2′ hydroxyl and a γ-phosphate oxygen could destabilize the U-shaped conformation of ATP required for efficient agonist action. In terms of rat P2X2 receptors, Gasparri et al. found that for 3′-dATP the EC50 was increased by a factor of 3, but that 2′-dATP displayed very little agonist activity33. Similarly in our study, both 3′- and 2′-dATP displayed reduced activity compared to ATP, with 2′-dATP having the lowest activity, suggesting broad agreement between the agonist profiles of P2X1 and P2X2. However, in our docking simulations, we observed two 2′-dATP binding modes to rat P2X2; one where the U-shaped conformation was lost, and one where maintenance of the U-shaped conformation required an intramolecular hydrogen bond between the γ-phosphate oxygen and the 3′-hydroxyl of the ribose, which induced a significant translation in the adenine ring. One intriguing possibility which emerges from our docking simulations is that ATP may adopt different conformations within the binding pocket. For example, our docking simulations with ATP never showed the requirement of the intramolecular hydrogen bond, instead, the 2′-hydroxyl of the ribose formed an intermolecular hydrogen bond with the main chain of Leu-211; this was observed in multiple poses. However, in our docking simulations with 2F-ATP, we observed the intramolecular bond in every conformation where the adenine ring and the triphosphate positions were preserved. While it seems critical for agonist action that the phosphates (particularly the γ-phosphate) and adenine ring are in the correct conformation, it may be possible that the ribose can be stabilized in different conformations in the binding pocket either by intermolecular or intramolecular hydrogen bonds. In either situation, loss of this hydrogen bond (by loss of the 2′-hydroxyl group) would have a negative effect on the stability of the agonist in the binding pocket. An alternative explanation, offered by Gasparri et al., based upon their observations that both 2′-F-dATP and the C3′-endo-locked LNA-ATP displayed significant agonist activity, is that it is the sugar pucker conformation of the ribose, rather than the propensity for intramolecular hydrogen-bonding, that is important for agonist recognition33, and our docking simulations do not discount this.
In our study, rat P2X2 exhibited a relatively broad response profile among the 80 adenosine nucleotides, which ranged smoothly from no response to full agonist activity. The solved crystal structures show that ATP is bound at the extracellular loop interface between two subunits within a trimer25,26,57,58, as was also suggested by previous research59,60. While the holoprotein can thereby bind three ATP molecules simultaneously, only two functional binding sites are required to be occupied in order to facilitate channel opening61. Careful early work on single channel opening kinetics had already suggested multiple binding steps and co-operative binding of the three ATP molecules to P2X253. It is now apparent from the work of Browne and North62 that co-operativity extends to nucleotides other than ATP. Thus, nucleotides such as CTP, UTP and even ADP are able to affect channel opening in the presence of very low concentrations of ATP, as inferred from whole-cell current responses, suggesting that binding of one molecule of ATP results in an intermediate closed channel state that permits further binding and activation of the channel by other nucleotides41. This result implies that the P2X2 binding pocket can accommodate diverse nucleotides and that these nucleotides can contribute to channel opening. Indeed, the sensitization of P2X receptors by a priming ATP stimulus has recently been used to probe the binding affinity of P2X for other nucleotides63. Therefore, one interpretation of our results is that the adenosine nucleotides were applied against a low background of ATP present in the extracellular space, as discussed above, so that we were able to obtain responses from a broader range of nucleotides than we would get from a naïve receptor. However, for the range of adenosine nucleotides tested here, there is no comparable study in the literature, so it is rather difficult to assess the extent to which the ligand specificity of rat P2X2 in Drosophila recapitulates its function in other heterologous systems. It is intriguing to speculate that a receptor such as P2X2 may have more than one palette of ligands to which it responds when its binding sites are successively occupied.
Methods
Drosophila stocks
Transgenic constructs were injected into embryos of a phiC31 expressing attp40 stock (stock 13–20 from the Cambridge Fly Facility) to obtain inserts in a 2nd chromosome landing site. Transgenic flies were crossed to flies carrying Gr5a-GAL4 (a gift from Anupama Dahanukar) and to flies of C155, which is an enhancer trap line expressing GAL4 under control of the elav promoter64, to obtain flies that drive expression of rat P2X2 under control of the Gr5a promoter in the sugar cell of the Drosophila taste sensilla (w; UAS-ratP2X2; Gr5a-GAL4) or pan-neurally (elav-GAL4; UAS-ratP2X2). Adult flies having pan-neural expression of rat P2X2 developed a shaking behavioral phenotype 3–5 days after eclosion, so flies were used for electrophysiology on the day after eclosion only. Adult flies expressing a mutant copy of rat P2X2, which causes an amino acid substitution in the predicted ATP binding pocket of the channel (T184I: see below), did not exhibit such a phenotype and were used 5–10 days after eclosion. As a wild-type stock, Oregon-R was used.
Construction of transgenes
The coding sequence of rat P2X2 in pcDNA3.1 (clone p698, a gift from Alan North) was amplified by PCR and ligated upstream and in-frame with mCherry in a pUAST-attB(x19) vector, which is the pUAST-attB vector modified to contain 19 UAS sites in tandem for enhanced expression (gift of Sonia Lopez de Quinto, Cardiff University, U.K.). The coding region for human P2X2 variant A (also called variant 1: NCBI reference sequence NM_170682.4) was obtained by synthesis (Life Technologies) and ligated in the pUAST-attB(x19) vector with mCherry to generate a construct encoding human P2X2 with a C-terminal mCherry fusion tag. All constructs were verified by sequencing. In addition to a construct with the wild type (WT) rat P2X2 sequence, a mutant clone encoding T184I in the rat P2X2 sequence was generated serendipitously. This mutant was ligated in place of zebra fish P2X4 in the zfP2X4.1 construct (kindly provided by Eric Gouaux), whereupon the rat P2X2-GFP-His tag sequence was amplified by PCR and ligated into pUAST-attB(x19).
Electrophysiology
Flies were prepared for electrophysiology (Fig. 1A) as described in Benton and Dahanukar65. Chemical stimuli were presented and action potentials were recorded from gustatory neurons using standard methods and apparatus, as described previously43. Briefly, the open tip (<20 μm outer diameter) of a recording electrode containing the stimulus solution was placed over the distal end of an L3,4 or L7–9 labellar taste sensillum (nomenclature following66) so that action potentials from the neurons were recorded extracellularly as the stimulus was presented. Stimulus solutions were prepared by diluting 1 mM stock solutions of chemicals from a library of 80 adenosine nucleotides (Jena Biosciences) to 100 μM in water containing 30 mM tricholine citrate. Tricholine citrate in the solvent provides conductance and suppresses activity of the water neuron, but has no appreciable effect on the other taste neurons67. Stock solutions were stored frozen at −20°C, and the 100 μM solutions were prepared daily; during experiments they were kept cold on crushed ice until required. PPADS (pyridoxal phosphate-6-azo(benzene-2,4-disulfonic acid) tetrasodium salt was obtained from Sigma-Aldrich. Recordings were band-pass filtered between 50 Hz and 5 KHz and digitized at 10 KHz. Basic analysis consisted of counting the maximum number of impulses in a 1 s interval within the first 5 s of a recording using Autospike 32 software (Syntech, Germany). For the T184S mutant, which exhibited slower onset of response and lower impulse rates, the response was obtained by taking the average number of impulses per s between 10–15 s after stimulus onset. For screening the library of adenosine nucleotides, up to 10 stimulus chemicals were presented per fly; each chemical was tested on 3–6 sensilla on at least two flies. Responses were normalized to the response to 100 μM ATP (Sigma-Aldrich), which was presented after every third stimulus.
To validate agonist activity of selected chemicals, whole-cell transmembrane current responses were recorded in the whole-cell voltage-clamp configuration. HEK 293 cells heterologously expressing rat P2X2 receptor were held at a voltage of −60 mV and nucleotides were presented using a gravity-fed Rapid Perfusion System (RSC, Biologic) which was capable of exchanging solutions within 20 ms. Complete details of the methods and equipment employed for recording were as described by Wilkinson et al.47.
Homology modeling and ligand docking
Homology modeling of rat P2X2 receptor was performed using Modeller (https://salilab.org/modeller/; Version 9.10)68 on the zebrafish P2X4 crystal structure in the ATP-bound open state (PDB ID 4DW1)25 as a template. Docking was performed using Schrödinger software (www.schrodinger.com; LigPrep, Epik, Glide-XP and Maestro: all from release 2015–1). Ligands were prepared (with LigPrep using the OPLS_2005 force field and Epik to generate ionization states at pH 7.0 ± 2.0), and docked into the ATP binding site of the molecular model using Glide-XP within a box of 22 Å. Poses were inspected visually and selected according to the lowest docking score values and maintenance of interactions known to be critical for agonist action. Images of docking poses were generated using UCSF Chimera (https://www.cgl.ucsf.edu/chimera/; Version 1.11)69.
Acknowledgements
We would like to thank Eric Gouaux (Vollum Institute, Oregon Health and Science University, USA), Alan North (University of Manchester, UK), and Sonia Lopez de Quinto (Cardiff University, UK) for plasmids used in this study and Anupama Dahanukar (University of California, USA) for the Gr5a-GAL4 flies. We thank Pete Watson (Cardiff University, UK) for assistance using the light sheet microscope, which was purchased through the Infrastructure Fund (Cardiff University), in the Bioimaging Research Hub at the Cardiff School of Biosciences. Molecular graphics and analyses were preformed with UCSF Chimera, developed by the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco, with support from NIH P41-GM103311. L.G. was funded by a joint BBSRC/MRC PhD studentship.
Author contributions
L.G., J.G., G.P. and W.V.N. performed the experiments. M.Y. and W.V.N. designed the research study. A.B., P.K., M.Y. and W.V.N. supervised the project. L.G., J.G., G.P., P.K., A.B and W.V.N. analysed the data. L.G., J.G., G.P. and W.V.N. produced the figures. M.Y. and W.V.N. wrote the manuscript. The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mark T. Young, Email: YoungMT@cardiff.ac.uk
Wynand van der Goes van Naters, Email: VanderGoesvanNatersWM@cardiff.ac.uk.
References
- 1.Chizh BA, Illes P. P2X receptors and nociception. Pharmacol. Rev. 2001;53:553–568. [PubMed] [Google Scholar]
- 2.Adinolfi E, Capece M, Amoroso F, De Marchi E, Franceschini A. Emerging roles of P2X receptors in cancer. Curr. Med. Chem. 2015;22:878–890. doi: 10.2174/0929867321666141012172913. [DOI] [PubMed] [Google Scholar]
- 3.Labasi JM, et al. Absence of the P2X7 receptor alters leukocyte function and attenuates an inflammatory response. J. Immunol. 2002;168:6436–6445. doi: 10.4049/jimmunol.168.12.6436. [DOI] [PubMed] [Google Scholar]
- 4.Broom DC, et al. Characterization of N-(adamantan-1-ylmethyl)-5-[(3R-amino-pyrrolidin-1-yl)methyl]-2-chloro-benzamide, a P2X7 antagonist in animal models of pain and inflammation. J. Pharmacol. Exp. Ther. 2008;327:620–633. doi: 10.1124/jpet.108.141853. [DOI] [PubMed] [Google Scholar]
- 5.Ke HZ, et al. Deletion of the P2X7 nucleotide receptor reveals its regulatory roles in bone formation and resorption. Mol. Endocrinol. 2003;17:1356–1367. doi: 10.1210/me.2003-0021. [DOI] [PubMed] [Google Scholar]
- 6.Pijacka W, et al. Purinergic receptors in the carotid body as a new drug target for controlling hypertension. Nat. Med. 2016;22:1151–1159. doi: 10.1038/nm.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khakh BS, North RA. P2X receptors as cell-surface ATP sensors in health and disease. Nature. 2006;442:527–532. doi: 10.1038/nature04886. [DOI] [PubMed] [Google Scholar]
- 8.Lazarowski ER. Vesicular and conductive mechanisms of nucleotide release. Purinergic Signal. 2012;8:359–373. doi: 10.1007/s11302-012-9304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lazarowski ER, Boucher RC, Harden TK. Constitutive release of ATP and evidence for major contribution of ecto-nucleotide pyrophosphatase and nucleoside diphosphokinase to extracellular nucleotide concentrations. J. Biol. Chem. 2000;275:31061–31068. doi: 10.1074/jbc.M003255200. [DOI] [PubMed] [Google Scholar]
- 10.Fountain, S. J. et al. An intracellular P2X receptor required for osmoregulation in Dictyostelium discoideum. Nature448, 200–203 (2007). [DOI] [PMC free article] [PubMed]
- 11.Parkinson K, et al. Calcium-dependent regulation of Rab activation and vesicle fusion by an intracellular P2X ion channel. Nat. Cell Biol. 2014;16:87–98. doi: 10.1038/ncb2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ren J, et al. P2X2 subunits contribute to fast synaptic excitation in myenteric neurons of the mouse small intestine. J. Physiol. 2003;552:809–821. doi: 10.1113/jphysiol.2003.047944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mateo J, Garcia-Lecea M, Miras-Portugal MT, Castro E. Ca2+ signals mediated by P2X-type purinoceptors in cultured cerebellar Purkinje cells. J. Neurosci. 1998;18:1704–1712. doi: 10.1523/JNEUROSCI.18-05-01704.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bardoni R, Goldstein PA, Lee CJ, Gu JG, MacDermott AB. ATP P2X receptors mediate fast synaptic transmission in the dorsal horn of the rat spinal cord. J. Neurosci. 1997;17:5297–5304. doi: 10.1523/JNEUROSCI.17-14-05297.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Villalobos C, Alonso-Torre SR, Nunez L, Garcia-Sancho J. Functional ATP receptors in rat anterior pituitary cells. Am. J. Physiol. 1997;273:C1963–1971. doi: 10.1152/ajpcell.1997.273.6.C1963. [DOI] [PubMed] [Google Scholar]
- 16.Tomic M, Jobin RM, Vergara LA, Stojilkovic SS. Expression of purinergic receptor channels and their role in calcium signaling and hormone release in pituitary gonadotrophs. Integration of P2 channels in plasma membrane- and endoplasmic reticulum-derived calcium oscillations. J. Biol. Chem. 1996;271:21200–21208. doi: 10.1074/jbc.271.35.21200. [DOI] [PubMed] [Google Scholar]
- 17.Finger TE, et al. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 2005;310:1495–1499. doi: 10.1126/science.1118435. [DOI] [PubMed] [Google Scholar]
- 18.Jiang LH, Rassendren F, Surprenant A, North RA. Identification of amino acid residues contributing to the ATP-binding site of a purinergic P2X receptor. J. Biol. Chem. 2000;275:34190–34196. doi: 10.1074/jbc.M005481200. [DOI] [PubMed] [Google Scholar]
- 19.Ennion S, Hagan S, Evans RJ. The role of positively charged amino acids in ATP recognition by human P2X(1) receptors. J. Biol. Chem. 2000;275:29361–29367. doi: 10.1074/jbc.M003637200. [DOI] [PubMed] [Google Scholar]
- 20.Roberts JA, Valente M, Allsopp RC, Watt D, Evans RJ. Contribution of the region Glu181 to Val200 of the extracellular loop of the human P2X1 receptor to agonist binding and gating revealed using cysteine scanning mutagenesis. J. Neurochem. 2009;109:1042–1052. doi: 10.1111/j.1471-4159.2009.06035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberts JA, Evans RJ. ATP binding at human P2X1 receptors. Contribution of aromatic and basic amino acids revealed using mutagenesis and partial agonists. J. Biol. Chem. 2004;279:9043–9055. doi: 10.1074/jbc.M308964200. [DOI] [PubMed] [Google Scholar]
- 22.Roberts JA, Evans RJ. Cysteine substitution mutants give structural insight and identify ATP binding and activation sites at P2X receptors. J. Neurosci. 2007;27:4072–4082. doi: 10.1523/JNEUROSCI.2310-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberts JA, et al. Cysteine substitution mutagenesis and the effects of methanethiosulfonate reagents at P2X2 and P2X4 receptors support a core common mode of ATP action at P2X receptors. J. Biol. Chem. 2008;283:20126–20136. doi: 10.1074/jbc.M800294200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawate T, Michel JC, Birdsong WT, Gouaux E. Crystal structure of the ATP-gated P2X(4) ion channel in the closed state. Nature. 2009;460:592–598. doi: 10.1038/nature08198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hattori M, Gouaux E. Molecular mechanism of ATP binding and ion channel activation in P2X receptors. Nature. 2012;485:207–212. doi: 10.1038/nature11010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mansoor SE, et al. X-ray structures define human P2X(3) receptor gating cycle and antagonist action. Nature. 2016;538:66–71. doi: 10.1038/nature19367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chataigneau T, Lemoine D, Grutter T. Exploring the ATP-binding site of P2X receptors. Front. Cell. Neurosci. 2013;7:273. doi: 10.3389/fncel.2013.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grimes L, Young MT. Purinergic P2X receptors: structural and functional features depicted by X-ray and molecular modelling studies. Curr. Med. Chem. 2015;22:783–798. doi: 10.2174/0929867321999141212131457. [DOI] [PubMed] [Google Scholar]
- 29.Pasqualetto G, Brancale A, Young MT. The Molecular Determinants of Small-Molecule Ligand Binding at P2X Receptors. Front. Pharmacol. 2018;9:58. doi: 10.3389/fphar.2018.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coddou C, Yan Z, Obsil T, Huidobro-Toro JP, Stojilkovic SS. Activation and regulation of purinergic P2X receptor channels. Pharmacol. Rev. 2011;63:641–683. doi: 10.1124/pr.110.003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacobson, K. A. & Muller, C. E. Medicinal chemistry of adenosine, P2Y and P2X receptors. Neuropharmacology (2015). [DOI] [PMC free article] [PubMed]
- 32.Lambertucci C, et al. Medicinal chemistry of P2X receptors: agonists and orthosteric antagonists. Curr. Med. Chem. 2015;22:915–928. doi: 10.2174/0929867321666141215093513. [DOI] [PubMed] [Google Scholar]
- 33.Gasparri F, Wengel J, Grutter T, Pless SA. Molecular determinants for agonist recognition and discrimination in P2X2 receptors. J. Gen. Physiol. 2019;151:898–911. doi: 10.1085/jgp.201912347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van der Goes van Naters W, Carlson JR. Insects as chemosensors of humans and crops. Nature. 2006;444:302–307. doi: 10.1038/nature05403. [DOI] [PubMed] [Google Scholar]
- 35.Dahanukar A, Hallem EA, Carlson JR. Insect chemoreception. Curr. Opin. Neurobiol. 2005;15:423–430. doi: 10.1016/j.conb.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 36.King BF, Wildman SS, Ziganshina LE, Pintor J, Burnstock G. Effects of extracellular pH on agonism and antagonism at a recombinant P2X2. receptor. Brit. J. Pharm. 1997;121:1445–1453. doi: 10.1038/sj.bjp.0701286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pintor J, King BF, Miras-Portugal MT, Burnstock G. Selectivity and activity of adenine dinucleotides at recombinant P2X2 and P2Y1 purinoceptors. Brit. J. Pharm. 1996;119:1006–1012. doi: 10.1111/j.1476-5381.1996.tb15771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khakh BS, et al. Activation-dependent changes in receptor distribution and dendritic morphology in hippocampal neurons expressing P2X2-green fluorescent protein receptors. Proc. Natl. Acad. Sci. USA. 2001;98:5288–5293. doi: 10.1073/pnas.081089198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kowalski M, et al. Flexible subunit stoichiometry of functional human P2X2/3 heteromeric receptors. Neuropharmacology. 2015;99:115–130. doi: 10.1016/j.neuropharm.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 40.Grote A, Boldogkoi Z, Zimmer A, Steinhauser C, Jabs R. Functional characterization of P2X3 receptors fused with fluorescent proteins. Mol. Membr. Biol. 2005;22:497–506. doi: 10.1080/09687860500370638. [DOI] [PubMed] [Google Scholar]
- 41.Fryatt AG, Dayl S, Cullis PM, Schmid R, Evans RJ. Mechanistic insights from resolving ligand-dependent kinetics of conformational changes at ATP-gated P2X1R ion channels. Sci. Rep. 2016;6:32918. doi: 10.1038/srep32918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Freeman EG, Wisotsky Z, Dahanukar A. Detection of sweet tastants by a conserved group of insect gustatory receptors. Proc. Natl. Acad. Sci. USA. 2014;111:1598–1603. doi: 10.1073/pnas.1311724111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dahanukar A, Foster K, van der Goes van Naters WM, Carlson JR. A Gr receptor is required for response to the sugar trehalose in taste neurons of. Drosophila. Nat. Neurosci. 2001;4:1182–1186. doi: 10.1038/nn765. [DOI] [PubMed] [Google Scholar]
- 44.Sato K, Tanaka K, Touhara K. Sugar-regulated cation channel formed by an insect gustatory receptor. Proc. Natl. Acad. Sci. USA. 2011;108:11680–11685. doi: 10.1073/pnas.1019622108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bo X, et al. Pharmacological and biophysical properties of the human P2X5 receptor. Mol. Pharmacol. 2003;63:1407–1416. doi: 10.1124/mol.63.6.1407. [DOI] [PubMed] [Google Scholar]
- 46.Egan TM, Khakh BS. Contribution of calcium ions to P2X channel responses. J. Neurosci. 2004;24:3413–3420. doi: 10.1523/JNEUROSCI.5429-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilkinson WJ, et al. Carbon monoxide is a rapid modulator of recombinant and native P2X(2) ligand-gated ion channels. Brit. J. Pharm. 2009;158:862–871. doi: 10.1111/j.1476-5381.2009.00354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lima SQ, Miesenbock G. Remote control of behavior through genetically targeted photostimulation of neurons. Cell. 2005;121:141–152. doi: 10.1016/j.cell.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 49.Burnstock G. Purinergic Mechanisms and Pain. Adv. Pharmacol. 2016;75:91–137. doi: 10.1016/bs.apha.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 50.Tsuda M, Tozaki-Saitoh H, Inoue K. Pain and purinergic signaling. Brain Res. Rev. 2010;63:222–232. doi: 10.1016/j.brainresrev.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 51.Hodgson ES, Lettvin JY, Roeder KD. Physiology of a primary chemoreceptor unit. Science. 1955;122:417–418. doi: 10.1126/science.122.3166.417-a. [DOI] [PubMed] [Google Scholar]
- 52.Fountain, S. J. & Burnstock, G. An evolutionary history of P2X receptors. Purinergic Signal. 5 (2009). [DOI] [PMC free article] [PubMed]
- 53.Ding S, Sachs F. Single channel properties of P2X2 purinoceptors. J. Gen. Physiol. 1999;113:695–720. doi: 10.1085/jgp.113.5.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cao L, Young MT, Broomhead HE, Fountain SJ, North RA. Thr339-to-serine substitution in rat P2X2 receptor second transmembrane domain causes constitutive opening and indicates a gating role for Lys308. J. Neurosci. 2007;27:12916–12923. doi: 10.1523/JNEUROSCI.4036-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Evans RJ, et al. Pharmacological characterization of heterologously expressed ATP-gated cation channels (P2x purinoceptors) Mol. Pharmacol. 1995;48:178–183. [PubMed] [Google Scholar]
- 56.Evans RJ. Orthosteric and allosteric binding sites of P2X receptors. Eur. Biophys. J. 2009;38:319–327. doi: 10.1007/s00249-008-0275-2. [DOI] [PubMed] [Google Scholar]
- 57.Karasawa A, Kawate T. Structural basis for subtype-specific inhibition of the P2X7 receptor. eLife. 2016;5:e22153. doi: 10.7554/eLife.22153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kasuya G, et al. Structural Insights into Divalent Cation Modulations of ATP-Gated P2X Receptor Channels. Cell Rep. 2016;14:932–944. doi: 10.1016/j.celrep.2015.12.087. [DOI] [PubMed] [Google Scholar]
- 59.Wilkinson WJ, Jiang LH, Surprenant A, North RA. Role of ectodomain lysines in the subunits of the heteromeric P2X2/3 receptor. Mol. Pharmacol. 2006;70:1159–1163. doi: 10.1124/mol.106.026658. [DOI] [PubMed] [Google Scholar]
- 60.Marquez-Klaka B, Rettinger J, Bhargava Y, Eisele T, Nicke A. Identification of an intersubunit cross-link between substituted cysteine residues located in the putative ATP binding site of the P2X1 receptor. J. Neurosci. 2007;27:1456–1466. doi: 10.1523/JNEUROSCI.3105-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stelmashenko O, et al. Activation of trimeric P2X2 receptors by fewer than three ATP molecules. Mol. Pharmacol. 2012;82:760–766. doi: 10.1124/mol.112.080903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Browne LE, North RA. P2X receptor intermediate activation states have altered nucleotide selectivity. J. Neurosci. 2013;33:14801–14808. doi: 10.1523/JNEUROSCI.2022-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kasuya G, et al. Structural insights into the nucleotide base specificity of P2X receptors. Sci. Rep. 2017;7:45208. doi: 10.1038/srep45208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lin DM, Goodman CS. Ectopic and increased expression of Fasciclin II alters motoneuron growth cone guidance. Neuron. 1994;13:507–523. doi: 10.1016/0896-6273(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 65.Benton R, Dahanukar A. Electrophysiological recording from Drosophila taste sensilla. Cold Spring Harb. Protoc. 2011;2011:839–850. doi: 10.1101/pdb.prot5631. [DOI] [PubMed] [Google Scholar]
- 66.Weiss LA, Dahanukar A, Kwon JY, Banerjee D, Carlson JR. The molecular and cellular basis of bitter taste in Drosophila. Neuron. 2011;69:258–272. doi: 10.1016/j.neuron.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wieczorek H, Wolff G. The labellar sugar receptor of Drosophila. J. Comp. Physiol. [A] 1989;164:825–834. doi: 10.1007/BF00616754. [DOI] [Google Scholar]
- 68.Sali A, Blundell TL. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 1993;234:779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- 69.Pettersen EF, et al. UCSF Chimera – a visualisation system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]