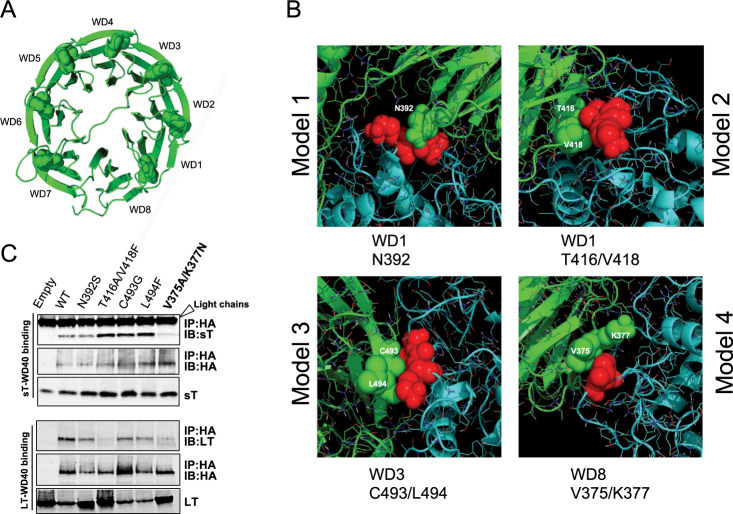
Fig. 2. Identification of new allosteric inhibitory sites responsible for sT FBW7 targeting.
a FBW7 WD40 domain. Ribbon diagram of the 8 WD-repeats in FBW7 WD40. Tryptophan-aspartic acid (WD) dipeptides are depicted as spheres. b Inter-residue interactions in MCV sT and WD40 domain docking models. To select and interpret correlated mutations in the WD40, MCV sT (cyan)-WD40 (green) complex structures from 4 potential docking models were analyzed and visualized based on the use of inter-residue contacts (green spears) with amino acids LK (red spears) in LSD. c WD8 blade beta propeller is a critical site for both sT and LT interactions. Co-immunoprecipitation assay shows that both MCV LT and sT binding were reduced by FBW7-WD40 mutations in residues V375 and K377 (V375A/K377N). This mutation is located within the WD8 blade.