Abstract
Compared with other racial/ethnic groups, African Americans have the highest colorectal cancer (CRC) morbidity and mortality rates. It is critical to help improve African Americans’ CRC prevention efforts in order to reduce the burden of CRC in this community. The aim of this study was to develop and field test a tablet app, called e-Motivate, designed to improve African Americans’ screening colonoscopy rates. The e-Motivate app was field tested, using an iterative approach. The first version of the app, e-Motivate 1.0, was field tested on 20 African Americans over the age of 50. Participants engaged in a think aloud exercise and provided feedback regarding the app’s usability and acceptability. The results of the first field test were used to modify the app and develop e-Motivate 2.0. The field test procedures were repeated on a new group of participants (N = 18). The results from the second field test were used to make final modifications to the app. Overall, participants responded positively to the app. Qualitative analyses showed that participants found the app to be easy to use and helpful. Furthermore, descriptive statistics revealed that participants found the app to be highly usable and acceptable, exceeding recommended benchmarks for usability and acceptability. Critiques of the app were used to modify and finalize the intervention. The results from the proposed study suggest that the e-Motivate app is highly feasible and acceptable. The next step in this line of research is to conduct a randomized clinical trial to formally test the efficacy of the e-Motivate app for improving screening colonoscopy rates among African Americans.
Keywords: Colorectal cancer, Digital health, Cancer prevention, African Americans
Implications
Practice: If the e-Motivate app proves efficacious, there is a strong argument to integrate the app into standard clinical care to help improve African Americans’ screening colonoscopy rates.
Policy: Hospitals and payers should explore digital health interventions designed to reduce colorectal cancer disparities between African Americans and whites, as these interventions are proving to be highly acceptable and feasible.
Research: A future randomized clinical trial needs to formally evaluate the efficacy of the e-Motivate app for improving African Americans’ screening colonoscopy rates.
INTRODUCTION
Colorectal cancer (CRC) remains the third leading cause of cancer death in men and women in the USA [1]. Compared with whites, African Americans are the more likely to be diagnosed with and die from CRC [2]. Guidelines recommend that average risk adults undergo regular CRC screening [3]. Of the recommended CRC screening tests, a colonoscopy is often the preferred method because it allows for the detection and removal of precancerous and cancerous growths [4, 5]. Epidemiological research has found an association between increased screening colonoscopy rates and reduced mortality rates [6]. Although screening colonoscopies can detect and prevent CRC, more than one-third of African Americans have not received a screening colonoscopy within the recommended time frame [7].
The current literature suggests that a motivational interviewing (MI)–based intervention may help improve screening colonoscopy uptake. MI is defined as “a collaborative conversation style for strengthening a person’s own motivation and commitment to change [8, p. 29].” MI has four sequential yet fluid processes: engaging, focusing, evoking, and planning [8]. During the engaging process, the clinician and client form a respectful and collaborative working relationship. To do so, the clinician expresses empathy and acceptance, while supporting the client’s autonomy. During the focusing process, the client and clinician work together to agree upon the focus or goal of the work. During the evoking process, the clinician helps evoke or draw out the client’s own motivations for change. Finally during the planning process, the client and clinician take active steps to plan for change. Core MI skills include asking open-ended questions, affirming, reflecting, and summarizing the clients’ thoughts.
MI has proven efficacious for a wide range of health behaviors including alcohol and nicotine use [9–12], diet [13–15], exercise [13], and medication adherence [16]. A systematic review of the literature suggests that MI may help improve the uptake of health screening (e.g., mammography and HIV testing) tests [17]. A 2018 study found that a phone-based MI intervention significantly improved colonoscopy uptake among individuals who had a first-degree relative with CRC [18]. Extensive empirical evidence supports the use of MI with African Americans to improve health behaviors including increasing HIV testing [19], improving medication adherence [20,21], and increasing fruit and vegetable intake [22].
Traditionally, MI is delivered live, where individuals meet with a professional either in person or over the telephone for a one-on-one intervention. Although efficacious, live-MI is not without limitations. Of greatest concern, live-MI requires both staffing and economic resources, limiting its potential for wide dissemination. As such, many studies have examined the efficacy of e-MI, that is, MI interventions delivered via electronic media (e.g., tablet, computer, and smartphone). A 2016 systematic review [23] of technology-delivered adaptations of MI interventions yielded 41 studies that evaluated the impact of e-MI. Approximately half of the studies targeted substance abuse and the remaining studies targeted other health outcomes such as improving medication adherence, decreasing risky sexual behaviors, and improving diet and exercise. Two studies included in the review examined e-MI interventions for improving health screening uptake. One study established the feasibility of a computer-assisted telephone interview (CATI) that used MI-consistent computer prompts to guide a telephone counseling call encouraging women to get a mammogram [24]. A second study conducted a randomized clinical trial that established the efficacy of an e-MI “talking laptop” for improving HIV testing among predominantly African American individuals in the criminal justice system [25]. These studies provide initial support for the feasibility and acceptability of conducting e-MI interventions within the context of cancer prevention.
Despite the promise of e-MI, no research to date has examined the efficacy of an e-MI intervention to help improve African Americans’ screening colonoscopy rates. It is hypothesized that this type of intervention may help improve African Americans’ screening colonoscopy rates, and in doing so, reduce the CRC disparities between African Americans and whites. The purpose of the presented study was to develop and field test an MI-informed tablet–delivered intervention designed to improve African Americans’ screening colonoscopy uptake. The results from this study were used to build a final version of the app that will be tested in a future randomized clinical trial.
METHODS
All study procedures received approval from Icahn School of Medicine at Mount Sinai's Program for the Protection of Human Subjects.
Preliminary app development
According to the U.S. Department of Health and Human Services’ (HHS) Research-Based Web Design and Usability Guidelines [26], it is important to use an iterative approach (i.e., test, revise, test, and revise) when developing a digital health intervention. Research has found that using an iterative approach can help improve, efficiency, user satisfaction, and overall user engagement [26]. Drawing directly from these guidelines, the proposed study aimed to develop an app, called e-Motivate, using an iterative approach.
Before developing the first iteration of the app, an interactive online prototype was created. The development of the prototype was guided by the most recent MI literature and included the four MI processes, MI-informed exercises (e.g., decisional balance) and implemented MI-consistent techniques (e.g., rolling with resistance and developing discrepancies). The prototype was presented to two focus groups to gather initial feedback and suggestions. The first focus group (N = 5) consisted of African American community members over the age of 50. The second focus group (N = 9) consisted of gastroenterology and primary care providers (e.g., nurses, residents, and fellows). The focus group members provided their feedback regarding the development of the app. In particular, they emphasized the importance of using video content, rather than written content, so that the app could be appropriate for individuals with varying levels of literacy. They also recommended the incorporation of game-like features in order to improve potential user-engagement.
Description of E-Motivate 1.0
The first version of the app, called e-Motivate 1.0, was developed based on the focus group feedback and the recent published MI literature. The app was designed to be completed in a primary care clinic, on a on a clinic-provided tablet, immediately after an African American patient receives a referral for a screening colonoscopy. Depending on the clinic, the app could be introduced or “prescribed” to patients by the referring physician, nurse, or patient navigator.
E-Motivate 1.0 aimed to incorporate the four processes of MI (i.e., engaging, focusing, eliciting, and planning). During the engaging process, a video featuring an African American health educator used nonconfrontational and autonomy supportive language to welcome users to the app. For example, at the beginning of the video, the health educator stated “some people feel unsure about whether or not they want to have a screening colonoscopy. This app is designed to help you decide whether or not having a screening colonoscopy is right for you.”
Then, during the focusing process, users were asked to reaffirm their willingness to discuss their decision to have a screening colonoscopy. If users agreed on the focus, the app elicited the users’ previous knowledge about CRC and its prevention. Specifically, users were asked whether statements were “myths” or “facts.” The statements covered topics including CRC and African Americans, CRC screening, physical discomfort during a colonoscopy, colonoscopy preparation, and other CRC screening options (e.g., fecal immunochemical test). Users answered one myth/fact statement at time. After identifying whether a statement was a myth or a fact, they received feedback about the response and were given the option to view a short video that provided more detailed information about the topic. If users did not want to view the video, they clicked “next” to view the next myth/fact statement.
During the evoking phase, the e-Motivate 1.0 app evoked the users’ own motivations for change through the use of MI-informed exercises (e.g., decisional balance). Users were asked to rate their perceived importance of having a screening colonoscopy as well as their perceived confidence in their ability to have the procedure. Based on their responses, the app cued the individuals to engage in MI-informed exercises aimed at evoking “change talk.” For example, during a decisional balance exercise, users were encouraged to identify reasons for wanting to have a screening colonoscopy. Throughout the evoking phase, individuals’ responses were summarized and reflected back to the users via text and video content. For example, if a user indicated that they were “not at all confident” in their ability to have a colonoscopy, they were prompted to participate in a video exercise (i.e., identifying previous successes). At the beginning of the video, the health educator stated “Many people do not feel confident in their ability to have a colonoscopy… it sounds like you don’t feel completely confident in your ability to have a colonoscopy.”
Finally, during the action phase, the users viewed a video summarizing the steps needed to complete a screening colonoscopy (e.g., schedule the appointment, identify a person to bring them home after the procedure, and take laxatives). They were then asked to type the name of a person who could take them home after the colonoscopy procedure. At the end of the app, individuals were given a printout that summarized the tailored information covered in the app.
The app was designed to be tailored to each user. Users have the option to view multiple educational videos and are also prompted to participate in various exercises, based on their reported levels of importance/confidence. Therefore, the time to complete the app can vary from 5 to 25 min.
Study sample and recruitment
Eligibility included (a) aged 50 years or older; (b) self-identified as black/African American; and (c) English speaking. Participants were excluded if they were hearing or vision impaired. Furthermore, if individuals participated in the first field test, they were excluded from participating in the second field test. Participants were recruited from a primary care waiting room and written informed consent was obtained from all participants. Participants received a US$25 gift card as compensation for their participation.
Field testing
First, the e-Motivate 1.0 app was field tested on 20 participants in order to gather feedback regarding the app’s acceptability and usability. Each participant was instructed to engage in a think aloud exercise while using the app. During the think aloud exercise, participants were encouraged to verbalize their thoughts while engaging with the app, in real-time. Think aloud exercises [27] are increasingly being used to conduct user testing of digital health interventions (e.g., 28–30). The research assistants took notes during the think aloud exercises. They were also instructed to have minimal to no involvement in the think aloud exercise (e.g., avoid showing the participant how to interact with the app). When indicated, the research assistants prompted users to comment on the positive and negative aspects of the app’s content, its cultural appropriateness (e.g., “Is the language relatable?”), and the overall functionality. For example, if a user was struggling with an aspect of the app, the user may be asked “How could we make this part easier?”
The sessions were audio recorded, transcribed, and then coded based on three general categories: positive comment, negative comment, and research assistant involvement (e.g., prompting the user to engage in the app, explaining content of the app, demonstrating how to use the app, and answering users’ questions). Both negative comments and research assistant involvement directly informed the identification of “problems” with the app. The research team then identified potential solutions to address each problem. Then, a second iteration of the app was created (e-Motivate 2.0) and field tested on a different set of 20 participants. Field test results were used to modify and finalize the app.
Measures
Demographics
A demographics questionnaire adapted from previous studies [31] was used to assess demographics and medical history.
App usability
The System Usability Scale (SUS) [32] was used to evaluate the usability of the intervention. The SUS includes 10 items scored on a 5-point Likert scale (1 = strongly disagree; 5 = strongly agree) that assessed the app’s usability (Cronbach alpha = .91) [33]. To adapt the original scale to the current study, the word “system” was replaced with “app” in each item (e.g., “I thought the app was easy to use” and “I felt very confident using the app”).
App acceptability
The Acceptability E-Scale [34] was used to assess the acceptability of the app’s function (e.g., ease of use) and the app’s content (e.g., helpfulness of app). The scale is composed of six items scored on a 5-point Likert scale, with higher scores indicating higher acceptability. The scale demonstrates acceptable reliability (Cronbach’s alpha = .76) [34].
Data analysis
To analyze the app’s usability, a contribution score for each item on the SUS was calculated and then summed. Then, the total score was multiplied by 2.5 to produce an overall score ranging from 0 to 100. According to previous research, an SUS score greater than 70 is considered acceptable usability [33].
To analyze the app’s acceptability, the scores on the Acceptability E-Scale were summed to produce an overall score ranging from 6 to 30. According to the literature, a score of 80% or higher (total score of 24 or higher) is considered acceptable [35].
Qualitative feedback was elicited using think aloud exercises. To analyze the data, first the audio recordings of the field tests were transcribed. Then, two independent reviewers coded the data for three themes: positive comment, negative comment, and research assistant involvement. Discrepancies in coding were resolved with a third coder.
RESULTS
Sample characteristics
Twenty participants completed the first field test and an additional 20 participants completed the second field test. Two participants were screen failures (completed the study multiple times) and thus were not included in the analysis. See Table 1 for sample characteristics. Field test 1 participants and field test 2 participants did not statistically differ on demographic variables (p > .05).
Table 1.
Participant demographics
| Age | Field test 1 (N = 20) | Field test 2 (N = 18) |
|---|---|---|
| M = 58.9; SD = 4.90 | M = 59.7; SD = 7.6 | |
| Race | ||
| Black/African American | 85.0% (17/20) | 66.7% (12/18) |
| Black/Caribbean | 5.0% (1/20) | 22.2% (4/18) |
| Black/African | 5.0% (1/20) | 0.0% (0/18) |
| Black/Latino | 0.0% (0/20) | 5.6% (1/18) |
| Other | 0.0% (0/20) | 5.6% (1/18) |
| Missing | 5.0% (1/20) | 0.0% (0/18) |
| Gender | ||
| Male | 30.0% (6/20) | 22.2% (4/18) |
| Female | 70.0% (14/20) | 77.8% (14/18) |
| Education | ||
| Grade school | 15.0% (3/20) | 0.0% (0/18) |
| High school | 50.0% (10/20) | 66.7% (12/18) |
| College | 25.0% (5/20) | 27.8% (5/18) |
| Post graduate | 5.0% (1/20) | 0.0% (0/18) |
| Missing | 5.0% (1/20) | 5.6% (1/18) |
| Income | ||
| Less than $19,999 | 60.0% (12/20) | 61.1% (11/18) |
| $20,000–$39,999 | 15.0% (3/20) | 16.7% (3/18) |
| $40,000+ | 10.0% (2/20) | 16.7% (3/18) |
| Missing | 15.0% (3/20) | 5.6% (1/18) |
| Previous colonoscopy | ||
| Yes | 50.0% (10/20) | 66.7 (12/18) |
| No | 50.0% (10/20) | 33.3 (6/18) |
Field test one
Overall, the participants responded positively to the app. An analysis of the qualitative data revealed that the participants found the app to be easy to use. Some positive comments regarding the app’s usability included, “I thought that app would be harder to use, but it’s really simple” and “The app was simple to navigate, if you needed to get some information, it was provided.” Participants also commented on the app’s utility and overall acceptability. Positive comments included, “Now if I had this, I would not have cancelled three colonoscopy appointments [in the past] because I didn’t know what to expect” and “I think that if more people did this, they wouldn’t be as afraid ….”
Negative comments about the app included: “There needs to be instructions on the slider to clarify how to slide” and “I’m struggling a little with the next button.” Additionally, several users required assistance using the app. In particular, users needed assistance with (a) using the app functions (e.g., next button and slider button); (b) reading the questions and instructions; (c) understanding the terms “myth/fact”; and (d) identifying a person to take them home after the procedure. It was also noted that some users skipped the introduction video and, on two occasions the print function did not work correctly. The negative comments and research assistants’ involvement were analyzed to identify “problems” with the app. Solutions were created to address each problem identified in the app. See Table 2.
Table.
Field test one: identified problems and solutions
| Field test 1 | |
|---|---|
| Problem | Solution |
| Required research staff to read written content | Added audio option on written instructions |
| Difficulty interacting with app features • Sliding confidence ruler • Playing videos • Pressing next |
Added demonstration video |
| Skipping the introduction video | Delayed the appearance of the “next” button by 7 s |
| Confusion over the term “myth” | Changed terms myth/fact to true/false |
| Difficulty identifying someone to take them home after the procedure | Deleted that section |
| Technical difficulty with print function | Corrected technical error |
| Field test 2 | |
| Problem | Solution |
| Confusion with the demonstration video | Recreated demonstration video |
| Confusion with true/false exercise | Added audio option on true/false answers |
| Confusion with the confidence question | Reworded the confidence question |
The SUS total (M = 86.62; SD = 20.35) indicated high usability and the mean Acceptability E-Scale score of 29.30 (SD = 1.49) indicated high overall acceptability.
Field test two
The e-Motivate 1.0 app was modified to create e-Motivate 2.0 (see Table 2 for modifications). E-Motivate 2.0 was then field tested on additional 18 participants.
Overall, participants responded favorably to the second iteration of app. Consistent with the first field test, participants found the app to be easy to use (e.g., “They were very clear, you know, the language was very clear and it was very informative like, very clear English, not too many medical terms...”) and useful (e.g., “I think it’s a good way of getting to a lot of patients ... that are afraid and make it more relaxing and now it makes me want to do one”).
Participants indicated some problems with the app such as “the confidence question was confusing” and “I just thought that one particular question was unclear.” Also, several participants required assistance while watching the demonstration video. In particular, the video showed screen grabs of the app and used a shaded circle to demonstrate how to use the various functions of the app (e.g., use a sliding scale and push the next button). Multiple participants attempted to interact with the video (e.g., push buttons on the video screen). The research assistants had to prompt the users to watch the video and also had to provide an explanation of the purpose of the video. Negative comments and research assistants’ involvement were analyzed together to identify “problems” with the app. Modifications were made to address each identified problem. See Table 2.
The SUS total (M = 89.56; SD = 10.62) indicated high usability for the second iteration of the app. Furthermore, the mean total Acceptability E-scale score of 28.83 (SD = 2.35) indicated high overall acceptability of the app.
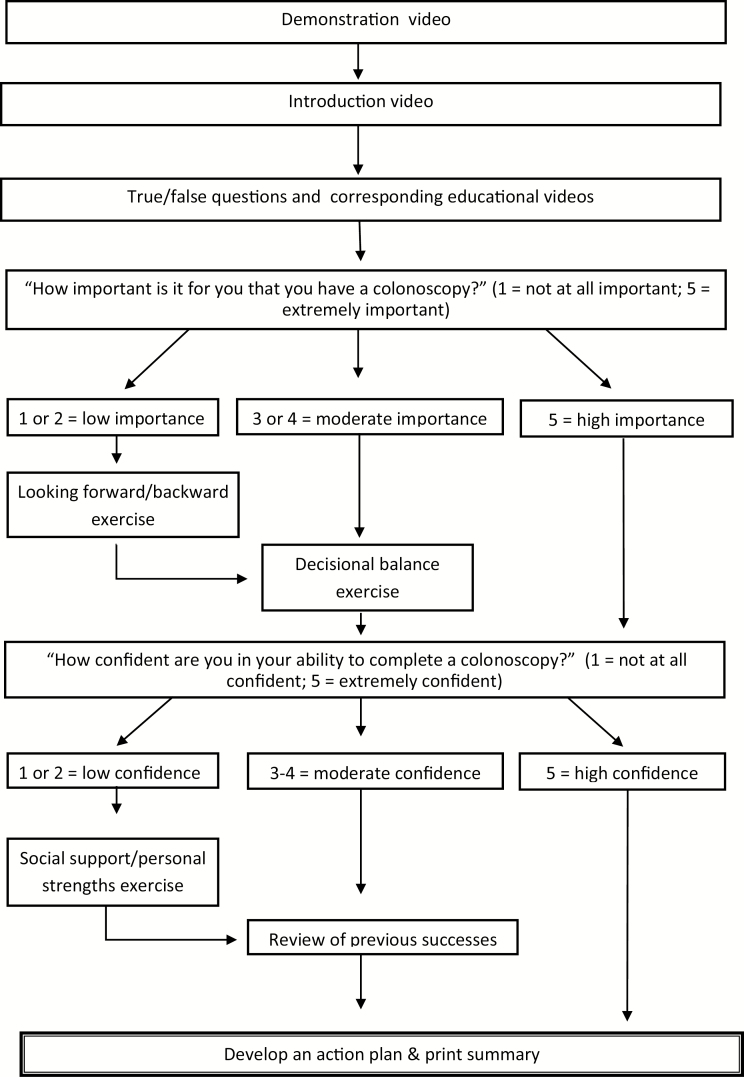
Based on the second field test results, additional modifications were made to the app. As previous discussed, during the second field test, several participants had difficulty understanding the demonstration video. To resolve this issue, a second demonstration video was created that showed a person interacting with the app and using the app functions. To confirm the acceptability of the second demonstration video, both videos were presented at the institution’s quarterly community advisory board meeting. The meeting consists of up to 20 East Harlem community members who are predominantly African American and Latino. The community members unanimously agreed that the new demonstration video was more user-friendly and clearly demonstrated how to use the various functions of the app. The final e-Motivate app included the new demonstration video. See Fig. 1 for the flow of the finalized e-Motivate app. See Fig. 2 for sample screenshots.
Fig. 1.
The decision tree demonstrates the videos and exercises incorporated in the e-Motivate app. The exercises are tailored to each user’s response to the importance and confidence questions.
Fig. 2.
The first screenshot illustrates the demonstration video that plays at the beginning of the app. The second and third screenshot depict the true/false exercise and the corresponding feedback/information. The final screenshot shows the importance ruler. The screenshots have been edited to remove institutional logos. The content of the app drew from MI literature [8], CRC literature [2] and previous work promoting CRC screening among African Americans [36].
DISCUSSION
CRC is the third leading cause of cancer death in men and women in the United States and African Americans are disproportionally affected by the disease. The e-Motivate app was developed to help improve African Americans’ screening colonoscopy uptake, and in doing so reduce the burden of CRC in this community.
The first iteration of the app was informed by MI literature and focus group feedback. The e-Motivate app underwent iterative field testing with African Americans over the age of 50. Overall, participants responded positively to the app and the qualitative analyses revealed that participants found the app to be helpful, user-friendly, and highly acceptable. These qualitative results were corroborated by quantitative data which found that both iterations of the app demonstrated high usability and acceptability. The negative comments and research assistants’ involvement that occurred during both field tests directly informed the modifications that were made to the app. Overall, the feedback from the participants helped ensure that the final version of the app was user-friendly and appropriate for the targeted population.
Limitations
There are limitations to the study that should be considered when interpreting the results. First, it is unknown whether MI can be accurately implemented via technology. For example, a key component of MI is establishing a strong therapeutic alliance between the client and MI interventionist (e.g., engaging phase) [8]. In the absence of a live interventionist, it is unclear whether the e-Motivate app can create a strong therapeutic alliance. However, a systematic review of the therapeutic alliance in e-therapy suggests that technology-based interventions may foster a working alliance comparable to face-to-face interventions [35]. Another essential component of MI is the elicitation of “change talk.” In the e-Motivate app, change talk is elicited through multiple choice questions (e.g., questions eliciting users’ reasons for wanting a screening colonoscopy) and videos content (e.g., videos encouraging individuals to think about a time in their life when they overcame barriers). The e-Motivate app was designed to be user-friendly and require low technology literacy, and therefore, the app does not require individuals to write or say any responses. However, it is unclear whether this type of change talk elicitation can lead to behavior change. Finally, live-MI is typically a fluid conversation between a client and interventionist. In fact, MI is often referred to as a “dance” between the interventionist and client, as opposed to a “wrestle” [8]. Although MI implements sequential processes (i.e., engaging, focusing, evoking, and action planning), MI is not a manualized treatment. The e-Motivate app, on the other hand, is structured and has a predetermined decision tree. It is unknown whether this type of adapted and structured intervention will be as effective as traditional MI.
Another limitation is that the current study did not evaluate the impact of the e-Motivate app on intentions to get screened or actual screening behavior. Future pilot studies and randomized clinical trials should formally evaluate the efficacy of the app for improving CRC screening uptake.
Conclusions
Despite published research proving the adoption of technology among older adults [37], there remains skepticism regarding the implementation of digital health interventions among this population. There are also concerns that digital health interventions may inadvertently exacerbate digital divides among racial minorities and whites. Given these concerns, the e-Motivate app was specifically designed to require low health, technology, and general literacy. For example, the app incorporates significant video and audio content and thus requires minimal reading and writing skills. Furthermore, the video and written content avoids the use of medical jargon. The app was also designed to be highly accessable. By administering the app in a primary care office on a clinic-provided tablet, the app can be accessed by inidivudals who do not own personal tablets and/or smartphones and users are not required to use their personal data plans and/or storage. The app was also designed to be culturally appropriate for African Americans. For example, the app provides information specific to African Americans (e.g., CRC and African Americans information video) and the videos feature African American health educators. The results from the field test provide initial support of the overall acceptability and usability of the e-Motivate app among African Americans adults over the age of 50.
The next critical step in this line of research is to formally test the efficacy of the app for improving screening colonoscopy uptake as well as improving secondary outcomes such as prep quality, time to complete the test, and the sharing of health information with peers/family. Future research should also evaluate potential mediators (e.g., confidence in one’s ability to have a colonoscopy) and moderators (e.g., previous colonoscopy completion) of the intervention. Furthermore, as technology is rapidly changing, it is important to continually assess the usability and acceptability of the app in order to make refinements and modifications when necessary.
If this line of research proves the e-Motivate app efficacious, there is a strong argument to integrate the app into standard clinical practice with the ultimate goal of reducing the burden of CRC in the African American community.
Acknowledgments
This study was funded by the National Cancer Institute (K07CA190726 and P30CA196521). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. Preliminary analyses of the reported data were presented as a poster at the Connected Health Conference 2017 [38]. We would like to thank the Mount Sinai AppLab for their help programming all iterations of the e-Motivate app. We would also like to thank the study participants for providing us with valuable feedback about the app.
Compliance with Ethical Standards
Conflict of Interest: Authors Sarah J. Miller, Jamilia R. Sly, Kemi Bolutayo, Zhiye Jiang, Brittney Henry, and Lina Jandorf declare that they have no conflict of interest to report.
Authors’ Contribution: All authors contributed to editing the manuscript. In addition, S.J.M. was the PI on the study and was involved in all aspects of the study including design, app content development, data analysis, and manuscript preparation; J.R.S. assisted with study design, data analysis and manuscript preparation; K.B.G. assisted with study design, participant recruitment, app content development, and qualitative data coding; Z.J. assisted with participant recruitment, app content development, qualitative data coding, and manuscript preparation; B.H. assisted with participant recruitment and qualitative data coding; L.J. was the primary mentor on the study and assisted with study design, data analysis and manuscript preparation.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed Consent: Informed consent was obtained from all individual participants included in the study.
References
- 1. American Cancer Society. Cancer Facts & Figures 2018. Atlanta: American Cancer Society; 2018. [Google Scholar]
- 2. American Cancer Society. Colorectal Cancer Facts & Figures 2017–2019. Atlanta: American Cancer Society; 2017. [Google Scholar]
- 3. Wolf AMD, Fontham ETH, Church TR, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin. 2018;68(4):250–281. [DOI] [PubMed] [Google Scholar]
- 4. Rex DK; ACG Board of Trustees American College of Gastroenterology action plan for colorectal cancer prevention. Am J Gastroenterol. 2004;99(4):574–577. [DOI] [PubMed] [Google Scholar]
- 5. Rex DK. Colonoscopy: the dominant and preferred colorectal cancer screening strategy in the United States. Mayo Clin Proc. 2007;82(6):662–664. [DOI] [PubMed] [Google Scholar]
- 6. Rabeneck L, Paszat LF, Saskin R, Stukel TA. Association between colonoscopy rates and colorectal cancer mortality. Am J Gastroenterol. 2010;105(7):1627–1632. [DOI] [PubMed] [Google Scholar]
- 7. Steele CB, Rim SH, Joseph DA, King JB, Seeff LC; Centers for Disease Control and Prevention (CDC) Colorectal cancer incidence and screening - United States, 2008 and 2010. MMWR Suppl. 2013;62(3):53–60. [PubMed] [Google Scholar]
- 8. Miller WR, Rollnick S.. Motivational Interviewing: Helping People Change. 3rd ed. New York, NY: The Guilford Press; 2012. [Google Scholar]
- 9. Murphy JG, Dennhardt AA, Skidmore JR, Martens MP, McDevitt-Murphy ME. Computerized versus motivational interviewing alcohol interventions: impact on discrepancy, motivation, and drinking. Psychol Addict Behav. 2010;24(4):628–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lundahl B, Burke BL. The effectiveness and applicability of motivational interviewing: a practice-friendly review of four meta-analyses. J Clin Psychol. 2009;65(11):1232–1245. [DOI] [PubMed] [Google Scholar]
- 11. Hettema J, Steele J, Miller WR. Motivational interviewing. Annu Rev Clin Psychol. 2005;1:91–111. https://www.ncbi.nlm.nih.gov/pubmed/17716083 [DOI] [PubMed] [Google Scholar]
- 12. Vasilaki EI, Hosier SG, Cox WM. The efficacy of motivational interviewing as a brief intervention for excessive drinking: a meta-analytic review. Alcohol Alcohol. 2006;41(3):328–335. [DOI] [PubMed] [Google Scholar]
- 13. Martins RK, McNeil DW. Review of motivational interviewing in promoting health behaviors. Clin Psychol Rev. 2009;29(4):283–293. [DOI] [PubMed] [Google Scholar]
- 14. Alexander GL, McClure JB, Calvi JH, et al. ; MENU Choices Team A randomized clinical trial evaluating online interventions to improve fruit and vegetable consumption. Am J Public Health. 2010;100(2):319–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. VanWormer JJ, Boucher JL. Motivational interviewing and diet modification: a review of the evidence. Diabetes Educ. 2004;30(3):404–406, 408–410, 414-406 passim. doi:10.1177/014572170403000309 [DOI] [PubMed] [Google Scholar]
- 16. Julius RJ, Novitsky MA, Jr., Dubin WR. Medication adherence: a review of the literature and implications for clinical practice. J Psychiatr Pract. 2009;15(1):34–44. [DOI] [PubMed] [Google Scholar]
- 17. Miller SJ, Foran-Tuller K, Ledergerber J, Jandorf L. Motivational interviewing to improve health screening uptake: a systematic review. Patient Educ Couns. 2017;100(2):190–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Salimzadeh H, Khabiri R, Khazaee-Pool M, Salimzadeh S, Delavari A. Motivational interviewing and screening colonoscopy in high-risk individuals. A randomized controlled trial. Patient Educ Couns. 2018;101(6):1082–1087. [DOI] [PubMed] [Google Scholar]
- 19. Outlaw AY, Naar-King S, Parsons JT, Green-Jones M, Janisse H, Secord E. Using motivational interviewing in HIV field outreach with young African American men who have sex with men: a randomized clinical trial. Am J Public Health. 2010;100(Suppl 1):S146–151. doi:10.2105/ajph.2009.166991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Holstad MM, DiIorio C, Kelley ME, Resnicow K, Sharma S. Group motivational interviewing to promote adherence to antiretroviral medications and risk reduction behaviors in HIV infected women. AIDS Behav. 2011;15(5):885–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ogedegbe G, Chaplin W, Schoenthaler A, et al. A practice-based trial of motivational interviewing and adherence in hypertensive African Americans. Am J Hypertens. 2008;21(10):1137–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Resnicow K, Jackson A, Wang T, et al. A motivational interviewing intervention to increase fruit and vegetable intake through Black churches: results of the Eat for Life trial. Am J Public Health. 2001;91(10):1686–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shingleton RM, Palfai TP. Technology-delivered adaptations of motivational interviewing for health-related behaviors: a systematic review of the current research. Patient Educ Couns. 2016;99(1):17–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Costanza ME, Luckmann R, White MJ, Rosal MC, LaPelle N, Cranos C. Moving mammogram-reluctant women to screening: a pilot study. Ann Behav Med. 2009;37(3):343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alemagno SA, Stephens RC, Stephens P, Shaffer-King P, White P. Brief motivational intervention to reduce HIV risk and to increase HIV testing among offenders under community supervision. J Correct Health Care. 2009;15(3):210–221. doi:10.1177/1078345809333398 [DOI] [PubMed] [Google Scholar]
- 26. U.S. Dept. of Health and Human Services. 2006. The Research-Based Web Design & Usability Guidelines, Enlarged/Expanded edition. Washington: U.S. Government Printing Office. [Google Scholar]
- 27. Ericsson KAS, HA. Verbal reports as data. Psychol Rev. 1980;87(3): 215–251. [Google Scholar]
- 28. Crane D, Garnett C, Brown J, West R, Michie S. Factors influencing usability of a smartphone app to reduce excessive alcohol consumption: think aloud and interview studies. Front Public Health. 2017;5:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Odukoya OK, Chui MA. Using think aloud protocols to assess E-prescribing in community pharmacies. Innov Pharm. 2012;3(3):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Taylor S, Allsop MJ, Bennett MI, Bewick BM. Usability testing of an electronic pain monitoring system for palliative cancer patients: a think-aloud study. Health Informatics J. 2017;1460458217741754. doi:10.1177/1460458217741754 [DOI] [PubMed] [Google Scholar]
- 31. Jandorf L, Braschi C, Ernstoff E, et al. Culturally targeted patient navigation for increasing african americans’ adherence to screening colonoscopy: a randomized clinical trial. Cancer Epidemiol Biomarkers Prev. 2013;22(9):1577–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brooke JB. A “quick and dirty” usability scale. In: Jordan PWT, Weerdmeester BA, McClelland AL (eds.). Usability Evaluation in Industry. London: Taylor and Francis; 1996. [Google Scholar]
- 33. Bangor A, Kortum PT, Miller JT. An empirical evaluation of the system usability scale. Int J Hum Comput Interact. 2008;24(6):574–594. doi:10.1080/10447310802205776 [Google Scholar]
- 34. Tariman JD, Berry DL, Halpenny B, Wolpin S, Schepp K. Validation and testing of the Acceptability E-scale for web-based patient-reported outcomes in cancer care. Appl Nurs Res. 2011;24(1):53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sucala M, Schnur JB, Constantino MJ, Miller SJ, Brackman EH, Montgomery GH. The therapeutic relationship in e-therapy for mental health: a systematic review. J Med Internet Res. 2012;14(4):e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ellis E, Erwin DO, Jandorf L, Saad-Harfouche F, Clark N, Dauphin C, Johnson D, Klasko-Foster LB, Martinez C, Sly J, White D, Winkel G, & Kiviniemi MT. Designing a randomized controlled trial to evaluate a community-based narrative intervention for improving colorectal cancer screening for African Americans. Contem Clin Trials, 2018;65:8–18. doi: 10.1016/j.cct.2017.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Anderson M, Perrin A (2017). Tech Adoption Climbs Among Older Adults Available at: http://www.pewinternet.org/2017/05/17/tech- adoption-climbs-among-older-adults/. Accessed 7 May 2018.
- 38. Miller S, Atreja A, Fasihuddin F, Ramireddy K, Zlatopolsky R, Deorocki A, Hassanzadeh N, Farahani A, Lodhi S, Otobo E, Rogers J, Jandorf L. E-motivate: Development of an App To Improve African Americans’ Screening Colonoscopy Rates, iproc 2017;3(1):e45. doi:10.2196/iproc.8462 [Google Scholar]