Abstract
Introduction
Idarucizumab, a monoclonal antibody fragment, was developed to reverse the anticoagulant effect of dabigatran, and it was approved in Japan in September 2016. An all-case post-marketing surveillance is ongoing to collect data in Japanese patients treated with idarucizumab who had serious bleeding (Group A) or required an urgent procedure (Group B).
Methods
The primary endpoint was the incidence of adverse drug reactions (ADRs). The secondary endpoint was the maximum extent of reversal of the anticoagulant effect of dabigatran based on activated partial thromboplastin time (aPTT) within 4 h after idarucizumab administration.
Results
This interim analysis included 262 patients who received idarucizumab. Eighteen patients (6.9%) experienced ADRs within 4 weeks. The reversal effect of idarucizumab based on aPTT within 4 h after idarucizumab administration was assessed in 30 patients and the median maximum percentage reversal was 100%. In Group A, the median time to bleeding cessation in patients without intracranial bleeding was 3.3 h. In Group B, normal intraoperative hemostasis was reported in 63 patients (72.4%).
Conclusions
The results of this interim analysis suggest that idarucizumab is safe and effective for the reversal of dabigatran in Japanese patients in a real-world setting, and support the continued use of idarucizumab.
Trial Registration
ClinicalTrials.gov identifier, NCT02946931.
Video Abstract
Electronic supplementary material
The online version of this article (10.1007/s40119-020-00165-8) contains supplementary material, which is available to authorized users.
Keywords: Anticoagulant, Dabigatran, Emergency surgery, Hemorrhage, Idarucizumab, Japan, Nonvalvular atrial fibrillation, Post-marketing surveillance, Reversal
Key Summary Points
| Why carry out this study? |
| Patients who are receiving long-term anticoagulation with dabigatran may require rapid reversal of the anticoagulant in the event of severe bleeding or urgent surgery. |
| Idarucizumab was developed to specifically reverse the anticoagulant effects of dabigatran, and neither promotes nor inhibits thrombosis. |
| This post-marketing surveillance study assessed the safety and effectiveness of idarucizumab when used according to the Japanese package insert in 262 patients. |
| What was learned from the study? |
| Adverse drug reactions occurred in 18 (6.9%) patients, and the median maximum percentage reversal of idarucizumab was 100%, based on activated partial thromboplastin time within 4 h after administration. |
| The safety and effectiveness data from this study support the continued use of idarucizumab in Japanese clinical practice, and no new safety concerns have been identified so far. |
Introduction
Dabigatran etexilate (dabigatran) is an oral thrombin inhibitor approved for the prevention of stroke in patients with nonvalvular atrial fibrillation [1]. Clinical trials and post-marketing surveillance (PMS) studies, including those in Japanese and Asian populations, have shown the effectiveness and safety of dabigatran compared with warfarin [2–6]. Dabigatran is associated with fewer bleeding events than warfarin [7] and in particular, fewer events of intracranial hemorrhage for both approved doses. However, anticoagulant reversal is central to the management of uncontrolled bleeding in anticoagulated patients and, where possible, in those on anticoagulants who require emergency surgery or other invasive procedures.
Idarucizumab was developed to specifically reverse the anticoagulant effects of dabigatran during uncontrolled bleeding or emergency surgery. It is a humanized antibody fragment that specifically binds unbound and thrombin-bound dabigatran and its active glucuronide metabolites [8]. As idarucizumab is specific to dabigatran, it does not promote or inhibit thrombosis [9, 10]. Phase I studies in Caucasian and Japanese subjects [9, 11, 12] and the global phase III study (RE-VERSE AD) [13] showed that idarucizumab rapidly reverses the anticoagulant effect of dabigatran.
Based on RE-VERSE AD, idarucizumab was shown to be effective for dabigatran reversal among patients who have uncontrolled bleeding or will be undergoing urgent surgery. Idarucizumab was approved in the US [14] and EU [15] in 2015, and it was approved in Japan [16] in September 2016, and became available from November onward. An all-case PMS is in progress to collect data from Japanese patients treated with idarucizumab as requested by the Japanese authority, because a limited number of Japanese patients were treated in clinical trials for the new drug application.
Here, we present the interim results of this all-case PMS. The objective of this PMS is to assess the safety and effectiveness of idarucizumab when used according to the Japanese package insert in a Japanese clinical setting, and to investigate the characteristics of patients treated with idarucizumab.
Methods
Ethical Statement
This PMS study is fully compliant with Japanese Good Post-marketing Study Practice regulations. The protocol was approved by the Ministry of Health, Labour and Welfare of the Japanese Government. This study involved the collection of anonymous data from clinical settings and, therefore, it was not necessary to obtain informed consent from patients. All medical institutions who agreed to provide these anonymized data signed a contract with Nippon Boehringer Ingelheim Co., Ltd.
Study Design
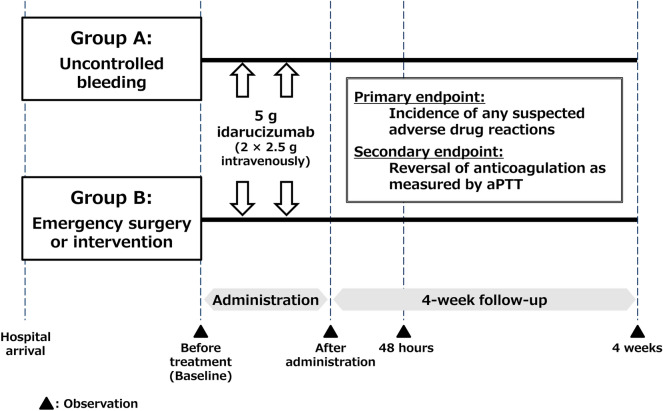
This PMS is a multicenter, open-label, uncontrolled, all-case, non-interventional surveillance (Fig. 1). The target number of patients is 300, but registration will continue until the conditions of the approval are satisfied according to the Japanese authority. Enrollment began in November 2016. All sites in Japan in which idarucizumab has been administered are included. This study was registered with ClinicalTrials.gov (NCT02946931).
Fig. 1.
Study design. The treatment period was specified as 5 days after administration. aPTT activated partial thromboplastin time
Patients
This study includes all patients treated with idarucizumab (according to the Japanese package insert) after idarucizumab was approved in Japan. Patients are classed into two groups: Group A includes patients who present life-threatening or uncontrolled bleeding, and Group B includes patients undergoing emergency surgery or an invasive procedure where significant bleeding is anticipated. According to the Japanese package insert, idarucizumab is given intravenously at 5 g/dose (2 × 2.5 g/50 ml) as two consecutive infusions over 5–10 min each or as a bolus injection. Patients are registered using paper forms, which are faxed to the sponsor. There are no exclusion criteria. Observations were made at the following time points: baseline before the first administration, during drug administration, 48 h after drug administration, and 4 weeks after drug administration or discontinuation.
Study Endpoints
The primary endpoint is the incidence of adverse drug reactions (ADRs). The secondary endpoint is the maximum extent of reversal of the anticoagulant effect of dabigatran based on activated partial thromboplastin time (aPTT) within 4 h after idarucizumab administration. A 100% reversal was defined as any value below the upper limit of normal range used by each participating hospital. Other endpoints include serious adverse events (AEs), hypersensitivity and thrombotic events, time to recorded cessation of bleeding since first infusion (in patients who developed life-threatening or uncontrolled bleeding; Group A), periprocedural hemostasis (in patients who required emergency surgery or an urgent invasive procedure; Group B), frequency of restarting anticoagulant therapy, time to restart of anticoagulant therapy, and re-exposure of patients to idarucizumab.
Data Collection and Management
Physicians use paper case report forms (CRFs) to collect patient data. Data are recorded as soon as possible after the medical examination and at the specified points (baseline, idarucizumab administration, 48 h after administration, and at 4 weeks or discontinuation). The data collected are summarized here and more details of observations and evaluations can be found in Table S1 in the Supplementary Information. Data include patient background, bleeding assessment, surgery/intervention, idarucizumab administration, coagulation tests, concomitant medications/therapy, blood pressure and pulse rate, laboratory tests, AEs, and restart of anticoagulant therapy. Data are being managed by CAC Croit Corporation (Tokyo, Japan).
Data Analysis
The safety analysis for this interim report included all patients who were registered, received idarucizumab, and who had available CRFs. AEs were coded using Version 20.0 of the Medical Dictionary for Regulatory Activities (MedDRA®) [17], the international medical terminology developed under the auspices of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. All AEs were recorded from the first administration of idarucizumab (i.e., baseline visit) to 4 weeks after the first administration. ADRs were defined as AEs that the investigator or sponsor assessed as related to idarucizumab.
The effectiveness analysis included all patients in the safety analysis who had at least one item of available effectiveness data. The effectiveness analysis items included: extent of reversal anticoagulation effect assessed by aPTT, time to recorded cessation of bleeding since first infusion (Group A), periprocedural hemostasis (Group B), frequencies of restarting anticoagulant therapy, and time to restart anticoagulant therapy.
Statistical Analysis
It was estimated that 250–300 cases were required to ensure the same accuracy of the estimation [95% confidence interval: 2.8–9.0% (thrombosis), 8.4–17.1% (hypersensitivity)] in the Report on the Deliberation Results from the Japanese Ministry of Health, Labour & Welfare [16] Additionally, in a sample size of 300 patients, any ADR with a frequency of 1% or higher can be detected with a probability of 95% or greater in at least one patient. Descriptive statistics were used and included means, standard deviations, ranges, medians and interquartile ranges (IQR), frequencies, and percentages.
Results
Patient Characteristics
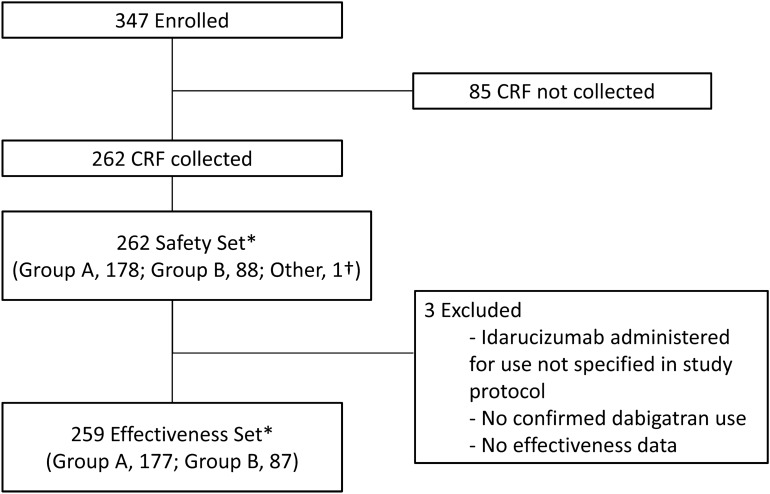
From November 2016 through April 2018, 347 patients have been enrolled and CRFs have been collected from 262 patients across 191 medical institutions (Fig. 2). The safety analysis included 262 patients (178 in Group A and 88 in Group B) and the effectiveness analysis included 259 patients (177 in Group A and 87 in Group B). Five patients were included in both Groups A and B; one patient diagnosed with abnormal coagulation accompanied by severe multi-organ disorder was classified as “other” (neither Group A nor B). In this study, enrollment of patients in Group A, Group B, or “other” was determined by the investigators. The key demographic characteristics of patients are shown in Table 1. The median age was 78.0 years, 82.8% of patients were aged 70 years or older. The median creatinine clearance was 49.0 ml/min. Overall, the duration of dabigatran use was unknown in slightly more than half (53.4%) of patients. The daily dabigatran dose was 220 mg and 300 mg in 66.0% and 16.4% of patients, respectively. The median patient-reported time from the last dose of dabigatran to the first infusion of idarucizumab was 9.4 h in Group A and 8.6 h in Group B. Overall, baseline coagulation tests were carried out in 76.3% of the patients. aPTT prolongation at baseline was reported in 47.7% of the patients.
Fig. 2.
Patient disposition. Group A included patients who had uncontrolled bleeding, and Group B included patients who required urgent surgery or intervention. *Five patients were included in both Groups A and B. †One patient was classified as “other” (neither Group A nor B). This patient was prescribed idarucizumab for abnormal coagulation accompanied by severe multi-organ disorder. CRF case report form
Table 1.
Baseline characteristics
| Characteristic | Group A (n = 178) | Group B (n = 88) | Totala (n = 262) |
|---|---|---|---|
| Age, years | |||
| Median [range]b | 78.0 [52–101] | 79.0 [44–93] | 78.0 [44–101] |
| Age ≥ 70 | 148 (83.2) | 73 (83.0) | 217 (82.8) |
| Age class | |||
| < 65 | 11 (6.2) | 8 (9.1) | 19 (7.3) |
| 65 – < 75 | 47 (26.4) | 19 (21.6) | 66 (25.2) |
| 75 – < 85 | 80 (44.9) | 39 (44.3) | 118 (45.0) |
| ≥85 | 38 (21.4) | 22 (25.0) | 57 (21.8) |
| Missing | 2 (1.1) | 0 (0.0) | 2 (0.8) |
| Sex, male | 115 (64.6) | 64 (72.7) | 176 (67.2) |
| BMIc, kg/m2 | |||
| Mean ± SD | 22.2 ± 3.6 | 22.6 ± 4.1 | 22.4 ± 3.7 |
| Creatinine clearance, ml/min | |||
| Median [range]d | 50.0 [2.8–150.9] | 48.8 [4.1–149.0] | 49.0 [2.8–150.9] |
| Distribution | |||
| ≥ 80 | 23 (12.9) | 10 (11.4) | 33 (12.6) |
| 50 – < 80 | 44 (24.7) | 15 (17.1) | 58 (22.1) |
| 30 – < 50 | 35 (19.7) | 21 (23.9) | 54 (20.6) |
| < 30 | 31 (17.4) | 8 (9.1) | 38 (14.5) |
| Unknown | 45 (25.3) | 34 (38.6) | 79 (30.2) |
| Daily dose of dabigatran, | |||
| 150 mg twice daily | 28 (15.7) | 16 (18.2) | 43 (16.4) |
| 110 mg twice daily | 120 (67.4) | 55 (62.5) | 173 (66.0) |
| Other | 4 (2.3) | 3 (3.4) | 7 (2.7) |
| Unknown/Missing | 26 (14.6) | 14 (15.9) | 39 (14.9) |
| Duration of dabigatran use, days | |||
| < 14 | 31 (17.4) | 18 (20.5) | 48 (18.3) |
| 14 – < 30 | 5 (2.8) | 2 (2.3) | 7 (2.7) |
| 30 – < 91 | 8 (4.5) | 2 (2.3) | 10 (3.8) |
| ≥91 | 41 (23.0) | 17 (19.3) | 57 (21.8) |
| Unknown/Missing | 93 (52.2) | 49 (55.7) | 140 (53.4) |
| Time from last dabigatran dose to first administration of idarucizumab, hours | |||
| Median [range]e | 9.4 [0.0–133.3] | 8.6 [0.0–31.0] | 9.1 [0.0–133.3] |
| Distribution | |||
| < 12 | 52 (29.2) | 27 (30.7) | 77 (29.4) |
| 12 – < 24 | 20 (11.2) | 10 (11.4) | 30 (11.5) |
| 24 – < 48 | 10 (5.6) | 5 (5.7) | 15 (5.7) |
| ≥48 | 1 (0.6) | 0 (0.0) | 1 (0.4) |
| Exact timing unknown | 95 (53.3) | 46 (52.3) | 139 (53.1) |
| Day before administration of idarucizumab | 35 (19.7) | 21 (11.8) | 56 (21.4) |
| Day of administration of idarucizumab | 32 (18.0) | 13 (14.8) | 44 (16.8) |
| ≥2 days before administration of idarucizumab | 4 (2.2) | 1 (1.1) | 5 (1.9) |
| Unknown/Missing | 24 (13.5) | 11 (12.5) | 34 (13.0) |
| Departmentf | |||
| Neurosurgery | 63 (35.4) | 42 (47.7) | 100 (38.2) |
| Cardiovascular | 40 (22.5) | 7 (8.0) | 47 (17.9) |
| Emergency | 29 (16.3) | 4 (4.6) | 34 (13.0) |
| Neurology | 12 (6.7) | 5 (5.7) | 17 (6.5) |
| Gastroenterology | 8 (4.5) | 3 (3.4) | 11 (4.2) |
| Other | 28 (15.7) | 28 (31.8) | 56 (21.4) |
| Performed coagulation test | 149 (83.7) | 55 (62.5) | 200 (76.3) |
| Elevated aPTT at baseline | |||
| > ULN in each site | 88 (49.4) | 38 (43.2) | 125 (47.7) |
| ≤ULN in each site | 54 (30.3) | 15 (17.1) | 66 (25.2) |
| Unknown | 36 (20.2) | 35 (39.8) | 71 (27.1) |
| PT-INR, median [range]g | 1.3 [0.9–27.7] | 1.3 [1.0–2.91] | 1.3 [0.9–27.7] |
| Fibrinogen, mg/dl, median [range]h | 282.0 [107.0–936.0] | 297.0 [129.0–795.0] | 286.0 [107.0–936.0] |
| Fibrin degradation products, µg/ml, median [range]i | 5.8 [0.6–173.6] | 8.2 [2.2–183.6] | 6.0 [3.5–183.6] |
| D-dimer, ng/dl, median [range]j | 1.6 [0.0–89.8] | 1.3 [0.5–35.9] | 1.4 [0.0–89.8] |
| Coexisting condition | |||
| Hypertension | 93 (52.3) | 46 (52.3) | 135 (51.5) |
| Congestive heart failure | 6 (3.4) | 4 (4.6) | 9 (3.4) |
| Diabetes | 39 (21.9) | 14 (15.9) | 50 (19.1) |
| Previous stroke | 4 (2.3) | 0 (0.0) | 4 (1.5) |
| Previous TIA | 1 (0.6) | 0 (0.0) | 1 (0.4) |
| Previous systemic embolism | 3 (1.7) | 0 (0.0) | 3 (1.2) |
| Concomitant treatment with antiplatelet drug | 25 (14.0) | 10 (11.4) | 34 (13.0) |
Data are shown as n (%) except where otherwise specified
Group A, patients with uncontrolled bleeding; Group B, patients requiring urgent surgery or intervention. Five patients were included in both Group A and Group B
BMI body mass index, aPTT activated partial thromboplastin time, ULN upper limit of normal, PMS post-marketing surveillance, PT-INR prothrombin time international normalized ratio, TIA transient ischemic attack
aTotal contains one patient who was classified as “other” (neither Group A nor B). This patient was prescribed idarucizumab for abnormal coagulation accompanied by severe multi-organ disorder
b–e,g–jData were available for 260 patients (176 in Group A, and 88 in Group B), 224 patients (150 in Group A, and 78 in Group B), 183 patients (133 in Group A, and 54 in Group B), 123 patients (83 in Group A, and 42 in Group B), 191 patients (142 in Group A, and 53 in Group B), 81 patients (59 in Group A, and 23 in Group B), 46 patients (37 in Group A, and 9 in Group B), 108 patients (84 in Group A, and 28 in Group B), respectively
fPatients may have been treated in more than one department
Of the 178 patients in Group A, 84 (47.2%) had intracranial bleeding, 49 (27.5%) had gastrointestinal bleeding, 16 (9.0%) had intra-pericardial bleeding, and 50 (28.1%) had trauma-related bleeding (Table 2). Sixty-eight patients (38.2%) had hemodynamic instability in Group A. Eighty-eight patients were classified into Group B, and the most frequent urgent surgery/intervention was a neurological procedure in 49 patients (55.7%), followed by abdominal surgery/intervention in 18 patients (20.5%); cardiovascular surgery/intervention in 15 patients (17.0%); genitourinary surgery/intervention in three patients (3.4%); and respiratory tract surgery/intervention in two patients (2.3%) (Table 2).
Table 2.
Idarucizumab indication for reversal effect of dabigatran
| Group A (n = 178) | |
|---|---|
| Bleeding location | |
| Intracranial | 84 (47.2) |
| Subdural | 34 (19.1) |
| Subarachnoid | 25 (14.0) |
| Intracerebral | 47 (26.4) |
| Gastrointestinal | 49 (27.5) |
| Lower | 26 (14.6) |
| Upper | 18 (10.1) |
| Unknown | 12 (6.7) |
| Intra-pericardial | 16 (9.0) |
| Retroperitoneal | 5 (2.8) |
| Intramuscular | 3 (1.7) |
| Other | 33 (18.5) |
| Trauma-related | 50 (28.1) |
| Group B (n = 88) | |
|---|---|
| Therapeutic area of surgery/intervention | |
| Neurological surgery/intervention: craniotomy/drainage for intracranial hemorrhage (35), thrombolysis, thrombectomy or bypass for stroke (9), tumor (2), abscess (2), hydrocephalus (1) | 49 (55.7) |
| Abdominal surgery/intervention: cholecystitis/cholangitis (7), gastrointestinal perforation (3), incarcerated hernia (3), intestinal obstruction (2), appendicitis (1), ERCP (1), unknown (1) | 18 (20.5) |
| Cardiovascular surgery/intervention: aortic dissection/aortic aneurysm rupture (11), cardiac tamponade/pericardial effusion (2), aneurysm (1), TAVI (1) | 15 (17.0) |
| Genitourinary surgery/intervention: ovarian tumor (1), renal failure (1), glomerulonephritis (1) | 3 (3.4) |
| Respiratory tract surgery/intervention: empyema (1), lung biopsy for lung cancer (1) | 2 (2.3) |
Data are shown as n (%). Patients may have had more than one type of bleeding. Surgery is not identified in one patient of Group B
TAVI transcatheter aortic valve implantation, ERCP endoscopic retrograde cholangiopancreatography
Safety
ADRs within 4 weeks after administration of idarucizumab were reported in 18 patients (6.9%) (Table 3). By MedDRA System Organ Class, those most frequently reported were “Nervous system disorders” in six patients, followed by "Infections and infestations" and “Injury, poisoning and procedural complications” in three patients each. The most common events reported according to MedDRA preferred term were “subdural hematoma” in two patients, “cerebral infarction” in two patients, and other events reported in one patient each. ADRs within 5 days after administration of idarucizumab were reported in 11 patients (4.2%) and the most frequently reported was “Nervous system disorders” in four patients.
Table 3.
Adverse events judged by investigators to be related to idarucizumab
| Group A (n = 178) | Group B (n = 88) | Totala (n = 262) | |
|---|---|---|---|
| Any ADR | 9 (5.1) | 9 (10.2) | 18 (6.9) |
| Infections and infestations | 2 (1.1) | 1 (1.1) | 3 (1.2) |
| Mediastinitis | 1 (0.6) | 0 (0.0) | 1 (0.4) |
| Systemic candida | 1 (0.6) | 0 (0.0) | 1 (0.4) |
| Infectious pleural effusion | 0 (0.0) | 1 (1.1) | 1 (0.4) |
| Nervous system disorders | 3 (1.7) | 3 (3.4) | 6 (2.3) |
| Cerebral infarction | 1 (0.6) | 1 (1.1) | 2 (0.8) |
| Brain stem hemorrhage | 1 (0.6) | 0 (0.0) | 1 (0.4) |
| Hydrocephalus | 0 (0.0) | 1 (1.1) | 1 (0.4) |
| Seizure | 0 (0.0) | 1 (1.1) | 1 (0.4) |
| Embolic cerebral infarction | 1 (0.6) | 0 (0.0) | 1 (0.4) |
| Cardiac disorders | 2 (1.1) | 0 (0.0) | 2 (0.8) |
| Acute myocardial infarction | 1 (0.6) | 0 (0.0) | 1 (0.4) |
| Cardio-respiratory arrest | 1 (0.6) | 0 (0.0) | 1 (0.4) |
| Vascular disorders | 0 (0.0) | 1 (1.1) | 1 (0.4) |
| Arterial occlusive disease | 0 (0.0) | 1 (1.1) | 1 (0.4) |
| Respiratory, thoracic, and mediastinal disorders | 1 (0.6) | 0 (0.0) | 1 (0.4) |
| Pneumonia aspiration | 1 (0.6) | 0 (0.0) | 1 (0.4) |
| Gastrointestinal disorders | 1 (0.6) | 1 (1.1) | 2 (0.8) |
| Abdominal discomfort | 0 (0.0) | 1 (1.1) | 1 (0.4) |
| Hemorrhoidal hemorrhage | 1 (0.6) | 0 (0.0) | 1 (0.4) |
| General disorders and administration site conditions | 0 (0.0) | 1 (1.1) | 1 (0.4) |
| Malaise | 0 (0.0) | 1 (1.1) | 1 (0.4) |
| Investigations | 1 (0.6) | 0 (0.0) | 1 (0.4) |
| International normalized ratio increased | 1 (0.6) | 0 (0.0) | 1 (0.4) |
| Injury, poisoning, and procedural complications | 1 (0.6) | 2 (2.3) | 3 (1.2) |
| Subdural hematoma | 0 (0.0) | 2 (2.3) | 2 (0.8) |
| Extradural hematoma | 1 (0.6) | 0 (0.0) | 1 (0.4) |
| Subdural hemorrhage | 1 (0.6) | 0 (0.0) | 1 (0.4) |
Data are reported as n (%). ADRs were coded using Version 20.0 of the Medical Dictionary for Regulatory Activities
ADR adverse drug reaction
aTotal contains one patient who was classified as “other” (neither Group A nor B). This patient was prescribed idarucizumab for abnormal coagulation accompanied by severe multi-organ disorder
Within 4 weeks after the administration of idarucizumab, 76 patients (29.0%) reported serious AEs. The most commonly observed AEs were “pneumonia aspiration” in ten patients, followed by “subdural hematoma”, eight patients; “cerebral infarction”, seven patients; “cerebral hemorrhage”, four patients; and “brain edema”, four patients. Other serious AEs were reported in three or fewer patients. Most of the events appeared to be a worsening of the index event or a coexisting condition. No other consistent pattern emerged.
The number of deaths that occurred within 4 weeks after treatment was 40 of 262 patients; 16.5% estimated by the Kaplan–Meier method, corresponding to 29 (17.8%) in Group A and 11 (13.4%) in Group B. The number of deaths that occurred within 5 days after treatment was 19 (11.0%) in Group A and six (6.9%) in Group B. The majority of events that resulted in death appeared to be a worsening of the initial event or were associated with coexisting conditions. Table 4 shows the characteristics of patients who died in each group.
Table 4.
Patients with adverse events leading to death within 5 days of idarucizumab treatment
| Groupa | Age (years) | Sex | AE (PT) | Time to death (days) | Reason of administration of idarucizumab |
|---|---|---|---|---|---|
| A | 74 | F | Epilepsy | 3 | Gastrointestinal bleeding |
| Pneumonia aspiration | |||||
| Cardiac failure congestive | |||||
| Brain herniation | |||||
| 75 | M | Respiratory failure | 3 | Bronchial bleeding | |
| Bronchial hemorrhage | |||||
| 71 | F | Cerebral hemorrhage | 1 | Intracranial bleeding | |
| 85 | M | Subdural hematoma | 4 | Intracranial bleeding | |
| 82 | M | Disseminated intravascular coagulation | 3 | Gastrointestinal, bronchial, and urinary tract bleeding | |
| Shock hemorrhagic | |||||
| 80 | M | Aortic aneurysm rupture | 1 | Retroperitoneal bleeding | |
| 89 | M | Embolic stroke | 2 | Intracranial bleeding | |
| 78 | F | Renal failure | 2 | Gastrointestinal bleeding | |
| Cardiac failure chronic | |||||
| Malignant neoplasm progression | |||||
| 83 | M | Subdural hematoma | 3 | Intracranial bleeding | |
| 81 | M | Cerebral hemorrhage | 2 | Intracranial bleeding | |
| 68 | M | Brain stem hemorrhage | 3 | Intracranial bleeding | |
| Brain edema | |||||
| 82 | F | Multiple organ dysfunction syndrome | 1 | Gastrointestinal bleeding | |
| Shock hemorrhagic | |||||
| Lower gastrointestinal hemorrhage | |||||
| 83 | F | Hemorrhage intracranial | 4 | Intracranial bleeding | |
| 60 | M | Pyelonephritis | 2 | Gastrointestinal bleeding | |
| Septic shock | |||||
| 70 | M | Head injury | 3 | Intracranial bleeding | |
| 89 | M | Pulmonary alveolar hemorrhage | 3 | Gastrointestinal bleeding, alveolar hemorrhage | |
| Respiratory failure | |||||
| 84 | M | Acute respiratory distress syndrome | 2 | Gastrointestinal bleeding | |
| Pneumonia aspiration | |||||
| 73 | F | Brain stem hemorrhage | 2 | Intracranial bleeding | |
| 82 | M | Road traffic accident | 4 | Pleural hemorrhage | |
| Traumatic hemorrhage | |||||
| B | 86 | M | Peritonitis | 1 | Missing |
| 75 | M | Hemorrhage | 1 | Blood vessel prosthesis implantation for aortic dissection | |
| Aortic dissection | |||||
| 50 | M | Hemorrhagic cerebral infarction | 3 | Craniotomy for intracranial bleeding | |
| 72 | F | Acute myocardial infarction | 2 | Blood vessel prosthesis implantation for aortic dissection | |
| Aortic dissection | |||||
| 80 | M | Sepsis | 1 | Colectomy | |
| 84 | M | Brain herniation | 4 | Craniotomy for intracranial bleeding |
AEs were coded using Version 20.0 of the Medical Dictionary for Regulatory Activities
AE adverse event, F female, M male, PT preferred term
aGroup A, uncontrolled bleeding; Group B, urgent surgery
Thrombotic Events
Thrombotic events occurred in 16 of the 262 patients (6.1%; 11 in Group A and five in Group B) within 4 weeks after treatment. Only three of the 16 patients were receiving anticoagulant therapy when the events occurred. Details of patients who presented thrombotic events in each group are provided in Table 5. During the 4-week follow-up of patients treated with idarucizumab, anticoagulant therapy was restarted in 45.8% of the patients in Group A and in 61.6% in Group B (Table 6), at a median of 5.3 days and 4.6 days, respectively, after the administration of idarucizumab.
Table 5.
Patients with thrombotic events occurring within 28 days after administration of idarucizumab
| Groupa | Sex | Age (years) | Index event | Thrombotic event | Time to thrombotic event after treatment | Outcome | OAC |
|---|---|---|---|---|---|---|---|
| A | M | 77 | Urinary tract bleeding | Acute myocardial infarction | < 24 h | Recovered | No |
| M | 89 | Intracranial hemorrhage | Embolic stroke | < 24 h | Fatal | Unknown | |
| M | 87 | Gastrointestinal bleeding | Myocardial infarction | < 24 h | Fatal | No | |
| F | 70 | Intracranial hemorrhage by trauma | Cerebral infarction | 1 d | Fatal | No | |
| M | 76 | Multiple bleeding by trauma | Cerebral infarction | 1 d | Recovered | Yes | |
| M | 63 | Gastrointestinal bleeding | Peripheral arterial occlusive disease | 5 d | Recovered | No | |
| M | 80 | Intracranial hemorrhage by trauma | Cerebral infarction | 6 d | Not recovered | No | |
| F | 80 | Intraperitoneal bleeding | Embolic cerebral infarction | 7 d | Recovered | No | |
| F | 101 | Gastrointestinal bleeding | Arterial occlusive disease | 9 d | Recovered | No | |
| F | 83 | Intracranial hemorrhage by trauma | Pulmonary embolism | 16 d | Unknown | Yes | |
| M | 78 | Intracranial hemorrhage by trauma | Cerebral infarction | 23 d | Unknown | No | |
| B | F | 72 | Blood vessel prosthesis implantation for aortic dissection | Acute myocardial infarction | < 24 h | Fatal | No |
| M | 70 | STA-MCA anastomosis for stroke | Cerebral infarction | 1 d | Not recovered | No | |
| F | 81 | Thrombolysis for stroke | Cerebral infarction | 2 d | Recovered | Yes | |
| M | 57 | Thrombolysis for stroke | Arterial occlusive disease | 2 db | Recovered | Unknown | |
| F | 77 | Blood vessel prosthesis implantation for aortic dissection | Cerebral infarction | 19 d | Fatal | No | |
| Carotid artery occlusion |
F female, M male, OAC oral anticoagulant, STA-MCA superficial temporal artery to middle cerebral artery
aGroup A, uncontrolled bleeding; Group B, urgent surgery
bEvent may have occurred before administration but was diagnosed 2 days after treatment
Table 6.
Blood product use and resumption of anticoagulant therapy
| Blood product use | Group A (n = 178) | Group B (n = 88) | Totala (n = 262) |
|---|---|---|---|
| Blood products/transfusions | 81 (45.5) | 30 (34.1) | 112 (42.8) |
| FFP | 33 (18.5) | 19 (21.6) | 53 (20.2) |
| Packed RBCs | 59 (33.2) | 22 (25.0) | 82 (31.3) |
| Platelets | 13 (7.3) | 12 (13.6) | 26 (9.9) |
| Cryoprecipitate | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Whole blood | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| PCC | 1 (0.6) | 0 (0.0) | 1 (0.4) |
| Factor VIIa | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Volume expanders | 2 (1.1) | 1 (1.1) | 3 (1.2) |
| Tranexamic acid | 24 (13.5) | 2 (2.3) | 26 (9.9) |
| Other | 13 (7.3) | 10 (11.4) | 23 (8.8) |
| Resumption of anticoagulant therapyb | |||
|---|---|---|---|
| Any anticoagulant therapy | 81 (45.8) | 53 (61.6) | 133 (51.6) |
| Warfarin | 3 (3.7) | 1 (1.9) | 4 (3.0) |
| Heparin | 9 (11.1) | 9 (17.0) | 18 (13.5) |
| Dabigatran | 36 (44.4) | 31 (58.5) | 67 (50.4) |
| Rivaroxaban | 3 (3.7) | 2 (3.8) | 5 (3.8) |
| Apixaban | 19 (23.5) | 5 (9.4) | 23 (17.3) |
| Edoxaban | 11 (13.6) | 4 (7.6) | 15 (11.3) |
| Time to resumption of anticoagulant therapy | |||
| Estimated patients | 76 | 48 | 123 |
| Median (days) | 5.3 | 4.6 | 4.9 |
Data are presented as n (%)
FFP fresh frozen plasma, RBC red blood cells, PCC prothrombin complex concentrate
aTotal contains one patient who was classified as “other” (neither Group A nor B). This patient was prescribed idarucizumab for abnormal coagulation accompanied by severe multi-organ disorder
bData were available for 258 patients (177 in Group A, and 86 in Group B)
Potential Hypersensitivity
Of the AEs of special interest, potential hypersensitivity occurred in two patients in Group A, whereby circulatory collapse and urticaria were reported. These events were not considered ADRs and resolved.
Re-Exposure to Idarucizumab
Only one of the 262 patients (0.4%) received a second dose of idarucizumab (5 g) after the initial administration of idarucizumab and was later able to restart dabigatran therapy. The patient was a 73-year-old man in Group B who received an initial dose of 5 g of idarucizumab for laparoscopic cholecystectomy. Dabigatran therapy was reinitiated 40 h after the administration of idarucizumab. Forty days after the administration of idarucizumab, the patient received the second dose of idarucizumab (5 g) before undergoing urgent endoscopic retrograde cholangiopancreatography. No AEs were reported after the first or second administration of idarucizumab.
Effectiveness
aPTT
As a secondary endpoint, we evaluated the maximum reversal of the anticoagulant effect of dabigatran based on aPTT within 4 h after idarucizumab administration. The number of patients with coagulation test data at baseline was 149 (83.7%) in Group A, 55 (62.5%) in Group B, and 200 (76.3%) in total. The number of patients with aPTT prolongation at baseline was 88 (49.4%) in Group A, 38 (43.2%) in Group B, and 125 (47.7%) in total (Table 1). Only 30 patients had aPTT data at baseline and within 4 h after administration of idarucizumab, and these data were used for the calculation of the maximum reversal effect. A complete reversal effect was observed in 20 of 30 patients based on aPTT and the median maximum percentage reversal within 4 h after the administration of idarucizumab was 100%. The effectiveness of idarucizumab based on aPTT of each patient is shown in Table 7. The use of blood products and volume expanders is described in Table 6.
Table 7.
Reversal effect of idarucizumab by aPTT in Groups A and B
| Groupa | Bleeding/surgery | Age (years) | Sex | Time to idarucizumab from last administration of dabigatran (hours) | CrCl (ml/min) | Daily dose of dabigatran (mg) | ULN | Pre-administration aPTT (s) | Post-administration aPTT (s) | Reversal effectb (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| A | Gastrointestinal | 78 | Female | Exact timing unknownc | 15.4 | 220 | 39.0 | 112.1 | 31.6 | 100 |
| Gastrointestinal | 78 | Male | 133.3 | 23.8 | 220 | 36.0 | 38.9 | 27.2 | 100 | |
| Gastrointestinal | 84 | Male | 15.0 | 22.8 | 220 | 36.0 | 64.8 | 33.3 | 100 | |
| Gastrointestinal | 91 | Female | 6.7 | 38.1 | 220 | 34.0 | 48.4 | 27.2 | 100 | |
| Intracranial | 82 | Male | 5.8 | 40.3 | 220 | 39.8 | 46.2 | 27.3 | 100 | |
| Intracranial | 82 | Female | Unknown | 45.2 | 220 | 34.5 | 49.3 | 34.1 | 100 | |
| Intracranial | 76 | Male | 13.3 | 54.8 | 220 | 38.0 | 45.7 | 36.0 | 100 | |
| Intramuscular | 74 | Male | 6.6 | 111.8 | 220 | 36.0 | 48 | 32.0 | 100 | |
| Intramuscular | 71 | Male | 9.4 | 87.2 | 220 | 40.0 | 62.4 | 35.0 | 100 | |
| Other | 76 | Male | Exact timing unknownc | 93.1 | 220 | 36.1 | 41.1 | 35.8 | 100 | |
| Other | 80 | Male | Exact timing unknownc | Missing | 110 | 39.7 | 44.7 | 37.2 | 100 | |
| Other | 86 | Male | Exact timing unknowne | 25.4 | 220 | 35.2 | 72.8 | 33.2 | 100 | |
| Intracranial | 81 | Female | Unknown | 41.9 | 220 | 40.0 | 91.3 | 37.6 | 100 | |
| Gastrointestinal | 78 | Female | 41.5 | 7.8 | 220 | 40.0 | 144 | 42.7 | 97.4 | |
| Gastrointestinal | 91 | Female | Exact timing unknownc | 26.4 | 220 | 39.0 | 152.7 | 58.9 | 82.5 | |
| Intra-pericardial | 78 | Female | 9.6 | 55.1 | 220 | 32.0 | 35.4 | 33.3 | 61.8 | |
| Intracranial | 85 | Male | Exact timing unknownd | 44.1 | 220 | 34.0 | 38.7 | 37.1 | 34.0 | |
| Gastrointestinal | 73 | Male | Exact timing unknownc | Missing | 150 | 38.0 | 50.9 | 47.7 | 24.8 | |
| Gastrointestinal | 60 | Male | Unknown | 101.5 | 300 | 32.0 | 142.1 | 135.6 | 5.9 | |
| Gastrointestinal | 66 | Female | Exact timing unknownd | Missing | 300 | 38.1 | 44.8 | 61.5 | 0 | |
| B | Burr hole drainage for subdural hemorrhage | 73 | Male | 5.0 | 69.6 | 300 | 40.0 | 47.2 | 27.8 | 100 |
| Craniotomy for brain tumoral hemorrhage | 80 | Male | 7.1 | 76.2 | 220 | 38.9 | 39.8 | 30.1 | 100 | |
| Decompressive craniectomy | 50 | Male | 12.3 | 90.8 | 220 | 40.0 | 54.9 | 35.8 | 100 | |
| Renal failure | 54 | Male | Exact timing unknownc | 25.9 | 220 | 35.6 | 58.4 | 23.4 | 100 | |
| Cholecystectomy | 81 | Female | 8.5 | 87.5 | 220 | 40.0 | 60.8 | 38.0 | 100 | |
| Brain tumor resection | 75 | Female | 31.0 | 67.8 | 75 | 34.3 | 38.5 | 29.6 | 100 | |
| Drainage for brain abscess | 85 | Male | Exact timing unknownd | 60.5 | 220 | 38.1 | 45.1 | 28.0 | 100 | |
| Suture for gastric perforation | 67 | Male | Exact timing unknownd | 38.3 | 300 | 35.0 | 98.7 | 43.1 | 87.3 | |
|
Colectomy for intestinal obstruction |
80 | Male | Missing | Missing | 110 | 35.0 | 161.9 | 68.6 | 73.5 | |
| Procedure for pericardial effusion | 88 | Male | 12.9 | 48.8 | 300 | 25.0 | 63.8 | 40.2 | 60.8 |
Data are presented as n (%)
ULN upper limit of normal at site, aPTT activated partial thromboplastin time, CrCl creatinine clearance
aGroup A, uncontrolled bleeding; Group B, urgent surgery
bMaximum reversal is calculated as [(predose aPTT − minimum postdose aPTT)/(predose aPTT − ULN)] × 100%
cExact time to idarucizumab from last administration of dabigatran was unknown. Last dose of dabigatran was administered at the day before administration of idarucizumab
dExact time to idarucizumab from last administration of dabigatran was unknown. Last dose of dabigatran was administered at the day of administration of idarucizumab
eExact time to idarucizumab from last administration of dabigatran was unknown. Last dose of dabigatran was administered ≥ 2 days before administration of idarucizumab
Time to Cessation of Bleeding
Cessation of bleeding judged by investigators was confirmed in 119 (67.2%) of the 177 patients analyzed in Group A. Of these 119 patients, time to cessation of bleeding was reported for 100 patients. The median time to recorded cessation of bleeding was 5.8 h (IQR 1.7–19.6 h). In many intracranial hemorrhage cases, time to recorded cessation of bleeding may be more prolonged as early follow-up imaging by computed tomography or magnetic resonance imaging was not mandated in this PMS. Therefore, we also evaluated the time to recorded cessation of bleeding in 62 nonintracranial hemorrhage patients. The median time to recorded cessation of nonintracranial hemorrhage was 3.3 h (IQR 0.8–15.0 h).
Periprocedural Hemostasis
Among 87 patients in Group B who underwent surgery or an intervention, periprocedural hemostasis was determined to be normal in 63 patients (72.4%), mildly abnormal in six patients (6.9%), moderately abnormal in nine patients (10.3%), severely abnormal in five patients (5.7%), and missing in four patients (4.6%).
Discussion
For this interim analysis, demographic characteristics and the safety and effectiveness of idarucizumab were evaluated in 262 Japanese patients receiving dabigatran who had either uncontrolled bleeding or were about to undergo an urgent procedure. Idarucizumab safely and effectively resulted in the reversal of the effects of dabigatran. Eighteen patients (6.9%) experienced ADRs within 4 weeks, while the reversal effect of idarucizumab based on aPTT within 4 h after administration (assessed in 30 patients) yielded a median maximum percentage reversal of 100%. Median time to bleeding cessation in patients with uncontrolled bleeding (without intracranial bleeding) was 3.3 h, and normal intraoperative hemostasis was reported in 63/87 patients (72.4%) scheduled for urgent surgery.
While this is an interim analysis, and the study designs differ, our results to date are consistent with the results of the RE-VERSE AD study [13]. The overall median age of patients in our study was 78 years, which is the same as RE-VERSE AD [13], and more than 80% of patients treated with idarucizumab were aged ≥ 70 years. This result is consistent with the previous finding that the majority of nonvalvular atrial fibrillation patients treated with dabigatran are elderly [2]. The median creatinine clearance was 49.0 ml/min in our study; in RE-VERSE AD, 43.3% of patients had a creatinine clearance of < 50 ml/min [13]. In the present interim analysis, the exact timing of the last dose of dabigatran was unknown in approximately 50% of the patients. However, the median patient-reported time from the last dose of dabigatran to the first infusion of idarucizumab was 9.1 h, which was a shorter period of time compared with the time in RE-VERSE AD (14.6 h in Group A and 18.0 h in Group B) [13]. Most patients in our study and RE-VERSE AD received the twice-daily 110-mg dose of dabigatran.
A total of 178 patients were enrolled in Group A because of life-threatening or uncontrolled bleeding. The most common bleeding event in Group A was intracranial hemorrhage followed by gastrointestinal bleeding. Patients with intracranial hemorrhage accounted for 47.2% of cases of uncontrolled bleeding, whereas patients with gastrointestinal hemorrhage accounted for 27.5% of cases of uncontrolled bleeding. Compared with the proportion of bleeding by location in RE-VERSE AD [13], the proportion of gastrointestinal bleeding in Group A was lower, and the proportion of intracranial hemorrhage was higher in this interim analysis. This is consistent with epidemiological studies that have shown intracranial hemorrhage to be more common in Asian than non-Asian patients [18]. In cases where the source of gastrointestinal bleeding can be identified by gastrointestinal endoscopy, cessation of bleeding without administration of idarucizumab could be achieved by clipping or cauterizing the blood vessel. While there may be differences in the medical environment for treatment of gastrointestinal bleeding, most hospitals in Japan can perform endoscopy.
In Group A, 9% of patients had cardiac tamponade during catheter ablation. Cardiac tamponade is a potentially fatal complication and is among the most frequently observed complications of catheter ablation [19]. Although the results of the RE-CIRCUIT study [20] show the benefit of uninterrupted anticoagulation with dabigatran during catheter ablation, administration of idarucizumab might also be useful for patients with cardiac tamponade, as demonstrated in an idarucizumab case series in Japan [21]. In a retrospective analysis of 21 patients with cardiac tamponade who received idarucizumab, hemostasis was restored at a median of 205.6 ± 14.8 min, and no thromboembolic events had occurred within 72 h after the idarucizumab administration [21].
Group B included 88 (33.6%) patients who underwent emergency surgery or an urgent intervention. In this analysis, classification of A or B was determined by investigators, and several patients who required an emergency surgery/intervention such as craniotomy or drainage of subdural hematoma were enrolled in Group B, even if they had bleeding. The most common emergency surgery or urgent intervention was neurological, including craniotomy for intracranial hemorrhage and thrombolysis/thrombectomy for ischemic stroke, which were performed in 49 patients (55.7%). Other common emergency surgeries or urgent interventions included abdominal surgery/intervention in 18 patients (20.5%) and cardiovascular surgery/intervention in 15 patients (17.0%). In RE-VERSE AD [13], the most common urgent surgery/intervention was abdominal condition or infection, followed by fracture or septic arthritis. Compared with the reasons for surgery in RE-VERSE AD [13], the proportion of neurological surgery/intervention in Group B was higher and there was no fracture or septic arthritis in this interim analysis. This difference may be attributable to the indications of dabigatran. In Japan, dabigatran is not indicated for the prevention or treatment of venous thromboembolism. In this interim analysis, nine patients were treated with idarucizumab before treatment of ischemic stroke (thrombolysis, thrombectomy, or bypass for ischemic stroke). Several case reports suggest that thrombolysis and thrombectomy can be performed safely after dabigatran reversal with idarucizumab [22, 23]. Although these data are limited, recommendations have been published regarding acute reperfusion therapy with idarucizumab for patients with ischemic stroke receiving dabigatran [24, 25].
In this interim analysis, about 30% of the patients in Group A had trauma-related bleeding. In addition to trauma-related bleeding in Group A, several patients in Group B required emergency surgery for trauma-related injury. However, we could not evaluate the number of patients accurately because it was not mandatory to collect trauma-related data in Group B. In Group B, the most frequent event was a surgery/intervention for subdural hematoma, which was secondary to a fall. Patients receiving anticoagulant therapy are at increased risk for intracranial hemorrhage after minor head injury, even in cases where initial computed tomography scans following the injury are negative [26]. There have been reports of patients who develop life-threatening intracranial hematoma in the days following even minor traumatic brain injury [27, 28]. Therefore, a drug that can rapidly reverse the anticoagulant effect may be useful in treating such patients and preventing further injury.
ADRs, serious AEs, and AEs leading to death within 4 weeks after administration of idarucizumab were reported in 18 patients (6.9%), 76 patients (29.0%), and 40 patients (15.3%) in this interim analysis, respectively. The majority of events appeared to be a worsening of the index event or were associated with coexisting conditions; these results were similar to those of RE-VERSE AD [13]. In the present interim analysis, the rate of thrombotic events was 6.1% within 4 weeks after treatment and the rate was similar in Groups A and B (6.2 and 5.7%, respectively). Eleven patients (68.8%) who presented thrombotic events had not reinitiated anticoagulation therapy when the events occurred. The rate of restarting anticoagulation was 51.6%. This low rate of restarting anticoagulation may have contributed to the thrombotic events because thrombotic events are more likely to reflect the underlying prothrombotic state than to be a direct effect of reversal. In RE-VERSE AD [13], thrombotic events occurred in 4.8% of patients overall within 30 days after treatment. These results underscore the importance of restarting anticoagulation therapy at an appropriate time for patients in whom it is indicated.
In this interim analysis, of the 262 patients, 25.2% had a normal range of aPTT and approximately 27% were lacking aPTT data at baseline. As this was an observational study, measurement and assessment of coagulation tests before and after administration of idarucizumab were not mandatory. Idarucizumab is generally used in urgent cases. It is likely that some physicians may have decided to administer idarucizumab to patients treated with dabigatran without measurement and/or assessment of coagulation parameters to avoid a delay in treatment. In RE-VERSE AD [13], even if patients had little or no circulating dabigatran, the use of idarucizumab was shown to be safe.
In RE-VERSE AD [13], reversal of the anticoagulant effect of dabigatran was assessed based on the diluted thrombin time (dTT) or the ecarin clotting time (ECT). In Japanese clinical settings, dTT, and ECT are not available. As aPTT is available in Japanese clinical settings, the reversal effect based on aPTT measurement was chosen as a secondary endpoint in this study. The reversal effect based on aPTT was evaluated in only 30 patients (11.5%) who had aPTT data before and after treatment with idarucizumab. The aPTT values in patients after treatment were below the normal range in 20 (66.7%) of the 30 patients. The remaining ten patients still showed prolongation of aPTT values after administration of idarucizumab; these patients included those with coagulopathy due to massive bleeding or those who had received heparin during the perioperative period. Notably, aside from dabigatran treatment, these patients seemed to have other factors that contributed to prolongation of aPTT values.
Among patients with non-intracranial bleeding who could be evaluated, the median time to the cessation of bleeding was 3.3 h. In RE-VERSE AD [13], among patients with overt bleeding who could be evaluated in the first 24 h, the median time to the cessation of bleeding was 2.5 h. The time to the cessation of bleeding in our study is similar to the time to cessation of bleeding in RE-VERSE AD [13]. In Group B of RE-VERSE AD [13], 95% of patients had normal or mildly abnormal hemostasis during the procedure, which is consistent with the hemostasis observed during the procedures in this study. Therefore, the use of idarucizumab permitted safe intervention in the majority of patients.
Data related to the re-exposure to idarucizumab are lacking as evidence of re-exposure of idarucizumab is limited in clinical trials. In this interim analysis, only one of the 262 patients (0.4%) received a second dose of 5-g idarucizumab after first administration of idarucizumab and resumption of dabigatran. The reversal effect after the first and second administration of idarucizumab could not be evaluated because aPTT data were missing. Regarding safety, no AEs were reported after the first and second administration of idarucizumab. Further clinical data are needed to fully determine the impact of repeated administration of idarucizumab.
Limitations
This study has some limitations inherent to PMS studies in general. We were unable to collect detailed information such as that obtained in clinical trials. Additionally, this study lacks a control arm. Evaluation of the reversal effect is limited because there are no data on the concentration of dabigatran, dTT, and ECT. The 4-week observation period could not be completed for some patients due to hospital discharge or transfer. Finally, this PMS is in progress, so these interim results should be interpreted with caution.
Conclusions
The results of this interim analysis of this PMS suggest that idarucizumab can safely and effectively reverse the effects of dabigatran in Japanese patients in real-world clinical practice. The safety and effectiveness data support the continued use of idarucizumab, and no new safety concerns have been identified thus far. These interim findings, based on the data from 262 patients, further corroborate the favorable safety profile of idarucizumab as previously shown in RE-VERSE AD.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank all the patients and the investigators involved in this PMS.
Funding
This post-marketing surveillance study and the journal’s Rapid Service Fee were sponsored by Nippon Boehringer Ingelheim Co., Ltd.
Medical Writing, Editorial, and Other Assistance
We thank Keyra Martinez Dunn, MD, of Edanz Medical Writing for providing medical writing support, which was funded by Nippon Boehringer Ingelheim through EMC K.K. in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3). Safety review and editorial support were provided by Yoshihide Yamamoto of Nippon Boehringer Ingelheim Co., Ltd.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript. YO, KO, and DN contributed to this PMS design, KO analyzed the data, and all authors contributed to the interpretation of the data. MY and DN led the drafting of the manuscript, all authors contributed to critically revising the manuscript, and all authors read and approved the final manuscript.
Disclosures
Masahiro Yasaka has received lecture, advisory, and travel fees from Bristol-Myers Squibb, Nippon Boehringer Ingelheim, Bayer, Daiichi Sankyo, CSL Behring, as well as scholarship funds or non-restricted grants from Nippon Boehringer Ingelheim. Hiroyuki Yokota has received lecture, consulting fees, and grants from Eisai, Nihon Kohden, Stryker Japan, IMI, Daiichi Sankyo, Asahi Kasei ZOLL Medical, Asahi Kasei Pharma, Astellas, Pfizer, and Otsuka Pharmaceutical, and Otsuka Pharmaceutical Factory. Michiyasu Suzuki has received lecture fees from Nippon Boehringer Ingelheim and CSL Berling. Hidesaku Asakura has received lecture fees from Bristol-Myers Squibb, Pfizer, Daiichi Sankyo, Bayer, Nippon Boehringer Ingelheim; scholarship from Pfizer, Daiichi Sankyo, Nippon Boehringer Ingelheim, CSL Berling; grants from Bristol-Myers Squibb. Teiichi Yamane has received lecture and consulting fees from Daiichi Sankyo, Nippon Boehringer Ingelheim, Abbott Japan, Bristol-Myers Squibb, Bayer Pharmaceutical Company, Medtronic Japan. Yukako Ogi and Daisuke Nakayama are employees of Nippon Boehringer Ingelheim. Kaori Ochiai is an employee of EPS Corporation.
Compliance with Ethics Guidelines
This PMS study is fully compliant with Japanese Good Post-marketing Study Practice regulations. The protocol was approved by the Ministry of Health, Labour and Welfare of the Japanese Government. This study involved the collection of anonymous data from clinical settings and, therefore, it was not necessary to obtain informed consent from patients. All medical institutions who agreed to provide these anonymized data signed a contract with Nippon Boehringer Ingelheim Co., Ltd.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Footnotes
Digital Features
This article is published with digital features, including a summary slide, slide deck and video abstract, to facilitate understanding of the article. To view digital features for this article go to 10.6084/m9.figshare.11763183.
Change history
6/1/2024
A peer-reviewed video abstract was retrospectively added to this publication.
References
- 1.Hankey GJ, Eikelboom JW. Dabigatran etexilate: a new oral thrombin inhibitor. Circulation. 2011;123:1436–1450. doi: 10.1161/CIRCULATIONAHA.110.004424. [DOI] [PubMed] [Google Scholar]
- 2.Inoue H, Uchiyama S, Atarashi H, J-Dabigatran Surveillance Investigators et al. Effectiveness and safety of long-term dabigatran among patients with non-valvular atrial fibrillation in clinical practice: J-dabigatran surveillance. J Cardiol. 2019;73:507–514. doi: 10.1016/j.jjcc.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 3.Hori M, Connolly SJ, Zhu J, RE-LY Investigators et al. Dabigatran versus warfarin: effects on ischemic and hemorrhagic strokes and bleeding in Asians and non-Asians with atrial fibrillation. Stroke. 2013;44:1891–1896. doi: 10.1161/STROKEAHA.113.000990. [DOI] [PubMed] [Google Scholar]
- 4.Hori M, Connolly SJ, Ezekowitz MD, Reilly PA, Yusuf S, Wallentin L, RE-LY Investigators Efficacy and safety of dabigatran vs. warfarin in patients with atrial fibrillation–sub-analysis in Japanese population in RE-LY trial. Circ J. 2011;75:800–805. doi: 10.1253/circj.CJ-11-0191. [DOI] [PubMed] [Google Scholar]
- 5.Naganuma M, Shiga T, Nagao T, Suzuki A, Murasaki K, Hagiwara N. Effectiveness and safety of dabigatran versus warfarin in “real-world” Japanese patients with atrial fibrillation: a single-center observational study. J Arrhythm. 2017;33:107–110. doi: 10.1016/j.joa.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Connolly SJ, Ezekowitz MD, Yusuf S, RE-LY Steering Committee and Investigators et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 7.Lip GY, Clemens A, Noack H, Ferreira J, Connolly SJ, Yusuf S. Patient outcomes using the European label for dabigatran. A post hoc analysis from the RE-LY database. Thromb Haemost. 2014;111:933–942. doi: 10.1160/TH13-09-0734. [DOI] [PubMed] [Google Scholar]
- 8.Schiele F, van Ryn J, Canada K, et al. A specific antidote for dabigatran: functional and structural characterization. Blood. 2013;121:3554–3562. doi: 10.1182/blood-2012-11-468207. [DOI] [PubMed] [Google Scholar]
- 9.Glund S, Moschetti V, Norris S, et al. A randomised study in healthy volunteers to investigate the safety, tolerability and pharmacokinetics of idarucizumab, a specific antidote to dabigatran. Thromb Haemost. 2015;113:943–951. doi: 10.1160/TH14-12-1080. [DOI] [PubMed] [Google Scholar]
- 10.Schmohl M, Glund S, Harada A, et al. Idarucizumab does not have procoagulant effects: assessment of thrombosis biomarkers in healthy volunteers. Thromb Haemost. 2017;117:269–276. doi: 10.1160/TH16-05-0385. [DOI] [PubMed] [Google Scholar]
- 11.Yasaka M, Ikushima I, Harada A, et al. Safety, pharmacokinetics and pharmacodynamics of idarucizumab, a specific dabigatran reversal agent in healthy Japanese volunteers: a randomized study. Res Pract Thromb Haemost. 2017;1:202–215. doi: 10.1002/rth2.12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glund S, Stangier J, Schmohl M, et al. Safety, tolerability, and efficacy of idarucizumab for the reversal of the anticoagulant effect of dabigatran in healthy male volunteers: a randomised, placebo-controlled, double-blind phase 1 trial. Lancet. 2015;386:680–690. doi: 10.1016/S0140-6736(15)60732-2. [DOI] [PubMed] [Google Scholar]
- 13.Pollack CV, Jr, Reilly PA, van Ryn J, et al. Idarucizumab for dabigatran reversal—full cohort analysis. N Engl J Med. 2017;377:431–441. doi: 10.1056/NEJMoa1707278. [DOI] [PubMed] [Google Scholar]
- 14.US Food and Drug Administration. Praxbind (idarucizumab) Drug Approval History. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/0761025Orig1000TOC.cfm. Accessed 20 Feb 2020.
- 15.European Medicines Agency. Pradaxa. Product information. https://www.ema.europa.eu/en/medicines/human/EPAR/pradaxa. Accessed 20 Feb 2020.
- 16.Japanese Ministry of Health, Labour & Welfare. Report on the Deliberation Results. 2016. http://www.pmda.go.jp/files/000222407.pdf Accessed 20 Feb 2020.
- 17.International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. Medical Dictionary for Regulatory Activities version 20. https://www.meddra.org. Accessed 20 Feb 2020.
- 18.Krishnamurthi RV, Feigin VL, Forouzanfar MH, et al. Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet Glob Health. 2013;1:e259–e281. doi: 10.1016/S2214-109X(13)70089-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pedersen MEF, Leo M, Kalla M, et al. Management of tamponade complicating catheter ablation for atrial fibrillation: early removal of pericardial drains is safe and effective and reduces analgesic requirements and hospital stay compared to conventional delayed removal. JACC Clin Electrophysiol. 2017;3:367–373. doi: 10.1016/j.jacep.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 20.Calkins H, Willems S, Gerstenfeld EP, et al. Uninterrupted dabigatran versus warfarin for ablation in atrial fibrillation. N Engl J Med. 2017;76:1627–1636. doi: 10.1056/NEJMoa1701005. [DOI] [PubMed] [Google Scholar]
- 21.Okishige K, Yamauchi Y, Hanaki Y, et al. Clinical experience of idarucizumab use in cases of cardiac tamponade under uninterrupted anticoagulation of dabigatran during catheter ablation of atrial fibrillation. J Thromb Thrombolysis. 2019;47:487–494. doi: 10.1007/s11239-019-01835-8. [DOI] [PubMed] [Google Scholar]
- 22.Mutzenbach JS, Pikija S, Otto F, Halwachs U, Weymayr F, Sellner J. Intravenous thrombolysis in acute ischemic stroke after dabigatran reversal with idarucizumab—a case report. Ann Clin Transl Neurol. 2016;3:889–892. doi: 10.1002/acn3.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pikija S, Sztriha LK, Mutzenbach S, Golaszewski SM, Sellner J. Idarucizumab in dabigatran-treated patients with acute ischemic stroke receiving alteplase: a systematic review of the available evidence. CNS Drugs. 2017;31:747–757. doi: 10.1007/s40263-017-0460-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toyoda K, Yamagami H, Koga M. Consensus guides on stroke thrombolysis for anticoagulated patients from Japan: application to other populations. J Stroke. 2018;20:321–331. doi: 10.5853/jos.2018.01788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diener HC, Bernstein R, Butcher K, et al. Thrombolysis and thrombectomy in patients treated with dabigatran with acute ischemic stroke: expert opinion. Int J Stroke. 2017;12:9–12. doi: 10.1177/1747493016669849. [DOI] [PubMed] [Google Scholar]
- 26.Menditto VG, Lucci M, Polonara S, Pomponio G, Gabrielli A. Management of minor head injury in patients receiving oral anticoagulant therapy: a prospective study of a 24-h observation protocol. Ann Emerg Med. 2012;59:451–455. doi: 10.1016/j.annemergmed.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Hadjigeorgiou GF, Anagnostopoulos C, Chamilos C, Petsanas A. Patients on anticoagulants after a head trauma: is a negative initial CT scan enough? Report of a case of delayed subdural haematoma and review of the literature. J Korean Neurosurg Soc. 2014;55:51–53. doi: 10.3340/jkns.2014.55.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Itshayek E, Rosenthal G, Fraifeld S, Perez-Sanchez X, Cohen JE, Spektor S. Delayed posttraumatic acute subdural hematoma in elderly patients on anticoagulation. Neurosurgery. 2006;58:E851–E856. doi: 10.1227/01.NEU.0000209653.82936.96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.