Abstract
Introduction
As HIV has become a manageable chronic condition, a renewed and increased interest in challenging traditional three-drug HIV therapies and moving toward two-drug regimens (2DR) for initial or maintenance treatment in people living with HIV (PLWH) has developed. As PLWH are living longer, continual advancements in antiretroviral regimens have been a focus to provide optimal life-long therapy options. Although early studies may have shown poor outcomes in virologic suppression with 2DR, newer studies and treatment options have emerged to show promise in the management of HIV. The purpose of this review is to evaluate current literature and assess the efficacy of two-drug (2DR) antiretroviral therapy in treatment-naïve and -experienced people living with HIV.
Methods
A systematic search was performed between January 2009 to January 2020, using EMBASE, MEDLINE, Google Scholar, and bibliographies. Combinations of the following search terms were used: HIV-1 infection, antiretroviral therapy, dual therapy, two-drug regimen, two-drug therapy, two-drug regimen, and 2DR. Included studies were those in the adult population with at least one active comparator, outcomes assessing HIV-1 RNA viral load while on treatment, and written in English.
Results
Thirty-three studies were included, 13 where 2DRs were evaluated as initial therapy (3 studies with extension data) and 15 where 2DRs were evaluated as maintenance or switch therapy (2 studies with extension data).
Conclusion
Although 2DRs may not be appropriate in all patient populations, they are being utilized more frequently and have the potential to reduce costs, adverse effects, and drug interactions.
Keywords: Antiretroviral therapy, Dual therapy, HIV, HIV treatment, Two-drug regimen (2DR)
Key Summary Points
| HIV treatment has evolved from a time where two-drug regimens (2DRs) were once considered a novel concept to current times where they are a reality. |
| Over the past year, national guidelines recommend 2DRs for initial consideration in the management of HIV for patients meeting certain criteria. |
| When compared with three-drug regimens, many 2DRs have demonstrated noninferiority, in terms of virologic efficacy, in both the treatment-naïve and -experienced populations. |
Introduction
With continued advancement of antiretroviral therapy (ART), human immunodeficiency virus (HIV) has evolved from an acute and fatal diagnosis to a chronic condition where many patients are able to live a long and healthy life. Although there is currently no cure available for HIV, highly toxic regimens requiring high pill burden and dosing frequency are no longer the standard in care. Antiretroviral medications are now more readily available with durable virologic efficacy, high genetic barriers to resistance, more tolerable side effect profiles, and reduced pill burden. To understand the future of HIV medicine, it is important to review the past lessons learned.
In 1987, the first agent to manage HIV, zidovudine (AZT), was approved as monotherapy in the treatment of people living with HIV (PLWH) [1]. However, the limitations of monotherapy soon became apparent as researchers found the regimen could not sustain virologic suppression and led to the rapid development of drug resistance [1].
In the 1990s, researchers began to shift their focus toward three-drug regimens (3DRs) for the treatment of HIV. This idea ultimately changed and shaped the course of HIV management. In 1996, the combination of a protease inhibitor (PI) with two NRTIs was shown to rapidly reduce HIV RNA levels and improve immune function in patients by targeting different steps within the HIV life cycle [2].
Since the approval of the AZT in 1987, there have been over 30 drugs and 7 different mechanistic drug classes approved for the management of PLWH [3]. Historically, initial ART for treatment-naïve individuals consisted of a two-drug NRTI backbone plus a drug from another drug class such as a non-nucleoside reverse transcriptase inhibitor (NNRTI), integrase strand transfer inhibitor (INSTI), or boosted PI [4]. Several clinical trials and evaluations over the last decade have shown that this three-drug treatment strategy for treatment-naïve patients results in HIV viral suppression and increased immunologic function [4].
The European AIDS Clinical Society (EACS) 2019 guidelines recommend initial regimens in treatment-naïve patients including unboosted INSTI-based regimens, NNRTI-based with rilpivirine (RPV) or doravirine (DOR) or PI-based with boosted darunavir (DRV) [5]. Interestingly, the EACS guidelines were the first to recommend the two-drug regimen (2DR) dolutegravir (DTG)/lamivudine (3TC) for consideration as a recommended regimen in patients whose hepatitis B surface antigen (HBsAg) is negative, HIV-1 RNA viral load < 500,000 copies/ml, and CD4 > 200 cells/mm3 [5]. Shortly thereafter, US-based guidelines also adopted this recommendation.
While 3DRs have been the mainstay of treatment over the last 2 decades, attempts have been made to further reduce medication and pill burden while preventing long-term toxicities and increasing tolerability. Studies have shown that simplifying ART regimens to monotherapy in virologically suppressed PLWH is not a reliable or effective option. In an open-label, phase 2, randomized non-inferiority trial, DTG monotherapy was non-inferior to combination ART at 24 weeks; however, viriologic failure was seen after 24 weeks with continued monotherapy, which led to DTG resistance [6]. The switch to certain monotherapy treatments occurred in patients already virologically suppressed; these regimens have largely failed to maintain viral suppression for an extended duration, with frequent cases of virologic rebound and emerging resistance [6–8].
In November of 2017, DTG/RPV was approved as the first dual ARV single-tablet regimen (STR) for the maintenance therapy of HIV-1 infection [9]. Although DTG/RPV studies have shown efficacy, safety, and tolerability in treatment-experienced patients with sustained virologic suppression, there is still the concern for arising ARV resistance [9]. Overall, DTG/RPV has opened the doors to researching the use of 2DR in in treatment-experienced and -naïve PLWH. Therefore, this article will focus reviewing the efficacy of 2DR as initial or maintenance/switch treatment in PLWH. This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Methods
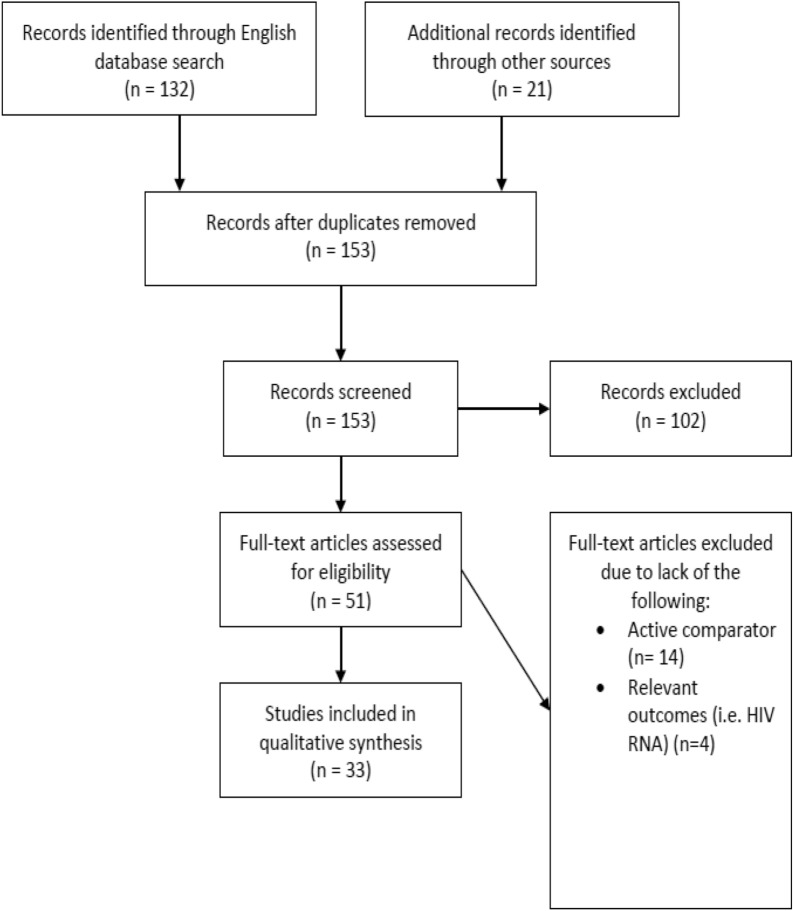
Manuscripts and abstracts were systematically searched between January 2009 and January 2020, using PubMed, EMBASE, MEDLINE, Google Scholar, conference proceedings, and bibliographies. Combinations of the following search terms were used: HIV-1 infection, antiretroviral therapy, dual therapy, two-drug regimen, two-drug therapy, two-drug regimen, and 2DR. Studies were screened by abstract review for relevance, and only those with at least one active comparator and outcomes assessing HIV-1 RNA viral load while on treatment were included (Fig. 1). In addition, article selection was limited to the English language and adult populations only. Two authors independently replicated the initial literature search to confirm the eligibility of identified studies and screened titles and abstracts of those identified for inclusion in the review with no disagreement.
Fig. 1.
Study selection process
Information taken from each study included ART regimens, trial design, outcomes including virologic suppression or failure, and the development of resistance. The data from each study were then organized into two main categories of whether patients were treatment naïve or treatment experienced and then further subdivided based on whether they were receiving a regimen that was NRTI inclusive or NRTI sparing.
Results
Initial HIV Treatment Population
NRTI Inclusive
Four randomized, non-inferiority trials were identified of NRTI-inclusive 2DRs in the treatment-naïve population (Table 1) [10–14]. A small study of (lopinavir/ritonavir) LPV/r + tenofovir disoproxil fumarate (TDF) vs. LPV/r + two non-TDF NRTIs failed to achieve non-inferiority, but was notably underpowered. Unusually high discontinuation rates were also observed among both groups (2DR 42%; 3DR 44%) in addition to poor efficacy (2DR 51%; 3DR 53%) [14].
Table 1.
Summary of trials comparing two-drug regimens as initial treatment in people living with HIV-1
| Study | Study agents | Study design | Virologic outcomes | Protocol-defined virologic failure | Additional comments |
|---|---|---|---|---|---|
| NRTI-inclusive regimens | |||||
| GEMINI 1/GEMINI 2 [10] | GEMINI-1 | Two parallel, phase 3 randomized, non-inferiority studies performed in 21 countries | VL < 50 copies/ml W48 (ITT): | DTG/3TC: 0/6 | Non-inferiority met |
| DTG/3TC (n = 356) | |||||
| DTG + TDF/FTC: 0/4 | |||||
| Response rate lower in the DTG/3TC patients with CD4 < 200 cells/mm3 | |||||
| DTG + TDF/FTC (n = 358) | GEMINI-1: | ||||
| DTG/3TC: 90% | |||||
| GEMINI-2 | DTG + TDF/FTC: 93% | ||||
| GEMINI-2: | |||||
| DTG/3TC (n = 360) | DTG/3TC: 93% | ||||
| DTG + TDF/FTC: 94% | |||||
| DTG + TDF/FTC (n = 359) | |||||
| GEMINI 1/GEMI NI 2 (96 weeks) [11] | Pooled data | VL < 50 copies/ml W96 | DTG/3TC: 0/11 | ||
| DTG/TDF/FTC: 0/7 | |||||
| DTG/3TC: 86% | |||||
| DTG/TDF/FTC: 90% | |||||
| ANDES [12] | DRV/r + 3TC (n = 70) | Phase 4, open-label, randomized, non-inferiority study performed in Argentina | VL < 50 copies/ml at W48 (ITT): | 3DR group: 0/1 | Non-inferiority met |
| DRV/r + TDF/FTC (n = 75) | |||||
| Generic DRV/r fixed-dose combination product (Virontar™) not available in US | |||||
| 2DR: 93% | |||||
| 3DR: 94% | |||||
| GARDEL [13] | LPV/r + 3TC (n = 217) | Phase 3, open-label, randomized, non-inferiority study performed in Argentina, Chile, Mexico, Peru, Spain, and the USA | VL < 50 copies/ml at W48 (ITT): | 2DR group: 2/10 | Non-inferiority met |
| LPV/r + 2NRTIs (n = 209) | |||||
| More treatment-related discontinuations in 3DR group, primarily due to AZT | |||||
| 2DR: 88% | NRTI: 2 | ||||
| 3DR group: 0/12 | |||||
| 3DR: 84% | |||||
| KALEAD Study [14] | LPV/r +TDF (n = 72) | Open-label, randomized, non-inferiority study performed in Italy | VL < 50 copies/ml at W72 (ITT): | 2DR group: 1/21 | Study was underpowered |
| LPV/r + two non-TDF NRTIs (n = 80) | High discontinuation rates (42% and 44%) | ||||
| PI: 1 | |||||
| 2DR: 51% | 3DR group: 2/12 | ||||
| 3DR: 53% | |||||
| NRTI: 2 | |||||
| NRTI-sparing regimens | |||||
| FLAIR [15] | 20-week induction: | Phase 3, randomized, open-label, non-inferiority study, multicenter | VL < 50 copies/ml at W48 (ITT): | LA CAB/RPV: 3/4 | Non-inferiority met |
| DTG/ABC/3TC | |||||
| *Required 4-week lead-in of oral CAB 30 mg + RPV for LA CAB/RPV group | |||||
| NNRTI: 3 | |||||
| LA CAB/RPV: 94% | |||||
| INSTI: 3 | |||||
| LA CAB/RPV every 4 weeks (n = 283)* | |||||
| DTG/ABC/3TC: 0/3 | |||||
| DTG/ABC/3TC: 93% | |||||
| DTG/ABC/3TC (n = 283) | |||||
| LATTE-2 [16] | 20-week induction: | Phase 2b, randomized, open-label, non-inferiority study performed in the USA, Canada, Spain, France, and Germany | VL < 50 copies/ml at W96 (ITT): | LA CAB/RPV 8-week group: 1/2 | Non-inferiority met |
| Oral CAB 30 mg + ABC/3TC | |||||
| LA CAB/RPV 4-week group: 87% | NNRTI: 1 | ||||
| LA CAB 400 mg/RPV 600 mg 4-week group (n = 115) | INSTI: 1 | ||||
| Oral CAB group: 0/1 | |||||
| LA CAB/RPV 8-week group: 94% | |||||
| LA CAB 600 mg/RPV 900 mg 8-week group (n = 115) | |||||
| Oral CAB + ABC/3TC: 84% | |||||
| Oral CAB 30 mg + ABC/3TC (n = 56) | |||||
| LATTE-2 (160 weeks) [17] | 4-week group (n = 115) | Phase 2b, randomized, open-label extension, non-inferiority study performed in the USA, Canada, Spain, France, and Germany | VL < 50 copies/ml at W96 (ITT): | No new cases of virologic failure among any groups after original 96-week period | LATTE-2 oral group “optimized” after week 96 and switched to every 4-week or 8-week LA CAB/RPV |
| 8-week group (n = 115) | |||||
| 4-week group: 83% | |||||
| 8-week group: 90% | |||||
| Optimized 4-week group (n = 10) | Optimized 4-week group: 100% | ||||
| Original 4 and 8-week LA groups were continued | |||||
| Optimized 8-week group: 97% | |||||
| Optimized 8-week group (n = 34) | |||||
| NEAT 001/ANRS 143 [18] | DRV/r + RAL (n = 401) | Phase 3, open-label, randomized, non-inferiority study performed in Europe | VL < 50 copies/ml at W96 (ITT): | DRV/r + RAL: 18/61 | Non-inferiority met |
| NRTI: 2 | |||||
| 2DR group had higher failure rates when baseline CD4 < 200 cells/mm3 | |||||
| DRV/r + RAL: 79% | PI: 1 | ||||
| DRV/r + TDF/FTC (n = 404) | DRV/r + TDF/FTC: 82% | INSTI:15 | |||
| DRV/r + TDF/FTC: 0/49 | |||||
| MODERN [19] | Daily MVC (150 mg) + DRV/r (n = 396) | Phase 3, double-blind, randomized, non-inferiority study performed in Europe, the USA, Australia, and Canada | VL < 50 copies/ml at W48 (FAS): | MVC + DRV/r: 0/37 | MVC 2DR found to be inferior to 3DR |
| DRV/r + TDF/FTC: 0/10 | |||||
| DRV/r + TDF/FTC (n = 401) | |||||
| MVC + DRV/r: 77% | Study terminated early due to virologic inferiority of the MVC arm | ||||
| DRV/r + TDF/FTC: 87% | |||||
| VEMAN [20] | Daily MVC (150 mg) + LPV/r twice daily (n = 26) | Prospective, open-label, randomized, proof-of concept, study performed in Italy | VL < 50 copies/ml W48 (ITT): | Not assessed | Similar virologic outcomes |
| Greater immunologic benefit in MVC + LPV/r group (p = 0.033) | |||||
| LPV/r + MVC: 100% | |||||
| Daily TDF/FTC + LPV/r twice daily (n = 24) | LPV/r + TDF/FTC: 96% | ||||
| LATTE [21] | 24-week Induction: Oral CAB 10 mg (n = 60), 30 mg (n = 60), 60 mg (n = 61) mg or EFV 600 mg (n = 62), + 2 NRTIs | Phase 2b, randomized, dose-finding study performed in the USA and Canada | VL < 50 copies/ml at W96 (ITT): | Oral CAB 10 mg: 2/2 | Dose finding study |
| INSTI: 1 | |||||
| NNRTI: 2 | |||||
| CAB 10 mg: 68% | Oral CAB 30 mg: 0/1 | ||||
| CAB 30 mg: 75% | |||||
| CAB 60 mg: 84% | EFV + 2 NRTIs: 0/2 | ||||
| EFV + 2 NRTIs: 63% | |||||
| Maintenance: | |||||
| Oral CAB + RPV | |||||
| EFV + 2 NRTIs | |||||
| LATTE (144 weeks) [22] | CAB 30 mg (n = 181) | Phase 2b, OLE performed in the USA and Canada | VL < 50 copies/ml at W144 (ITT): Stratified by original randomization | New cases after original 96-week period | |
| Stratified by original randomization | |||||
| CAB 10 mg: 2/2 | |||||
| CAB 10 mg (n = 60) | |||||
| CAB 30 mg (n = 60) | INSTI: 1 | ||||
| CAB 60 mg (n = 61) | NNRTI: 2 | ||||
| CAB 10 mg 58% | |||||
| CAB 30 mg: 0/1 | |||||
| CAB 30 mg 67% | |||||
| CAB 60 mg 77% | |||||
| RADAR [23] | DRV/r + RAL (n = 42) | Open-label, randomized, non-inferiority study performed in Dallas, TX, USA | VL < 48 copies/ml W48 (ITT): | DRV/r + RAL: 0/3 | NRTI-sparing regimen inferior at W48 |
| DRV/r + TDF/FTC (n = 43) | |||||
| DRV/r + RAL: 63% | |||||
| DRV/r + TDF/FTC: 84% | |||||
| PROGRESS [24] | LPV/r + RAL (n = 101) | Open-label, randomized, pilot study performed in the USA, Poland, Puerto Rico, Italy, and Spain | VL < 40 copies/ml at W96 (ITT): | LPV/r + RAL: 3/8 | Non-inferiority met |
| LPV/r + TDF/FTC (n = 105) | INSTI: 3 | ||||
| PI: 1 | |||||
| LPV/r + TDF/FTC: 1/5 | |||||
| LPV/r + RAL: 71% | |||||
| LPV/r + TDF/FTC: 71% | |||||
| SPARTAN [25] | ATV + RAL (n = 63) | Phase 2b, open-label, randomized, non-inferiority, pilot study performed in New Haven, CT, USA | VL < 50 copies/ml at W24 (mITT): | ATV + RAL: 4/6 | Study terminated early due to hyperbilirubinemia and RAL resistance emergence in ATV + RAL |
| ATV/r +TDF/FTC (n = 31) | INSTI: 4 | ||||
| ATV/r +TDF/FTC: 0/1 | |||||
| ATV + RAL: 75% | |||||
| ATV/r +TDF/FTC: 63% | |||||
2DR two-drug regimen, 3DR three-drug regimen, 3TC lamivudine, ABC abacavir, ATV/r ritonavir boosted atazanavir, AZT zidovudine, CAB cabotegravir, DRV/r ritonavir boosted darunavir, DTG dolutegravir, EFV efavirenz, FAS full analysis set, FTC emtricitabine, INSTI integrase strand transfer inhibitors, ITT intention-to-treat, LA long acting, LPV/r ritonavir-boosted lopinavir, mITT modified intent-to-treat, MVC maraviroc, NRTI nucleoside reverse transcriptase inhibitor, NNRTI non- nucleoside reverse transcriptase inhibitors, PI protease inhibitor, OLE open-label extension, RAL raltegravir, RAM resistance associated mutations, RCT randomized controlled trial, RPV rilpivirine, TDF tenofovir disoproxil fumarate, VL viral load, W48 week 48, W96 week 96, W144 week 144
Three studies included lamivudine (3TC) as the single NRTI of their 2DR, with two studies including a boosted PI and one including DTG [10, 12, 13]. High rates of virolologic suppression at 48 weeks were achieved among all three studies, with comparable efficacy shown between groups ranging from 88 to 93% for 2DRs vs. 84–94% for 3DRs. No cases of treatment-emergent resistance were found among the GEMINI study of DTG + 3TC [10] or the ANDES study of darunavir (DRV)/r + 3TC [12]. Ninety-six-week pooled data from the GEMINI study were recently presented, and non-inferiority was met between 2DR and 3DR [11]. Although 11 people in the 2DR arm and 7 in the 3DR arm met protocol-defined failure, no participant developed treatment-emergent resistance through 96 weeks.
NRTI Sparing
Of nine non-inferiority studies including a variety of 2DR combinations, non-inferiority was achieved in five studies [15–18, 24] (Table 1) [15–25]. Two studies included maraviroc (MVC) + boosted PI [19, 20]. The 2DR of daily MVC + DRV/r was terminated early because of poor comparative efficacy [19]. However, non-inferiority was achieved in a subsequent small, proof-of-concept study of patients with lower baseline VL (i.e., < 100,000 copies/ml) initiated on daily MVC + twice daily LPV/r [20]. It should be noted that these findings were not seen in a larger study comparing MVC with a boosted PI in participants with baseline HIV RNA > 100,000 copies/ml [19]. Although data exist for once-daily maraviroc in treatment-experienced patients with R5 virus, the dose is 300 mg daily instead of 150 mg daily [26]. The lack of the typical 300 mg daily dosing of MVC may have reduced its efficacy and use in this setting.
Three studies included raltegravir (RAL) + boosted PI [23–25]. One study of RAL + LPV/r achieved non-inferiority and reported more favorable changes from the baseline estimated glomerular filtration rate (eGFR) and bone mineral density (BMD) relative to LPV/r + TDF/emtricitabine (FTC) [24]. A small study of DRV/r + RAL vs. DRV/r + TDF/FTC failed to achieve non-inferiority [23]. However, a much larger study of the same regimens achieved non-inferiority at 96 weeks and found increased rates of resistance and treatment failure among patients with low CD4 counts (i.e., < 200 cells/mm3) or high baseline HIV-RNA viral loads (i.e., > 100,000 copies/ml) [18]. A single pilot study of RAL + unboosted atazanavir (ATV) failed to achieve non-inferiority and was terminated early because of high rates of resistance and toxicities emerging from the once-daily dosing regimen of ATV (SPARTAN) [25].
Three studies included the new INSTI being evaluated for approval, cabotegravir (CAB), plus the NNRTI RPV [15–17, 21, 22]. Of those studies, one was a dose-finding study for oral formulations and two were of the novel, long-acting (LA) formulations. Data from LATTE demonstrated patients receiving oral CAB 30 mg + RPV 25 mg daily experienced high rates of viral suppression at week 96 (75%) and tolerated the regimen well without any development of resistance [21, 22]. Based on these results, the CAB 30 mg once-daily dose was selected for continued evaluation. LATTE-2 utilized the 30 mg CAB dose as part of a 20-week, 3DR induction phase, which was followed by a novel 2DR of LA, injectable formulation of CAB and RPV given once every 4 or 8 weeks [16, 17]. Non-inferiority at 96 weeks was achieved in both groups relative to the continuation of the induction phase 3DR, with minimal resistance emerging in the LA 8-week group. The LA formulations dosed every 4 and 8 weeks were well tolerated, with frequent, yet expected, mild injection site reactions. Patient survey data also demonstrated increases in treatment satisfaction and preference for the LA 2DR formulation. Comprehensive data from the FLAIR (treatment-naïve) and ATLAS (treatment-experienced) studies demonstrated virologic suppression in 93.1% in the LA CAB/RPV arm and 94.4% in the current antiretroviral regimen (CAR) arm, which was 3DR at week 48 and met non-inferiority. Although seven participants in the FLAIR study experienced confirmed virologic failure (CVF), three in the 2DR arm and four in the 3DR arm, three out of four in the CAR/3DR arm did not have treatment-emergent resistance (resistance-associated mutation (RAM) was not reported) [15]. Interestingly, participants who developed resistance in the LA CAB/RPV group were all from Russia and were found to have the polymorphism L74I at baseline, which is associated with INSTI resistance.
Maintenance/Switch Treatment Population
NRTI Inclusive
Seven non-inferiority studies of NRTI-inclusive 2DRs in treatment-experienced populations were identified (Table 2) [27–34]. The COOL study evaluating EFV + TDF vs. EFV + TDF/3TC failed to achieve non-inferiority [34]. The remaining six studies each included the NRTI 3TC as part of the 2DR and met non-inferiority at 48 weeks without any cases of emergent resistance. Four of the studies included a boosted PI + 3TC (two studied ATV/r, one LPV/r, and one DRV/r) [29–33], while two studies included DTG + 3TC [27, 28]. While safety outcomes from the studies were consistent with previous findings, studies including ATV produced more cases of hyperbilirubinemia, scleral icterus, and elevations in liver function tests (LFTs) and bilirubin [29, 31, 32], while the study including LPV/r experienced more frequent GI upset [31].
Table 2.
Summary of trials comparing two-drug regimens as maintenance/switch treatment in people living with HIV-1
| Title | Study agents | Study design | Outcomes | Emergent resistance | Additional comments |
|---|---|---|---|---|---|
| NRTI-inclusive regimens | |||||
| TANGO [27] | DTG + 3TC (n = 369) | Phase 3, open-label, randomized non-inferior, switch study performed in the USA, Spain, UK, The Netherlands, Germany, Japan, France, Canada, Belgium, Australia | VL < 50 copies/ml at W48 (ITT): | TAF-based regimen: 0/1 | Non-inferiority met |
| TAF-containing 3 or 4DR (n = 372) | |||||
| 2DR: 93% | |||||
| 3 or 4DR: 93% | |||||
| ASPIRE [28] | DTG + 3TC (n = 45) | Open-label, randomized, non-inferiority study performed in the USA | VL < 50 copies/ml at W48 (ITT): | 2DR group: 0/1 | Non-inferiority met |
| Continue 3DR (n = 45) | |||||
| 2DR: 91% | |||||
| 3DR: 89% | |||||
| ATLAS-M [29] | ATV/r + 3TC (n = 133) | Open-label, randomized, non-inferiority study performed in Italy | VL < 50 copies/ml at W48 (ITT): | 2DR group: 0/2 | Non-inferiority met |
| Superiority identified in post hoc analysis for 2DR group | |||||
| Continue ATV/r + 2 NRTIs (n = 133) | 3DR group: 0/6 | ||||
| 2DR: 90% | |||||
| 3DR: 80% | |||||
| DUAL GESIDA [30] | DRV/r + 3TC (n = 129) | Phase 4, open-label, randomized, non-inferiority study performed in Spain | VL < 50 copies/ml W48 (ITT): | 2DR group: 0/4 | Non-inferiority met |
| DRV/r + 2NRTIs (n = 128) | 3DR group: 0/2 | ||||
| 2DR: 89% | |||||
| 3DR: 93% | |||||
| OLE [31] | LPV/r + 3TC (n = 123) | Open-label, randomized, non-inferiority trial performed in Spain and France | VL < 50 copies/ml at W48 (ITT): | 2DR group: 0/3* | Non-inferiority met |
| *One case of NRTI resistance in 2DR group found to be a previously archived mutation | |||||
| LPV/r + 2NRTIs (n = 127) | 3DR group: 0/3 | ||||
| 2DR: 88% | |||||
| 3DR: 87% | |||||
| SALT [32] | ATV/r + 3TC (n = 143) | Open-label, randomized, non-inferiority study performed in Spain | VL < 50 copies/ml at W48 (ITT): | 2DR group: 0/6 | Non-inferiority met |
| ATV/r + 2NRTIs (n = 143) | 3DR group: 1/4 | ||||
| 2DR: 77% | |||||
| 3DR: 76% | |||||
| NRTI: 1 | |||||
| SALT (96 weeks) [33] | VL < 50 copies/ml at W96 (ITT): | 2DR group: 0/9 | Non-inferiority met | ||
| 3DR group: 1/5 | |||||
| 2DR: 74% | |||||
| 3DR: 74% | NRTI: 1 | ||||
| COOL [34] | EFV + TDF (n = 74) | Open-label, randomized, non-inferiority study performed in France | VL < 50 copies/ml at W48 (ITT): | 2DR group: 3/3 | 2DR failed to meet non-inferiority |
| EFV + TDF + 3TC (n = 74) | NNRTI: 3 | ||||
| 2DR: 82% | |||||
| 3DR: 97% | |||||
| NRTI-sparing regimens | |||||
| DUALIS [35] | DTG + DRV/r (n = 131) | Phase 3b, open-label, randomized, non-inferiority, switch study performed in Germany | VL < 50 copies/ml at W48 (ITT): | Not reported but no treatment emergent resistance | Non-inferiority met |
| Continue 3DR (n = 132) | Premature termination of recruitment due to slow recruitment | ||||
| DTG + DRV/r: 86% | |||||
| Continued 3DR: 88% | |||||
| SWORD 1/SWORD 2 [36] | SWORD-1 | Phase 3, open-label, parallel-group, randomized, non-inferiority switch study performed in 12 countries | VL < 50 copies/ml at W48 (ITT): | 2DR group: 1/3 | Non-inferiority met |
| DTG/RPV (n = 252) | |||||
| NNRTI: 1 | |||||
| Current 3DR (n = 256) | SWORD-1 | 3DR group: 0/6 | |||
| 2DR: 95% | |||||
| SWORD-2 | 3DR: 96% | ||||
| DTG/RPV (n = 261) | SWORD-2 | ||||
| 2DR: 94% | |||||
| Current 3DR (n = 255) | 3DR: 94% | ||||
| SWORD 1/SWORD 2 148-Week Open-Label Extension Data [37] | Early switch-original SWORD 1/SWORD 2 DTG/RPV cohort | Phase 3, OLE performed in 12 countries | VL < 50 copies/ml at W148 (ITT): | Early switch: 4/14 | Non-inferiority met |
| NNRTI: 4 | |||||
| Early switch: 84% | Late switch: 2/11 | ||||
| Late switch-3DR patients virologically suppressed at W48 switched to DTG/RPV at W52 | |||||
| Late switch: 90% | NNRTI: 2 | ||||
| DTG/RPV early switch (n = 513) | |||||
| DTG/RPV late switch (n = 477) | |||||
| ATLAS [38] | 4-week induction for IM CAB/RPV group: oral CAB 25 mg + RPV 25 mg | Open-label, randomized, non-inferiority, switch study performed in the USA, Italy, Germany, Spain, South Africa, Russia, Canada, and Belgium | VL < 50 copies/ml at W48 (ITT): | 2DR group: 3/3 | Non-inferiority met |
| Patient satisfaction survey reported 97% of patients were more satisfied with IM regimen over oral regimen during lead-in phase | |||||
| NNRTI: 3 | |||||
| INSTI: 1 | |||||
| IM CAB/RPV: 93% | 3DR group: 3/4 | ||||
| 3DR: 96% | |||||
| LA CAB/RPV (400/600 mg) every 4 weeks (n = 308) | NRTI: 2 | ||||
| NNRTI: 2 | |||||
| NNRTI/PI/INSTI + 2 NRTIs (n = 308) | |||||
| PROBE [39] | DRV/r + RPV (n = 30) | Open-label, randomized, non-inferiority, switch study performed in Italy | VL < 50 copies/ml at W48 (ITT): | None in either group | Non-inferiority met |
| Continue 3DR (n = 30) | 3DR limited to ATV or DRV/r + 2NRTIs (most common NRTI-backbone TDF/FTC) | ||||
| 2DR: 97% | |||||
| 3DR: 93% | |||||
| MARCH [40] | MVC + PI/r (n = 158) | Open-label, randomized, non-inferiority, switch study performed in various countries | VL < 50 copies/ml at W48 (ITT): | MVC + PI/r: 4/18 | Switch to NRTI-sparing 2DR found to be inferior and was discontinued from the complete 96-week study period |
| NNRTI: 1 | |||||
| MCV + PI/r: 78% | |||||
| PI: 1 | |||||
| MVC + 2NRTIs: 92% | |||||
| MVC + 2NRTIs (n = 157) | CXCR4 tropic: 3 | ||||
| Continued 3DR: 95% | |||||
| MVC + 2NRTIs: 5/6 | |||||
| Continue 3DR (n = 82) | |||||
| NNRTI: 2 | |||||
| NRTI: 5 | |||||
| PI: 1 | |||||
| Continued 3DR: 1/1 | |||||
| NNRTI: 1 | |||||
| HARNESS [41] | ATV/r + RAL (n = 72) | Open-label randomized, pilot switch study performed in the USA, UK, Germany, Spain, Italy, France, and Poland | VL < 50 copies/ml at W48 (ITT): | 2DR group: 2/9 | Study terminated due to increased virologic rebound and emergent resistance in 2DR |
| ATV/r + TDF/3TC (n = 37) | |||||
| 2DR: 69% | |||||
| 3DR: 87% | PI: 1 | ||||
| INSTI: 2 | |||||
| 3DR group: 0/1 | |||||
| SECOND-LINE [42] | LPV/r + RAL (n = 270) | Phase 3b/4, randomized, open-label, non-inferiority study performed in Africa, Asia, Europe, and Latin America | VL < 50 copies/ml at W96 (ITT): | 2DR group: 23/83 | Non-inferiority met |
| LPV/r + 2 NRTIs (n = 271) | NRTI: 2 | Limited to treatment-experienced patients with evidence of virologic failure on NNRTI + dual NRTI regimen | |||
| 2DR: 70% | PI: 1 | ||||
| 3DR: 68% | INSTI: 20 | ||||
| 3DR group: 11/82 | |||||
| NRTI: 8 | |||||
| PI: 2 | |||||
| INSTI: 1 | |||||
| KITE [43] | LPV/r + RAL (n = 40) | Open-label, randomized, pilot switch study performed in Atlanta, GA, USA | VL < 50 copies/ml at W48 (ITT): | 2DR group: */1 | *Treatment-emergent resistance among cases of virologic failure not assessed |
| Current 3DR (n = 20) | 3DR group: */2 | ||||
| 2DR: 92% | |||||
| 3DR: 88% | |||||
2DR two-drug regimen, 3DR three-drug regimen, 3TC lamivudine, ABC abacavir, ATV/r ritonavir-boosted atazanavir, AZT zidovudine, CAB cabotegravir, DRV/r ritonavir-boosted darunavir, DTG dolutegravir, EFV efavirenz, FAS full analysis set, FTC emtricitabine, INSTI integrase strand transfer inhibitors, ITT intention to treat, LA long acting, LPV/r ritonavir boosted lopinavir, mITT modified intent to treat, MVC maraviroc, NRTI nucleoside reverse transcriptase inhibitor, NNRTI non- nucleoside reverse transcriptase inhibitors, PI protease inhibitor, OLE open-label extension, RAL raltegravir, RAM resistance associated mutations, RCT randomized controlled trial, RPV rilpivirine, TDF tenofovir disoproxil fumarate, VL viral load, W48 week 48, W96 week 96, W148 week 148
NRTI Sparing
Eight studies were identified on NRTI-sparing regimens used in treatment-experienced patients (Table 2) [35–43]. All but one study evaluated patients that were virologically suppressed and on a stable ART regimen prior to randomization, although they varied in definition and required duration of viral suppression. Of the six non-inferiority studies, including a variety of 2DR combinations, non-inferiority was met in five studies [35–39]. The switch strategy of replacing a dual NRTI backbone with MVC in a boosted-PI regimen was found to be inferior to continuing a 3DR [40].
The SWORD studies and ATLAS studies included INSTI + NNRTI 2DRs [36–38]. SWORD 1 and 2 demonstrated the 2DR of DTG/RPV met the pre-specified non-inferiority criteria to standard 3DR [36]. The open-label extension of SWORD 1 and 2 also demonstrated durable efficacy in both early- and late-switch DTG/RPV groups [37]. There was one case of treatment-emergent NNRTI resistance among the 2DR arm in the first 48 weeks and five cases of NNRTI resistance in the early- and late-switch groups.
The ATLAS study demonstrated high rates of virologic suppression at 48 weeks in individuals with a suppressed viral load who were switched to a 4-week lead-in of oral CAB + RPV followed by a once-monthly injectable LA CAB/RPV regimen [38]. The study showed good tolerability, minimal emergence of treatment-related resistance, and high patient satisfaction of a once-monthly injectable 2DR. ATLAS-2 M is an ongoing non-inferiority study evaluating LA CAB/RPV administered every 8 weeks compared with every 4 weeks in virologically suppressed patients [44]. The study demonstrated LA CAB/RPV given every 8 weeks was non-inferior to 4 week dosing through 48 weeks. One study utilized a PI + NNRTI 2DR, which showed favorable results. In a small proof-of-concept study DRV/r + RPV was found to be non-inferior and well tolerated without emergence of resistance [39].
Three studies included RAL + boosted PI [41–43]. A pilot study of LPV/r + RAL was shown to be a viable switch strategy out to 48 weeks [43]; however, a study of ATV/r + RAL was terminated early because of increased rates of virologic rebound and treatment-emergent resistance [41]. Another study included treatment-experienced patients with evidence of virologic failure on an NNRTI + dual NRTI regimen. The use of LPV/r + RAL as second-line therapy showed promising results relative to a 3DR containing LPV/r + dual NRTIs out to 96 weeks [42].
Discussion
In both treatment-naïve and -experienced populations, 3TC was the most commonly used NRTI in 2DRs that met non-inferiority. Although 3TC and FTC are viewed in clinical practice as therapeutically interchangeable cytidine analogs, FTC has yet to be studied in the context of NRTI-inclusive 2DRs. Two studies of NRTI-inclusive 2DRs containing TDF as opposed to the more robustly studied 3TC both failed to achieve non-inferiority [11, 34]. Thus, the data to date, do not support the use of TDF as a single NRTI agent in a 2DR. With the more recent approval date, TAF remains virtually unstudied by comparison or inclusion in the 2DR space.
3TC in combination with boosted PIs has demonstrated efficacy in treatment-naïve individuals. In the ANDES and GARDEL studies, 3TC + DRV/r and 3TC + LPV/r were non-inferior to 3DRs [12, 13]. Boosted PI and 3TC regimens have also been shown to be effective 2DRs in treatment-experienced populations. ATV/r + 3TC regimens have consistently met non-inferiority, evidenced in both simplification from a previous ATV/r-based regimen and switching from a variety of PI and NNRTI-based 3DRs (ATLAS-M, SALT) [29, 32, 33]. There is limited evidence studying DRV in this population, although results from the DUAL-GESIDA study are promising [30]. These studies have not been associated with treatment-emergent resistance and have similar rates of viral suppression to comparator 3DRs. Although a boosted PI + 3TC may be an effective 2DR in treatment-naïve and -experienced populations, PI-associated toxicities including metabolic disorders and laboratory abnormalities found even with the newer, more well-tolerated PIs such as DRV may be a limitation as well as significant drug interactions. These limitations may hinder the use of boosted PI + 3TC regimens in real-world settings.
The most promising NRTI-inclusive 2DR in patients initiating ART is currently DTG + 3TC. Recent guideline updates now recommend the co-formulated 2DR, DTG + 3TC, as a consideration in the treatment-naïve population with the exception of individuals with pre-treatment HIV RNA > 500,000 copies/ml, active hepatitis B virus (HBV) coinfection, or initiation of ART prior to the availability of HIV reverse transcriptase genotype or HBV testing [4, 5]. Results from the TANGO study were recently presented and demonstrated non-inferiority of DTG + 3TC compared with TAF-based 3DR in virologically suppressed patients [4, 5]. Although not approved, switch data look promising for the use of DTG + 3TC in the treatment-experienced population. DTG/3TC demonstrated excellent virologic suppression without emergence of resistance in the treatment-experienced population [28], in line with the large body of evidence that was established by the GEMINI studies [10, 11]. More data are needed, but DTG + 3TC likely represents the most promising NRTI-inclusive 2DR in treatment-experienced patients, avoiding the issues of drug interactions and more severe side effect profiles of boosted PI + 3TC regimens. Recent 48-week data from the ANRS 167 LAMIDOL single-arm study further support switching to DTG/3TC in select, virologically suppressed patients [45]. Similarly, the DOLAM triple-armed study showed few cases of treatment failure among patients switched to DTG + 3TC as continuing current 3DR, without emergence of resistance [46].
In both treatment-naïve and -experienced populations, integrase inhibitors play a large role in NRTI-sparing 2DRs. Raltegravir was studied in many 2DRs as it was the first INSTI on the market [18, 24, 25, 42]. However, data from randomized-controlled studies largely do not support any additional benefit from the use of a boosted PI with RAL since this regimen is quickly becoming obsolete given that RAL is a first-generation INSTI that is less potent than newer agents such as DTG and BIC. In addition, RAL suffers from issues of increased pill burden and cross-resistance with elvitegravir (EVG).
Dolutegravir has been studied in both NRTI-inclusive and -sparing 2DRs. Unlike RAL, DTG has a long half-life and is efficacious in the presence of other INSTI resistance such as the Q148 pathway selected for with RAL or EVG. In NRTI-sparing 2DRs, it has been studied with both PIs and NNRTIs in mostly treatment-experienced populations. A regimen commonly considered in clinical practice consists of DRV/r + DTG. This regimen combines two highly potent agents with high genetic barriers to resistance. This regimen is currently being investigated in treatment-experienced populations, as both a simplification strategy for virologically suppressed patients and a salvage therapy [35, 47, 48]. Recent data from a subanalysis of the DUALIS study met non-inferiority. At week 48, 98.1% of patients (n = 113) in the observational TIVISTA study achieved virologic suppression (< 50 copies/ml) despite 18 subjects experiencing reduced sensitivity to darunavir at baseline. No participant developed new drug resistance mutations throughout the study [49]. Additional observational studies have demonstrated similar results although DRV/r and/or DTG was allowed twice daily [48] while another retrospective study demonstrated the feasibility of cobicistat-boosted DRV with DTG once daily in treatment-experienced patients [50].
Dolutegravir/RPV is the simplest option with the lowest pill burden for virologically suppressed PLWH, who do not have underlying resistance to either agent. Patients must be HBV-negative with demonstrated virologic suppression for at least 6 months with a CD4 count of > 200 cells/mm3. In addition, the ideal patient for this regimen must be able to take this regimen with a full meal and avoid proton pump inhibitors. SWORD 1/2 studies met non-inferiority between DTG/RPV and 3DRs [36]. Long-term extension data have shown 2DR durability with minimal development of resistance [37]. Two observational studies of DTG/RPV used in treatment-experienced patients have also demonstrated good efficacy among a real-world population [51, 52].
The most robust and emerging data of an INSTI containing 2DR are derived from studies of CAB and RPV as oral and LA intramuscular formulations. An open-label extension of LATTE demonstrated the durability of CAB 30 mg + RPV at 144 weeks [22]. It is important to note that patients studied in LATTE were required to undergo a 24-week induction phase on oral CAB + 2 NRTIs prior to switching to a regimen of RPV as well as continuing their randomized CAB dose. In treatment-naïve patients, LA CAB/RPV has demonstrated the promising results in the achievement and durability of high rates of viral suppression, tolerability, and lack of significant treatment-emergent resistance. Furthermore, the LATTE-2 study also demonstrated durable viral suppression out to 160 weeks in the long-term extension data, with minimal emergence of resistance [17]. LA CAB/RPV represents a potential option in the future for PLWH. However, as such therapies become available, a key consideration will be the necessity of an induction phase of oral CAB and how to best assess for initial safety and tolerability because of the long half-life and gap in care between injections. Furthermore, adherence during this induction phase will be crucial. Although safety and efficacy were demonstrated in clinical trials, the Food and Drug Administration denied approval of this co-formulated injectable secondary to chemistry manufacturing and controls [53].
Non-INSTI containing 2DRs that have shown positive results include a PI + NNRTI regimen. The 2DR of DRV/r + RPV appears promising from limited prospective data [39]. These results were confirmed with recent, real-world observational data, with viral suppression < 50 copies/ml achieved in 83% of patients (RIDAR) [54]. Several drawbacks of this regimen include the lack of a STR, increased adverse effects seen with PIs, and an increased potential for drug-drug interactions.
Although MVC was initially approved for management of resistant HIV virus, its recommended twice-daily dosing and numerous drug interactions leave it as an alternative treatment option. In addition, patients need to have a CCR5-tropic virus prior to initiation of therapy. Furthermore, maraviroc 2DRs in combination with PIs were shown to be inferior to 3DRs in studies in both treatment-naïve and -experienced populations.
The “Ideal” 2DR
Antiretroviral selection is key to ensuring a patient’s success. Modern regimens achieve rapid viral suppression. However, the issue remains of the long-term durability of a regimen in maintaining virologic suppression without the emergence of resistance, particularly when considering incomplete adherence to an ART regimen. The optimal 2DR will have a low pill burden, high efficacy, minimal toxicity, reduced cost, and a high genetic barrier to resistance in both treatment-naïve and -experienced populations. Furthermore, as PLWH are living longer, it would also be beneficial for the ideal 2DR to have limited drug-drug interactions and not require renal or hepatic dose adjustments.
When considering 2DRs as initial or switch therapy, it is essential to assess for baseline and transmitted drug resistance. A major concern of a 2DR containing a single NRTI agent is the potential for the emergence of NRTI RAMs such as M184I/V, which greatly reduces susceptibility to 3TC and FTC. Recent data demonstrate effective use of 3TC as a single NRTI in a 2DR, with minimal emergence of NRTI resistance, including M184I/V mutations, in cases of virologic failure [10, 11]. It should be noted that no difference was observed in treatment-naïve or -experienced 2DR studies in the development of M184I/V mutations. Another benefit of 3TC-containing regimens is the high tolerability. By utilizing 3TC as a single NRTI backbone, and avoiding or removing the use of TDF, negative effects on renal function and bone biomarkers may be eliminated or ameliorated.
The role of INSTIs has revolutionized ART based on their rapid achievement of virologic suppression, enhanced tolerability, and reduced drug interaction potential compared with PIs and NNRTIs.
The first-generation INSTIs, RAL and EVG, often display cross-resistance and have a lower genetic barrier to resistance; however, DTG was developed as a second-generation INSTI with improved resistance profile and high efficacy. Dolutegravir has demonstrated efficacy in 2DRs that are both NRTI-inclusive and sparing. Although BIC also has a high genetic barrier to resistance and demonstrates similar safety and tolerability to DTG, it is only available as a co-formulated single-tablet 3DR. Cabotegravir has been studied as a LA 2DR, in combination with RPV, in both treatment-naïve and -experienced populations. This 2DR appears very promising, especially with its novel formulation; however, its approval is still pending based on formulation concerns.
The emergence of 2DRs as initial therapy in treatment-naïve individuals and as simplification strategies in treatment-experienced populations has become a reality and is creating a paradigm shift in the management of HIV. Dual therapies are largely prescribed as simplification strategies in European countries and is becoming more favorable in the USA. Unfortunately, most studies evaluated standard 3DRs that were considered first line at the time of the study initiation. More modern comparators should be included in 2DR studies since most of the available head-to-head, randomized clinical trial data include TDF-based regimens.
Although 2DRs are being utilized more frequently, their use may not be appropriate in all patient populations. Concerns exist about the use of 2DRs in PLWH who have low CD4 counts (< 200 cells/mm3) or high baseline viral loads (< 100,000 or 500,000 copies/ml based on study design) prior to drug initiation [10, 11, 18]. Subgroup analyses of failure rates in these populations are often limited because of the small sample size and often not attributed to true virologic failure. Most studies, with the exception of rescue/salvage therapy aside, screen for and exclude patients with underlying baseline resistance to the regimen that they will be initiated or switched to. Other populations in which 2DRs should be avoided include pregnant individuals and those with hepatitis B coinfection.
While the overall picture of evidence on 2DRs remains mixed, it is important to note the significant changes in the HIV treatment landscape that have taken place over the last decade. Another question remains about how the data from switch studies are reported and applied to the management of HIV. Particularly in switch studies evaluating treatment-naïve and -experienced patients, is accepting “non-inferiority” enough? There has been a recent change in reporting outcomes for assessing the non-inferiority of HIV switch strategies. The US Food and Drug Administration recommends primary end points of switch trials to report the rate of virologic failure in each treatment group where non-inferiority would be a margin of ≤ 4% [55].
Conclusion
HIV treatment has evolved from a time where 2DRs were once considered a novel concept to current times where they are a reality. As 2DRs are reducing the number of medications required to manage HIV, while maintaining durable efficacy, 3DR may no longer remain the standard of care but become the antiquated way of the past.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Disclosures
Melissa Badowski, Sarah Perez, David Silva, and Andrea Lee have no disclosures to report.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.
Footnotes
Digital Features
To view digital features for this article go to 10.6084/m9.figshare.11961933.
References
- 1.Broder S. The development of antiretroviral therapy and its impact on the HIV-1/AIDS pandemic. Antivir Res. 2010;85:1–18. doi: 10.1016/j.antiviral.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pau AK, George JM. Antiretroviral therapy: current drugs. Infect Dis Clin N Am. 2014;28:371–402. doi: 10.1016/j.idc.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.U.S. Department of Health and Human Services. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV. http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. Accessed 23 Jan 2020.
- 4.US Department of Health and Human Services. What to start: initial combination regiments for the antiretroviral-naïve patient. https://aidsinfo.nih.gov/guidelines/brief-html/1/adult-and-adolescent-arv/11/what-to-start. Accessed 23 Jan 2020.
- 5.European AIDS Clinical Society. EACS guidelines version 10.0 (Nov 2019). https://eacs.sanfordguide.com/. Accessed 23 Jan 2020.
- 6.Wijting I, Rokx C, Boucher C, et al. Dolutegravir as maintenance monotherapy for HIV (DOMONO): a phase 2, randomised non-inferiority trial. Lancet HIV. 2017;4:e547–e554. doi: 10.1016/S2352-3018(17)30152-2. [DOI] [PubMed] [Google Scholar]
- 7.Hocqueloux L, Raffi F, Prazuck T, et al. Dolutegravir monotherapy versus dolutegravir/abacavir/lamivudine for virologically suppressed people living with chronic HIV infection: the randomized non-inferiority MONCAY trial. Clin Infect Dis. 2019;69:1498–1505. doi: 10.1093/cid/ciy1132. [DOI] [PubMed] [Google Scholar]
- 8.Galli L, Spagnuolo V, Bigoloni A, et al. Atazanavir/ritonavir monotherapy: 96 week efficacy, safety and bone mineral density from the MODAt randomized trial. J Antimicrob Chemother. 2016;71:1637–1642. doi: 10.1093/jac/dkw031. [DOI] [PubMed] [Google Scholar]
- 9.Juluca [package insert]. Research Triangle Park, NC. ViiV Healthcare; 2019.
- 10.Cahn P, Madero JS, Arribas JR, et al. Dolutegravir plus lamivudine versus dolutegravir plus tenofovir disoproxil fumarate and emtricitabine in antiretroviral-naive adults with HIV-1 infection (GEMINI-1 and GEMINI-2): week 48 results from two multicentre, double-blind, randomised, non-inferiority, phase 3 trials. Lancet. 2019;393:143–155. doi: 10.1016/S0140-6736(18)32462-0. [DOI] [PubMed] [Google Scholar]
- 11.Cahn, P et al. Durable efficacy of dolutegravir (DTG) plus lamivudine (3TC) in antiretroviral treatment-naïve adults with HIV-1 infection—96-week results from the GEMINI studies. Presented at: 10th International AIDS Conference on HIV Science (IAS 2019); July 21–24, 2019, Mexico City, Mexico.
- 12.Figueroa MI, Sued OG, Gun AM, Belloso WH, Cecchini DM, Lopardo G. DRV/R plus 3TC for HIV-1 treatment naïve patients: week 48 results of the ANDES study. Presented at: 2018 Conference on Retroviruses and Opportunistic Infections (CROI); March 4–7, 2018. Boston, MA, USA
- 13.Cahn P, Andrade-Villanueva J, Arribas JR, et al. Dual therapy with lopinavir and ritonavir plus lamivudine versus triple therapy with lopinavir and ritonavir plus two nucleoside reverse transcriptase inhibitors in antiretroviral-therapy-naive adults with HIV-1 infection: 48 week results of the randomised, open label, non-inferiority GARDEL trial. Lancet Infect Dis. 2014;14:572–580. doi: 10.1016/S1473-3099(14)70736-4. [DOI] [PubMed] [Google Scholar]
- 14.Pinola M, Lazzarin A, Antinori A, et al. Lopinavir/ritonavir + tenofovir dual therapy versus lopinavir/ritonavir-based triple therapy in HIV-infected antiretroviral naïve subjects: the Kalead Study. J Antivir Antiretrovir. 2010;2:56–62. doi: 10.4172/jaa.1000024. [DOI] [Google Scholar]
- 15.Orkin C, Arasteh K, Hernandez-Mora MG, et al. Long-acting cabotegravir + rilpivirine for HIV maintenance: FLAIR week 48 results. Presented at: 2019 CROI. March 4–7, 2019; Seattle, WA, USA
- 16.Margolis DA, Gonzalez-Garcia J, Stellbrink HJ, et al. Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, non-inferiority trial. Lancet. 2017;390:1499–1510. doi: 10.1016/S0140-6736(17)31917-7. [DOI] [PubMed] [Google Scholar]
- 17.Margolis DA, et al. Safety, efficacy and durability of long-acting CAB and RPV as two drug IM maintenance therapy for HIV-1 infection: LATTE-2 week 160 results. Presented at: Glasgow HIV. October 28–31, 2018; Glasgow, UK.
- 18.Lambert-Niclot S, George EC, Pozniak A, et al. Antiretroviral resistance at virological failure in the NEAT 001/ANRS 143 trial: raltegravir plus darunavir/ritonavir or tenofovir/emtricitabine plus darunavir/ritonavir as first-line ART. J Antimicrob Chemother. 2016;71:1056–1062. doi: 10.1093/jac/dkv427. [DOI] [PubMed] [Google Scholar]
- 19.Stellbrink HJ, Le Fevre E, Carr A, et al. Once-daily maraviroc versus tenofovir/emtricitabine each combined with darunavir/ritonavir for initial HIV-1 treatment. AIDS. 2016;30:1229–1238. doi: 10.1097/QAD.0000000000001058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nozza S, Galli L, Antinori A, et al. Maraviroc 150 mg daily plus lopinavir/ritonavir, a nucleoside/nucleotide reverse transcriptase inhibitor-sparing regimen for HIV-infected naive patients: 48-week final results of VEMAN study. Clin Microbiol Infect. 2015;21(510):e1–e9. doi: 10.1016/j.cmi.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 21.Margolis DA, Brinson CC, Smith GHR, et al. Cabotegravir plus rilpivirine, once a day, after induction with cabotegravir plus nucleoside reverse transcriptase inhibitors in antiretroviral-naive adults with HIV-1 infection (LATTE): a randomised, phase 2b, dose-ranging trial. Lancet Infect Dis. 2015;15:1145–1155. doi: 10.1016/S1473-3099(15)00152-8. [DOI] [PubMed] [Google Scholar]
- 22.Margolis DA, Brinson C, Smith GH, et al. Long-term safety and efficacy of CAB and RPV as 2-drug oral maintenance therapy. Poster presented at: 2017 CROI; February 13–16, 2017; Seattle, WA, USA
- 23.Bedimo RJ, Drechsler H, Jain M, et al. The RADAR study: week 48 safety and efficacy of RAltegravir combined with boosted DARunavir compared to tenofovir/emtricitabine combined with boosted darunavir in antiretroviral-naive patients. Impact on bone health. PLoS One. 2014;9:e106221. doi: 10.1371/journal.pone.0106221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reynes J, Trinh R, Pulido F, et al. Lopinavir/ritonavir combined with raltegravir or tenofovir/emtricitabine in antiretroviral-naive subjects: 96-week results of the PROGRESS study. AIDS Res Hum Retroviruses. 2013;29:256–265. doi: 10.1089/aid.2011.0275. [DOI] [PubMed] [Google Scholar]
- 25.Kozal MJ, Lupo S, Dejesus E, et al. A nucleoside- and ritonavir-sparing regimen containing atazanavir plus raltegravir in antiretroviral treatment-naïve HIV-infected patients: SPARTAN study results. HIV Clin Trials. 2012;13:119–130. doi: 10.1310/hct1303-119. [DOI] [PubMed] [Google Scholar]
- 26.Saumoy M, Llibre JM, Terron A, et al. Short communication: maraviroc once-daily: experience in routine clinical practice. AIDS Res Hum Retrovir. 2017;33:29–32. doi: 10.1089/aid.2015.0386. [DOI] [PubMed] [Google Scholar]
- 27.van Wyk J, Ajana F, Bisshop F, et al. Switching to DTG/3TC fixed-dose combination (FDC) is non-inferior to continuing a TAF-based regimen in maintaining virologic suppression through 48 weeks (TANGO study). Presented at: IAS 2019; July 21-24, 2019; Mexico City, México.
- 28.Taiwo BO, Marconi VC, Berzins B, et al. Dolutegravir plus lamivudine maintains human immunodeficiency virus-1 suppression through week 48 in a pilot randomized trial. Clin Infect Dis. 2018;66:1794–1797. doi: 10.1093/cid/cix1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Di Giambenedetto S, Fabbiani M, Quiros Roldan E, et al. Treatment simplification to atazanavir/ritonavir + lamivudine versus maintenance of atazanavir/ritonavir + two NRTIs in virologically suppressed HIV-1-infected patients: 48 week results from a randomized trial (ATLAS-M) J Antimicrob Chemother. 2017;72:1163–1171. doi: 10.1093/jac/dkw557. [DOI] [PubMed] [Google Scholar]
- 30.Pulido F, Ribera E, Lagarde M, et al. Dual therapy with darunavir and ritonavir plus lamivudine vs triple therapy with darunavir and ritonavir plus tenofovir disoproxil fumarate and emtricitabine or abacavir and lamivudine for maintenance of human immunodeficiency virus type 1 viral suppression: randomized, open-label, noninferiority DUAL-GESIDA 8014-RIS-EST45 trial. Clin Infect Dis. 2017;65(12):2112–2118. doi: 10.1093/cid/cix734. [DOI] [PubMed] [Google Scholar]
- 31.Arribas JR, Girard PM, Landman R, et al. Dual treatment with lopinavir-ritonavir plus lamivudine versus triple treatment with lopinavir-ritonavir plus lamivudine or emtricitabine and a second nucleos(t)ide reverse transcriptase inhibitor for maintenance of HIV-1 viral suppression (OLE): a randomised, open-label, non-inferiority trial. Lancet Infect Dis. 2015;15:785–792. doi: 10.1016/S1473-3099(15)00096-1. [DOI] [PubMed] [Google Scholar]
- 32.Perez-Molina JA, Rubio R, Rivero A, et al. Dual treatment with atazanavir-ritonavir plus lamivudine versus triple treatment with atazanavir-ritonavir plus two nucleos(t)ides in virologically stable patients with HIV-1 (SALT): 48 week results from a randomised, open-label, non-inferiority trial. Lancet Infect Dis. 2015;15:775–784. doi: 10.1016/S1473-3099(15)00097-3. [DOI] [PubMed] [Google Scholar]
- 33.Perez-Molina JA, Rubio R, Rivero A, et al. Simplification to dual therapy (atazanavir/ritonavir + lamivudine) versus standard triple therapy [atazanavir/ritonavir + two nucleos(t)ides] in virologically stable patients on antiretroviral therapy: 96 week results from an open-label, non-inferiority, randomized clinical trial (SALT study) J Antimicrob Chemother. 2017;72:246–253. doi: 10.1093/jac/dkw379. [DOI] [PubMed] [Google Scholar]
- 34.Girard PM, Cabié A, Michelet C, et al. A randomized trial of two-drug versus three-drug tenofovir-containing maintenance regimens in virologically controlled HIV-1 patients. J Antimicrob Chemother. 2009;64:126–134. doi: 10.1093/jac/dkp141. [DOI] [PubMed] [Google Scholar]
- 35.Spinner CD, Kummerle T, Schneider J, et al. A switch to dolutegravir in combination with boosted darunavir is safe and effective in suppressed patients with HIV—a subanalysis of the DUALIS study. Presented at: IAS 2019: Conference on HIV Pathogenesis Treatment and Prevention; July 21–24, 2019; Mexico City, Mexico.
- 36.Llibre JM, Hung CC, Brinson C, et al. Efficacy, safety, and tolerability of dolutegravir-rilpivirine for the maintenance of virological suppression in adults with HIV-1: phase 3, randomised, non-inferiority SWORD-1 and SWORD-2 studies. Lancet. 2018;391:839–849. doi: 10.1016/S0140-6736(17)33095-7. [DOI] [PubMed] [Google Scholar]
- 37.van Wyk J, Orkin C, Rubio R, et al. Durable suppression and low rate of virologic failures 3 years after switch to DTG + RPV 2DRug Regimen: SWORD 1 and 2 Studies. Presented at: 25th Annual Conference of the British HIV Association; April 2–5, 2019; Bournemouth, UK.
- 38.Swindells S, Andrade-Villanueva JF, Richmond GJ, et al. Long-acting cabotegravir rilpivirine maintenance therapy: ATLAS week 48 results. Presented at: 2019 CROI; March 4–7, 2019; Seattle, WA, USA
- 39.Maggiolo F, Di Filippo E, Valenti D, Serna Ortega PA, Callegaro A. NRTI sparing therapy in virologically controlled HIV-1 infected subjects: results of a controlled, randomized trial (Probe) J Acquir Immune Defic Syndr. 2016;72:46–51. doi: 10.1097/QAI.0000000000000966. [DOI] [PubMed] [Google Scholar]
- 40.Pett SL, Amin J, Horban A, et al. Maraviroc, as a switch option, in HIV-1-infected individuals with stable, well-controlled HIV replication and R5-tropic virus on their first nucleoside/nucleotide reverse transcriptase inhibitor plus ritonavir-boosted protease inhibitor regimen: week 48 results of the randomized, Multicenter MARCH Study. Clin Infect Dis. 2016;63:122–132. doi: 10.1093/cid/ciw207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Lunzen J, Pozniak A, Gatell JM, et al. Brief report: switch to ritonavir-boosted atazanavir plus raltegravir in virologically suppressed patients with HIV-1 infection: a randomized pilot study. J Acquir Immune Defic Syndr. 2016;71:538–543. doi: 10.1097/QAI.0000000000000904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amin J, Boyd MA, Kumarasamy N, et al. Raltegravir non-inferior to nucleoside based regimens in second-line therapy with lopinavir/ritonavir over 96 weeks: a randomised open label study for the treatment of HIV-1 infection. PLoS One. 2015;10:e0118228. doi: 10.1371/journal.pone.0118228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ofotokun I, Sheth AN, Sanford SE, et al. A switch in therapy to a reverse transcriptase inhibitor sparing combination of lopinavir/ritonavir and raltegravir in virologically suppressed HIV-infected patients: a pilot randomized trial to assess efficacy and safety profile: the KITE study. AIDS Res Hum Retrovir. 2012;28:1196–1206. doi: 10.1089/aid.2011.0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.ViiV Healthcare. ViiV Healthcare reports positive phase III study results of investigational, long-acting, injectable HIV-treatment regimen administered every 2 months. https://viivhealthcare.com/en-gb/media/press-releases/2019/august/viiv-healthcare-reports-positive-phase-iii-study-results-of-inve/. Accessed 6 Dec 2019.
- 45.Joly V, Burdet C, Landman R, et al. Dolutegravir and lamivudine maintenance therapy in HIV-1 virologically suppressed patients: results of the ANRS 167 trial (LAMIDOL) J Antimicrob Chemother. 2019;74:739–745. doi: 10.1093/jac/dky467. [DOI] [PubMed] [Google Scholar]
- 46.Blanco JL, Rojas J, Paredes R, et al. Dolutegravir-based maintenance monotherapy versus dual therapy with lamivudine: a planned 24 week analysis of the DOLAM randomized clinical trial. J Antimicrob Chemother. 2018;73:1965–1971. doi: 10.1093/jac/dky093. [DOI] [PubMed] [Google Scholar]
- 47.Capetti AF, Sterrantino G, Cossu MV, Cenderello G, Cattelan AM, De Socio GV, Rusconi S, Riccardi N, Baldin GM, Cima S, Niero FP, Rizzardini G, Sasset L. Salvage therapy or simplification of salvage regimens with dolutegravir plus ritonavir-boosted darunavir dual therapy in highly cART-experienced subjects: an Italian cohort. Antivir Ther. 2017;22:257–262. doi: 10.3851/IMP3095. [DOI] [PubMed] [Google Scholar]
- 48.Capetti AF, Cossu MV, Orofino G, Sterrantino G, Cenderello G, De Socio GV, Cattelan AM, Soria A, Rusconi S, Riccardi N, Baldin GM, Niero FP, Barbarini G, Rizzardini G. A dual regimen of ritonavir/darunavir plus dolutegravir for rescue or simplification of rescue therapy: 48 weeks’ observational data. BMC Infect Dis. 2017;17:658. doi: 10.1186/s12879-017-2755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sterrantino G. Dolutegravir plus ritonavir-boosted darunavir in highly cART-experienced subjects. http://regist2.virology-education.com/2016/14EU/05_Sterrantino.pdf. Accessed 14 Jan 2020.
- 50.Lee SA, Kim SW, Chang HH, et al. Effectiveness, safety, and tolerability of a switch to dual therapy with dolutegravir plus cobicistat-boosted darunavir in treatment-experienced patients with human immunodeficiency virus. Infect Chemother. 2018;50:252–262. doi: 10.3947/ic.2018.50.3.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gantner P, Cuzin L, Allavena C, et al. Efficacy and safety of dolutegravir and rilpivirine dual therapy as a simplification strategy: a cohort study. HIV Med. 2017;18:704–708. doi: 10.1111/hiv.12506. [DOI] [PubMed] [Google Scholar]
- 52.Capetti AF, Cossu MV, Sterrantino G, et al. Dolutegravir plus rilpivirine as a switch option in cART-experienced patients: 96-week data. Ann Pharmacother. 2018;52:740–746. doi: 10.1177/1060028018761600. [DOI] [PubMed] [Google Scholar]
- 53.ViiV Healthcare. ViiV Healthcare receives complete response letter from US FDA for use of investigational cabotegravir and rilpivirine long-acting regimen in the treatment of HIV. https://viivhealthcare.com/en-gb/media/press-releases/2019/december/complete-response-letter-from-us-fda/. Accessed 30 Dec 2019.
- 54.Pasquau J, De Jesus SE, Arazo P, et al. Effectiveness and safety of dual therapy with rilpivirine and boosted darunavir in treatment-experienced patients with advanced HIV infection: a preliminary 24 week analysis (RIDAR study) BMC Infect Dis. 2019;19:207. doi: 10.1186/s12879-019-3817-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.US Food and Drug Administration. Human immunodeficiency virus-1 infection: developing antiretroviral drugs for treatment. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/human-immunodeficiency-virus-1-infection-developing-antiretroviral-drugs-treatment. Accessed 24 Jan 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.