Abstract
Preeclampsia (PE) is a placental disorder with different phenotypic presentations. In malaria-endemic regions, high incidence of PE is reported, with debilitating foeto-maternal effects, particularly among primigravid women. However, the relationship between placental pathology and Plasmodium falciparum infection in the placenta with PE is underexplored. Placentas from 134 pregnant women were examined after delivery for pathological lesions and placental malaria (PM). They comprised of 69 women without PE (non-PE group) and 65 women diagnosed with PE (PE group). The presence of placental pathology increased the risk of PE, with particular reference to syncytial knots. Placental malaria was 64 (48.1%) and 21 (15.8%) respectively for active and past infections and these proportions were significantly higher in the PE group compared to the non-PE group. Further multivariate analyses showed placental pathology (adjusted (aOR) 3.0, 95% CI = 1.2–7.5), active PM (aOR 6.7, 95% CI = 2.3–19.1), past PM (aOR 12.4, 95% CI = 3.0–51.0) and primigravidity (aOR 6.6, 95% CI 2.4–18.2) to be associated with PE. Our findings suggest that placental histological changes and PM are independent risk factors for PE particularly in primigravida. These findings might improve the management of PE in malaria-endemic regions.
Subject terms: Diseases, Health care, Pathogenesis, Risk factors
Introduction
The maternal syndrome of preeclampsia (PE) complicates 2–10% of pregnancies worldwide1 and contributes significantly to maternal and foetal morbidity and mortality2. It is clinically characterised by the new onset of hypertension and proteinuria after 20 weeks of gestation in previously normotensive women3. However, the risk is also increased in women with chronic hypertension and other metabolic conditions such as diabetes4. In Ghana, maternal mortality due to PE and other hypertensive disorders in pregnancy has doubled over the past decade (9% in 2007 to 18% in 2017)5. Subsequently, these disorders have emerged as the leading cause of maternal mortality in the tertiary centres of the country6,7.
While the search for aetiological links to PE continues, significant advancements have been made in understanding the pathophysiologic role of the placenta in the development of PE.
Histopathological examination of placental biopsies from pregnancies complicated by PE and foetal growth restriction revealed inadequate trophoblast invasion and impaired remodelling of maternal spiral arteries8,9. Further on, the two-stage model of PE by Robert and Hubel10 suggests a causal association between placental insufficiencies (stage 1) and the development of clinical symptoms (stage 2). Poor placental perfusion has been associated with placental histopathological lesions such as infarcts, increase in syncytial knots, fibrin deposits, atherosis of the arterial wall, accelerated villous maturation and calcifications11–16. These changes result from ischaemic and hypoxic mechanisms secondary to poor perfusion17. Additionally, variable effects on the clinical severity of PE and foetal outcomes have been shown to implicate several placental factors in the pathogenesis of PE14,16.
In malaria-endemic regions, women exposed to placental parasites could be at an increased risk of PE compared to those in non-malarious regions. Although there are conflicting reports, most studies have confirmed this theory18–21. Sequestration of infected erythrocytes in the placenta is the hallmark of placental malaria pathogenesis and its effects have been associated with the pathogenesis of PE22,23. Consequential adverse effects such as low birth weight and foetal death resulting from placental pathological alterations have been reported in both conditions24–27. These alterations, such as excessive fibrinoid deposits associated with syncytiotrophoblast damage, and ultra-structural damage lead to basal lamina thickening in placentas affected by placental malaria (PM)28. Immunopathologic processes are also related to these placental changes. Specifically, heightened inflammation has been reported in murine model of PM leading to tissue disorganisation, reduced vascular spaces and blood supply in placental tissues29. Markers of hypoxia such as hypoxia-inducible factors and tissue damage are also increased in women with sub-microscopic PM infections30.
Over the years, the effects of placental changes during PM have focused mainly on foetal outcome, maternal anaemia and death. Maternal death due to PM is believed to be indirect. Besides, most women with placental parasite infections are either asymptomatic and or lack sensitivity to peripheral blood test31. The silent placental infection may be associated with pathological changes, including localised placental inflammation, which may compromise placental function. In effect, PM may induce or exacerbate pregnancy-associated syndrome such as PE. The possible link between placental pathological lesions, PM and the outcome of PE has been investigated in this study by testing the hypothesis that more women with PM and its associated histological lesions suffer from the PE syndrome.
Results
Characteristics and perinatal outcome of study participants
A total of 134 placentas were sampled at delivery comprising 65 from women diagnosed with PE (PE group) and 69 without PE (non-PE group) (Table 1). The age of the pregnant women ranged between 18–42 years and was similar between the two groups. Body mass index (BMI) and intermittent preventive treatment in pregnancy (IPTp) coverage were also similar between the PE and the non-PE groups (P > 0.05 in all cases). Most women (91%) received between one to four doses of IPTp during gestation. Gravidity was different between the groups (P = 0. 003). Systolic blood pressure (SBP) and diastolic blood pressure (DBP) at sampling were significantly higher in the PE (mean SBP = 150.7 ± 18.1, mean DBP = 95.7 ± 14.3; P < 0.0001) compared to non-PE group (mean SBP 122.2 ± 14.2, mean DBP = 75.5 ± 8.8; P < 0.0001). For perinatal outcome, the PE group had significantly lower gestational age at delivery (36.2 ± 4 weeks), vaginal deliveries (23.1% of 65), and birth weight (2.4 ± 1 kg) compared to the non-PE group [(gestational age at delivery 39.5 ± 2 weeks, P < 0.0001); vaginal deliveries (100% of 69, P < 0.0001); birth weight (3.2 ± 0.5 kg, P < 0.0001) (Table 1). Apgar score which measures the physical characteristics of the newborn was low resulting in a higher number of neonatal intensive care unit (NICU) admissions among the PE group deliveries (57.8% of 65, P < 0.0001).
Table 1.
Demographic and clinical characteristics among women diagnosed with or without preeclampsia.
| Characteristic | Non-PE n = 69 | PE n = 65 | Total n = 134 | P-value |
|---|---|---|---|---|
| Maternal age (years)# | 29 ± 6 | 28 ± 6 | 28 ± 6 | 0.27 |
| Primigravid* | 16 (23.2%) | 32 (49.2%) | 48 (35.8%) | 0.003 |
| Multigravid* | 53 (76.8%) | 33 (50.8%) | 86 (64.2%) | |
| Mean BMI (kg/m2)# | 26 ± 4.9 | 27.7 ± 5.6 | 26.8 ± 5.3 | 0.08 |
| Sampling Systolic BP (mmHg)# | 122.2 ± 14.2 | 150.7 ± 18.1 | 136.0 ± 21.6 | <0.0001 |
| Sampling Diastolic BP (mmHg)# | 75.5 ± 8.8 | 95.7 ± 14.3 | 85.2 ± 15.5 | <0.0001 |
| IPTp use* | ||||
| Dose 4 | 1 (1.5%) | 2 (3.4%) | 3 (2.4%) | 0.78 |
| Dose 3 | 36 (55.4%) | 27 (46.6%) | 63 (51.2%) | |
| Dose 2 | 10 (15.4%) | 13 (22.4%) | 23 (18.7%) | |
| Dose 1 | 12 (18.5%) | 11 (19.0%) | 25 (18.7%) | |
| Dose 0 | 6 (9.2%) | 5 (8.6%) | 11 (8.9%) | |
| No record | 4 | 7 | 11 | |
| Delivery age (weeks)# | 39.5 ± 2 | 36.2 ± 4 | 37.9 ± 4 | <0.0001 |
| Mode of delivery* | ||||
| Vaginal | 69 (100%) | 15 (23.1%) | 84 (62.7%) | <0.0001 |
| C section | 0 | 50 (76.9%) | 50 (37.3%) | |
| Preterm delivery* | 3 (4.3%) | 30 (46.3%) | 33 (24.6%) | <0.0001 |
| Birth weight (kg)# | 3.2 ± 0.5 | 2.4 ± 1.0 | 2.8 ± 0.8 | <0.0001 |
| Apgar at 1 min# | 7.1 (±1.1) | 6.3 (±1.3) | 6.7 (±1.3) | <0.002 |
| Apgar at 5 min# | 8.3 (±0.9) | 7.6 (±1.2) | 8.0 (±1.1) | <0.001 |
| Sex of babya* | ||||
| Male | 33 (47.8%) | 30 (46.9%) | 63 (47.4%) | 1.00 |
| Female | 36 (52.2%) | 34 (53.1%) | 70 (52.6%) | |
| NICU admissionb* | ||||
| Yes | 7 (10.1%) | 37 (57.8%) | 44 (33.1%) | <0.0001 |
| No | 62 (89.9%) | 27 (42.2%) | 89 (66.9%) | |
#Mean ± SD; *n = %; a n = 64 (PE group); bn = 68 (both non-PE and PE groups) and PE NICU admission; BMI = Body mass index; BP = Blood pressure; IPTp = Intermittent preventive treatment in pregnancy; NICU = Neonatal intensive care unit. P-values were generated using Student’s t-test for continuous data and Fisher’s exact test for categorical data.
Placental histological findings in women diagnosed with and without preeclampsia
Unique pathological features were observed and reported by histology from the placentas of non-PE and PE pregnancies. Of the 134 placentas, 51 (38.1%) showed histopathologic lesions and this comprised 20 (29% of 69) in the non-PE group and 31 (47.7% of 65) in the PE group. Calcifications, syncytial knots, infarctions, atherosis, accelerated maturation of villi and a combination of 2 or more of these pathologies (mixed) were the major changes observed in the sampled placentas (Fig. 1). The percentage of placentas with calcifications was pronounced in the non-PE group (31.4% of 51) compared to the PE group (13.7% of 51). Syncytial knots were prominent in the PE group (27.5% of 51) than the non-PE group (3.9% of 51). The mixed group comprising 2 or more observed pathologies within a placenta together with atherosis were not different between the non-PE and PE groups. Accelerated villous maturation and infarctions were observed in the PE group only (Fig. 2). The placental pathologic effects on gravidity and perinatal outcomes were assessed in PE and non-PE pregnancies. Percentage pathology by gravidity is shown in Supplementary Fig. S1a. In the PE group, women with both normal and pathologic placentas had a lower mean delivery age compared to the non-PE group (Supplementary Fig. S1b). Similarly, the mean birth weight of babies delivered to PE women with both normal and pathologic placentas was lower compared to the mean birth weight from the non-PE group (Supplementary Fig. S1c).
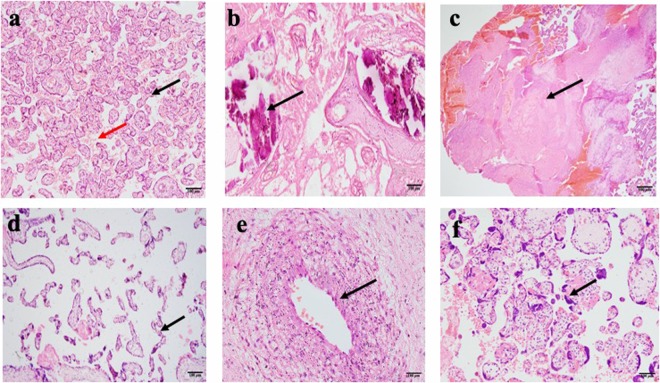
Figure 1.
Photomicrographs of placental changes observed among pregnant women from the study. (a) A non-PE placenta showing normal villi (black arrow) and intervillous spaces (red arrow). (b) Calcifications (black arrow) in a 33-week old PE placenta. (c) Infarction (coagulative necrosis) of large area of the placenta from ischaemia (black arrow) in 29-week placenta with intrauterine foetal death). (d) Accelerated villous maturation (thin finger-like or slender villi with reduced branching). (e) Atherosis showing accumulation of lipid laden macrophages within sub-endothelial area of arterial wall. (f) Increased syncytial knots showing densely stained and closely packed nuclei.
Figure 2.
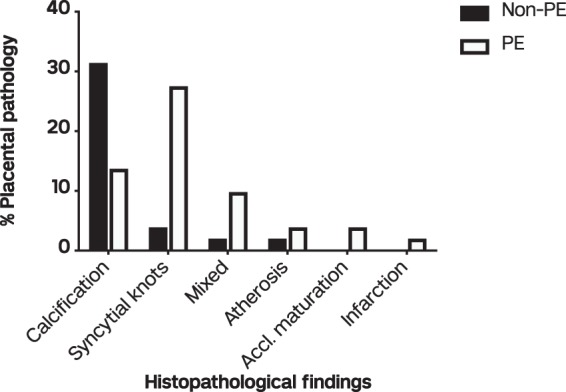
Percentage of histological findings in normal and pathologic placentas. Bars represent non-PE (white bars) and PE (dark bars) women. Mixed = 2 or more placental abnormalities; accl. maturation = accelerated villous maturation.
Risk factors associated with pathology in non-preeclamptic and preeclamptic pregnancies
Generally, placental pathology was significantly associated with PE. Presence of placental pathology increased the odds of PE (odds ratio (OR) 2.2, 95% CI 1.1–4.6; P = 0.027). Syncytial knots were the only specific pathology that increased the risk of PE (OR 10.1, 95% CI 2.2–47.3; P = 0.003). Calcification, mixed pathology, infarction, accelerated villous maturation and atherosis did not show significant effects. Primigravidity increased the odds of developing PE in both the univariate (OR: 3.0, 95% CI (1.5–6.3), P = 0.003 respectively) and multivariate (OR: 4.5, 95% CI (2.0–10.7), P < 0.0001) analyses (Supplementary Table S1).
Plasmodium falciparum placental exposure and the risk of preeclampsia
A total of 133 placentas were evaluable for PM. The classification guide by Bulmer et al.32, was used to score the absence of infection, acute, chronic and past infections for all the 133 placentas (Supplementary Table S2 and Fig. 3). Overall, 64 (48.1% of 133) placentas had active parasites (acute and chronic infections), 21(15.8% of 133) with past infections and 48 (36.1% of 133) with no infections (Fig. 4). A higher proportion of active and past placental malaria were observed in women diagnosed PE (61.0% and 23.4% of 64 respectively) compared to non-PE diagnosed women (36.2% and 8.7% of 69 respectively). Women with no parasite infections were 10 (15.6% of 64) in PE diagnosed placentas and 38 (55.1% of 69) in non-PE diagnosed placentas.
Figure 3.
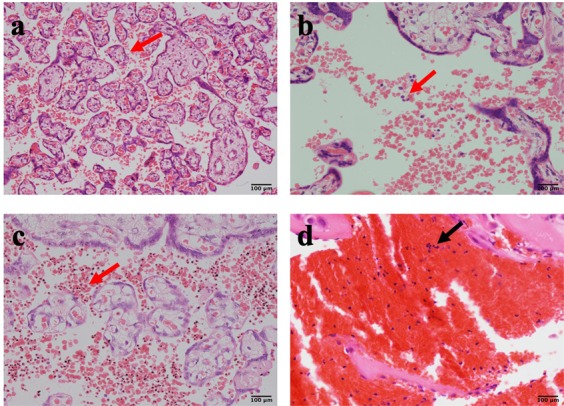
Placental infections observed among pregnant women from the study. (a) Normal placental architecture with uninfected RBCs in the intervillous spaces. (b) Acute malaria infection with only parasitized red cells and no parasite pigments (c) Chronic malaria infection with parasites and parasite pigments present in red cells in a 38-week placenta (d) Past malaria infection showing pigments in fibrin.
Figure 4.
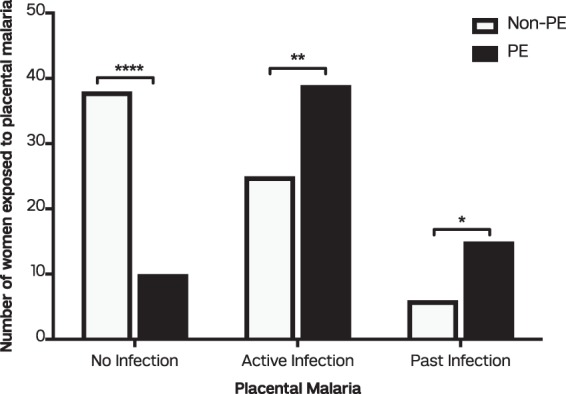
Measure of exposure to placental malaria in women diagnosed with non-PE (white bars) and PE (dark bars). Data presented as proportions between active, past and no infection in non-PE and PE placentas by Fisher’s exact test. *(P < 0.05), **(P < 0.01); ****(P < 0.0001).
Placental exposure, maternal and pregnancy outcomes in preeclampsia
Placental malaria was further evaluated based on gravidity (Table 2). At delivery, the presence of active and past PM was assessed against placentas without parasite infections. In the primigravid women (n = 47), 19 active placental infections (82.6% of 23), 5 past placental infections (71.4% of 7) and 7 with no placental infections (41.2% of 17) was reported for the PE group. In the non-PE group, 4 active placental infections (17.4% of 24), 2 past placental infections (28.6% of 7) and 10 with no placental infections (58.8% of 17; P = 0.02). In the multigravid women (n = 86), 20 active placental infections (48.8% of 41), 10 past placental infections (71.4% of 14) and 3 with no placental infections (9.7% of 31) was observed in PE placentas. Placentas from non-PE woman had 21active placental infections (51.2% of 41), 4 past placental infections (28.6% of 14) and 28 with no placental infections (90.3% of 31). The maternal age at delivery was significantly lower in the PE women with active infections (35.7 ± 3.98 weeks) compared to non-PE women (39.4 ± 1.60 weeks; P = 0.0002). However, the mean delivery age in women with past infections or no infections did not differ between the groups. (Supplementary Fig. S2a). Also, the mean birth weight of babies was significantly lower amongst PE women with active (2.4 ± 0.96 kg) and past infection (2.4 ± 0.86 kg) compared to those in non-PE women (active infections 3.2 ± 0.50 kg, P = 0.0003; past infections 3.3 ± 0.29 kg, P = 0.03) (Supplementary Fig. S2b).
Table 2.
Distribution of parasite infection status by gravidity between study groups.
| No Infection n (%) | Active Infection n (%) | Past-Infection n (%) | Total n (%) | P-value | |
|---|---|---|---|---|---|
| Primigravid | |||||
| Non-PE | 10 (58.8) | 4 (17.4) | 2 (28.6) | 16 (32.0) | 0.02 |
| PE | 7 (41.2) | 19 (82.6) | 5 (71.4) | 31 (68.0) | |
| Multigravid | |||||
| Non-PE | 28 (90.3) | 21 (51.2) | 4 (28.6) | 53 (58.9) | <0.0001 |
| PE | 3 (9.7) | 20 (48.8) | 10 (71.4) | 33 (41.1) | |
| Total | 48 | 64 | 21 | 133 | |
Data presented as proportions between non-PE and PE groups in primigravid and multigravid women. P-value obtained by Fisher’s exact test.
Placental malaria, pathology and pregnancy factors as predictors of preeclampsia
The study compared placental infections, pathology and other significant maternal risk factors for PE development using a logistic regression model (Table 3). The risk of PE was increased in women with active and past placental infections [odds ratio: 5.9 (2.6–14.0), P < 0.0001 and 9.5 (2.9–30.8), P < 0.0001 respectively]. In a multivariate analysis, the risk of PE was still increased in women with active or past placental infections [adjusted odds ratio: 6.7 (2.3–19.1), P < 0.0001; 12.4 (3.0–51.0), P < 0.0001 respectively]. This risk was further increased in primigravid women [adjusted odds ratio: 6.6 (2.4–18.2), P < 0.0.0001] with placental pathological lesions [adjusted odds ratio: 3.0 (1.2–7.5), P = 0.019].
Table 3.
Crude odds ratio (OR) and adjusted odds ratio (AOR) for factors associated with placental malaria and the risk of PE.
| OR (95% CI) | P-value | AOR (95% CI) | P-value | |
|---|---|---|---|---|
| PM | ||||
| No Infection | Ref | Ref | Ref | |
| Active Infection | 5.9 (2.6–14.0) | <0.0001 | 6.7 (2.3–19.1) | <0.0001 |
| Past Infection | 9.5 (2.9–30.8) | <0.0001 | 12.4 (3.0–51.0) | <0.0001 |
| Pathology | ||||
| No | Ref | Ref | Ref | |
| Yes | 2.2 (1.1–4.6) | 0.027 | 3.0 (1.2–7.5) | 0.019 |
| 1st visit SBP (mmHg) | 1.0 (1.0–1.1) | 0.010 | 1.04 (1.0–1.1) | 0.083 |
| 1st visit DBP (mmHg) | 1.0 (1.0–1.1) | 0.042 | 1.0 (0.9–1.1) | 0.65 |
| Gravidity | ||||
| Multigravid | Ref | Ref | Ref | |
| Primigravid | 3.0 (1.5–6.3) | 0.003 | 6.6 (2.4–18.2) | <0.0001 |
Logistic regression model used. P value is significant if <0.05. PM = placental malaria, PE = preeclampsia, SBP and DBP = systolic and diastolic blood pressure respectively.
Discussion
Placental examination is an important, non-invasive means to continuously decipher pathological conditions that improve maternal and foetal health. Therefore, histological examination of placentas after delivery from PE and non-PE diagnosed women in a malaria-endemic region will provide further insight into the pathophysiology of the disorder. From the study, histological alterations occurred more frequently in PE placentas than non-PE placentas. This reflects the placental origin and the pathophysiologic involvement of the placenta in PE pathogenesis. Reports of placental alterations in PE may either be specific33 or non-specific17 to PE suggesting that these alterations may contribute either directly or indirectly to PE pathogenesis.
Of the specific alterations associated with PE in this study, syncytial knots were identified as the main contributor to placental pathology in women diagnosed with PE. It was about ten-fold more frequent in PE than non-PE placentas. These aggregates of syncytial nuclei are associated with conditions of uteroplacental malperfusion34 and hypoxia35 such as PE. The syncytiotrophoblasts mainly serve as a protective barrier against harmful effects to the foetus as well as a foeto-maternal exchange portal for nutrients and waste products. Functional deficits of the syncytiotrophoblasts result in the formation of these knots which support the ‘Tenney-Parker’ changes of the placenta used as an index of well-being36–39. Our data also confirm findings from a previous report of excessive syncytial knots in PE placentas compared to normotensive women39. Increased syncytial knots have also been associated with PE severity39,40. The biological inference here is that increased syncytial knots may result in sporadic release of factors into the localized placental environment that cause further damage to the placenta. Bioactive substances released such as soluble vascular endothelial growth factor receptor one (sVEGFR1) may be exported into maternal circulation to cause clinical effects of PE.
Accelerated villous maturations and infarctions were reported in the PE group only hence statistical significance could not be established. These findings which have been shown elsewhere to increase the risk of PE16 clearly emphasizes PE as a syndrome of placental dysfunction. Calcifications, atherosis and presence of mixed pathologies were not different between the groups. Although these are biologically significant pathologic alterations in PE pregnancies, literature reports a discordance in their pathophysiologic ramifications11,15. Despite these reports, the overall association between placental pathology and PE as shown in this study suggests that these placental lesions may have a contributory effect to the PE syndrome.
Maternal risk factors for PE such as twin gestation, chronic hypertension and diabetes were excluded at recruitment to limit confounders. Other risk factors such as high BMI were not different between the PE and non-PE groups as shown in the demographic characteristics. Primigravidity and blood pressure were independent risk factors for PE in the univariate analysis. These were also evident in a multivariate analysis that included the presence of placental pathology except diastolic blood pressure at booking. Increased blood pressure has been linked to the development of hypertensive disorders in pregnancy (HDP)41. Krielessi correlated the extent of placental lesions with the level of hypertensive disorders and found that extensive placental lesions were associated with a higher level of hypertension42. However high blood pressure alone, is indicative of gestational hypertension, a different phenotype from PE and may not present with placental pathology as reported elsewhere43,44. Primigravidity is also a known risk factor for PE45,46. Our study has shown placental pathology, primigravidity but not diastolic blood pressure at booking as contributors to PE and these may discriminate it from other HDP. Our data also support earlier findings regarding adverse pregnancy and foetal outcomes such as low birth weight and pre-term birth associated with PE.
Malaria endemicity may be an added risk factor for PE pathogenesis as demonstrated in earlier studies18,20,21,47. The histological grading system for PM showed a high exposure rate in PE than non-PE women. Consistent with earlier studies, PM is an independent risk factor for PE with adverse outcomes such as low birth weight. Approximately nine and six fold increases in the risk for PE were observed for past and active parasite infections respectively, with absence of infections as a reference. In a similar study in Sudan, PM was associated with PE mostly by past malaria infection in placental histological grading20. In Senegal, histological diagnosis was made but the study did not present data based on the histological grading system as done in Sudan and this current study47. Therefore, conclusions cannot be drawn on whether the association was based on only active infections, past infections or both. Other studies generally associated PM with HDP and not PE only18,19 as this present study has shown. Placental pathology was not specifically related to PM and other factors in these studies. Muehlenbachs et al.18, however showed elevated levels of sVEGFR-1, a PE biomarker, in primigravids with PM, hypertension or both. They concluded that this marker, associated with endothelial or placental dysfunction, may be under selective pressure during first pregnancies in malaria endemic regions. Our study has brought to bear the link between placental pathology, PM (active or past infections) and PE development. In the multiple comparison model, blood pressure at booking which is prior to PE diagnosis was not predictive of the disorder. Others have reported an inverse relationship between parasite density and blood pressure18. Similarly, a lack of association between PM and non-proteinuric hypertension has been reported19. In view of this, our investigation suggests that a compromised placenta plays a critical role in PE pathogenesis and this is particularly significant in primigravidae exposed to PM. Although over 90% of participants received at least one dose of IPTp, our study did not find any relationship between placental pathology, IPTp use and PE development. However, it has recently been shown that high uptake of IPTp improves birth weight48. Further studies are needed to ascertain if high uptake of IPTp reduces placental pathology and the risk of PE in malaria endemic regions.
Limitations
Our study was a single site study that has given a snapshot of the possible pathological effects of PM during PE. We propose a longitudinal multicenter study to examine pathological variations of PM together with other risk factors to further understand the heterogeneity of PE.
Conclusion
Our study has shown for the first time among Ghanaian women pathological effects of a PE placenta in women exposed to PM. This may be an important consideration in future management guidelines for PE in malaria endemic regions. Furthermore, mechanisms that may induce PM specific PE should be elucidated.
Methods
Study site
The study was conducted at the Department of Obstetrics and Gynaecology of the Korle-Bu Teaching Hospital (KBTH) in Accra, Ghana. KBTH is a tertiary hospital and a leading national referral centre and hosts the University of Ghana School of Medicine and Dentistry. The Department of Obstetrics and Gynaecology is the largest department in the hospital with a 275-bed capacity for obstetric care (~10,000 deliveries/year). It has the capacity and medical expertise to manage major obstetric complications hence serves as a pivot for obstetric referrals from the southern half of the country.
Study design and target population
The study was carried out between January and December 2017. Pregnant women diagnosed with PE and presenting for delivery at the hospital were screened for inclusion into the study. Healthy pregnancies (non-PE) were concurrently screened for inclusion from the same hospital. Informed consent was obtained from each participant recruited. Case report forms were appropriately completed to capture both demographic and clinical information. Each participant was assigned a unique identification code prior to sampling. Laboratory data were collected at delivery and transported to the Pathology Department of the Korle-Bu Teaching Hospital for analysis. Both primigravidae and multigravidae within the age range of 18–45 years were enrolled into the study.
Inclusion criteria for preeclampsia (PE)
Clinical PE was defined as pregnant woman who presented with a history of sustained hypertension (blood pressure ≥140 mmHg systolic or ≥90 mmHg diastolic at least 4 hours apart) with proteinuria (two readings of 1+ or higher on urinalysis) ≥20 weeks of gestation as diagnosed by an obstetrician. This definition is in accordance with the International Society for the Study of Hypertension in Pregnancy (ISSHP) for the diagnosis of clinical PE3,49.
Inclusion criteria for healthy or non-preeclamptic pregnancy (non-PE)
This was defined as pregnant woman at ≥20 weeks of gestation with a history of sustained blood pressure <140 mmHg systolic or <90 mmHg diastolic) without proteinuria at time of enrolment as confirmed by a clinician/midwife and medical records.
General exclusion criteria
Women with multiple gestation, pre-existing cardiovascular disease, chronic hypertension, diabetes, infections such as HIV and hepatitis, haematological and immunological disorders were excluded.
Ethical considerations
All methods were carried out in accordance with relevant guidelines and regulations. Approval to conduct this study was obtained from the Institutional Review Board of the NMIMR (IRB# 00001276)) registered with OHRP (FWA 00001824) and the Ethical and Protocol Review Committee of the KBTH (KBTH/MD/G3/17). Demographic data and biological specimen were obtained following informed consent from selected participants. Participation was strictly voluntary with no restrictions if participant decided to opt out of study.
Measure of exposure to placental malaria
Placental Malaria (PM) was defined as the presence of parasites in the placental blood and/or parasite pigment deposition in placental biopsies after delivery as previously described18,32.
Placental tissue sampling and processing
To investigate the association between placental pathology and Plasmodium falciparum infection, placental tissues were sampled from PE and non-PE women after delivery. A portion of excised tissue was washed in physiological saline to remove excess blood and immediately placed in a well labelled container with 100 ml of 10% neutral buffered formalin for tissue fixation. This was to maintain tissue integrity and minimize the formation of formalin pigment in the tissue. Biopsy was immediately transported to the Department of Pathology, KBTH, where tissue processing was carried out. Fixative was changed from time to time until a clear fixative was obtained that ensured that the tissue was well fixed before processing. Processing of fixed tissue was carried out within 72 hours of collection.
Placental tissue processing
Placental tissues were processed using Leica TP1020 (Leica Biosystems, Germany) tissue processor. Briefly, a 3 cm3 (3 × 1 × 1 cm) portion of the placental tissue was grossed and placed into a well labelled tissue processing cassette and fixed in 10% neutral buffered formalin for about an hour. The tissue portion in the cassette was dehydrated in ascending grades of ethanol (60%, 70%, 80% and 90%) for 90 minutes. This was then placed in three changes of absolute ethanol to achieve full dehydration. After dehydration, tissue was cleared in two changes of xylene and transferred into molten paraffin wax at a melting temperature of 56 °C. Infiltration was carried out for 2 hours. For paraffin embedding, Leica EG1150, (Leica Biosystems, Germany) modular tissue embedder was used to form tissue blocks.
Placental tissue sectioning and staining
Placental tissue block was trimmed at 10 microns and placed on ice to cool, after which 3 microns thick of tissue was sectioned into ribbons using a rotary microtome (Leica RM2235, Leica Biosystems, Germany). Ribbons were floated onto a protein-free water bath (Boekel Scientific 14793, USA) at a temperature of 50 °C. Two floating ribbons from each placental tissue were mounted onto well labelled grease-free frosted end glass slides. Subsequently, the slides were air dried and heat fixed on a hot plate. Placental tissue sections mounted on each slide were stained using standard haematoxylin and eosin (H&E) stains and Giemsa respectively. The H & E staining was carried out to differentiate cellular components of placental tissues according to Mayer’s staining protocol50. Briefly, sections were deparaffinized in xylene, rehydrated in absolute alcohol, conditioned in varying gradients of alcohol and stained with haematoxylin. Sections were counter stained in eosin, dehydrated, cleared and mounted with a xylene based mounting medium (DPX 44581, Sigma). Similar procedure was carried out for Giemsa staining.
Microscopic examination of histologically stained placental tissues
Slides were blindly examined using a standard light microscope and a histological grading system adapted from Muehlenbachs and group51. Presence or absence of malaria parasites, hemozoin, inflammation and unique placental changes were recorded under x4, x10, x20 or x40 using the Olympus BX 43 microscope. Images were captured on the cellSens standard software using d27 camera. Infection state was classified into four groups namely acute infection (presence of parasites and absence of hemozoin), chronic infection (presence of parasites and significant amount of hemozoin in fibrin or macrophages), past infection (absence of parasites with presence of hemozoin in fibrin or macrophages) and no infection (absence of both parasites and hemozoin in fibrin or macrophages)32,51. Unique pathologies observed during the examination of the placenta were also reported.
Statistical analysis
Data were analysed using GraphPad prism version 6 (California, USA) and R version 3.5.1 (R Development Core team). Means were compared between two continuous variables using Student’s t-test. One-way analysis of variance (ANOVA) test was used to compare means for more than two continuous variables. Pair-wise post-hoc comparisons were done using Dunn’s multiple comparison test. Categorical data was analysed using Chi-square test or Fisher’s exact test for association. Univariate and multivariate binary logistic regression analyses were carried out to predict the risk of PE. For all analyses, P < 0.05 was considered statistically significant.
Supplementary information
Acknowledgements
We express our gratitude to the study volunteers and staff of the Pathology, Obstetrics and Gynaecology Departments of the Korle-Bu Teaching Hospital for active participation in the study. We also thank Thomas Addison, Richard Darko, Emmanuel Sackey, Andrew Osei Obese, Jacob Quartey and Nii Ocquaye Kwabena Yeboah of the Immunology Department, Noguchi Memorial Institute for Medical Research and fellows of the Infectious Disease Research Lab of the West African Centre for Cell Biology of Infectious Pathogens of the University of Ghana for the technical and laboratory support; Dr. Lawrence Edusei for expert pathological advice and Dr. Selorme Adukpo for reviewing the manuscript. Dorotheah Obiri was supported by a PhD fellowship from the World Bank African Centres of Excellence Grant (ACE02-WACCBIP: Awandare).
Author contributions
D. Obiri conceived the idea, B.A.G., D. Oduro, K.A.B., K.A.K. and M.F.O. supervised the work. K.A.B. and I.J.E. provided expert’s advice. D. Obiri, I.J.E., K.A.B. and J.A. carried out the experiment and obtained the data. D. Obiri I.J.E. and K.A.K. analysed the data. D. Obiri wrote the first draft of the manuscript. All authors reviewed, read and approved the final manuscript.
Data availability
The data generated during the current study is included in this article and its supplementary files.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Dorotheah Obiri, Email: dorotheahobiri@gmail.com.
Michael Fokuo Ofori, Email: mofori@noguchi.ug.edu.gh.
Supplementary information
is available for this paper at 10.1038/s41598-020-64736-4.
References
- 1.Stevens W, et al. Short-term costs of preeclampsia to the United States health care system. Am. J. Obstet. Gynecol. 2017;217(3):237–248. e16. doi: 10.1016/j.ajog.2017.04.032. [DOI] [PubMed] [Google Scholar]
- 2.Brown MA, et al. Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension. 2018;72(1):24–43. doi: 10.1161/HYPERTENSIONAHA.117.10803. [DOI] [PubMed] [Google Scholar]
- 3.Tranquilli A, et al. The classification, diagnosis and management of the hypertensive disorders of pregnancy: A revised statement from the ISSHP. Pregnancy. Hypertension: An International Journal of Women’s Cardiovascular Health. 2014;4(2):97–104. doi: 10.1016/j.preghy.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Bartsch E, Medcalf KE, Park AL, Ray JG. Clinical risk factors for pre-eclampsia determined in early pregnancy: systematic review and meta-analysis of large cohort studies. BMJ. 2016;353:i1753. doi: 10.1136/bmj.i1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghana Statistical Service (GSS), G.H.S.G., and ICF. Ghana Maternal Health Survey 2017. Accra: Ghana Demogr Health Surv 2018; Available from: https://dhsprogram.com/pubs/pdf/FR340/FR340.pdf (Accessed: 15th August 2019).
- 6.Adu-Bonsaffoh K, Oppong SA, Binlinla G, Obed SA. Maternal deaths attributable to hypertensive disorders in a tertiary hospital in Ghana. Int. J. Gynaecol. Obstet. 2013;123(2):110–113. doi: 10.1016/j.ijgo.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 7.Dassah ET, Kusi-Mensah E, Morhe ESK, Odoi AT. Maternal and perinatal outcomes among women with hypertensive disorders in pregnancy in Kumasi, Ghana. Plos One. 2019;14(10):e0223478–e0223478. doi: 10.1371/journal.pone.0223478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerretsen G, Huisjes H, Elema J. Morphological changes of the spiral arteries in the placentae bed in relation to pre‐eclampsia and fetal growth retardation. BJOG. 1981;88(9):876–881. doi: 10.1111/j.1471-0528.1981.tb02222.x. [DOI] [PubMed] [Google Scholar]
- 9.Khong T, De Wolf F, Robertson W, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre‐eclampsia and by small‐for‐gestational age infants. BJOG. 1986;93(10):1049–1059. doi: 10.1111/j.1471-0528.1986.tb07830.x. [DOI] [PubMed] [Google Scholar]
- 10.Roberts JM, Hubel CA. The two stage model of preeclampsia: variations on the theme. Placenta. 2009;30:32–37. doi: 10.1016/j.placenta.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Staff AC, Dechend R, Pijnenborg R. Learning from the placenta: acute atherosis and vascular remodeling in preeclampsia–novel aspects for atherosclerosis and future cardiovascular health. Hypertension. 2010;56(6):1026–1034. doi: 10.1161/HYPERTENSIONAHA.110.157743. [DOI] [PubMed] [Google Scholar]
- 12.Vinnars MT, Nasiell J, Ghazi S, Westgren M, Papadogiannakis N. The severity of clinical manifestations in preeclampsia correlates with the amount of placental infarction. Acta Obstet. Gynecol. Scand. 2011;90(1):19–25. doi: 10.1111/j.1600-0412.2010.01012.x. [DOI] [PubMed] [Google Scholar]
- 13.Souza DAD, Bezerra AFDS, Wanderley DC, Souto CMB. Increase in perivillous fibrinoid material in placentas from pregnancies with preeclampsia. Jornal Brasileiro de Patologia e Medicina Laboratorial. 2011;47(1):70–77. doi: 10.1590/S1676-24442011000100010. [DOI] [Google Scholar]
- 14.Stevens D, Al-Nasiry S, Bulten J, Spaanderman M. Decidual vasculopathy in preeclampsia: lesion characteristics relate to disease severity and perinatal outcome. Placenta. 2013;34(9):805–809. doi: 10.1016/j.placenta.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 15.Wallingford MC, Benson C, Chavkin NW, Chin MT, Frasch MG. Placental vascular calcification and cardiovascular health: It is time to determine how much of maternal and offspring health is written in stone. Front. Physiol. 2018;9:1044. doi: 10.3389/fphys.2018.01044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ezeigwe, C.O., C.I. Okafor, G.U. Eleje, G.O. Udigwe, &D.C. Anyiam, Placental Peripartum Pathologies in Women with Preeclampsia and Eclampsia. Obstet. Gynecol., 2018 (2018). [DOI] [PMC free article] [PubMed]
- 17.Roberts JM, Escudero C. The placenta in preeclampsia. Pregnancy Hypertension: An International Journal of Women’s Cardiovascular Health. 2012;2(2):72–83. doi: 10.1016/j.preghy.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muehlenbachs A, Mutabingwa TK, Edmonds S, Fried M, Duffy PE. Hypertension and maternal–fetal conflict during placental malaria. PLoS Med. 2006;3(11):e446. doi: 10.1371/journal.pmed.0030446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ndao, C. T. et al. Placental malarial infection as a risk factor for hypertensive disorders during pregnancy in Africa: a case-control study in an urban area of Senegal, West Africa. Am. J. Epidemiol., kwp207 (2009). [DOI] [PubMed]
- 20.Adam I, Elhassan EM, Mohmmed AA, Salih MM, Elbashir MI. Malaria and pre-eclampsia in an area with unstable malaria transmission in Central Sudan. Malar J. 2011;10:258. doi: 10.1186/1475-2875-10-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Etard J-F, Kodio B, Ronsmans C. Seasonal variation in direct obstetric mortality in rural Senegal: role of malaria? Am. J. Trop. Med. Hyg. 2003;68(4):503–504. doi: 10.4269/ajtmh.2003.68.503. [DOI] [PubMed] [Google Scholar]
- 22.Brabin BJ, Johnson PM. Placental malaria and pre-eclampsia through the looking glass backwards? Reprod. Immunol. 2005;65(1):1–15. doi: 10.1016/j.jri.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Etyang AO, Smeeth L, Cruickshank JK, Scott JAG. The Malaria-High Blood Pressure HypothesisNovelty and Significance. Circ. Res. 2016;119(1):36–40. doi: 10.1161/CIRCRESAHA.116.308763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dorman E, et al. Impaired uteroplacental blood flow in pregnancies complicated by falciparum malaria. Ultrasound Obstet. Gynecol. 2002;19(2):165–170. doi: 10.1046/j.0960-7692.2001.00545.x. [DOI] [PubMed] [Google Scholar]
- 25.Ofori MF, et al. Pregnancy-associated malaria in a rural community of Ghana. Ghana Med. J. 2009;43(1):13. [PMC free article] [PubMed] [Google Scholar]
- 26.Kapisi J, et al. Relationships between infection with Plasmodium falciparum during pregnancy, measures of placental malaria, and adverse birth outcomes. Malar. J. 2017;16(1):400. doi: 10.1186/s12936-017-2040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lufele E, et al. Risk factors and pregnancy outcomes associated with placental malaria in a prospective cohort of Papua New Guinean women. Malar. J. 2017;16(1):427. doi: 10.1186/s12936-017-2077-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walter PR, Garin Y, Blot P. Placental pathologic changes in malaria. A histologic and ultrastructural study. Am. J. Pathol. 1982;109(3):330. [PMC free article] [PubMed] [Google Scholar]
- 29.Neres R, Marinho CR, Gonçalves LA, Catarino MB, Penha-Gonçalves C. Pregnancy outcome and placenta pathology in Plasmodium berghei ANKA infected mice reproduce the pathogenesis of severe malaria in pregnant women. Plos One. 2008;3(2):e1608. doi: 10.1371/journal.pone.0001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agudelo OM, et al. Submicroscopic infection of placenta by Plasmodium produces Th1/Th2 cytokine imbalance, inflammation and hypoxia in women from north-west Colombia. Malar. J. 2014;13(1):122. doi: 10.1186/1475-2875-13-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blay EA, et al. Congenital toxoplasmosis and pregnancy malaria detection post-partum: Effective diagnosis and its implication for efficient management of congenital infection. Parasitol. Int. 2015;64(6):603–608. doi: 10.1016/j.parint.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 32.Bulmer J, Rasheed F, Francis N, Morrison L, Greenwood B. Placental malaria. I. Pathological classification. Histopathology. 1993;22(3):211–218. doi: 10.1111/j.1365-2559.1993.tb00110.x. [DOI] [PubMed] [Google Scholar]
- 33.Falco M, Sivanathan J, Laoreti A, Thilaganathan B, Khalil A. Placental histopathology associated with pre‐eclampsia: systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2017;50(3):295–301. doi: 10.1002/uog.17494. [DOI] [PubMed] [Google Scholar]
- 34.Loukeris K, Sela R, Baergen RN. Syncytial knots as a reflection of placental maturity: reference values for 20 to 40 weeks’ gestational age. Pediatr. Dev. Pathol. 2010;13(4):305–309. doi: 10.2350/09-08-0692-OA.1. [DOI] [PubMed] [Google Scholar]
- 35.Heazell A, Moll S, Jones C, Baker P, Crocker I. Formation of syncytial knots is increased by hyperoxia, hypoxia and reactive oxygen species. Placenta. 2007;28:S33–S40. doi: 10.1016/j.placenta.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 36.Tenney B, Jr., Parker F., Jr. The placenta in toxemia of pregnancy. Am. J. Obstet. Gynecol. 1940;39(6):1000–1005. doi: 10.1016/S0002-9378(40)90458-6. [DOI] [Google Scholar]
- 37.Benirschke, K., Burton, G. J. & Baergen, R. N. Early development of the human placenta, in Pathology of the human placenta. 41–53. Springer, 2012).
- 38.Fogarty NM, Ferguson-Smith AC, Burton GJ. Syncytial knots (Tenney-Parker changes) in the human placenta: evidence of loss of transcriptional activity and oxidative damage. The American journal of pathology. 2013;183(1):144–152. doi: 10.1016/j.ajpath.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 39.Devisme L, et al. A case–control study of placental lesions associated with pre‐eclampsia. International Journal of Gynecology & Obstetrics. 2013;120(2):165–168. doi: 10.1016/j.ijgo.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 40.Weel IC, et al. Association between placental lesions, cytokines and angiogenic factors in pregnant women with preeclampsia. Plos One. 2016;11(6):e0157584. doi: 10.1371/journal.pone.0157584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boghossian NS, Albert PS, Mendola P, Grantz KL, Yeung E. Delivery blood pressure and other first pregnancy risk factors in relation to hypertensive disorders in second pregnancies. Am. J. Hypertens. 2015;28(9):1172–1179. doi: 10.1093/ajh/hpv001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krielessi, V. et al. Placental pathology and blood pressure’s level in women with hypertensive disorders in pregnancy. Obstet. Gynecol. Int, 2012 (2012). [DOI] [PMC free article] [PubMed]
- 43.Melamed N, Ray JG, Hladunewich M, Cox B. Gestational hypertension and preeclampsia: are they the same disease? J. Obstet. Gynaecol. Can. 2014;36(7):642–647. doi: 10.1016/S1701-2163(15)30545-4. [DOI] [PubMed] [Google Scholar]
- 44.Stanek, J., Placental pathology varies in hypertensive conditions of pregnancy. Virchows Arch., 1–9 (2017). [DOI] [PubMed]
- 45.Duckitt K, Harrington D. Risk factors for pre-eclampsia at antenatal booking: systematic review of controlled studies. BMJ. 2005;330(7491):565. doi: 10.1136/bmj.38380.674340.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hernández-Díaz S, Toh S, Cnattingius S. Risk of pre-eclampsia in first and subsequent pregnancies: prospective cohort study. BMJ. 2009;338:b2255. doi: 10.1136/bmj.b2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sartelet H, Rogier C, Milko-Sartelet I, Angel G, Michel G. Malaria associated pre-eclampsia in Senegal. Lancet. 1996;347(9008):1121. doi: 10.1016/S0140-6736(96)90321-9. [DOI] [PubMed] [Google Scholar]
- 48.Quakyi I, et al. High uptake of Intermittent Preventive Treatment of malaria in pregnancy is associated with improved birth weight among pregnant women in Ghana. Sci. Rep. 2019;9(1):19034–19034. doi: 10.1038/s41598-019-55046-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.ACOG Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on hypertension in pregnancy. Obstet. Gynecol. 2013;122(5):1122. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 50.Fischer, A. H., Jacobson, K. A., Rose, J. & Zeller, R. Hematoxylin and eosin staining of tissue and cell sections. Cold Spring Harbor Protocols, 2008(5), pdb. prot4986 (2008). [DOI] [PubMed]
- 51.Muehlenbachs A, et al. A novel histological grading scheme for placental malaria applied in areas of high and low malaria transmission. The Journal of infectious diseases. 2010;202(10):1608–1616. doi: 10.1086/656723. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated during the current study is included in this article and its supplementary files.