Methionine is an essential amino acid involved in both biosynthetic and regulatory processes in the bacterial cell. To ensure an adequate supply of methionine, bacteria have evolved multiple systems to synthesize, import, and recover this amino acid. To explore the importance of methionine synthesis, transport, and recovery in any environment, all of these systems must be identified and mutagenized. Here, we have mutagenized every high-affinity methionine uptake system and methionine sulfoxide reductase encoded in the genome of the diarrheal pathogen V. cholerae. We use this information to determine that high-affinity methionine uptake systems are sufficient to acquire methionine in the intestine of the model arthropod Drosophila melanogaster but are not involved in virulence and that the intestinal concentration of methionine must be between 0.05 mM and 0.5 mM.
KEYWORDS: Drosophila, invertebrate host, microbiology, Vibrio cholerae, methionine, methionine sulfoxide
ABSTRACT
While only a subset of Vibrio cholerae strains are human diarrheal pathogens, all are aquatic organisms. In this environment, they often persist in close association with arthropods. In the intestinal lumen of the model arthropod Drosophila melanogaster, methionine and methionine sulfoxide decrease susceptibility to V. cholerae infection. In addition to its structural role in proteins, methionine participates in the methionine cycle, which carries out synthetic and regulatory methylation reactions. It is, therefore, essential for the growth of both animals and bacteria. Methionine is scarce in some environments, and the facile conversion of free methionine to methionine sulfoxide in oxidizing environments interferes with its utilization. To ensure an adequate supply of methionine, the genomes of most organisms encode multiple high-affinity uptake pathways for methionine as well as multiple methionine sulfoxide reductases, which reduce free and protein-associated methionine sulfoxide to methionine. To explore the role of methionine uptake and reduction in V. cholerae colonization of the arthropod intestine, we mutagenized the two high-affinity methionine transporters and five methionine sulfoxide reductases encoded in the V. cholerae genome. We show that MsrC is the sole methionine sulfoxide reductase active on free methionine sulfoxide. Furthermore, in the absence of methionine synthesis, high-affinity methionine uptake but not reduction is essential for V. cholerae colonization of the Drosophila intestine. These findings allow us to place a lower limit of 0.05 mM and an upper limit of 0.5 mM on the methionine concentration in the Drosophila intestine.
IMPORTANCE Methionine is an essential amino acid involved in both biosynthetic and regulatory processes in the bacterial cell. To ensure an adequate supply of methionine, bacteria have evolved multiple systems to synthesize, import, and recover this amino acid. To explore the importance of methionine synthesis, transport, and recovery in any environment, all of these systems must be identified and mutagenized. Here, we have mutagenized every high-affinity methionine uptake system and methionine sulfoxide reductase encoded in the genome of the diarrheal pathogen V. cholerae. We use this information to determine that high-affinity methionine uptake systems are sufficient to acquire methionine in the intestine of the model arthropod Drosophila melanogaster but are not involved in virulence and that the intestinal concentration of methionine must be between 0.05 mM and 0.5 mM.
INTRODUCTION
The Gram-negative, halophilic, diarrheagenic bacterium Vibrio cholerae is found in brackish and salt water environments where only a subset of strains carries the virulence determinants that are associated with the human diarrheal disease cholera (1). In these environments, V. cholerae is found in association with aquatic arthropods, such as zooplankton and other crustaceans. In the environment, V. cholerae colonizes the intestines of aquatic arthropods and, in the laboratory, the intestines of model arthropods (2–5). Studies in our laboratory demonstrated that the rate of stem cell division in the intestine of the model arthropod Drosophila melanogaster is linked to luminal methionine availability (6). In addition, these studies suggested that, in the intestinal environment, methionine sulfoxide (MetO), an oxidized form of methionine, plays a role in competitively inhibiting repair of damaged host proteins required for V. cholerae pathogenesis. We questioned whether V. cholerae uptake of methionine and MetO might promote virulence by decreasing the abundance of these amino acids in the arthropod intestine. Therefore, we set out to test the impact of intestinal methionine synthesis, methionine uptake, and MetO reduction on V. cholerae colonization and growth in the intestine of this host.
The sulfur-containing amino acid methionine participates in multiple essential synthetic and regulatory pathways central to bacterial physiology (7). Methionine exists in dextrorotatory (d) and levorotatory (l) forms due to its chiral α atom. These enantiomers have distinct functions in bacteria (8). l-Methionine is a building block of many proteins, and formyl-methionine is required to initiate protein translation. Furthermore, l-methionine and ATP are converted to S-adenosyl-l-methionine (SAM) through the action of SAM synthase. SAM is essential for synthetic methylation reactions that give rise to formylated Met-tRNA, pantothenate, thymidylate, thiamine, and purines and is responsible for glycine and serine interconversion. In addition, methylation of DNA, RNA, and proteins by SAM serves to regulate transcription, translation, and protein function. d-Methionine is also thought to play a role in bacterial physiology. It can be incorporated into peptidoglycan, and there is evidence that this inhibits peptidoglycan synthesis (9, 10).
Free methionine is easily oxidized by reactive oxygen species (ROS) to one of the diastereoisomers methionine-S-sulfoxide (Met-S-O) or methionine-R-sulfoxide (Met-R-O). Bacteria can use these compounds as a source of methionine after MetO reduction by methionine sulfoxide reductases (Msrs) (11–13). Protein function is also modulated by methionine oxidation, and a large body of evidence suggests that reversible oxidation of protein-associated methionine is a mechanism by which cells adjust their physiology in response to ROS (14). As one might predict, MetO reduction is essential for the virulence of many pathogens (15–20).
Because methionine participates in so many essential processes, bacteria have evolved methionine synthesis pathways, multiple uptake systems for this amino acid, and multiple Msrs (7, 21). Methionine synthesis from homocysteine is carried out by either MetE or MetH (22, 23). When environmental methionine is scarce, the LysR-type transcription factor MetR binds to the promoters of the metE and metH genes to activate their transcription and thus methionine synthesis (23, 24). Two high-affinity methionine uptake systems have been described in bacteria. The metD locus encodes an ATP-binding cassette (ABC) transporter composed of three proteins as follows: an ATPase (MetN), a permease (MetI), and a substrate-binding protein (MetQ) (25). This system transports both the l and d isomers of methionine as well as MetO (26, 27). The well-conserved MetT protein is a cation/methionine antiporter (28). MsrA, MsrB, and MsrC, three Msrs without sequence homology to each other, which are broadly conserved in bacteria, archaea, and eukaryotes, reduce MetO to methionine (29–31). MsrA, the first identified methionine sulfoxide reductase, is distinguished by a protein methionine sulfoxide reductase or peptide methionine sulfoxide reductase (PMSR) domain (32, 33). MsrA homologs are generally active on free and protein-associated Met-S-O (34). MsrB proteins possess SelR domains that coordinate zinc via a CXXC motif (35, 36). A subset of MsrBs utilize a rare catalytic selenocysteine amino acid for their function. MsrB homologs are generally active on protein-associated Met-R-O alone (37). Some organisms also encode fused forms of MsrA and B. MsrC proteins encode a GAF domain that is named for the proteins in which it is found, namely, cGMP-specific phosphodiesterases, adenylyl cyclases, and FhlA, the formate-hydrogen lyase transcriptional activator protein (31, 38). In some of these proteins, the GAF domain has been demonstrated to bind cyclic nucleotides. MsrC homologs are active on free Met-R-O.
Very little is known about V. cholerae methionine synthesis and acquisition systems except that the V. cholerae genome encodes a MetR homolog that is essential for growth on defined medium lacking methionine (39). This transcription factor activates methionine synthesis by binding to promoters that direct methionine synthesis when environmental availability is inadequate. MetR was originally studied due to its role in colonization of the mammalian intestine (39). However, MetR activation of glycine catabolism rather than methionine synthesis was ultimately found to be critical in this environment.
To explore methionine acquisition by V. cholerae in the arthropod intestine, we first identified and deleted genes encoding two high-affinity methionine transporters and five Msrs. This allowed us to demonstrate that, similar to the mammalian intestine, methionine abundance in the arthropod gut is sufficient to support growth of V. cholerae in the absence of bacterial synthesis or dietary supplementation (39). Furthermore, we showed that, when synthesis is blocked by deletion of metR, high-affinity methionine transport but not MetO reduction is essential for colonization of the Drosophila intestine. Our findings suggest that the intestinal concentration of methionine is between 0.05 mM and 0.5 mM. Therefore, while methionine synthesis is required in the host environments of invasive pathogens (40, 41), this may not be the case for pathogens that cause disease from the intestinal lumen.
RESULTS
Methionine synthesis is not essential for virulence or growth in the Drosophila intestine.
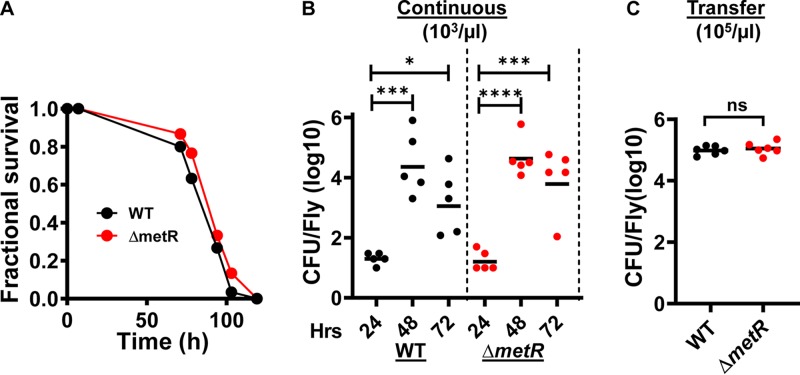
V. cholerae is a methionine auxotroph in the absence of the regulator MetR (39). To test the requirement for methionine synthesis in the arthropod gut, we constructed a strain carrying an in-frame deletion in metR and then fed wild-type V. cholerae as well as the ΔmetR mutant resuspended in Luria-Bertani (LB) broth to the control Drosophila strain Oregon R (42). Flies infected with either wild-type V. cholerae or the ΔmetR mutant died at the same rate (Fig. 1A). To determine whether MetR was essential for colonization of the fly intestine, we resuspended these strains at a density of 1,000 CFU/μl in phosphate-buffered saline (PBS), which is just above the infectious dose (5), and allowed the control Drosophila strain Oregon R to ingest this suspension. We quantified bacterial burden at 24, 48, and 72 h. As shown in Fig. 1B, at 24 h, the mean CFU/fly was only 20. Over the next 24 h, the intestinal load of wild-type V. cholerae increased to approximately 200,000 CFU/fly and then remained constant. The ΔmetR mutant colonized the Drosophila intestine equally rapidly and abundantly. This pattern of growth is similar to what has been observed previously (5). We hypothesize that minimal accumulation of V. cholerae in the fly gut on day 1 results from a bottle neck consisting of the innate immune response of the anterior midgut and the acidity of the middle midgut (43–45). V. cholerae bacteria that survive these compartments and reach the posterior midgut and rectum form biofilms and multiply on the surfaces of these compartments (5).
FIG 1.
Methionine synthesis is not essential for V. cholerae growth in the fly gut. (A) Fractional survival of flies fed wild-type V. cholerae (WT) or a ΔmetR mutant in LB broth. The difference in survival is not significant. (B) Colonization of the fly gut by wild-type V. cholerae (WT) and a ΔmetR mutant during continuous feeding on PBS inoculated with V. cholerae at a density of 103 CFU/μl for 72 h. (C) Colonization of the fly gut by wild-type V. cholerae and a ΔmetR mutant during continuous feeding on PBS inoculated with 105 CFU/μl of V. cholerae for 48 h followed by transfer to sterile PBS for 24 h. In panels B and C, each data point represents the V. cholerae burden of an individual fly. Data was log transformed. In panel B, the statistical significance was determined by applying a one-way ANOVA with Dunnett’s multiple-comparison test to the log-transformed data. In panel C, a Student's t test was applied to the log-transformed data. *, P < 0.05; ***, P < 0.001; ****, P < 0.0001; ns, not significant.
To ensure that bacteria that had accumulated as of day 2 were adherent to the intestine, we incorporated a 24-h washout period on sterile PBS at the end of our experiment. In this experiment, flies were fed V. cholerae at a density of 105 bacteria/μl, which is well above the minimum infectious dose, for 48 h prior to washout. We observed no difference in the ability of wild-type V. cholerae and the ΔmetR mutant to adhere to the fly gut (Fig. 1C). Therefore, we conclude that methionine supplied by the host is adequate to support V. cholerae colonization and growth in the absence of methionine synthesis.
MetT and MetNIQ transport l-methionine.
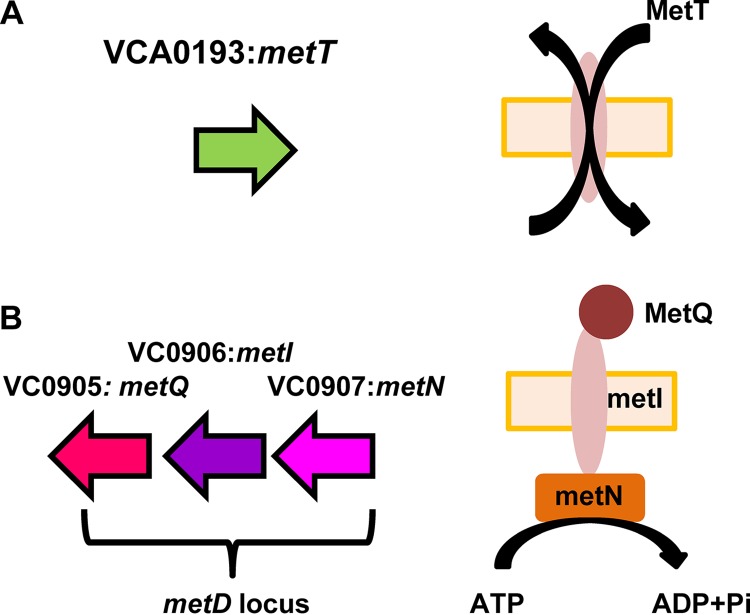
To identify high-affinity transporters of methionine that might be operative in the intestine, we set out to mutagenize all putative methionine transporters. The V. cholerae genome encodes two predicted methionine transport systems, MetNIQ and MetT (Fig. 2). Interestingly, the V. cholerae MetT homolog is more closely related to that of Bacillus subtilis (45% identity, 66% similarity) than to that of Escherichia coli (23% identity, 45% similarity).
FIG 2.
Two V. cholerae loci that encode homologs of bacterial methionine transport systems. (A) MetT is a cation/methionine antiporter. (B) The metD locus encodes an ABC transporter comprised of the periplasmic methionine-binding protein MetQ, the methionine inner membrane permease MetI, and the ATPase MetN. In this study, the metD locus is inactivated by an in-frame deletion in metI.
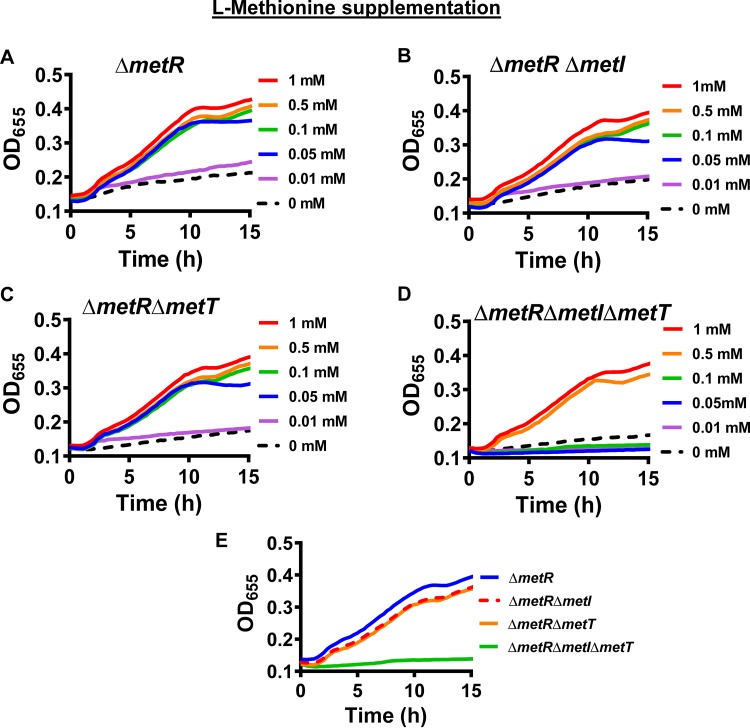
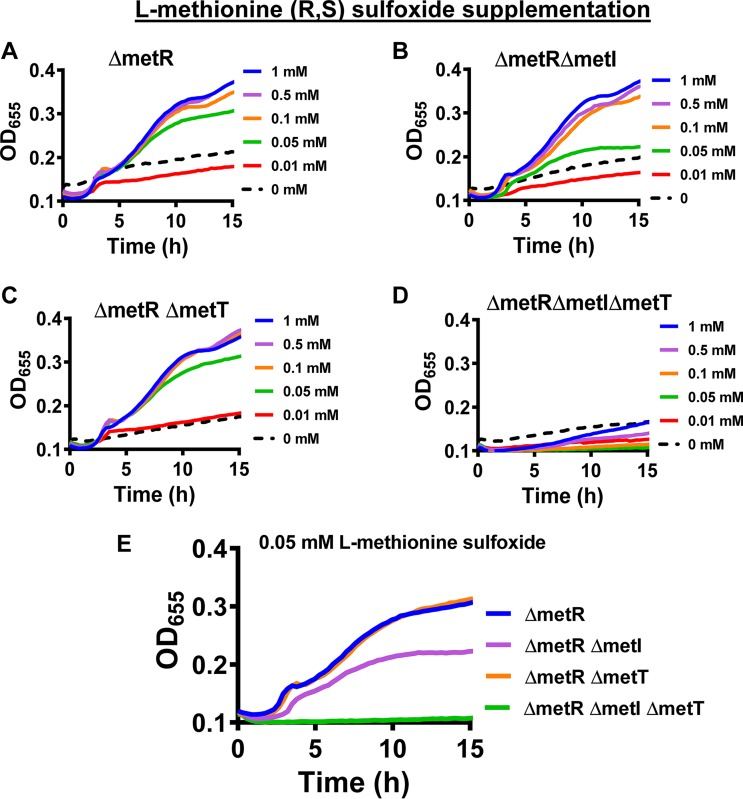
To explore the role of MetNIQ and MetT in V. cholerae methionine uptake, we created in-frame deletions in metI and metT in a ΔmetR mutant background. We then tested the growth of these mutants in a defined medium supplemented with various concentrations of l-methionine. As shown in Fig. 3A, the ΔmetR mutant only grew in defined medium if supplemented with at least 0.05 mM l-methionine. The double mutants ΔmetR ΔmetI and ΔmetR ΔmetT demonstrated an l-methionine dependence very similar to that of the ΔmetR mutant (Fig. 3B and C). However, the ΔmetR ΔmetI ΔmetT triple mutant required 0.5 mM l-methionine for growth (Fig. 3D). Because the ΔmetR ΔmetI and ΔmetR ΔmetT mutants grow equally well with l-methionine supplementation, we conclude that they are both capable of transporting this substrate (Fig. 3E). Furthermore, because growth of the triple mutant is rescued by supplementation with high concentrations of l-methionine, we conclude that the observed growth defect results from the absence of high-affinity methionine transport and that one or more additional low-affinity transporters exists.
FIG 3.
MetI or MetT is required for growth of a V. cholerae ΔmetR mutant at concentrations of l-methionine less than or equal to 0.01 mM. (A to D) Growth of the indicated V. cholerae mutants in defined medium supplemented with l-methionine in the concentrations indicated at the right. (E) A comparison of the growth of indicated mutants in defined medium supplemented with 0.1 mM l-methionine. Growth curves represent the mean of technical duplicates and are representative of three biological experiments.
d-Methionine has opposing effects on V. cholerae growth.
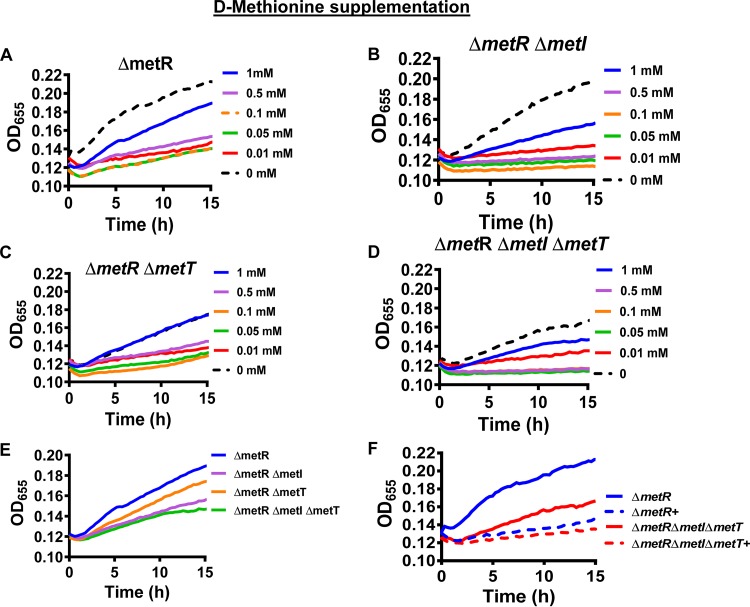
Periplasmic d-methionine has previously been reported to repress growth of V. cholerae as the result of incorporation into peptidoglycan (10). We hypothesized that d-methionine might also be transported into the cytoplasm by MetI and MetT and used to support growth. To assess the periplasmic and cytoplasmic roles of d-methionine, we measured the growth of ΔmetR, ΔmetR ΔmetI, ΔmetR ΔmetT, and ΔmetR ΔmetI ΔmetT mutants over time in defined medium supplemented with this amino acid (Fig. 4). As shown in Fig. 4A, concentrations of 0.01 to 1 mM d-methionine decreased growth of the ΔmetR mutant in defined medium. However, growth did not decrease steadily as the concentration of d-methionine was increased. Instead, the growth suppression was comparable for concentrations of 0.01 to 0.5 mM but less at the higher concentration of 1 mM d-methionine.
FIG 4.
d-methionine decreases growth of a V. cholerae methionine auxotroph (ΔmetR) in a transport-independent fashion. (A to D) Growth of the indicated mutants in defined medium supplemented with the indicated concentrations of d-methionine. (E) Growth of the indicated mutants in defined medium supplemented with 1 mM d-methionine demonstrating that transport by MetNIQ augments growth. (F) Growth of the indicated mutants in defined medium alone or supplemented with 0.01 mM d-methionine (+) illustrating transport-independent growth suppression by d-methionine. Growth curves represent the mean of technical duplicates and are representative of three biological experiments.
We then tested the impact on growth of d-methionine transport through MetI and MetT. In the ΔmetR background, growth in unsupplemented defined medium was unchanged by deletion of metI (Fig. 4A and B) but decreased by deletion of metT (Fig. 4A and C), suggesting that MetT may play a minor role in transport of another amino acid. Furthermore, as compared with the parental ΔmetR mutant, deletion of metI decreased growth in defined medium supplemented with 0.05 mM to 1 mM d-methionine (Fig. 4B to E). Growth inhibition by 0.01 mM d-methionine was observed both in the presence and absence of MetT and MetI (Fig. 4F). These observations are consistent with a model in which d-methionine has opposing effects on growth, inhibiting growth from the periplasm and increasing growth after MetI-mediated transport into the cytoplasm.
MetI and MetT transport methionine sulfoxide.
In oxidizing environments, exogenous methionine may be present principally in the form of MetO. This oxidized form of methionine must be transported and reduced prior to use. E. coli MetNIQ has been shown to transport MetO as well as methionine (26). To determine whether V. cholerae MetI and MetT are involved in l-MetO transport, we measured growth of the ΔmetR mutant as well as derivative transport mutants in the presence and absence of a variety of concentrations of l-MetO. As shown in Fig. 5A, the ΔmetR mutant was able to grow on concentrations of l-MetO as low as 0.05 mM. The ΔmetR ΔmetI mutant could only grow on concentrations of 0.1 mM or higher, suggesting that, as previously observed for E. coli, metT transports l-MetO (Fig. 5B). Deletion of metT reduced V. cholerae growth only when MetI was absent (Fig. 5C and D). This suggests that, while MetI plays a more important role in transport of l-MetO at low substrate concentrations, MetT also transports l-MetO (Fig. 5E).
FIG 5.
MetI and MetT are essential for growth of a V. cholerae methionine auxotroph on l-methionine (R,S) sulfoxide. (A to D) Growth of the indicated mutants in defined medium supplemented with the indicated concentrations of l-methionine sulfoxide. (E) Growth of the indicated mutants in defined medium supplemented with 0.05 mM l-methionine sulfoxide to illustrate the relative roles of MetI and MetT in transport. Growth curves represent the mean of technical duplicates and are representative of three biological experiments.
V. cholerae encodes five methionine sulfoxide reductases.
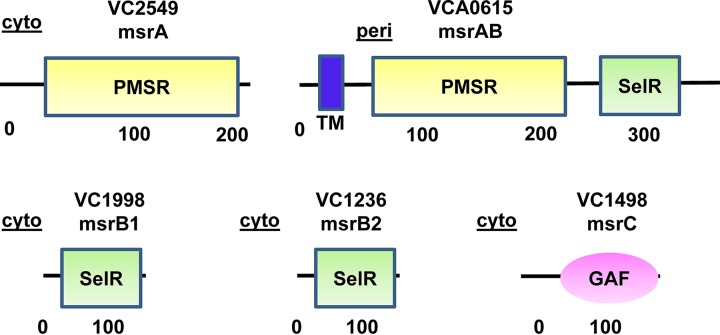
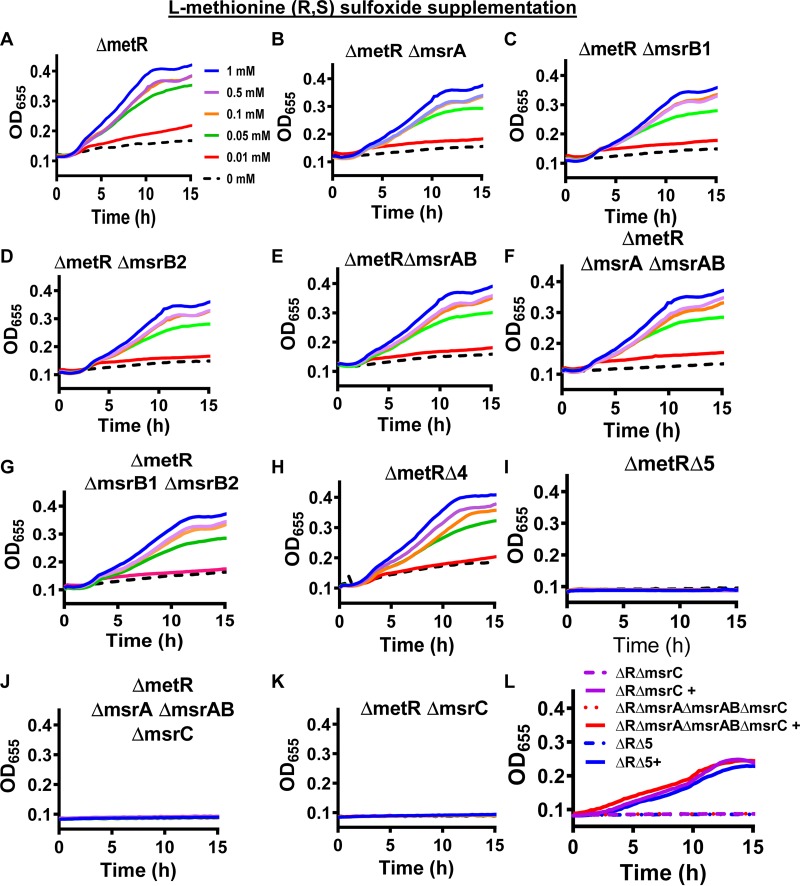
A previous publication identified the presence of one gene encoding a homolog of MsrA (VC2549), two genes encoding homologs of MsrB (MsrB1, VC1998; MsrB2, VC1236), and one gene encoding a fusion of MsrA and MsrB (MsrAB, VCA0615) (Fig. 6) (12). Upon further in silico research, we identified a homolog of MsrC (VC1498) in the V. cholerae proteome (Fig. 6). Only the MsrAB homolog included a signal sequence and transmembrane domain, suggesting that it is anchored in the inner membrane and functions in the periplasm. We discovered that in-frame deletions in the genes encoding the MsrAs and MsrBs either singly or in combination did not alter growth on l-Met-R,S-O (Fig. 7A to H). However, when msrC was deleted in any of these multiple deletion mutant backgrounds, V. cholerae was no longer able to grow on l-Met-R,S-O (Fig. 7I to K). Importantly, all mutants that were unable to grow on l-Met-R,S-O could be rescued by methionine supplementation (Fig. 7L), demonstrating that the growth defect is due to an inability to utilize l-Met-R,S-O. MsrA homologs are thought to be active on free and protein-associated Met-S-O, while MsrC homologs are active on free Met-R-O (34). Our results demonstrate that, of the five V. cholerae Msr homologs, only MsrC reduces l-Met-O. Because we are supplementing our medium with the diastereomeric mixture l-Met-R,S-O, we cannot determine which diastereomer is reduced by MsrC.
FIG 6.
Five methionine sulfoxide reductases are encoded in the V. cholerae genome. In silico prediction of the domains and subcellular locations based on transmembrane domains and signal sequence predictions of the five methionine sulfoxide reductases of V. cholerae. MsrA, which has a peptide methionine sulfoxide reductase (PMSR) domain, is predicted to be active on free and protein-associated l-Met-S-O. MsrB has a SelR domain that coordinates Zn2+ via a CXXC motif and is predicted to be active on protein-associated l-Met-R-O. MsrC has a GAF domain that, in some proteins, binds cyclic nucleotides and is predicted to be active on free l-Met-R-O. Peri, periplasm; cyto, cytoplasm.
FIG 7.
Methionine sulfoxide reductase C is essential for growth of a V. cholerae ΔmetR mutant in defined medium supplemented with l-methionine-R,S-sulfoxide but not methionine. (A to K) Growth of the indicated V. cholerae mutants in defined medium supplemented with the indicated amount of l-methionine-R,S-sulfoxide. ΔmetR ΔmsrA ΔmsrB1 ΔmsrB2 ΔmsrAB, ΔmetR Δ4; ΔmetR ΔmsrA ΔmsrB1 ΔmsrB2 ΔmsrAB ΔmsrC, ΔmetR Δ5. (L) Growth of the indicated mutants in the ΔmetR background (ΔR) in defined medium alone or supplemented with 0.1 mM methionine (+). Growth curves represent the mean of technical duplicates and are representative of three biological experiments.
High-affinity methionine transport but not methionine sulfoxide reduction is essential for V. cholerae replication in the Drosophila intestine.
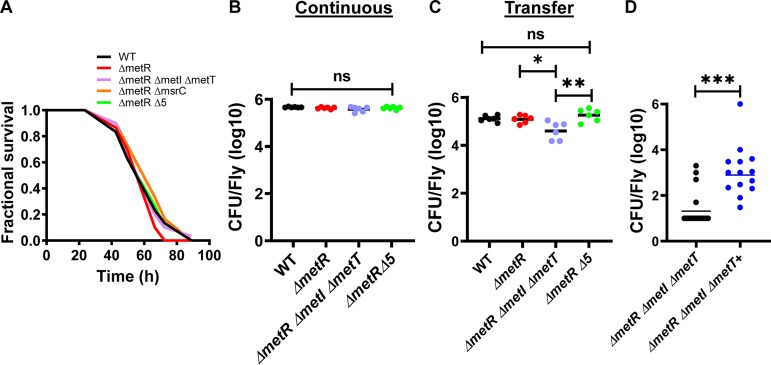
When V. cholerae is delivered in LB broth, its ingestion suppresses intestinal stem cell divisions and is lethal to the model arthropod host Drosophila melanogaster (42). Increased MetO concentrations in the intestinal lumen decrease host mortality, and methionine increases intestinal stem cell divisions, which has also been correlated with prolonged host survival (6, 46). We hypothesized that, if V. cholerae methionine uptake, MetO uptake, or MetO reduction significantly altered the concentrations of these amino acids in the Drosophila intestinal lumen, mutation of V. cholerae genes encoding transport systems and/or Msrs should decrease virulence. We, therefore, compared fly survival after infection with wild-type V. cholerae, a ΔmetR mutant, a ΔmetR ΔmetI ΔmetT mutant, a ΔmetR ΔmsrC mutant, and a V. cholerae ∆metR mutant also devoid of all Msrs (∆5) in LB broth. As shown in Fig. 8A, none of these mutations resulted in a virulence defect. This suggests that V. cholerae methionine uptake, MetO uptake, and MetO reduction do not significantly impact the concentrations of these amino acids in the Drosophila intestinal lumen. We then questioned whether methionine and MetO uptake and MetO reduction are required for V. cholerae colonization in the Drosophila intestine. To test this, we compared colonization by the ΔmetR ΔmetI ΔmetT and the Δ5 mutant with that by wild-type V. cholerae and the ΔmetR mutant both during continuous feeding on 105 V. cholerae/μl in PBS and after a washout period. As shown in Fig. 8B, there was no difference in colonization between wild-type V. cholerae and these mutants after continuous feeding. In contrast, after a washout period, the ΔmetR ΔmetI ΔmetT mutant but not the Δ5 mutant showed a colonization defect (Fig. 8C). To demonstrate that this colonization defect is the result of an inability to acquire methionine through the high-affinity methionine transporters MetI and MetT, we provided flies with 103 V. cholerae/μl in PBS alone or supplemented with 1 mM methionine and measured pathogen burden after 72 h. While few ΔmetR ΔmetI ΔmetT mutants were detected in the Drosophila intestine under these conditions, provision of 1 mM methionine to this mutant significantly rescued intestinal numbers (Fig. 8D). We conclude that high-affinity methionine transport is essential for V. cholerae growth in the Drosophila intestine in the absence of synthesis, but reduction of MetO is not. Furthermore, because the high-affinity methionine transporters MetI and MetT allow for V. cholerae growth in the range of 0.05 to 0.5 mM methionine, we conclude that the concentration of methionine in the fly gut is between 0.05 and 0.5 mM.
FIG 8.
Methionine concentrations in the Drosophila intestine support colonization and growth of a V. cholerae methionine auxotroph capable of high affinity methionine transport. (A) Survival of Drosophila during ingestion of wild-type V. cholerae (WT), ΔmetR, ΔmetR ΔmetI ΔmetT, ΔmetR ΔmsrC, and ΔmetR ΔmsrA ΔmsrB1 ΔmsrB2 ΔmsrAB ΔmsrC (ΔmetR Δ5) mutants administered in LB broth. Log-rank analysis was used to determine that these survival curves are not significantly different. (B) Colonization of the fly gut by wild-type V. cholerae and the indicated mutants during continuous feeding for 72 h on PBS inoculated with 105 CFU V. cholerae/μl. (C) Colonization of the fly gut by wild-type V. cholerae and the indicated mutants during continuous feeding for 48 h on PBS inoculated with 105 CFU V. cholerae/μl followed by transfer to sterile PBS for 24 h. (D) Colonization of the fly gut by the indicated V. cholerae mutants during continuous feeding at a concentration of 103 CFU/μl in PBS alone or supplemented with 1 mM methionine (+) for 72 h. A one-way ANOVA was used to calculate significance in panels B and C. A Mann-Whitney test was used in panel D. ***, P < 0.001; *, P < 0.05; ns, not significant. Each data point represents colonization of an individual fly.
DISCUSSION
The amino acid methionine is critical for many cellular functions including translation, transcription, and posttranscriptional methylation reactions. To understand how V. cholerae acquires methionine from its environment, we sought to identify methionine transporters and methionine sulfoxide reductases. In this work, we identify two transporters, MetI (VC0906) and MetT (VCA0193), which transport l-methionine, d-methionine, and MetO. Furthermore, we have identified five methionine sulfoxide reductases (VC1236, VC1498, VC1998, VC2549, and VCA0615). Only one of these, MsrC, is essential for reduction of free MetO to methionine. The identification of these genes allows us to establish the importance of high-affinity methionine uptake but not methionine sulfoxide reduction for colonization and growth in the arthropod intestine.
Methionine is essential for V. cholerae viability regardless of whether the environment is an estuary or the intestinal lumen of a mammal or an arthropod. V. cholerae is capable of synthesizing methionine when necessary, but uptake is favored when methionine is available in the environment (39). Because synthesis is dispensable for survival in the infant mouse intestine, it is likely that this intestinal environment is not methionine limited (39). Similarly, we found that methionine synthesis was not essential for V. cholerae replication in the arthropod intestine, but that, in the absence of synthesis, high-affinity transporters were. This allowed us to place a lower limit of 0.05 mM and an upper limit of 0.5 mM on the concentration of l-methionine in the intestine. In both the infant mouse and arthropod infection models, V. cholerae is restricted to the intestinal lumen, where observations suggest that methionine is not limiting. However, methionine is limiting in some host environments. For instance, the concentration of methionine in human plasma is estimated to be 3 to 30 μM (41, 47, 48), and methionine biosynthesis is essential for intracellular replication of Mycobacterium tuberculosis in human macrophages and for pulmonary infection and dissemination in an immunocompromised mouse model (41). Salmonella enterica serovar Typhimurium infection of mice, which involves intracellular replication and dissemination from the intestine to the spleen and liver, is also dependent on methionine biosynthesis and high-affinity methionine acquisition (40, 49). For invasive pathogens, such as Staphylococcus aureus, Pseudomonas aeruginosa, group B Streptococcus, and E. coli, proliferation in plasma is dependent on high-affinity methionine uptake systems (21, 47). For this reason, bacterial methionine synthesis and high-affinity uptake have been suggested as antibacterial targets.
Methionine is easily oxidized to MetO. Free MetO must be reduced to methionine prior to utilization, and reversible oxidation of methionine in proteins modulates their function. Msrs are, therefore, absolutely required for bacterial growth on MetO and for survival of oxidative stress, and the impact of Msr deletion on pathogen virulence reflects the level of oxidative stress in the host niche. Here, we show that V. cholerae acquires methionine and MetO through the same transporters. Furthermore, we show an unusual amount of redundancy in V. cholerae Msrs, suggesting that it is adapted to oxidizing environments. Msrs do not appear to be necessary for utilization of luminal methionine by a V. cholerae ΔmetR mutant in the Drosophila intestine, suggesting that this is not a highly oxidizing environment. Furthermore, while we previously showed that luminal methionine and MetO attenuate V. cholerae virulence in this environment, deletion of V. cholerae MetI and MetT or all Msrs had no effect on virulence (6). This suggests that V. cholerae methionine uptake and MetO reduction do not significantly impact concentrations of these amino acids in the Drosophila gut under the conditions of our infection experiments. Survival of other pathogens in host gastrointestinal environment is dependent on Msrs. For instance, the genome of Helicobacter pylori encodes only one MsrAB protein found principally in the membrane. Upon deletion of MsrAB, H. pylori is no longer able to colonize the murine gastric mucosa (50). A Lactobacillus reuteri ΔmsrB mutant displays a defect in competition with wild-type strains in the mouse intestine (51). Msrs may also be important in systemic bacterial infections. S. aureus produces three MsrAs and one MsrB (18, 52). However, only one of these, designated MsrA1, plays a role in survival in a mouse model of systemic infection. Defects in virulence upon deletion of Msrs have also been recorded for Enterococcus faecalis and Francisella tularensis (15, 53).
The amino acid methionine is an integral component of several essential bacterial synthetic, metabolic, and regulatory pathways. In this work, we have fully defined the pathways used by V. cholerae to acquire methionine. These pathways are characterized by remarkable redundancy. While methionine is a prerequisite for many essential bacterial processes, the redundant pathways for methionine synthesis and acquisition along with the availability of methionine or methionine sulfoxide in the mammalian intestine make this a poor target for the development of therapeutics against intestinal pathogens and an unlikely target for treatment or prevention of cholera.
MATERIALS AND METHODS
Bacterial strains and media.
The bacterial strains used in these experiments are listed in Table 1. E. coli strains were grown in Luria-Bertani (LB) broth supplemented with ampicillin (100 μg/ml) at 37°C. Vibrio cholerae strains were routinely cultured in LB broth (Difco) or on LB agar supplemented with streptomycin (100 μg/ml) at 27°C. Growth was assessed in defined medium. This previously described defined medium was prepared with the omission of methionine and supplementation with 0.5% glucose (54) (referred to as minimal medium in the previous publication). In addition, the indicated concentrations of l-methionine (M5308; Sigma), d-methionine (M9375; Sigma), or l-methionine-R,S-sulfoxide (M1126; Sigma) were added.
TABLE 1.
Strains, plasmids, and oligonucleotides
| Strain, plasmid, or oligonucleotide | Genotype, description, or sequence | Reference or source |
|---|---|---|
| Bacterial strain | ||
| PW724 | V. cholerae MO10; Smr | 60 |
| PW1864 | MO10 ΔmetI; Smr | This study |
| PW1918 | MO10 ΔmetT; Smr | This study |
| PW1923 | MO10 ΔmetI ΔmetT; Smr | This study |
| PW1865 | MO10 ΔmsrAB; Smr | This study |
| PW1866 | MO10 ΔmsrA; Smr | This study |
| PW1899 | MO10 ΔmsrB1; Smr | This study |
| PW1919 | MO10 ΔmsrB2; Smr | This study |
| PW1976 | MO10 ΔmsrC; Smr | This study |
| PW1874 | MO10 ΔmsrAB ΔmsrA; Smr | This study |
| PW1924 | MO10 ΔmsrB1 ΔmsrB2; Smr | This study |
| PW1950 | MO10 ΔmsrAB ΔmsrA ΔmsrB1 ΔmsrB2; Smr | This study |
| PW1977 | MO10 ΔmsrAB ΔmsrA ΔmsrB1 ΔmsrB2 ΔmsrC; Smr | This study |
| PW1822 | MO10 ΔmetR; Smr | This study |
| PW1867 | MO10 ΔmetR ΔmetI; Smr | This study |
| PW1944 | MO10 ΔmetR ΔmetT; Smr | This study |
| PW1945 | MO10 ΔmetR ΔmetI ΔmetT; Smr | This study |
| PW1868 | MO10 ΔmetR ΔmsrAB; Smr | This study |
| PW1986 | MO10 ΔmetR ΔmsrA; Smr | This study |
| PW1952 | MO10 ΔmetR ΔmsrB1; Smr | This study |
| PW2210 | MO10 ΔmetR ΔmsrC; Smr | This study |
| PW1953 | MO10 ΔmetR ΔmsrB2; Smr | This study |
| PW1895 | MO10 ΔmetR ΔmsrAB ΔmsrA; Smr | This study |
| PW2211 | MO10 ΔmetR ΔmsrAB ΔmsrA ΔmsrC; Smr | This study |
| PW1946 | MO10 ΔmetR ΔmsrB1 ΔmsrB2; Smr | This study |
| PW1955 | MO10 ΔmetR ΔmsrAB ΔmsrA ΔmsrB1 ΔmsrB2; Smr | This study |
| PW1978 | MO10 ΔmetR ΔmsrAB ΔmsrA ΔmsrB1 ΔmsrB2 ΔmsrC; Smr | This study |
| SM10λpir | E. coli thi thr leu tonA lacY supE recA::RP4-2-Tc::Mu (λpirR6K) | 61 |
| Plasmid | ||
| pWM91 | oriR6K mobRP4 lacI pTac tnp mini-Tn10; Kmr Apr | 62 |
| pWM91::ΔmetI | pWM91 carrying an unmarked, in-frame deletion in VC0906 | This study |
| pWM91::ΔmetT | pWM91 carrying an unmarked, in-frame deletion in VCA0193 | This study |
| pWM91::ΔmsrAB | pWM91 carrying an unmarked, in-frame deletion in VCA0615 | This study |
| pWM91::ΔmsrA | pWM91 carrying an unmarked, in-frame deletion in VC2549 | This study |
| pWM91::ΔmsrB1 | pWM91 carrying an unmarked, in-frame deletion in VC1998 | This study |
| pWM91::ΔmsrB2 | pWM91 carrying an unmarked, in-frame deletion in VC1236 | This study |
| pWM91::ΔmsrC | pWM91 carrying an unmarked, in-frame deletion in VC1498 | This study |
| pWM91::ΔmetR | pWM91 carrying an unmarked, in-frame deletion in VC1706 | This study |
| Oligonucleotide | ||
| ΔVC0906 | ||
| metI#1 | 5'-GATGACGCAAATTGCTCGCA | This study |
| metI#2 | 5'-TAACGAGCGGCCGCACATAACCCAGCACCTCTACT | This study |
| metI#3 | 5'-TGCGGCCGCTCGTTATAAGCAAAACAAAAGCCTTT | This study |
| metI#4 | 5'-ATGCTGCGTTTGGTGTTACG | This study |
| ΔVCA0193 | ||
| metT#1 | 5'-CCTGCAGCCCGGGGGATCCACATCCCCACCGTATGTTCG | This study |
| metT#2 | 5'-CATGATTTGATATTTGCATGTGAATTAGGCGTTGCGATTG | This study |
| metT#3 | 5'-CAATCGCAACGCCTAATTCACATGCAAATATCAAATCATG | This study |
| metT#4 | 5'-ATTGACGGCTCTAGAACTAGGCTCTCTGCGGCGTTTGTA | This study |
| ΔVCA0615 | ||
| msrAB#1 | 5'-AGTGATAAAGCCGCACAGCA | This study |
| msrAB#2 | 5'-TAACGAGCGGCCGCACAAACCGGATCAGAAATTTC | This study |
| msrAB#3 | 5'-TGCGGCCGCTCGTTATAATTAGCTATACCAACCCT | This study |
| msrAB#4 | 5'-GTCGGATGATGTGGCAGTCA | This study |
| ΔVC2549 | ||
| msrA#1 | 5'-AATGCAACCCTGCGTCTGTA | This study |
| msrA#2 | 5'-TAACGAGCGGCCGCACATGATGTTTCCTTACTGAA | This study |
| msrA#3 | 5'-TGCGGCCGCTCGTTATAAAGACATCTCAATGAGGG | This study |
| msrA#4 | 5'-CCATCAGTGGCGCATTCATC | This study |
| ΔVC1998 | ||
| msrB1#1 | 5'-CCTGCAGCCCGGGGGATCCAAGCACGGAAAACGAAACGAC | This study |
| msrB1#2 | 5'-CAAGTTTTTCACAAAAAATGTGAATATTGCCAGACGACTT | This study |
| msrB1#3 | 5'-AAGTCGTCTGGCAATATTCACATTTTTTGTGAAAAACTTG | This study |
| msrB1#4 | 5'-ATTGACGGCTCTAGAACTAGAGCAATGGTCAACCCGTCAA | This study |
| ΔVC1236 | ||
| msrB2#1 | 5'-CCTGCAGCCCGGGGGATCCACCATTGCCGACGATAGCAAC | This study |
| msrB2#2 | 5'-CAACAAGAGGAGAGAGCAATGTAAATATACCCTTCCTACTTG | This study |
| msrB2#3 | 5'-CAAGTAGGAAGGGTATATTTACATTGCTCTCTCCTCTTGTTG | This study |
| msrB2#4 | 5'-ATTGACGGCTCTAGAACTAGGGCACAGTCTACTCGCTCTG | This study |
| ΔVC1498 | ||
| msrC#1 | 5'-CCTGCAGCCCGGGGGATCCACAATGGCGGTACCAATCACA | This study |
| msrC#2 | 5'-CACAGGCAAAGGGCAATTTACACTATTAGCCGCTCTTGGC | This study |
| msrC#3 | 5'-GCCAAGAGCGGCTAATAGTGTAAATTGCCCTTTGCCTGTG | This study |
| msrC#4 | 5'-ATTGACGGCTCTAGAACTAGTTCGCCTTTGGCTTGTTCG | This study |
| ΔVC1706 | ||
| metR#1 | 5'-CCTCAGCACATCGAAAGATG | This study |
| metR#2 | 5'-TAACGAGCGGCCGCACATGAACTCTCCTCACTTATC | This study |
| metR#3 | 5'-TGCGGCCGCTCGTTATAGGCATCACTACCGAGCA | This study |
| metR#4 | 5'-GGAGTGATGTGATGGATCTG | This study |
V. cholerae strain construction.
V. cholerae mutants used in this work were engineered by construction of a suicide plasmid carrying a mutant allele. This mutant allele was inserted into the chromosome by double homologous recombination as previously described (55). Briefly, approximately 500-bp fragments upstream and downstream of the target gene and including the start and stop codons, respectively, were amplified by the PCR using the primers listed in Table 1. For ΔmetR, ΔmetI, ΔmsrAB, and ΔmsrA mutant constructions, the overlapping fragments included 15-bp complementary sequences at their 3′ and 5′ ends. These were joined using the splicing by overlap extension (SOE) technique, resulting in the construction of a fragment with an in-frame deletion in the gene of interest (56, 57). The fragment containing the deletion was ligated into the suicide plasmid pWM91 to create the plasmids listed in Table 1. For ΔmetT, ΔmsrB1, ΔmsrB2, and ΔmsrC, inner primers included a complementary 20-bp sequence at their 3′ and 5′ ends to permit Gibson assembly. These two fragments were ligated with the pWM91 suicide vector by Gibson assembly as per the manufacturer’s instructions (New England Biolabs) to create the plasmids listed in Table 1 (58). These plasmids were used to create gene deletions in the relevant strains by double homologous recombination and sucrose selection as previously described (59).
Drosophila virulence and colonization assays.
For virulence assays, ten 5- to 10-day-old Oregon R male flies were placed in each of three fly vials. These vials were prepared with a cellulose acetate plug infiltrated with 3 ml of LB broth inoculated with a 10-fold dilution of an overnight culture of the indicated V. cholerae strain. Mortality was assessed at least once each day.
For colonization assays, V. cholerae strains were prepared by culturing overnight in LB broth, washing in sterile PBS supplemented with 100 μg/ml streptomycin, and resuspending in an equal volume of PBS. The bacterial resuspension was diluted in a 1:10 (105/μl) or 1:1,000 (103/μl) ratio in PBS, and 3 ml of the diluted culture was added to a sterile cellulose acetate plug placed at the bottom of a standard fly vial. Ten male Oregon R flies aged between 5 and 10 days were placed in this vial inoculated with V. cholerae and either allowed to ingest this suspension for 72 h or transferred to sterile PBS supplemented with streptomycin after 48 h and maintained in these vials for 24 h. At this point, the flies in each vial were anesthetized with CO2, and six live flies, chosen at random, were placed singly in Eppendorf tubes on ice. After addition of 200 μl of sterile PBS, flies were homogenized. The homogenate was allowed to settle briefly, and the supernatant was serially diluted in PBS. Dilutions were plated on LB agar plates supplemented with 100 μg/ml streptomycin, and CFU were counted after overnight incubation at 27°C. The V. cholerae burden was measured for at least six infected flies for each test condition, and each experiment was repeated with similar results.
Growth curves.
V. cholerae wild-type bacteria and mutants were grown overnight in LB at 27°C. One milliliter of culture was pelleted by centrifugation (2,300 × g for 6 min), washed 3 times in PBS, and then diluted to an optical density at 655 nm (OD655) of 1 in PBS. The resulting suspension was diluted 1:100 in the indicated growth medium, and 100 μl was aliquoted into each of three wells of a 96-well plate to represent biological triplicates. The plates were incubated in a microplate reader (Infinite 200; Tecan), and the OD655 was measured every 15 min after agitation.
Statistical analyses.
CFU were measured for at least 5 flies per condition. Each fly was considered to be an independent biological replicate. Experiments were repeated once. Horizontal lines shown represent the geometric mean, and log-transformed data were used in calculations of significance. Statistical significance was calculated using either a Student's t test or a one-way analysis of variance (ANOVA) followed by a Tukey’s or Dunnett’s multiple-comparison test as appropriate. Technical duplicates were performed for growth curves, and experiments were repeated three times. Graphs of growth curves represent the mean of technical duplicate measurements and are representative of the three experiments.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants R21 AI109436 and R01 AI112652 (P.I.W.).
REFERENCES
- 1.Faruque SM, Nair GB. 2002. Molecular ecology of toxigenic Vibrio cholerae. Microbiol Immunol 46:59–66. doi: 10.1111/j.1348-0421.2002.tb02659.x. [DOI] [PubMed] [Google Scholar]
- 2.Shukla BN, Singh DV, Sanyal SC. 1995. Attachment of non-culturable toxigenic Vibrio cholerae O1 and non-O1 and Aeromonas spp. to the aquatic arthropod Gerris spinolae and plants in the River Ganga, Varanasi. FEMS Immunol Med Microbiol 12:113–120. doi: 10.1111/j.1574-695X.1995.tb00182.x. [DOI] [PubMed] [Google Scholar]
- 3.Huq A, Huq SA, Grimes DJ, O’Brien M, Chu KH, Capuzzo JM, Colwell RR. 1986. Colonization of the gut of the blue crab (Callinectes sapidus) by Vibrio cholerae. Appl Environ Microbiol 52:586–588. doi: 10.1128/AEM.52.3.586-588.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Magny GC, Mozumder PK, Grim CJ, Hasan NA, Naser MN, Alam M, Sack RB, Huq A, Colwell RR. 2011. Role of zooplankton diversity in Vibrio cholerae population dynamics and in the incidence of cholera in the Bangladesh Sundarbans. Appl Environ Microbiol 77:6125–6132. doi: 10.1128/AEM.01472-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Purdy AE, Watnick PI. 2011. Spatially selective colonization of the arthropod intestine through activation of Vibrio cholerae biofilm formation. Proc Natl Acad Sci U S A 108:19737–19742. doi: 10.1073/pnas.1111530108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vanhove AS, Hang S, Vijayakumar V, Wong AC, Asara JM, Watnick PI. 2017. Vibrio cholerae ensures function of host proteins required for virulence through consumption of luminal methionine sulfoxide. PLoS Pathog 13:e1006428. doi: 10.1371/journal.ppat.1006428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hondorp ER, Matthews RG. 2006. Methionine. EcoSal Plus 2006 doi: 10.1128/ecosalplus.3.6.1.7. [DOI] [PubMed] [Google Scholar]
- 8.Cava F, Lam H, de Pedro MA, Waldor MK. 2011. Emerging knowledge of regulatory roles of D-amino acids in bacteria. Cell Mol Life Sci 68:817–831. doi: 10.1007/s00018-010-0571-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caparros M, Pisabarro AG, de Pedro MA. 1992. Effect of D-amino acids on structure and synthesis of peptidoglycan in Escherichia coli. J Bacteriol 174:5549–5559. doi: 10.1128/jb.174.17.5549-5559.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lam H, Oh DC, Cava F, Takacs CN, Clardy J, de Pedro MA, Waldor MK. 2009. D-amino acids govern stationary phase cell wall remodeling in bacteria. Science 325:1552–1555. doi: 10.1126/science.1178123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moskovitz J. 2005. Methionine sulfoxide reductases: ubiquitous enzymes involved in antioxidant defense, protein regulation, and prevention of aging-associated diseases. Biochim Biophys Acta 1703:213–219. doi: 10.1016/j.bbapap.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Ezraty B, Aussel L, Barras F. 2005. Methionine sulfoxide reductases in prokaryotes. Biochim Biophys Acta 1703:221–229. doi: 10.1016/j.bbapap.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 13.Cooper S. 1966. Utilization of D-methionine by Escherichia coli. J Bacteriol 92:328–332. doi: 10.1128/JB.92.2.328-332.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drazic A, Winter J. 2014. The physiological role of reversible methionine oxidation. Biochim Biophys Acta 1844:1367–1382. doi: 10.1016/j.bbapap.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Saha SS, Hashino M, Suzuki J, Uda A, Watanabe K, Shimizu T, Watarai M. 2017. Contribution of methionine sulfoxide reductase B (MsrB) to Francisella tularensis infection in mice. FEMS Microbiol Lett 364:fnw260. doi: 10.1093/femsle/fnw260. [DOI] [PubMed] [Google Scholar]
- 16.Trivedi RN, Agarwal P, Kumawat M, Pesingi PK, Gupta VK, Goswami TK, Mahawar M. 2015. Methionine sulfoxide reductase A (MsrA) contributes to Salmonella typhimurium survival against oxidative attack of neutrophils. Immunobiology 220:1322–1327. doi: 10.1016/j.imbio.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 17.Hassouni ME, Chambost JP, Expert D, Van Gijsegem F, Barras F. 1999. The minimal gene set member msrA, encoding peptide methionine sulfoxide reductase, is a virulence determinant of the plant pathogen Erwinia chrysanthemi. Proc Natl Acad Sci U S A 96:887–892. doi: 10.1073/pnas.96.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh VK, Vaish M, Johansson TR, Baum KR, Ring RP, Singh S, Shukla SK, Moskovitz J. 2015. Significance of four methionine sulfoxide reductases in Staphylococcus aureus. PLoS One 10:e0117594. doi: 10.1371/journal.pone.0117594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Denkel LA, Horst SA, Rouf SF, Kitowski V, Bohm OM, Rhen M, Jager T, Bange FC. 2011. Methionine sulfoxide reductases are essential for virulence of Salmonella Typhimurium. PLoS One 6:e26974. doi: 10.1371/journal.pone.0026974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh VK, Singh K, Baum K. 2018. The role of methionine sulfoxide reductases in oxidative stress tolerance and virulence of Staphylococcus aureus and other bacteria. Antioxidants (Basel) 7:128. doi: 10.3390/antiox7100128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shelver D, Rajagopal L, Harris TO, Rubens CE. 2003. MtaR, a regulator of methionine transport, is critical for survival of group B Streptococcus in vivo. J Bacteriol 185:6592–6599. doi: 10.1128/jb.185.22.6592-6599.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Banerjee RV, Johnston NL, Sobeski JK, Datta P, Matthews RG. 1989. Cloning and sequence analysis of the Escherichia coli metH gene encoding cobalamin-dependent methionine synthase and isolation of a tryptic fragment containing the cobalamin-binding domain. J Biol Chem 264:13888–13895. [PubMed] [Google Scholar]
- 23.Maxon ME, Redfield B, Cai XY, Shoeman R, Fujita K, Fisher W, Stauffer G, Weissbach H, Brot N. 1989. Regulation of methionine synthesis in Escherichia coli: effect of the MetR protein on the expression of the metE and metR genes. Proc Natl Acad Sci U S A 86:85–89. doi: 10.1073/pnas.86.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Urbanowski ML, Stauffer LT, Plamann LS, Stauffer GV. 1987. A new methionine locus, metR, that encodes a trans-acting protein required for activation of metE and metH in Escherichia coli and Salmonella Typhimurium. J Bacteriol 169:1391–1397. doi: 10.1128/jb.169.4.1391-1397.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merlin C, Gardiner G, Durand S, Masters M. 2002. The Escherichia coli metD locus encodes an ABC transporter which includes Abc (MetN), YaeE (MetI), and YaeC (MetQ). J Bacteriol 184:5513–5517. doi: 10.1128/jb.184.19.5513-5517.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kadner RJ. 1977. Transport and utilization of D-methionine and other methionine sources in Escherichia coli. J Bacteriol 129:207–216. doi: 10.1128/JB.129.1.207-216.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Z, Feige JN, Chang AB, Anderson IJ, Brodianski VM, Vitreschak AG, Gelfand MS, Saier MH Jr. 2003. A transporter of Escherichia coli specific for L- and D-methionine is the prototype for a new family within the ABC superfamily. Arch Microbiol 180:88–100. doi: 10.1007/s00203-003-0561-4. [DOI] [PubMed] [Google Scholar]
- 28.Rodionov DA, Vitreschak AG, Mironov AA, Gelfand MS. 2004. Comparative genomics of the methionine metabolism in Gram-positive bacteria: a variety of regulatory systems. Nucleic Acids Res 32:3340–3353. doi: 10.1093/nar/gkh659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grimaud R, Ezraty B, Mitchell JK, Lafitte D, Briand C, Derrick PJ, Barras F. 2001. Repair of oxidized proteins: identification of a new methionine sulfoxide reductase. J Biol Chem 276:48915–48920. doi: 10.1074/jbc.M105509200. [DOI] [PubMed] [Google Scholar]
- 30.Rahman MA, Nelson H, Weissbach H, Brot N. 1992. Cloning, sequencing, and expression of the Escherichia coli peptide methionine sulfoxide reductase gene. J Biol Chem 267:15549–15551. [PubMed] [Google Scholar]
- 31.Lin Z, Johnson LC, Weissbach H, Brot N, Lively MO, Lowther WT. 2007. Free methionine-(R)-sulfoxide reductase from Escherichia coli reveals a new GAF domain function. Proc Natl Acad Sci U S A 104:9597–9602. doi: 10.1073/pnas.0703774104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moskovitz J, Weissbach H, Brot N. 1996. Cloning the expression of a mammalian gene involved in the reduction of methionine sulfoxide residues in proteins. Proc Natl Acad Sci U S A 93:2095–2099. doi: 10.1073/pnas.93.5.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moskovitz J, Rahman MA, Strassman J, Yancey SO, Kushner SR, Brot N, Weissbach H. 1995. Escherichia coli peptide methionine sulfoxide reductase gene: regulation of expression and role in protecting against oxidative damage. J Bacteriol 177:502–507. doi: 10.1128/jb.177.3.502-507.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee BC, Gladyshev VN. 2011. The biological significance of methionine sulfoxide stereochemistry. Free Radic Biol Med 50:221–227. doi: 10.1016/j.freeradbiomed.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee BC, Dikiy A, Kim HY, Gladyshev VN. 2009. Functions and evolution of selenoprotein methionine sulfoxide reductases. Biochim Biophys Acta 1790:1471–1477. doi: 10.1016/j.bbagen.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaya A, Lee BC, Gladyshev VN. 2015. Regulation of protein function by reversible methionine oxidation and the role of selenoprotein MsrB1. Antioxid Redox Signal 23:814–822. doi: 10.1089/ars.2015.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moskovitz J, Singh VK, Requena J, Wilkinson BJ, Jayaswal RK, Stadtman ER. 2002. Purification and characterization of methionine sulfoxide reductases from mouse and Staphylococcus aureus and their substrate stereospecificity. Biochem Biophys Res Commun 290:62–65. doi: 10.1006/bbrc.2001.6171. [DOI] [PubMed] [Google Scholar]
- 38.Etienne F, Spector D, Brot N, Weissbach H. 2003. A methionine sulfoxide reductase in Escherichia coli that reduces the R enantiomer of methionine sulfoxide. Biochem Biophys Res Commun 300:378–382. doi: 10.1016/S0006-291X(02)02870-X. [DOI] [PubMed] [Google Scholar]
- 39.Bogard RW, Davies BW, Mekalanos JJ. 2012. MetR-regulated Vibrio cholerae metabolism is required for virulence. mBio 3:e00236-12. doi: 10.1128/mBio.00236-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Husna AU, Wang N, Cobbold SA, Newton HJ, Hocking DM, Wilksch JJ, Scott TA, Davies MR, Hinton JC, Tree JJ, Lithgow T, McConville MJ, Strugnell RA. 2018. Methionine biosynthesis and transport are functionally redundant for the growth and virulence of Salmonella Typhimurium. J Biol Chem 293:9506–9519. doi: 10.1074/jbc.RA118.002592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berney M, Berney-Meyer L, Wong KW, Chen B, Chen M, Kim J, Wang J, Harris D, Parkhill J, Chan J, Wang F, Jacobs WR Jr. 2015. Essential roles of methionine and S-adenosylmethionine in the autarkic lifestyle of Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 112:10008–10013. doi: 10.1073/pnas.1513033112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blow NS, Salomon RN, Garrity K, Reveillaud I, Kopin A, Jackson FR, Watnick PI. 2005. Vibrio cholerae infection of Drosophila melanogaster mimics the human disease cholera. PLoS Pathog 1:e8. doi: 10.1371/journal.ppat.0010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watnick PI, Jugder BE. 2020. Microbial control of intestinal homeostasis via enteroendocrine cell innate immune signaling. Trends Microbiol 28:141–149. doi: 10.1016/j.tim.2019.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kamareddine L, Robins WP, Berkey CD, Mekalanos JJ, Watnick PI. 2018. The Drosophila immune deficiency pathway modulates enteroendocrine function and host metabolism. Cell Metab 28:449–462. doi: 10.1016/j.cmet.2018.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Overend G, Luo Y, Henderson L, Douglas AE, Davies SA, Dow JA. 2016. Molecular mechanism and functional significance of acid generation in the Drosophila midgut. Sci Rep 6:27242. doi: 10.1038/srep27242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Z, Hang S, Purdy AE, Watnick PI. 2013. Mutations in the IMD pathway and mustard counter Vibrio cholerae suppression of intestinal stem cell division in Drosophila. mBio 4:e00337-13. doi: 10.1128/mBio.00337-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jochim A, Shi T, Belikova D, Schwarz S, Peschel A, Heilbronner S. 2019. Methionine limitation impairs pathogen expansion and biofilm formation capacity. Appl Environ Microbiol 85:e00177-19. doi: 10.1128/AEM.00177-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoffman RM. 2019. Clinical studies of methionine-restricted diets for cancer patients. Methods Mol Biol 1866:95–105. doi: 10.1007/978-1-4939-8796-2_9. [DOI] [PubMed] [Google Scholar]
- 49.Leung KY, Finlay BB. 1991. Intracellular replication is essential for the virulence of Salmonella Typhimurium. Proc Natl Acad Sci U S A 88:11470–11474. doi: 10.1073/pnas.88.24.11470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alamuri P, Maier RJ. 2004. Methionine sulphoxide reductase is an important antioxidant enzyme in the gastric pathogen Helicobacter pylori. Mol Microbiol 53:1397–1406. doi: 10.1111/j.1365-2958.2004.04190.x. [DOI] [PubMed] [Google Scholar]
- 51.Walter J, Chagnaud P, Tannock GW, Loach DM, Dal Bello F, Jenkinson HF, Hammes WP, Hertel C. 2005. A high-molecular-mass surface protein (Lsp) and methionine sulfoxide reductase B (MsrB) contribute to the ecological performance of Lactobacillus reuteri in the murine gut. Appl Environ Microbiol 71:979–986. doi: 10.1128/AEM.71.2.979-986.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mei JM, Nourbakhsh F, Ford CW, Holden DW. 1997. Identification of Staphylococcus aureus virulence genes in a murine model of bacteraemia using signature-tagged mutagenesis. Mol Microbiol 26:399–407. doi: 10.1046/j.1365-2958.1997.5911966.x. [DOI] [PubMed] [Google Scholar]
- 53.Zhao C, Hartke A, La Sorda M, Posteraro B, Laplace JM, Auffray Y, Sanguinetti M. 2010. Role of methionine sulfoxide reductases A and B of Enterococcus faecalis in oxidative stress and virulence. Infect Immun 78:3889–3897. doi: 10.1128/IAI.00165-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Houot L, Watnick PI. 2008. A novel role for enzyme I of the Vibrio cholerae phosphoenolpyruvate phosphotransferase system in regulation of growth in a biofilm. J Bacteriol 190:311–320. doi: 10.1128/JB.01410-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Houot L, Chang S, Pickering BS, Absalon C, Watnick PI. 2010. The phosphoenolpyruvate phosphotransferase system regulates Vibrio cholerae biofilm formation through multiple independent pathways. J Bacteriol 192:3055–3067. doi: 10.1128/JB.00213-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Horton RM, Cai ZL, Ho SN, Pease LR. 1990. Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. Biotechniques 8:528–535. [PubMed] [Google Scholar]
- 57.Lefebvre B, Formstecher P, Lefebvre P. 1995. Improvement of the gene splicing overlap (SOE) method. Biotechniques 19:186–188. [PubMed] [Google Scholar]
- 58.Vijayakumar V, Vanhove AS, Pickering BS, Liao J, Tierney BT, Asara JM, Bronson R, Watnick PI. 2018. Removal of a membrane anchor reveals the opposing regulatory functions of Vibrio cholerae glucose-specific enzyme IIA in biofilms and the mammalian intestine. mBio 9:e00858-18. doi: 10.1128/mBio.00858-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haugo AJ, Watnick PI. 2002. Vibrio cholerae CytR is a repressor of biofilm development. Mol Microbiol 45:471–483. doi: 10.1046/j.1365-2958.2002.03023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Waldor MK, Mekalanos JJ. 1994. Emergence of a new cholera pandemic: molecular analysis of virulence determinants in Vibrio cholerae O139 and development of a live vaccine prototype. J Infect Dis 170:278–283. doi: 10.1093/infdis/170.2.278. [DOI] [PubMed] [Google Scholar]
- 61.Miller VL, Mekalanos JJ. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol 170:2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Metcalf WW, Jiang W, Daniels LL, Kim SK, Haldimann A, Wanner BL. 1996. Conditionally replicative and conjugative plasmids carrying lacZα for cloning, mutagenesis, and allele replacement in bacteria. Plasmid 35:1–13. doi: 10.1006/plas.1996.0001. [DOI] [PubMed] [Google Scholar]