The kinds of bacteria that form the collection of microbes (the microbiota) in the gut of human infants may influence health and well-being. Knowledge of how the composition of the infant diet influences the assemblage of the bacterial collection is therefore important because dietary interventions may offer opportunities to alter the microbiota with the aim of improving health. Bifidobacterium longum subspecies infantis is a well-known bacterial species, but under modern child-rearing conditions it may be disadvantaged in the gut. Modern formula milks often contain particular oligosaccharide additives that are generally considered to support bifidobacterial growth. However, studies of the ability of various bifidobacterial species to grow together in the presence of these oligosaccharides have not been conducted. These kinds of studies are essential for developing concepts of microbial ecology related to the influence of human nutrition on the development of the gut microbiota.
KEYWORDS: bifidobacteria, oligosaccharides
ABSTRACT
Bifidobacterial species are common inhabitants of the gut of human infants during the period when milk is a major component of the diet. Bifidobacterium breve, Bifidobacterium bifidum, Bifidobacterium longum subspecies longum, and B. longum subspecies infantis have been detected frequently in infant feces, but B. longum subsp. infantis may be disadvantaged numerically in the gut of infants in westernized countries. This may be due to the different durations of breast milk feeding in different countries. Supplementation of the infant diet or replacement of breast milk using formula feeds is common in Western countries. Formula milks often contain galacto- and/or fructo-oligosaccharides (GOS and FOS, respectively) as additives to augment the concentration of oligosaccharides in ruminant milks, but the ability of B. longum subsp. infantis to utilize these potential growth substrates when they are in competition with other bifidobacterial species is unknown. We compared the growth and oligosaccharide utilization of GOS and FOS by bifidobacterial species in pure culture and coculture. Short-chain GOS and FOS (degrees of polymerization [DP] 2 and 3) were favored growth substrates for strains of B. bifidum and B. longum subsp. longum, whereas both B. breve and B. longum subsp. infantis had the ability to utilize both short- and longer-chain GOS and FOS (DP 2 to 6). B. breve was nevertheless numerically dominant over B. longum subsp. infantis in cocultures. This was probably related to the slower use of GOS of DP 3 by B. longum subsp. infantis, indicating that the kinetics of substrate utilization is an important ecological factor in the assemblage of gut communities.
IMPORTANCE The kinds of bacteria that form the collection of microbes (the microbiota) in the gut of human infants may influence health and well-being. Knowledge of how the composition of the infant diet influences the assemblage of the bacterial collection is therefore important because dietary interventions may offer opportunities to alter the microbiota with the aim of improving health. Bifidobacterium longum subspecies infantis is a well-known bacterial species, but under modern child-rearing conditions it may be disadvantaged in the gut. Modern formula milks often contain particular oligosaccharide additives that are generally considered to support bifidobacterial growth. However, studies of the ability of various bifidobacterial species to grow together in the presence of these oligosaccharides have not been conducted. These kinds of studies are essential for developing concepts of microbial ecology related to the influence of human nutrition on the development of the gut microbiota.
INTRODUCTION
Bacteria belonging to the genus Bifidobacterium are commonly detected as predominant members of the gut microbiota of infants, especially prior to the introduction of complementary (weaning) foods to the diet (1–14). Four bifidobacterial species, Bifidobacterium breve, Bifidobacterium bifidum, Bifidobacterium longum subspecies longum, and B. longum subspecies infantis are characteristic of the infant gut microbiota in that they have been detected consistently in feces (2, 8, 15). However, from the results of more recent studies, there appears to be a paucity of B. longum subsp. infantis in the microbiota of infants inhabiting westernized countries (16–18). In theory, this may be due to the short duration of breast milk feeding in Western countries relative to that of other countries where B. longum subsp. infantis dominates the microbiota (17). B. longum subsp. infantis is well-equipped biochemically to internalize, degrade, and ferment human milk oligosaccharides (HMOs) (19). In the absence of HMOs, B. longum subsp. infantis lacks an important growth substrate and thus may be disadvantaged with respect to competitive growth with other bifidobacterial species that do not have the same reliance on HMOs for growth (20). In support of this view, in Indonesia, where breast milk feeding tends to be of long duration, the predominance of B. longum subsp. infantis is driven by the niche model of microbiota assemblage (trophic adaptation results in preferential colonization). In contrast, in New Zealand, where breast milk feeding is usually of shorter duration, B. longum subsp. infantis does not dominate the microbiota, and microbiota assemblage follows the neutral model (species randomly assemble and are functionally equivalent, so competition will be a feature of community assembly) (17). B. longum subsp. infantis can be detected as a minor component of the gut microbiota in Western infants, so it is not an extinct bacterial lineage but appears to be less capable of dominating the ecosystem under these circumstances (17, 18, 21).
Infant formula is a major nutritive source for infants when breast feeding is not possible, as well as during the complementary (weaning) feeding period (22). Modern formulas often contain prebiotics that increase the oligosaccharide content of the products, which are usually based on ruminant milk. Galacto- and fructo-oligosaccharides (GOS and FOS, respectively) are common prebiotics used for this purpose (23–37). Commercially available GOS contain oligosaccharides composed of galactose residues that are variously linked β-d-1,2, β-d-1,3, β-d-1,4, and β-d-1,6 and range in degrees of polymerization (DP) from 2 to 5 (designated herein according to the DP as G2 to G5) (38). Each oligosaccharide is terminated with a glucose residue. FOS contain two series of β-d-2,1-linked fructo-oligosaccharides, one of which contains a terminal glucose (GF series, with DPs of 2 to 6 and designated as GF2 to GF6) and one which does not (F series, with DPs of 2 to 6 and designated as F2 to F6). As such, GOS and FOS do not chemically resemble HMOs, which are complex, diverse molecules derived of glucose, galactose, N-acetylglucosamine, fucose, or sialic acid (39). GOS and FOS are nevertheless utilized for growth by at least some species of bifidobacteria (23–37). However, the relative abilities of bifidobacterial species to utilize GOS and FOS in coculture have not been tested. We wondered whether B. longum subsp. infantis could compete with other bifidobacteria when GOS and FOS were provided as growth substrates for cocultures. If they could not, formula feeding might be inimical to the growth of B. longum subsp. infantis in the gut. The purpose of our work, therefore, was to compare the in vitro growth profiles of Bifidobacterium bifidum, Bifidobacterium breve, B. longum subspecies infantis, and Bifidobacterium longum subspecies longum in batch and continuous cocultures in relation to the usage of GOS and FOS.
RESULTS
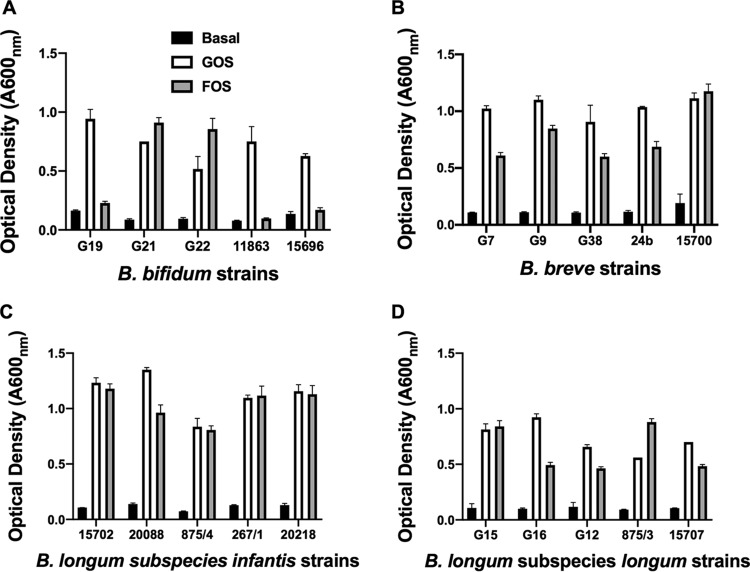
Growth of bifidobacterial species in culture medium containing GOS or FOS.
The variability of bifidobacterial strains to use GOS or FOS as growth substrates was tested using pure bacterial cultures. In general, after 24 h of incubation, the B. longum subspecies cultures showed similar, interstrain growth patterns in GOS and FOS cultures. A variety of growth patterns in FOS medium were evident among B. bifidum strains, whereas there was generally more growth in GOS cultures than in FOS cultures in the case of B. breve strains (Fig. 1). Four strains that utilized both GOS and FOS for growth were chosen for further experiments in coculture: B. bifidum G22, B. breve G9, B. longum subsp. infantis ATCC 15702, and B. longum subsp. longum G15.
FIG 1.
Growth of pure cultures of bifidobacterial species in basal medium or in basal medium containing GOS (0.2%, wt/vol) or FOS (0.2% wt/vol) incubated anaerobically for 24 h at 37°C, as indicated. Means and standard errors of optical density measurements of triplicate cultures are shown.
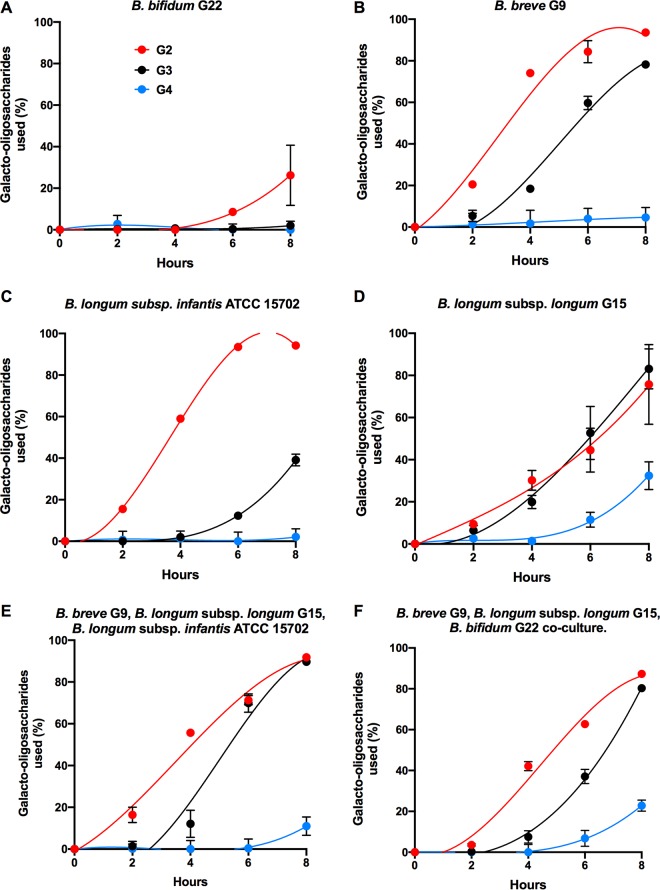
Growth of bifidobacterial species and utilization of oligosaccharides in batch culture.
The growth of the selected strains in pure culture in GOS/FOS medium was followed during an 8-h incubation in order to determine the oligosaccharide preferences of the bacteria. Under these conditions, B. breve and B. longum subsp. infantis attained the highest numbers of CFU/milliliter and optical densities at 8 h. B. bifidum tended to lag behind the other strains during the growth period (Fig. 2). The slower growth of the B. bifidum strain was associated with low usage of GOS; only GOS G2 was used to any extent (Fig. 3A). The other strains used GOS prioritized in order of DP, but the B. longum subsp. longum strain was the only one to utilize GOS G4 to an appreciable extent (Fig. 3D). The activity of this strain in relation to usage of GOS G4 was evident in cocultures (Fig. 3E and F). None of the individual strains or combinations of strains utilized FOS in 8-h batch culture, showing that GOS were the preferred growth substrates under these culture conditions. Since all four strains had been shown to grow in FOS medium with incubation for 24 h (Fig. 1), we determined the extent of GOS and FOS utilization in these cultures. B. bifidum mainly used GOS G2 and G3 for growth, with very little use of the FOS series (Table 1). B. longum subsp. longum used GOS G2, G3, and G4 as well as the shorter-chain-length FOS GF and F series. B. longum subsp. infantis utilized all of the GOS and FOS. Similarly, B. breve consumed all of the GOS and FOS although GOS G4 use was limited compared to that of the B. longum subsp. longum (Table 1).
FIG 2.

Growth of pure cultures of bifidobacterial strains. (A) Population levels of pure cultures of bifidobacterial strains representing four species in basal medium containing GOS and FOS (both at 0.2%, wt/vol) incubated anaerobically for 8 h at 37°C. Values are means and standard errors of triplicate cultures. (B) Optical densities of pure cultures as described for panel A. Means and standard errors of A600 measurements of triplicate cultures are shown.
FIG 3.
(A to D) Utilization of GOS by pure cultures of bifidobacterial strains representing four species in basal medium containing GOS and FOS (both at 0.2%, wt/vol) incubated anaerobically for 8 h at 37°C. (E and F) Utilization of GOS by cocultures. Values are means and standard errors of triplicate cultures.
TABLE 1.
Utilization of GOS and FOS by bifidobacterial species in pure cultures incubated for 24 h
| Oligosaccharide | Oligosaccharide utilization (%) by speciesa
|
|||
|---|---|---|---|---|
| B. bifidum G22 | B. breve G9 | B. longum subsp. infantis ATCC 15702 | B. longum subsp. longum G15 | |
| G2 | 100.0 | 100.0 | 100.0 | 100.0 |
| G3 | 96.0 | 100.0 | 100.0 | 98.0 |
| G4 | 5.7 | 65.6 | 100.0 | 100.0 |
| GF2 | 15.1 | 100.0 | 100.0 | 90.1 |
| GF3 | 2.2 | 99.5 | 100.0 | 10.0 |
| GF4 | 2.7 | 99.4 | 100.0 | 15.2 |
| GF5 | 3.3 | 100.0 | 100.0 | 16.8 |
| GF6 | 3.3 | 100.0 | 100.0 | 19.2 |
| F2 | 4.2 | 100.0 | 100.0 | 90.3 |
| F3 | 1.1 | 100.0 | 100.0 | 98.6 |
| F4 | 0.6 | 100.0 | 100.0 | 55.4 |
| F5 | 1.5 | 100.0 | 100.0 | 16.6 |
| F6 | 0.8 | 99.7 | 100.0 | 15.5 |
Values are means of duplicate assays.
Growth of bifidobacterial species and utilization of oligosaccharides in continuous coculture.
The growth and utilization of GOS and FOS under steady-state conditions (continuous culture) were investigated because the use of FOS did not occur during the relatively short log-phase growth period in batch culture. In continuous culture, all bacterial cells are maintained in the same physiological state, under substrate limiting conditions, during the entire experiment (40). Continuous cultures were inoculated with mixtures of either B. breve G9, B. longum subsp. longum G15, and B. bifidum G22 or of B. breve, B. longum subsp. longum, and B. longum subsp. infantis ATCC 15702. It was not possible to test the four strains in coculture because differential plate counts could not be used satisfactorily. Nevertheless, the combination of most interest was B. breve and the B. longum subspecies because these strains used GOS and FOS to a major extent for growth (Table 1) and would thus be potentially in competition. In general, apart from discrepancies in GOS G4 and FOS GF2 values in cocultures of B. breve, B. longum subsp. longum, and B. bifidum, there was good agreement between continuous culture runs with regard to usage of GOS and FOS by the cocultures (Fig. 4). FOS utilization was greater in cocultures containing B. longum subsp. infantis. Cocultures of the two combinations of strains produced similar population sizes (Fig. 5), including the numerical dominance of B. breve over the B. longum subspecies. That B. longum subsp. longum had less ability than B. breve to use longer-chain FOS was the most likely explanation for the differences in population sizes. However, B. longum subsp. infantis had an ability equal to that of B. breve to use all GOS and FOS for growth, yet B. breve was numerically dominant. This is probably explained by the more rapid utilization of GOS G3 by B. breve than by B. longum subsp. infantis (Fig. 3B and C) and the associated more rapid doubling time of the respective strains in GOS/FOS medium. B. breve G9 doubling time was 85 min, whereas that of B. longum subsp. infantis ATCC 15702 was almost twice as long at 156 min. Thus, despite the similar abilities of these strains to use oligosaccharides, temporal kinetics of utilization and doubling times under the same growth conditions were different and influenced population outcomes.
FIG 4.
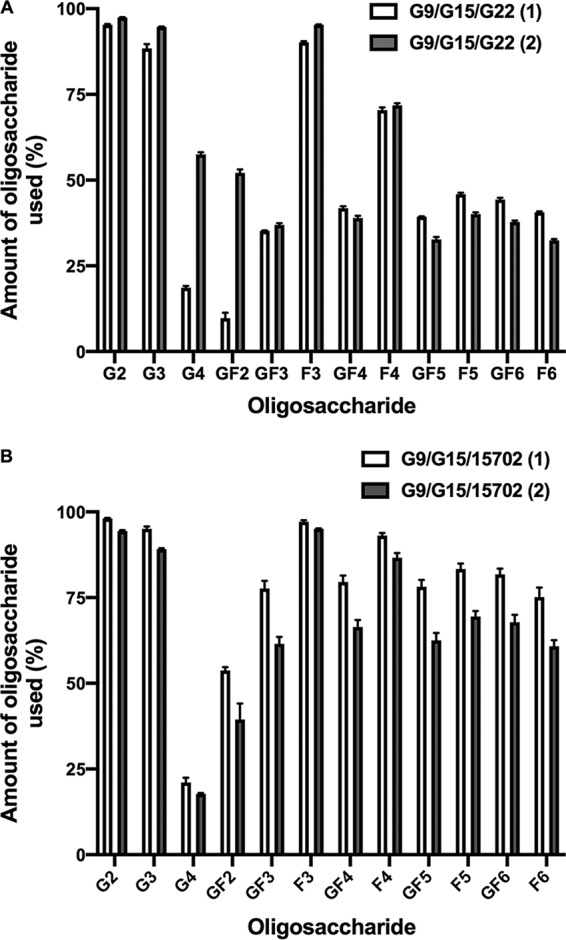
Utilization of GOS and FOS by continuous cocultures of combinations of bifidobacterial strains in basal medium containing GOS and FOS (both at 0.2%, wt/vol) incubated anaerobically at 37°C. Means and standard errors of triplicate samples collected from two chemostat runs (1 and 2) per combination of strains are shown.
FIG 5.
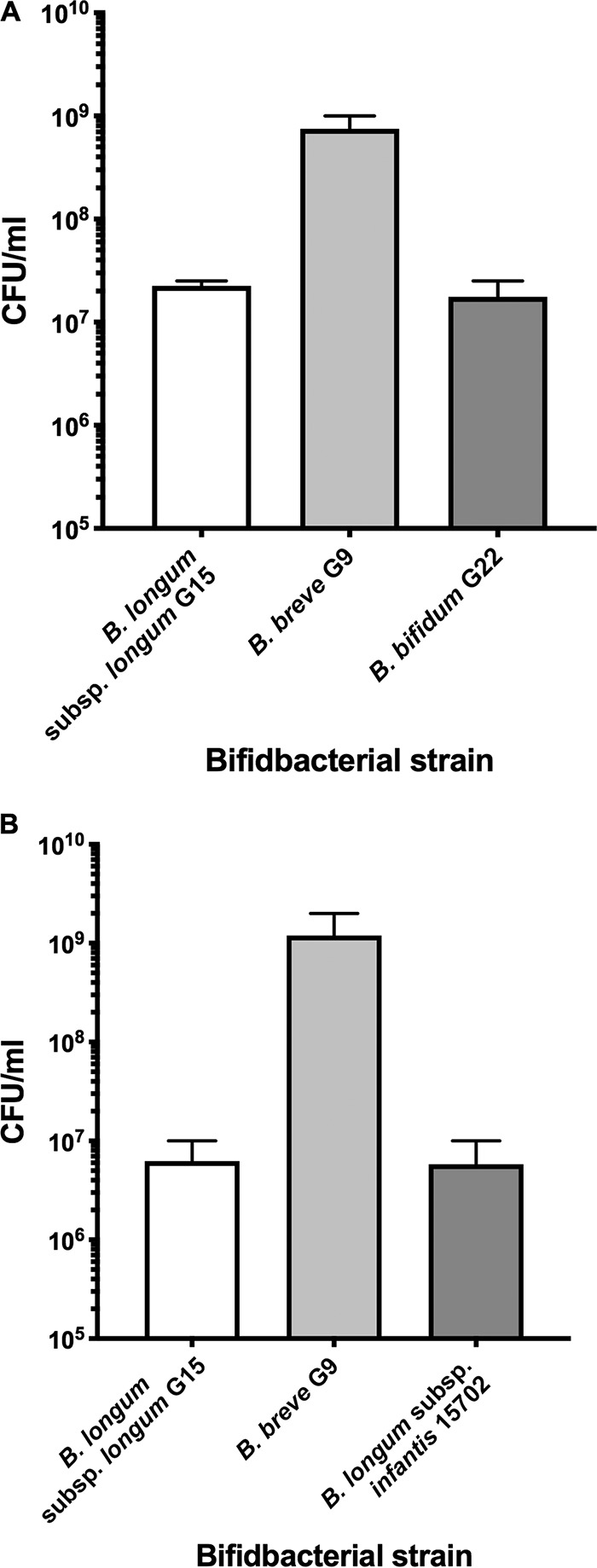
Population levels of bifidobacterial species in continuous coculture in GOS/FOS medium (both substrates at 0.2%, wt/vol). (A) Chemostat inoculated with a combination of strains G9, G15, and G22. (B) Chemostat inoculated with a combination of strains G9, G15, and ATCC 15702. Values are means and standard errors of duplicate chemostat runs.
DISCUSSION
The bacterial community resident in the large bowel of humans is daily presented with a mélange of potential growth substrates of various structural and chemical compositions. Even in the case of infants, where the diet before weaning is exclusively milk, a variety of carbohydrates are present as potential growth substrates for bacterial species. These substrates include lactose (some escapes digestion in the small bowel), carbohydrates linked to milk proteins (glycoproteins), and HMOs that pass to the large bowel undigested by the infant (39, 41). Lactose is a growth substrate for all bifidobacterial species characteristic of the gut (42); some B. longum subsp. longum, B. longum subsp. infantis, and B. breve strains have endoglycosidases that may be important in harvesting fermentable glycans from glycoproteins (43), while HMOs are most efficiently consumed by B. longum subsp. infantis (19, 39, 44). Other bifidobacterial species detected in the gut of infants are generally less efficient in the use of HMOs for growth although B. bifidum has an important hydrolytic capacity to provide sialic acid and fucose from HMOs and mucins as growth substrates for other bacteria (39, 40, 45–47). The addition of GOS/FOS to infant formulas provides yet further fermentable substrates to the bifidobacterial environment, and in vitro and in vivo testing has shown that at least some strains of the common species can use them to support growth (23–37). Oligosaccharides of different chain lengths are contained within the general categories GOS and FOS, yet little information about the effect of the degree of polymerization on the usage of these potential growth substrates has been reported (48). Knowledge of the prioritization of individual carbohydrates by gut bacteria is increasingly appreciated as fundamental to understanding how the microbiota degrades and ferments the complex mixtures of indigestible components of human food (49, 50). Use of carbohydrates in a preferential order by bacteria can consequently affect the emergent properties of the microbiota (51). Considering the widespread use of GOS and FOS in formulas and other foods, the lack of information about the preferential utilization of these substrates by bifidobacteria is surprising, especially in the potentially competitive setting of the infant gut where at least three bifidobacterial species commonly coexist (2, 5, 8, 15, 17).
The results of our study show that B. breve has the greatest trophic potential with respect to GOS/FOS to outcompete B. longum subsp. infantis. B. breve is a common member of the infant gut microbiota. However, the in vivo effect of GOS/FOS dietary supplementation on the proportions of the different species within bifidobacterial populations has not been a feature of the trials in which the effect of GOS/FOS-supplemented formulas has been compared to that of nonsupplemented formulas (31–37). This may be due to past difficulties of differentiating between bifidobacterial species using DNA-based methodologies (20, 21). Nevertheless, the species and subspecies can be easily differentiated using biochemical tests of cultured isolates (20). Clearly, there is a need to measure the abundances of bifidobacterial species in the feces of infants in association with dietary interventions so that new, detailed, ecological perspectives can be obtained. B. breve and GOS/FOS are used as a synbiotic combination, and this is certainly appropriate according to the results of our study (52–54). Synbiotics containing B. longum subsp. infantis might best combine the bacteria with synthetic HMOs that are now available (55).
Differences in abundances of bifidobacterial species in the gut of infants of different countries are unlikely to be fully explained by variation in breast milk composition (56), general feeding practices, and GOS/FOS supplementation of formulas. Shared environments and similar diets under conditions of communal living are important in the assembly and maintenance of the microbiota of the human body because they result in greater ease of dispersal (horizontal transmission) of gut commensals (57–60). Western methods of sanitation, water treatment, and hygiene might impair the dispersal of B. longum subsp. infantis (17). The genetic impact of the human host on the colonization of the gut by bacterial species might also be important. Heritable taxa, notably members of the family Christensenellaceae, have been recognized in studies of Canadians of European descent and of Koreans (61) but, to date, genome-wide association studies (GWASs) that aimed to associate human genetic variants (single nucleotide polymorphisms [SNPs]) with microbiota compositions have led to the conclusion that, overall, the genotype of the host probably has little impact on the composition of the adult microbiota (61, 62). The situation in infants, however, is unknown. Thus, currently, niche definitions based on trophic requirements of species appear to be the pertinent means of testing mechanisms that enable cohabitation of potentially competitive strains in microbial communities (63–65). Knowledge of substrate preferences and of the kinetics of their utilization by gut bacteria, such as bifidobacteria, is therefore important and continues to require investigation.
MATERIALS AND METHODS
Bifidobacterial strains.
Strain variability with respect to utilization of GOS and FOS was tested using pure cultures of five strains each of B. bifidum (G19, G21, G22, ATCC 11863, and ATCC 15696), B. breve (G7, G9, G38, 24b, and ATCC 15700T), B. longum subsp. infantis (ATCC 15702, DSM 20088T, 875/4, 267/1, and DSM 20218), and B. longum subsp. longum (G12, G15, G16, 875/3, and ATCC 15707T). Strains not originating from ATCC and DSM culture collections were isolated from infant feces and identified by 16S rRNA gene sequencing and biochemical tests, as described previously (66).
Cultures.
GOS and FOS media were composed of a previously described basal medium with the same formulation as lactobacillus deMan-Rogosa-Sharpe (MRS) medium (Difco), but with glucose omitted (67). GOS (β-d-1,4-linked oligosaccharides [68]) (Nissin Sugar Mfg., Japan) and FOS (Orafti P95; BENEO) were added to the medium at 0.2% (wt/vol). Medium was inoculated with bifidobacterial strains prepared in lactobacillus MRS medium, individually, at 1% (vol/vol) (Difco). The amounts of growth of the strains were similar in MRS medium (contains glucose as a fermentable carbohydrate), and each culture that was used as an inoculum contained ∼109 viable cells/ml. Culture manipulations and incubations were carried out in an anaerobic glove box. The optical density (A600) of cultures was determined after appropriate incubation times, and carbohydrate analysis was carried out on supernatants obtained after centrifugation of samples for 5 min at 15,000 × g, followed by filtration (0.45-μm pore size). Triplicate cultures were tested in all batch culture experiments.
Continuous cultures were prepared using Freter-type chemostats (30-ml volume), with a flow rate of 3 ml/h (giving a dilution rate [D] of 0.1 per h), and maintained in an anaerobic glove box, as described previously (40). The culture was considered to be in steady-state condition after 5 complete turnovers of reactor volume, and the culture was then sampled. Triplicate samples from duplicate runs were tested.
The doubling times in GOS/FOS medium of B. breve G9 and B. longum subsp. infantis ATCC 15702 were measured as described previously (63) in which single aliquots of triplicate cultures were removed at hourly intervals during the incubation period, and the optical density was determined. The optical density values were converted to natural logarithms and plotted against sampling times. Doubling times were calculated by log2/slope of the linear part of the graph.
Differential CFU count determination.
As described previously (39), samples of cultures were diluted in basal medium to 1 × 10−6 in 10-fold steps, and then aliquots of each dilution were spread-plated on basal medium agar containing carbohydrate substrates at 0.5% (wt/vol) to determine the number of CFU per milliliter on lacto-N-neotetraose (Glycom) for B. bifidum or B. longum subsp. infantis (40), on salicin (Sigma) for B. breve (42), and on arabinose for B. longum subsp. longum (42). Colonies were enumerated, and the number of CFU per milliliter was calculated after 48 h of anaerobic incubation at 37°C.
Chemical analysis of culture supernatants.
As described previously (69), concentrations of GOS and FOS in cell-free culture supernatants were determined by high-performance anion-exchange chromatography (HPAEC) using a Dionex ICS 3000 system (Dionex Corp., Sunnyvale, CA). Samples were diluted 400-fold with distilled water, injected (20 μl) onto a CarboPac PA-100 (4 by 250 mm) column equilibrated in 50 mM NaOH, and eluted with simultaneous gradients of NaOH (50 to 100 mM) and sodium acetate (0 to 90 mM) from 2.5 to 10 min at a flow rate of 1 ml/min. The eluant was monitored by pulsed amperometric detection using the Dionex standard carbohydrate waveform. The eluted sugars were identified from their elution times relative to those of standard sugars. Utilization (percent) of each oligosaccharide was calculated by reference to blank (uninoculated medium) values. Duplicate samples of batch cultures and triplicate samples from continuous cultures were analyzed.
ACKNOWLEDGMENTS
The research work was supported in part by MBIE Smart Idea (grant UOOX1202) and in part by Riddet Institute CORE funding. G.W.T. was supported by a James Cook Research Fellowship (Royal Society of New Zealand).
FOS (Orafti P95; BENEO) was a gift from Invita NZ, Ltd. (Auckland, NZ).
REFERENCES
- 1.Biavati B, Castagnoli P, Crociani F, Trovatelli LD. 1984. Species of the genus Bifidobacterium in the feces of infants. Microbiologica 7:341–345. [PubMed] [Google Scholar]
- 2.Matsuki T, Watanabe K, Tanaka R, Fukuda M, Oyaizu H. 1999. Distribution of bifidobacterial species in human intestinal microflora examined with 16S rRNA-gene-targeted species-specific primers. Appl Environ Microbiol 65:4506–4512. doi: 10.1128/AEM.65.10.4506-4512.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Favier CF, Vaughan EE, De Vos WM, Akkermans A. 2002. Molecular monitoring of succession of bacterial communities in human neonates. Appl Environ Microbiol 68:219–226. doi: 10.1128/AEM.68.1.219-226.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young SL, Simon MA, Baird MA, Tannock GW, Bibiloni R, Spencely K, Lane JM, Fitzharris P, Crane J, Town I, Addo-Yobo E, Murray CS, Woodcock A. 2004. Bifidobacterial species differentially affect expression of cell surface markers and cytokines of dendritic cells harvested from cord blood. Clin Diagn Lab Immunol 11:686–690. doi: 10.1128/CDLI.11.4.686-690.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Youn SY, Seo JM, Ji GE. 2007. Evaluation of the PCR method for identification of Bifidobacterium species. Lett Appl Microbiol 46:7–13. doi: 10.1111/j.1472-765X.2007.02263.x. [DOI] [PubMed] [Google Scholar]
- 6.Roger LC, Costabile A, Holland DT, Hoyles L, McCartney AL. 2010. Examination of faecal Bifidobacterium populations in breast- and formula-fed infants during the first 18 months of life. Microbiology 156:3329–3341. doi: 10.1099/mic.0.043224-0. [DOI] [PubMed] [Google Scholar]
- 7.Grönlund M-M, Grześkowiak Ł, Isolauri E, Salminen S. 2011. Influence of mother’s intestinal microbiota on gut colonization in the infant. Gut Microbes 2:227–233. doi: 10.4161/gmic.2.4.16799. [DOI] [PubMed] [Google Scholar]
- 8.Turroni F, Peano C, Pass DA, Foroni E, Severgnini M, Claesson MJ, Kerr C, Hourihane J, Murray D, Fuligni F, Gueimonde M, Margolles A, De Bellis G, O’Toole PW, van Sinderen D, Marchesi JR, Ventura M. 2012. Diversity of bifidobacteria within the infant gut microbiota. PLoS One 7:e36957. doi: 10.1371/journal.pone.0036957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Makino H, Kushiro A, Ishikawa E, Kubota H, Gawad A, Sakai T, Oishi K, Martin R, Ben-Amor K, Knol J, Tanaka R. 2013. Mother-to-infant transmission of intestinal bifidobacterial strains has an impact on the early development of vaginally delivered infant’s microbiota. PLoS One 8:e78331. doi: 10.1371/journal.pone.0078331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tannock GW, Lawley B, Munro K, Gowri Pathmanathan S, Zhou SJ, Makrides M, Gibson RA, Sullivan T, Prosser CG, Lowry D, Hodgkinson AJ. 2013. Comparison of the compositions of the stool microbiotas of infants fed goat milk formula, cow milk-based formula, or breast milk. Appl Environ Microbiol 79:3040–3048. doi: 10.1128/AEM.03910-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huda MN, Lewis Z, Kalanetra KM, Rashid M, Ahmad SM, Raqib R, Qadri F, Underwood MA, Mills DA, Stephensen CB. 2014. Stool microbiota and vaccine responses of infants. Pediatrics 134:e362–e372. doi: 10.1542/peds.2013-3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bäckhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva-Datchary P, Li Y, Xia Y, Xie H, Zhong H, Khan MT, Zhang J, Li J, Xiao L, Al-Aama J, Zhang D, Lee YS, Kotowska D, Colding C, Tremaroli V, Yin Y, Bergman S, Xu X, Madsen L, Kristiansen K, Dahlgren J, Wang J. 2015. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 17:690–703. doi: 10.1016/j.chom.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Milani C, Mancabelli L, Lugli GA, Duranti S, Turroni F, Ferrario C, Mangifesta M, Viappiani A, Ferretti P, Gorfer V, Tett A, Segata N, van Sinderen D, Ventura M. 2015. Exploring vertical transmission of bifidobacteria from mother to child. Appl Environ Microbiol 81:7078–7087. doi: 10.1128/AEM.02037-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin R, Makino H, Cetinyurek Yavuz A, Ben-Amor K, Roelofs M, Ishikawa E, Kubota H, Swinkels S, Sakai T, Oishi K, Kushiro A, Knol J. 2016. Early-life events, including mode of delivery and type of feeding, siblings and gender, shape the developing gut microbiota. PLoS One 11:e0158498. doi: 10.1371/journal.pone.0158498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagpal R, Kurakawa T, Tsuji H, Takahashi T, Kawashima K, Nagata S, Nomoto K, Yamashiro Y. 2017. Evolution of gut Bifidobacterium population in healthy Japanese infants over the first three years of life: a quantitative assessment. Sci Rep 7:10097. doi: 10.1038/s41598-017-10711-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frese SA, Hutton AA, Contreras LN, Shaw CA, Palumbo MC, Casaburi G, Xu G, Davis JCC, Lebrilla CB, Henrick BM, Freeman SL, Barile D, German JB, Mills DA, Smilowitz JT, Underwood MA. 2017. Persistence of supplemented Bifidobacterium longum subsp. infantis evc001 in breastfed infants. mSphere 2:e00501-17. doi: 10.1128/mSphere.00501-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lawley B, Otal A, Moloney-Geany K, Diana A, Houghton L, Heath A-L, Taylor RW, Tannock GW. 2019. Fecal microbiotas of Indonesian and New Zealand children differ in complexity and bifidobacterial taxa during the first year of life. Appl Environ Microbiol 85:e01105-19. doi: 10.1128/AEM.01105-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vatanen T, Plichta DR, Somani J, Münch PC, Arthur TD, Hall AB, Rudolf S, Oakeley EJ, Ke X, Young RA, Haiser HJ, Kolde R, Yassour M, Luopajärvi K, Siljander H, Virtanen SM, Ilonen J, Uibo R, Tillmann V, Mokurov S, Dorshakova N, Porter JA, McHardy AC, Lähdesmäki H, Vlamakis H, Huttenhower C, Knip M, Xavier RJ. 2019. Genomic variation and strain-specific functional adaptation in the human gut microbiome during early life. Nat Microbiol 4:470–479. doi: 10.1038/s41564-018-0321-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sela DA, Chapman J, Adeuya A, Kim JH, Chen F, Whitehead TR, Lapidus A, Rokhsar DS, Lebrilla CB, German JB, Price NP, Richardson PM, Mills DA. 2008. The genome sequence of Bifidobacterium longum subsp. infantis reveals adaptations for milk utilization within the infant microbiome. Proc Natl Acad Sci U S A 105:18964–18969. doi: 10.1073/pnas.0809584105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lawley B, Centanni M, Watanabe J, Sims I, Carnachan S, Broadbent R, Lee PS, Wong KH, Tannock GW. 2018. tuf gene sequence variation in Bifidobacterium longum subsp. infantis detected in the fecal microbiota of Chinese infants. Appl Environ Microbiol 84:e00336-18. doi: 10.1128/AEM.00336-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawley B, Munro K, Hughes A, Hodgkinson AJ, Prosser CG, Lowry D, Zhou SJ, Makrides M, Gibson RA, Lay C, Chew C, Lee PS, Wong KH, Tannock GW. 2017. Differentiation of Bifidobacterium longum subspecies longum and infantis by quantitative PCR using functional gene targets. PeerJ 5:e3375. doi: 10.7717/peerj.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leong C, Haszard JJ, Lawley B, Otal A, Taylor RW, Szymlek-Gay EA, Fleming EA, Daniels L, Fangupo LJ, Tannock GW, Heath AM. 2018. Mediation analysis as a means of identifying dietary components that differentially affect the fecal microbiota of infants weaned by modified baby-led and traditional approaches. Appl Environ Microbiol 84:e00914-18. doi: 10.1128/AEM.00914-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palframan RJ, Gibson GR, Rastall RA. 2003. Carbohydrate preferences of Bifidobacterium species isolated from the human gut. Curr Issues Intest Microbiol 4:71–75. [PubMed] [Google Scholar]
- 24.Boehm G, Moro G. 2008. Structural and functional aspects of prebiotics used in infant nutrition. J Nutr 138:1818S–1828S. doi: 10.1093/jn/138.9.1818S. [DOI] [PubMed] [Google Scholar]
- 25.Macfarlane GT, Steed H, Macfarlane S. 2008. Bacterial metabolism and health-related effects of galacto-oligosaccharides and other prebiotics. J Appl Microbiol 104:305–344. doi: 10.1111/j.1365-2672.2007.03520.x. [DOI] [PubMed] [Google Scholar]
- 26.Barboza M, Sela DA, Pirim C, Locascio RG, Freeman SL, German JB, Mills DA, Lebrilla CB. 2009. Glycoprofiling bifidobacterial consumption of galacto-oligosaccharides by mass spectrometry reveals strain-specific, preferential consumption of glycans. Appl Environ Microbiol 75:7319–7325. doi: 10.1128/AEM.00842-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oozeer R, van Limpt K, Ludwig T, Ben Amor K, Martin R, Wind RD, Boehm G, Knol J. 2013. Intestinal microbiology in early life: specific prebiotics can have similar functionalities as human-milk oligosaccharides. Am J Clin Nutr 98:561S–571S. doi: 10.3945/ajcn.112.038893. [DOI] [PubMed] [Google Scholar]
- 28.Watson D, O'Connell Motherway M, Schoterman MHC, van Neerven RJJ, Nauta A, van Sinderen D. 2013. Selective carbohydrate utilization by lactobacilli and bifidobacteria. J Appl Microbiol 114:1132–1146. doi: 10.1111/jam.12105. [DOI] [PubMed] [Google Scholar]
- 29.Sotoya H, Shigehisa A, Hara T, Matsumoto H, Hatano H, Matsuki T. 2017. Identification of genes involved in galactooligosaccharide utilization in Bifidobacterium breve strain YIT 4014T. Microbiology 163:1420–1428. doi: 10.1099/mic.0.000517. [DOI] [PubMed] [Google Scholar]
- 30.Valdés-Varela L, Ruas-Madiedo P, Gueimonde M. 2017. In vitro fermentation of different fructo-oligosaccharides by Bifidobacterium strains for the selection of synbiotic combinations. Int J Food Microbiol 242:19–23. doi: 10.1016/j.ijfoodmicro.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 31.Bakker-Zierikzee AM, Alles MS, Knol J, Kok FJ, Tolboom JJ, Bindels JG. 2005. Effects of infant formula containing a mixture of galacto- and fructo-oligosaccharides or viable Bifidobacterium animalis on the intestinal microflora during the first 4 months of life. Br J Nutr 94:783–790. doi: 10.1079/BJN20051451. [DOI] [PubMed] [Google Scholar]
- 32.Knol J, Scholtens P, Kafka C, Steenbakkers J, Gro S, Helm K, Klarczyk M, Schöpfer H, Böckler HM, Wells J. 2005. Colon microflora in infants fed formula with galacto- and fructo-oligosaccharides: more like breast-fed infants. J Pediatr Gastroenterol Nutr 40:36–42. doi: 10.1097/00005176-200501000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Holscher HD, Faust KL, Czerkies LA, Litov R, Ziegler EE, Lessin H, Hatch T, Sun S, Tappenden KA. 2012. Effects of prebiotic-containing infant formula on gastrointestinal tolerance and fecal microbiota in a randomized controlled trial. JPEN J Parenter Enteral Nutr 36:95S–105S. doi: 10.1177/0148607111430087. [DOI] [PubMed] [Google Scholar]
- 34.Vandenplas Y, De Greef E, Veereman G. 2014. Prebiotics in infant formula. Gut Microbes 5:681–687. doi: 10.4161/19490976.2014.972237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsuki T, Tajima S, Hara T, Yahagi K, Ogawa E, Kodama H. 2016. Infant formula with galacto-oligosaccharides (OM55N) stimulates the growth of indigenous bifidobacteria in healthy term infants. Benef Microbes 7:453–461. doi: 10.3920/BM2015.0168. [DOI] [PubMed] [Google Scholar]
- 36.Akkerman R, Faas MM, de Vos P. 2019. Non-digestible carbohydrates in infant formula as substitution for human milk oligosaccharide functions: effects on microbiota and gut maturation. Crit Rev Food Sci Nutr 59:1486–1497. doi: 10.1080/10408398.2017.1414030. [DOI] [PubMed] [Google Scholar]
- 37.Borewicz K, Suarez-Diez M, Hechler C, Beijers R, de Weerth C, Arts I, Penders J, Thijs C, Nauta A, Lindner C, Van Leusen E, Vaughan EE, Smidt H. 2019. The effect of prebiotic fortified infant formulas on microbiota composition and dynamics in early life. Sci Rep 9:2434. doi: 10.1038/s41598-018-38268-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Leeuwen SS, Kuipers BJ, Dijkhuizen L, Kamerling JP. 2016. Comparative structural characterization of 7 commercial galacto-oligosaccharide (GOS) products. Carbohydr Res 425:48–58. doi: 10.1016/j.carres.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 39.Smilowitz JT, Lebrilla CB, Mills DA, German JB, Freeman SL. 2014. Breast milk oligosaccharides: structure-function relationships in the neonate. Annu Rev Nutr 34:143–169. doi: 10.1146/annurev-nutr-071813-105721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Centanni M, Ferguson SA, Sims IM, Biswas A, Tannock GW. 2019. Bifidobacterium bifidum ATCC 15696 and Bifidobacterium breve 24b metabolic interaction based on 2’-O-fucosyl-lactose studied in steady-state cultures in a Freter-style chemostat. Appl Environ Microbiol 85:e02783-18. doi: 10.1128/AEM.02783-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karav S, Le Parc A, Leite Nobrega de Moura Bell JM, Frese SA, Kirmiz N, Block DE, Barile D, Mills DA. 2016. Oligosaccharides released from milk glycoproteins are selective growth substrates for infant-associated bifidobacteria. Appl Environ Microbiol 82:3622–3630. doi: 10.1128/AEM.00547-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sgorbati B, Biavati B, Palenzona D. 1995. The genus Bifidobacterium, p 279–308. In Holzapfel WHN, Wood JB (ed), The genera of lactic acid bacteria. Springer, Dordrecht, The Netherlands. [Google Scholar]
- 43.Garrido D, Nwosu C, Ruiz-Moyano S, Aldredge D, German JB, Lebrilla CB, Mills DA. 2012. Endo-β-N-acetylglucosaminidases from infant gut-associated bifidobacteria release complex N-glycans from human milk glycoproteins. Mol Cell Proteomics 11:775–785. doi: 10.1074/mcp.M112.018119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taft DH, Liu J, Maldonado-Gomez MX, Akre S, Huda MN, Ahmad SM, Stephensen CB, Mills DA. 2018. Bifidobacterial dominance of the gut in early life and acquisition of antimicrobial resistance. mSphere 3:e00441-18. doi: 10.1128/mSphere.00441-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turroni F, Bottacini F, Foroni E, Mulder I, Kim JH, Zomer A, Sánchez B, Bidossi A, Ferrarini A, Giubellini V, Delledonne M, Henrissat B, Coutinho P, Oggioni M, Fitzgerald GF, Mills D, Margolles A, Kelly D, van Sinderen D, Ventura M. 2010. Genome analysis of Bifidobacterium bifidum PRL2010 reveals metabolic pathways for host-derived glycan foraging. Proc Natl Acad Sci U S A 107:19514–19519. doi: 10.1073/pnas.1011100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Egan M, Motherway MO, Kilcoyne M, Kane M, Joshi L, Ventura M, van Sinderen D. 2014. Cross-feeding by Bifidobacterium breve UCC2003 during co-cultivation with Bifidobacterium bifidum PRL2010 in a mucin-based medium. BMC Microbiol 14:282. doi: 10.1186/s12866-014-0282-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Turroni F, Duranti S, Milani C, Lugli GA, van Sinderen D, Ventura M. 2019. Bifidobacterium bifidum: a key member of the early human gut microbiota. Microorganisms 7:544. doi: 10.3390/microorganisms7110544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scott KP, Martin JC, Duncan SH, Flint HJ. 2014. Prebiotic stimulation of human colonic butyrate-producing bacteria and bifidobacteria, in vitro. FEMS Microbiol Ecol 87:30–40. doi: 10.1111/1574-6941.12186. [DOI] [PubMed] [Google Scholar]
- 49.Hamaker BR, Tuncil YE. 2014. A perspective on the complexity of dietary fiber structures and their potential effect on the gut microbiota. J Mol Biol 426:3838–3850. doi: 10.1016/j.jmb.2014.07.028. [DOI] [PubMed] [Google Scholar]
- 50.Rogowski A, Briggs JA, Mortimer JC, Tryfona T, Terrapon N, Lowe EC, Baslé A, Morland C, Day AM, Zheng H, Rogers TE, Thompson P, Hawkins AR, Yadav MP, Henrissat B, Martens EC, Dupree P, Gilbert HJ, Bolam DN. 2015. Glycan complexity dictates microbial resource allocation in the large intestine. Nat Commun 6:7481. doi: 10.1038/ncomms8481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu Y, Heath AL, Galland B, Rehrer N, Drummond L, Wu XY, Bell TJ, Lawley B, Sims IM, Tannock GW. 2019. Substrate use prioritization by a coculture of five species of gut bacteria fed mixtures of arabinoxylan, xyloglucan, β-glucan, and pectin. Appl Environ Microbiol 86:e01905-19. doi: 10.1128/AEM.01905-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chua MC, Ben-Amor K, Lay C, Goh AEN, Chiang WC, Rao R, Chew C, Chaithongwongwatthana S, Khemapech N, Knol J, Chongsrisawat V. 2017. Effect of synbiotic on the gut microbiota of cesarean delivered infants: a randomized, double-blind, multicenter study. J Pediatr Gastroenterol 65:102–106. doi: 10.1097/MPG.0000000000001623. [DOI] [PubMed] [Google Scholar]
- 53.Kosuwon P, Lao-Araya M, Uthaisangsook S, Lay C, Bindels J, Knol J, Chatchatee P. 2018. A synbiotic mixture of scGOS/lcFOS and Bifidobacterium breve M-16V increases faecal Bifidobacterium in healthy young children. Benef Microbes 9:541–552. doi: 10.3920/BM2017.0110. [DOI] [PubMed] [Google Scholar]
- 54.Rigo-Adrover MDM, van Limpt K, Knipping K, Garssen J, Knol J, Costabile A, Franch À, Castell M, Pérez-Cano FJ. 2018. Preventive effect of a synbiotic combination of galacto- and fructooligosaccharides mixture with Bifidobacterium breve M-16V in a model of multiple rotavirus infections. Front Immunol 9:1318. doi: 10.3389/fimmu.2018.01318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marriage BJ, Buck RH, Goehring KC, Oliver JS, Williams JA. 2015. Infants fed a lower calorie formula with 2′FL show growth and 2′FL uptake like breast-fed infants. J Pediatr Gastroenterol Nutr 61:649–658. doi: 10.1097/MPG.0000000000000889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McGuire MK, Meehan CL, McGuire MA, Williams JE, Foster J, Sellen DW, Kamau-Mbuthia EW, Kamundia EW, Mbugua S, Moore SE, Prentice AM, Kvist LJ, Otoo GE, Brooker SL, Price WJ, Shafii B, Placek C, Lackey KA, Robertson B, Manzano S, Ruíz L, Rodríguez JM, Pareja RG, Bode L. 2017. What’s normal? Oligosaccharide concentrations and profiles in milk produced by healthy women vary geographically. Am J Clin Nutr 105:1086–1100. doi: 10.3945/ajcn.116.139980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martínez I, Stegen JC, Maldonado-Gómez MX, Eren AM, Siba PM, Greenhill AR, Walter J. 2015. The gut microbiota of rural Papua New Guineans: composition, diversity patterns, and ecological processes. Cell Rep 11:527–538. doi: 10.1016/j.celrep.2015.03.049. [DOI] [PubMed] [Google Scholar]
- 58.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, Egholm M, Henrissat B, Heath AC, Knight R, Gordon JI. 2009. A core gut microbiome in obese and lean twins. Nature 457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Song SJ, Lauber C, Costello EK, Lozupone CA, Humphrey G, Berg-Lyons D, Caporaso JG, Knights D, Clemente JC, Nakielny S, Gordon JI, Fierer N, Knight R. 2013. Cohabiting family members share microbiota with one another and with their dogs. Elife 2:e00458. doi: 10.7554/eLife.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vangay P, Johnson AJ, Ward TL, Al-Ghalith GA, Shields-Cutler RR, Hillmann BM, Lucas SK, Beura LK, Thompson EA, Till LM, Batres R, Paw B, Pergament SL, Saenyakul P, Xiong M, Kim AD, Kim G, Masopust D, Martens EC, Angkurawaranon C, McGready R, Kashyap PC, Culhane-Pera KA, Knights D. 2018. US immigration westernizes the human gut microbiome. Cell 175:962–972.e10. doi: 10.1016/j.cell.2018.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goodrich JK, Davenport ER, Clark AG, Ley RE. 2017. The relationship between the human genome and microbiome comes into view. Annu Rev Genet 51:413–433. doi: 10.1146/annurev-genet-110711-155532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rothschild D, Weissbrod O, Barkan E, Kurilshikov A, Korem T, Zeevi D, Costea PI, Godneva A, Kalka IN, Bar N, Shilo S, Lador D, Vila AV, Zmora N, Pevsner-Fischer M, Israeli D, Kosower N, Malka G, Wolf BC, Avnit-Sagi T, Lotan-Pompan M, Weinberger A, Halpern Z, Carmi S, Fu J, Wijmenga C, Zhernakova A, Elinav E, Segal E. 2018. Environment dominates over host genetics in shaping human gut microbiota. Nature 555:210–215. doi: 10.1038/nature25973. [DOI] [PubMed] [Google Scholar]
- 63.Tannock GW, Wilson CM, Loach D, Cook GM, Eason J, O'Toole PW, Holtrop G, Lawley B. 2012. Resource partitioning in relation to cohabitation of Lactobacillus species in the mouse forestomach. ISME J 6:927–938. doi: 10.1038/ismej.2011.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Widder S, Allen RJ, Pfeiffer T, Curtis TP, Wiuf C, Sloan WT, Cordero OX, Brown SP, Momeni B, Shou W, Kettle H, Flint HJ, Haas AF, Laroche B, Kreft JU, Rainey PB, Freilich S, Schuster S, Milferstedt K, van der Meer JR, Grosskopf T, Huisman J, Free A, Picioreanu C, Quince C, Klapper I, Labarthe S, Smets BF, Wang H, Isaac Newton Institute Fellows, Soyer OS. 2016. Challenges in microbial ecology: building predictive understanding of community function and dynamics. ISME J 10:2557–2568. doi: 10.1038/ismej.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tannock GW. 2017. Understanding the gut microbiota. John Wiley and Sons, Hoboken, NJ. [Google Scholar]
- 66.Tannock GW, Lawley B, Munro K, Sims IM, Lee J, Butts CA, Roy N. 2014. RNA-stable-isotope probing shows utilization of carbon from inulin by specific bacterial populations in the rat large bowel. Appl Environ Microbiol 80:2240–2247. doi: 10.1128/AEM.03799-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Centanni M, Hutchison JC, Carnachan SM, Daines AM, Kelly WJ, Tannock GW, Sims IM. 2017. Differential growth of bowel commensal Bacteroides species on plant xylans of differing structural complexity. Carbohydr Polym 157:1374–1382. doi: 10.1016/j.carbpol.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 68.Playne M, Crittenden R. 2009. Galacto-oligosaccharides and other products derived from lactose, p 121–201. In McSweeney P, Fox P (ed), Advanced dairy chemistry. Springer, New York, NY. [Google Scholar]
- 69.Centanni M, Carnachan SM, Bell TJ, Daines AM, Hinkley SFR, Tannock GW, Sims IM. 2019. Utilization of complex pectic polysaccharides from New Zealand plants (Tetragonia tetragonioides and Corynocarpus laevigatus) by gut Bacteroides species. J Agric Food Chem 67:7755–7764. doi: 10.1021/acs.jafc.9b02429. [DOI] [PubMed] [Google Scholar]