We have developed a novel preparation to kill mosquitoes from an abundant soil bacterium, Chromobacterium sp. Panama. This preparation is an air-dried powder containing no live bacteria, and it can be incorporated into an attractive bait and fed directly to mosquito larvae. We demonstrate that the preparation has broad spectrum activity against the larval form of the mosquitoes responsible for the transmission of malaria and the dengue, chikungunya, yellow fever, West Nile, and Zika viruses, as well as mosquito larvae that are already resistant to commonly used mosquitocidal chemicals. Our preparation possesses many favorable traits: it kills at a low dosage, and it does not lose activity when exposed to high temperatures, all of which suggest that this preparation could eventually become an effective new tool for controlling mosquitoes and the diseases they spread.
KEYWORDS: mosquito control, insecticide, biopesticide, Chromobacterium Csp_P, Aedes aegypti, Anopheles gambiae, Culex quinquefasciatus, semi-field trial
ABSTRACT
Given the continued high prevalence of mosquito-transmitted diseases, there is a clear need to develop novel disease and vector control strategies. Biopesticides of microbial origin represent a promising source of new approaches to target disease-transmitting mosquito populations. Here, we describe the development and characterization of a novel mosquito biopesticide, derived from an air-dried, nonlive preparation of the bacterium Chromobacterium sp. Panama (family: Neisseriaceae). This preparation rapidly and effectively kills the larvae of prominent mosquito vectors, including the dengue and Zika vector Aedes aegypti and the human malaria vector Anopheles gambiae. During semi-field trials in Puerto Rico, we observed high efficacy of the biopesticide against field-derived A. aegypti populations, and against A. aegypti and Culex species larvae in natural breeding water, indicating the suitability of the biopesticide for use under more natural conditions. In addition to high efficacy, the nonlive Csp_P biopesticide has a low effective dose, a long shelf life, and high heat stability and can be incorporated into attractive larval baits, all of which are desirable characteristics for a biopesticide.
IMPORTANCE We have developed a novel preparation to kill mosquitoes from an abundant soil bacterium, Chromobacterium sp. Panama. This preparation is an air-dried powder containing no live bacteria, and it can be incorporated into an attractive bait and fed directly to mosquito larvae. We demonstrate that the preparation has broad spectrum activity against the larval form of the mosquitoes responsible for the transmission of malaria and the dengue, chikungunya, yellow fever, West Nile, and Zika viruses, as well as mosquito larvae that are already resistant to commonly used mosquitocidal chemicals. Our preparation possesses many favorable traits: it kills at a low dosage, and it does not lose activity when exposed to high temperatures, all of which suggest that this preparation could eventually become an effective new tool for controlling mosquitoes and the diseases they spread.
INTRODUCTION
Parasites, viruses, and filarial worms that can be transmitted from person to person when mosquitoes feed on human blood represent a clear and present threat to human health around the world. The burden of malaria, a febrile illness caused by Plasmodium parasites that are transmitted by mosquitoes from the genus Anopheles, is particularly high. Unfortunately, even though unprecedented disease control efforts over the last 15 years have reduced the burden of disease (1), the human cost still remains high. Recent data suggest that there were an estimated 219 million cases of malaria and approximately 435,000 deaths in 2017, many of which were young children (2).
Mosquito-transmitted arboviral diseases also have a major impact on human health, with key viruses such as dengue virus, transmitted by mosquitoes from the genera Aedes and Culex, responsible for millions of infections each year (3–6). Critically, the incidence of arboviral disease has risen greatly over the last 20 years (7), as changes in climate, mosquito distribution, and human behavior have brought humans and mosquitoes into contact more frequently (8–10). These factors have also helped to promote the emergence of novel mosquito-transmitted viruses such as the chikungunya and Zika viruses (11), which have caused hundreds of thousands of infections during major outbreaks (4, 6).
Unfortunately, there are no effective, commercially available vaccines for most mosquito-transmitted diseases, and crucially, malaria parasites are rapidly developing resistance to drugs (12), while drugs for arboviruses do not currently exist. For this reason, mosquito control has long been the most common strategy employed to limit disease transmission, and historically the most common approach has been to utilize different chemical insecticides to rapidly and effectively kill mosquitoes (13). These insecticides can be used to target both larval and adult mosquito stages and can be used synergistically with mosquito bite prevention strategies (14). Many commonly used insecticides, including pyrethroids and organophosphates, kill by targeting the mosquito central nervous system (15–17). Others, including chitin synthesis inhibitors and juvenile hormone analogues such as methoprene, act to prevent development beyond larval stages (17, 18). Concerns about the environmental impacts of chemical insecticides have sharpened focus on the development of environment-friendly mosquitocidals (19). There are also a variety biologically derived insecticides, or biopesticides, used in mosquito control. These include the bacteria Bacillus thuringiensis subsp. israelensis and Lysinibacillus sphaericus, which produce highly durable spores that form a crystal protein which shreds the mosquito gut after ingestion (20, 21). There are also entomopathogenic fungi, such as Metarhizium anisopliae, which can target and kill specific mosquito species (22).
Regular exposure to chemical insecticides has led to genetic resistance becoming increasingly prevalent in mosquito populations (23, 24), complicating mosquito control efforts (25). The implication of insecticide resistance is that no single insecticide will offer perfect, long-term control of any mosquito population. Instead, effective, long-term control will likely come through multifaceted strategies that exploit synergies between different insecticides, thereby providing a greater chance of limiting or overcoming potential mechanisms of resistance (26). Consequently, novel mosquitocidal chemicals and biopesticides must continue to be developed, since they will provide new options to improve or supplement existing mosquito control programs.
The Chromobacterium species Panama (Csp_P) (class: Betaproteobacteria, family: Neisseriaceae) is a soil bacterium first isolated from Ae. aegypti midguts (27) which has a number of unique and useful properties for controlling mosquito-transmitted disease and mosquito populations. When Anopheles gambiae mosquitoes are fed low doses of Csp_P, they display reduced susceptibility to infection with the human malarial parasite Plasmodium falciparum (27), as Csp_P produces a depsipeptide called romidepsin, which kills the parasite (28). Similarly, Csp_P infection in Ae. aegypti reduces susceptibility to dengue virus (27), through production of an aminopeptidase that promotes degradation of the viral envelope protein (29). Critically, higher doses of Csp_P have potent adulticidal activity against many mosquito species when provided in sucrose and are also a very effective larvicide of Ae. aegypti and An. gambiae (27, 30).
Given these properties, Csp_P has great potential as an insecticide. However, to overcome potential regulatory, ecological, and epidemiological concerns about using an insecticide containing live bacteria, we sought to develop a Csp_P preparation that contained no live bacteria, which was easily prepared and had a long shelf life, while retaining insecticidal activity against a range of important mosquito vectors in the laboratory and the field.
RESULTS
Pellet design and attractants.
In order to facilitate the development of a nonlive insecticide based on the bacterium Chromobacterium species Panama Csp_P, we first developed an attractive larvicidal bait in the form of a pellet that could be used to deliver Csp_P to mosquito larvae. This bait needed to induce feeding among larvae and also maintain structural integrity within an aqueous environment so that mosquitoes could feed on it.
In a series of preliminary experiments (see File S1 in the supplemental material), we assessed the attractiveness of fishmeal (Dirty Gardener) and ground tropical fish flakes (Tetramin) when provided to Ae. aegypti larvae in pellets made with 20% gelatin as a stabilizing agent. In experiments testing the attractiveness of individual baits, we observed that 97.5% of larvae responded to fishmeal pellets within 30 min of exposure. In contrast, only 71.7% of larvae responded to pellets containing tropical flakes over the same time period. When both fishmeal- and tropical flake-containing baits were offered to larvae over 30 min in bait choice assays, an average of 80% responded to fishmeal-containing baits, while 13.33% chose tropical flake-containing baits, and 6.67% did not respond. Consequently, we decided to use fishmeal baits in all subsequent assays.
We then incorporated live Csp_P into pellets and fed it to Ae. aegypti Rockefeller (ROCK) larvae in order to examine the efficacy of pellets as a larvicide delivery tool. Pellets containing live Csp_P killed all larvae in a 300-ml container within 6 days, with an average time to death postexposure of 2.09 (± 0.05) days and an expected hazard ratio of 83.99 in comparison to pellets without bacteria (Cox regression: W = 180.64, df = 1, exp(β) = 83.99, P < 0.0001) (see Fig. S1 in the supplemental material).
Development and assessment of nonlive Csp_P preparations.
We developed five different culturing methods (nonlive_1 through nonlive_5) to produce large quantities of nonlive, air-dried, powdered Csp_P (see Materials and Methods for details on the culturing processes). Immediately after air drying, each of the nonlive powders was mixed with 100 μl of 1× phosphate-buffered saline (PBS), and three different dilutions of these mixtures were inoculated onto Luria-Bertani (LB) agar, followed by incubation at 30°C for 2 days. Across three experimental replicates, we did not observe any bacterial colonies for any of the five preparations at any dilution.
We compared the larvicidal activities of these preparations across three replicate experiments wherein 100 mg of each preparation was incorporated into a gelatin/fishmeal pellet and then offered to Ae. aegypti ROCK larvae. This dose was utilized in all subsequent assays, unless specified otherwise. We again observed that all five powders killed larvae; however, they were not equally effective (Table 1). Based on the results of these experiments, we selected the nonlive_1 preparation (Fig. 1) for further testing since it produced the shortest average time to death for exposed larvae (2.80 ± 0.10 days), and also produced the highest hazard ratio (Cox regression: W = 145.83, df = 1, exp(β) = 50.11, P < 0.0001) in comparison to the control treatment. This decision was also based on the consistency of the larvicidal activity that was observed for nonlive_1 powder across three replicates and the high yield of that powder compared to the other four. In the experiments described below, we refer to the nonlive_1 powder as nonlive Csp_P.
TABLE 1.
Larvicidal activity of different nonlive Csp_P preparations
| Treatment | na | Avg time to death (days) ± SEMb
|
exp(β)c | % survivald | |||
|---|---|---|---|---|---|---|---|
| R1 | R2 | R3 | Overall | ||||
| Control | 270 | 5.91 ± 0.39 | 7.56 ± 0.71 | 7.00 ± 1.22 | 6.93 ± 0.44 | NA | 87.78 |
| Live | 260 | 1.90 ± 0.06 | 2.36 ± 0.07 | 2.72 ± 0.09 | 2.32 ± 0.05 | 99.17 | 0.00 |
| Nonlive_1 | 253 | 2.52 ± 0.20 | 3.31 ± 0.17 | 2.55 ± 0.09 | 2.80 ± 0.10 | 50.11 | 0.79 |
| Nonlive_2 | 254 | 3.97 ± 0.18 | 6.36 ± 0.34 | 5.43 ± 0.30 | 5.19 ± 0.17 | 31.17 | 4.72 |
| Nonlive_3 | 262 | 3.98 ± 0.26 | 3.73 ± 0.23 | 3.56 ± 0.18 | 3.76 ± 0.13 | 30.44 | 0.38 |
| Nonlive_4 | 247 | 3.73 ± 0.22 | 2.40 ± 0.19 | 3.88 ± 0.21 | 3.31 ± 0.13 | 33.00 | 0.81 |
| Nonlive_5 | 255 | 3.09 ± 0.17 | 4.48 ± 0.30 | 1.81 ± 0.08 | 3.08 ± 0.13 | 44.38 | 0.39 |
Total larvae counted across three experiments.
Average time to death compiled across three replicate cages for replicate experiments 1 to 3 (R1 to R3).
exp(β) = hazard ratio (calculated using Cox regression).
Percentage of larvae surviving at 12 days after exposure to the pellet.
FIG 1.
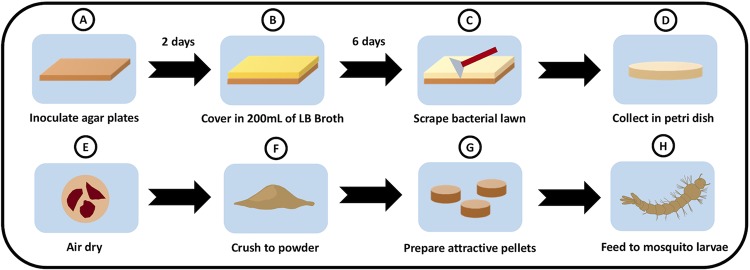
Workflow for preparing the nonlive Csp_P biopesticide. (A) Prepare large plates with 200 ml of LB agar and then inoculate each with 200 μl of Csp_P culture. (B) Allow plates to grow for 2 days at 30°C and then inoculate with 200 ml of LB broth. (C) Grow for 5 days and then decant the liquid phase. Let plates sit for 24 h and then scrape off the bacterial lawn. (D) Collect bacterial lawn in a petri dish. (E) Air dry preparation in fume hood. (F) Once dry, crush the preparation to powder with a mortar and pestle. (G) Incorporate nonlive Csp_P powder into gelatin/fishmeal attractive pellets. (H) Feed pellets containing 100 mg of powder to target mosquito larvae.
Nonlive Csp_P powder does not contain cyanide.
We tested each of the five powders for the presence of cyanide using a cyanide test kit, model CYN-3 (Hach, 2010-02), since cyanide toxicity is considered the likely means by which live Csp_P kill larvae when in suspension (30). We observed that the pH of each powder, resuspended in deionized (DI) water, was between 5 and 6. At the completion of the test, we did not observe a change in color for any sample, indicating that there was no evidence of the presence of cyanide species in any of the nonlive Csp_P powders.
Nonlive Csp_P powder has a larvicidal effect against different mosquito species.
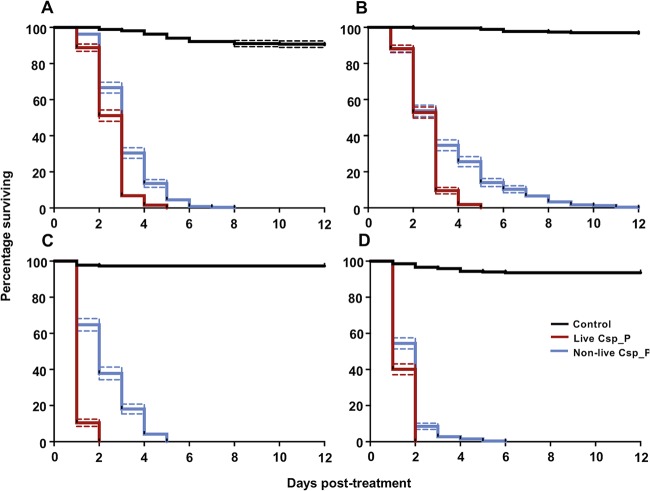
We examined the larvicidal efficacy of nonlive Csp_P powder against Ae. aegypti Rockefeller (Fig. 2A), pyrethroid-resistant Ae. aegypti (Fig. 2B), An. gambiae Keele (Fig. 2C), and Culex quinquefasciatus (Fig. 2D). In each of these assays, gelatin/fishmeal pellets containing 100 mg of nonlive Csp_P powder were fed to L2 larvae, and mortality rates compared against groups of larvae fed pellets containing either live Csp_P or no-bacterium controls. Rockefeller larvae challenged with live bacteria had an average time to death of 2.48 ± 0.05 days (Cox regression: W = 275.42, df = 1, exp(β) = 67.08, P < 0.0001), while those challenged with nonlive Csp_P powder had an average time to death of 3.13 ± 0.08 days (Cox regression: W = 233.35, df = 1, exp(β) = 42.68, P < 0.0001). Pyrethroid-resistant Ae. aegypti challenged with live Csp_P lived 2.52 ± 0.05 days on average postexposure (Cox regression: W = 202.04, df = 1, exp(β) = 295.64, P < 0.0001), while larvae from the same line fed on nonlive Csp_P lived 3.40 ± 0.13 days on average (Cox regression: W = 177.81, df = 1, exp(β) = 183.94, P < 0.0001). An. gambiae larvae fed live Csp_P had an average time to death of 1.10 ± 0.02 days (Cox regression: W = 162.63, df = 1, exp(β) = 272.37, P < 0.0001), while those challenged with nonlive Csp_P survived for 2.25 ± 0.09 days posttreatment, on average (Cox regression: W = 122.00, df = 1, exp(β) = 113.30, P < 0.0001). Finally, Cx. quinquefasciatus larvae challenged with live Csp_P lived 1.40 ± 0.03 days (Cox regression: W = 199.99, df = 1, exp(β) = 63.02, P < 0.0001) on average, compared to 1.68 ± 0.05 days on average for those challenged with nonlive Csp_P (Cox regression: W = 187.76, df = 1, exp(β) = 51.17, P < 0.0001). During the course of these experiments, no larvae of any species that were exposed to the Csp_P biopesticide pupated. In contrast, we observed that pupation among larvae fed on control bait occurred from days 3 through 7 after exposure to the bait.
FIG 2.
Nonlive Csp_P effectively kills the larvae of important mosquito vector species, including those resistant to common chemical insecticides. At 3 days posthatching, larvae from the Ae. aegypti ROCK strain (A), the pyrethroid-resistant Ae. aegypti strain NR-48830 (B), the An. gambiae Keele strain (C), and the Cx. quinquefasciatus JHB strain (D) were fed an attractive pellet containing fishmeal and 20% gelatin. Larvae were treated with one of three different types of pellets: no-bacterium controls (black lines), live Chromobacterium Csp_P (red lines), or nonlive Chromobacterium Csp_P (blue lines). Nonlive Csp_P was an effective larvicide for each line, with average times to death ± the standard errors of the mean (SEM) of 3.13 ± 0.08 days for ROCK, 3.40 ± 0.13 days for NR-48830, 2.25 ± 0.09 days for Keele, and 1.68 ± 0.05 days for JHB. Larvae were reared in groups of 25 to 30 in 300 ml of deionized water. Lines depict the percentages of larvae surviving at each day posttreatment (± SEM) for three experimental replicates, with each containing three cages per treatment.
Larvicidal dose-response of nonlive Csp_P powder.
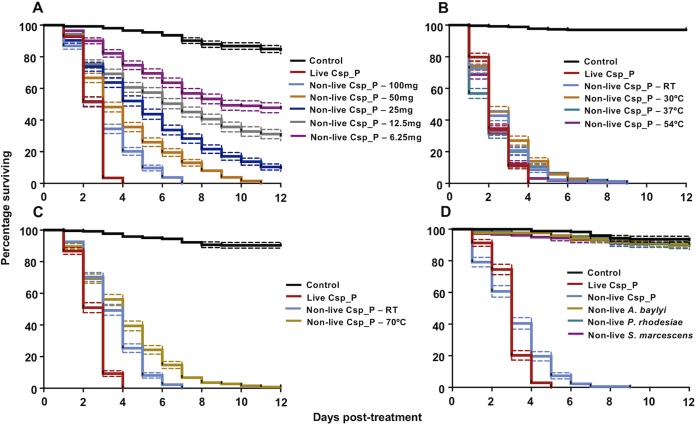
To assess the efficacy of nonlive Csp_P at different doses, we performed three experiments where we provided larvae with gelatin/fishmeal pellets containing 100, 50, 25, 12.5, or 6.25 mg of nonlive Csp_P powder (Fig. 3A). We observed that larvae treated with all five doses had significantly greater mortality than the control treatment (Cox regression: P < 0.0001 for all comparisons). One hundred percent mortality was observed with the 100-mg treatment, while >99% mortality was observed with the 50-mg treatment at 12 days postexposure. No pupation was observed in either of these conditions, while control larvae pupated 4 to 6 days after the start of the experiment. A small number of adults were observed in the 12.5- and 6.25-mg treatments, with average mortalities of 70.34 and 56.36% observed in these treatments, respectively, at 12 days posttreatment. Based on the results of these experiments, we calculated the 50% lethal dose (LD50) of nonlive Csp_P in our experimental setup (30 larvae in 300 ml of water) to be 3.40 mg of powder. This was equal to an LD50 of 11.35 mg of nonlive Csp_P powder per liter of water.
FIG 3.
The nonlive Csp_P biopesticide has a low effective dose and a durable active ingredient that is not produced by other common mosquito-associated bacteria. ROCK larvae were fed pellets containing different doses of nonlive Csp_P (A). Doses of 100 and 50 mg killed 100% of larvae, while doses of 25, 12.5, and 6.25 mg yielded partial mortality and delayed pupation. An LD50 was calculated at 11.35 mg per liter of water in the larval habitat. Nonlive Csp_P powder was heat treated at room temperature (22°C), 30, 37, or 54°C (B) and, in independent experiments, at room temperature (22°C) or 70°C (C) in accelerated shelf life tests in order to assess the durability of the active ingredient. The 30, 37, and 54°C treatments did not differ in efficacy from the room temperature treatment (Cox regression; P > 0.05), while the 70°C treatment still killed 100% of the larvae exposed but took significantly longer to do so (Cox regression: P < 0.0001), with these results suggesting that the active ingredient was highly heat stable and likely to have a long shelf life. (D) Three other common mosquito-associated bacterial samples were cultured and dried according to the same protocol used to produce nonlive Csp_P powder. Then, 100 mg of each of these powders was added to attractive pellets and provided to ROCK larvae. None of these three preparations caused mortality that was significantly different from that seen for larvae treated with no-bacterium control pellets (Cox regression: P > 0.05), suggesting that the larvicidal effect we observed in our results was not due to the culturing methods and not universal among all bacteria. In all experiments, larvae were reared in groups of 30 in 300 ml of deionized water. Lines depict the percentages of larvae surviving at each day posttreatment (± SEM) for three experimental replicates, with each containing three cages per treatment.
Nonlive Csp_P powder is highly heat stable.
In order to assess the potential shelf life of nonlive Csp_P powder, we performed accelerated shelf life tests, where we treated the powder at 30, 37, and 54°C (Fig. 3B) or 70°C (Fig. 3C) for 2 weeks and then assessed the impact on larvicidal activity by comparing these treatments against powder left at room temperature for 2 weeks, with powder from each treatment independently incorporated into gelatin/fishmeal pellets. We observed that the mortality of all of nonlive Csp_P treatments was significantly greater than that of the control treatment (Cox regression: at room temperature, W = 178.65, df = 1, exp(β) = 140.13, P < 0.0001; at 30°C, W = 170.88, df = 1, exp(β) = 126.57, P < 0.0001; at 37°C, W = 185.77, df = 1, exp(β) = 152.63, P < 0.0001; at 54°C, W = 189.91, df = 1, exp(β) = 164.63, P < 0.0001). Critically, we observed no difference in activity between the heat-treated powders and the powder left at room temperature (Cox regression: P > 0.05).
Experiments with nonlive Csp_P powder treated at 70°C were run independently due to incubator availability. In these experiments, we observed that both the room temperature and 70°C treatments had significantly greater mortality than the control treatment (Cox regression: at room temperature, W = 285.91, df = 1, exp(β) = 44.74, P < 0.0001; at 70°C, W = 254.31, df = 1, exp(β) = 32.41, P < 0.0001). We also observed a slight loss of larvicidal activity with the 70°C treatment, although 99.2% of the larvae that were exposed died within 12 days. The average mortality for the 70°C treatment was 4.03 ± 0.14 days compared to 3.48 ± 0.09 for the room temperature treatment (Cox regression: W = 12.83, df = 1, exp(β) = 0.72, P < 0.0001).
While conducting the above-described experiments, we noticed that our gelatin-based pellets dissolved within 2 to 3 days after being added to water. Consequently, we developed an agar-based pellet formulation that maintained structural integrity when left submerged in water for 14 days (see Fig. S2A in the supplemental material). Interestingly, when Ae. aegypti larvae were added to containers where the water had been preexposed to nonlive Csp_P agar pellets for 14 days, we observed significantly higher mortality than for larvae treated with freshly made nonlive Csp_P agar pellets (Cox regression: W = 31.031, df = 1, exp(β) = 2.07, P < 0.0001). However, there was no significant effect on mortality due to preexposure for nonbacterial control pellets (Cox regression: W = 0.59, df = 1, exp(β) = 0.67, P = 0.442). We then exposed control and nonlive Csp_P agar pellets to different temperatures (room temperature, 37°C, or 54°C) for 7 days in order to determine whether temperature treatment had an impact on the larvicidal activity of agar pellets (Fig. S2B). We observed no significant influence of temperature on the larvicidal activity of control pellets (Cox regression: W = 0.15, df = 2, P = 0.929) or nonlive Csp_P pellets (Cox regression: W = 2.80, df = 2, P = 0.246).
Effect with other bacteria.
We performed the same nonlive Csp_P culturing and drying procedure described above, with three other mosquito-associated bacteria: Acinetobacter baylyi, Serratia marcescens, and Pseudomonas rhodesiae, in order to demonstrate that the larvicidal effect we observed after feeding on nonlive Csp_P powder was not due to the culturing and/or drying processes and was not ubiquitous across all bacteria fed to mosquito larvae in this manner (Fig. 3D). Across three experiments, we observed that only nonlive Csp_P powder had significantly different mortality to the no-bacterium control treatment (Cox regression: W = 180.34, df = 1, exp(β) = 75.20, P < 0.0001). The hazard ratios associated with feeding powders derived from A. baylyi, S. marcescens, and P. rhodesiae cultures were 1.46, 1.56, and 1.51, respectively, compared to the control treatment, indicating that there was no significant larvicidal effect associated with powders derived from these three bacteria.
Nonlive Csp_P powder exerts larvicidal activity under field conditions.
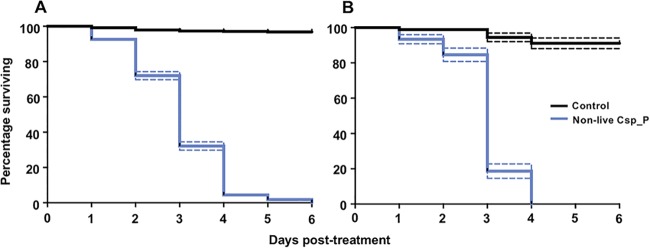
We assessed the efficacy of the nonlive Csp_P powder in a semi-field setting. These experiments were conducted at a semi-field facility in Gurabo, Puerto Rico. In the first of three trials, we tested the nonlive Csp_P powder against larvae from the Centers for Disease Control and Prevention (CDC) San Juan Ae. aegypti colony (Patillas strain) (Fig. 4A). Larvae were moved to small cups containing 300 ml of tap water, treated with gelatin/fishmeal pellets containing 200 mg of nonlive Csp_P powder, and the cups left in the semi-field cage, exposed to ambient environmental conditions. We observed that mortality was significantly increased in the treated cups compared to the control cups (Cox regression: W = 207.85, df = 1, exp(β) = 87.59, P < 0.0001), with 100% mortality within 6 days compared to 2.93% mortality in the control treatment cups over that time. The average time to death for insecticide-treated larvae was 3.03 ± 0.05 days. We then performed similar experiments with mosquitoes from the CDC Aedes mediovittatus colony (Fig. 4B) and observed that all larvae died within 4 days of treatment, with an average time to death of 2.97 ± 0.08 days (Cox regression: W = 64.39, df = 1, exp(β) = 20.64, P < 0.0001).
FIG 4.
Nonlive Csp_P powder effectively kills mosquito larvae under semi-field conditions. Ae. aegypti Patillas (A) and Ae. mediovittatus (B) larvae were reared under insectary conditions for 3 days and then transferred to a semi-field facility at Gurabo, Puerto Rico. Larvae were treated with a no-bacterium control pellet (black lines) or a pellet containing 200 mg of nonlive Csp_P (blue lines) and then left under ambient environmental conditions. We observed significant mortality induced by nonlive Csp_P for larvae from both species (Cox regression: P < 0.0001), indicating that the larvicide performed effectively under semi-field conditions. Larvae were reared in groups of 30 in 300 ml of tap water. Lines depict the percentages of larvae surviving at each day posttreatment (± SEM).
Next, we collected Ae. aegypti eggs using oviposition cups deployed at eleven different sites across Puerto Rico. These egg papers were returned to the laboratory and hatched in tap water. G1 larvae from these populations were transferred to the semi-field cage. The larvae from each population were split into two cups; half were fed a control pellet, and half were fed a nonlive Csp_P pellet. Survival was monitored in these cups for 6 days, at which point 100% of the insecticide-treated larvae had died (Fig. 5). Over this time period 6/143 (6.29%) of the control larvae had died (Cox regression: W = 112.61, df = 1, exp(β) = 49.17, P < 0.0001). For Csp_P-exposed mosquitoes across all 11 populations, there was an average time to death of 2.73 ± 0.08 days.
FIG 5.
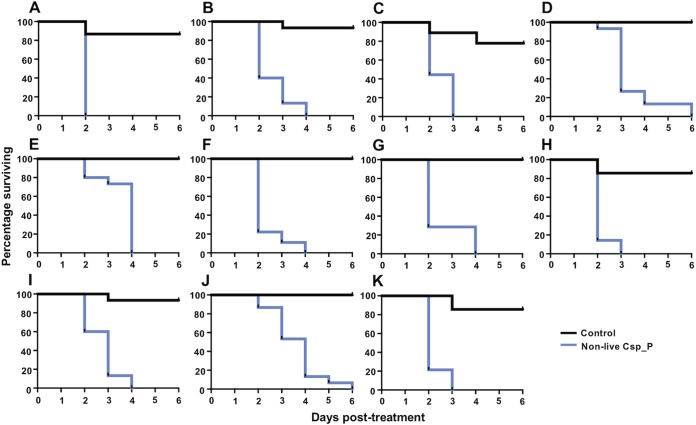
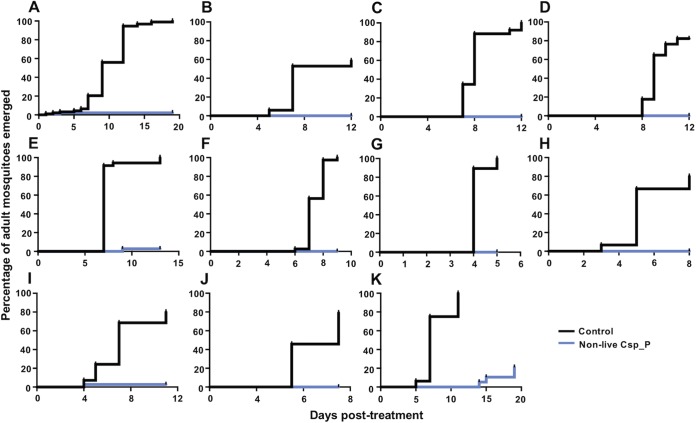
Nonlive Csp_P powder effectively kills larvae from different Ae. aegypti G1 field-derived populations under semi-field conditions. Ae. aegypti eggs were collected from 11 different sites around Puerto Rico using oviposition cups. Egg papers were hatched under insectary conditions, typed for mosquito species, and then transferred to the semi-field cage. Larvae from each population were divided in two, with half fed a control pellet (black lines) and half fed a pellet containing 200 mg of nonlive Csp_P (blue lines), and then left under ambient environmental conditions. A 100% mortality for all Csp_P-treated larvae was achieved in 2 to 6 days. Each panel depicts a different Ae. aegypti population: Bayamon (A), Catano (B), Guayanilla (C), Gurabo (D), Humacao (E), Juncos (F), Loiza (G), Ponce (H), Toa Alta (I), Trujillo Alto (J), and Yauco (K). Larvae were reared in groups of 7 to 15 in 300 ml of tap water. Lines depict the percentages of larvae surviving at each day posttreatment.
We then sought to assess the activity of the nonlive Csp_P powder against field-collected larvae in their natural breeding habitats. We collected larvae and water from 11 different breeding sites across eastern Puerto Rico. The sites included a variety of plastic containers, pails, and tires (Table 2). Larvae from these breeding sites were taxonomically identified and fell into three categories: (i) Aedes aegypti only, (ii) Aedes aegypti and Culex quinquefasciatus, and (iii) Culex quinquefasciatus only. The water and larvae within these breeding sites were transported to the semi-field facility and then divided into two cups, with half fed a control pellet and half fed a pellet containing nonlive Csp_P. In these experiments (Fig. 6), we monitored adult eclosion rather than larval mortality, since the breeding site water was typically too opaque to perform an accurate assessment of larval mortality. Across all breeding sites, there were 374 larvae involved in each of the control and nonlive Csp_P treatments. For the control treatment, 342 larvae eclosed as adults (91.44%) and 32 died during the course of the experiment. For nonlive Csp_P-treated cups, 9 adult mosquitoes eclosed (2.41%), 5 females and 4 males, and 365 larvae died (Fisher exact test: odds ratio = 433.4, P < 0.0001). Cox regression analysis indicated that treatment with nonlive Csp_P was a significant factor affecting the likelihood of adult eclosion, with larvae from a nonlive Csp_P-treated cup 125 times less likely to eclose than untreated larvae (Cox regression: W = 179.11, df = 1, exp(β) = 0.008, P < 0.0001).
TABLE 2.
Characteristics of the 11 breeding sites collected around Puerto Rico
| Site | Site type | Collection datea | Collection location | No. of larvae and/or pupaeb | Mosquito genus or genera |
|---|---|---|---|---|---|
| A | Tire | 14 Feb 18 | Caguas | 186 | Aedes/Culex |
| B | Paint bucket | 15 Mar 18 | Cataño | 20 | Aedes |
| C | Water meter | 15 Mar 18 | Cataño | 52 | Culex |
| D | Plastic cup | 22 Mar 18 | Cataño | 28 | Aedes |
| E | Plastic bucket | 21 Aug 18 | Puerto Nuevo | 70 | Aedes/Culex |
| F | Trash container | 22 Aug 18 | Cupey | 78 | Aedes/Culex |
| G | Paint bucket | 22 Aug 18 | Cupey | 38 | Aedes |
| H | Metal pipe | 18 Sep 18 | Puerto Nuevo | 24 | Aedes |
| I | Metal bucket | 25 Oct 18 | Salinas | 112 | Aedes |
| J | Plastic container | 25 Oct 18 | Salinas | 38 | Aedes/Culex |
| K | Wastewater tank | 25 Oct 18 | Salinas | 38 | Culex |
Breeding sites were translocated to the semi-field facility on this date and then treated with the nonlive Csp_P biopesticide.
Total larvae and pupae observed in the breeding site water.
FIG 6.
Nonlive Csp_P powder effectively prevents adult mosquito emergence from translocated breeding sites under semi-field conditions. Mosquito breeding sites were located at various sites around eastern Puerto Rico. The water and any larvae and pupae were removed from the breeding site and moved to the semi-field cage in sterile containers. Larvae and pupae were counted and, along with the water and any detritus, divided evenly between a control treatment (black lines) and a nonlive Csp_P treatment (blue lines). Adult emergence was monitored since it was too difficult to locate dead L1 and L2 larvae in the opaque breeding site water. Across eleven different breeding sites, we observed that 342/374 adults emerged from the control treatment compared to 9/374 from the nonlive Csp_P treatment (Fisher exact test: P < 0.0001). Breeding sites contained either Ae. aegypti larvae (n = 5), Cx. quinquefasciatus larvae (n = 2), or a mix of both (n = 4). Breeding sites were collected from the following receptacles: tire (A), paint pail (B), water meter (C), plastic cup (D), bucket (E), container (F), bucket (G), pipe (H), bucket (I), container (J), and wastewater tank (K). Lines depict the percentages of adults that had emerged from the breeding site water at different intervals posttreatment.
DISCUSSION
We have developed a novel insecticide based on a nonlive, air-dried preparation of the bacterium Chromobacterium species Panama (Csp_P). Previous reports from our group have demonstrated that live Csp_P is a highly effective mosquitocidal agent that can rapidly kill the larvae and adults of mosquito vectors of medically important pathogens, including An. gambiae and Ae. aegypti (27, 30). Given the potential environmental and human/animal health concerns associated with an insecticidal preparation based on live bacteria, as well as complications with storage and shelf life, we sought to develop a formulation containing nonlive Csp_P that also retained killing activity against mosquito larvae.
Although the development of a potential mosquitocidal formulation involving nonlive Csp_P is still in the early stages, the biopesticide that we have developed possesses many desirable properties for an insecticide. Our results confirm that no Csp_P cells survive the air-drying process used during preparation, indicating that releasing this product into the field would not spread live bacteria. The current formulation is fast-acting, and capable of killing mosquito larvae within an average of 2 to 3 days of postexposure. Interestingly, we observed that exposure to nonlive Csp_P halted larval development, which potentially facilitated a greater window of time for larvae to ingest the biopesticide and be killed. This was in line with what we observed in a previous study, where the larvae of An. gambiae females that survived treatment with live Csp_P experienced a developmental delay (31). We also determined that the powder had an LD50 of 11.36 mg per liter of larval rearing water under laboratory conditions. Since mass culturing of bacteria can be quite expensive, such a low effective dose is a highly beneficial trait.
In addition, the powder had broad larvicidal activity against a broad range of mosquito targets, including those of high epidemiological importance. This included the prominent mosquito vectors An. gambiae, Ae. aegypti, and Cx. quinquefasciatus, as well as Ae. mediovittatus. The Csp_P biopesticide was most effective against Cx. quinquefasciatus larvae, where we observed a shorter average time-to-death postexposure than for the other species. Mosquitoes of the Culex genus include prominent vectors of West Nile virus, Japanese encephalitis virus, and the nematodes that cause filariasis. All of these pathogens have significant impacts on human health. Nonlive Csp_P powder also effectively kills larvae from the pyrethroid-resistant Ae. aegypti NR-48830 line, which was expected given that this line had not previously been exposed to our insecticide. Given the widespread usage of pyrethroids in mosquito control programs around the world, there are high levels of pyrethroid resistance among mosquito populations (32, 33), and our data suggest that the biopesticide could prove to be a good candidate compound for a novel insecticide to target these resistant populations.
Nonlive Csp_P powder also appears to be highly temperature stable, with accelerated shelf life assays demonstrating that the larvicidal activity is unaffected by heat treatment at 54°C for 2 weeks, which is comparable to storage at room temperature for 1 year. Interestingly, while treatment at 70°C for 2 weeks did lead to slightly reduced activity, more than 95% of the larvae that were exposed were still killed. In addition, heat treatment of whole agar pellets for 7 days had no significant impact on larvicidal activity. All of these results indicate that the as-yet-uncharacterized active ingredient in our insecticide is highly heat stable and that nonlive Csp_P powder will likely have a shelf life in excess of 1 year.
Our data demonstrate that nonlive Csp_P powder is an effective larvicide against laboratory- and field-derived mosquitoes, under semi-field conditions. Experiments were conducted at a semi-field facility in Puerto Rico, mosquito larvae were kept in a contained environment, under a tarpaulin, but otherwise exposed to ambient environmental conditions. We observed that exposure to nonlive Csp_P powder was highly effective at killing laboratory-reared Ae. aegypti and Ae. mediovittatus larvae that were moved to the field site at 3 days posthatching. We saw similar efficacy when the biopesticide was trialed against G1 larvae from 11 different field-derived Ae. aegypti populations that were collected from around Puerto Rico, where all larvae died within 4 days of exposure. Critically, the Csp_P biopesticide was highly effective at preventing the emergence of adult Ae. aegypti and/or Culex mosquitoes from natural breeding site water, suggesting that it could be successfully deployed to target a range of different mosquito populations and larval habitats in the field.
The cause of the larvicidal activity observed with the nonlive Csp_P powder is still unclear. We observed larvicidal activity for fishmeal/gelatin pellets containing either live Csp_P or nonlive Csp_P. However, larvae that were fed on pellets containing live bacteria died approximately 1 to 2 days sooner on average than those fed on nonlive bacterial powder. It is possible that some factor involved in generating larvicidal activity was lost during the air drying process, that there were differences in concentrations of larvicidal factors between the live and nonlive treatments, or even that proliferation of live Csp_P in the larval gut could have led to increased levels of the insecticidal agent(s). Previous data from our group indicates that live Csp_P can mediate mosquito death through the production of hydrogen cyanide (30). However, we did not detect cyanide in any of the five nonlive, air-dried Csp_P powders that we tested. This result was not particularly surprising given that cyanide is a volatile compound that is known to be lost from Csp_P cultures during evaporation (30). These observations may indicate that the nonlive Csp_P powder might kill mosquito larvae through alternative mechanisms to the live bacteria or that some factors that mediate mosquito killing in live Csp_P are lost during the air-drying process. To fully elucidate these differences, it will likely be necessary to identify the active ingredient associated with larval killing in the nonlive Csp_P powder.
Our findings suggest that not all bacteria have larvicidal activity when fed to mosquitoes as nonlive powders, indicating that the larvicidal effects we observed with the nonlive Csp_P powder were not simply a by-product of the culturing protocol that we used. At this stage, it is still unclear whether this larvicidal active ingredient in the powder is something that is specific to Csp_P or something common among members of the genus Chromobacterium. The latter could be quite likely, given that many Chromobacterium species have larvicidal properties when fed live to mosquitoes (30). Interestingly, there is already a commercially available insecticide Grandevo (Marrone BioInovations), which was developed from a preparation of the bacterium Chromobacterium subtsugiae, that is used to target agricultural pest species (34, 35). It is unclear whether Grandevo shares a mechanism of action with nonlive Csp_P powder.
Study limitations and future directions.
While our results are encouraging, there are still multiple factors that need to be addressed to determine whether the nonlive Csp_P formulation can become an effective mosquitocidal tool. There is a need to develop a scale-up compatible culturing protocol that minimizes production time and costs and maximizes yield of the active ingredient without compromising the stability and the efficacy found in the current formulation. The current culturing protocol is unusual in that it involves both liquid and solid media. This method allowed the bacteria to form a very thick biofilm, which was potentially enriched for our unknown active ingredient(s). However, the culturing process was laborious and would be unlikely to be cost-effective or suitable for mass production. Consequently, there is a clear need to develop an optimized formulation for producing the biopesticide. Ideally, this should be based solely on liquid media, since this would simplify the mass production of the biopesticide using standard fermentation technology. Identification of the active ingredient and developing a method of quantifying levels of that ingredient in culture could potentially expedite the process and could allow for improved larvicidal activity. Making these changes could potentially offer scope to improve powder yield, while decreasing time costs associated with production.
We will also need to consider the issue of residual activity of our formulation. In our experiments we observed that gelatin-based pellets dissolved in water quite rapidly, while agar-based pellets had greater structural integrity and appeared to retain or improve larvicidal activity when left in water for 2 weeks prior to treatment. An effective larvicidal agent must not persist in the environment for too short a time, since it could necessitate more frequent treatment. Consequently, evaluating the stability and persistence of the biopesticide in water will be a key concern going forward. Ideally, it would not persist for too long, since this risks a loss of activity over time, meaning that larvae would get exposed to lower-than-optimal doses and then be more likely to develop resistance. For these reasons, we plan to investigate whether larvae can develop resistance to nonlive Csp_P powder and to investigate potential mechanisms of resistance in a future study.
We will seek to test an optimized formulation against adult mosquitoes and against other animal species, particularly agricultural pests and other vectors, since this will indicate whether the nonlive Csp_P biopesticide has a broader scope for potential use. We will perform rigorous testing of the biopesticide in line with WHO Pesticide Evaluation Scheme guidelines to determine whether the biopesticide affects nontarget species, including beneficial insects such as honeybees. We will also investigate potential ecological and health and safety concerns associated with deployments into the environment by testing the biopesticide against mammals. It should be noted that there are attractive toxic baits used to target mosquitoes, which can be developed in a way that prevents nontarget insects from feeding (36–38), and we will look at utilizing this technology for future trials with adult mosquitoes.
Bacteria from the genus Chromobacterium are highly abundant in nature and have been isolated from a wide variety of environments (39–44). As such, any insecticidal compounds they produce are likely already present in nature, although not at the levels that would be found during a field trial of a Chromobacterium-based biopesticide. It is important to remember that similar biopesticides are already in use in the field. In the case of Grandevo, it has been demonstrated that toxicity does not occur ubiquitously in all arthropods that are exposed (34), indicating that Chromobacterium-based biopesticides may not be universally toxic among arthropods.
Conclusions.
We have developed a novel biopesticide based on a nonlive air-dried preparation of the bacterium Chromobacterium sp. Panama, which is highly effective at killing the larvae of multiple mosquito species, including key vectors of malaria, and the dengue and Zika viruses. This insecticide is still in the early stages of development, but it displays many beneficial properties for an insecticide, including a low effective dosage and an active ingredient that appears to be highly heat stable. Critically, our data demonstrate that the nonlive Csp_P biopesticide is highly effective at preventing the emergence of adult mosquitoes under semi-field conditions.
MATERIALS AND METHODS
Mosquito lines.
Multiple mosquito lines were used in the experiments described here. The majority of the laboratory experiments were performed using the Aedes aegypti Rockefeller strain. The pyrethroid-resistant Ae. aegypti line (BEI Resources, NR-48830) was purchased from BEI Resources (Manassas, VA). Experiments involving this line were performed using F15 generation mosquitoes, one-generation postinsecticidal treatment. Additional laboratory experiments were performed using the Culex quinquefasciatus JHB strain (originally isolated in Johannesburg, South Africa; BEI Resources, NR-43025) and the Anopheles gambiae Keele strain, obtained from the Johns Hopkins University Malaria Research Institute Insectary. Laboratory mosquitoes were hatched in DI water mixed with our laboratory’s Aedes diet (one part tropical fish flakes [Ken’s Fish], one part rabbit chow [Nature’s Promise], and two parts liver powder [Now Foods]). At 2 days after hatching, L2-stage larvae were thinned to a density of 250 per 1.5 liters of DI water and then maintained on dry cat food pellets until the start of experiments. All laboratory mosquito strains were maintained in a climate-controlled insectary (temperature, 27°C ± 1°C; relative humidity [RH], 80% ± 10%), with a 14:10-h day-night cycle.
Experiments in the semi-field facility at Gurabo, PR, involved Ae. aegypti Patillas strain, and Ae. mediovittatus, both derived from previously described CDC San Juan mosquito colonies (45, 46). These colonies were maintained on 10% sucrose and were kept in an insectary facility at 25 to 27°C, a RH of approximately 75%, and a 12-h light-dark cycle. Eggs from both colonies were hatched in tap water and maintained on rabbit food. At 3 days posthatching, larvae were transferred to the semi-field facility for experiments. Field-derived Aedes and Culex mosquitoes were also used in experiments conducted at our semi-field facility. Eggs from 11 mosquito populations were collected from different neighborhoods around Puerto Rico (Bayamon, Catano, Guayanilla, Gurabo, Humacao, Juncos, Loiza, Ponce, Toa Alta, Trujillo Alto, and Yauco). At least three oviposition cups containing water or hay infusion and paper as an oviposition medium were left at each site. Cups were left out for approximately 7 days and then collected. Egg papers were returned to the CDC, dried, hatched, and reared on rabbit food until adulthood. Only Aedes aegypti mosquitoes from these collections were used in subsequent experiments. In another set of experiments, larvae and water were collected from breeding sites that were discovered during surveys of different neighborhoods in eastern Puerto Rico. These mosquitoes were transferred directly to the semi-field facility for experiments. Field-collected mosquitoes were identified to the genus and/or species level, where possible.
Larvicidal pellet formulation and laboratory experimental design.
For laboratory experiments, groups of 30 L3 larvae were moved to mosquito breeders (height, 19.5 cm, base diameter, 11 cm; Bioquip, catalog no. 1425) containing 300 ml of DI water at 3 days posthatching. Three replicate breeders were set up for each treatment. Breeders were treated with either a negative-control pellet, a live bacterial control pellet, or a pellet containing nonlive Csp_P powder (see below). The control pellets contained 100 mg of fishmeal (Dirty Gardener) as an attractant, 500 μl of LB broth (Lennox; Sigma-Aldrich, catalog no. L3022), and 500 μl of 20% gelatin solution (Sigma-Aldrich, catalog no. 53028) as a stabilizing agent. For control pellets, this formulation provided sufficient nutrients for 30 larvae to pupate and eclose. In place of LB broth, the live bacterial control pellets contained 500 μl of Csp_P grown in LB broth for 16 h on a shaker at 30°C at a speed of 200 rpm. The nonlive Csp_P pellets had the same composition as the negative-control pellet but also contained 100 mg of nonlive Csp_P powder. After preparation, pellets were allowed to set at 4°C for 1 to 2 h and then added to a mosquito breeder. Mosquito survival was then monitored daily for 12 days, with adults provided cotton soaked in 10% sucrose, which was refreshed daily. Each experiment was repeated three times and involved three replicate cages per treatment.
Nonlive Csp_P powder preparation.
Five different air-dried, nonlive Csp_P powders were evaluated for their ability to kill Rockefeller larvae. Three preparations (nonlive_1, nonlive_2, and nonlive_3) were derived from Csp_P cultures on sterile 400-cm2 petri dishes (Coning), each containing approximately 200 ml of LB agar (Sigma-Aldrich, catalog no. L2897). Each plate was inoculated with 2 ml of live Csp_P stock (1:1 in 50% glycerol solution, stored at −80°C) and then left to grow for 48 h in an incubator at 30°C. For the nonlive_2 preparation, bacterial cells were removed from the agar using a cell scraper (Sarstedt) and then transferred to a petri dish to dry. For the nonlive_1 and nonlive_3 preparations, 50 ml of LB broth was added to the surface of each plate. Plates were then incubated for a further 120 h at room temperature. At that point, the liquid on the surface of each plate was decanted and dried down to become the nonlive_3 preparation. Plates were left to dry for a further 24 h, and then the bacterial cells on the surface were removed with a scraper and dried to become the nonlive_1 preparation. The final two preparations, nonlive_4 and nonlive_5, were cultured in sterile 6-well plates (Costar, catalog no. 3506). Briefly, each well containing 5 ml of LB broth was inoculated with 5 μl of live Csp_P stock, and the plates were sealed in Parafilm and then left to grow for 72 h at room temperature. For the nonlive_5 preparation, biofilm was collected from the surface of each well, mixed with sterile 1× PBS and then dried. The nonlive_4 preparation contained the remaining material from the 6-well plate after the surface biofilm was removed. This, too, was air dried at room temperature. All preparations were dried under continuous airflow in a fume hood. Four preparations (nonlive 2 through 5) were completely dried over the course of 2 to 3 days. The final preparation (nonlive 1) required a longer period to dry completely due to a greater volume of material. After drying, each preparation was manually crushed to a fine powder using a mortar and pestle. Pellets containing the different powders were prepared, as described above, and then fed to Rockefeller larvae to assess their larvicidal activity.
To validate that each of these powders contained no live Csp_P cells, we collected 100 mg of each preparation immediately after the air-dried bacteria had been crushed to a powder. The powders were moved to sterile 1.5-ml tubes and then mixed with 1 ml of sterile 1× PBS. Three dilutions were prepared for each powder (100, 102, and 104) through serial dilution in sterile 1× PBS, and 100 μl from each of these tubes was inoculated onto sterile LB agar plates (without antibiotics) and spread using sterile glass beads. The plates were inverted and then placed in an incubator set to 30°C for 48 h, with these conditions being optimal for culturing live Csp_P. These experiments were performed three times, each from independent batches of air-dried powders.
We assayed for the presence of cyanide containing species in each of the five nonlive powders using a cyanide test kit (model CYN-3; Hach, catalog no. 2010-02). Each preparation was cultured, air dried, and crushed to powder, as described above, and then used for testing within 1 week after the air drying process was finished. A total of 10 mg of each powder was dissolved in 10 ml of MilliQ water in a 15-ml tube. These tubes were then mixed by hand until the contents went into suspension. Finally, 5 ml from each tube was used in the cyanide test, which was completed according to the manufacturer’s instructions (30).
Assaying nonlive Csp_P activity.
Unless specified below, all experiments utilized 100 mg of nonlive Csp_P powder, prepared according to the nonlive_1 Csp_P protocol, as described above. Pellets containing the biopesticide were prepared and then fed to Rockefeller larvae, pyrethroid-resistant Ae. aegypti ROCK larvae, Cx. quinquefasciatus larvae, and An. gambiae larvae, in order to assay whether the powder could kill multiple mosquito vector species and mosquitoes that were resistant to commonly used insecticides. In further experiments, pellets containing different quantities of nonlive_1 Csp_P powder (100, 50, 25, 12.5, and 6.25 mg) were offered to ROCK larvae in order to evaluate the larvicidal properties of lower doses and calculate the LD50 of that preparation. To assess whether the larvicidal activity occurred as a result of culturing method, we cultured the mosquito-associated bacteria Acinetobacter baylyi, Pseudomonas rhodesiae, and Serratia marcescens using the nonlive_1 protocol and fed these to Rockefeller larvae. These species were grown from frozen stocks that were already present in our laboratory, stored at −80°C in 50% glycerol and LB broth (47).
Accelerated shelf life testing.
The EPA guidelines for product development indicate that accelerated shelf life tests be performed to assess stability of the active agents in a product (48). Under the suggested guidelines, a product treated at 54°C for 2 weeks is comparable to 1 year spent at room temperature. Nonlive_1 Csp_P powder was transferred to 50-ml plastic tubes (Falcon) and wrapped in one layer of aluminum foil, with this setup serving as a mock commercial packaging. The tubes of powder were then left at room temperature, 30, 37, 54, or 70°C for 2 weeks in incubators. Due to incubator availability, the 70°C treatment was performed independently; however, comparisons in these experiments were made using a separate batch of room temperature nonlive_1 Csp_P powder that was prepared during the same period. Pellets were made from each preparation and fed to Rockefeller larvae.
Increasing pellet stability.
To improve pellet stability, we developed a revised pellet formulation with 1.5% agar (Sigma-Aldrich, A1296) substituting for gelatin as the stabilizing agent, and LB broth was excluded from the recipe. These agar pellets displayed increased integrity in water, remaining intact for weeks, as opposed to days for gelatin-based pellets. To assess the integrity of the revised formulation, we performed two experiments. In these experiments, control pellets contained 100 mg of fishmeal as an attractant, and 1 ml of 1.5% agar, while nonlive Csp_P pellets also contained 125 mg of nonlive_1 Csp_P powder. Assay conditions and sample size were as described for the gelatin pellet experiments. Each experiment was repeated three times, and these replicates contained two technical replicates of each pellet type/treatment. In the first experiment, we assessed the residual activity of agar pellets. One batch of agar pellets was prepared and immediately placed into individual mosquito breeders, each containing 300 ml of DI water. These breeders and pellets were left undisturbed for 14 days at room temperature. After this time, a further batch of pellets was prepared and added to mosquito breeders. Ae. aegypti larvae were then added to all breeders, and survival was monitored daily, as described above. Second, we assessed the activity of whole agar pellets after heat treatment. Control and nonlive Csp_P agar pellets were prepared as described above. Pellets were sealed in plastic wrap to prevent moisture loss, and then left at room temperature, at 37°C, or at 54°C for 7 days. Pellets were then fed to Ae. aegypti larvae, and survival was monitored daily, as described above.
Semi-field trials with CDC colony and field-derived mosquitoes.
For semi-field experiments, pellets were made, as above, at the semi-field site, except that LB broth and gelatin stocks were not prepared under sterile conditions. The dose of Csp_P powder in the pellets used in these experiments was increased to 200 mg to account for high larval numbers in some breeding sites. Nonlive Csp_P powder stocks used in these experiments were prepared at Johns Hopkins University and shipped to Puerto Rico. We first assessed the impact of the powder on Ae. aegypti Patillas and Ae. mediovittatus colony mosquitoes. Larvae were transported to the field cage at 3 days posthatching and then divided into small plastic cups containing 300 ml of tap water, 30 larvae to a cup, with four cups of each treatment used per experiment. Larval survival was then monitored daily for 6 days. Next, we assessed the efficacy of the nonlive Csp_P powder on G1 larvae from 11 Ae. aegypti populations collected around Puerto Rico. Eggs from each population were hatched and then taken to the field cage 2 days later. Since the G1 larval numbers were low, a maximum of 15 per cup were used, with one cup per treatment, per population. In these experiments, survival was monitored every 1 to 3 days.
For experiments involving larvae and water collected from breeding sites in the field, larvae and water were transferred to sterile plastic cups using sterile pipettes and then transported to the semi-field cage where experiments were conducted. The volume of water was measured using plastic measuring cups and divided evenly between two plastic containers. Larvae and pupae from each breeding site were divided into these containers. One cup was fed a negative-control pellet, while the other was fed a pellet containing nonlive Csp_P powder. Cups were then covered in mesh to prevent adults from escaping. In these experiments, adult eclosion was monitored every 1 to 3 days by counting and then removing adults in each cup.
Statistical analysis.
For all experiments, survival data were compared across replicate experiments using Cox proportional hazard models within SPSS v17 (IBM). For field breeding site experiments, the proportion of mosquitoes that eclosed was compared between treatment and control groups using the Fisher exact test (Prism v6.0h; GraphPad) and Cox proportional hazard models (SPSS v17; IBM). Figures were created using Prism v6.0h (GraphPad) and Microsoft PowerPoint for Mac (v16.19).
Supplementary Material
ACKNOWLEDGMENTS
We thank Manuel Amador and Veronica Acevedo, CDC Dengue branch, Puerto Rico, for assistance with mosquito identification. We are also grateful to Chris Kizito and the JHMRI insectary for providing Anopheles gambiae larvae, Hannah J. McLeod for providing Culex quinquefasciatus larvae, and members of the Dimopoulos Group for helpful discussions about the project.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.World Health Organization. 2018. World malaria report. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.World Health Organization. 2019. Malaria: fact sheet, 27 March 2019. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 3.World Health Organization. 2019. Dengue and severe dengue: fact sheet, 15 April 2019. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 4.World Health Organization. 2017. Chikungunya: fact sheet, 12 April 2017. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 5.World Health Organization. 2019. Yellow fever: fact sheet, 7 May 2019. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 6.World Health Organization. 2018. Zika virus: fact sheet, 20 July 2018. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 7.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons CP, Scott TW, Farrar JJ, Hay SI. 2013. The global distribution and burden of dengue. Nature 496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gould EA, Higgs S. 2009. Impact of climate change and other factors on emerging arbovirus diseases. Trans R Soc Trop Med Hyg 103:109–121. doi: 10.1016/j.trstmh.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gubler DJ. 2011. Dengue, urbanization and globalization: the unholy trinity of the 21st century. Trop Med Health 39:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weaver SC, Reisen WK. 2010. Present and future arboviral threats. Antiviral Res 85:328–345. doi: 10.1016/j.antiviral.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dutra HL, Caragata EP, Moreira LA. 2017. The re-emerging arboviral threat: hidden enemies: the emergence of obscure arboviral diseases, and the potential use of Wolbachia in their control. Bioessays 39:1600175. doi: 10.1002/bies.201600175. [DOI] [PubMed] [Google Scholar]
- 12.Conrad MD, Rosenthal PJ. 2019. Antimalarial drug resistance in Africa: the calm before the storm? Lancet Infect Dis 19:e338–e351. doi: 10.1016/S1473-3099(19)30261-0. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. 2011. Global insecticide use for vector-borne disease control, 5th ed World Health Organization, Geneva, Switzerland. [Google Scholar]
- 14.Killeen GF, Smith TA, Ferguson HM, Mshinda H, Abdulla S, Lengeler C, Kachur SP. 2007. Preventing childhood malaria in Africa by protecting adults from mosquitoes with insecticide-treated nets. PLoS Med 4:e229. doi: 10.1371/journal.pmed.0040229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Du YZ, Nomura Y, Satar G, Hu ZN, Nauen R, He SY, Zhorov BS, Dong K. 2013. Molecular evidence for dual pyrethroid-receptor sites on a mosquito sodium channel. Proc Natl Acad Sci U S A 110:11785–11790. doi: 10.1073/pnas.1305118110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller JE, Lindsay SW, Armstrong J. 1991. Experimental hut trials of bednets impregnated with synthetic pyrethroid or organophosphate insecticide for mosquito-control in the Gambia. Med Vet Entomol 5:465–476. doi: 10.1111/j.1365-2915.1991.tb00575.x. [DOI] [PubMed] [Google Scholar]
- 17.Rose RI. 2001. Pesticides and public health: integrated methods of mosquito management. Emerg Infect Dis 7:17–23. doi: 10.3201/eid0701.010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishiura JT, Ho P, Ray K. 2003. Methoprene interferes with mosquito midgut remodeling during metamorphosis. J Med Entomol 40:498–507. doi: 10.1603/0022-2585-40.4.498. [DOI] [PubMed] [Google Scholar]
- 19.Chandler D, Bailey AS, Tatchell GM, Davidson G, Greaves J, Grant WP. 2011. The development, regulation and use of biopesticides for integrated pest management. Philos Trans R Soc B 366:1987–1998. doi: 10.1098/rstb.2010.0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berry C. 2012. The bacterium, Lysinibacillus sphaericus, as an insect pathogen. J Invertebr Pathol 109:1–10. doi: 10.1016/j.jip.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 21.Lacey LA. 2007. Bacillus thuringiensis serovariety israelensis and Bacillus sphaericus for mosquito control. J Am Mosq Control Assoc 23:133–163. doi: 10.2987/8756-971X(2007)23[133:BTSIAB]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 22.Scholte EJ, Ng’habi K, Kihonda J, Takken W, Paaijmans K, Abdulla S, Killeen GF, Knols BG. 2005. An entomopathogenic fungus for control of adult African malaria mosquitoes. Science 308:1641–1642. doi: 10.1126/science.1108639. [DOI] [PubMed] [Google Scholar]
- 23.Macoris MD, Andrighetti MTM, Takaku L, Glasser CM, Garbeloto VC, Bracco JE. 2003. Resistance of Aedes aegypti from the State of Sao Paulo, Brazil, to organophosphates insecticides. Mem Inst Oswaldo Cruz 98:703–708. doi: 10.1590/S0074-02762003000500020. [DOI] [PubMed] [Google Scholar]
- 24.Protopopoff N, Matowo J, Malima R, Kavishe R, Kaaya R, Wright A, West PA, Kleinschmidt I, Kisinza W, Mosha FW, Rowland M. 2013. High level of resistance in the mosquito Anopheles gambiae to pyrethroid insecticides and reduced susceptibility to bendiocarb in north-western Tanzania. Malar J 12:149. doi: 10.1186/1475-2875-12-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ranson H, Lissenden N. 2016. Insecticide resistance in African Anopheles mosquitoes: a worsening situation that needs urgent action to maintain malaria control. Trends Parasitol 32:187–196. doi: 10.1016/j.pt.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 26.Beier JC, Keating J, Githure JI, Macdonald MB, Impoinvil DE, Novak RJ. 2008. Integrated vector management for malaria control. Malar J 7:S4. doi: 10.1186/1475-2875-7-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramirez JL, Short SM, Bahia AC, Saraiva RG, Dong Y, Kang S, Tripathi A, Mlambo G, Dimopoulos G. 2014. Chromobacterium Csp_P reduces malaria and dengue infection in vector mosquitoes and has entomopathogenic and in vitro anti-pathogen activities. PLoS Pathog 10:e1004398. doi: 10.1371/journal.ppat.1004398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saraiva RG, Huitt-Roehl CR, Tripathi A, Cheng YQ, Bosch J, Townsend CA, Dimopoulos G. 2018. Chromobacterium spp. mediate their anti-Plasmodium activity through secretion of the histone deacetylase inhibitor romidepsin. Sci Rep 8:6176. doi: 10.1038/s41598-018-24296-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saraiva RG, Fang J, Kang S, Anglero-Rodriguez YI, Dong Y, Dimopoulos G. 2018. Aminopeptidase secreted by Chromobacterium sp. Panama inhibits dengue virus infection by degrading the E protein. PLoS Negl Trop Dis 12:e0006443. doi: 10.1371/journal.pntd.0006443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Short SM, van Tol S, MacLeod HJ, Dimopoulos G. 2018. Hydrogen cyanide produced by the soil bacterium Chromobacterium sp. Panama contributes to mortality in Anopheles gambiae mosquito larvae. Sci Rep 8:8358. doi: 10.1038/s41598-018-26680-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Short SM, van Tol S, Smith B, Dong Y, Dimopoulos G. 2018. The mosquito adulticidal Chromobacterium sp. Panama causes transgenerational impacts on fitness parameters and elicits xenobiotic gene responses. Parasit Vectors 11:229. doi: 10.1186/s13071-018-2822-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith LB, Kasai S, Scott JG. 2016. Pyrethroid resistance in Aedes aegypti and Aedes albopictus: important mosquito vectors of human diseases. Pestic Biochem Physiol 133:1–12. doi: 10.1016/j.pestbp.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 33.Vontas J, Kioulos E, Pavlidi N, Morou E, della Torre A, Ranson H. 2012. Insecticide resistance in the major dengue vectors Aedes albopictus and Aedes aegypti. Pestic Biochem Physiol 104:126–131. doi: 10.1016/j.pestbp.2012.05.008. [DOI] [Google Scholar]
- 34.Ray HA, Hoy MA. 2014. Effects of reduced-risk insecticides on three orchid pests and two predacious natural enemies. Florida Entomologist 97:972–978. doi: 10.1653/024.097.0355. [DOI] [Google Scholar]
- 35.Shapiro-Ilan DI, Cottrell TE, Bock C, Mai K, Boykin D, Wells L, Hudson WG, Mizell RF III. 2017. Control of pecan weevil with microbial biopesticides. Environ Entomol 46:1299–1304. doi: 10.1093/ee/nvx144. [DOI] [PubMed] [Google Scholar]
- 36.Beier JC, Muller GC, Gu W, Arheart KL, Schlein Y. 2012. Attractive toxic sugar bait (ATSB) methods decimate populations of Anopheles malaria vectors in arid environments regardless of the local availability of favored sugar-source blossoms. Malar J 11:31. doi: 10.1186/1475-2875-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Müller GC, Junnila A, Qualls W, Revay EE, Kline DL, Allan S, Schlein Y, Xue RD. 2010. Control of Culex quinquefasciatus in a storm drain system in Florida using attractive toxic sugar baits. Med Vet Entomol 24:346–351. doi: 10.1111/j.1365-2915.2010.00876.x. [DOI] [PubMed] [Google Scholar]
- 38.Stewart ZP, Oxborough RM, Tungu PK, Kirby MJ, Rowland MW, Irish SR. 2013. Indoor application of attractive toxic sugar bait (ATSB) in combination with mosquito nets for control of pyrethroid-resistant mosquitoes. PLoS One 8:e84168. doi: 10.1371/journal.pone.0084168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blackburn MB, Farrar RR Jr, Sparks ME, Kuhar D, Mitchell A, Gundersen-Rindal DE. 2017. Chromobacterium sphagni sp. nov., an insecticidal bacterium isolated from sphagnum bogs. Int J Syst Evol Microbiol 67:3417–3422. doi: 10.1099/ijsem.0.002127. [DOI] [PubMed] [Google Scholar]
- 40.Blackburn MB, Farrar RR Jr, Sparks ME, Kuhar D, Mowery JD, Mitchell A, Gundersen-Rindal DE. 2019. Chromobacterium phragmitis sp. nov., isolated from estuarine marshes. Int J Syst Evol Microbiol 69:2681–2686. doi: 10.1099/ijsem.0.003508. [DOI] [PubMed] [Google Scholar]
- 41.Lima-Bittencourt CI, Astolfi-Filho S, Chartone-Souza E, Santos FR, Nascimento AM. 2007. Analysis of Chromobacterium sp. natural isolates from different Brazilian ecosystems. BMC Microbiol 7:58. doi: 10.1186/1471-2180-7-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lima-Bittencourt CI, Costa PS, Barbosa FA, Chartone-Souza E, Nascimento AM. 2011. Characterization of a Chromobacterium haemolyticum population from a natural tropical lake. Lett Appl Microbiol 52:642–650. doi: 10.1111/j.1472-765X.2011.03052.x. [DOI] [PubMed] [Google Scholar]
- 43.Santini AC, Magalhaes JT, Cascardo JC, Correa RX. 2016. Genetic variability in isolates of Chromobacterium violaceum from pulmonary secretion, water, and soil. Genet Mol Res 15. doi: 10.4238/gmr.15027955. [DOI] [PubMed] [Google Scholar]
- 44.Soby SD, Gadagkar SR, Contreras C, Caruso FL. 2013. Chromobacterium vaccinii sp. nov., isolated from native and cultivated cranberry (Vaccinium macrocarpon Ait.) bogs and irrigation ponds. Int J Syst Evol Microbiol 63:1840–1846. doi: 10.1099/ijs.0.045161-0. [DOI] [PubMed] [Google Scholar]
- 45.Barrera R, Mackay AJ, Amador M. 2013. A novel autocidal ovitrap for the surveillance and control of Aedes aegypti. J Am Mosq Control Assoc 29:293–296. doi: 10.2987/13-6345R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poole-Smith BK, Hemme RR, Delorey M, Felix G, Gonzalez AL, Amador M, Hunsperger EA, Barrera R. 2015. Comparison of vector competence of Aedes mediovittatus and Aedes aegypti for dengue virus: implications for dengue control in the Caribbean. PLoS Negl Trop Dis 9:e0003462. doi: 10.1371/journal.pntd.0003462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bahia AC, Dong YM, Blumberg BJ, Mlambo G, Tripathi A, BenMarzouk-Hidalgo OJ, Chandra R, Dimopoulos G. 2014. Exploring Anopheles gut bacteria for Plasmodium blocking activity. Environ Microbiol 16:2980–2994. doi: 10.1111/1462-2920.12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.EPA. 2002. OPPTS 830.6317: storage stability. U.S. Environmental Protection Agency, Washington, DC. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.