Dear Editor,
Since December 2019, a coronavirus disease-2019 (COVID-19) was first reported in Wuhan, China, which was caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and expeditiously spread to countries worldwide with massive mortality [1]. The response of lymphocytes in COVID-19 is characterized by a phenomenon of lymphopenia and hypercytokinemia, and is correlated with disease severity and prognosis [2]. Besides, interleukin-6 (IL-6) may be the key cytokine leading to inflammatory storm in COVID-19 [3]. Hence, we develop a simplified immune-dysregulation index—the level of IL-6 to lymphocytes count ratio—to define the immune-dysregulation in COVID-19. We aimed to evaluate the predictive value of IL-6/lymphocyte in patients with COVID-19.
We conducted a retrospective study based on patients with confirmed diagnosis of COVID-19 admitted to the intensive care units in Tongji Hospital. The inclusion and exclusion criteria were presented in the online supplement. IL-6 (pg/ml) and lymphocytes count (106/ml) within the first day after hospital admission were collected. IL-6/lymphocyte was calculated. We used the highest ratio if a variable was recorded more than once. The primary outcome was 28-day mortality. Multivariate regression was selected to characterize the association between IL-6, lymphocyte, IL-6/lymphocyte and 28-day mortality, respectively (description in the online supplement), we then model the nonlinear relationship between IL-6/lymphocyte and 28-day mortality using splines, and the predictive value of IL-6/lymphocyte, IL-6 and lymphocytes for 28-day mortality was compared according to receiver operating characteristic (ROC) curves, net reclassification improvement (NRI) and integrated discrimination improvement (IDI). We also attempt to stratified patients through the IL-6/lymphocyte. The study was approved by Tongji Hospital Ethics Committee.
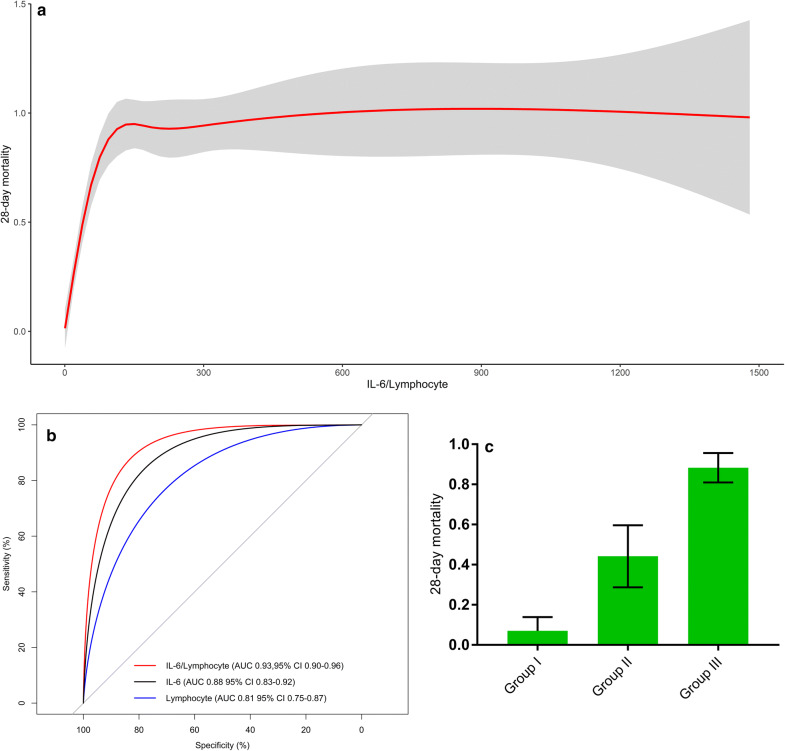
A total of 172 patients were included in our study (description in the online supplement). Overall 28- day mortality was 50.5%. Baseline characteristics between survivors and non-survivors are presented in eTable 1. Survivors had a higher lymphocytes count (0.94 (0.73–1.28) vs. 0.53 (0.38–0.74); p < 0.001) and a lower IL-6 (7.1 (2–21.6) vs. 61.1 (33.4–137.4); p < 0.001) compared non-survivors. Statistical analysis demonstrated that lymphocyte is negatively correlated with IL-6 (R = − 0.26; p < 0.001) (eFig. 1). IL-6/lymphocyte was significantly higher in non-survivors than that in survivors (125.5 (56.9–267.4) vs. 8.3 (2.1–23.4); p < 0.001). The logistic regression analyses showed a significantly association between lymphocyte (OR 0.03; 95% CI 0.01–0.17; p < 0.001), IL-6 (OR 1.03; 95% CI 1.01–1.05; p < 0.001); IL-6/lymphocyte (OR 1.05; 95% CI 1.03–1.08; p < 0.001) and 28-day mortality, respectively (eTables 2, 3 and 4). The nonlinear relationship between IL-6/lymphocyte and 28-day mortality is shown in Fig. 1a.
Fig. 1.
a Relationship between IL-6/lymphocyte and 28-day mortality for intensive care patients with COVID-19 using splines. Overlying red is fitted linear splines with 95% CI. b ROC curve analysis and comparison of the AUCs for IL-6/lymphocyte, IL-6 and lymphocytes in COVID-19. c Stratified mortality of groups based on the cutoff value of IL-6/lymphocyte in COVID-19. Bars show the percent of patients who died and 95% CI. COVID-19: coronavirus disease-2019; ROC: receiver operating characteristic; AUC: area under the curve
Figure 1b indicates that IL-6/lymphocyte had a higher area under the curve (AUC) (0.93 (95% CI 0.9–0.96)) than IL-6 (0.88 (95% CI 0.83–0.92); p < 0.001) and lymphocytes (0.81 (95% CI 0.75–0.87); p < 0.001). Compared with IL-6, IL-6/lymphocyte resulted in an additive NRI of 22.2% (95% CI: 9.7–34.7%; p < 0.001), and an IDI of 11.8% (95% CI: 8–15.6%; p < 0.001); compared with lymphocyte, IL-6/lymphocyte resulted in an additive NRI of 36.5% (95% CI 17.2–55.8%; p < 0.001) and an IDI of 20.2% (95% CI 11.8–28.6%; p < 0.001). According to the value of IL-6/lymphocyte, we divided patient into three groups: Group I (< 15); Group II (15–50); Group III (> 50), and the Group III had the highest 28-day mortality (Fig. 1c).
The degree of lymphopenia and the increase in inflammatory cytokine—especially IL-6—were associated with poor outcome in patients with COVID-19 [4]. Our study developed a novel marker to represent immune-dysregulation and concluded that IL-6/lymphocyte had an improved predictive value compared to IL-6 and lymphocytes alone. Additionally, IL-6/lymphocyte showed a potential value to identify high-risk patients who needed more timely therapy at the early phase of COVID-19. The limitations of study are the incomplete assessment of immune-dysregulation and a single-center experience. Further researches are needed to compare IL-6/lymphocyte and other immunological indicators, as well as the dynamic change of IL-6/lymphocyte.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank all patients included in this study.
Author contributions
JJ and YSL conceptualized the research aims, design the study, and take responsibility for the integrity of the data and the accuracy of the data analysis. HC, NS and JW contributed to the acquisition of data, HC and XBB performed the statistical analysis. HC wrote the first draft of the paper, and other authors provided comments and approved the final manuscript.
Funding
This work was supported by Fundamental Research Funds for the central Universities (HUST: 2017KFYXJJ113) and Wuhan Municipal Science and Technology Bureau (2017060201010173) and The Emergency Project for the Prevention and Control of the Novel Coronavirus Outbreak in Suzhou, Jiangsu Province, China, and Science Foundation of Jiangsu Commission of Health (H2018117).
Compliance with ethical standards
Conflicts of interest
The authors declare that they have no competing interests.
Ethic approval and consent to participate
The study was approved by Tongji Hospital Ethics Committee.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yongsheng Li, Email: dr_ysli@126.com.
Jun Jin, Email: jinjun0514@163.com.
References
- 1.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020 doi: 10.1016/s2213-2600(20)30076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu X, Han M, Li T, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci USA. 2020 doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020 doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.