Abstract
Aerobic exercise training in the heart failure (HF) population is supported by an extensive body of literature. The clinically accepted model for exercise prescription is currently moderate-intensity-aerobic continuous training (MI-ACT). Documented benefits from the literature include improvements in various aspects of physiologic function, aerobic exercise capacity and quality of life while the impact on morbidity and mortality is promising but requires further investigation. Recently, however, a body of evidence has begun to emerge demonstrating high-intensity-aerobic interval training (HI-AIT) can be performed safely with impressive improvements in physiology, functional capacity and quality of life. These initial findings have led some to question the long-standing clinical approach to aerobic exercise training in patients with HF (i.e., MI-ACT), implying it should perhaps be replaced with a HI-AIT model. This is a potentially controversial paradigm shift given the potential increase in adverse event risk associated with exercising at higher intensities, particularly in the HF population where the likelihood of an untoward episode is already at a heightened state relative to the apparently healthy population. The present review therefore addresses key issues related to HI-AIT in the HF population and makes recommendations for future research and current clinical practice.
Keywords: Rehabilitation, Cardiac, Exercise prescription, Moderate intensity, Continuous, Safety
Introduction
Evidence demonstrating the benefits of aerobic exercise training (ET) in patients diagnosed with heart failure (HF) began to surface more than 30 years ago [1]. Since that time, a wealth of original research has accumulated clearly demonstrating aerobic ET is a highly valuable intervention in this chronic disease population [2–7]. Documented benefits from the literature include improvements in various aspects of physiologic function, aerobic exercise capacity and quality of life while the impact on morbidity and mortality is promising but requires further investigation. Even so, the available data have resulted in leading professional organizations to strongly recommend that aerobic ET be considered a standard of care in HF [8, 9]. The vast majority of original investigations supporting aerobic ET have utilized programs that are continuous at a moderate intensity relative to an individual’s maximal capacity. Currently, these exercise prescription parameters are well accepted as the clinical standard in patients with HF.
Recently, however, initial evidence has begun to emerge demonstrating high-intensity-aerobic interval training (HI-AIT) may be performed safely and result in improvements in physiology, functional capacity and quality of life [10]. These initial findings have led some to question the long-standing clinical approach to aerobic ET in patients with HF, implying it should perhaps be replaced with a HI-AIT model. This is a potentially controversial paradigm shift given the potential increase in adverse event risk associated with exercising at higher intensities [11], particularly in the HF population where the likelihood of an untoward episode is already at a heightened state relative to the apparently healthy population. Nevertheless, initial evidence examining HI-AIT in patients with HF has produced positive findings, which warrants further consideration. The present review therefore addresses key issues related to HI-AIT in the HF population and makes recommendations for future research and current clinical practice.
Exercise prescription principles for high-intensity-aerobic interval training
Principals of high-intensity interval training
The fundamental principle behind HI-AIT is that periods of high-level ET are interspersed with periods of lower-intensity ET that permit enough recovery such that the individual is able to reengage in high-intensity ET bouts, or “intervals.” Historically, HI-AIT was thought to be applicable only to athletes; for example, repeated bouts of sprinting have been used for decades among competitive runners to optimize performance in middle- and long-distance events. However, when performed appropriately, this form of ET confers many of the same benefits among patients with cardiovascular (CV) disease, including patients with HF [12]. In patients with HF, interval training periods can take the form of walking, cycling, rowing, swimming or other forms of exercise; like any exercise prescription, the work rate employed is individualized and will differ considerably from that of healthy individuals. Available data suggest that it is the accumulated time engaged in the high-intensity intervals that determines physiological benefits with ET [12].
There have been several approaches to HI-AIT in the current body of research, admittedly a great deal of which has been performed in cohorts not diagnosed with HF, and it is unlikely that there is one approach that suits all patients. The alternating periods of reduced intensity ET are designed such that the cardiopulmonary system does not fully recover (i.e., return to near resting levels), while permitting the individual enough recovery to be able to repeat the period of high-intensity ET. The underlying physiologic concept behind interval training is that the metabolic rate is raised for a brief period that is considerably higher than that for a typical continuous ET program, which permits a longer duration of a given training period to be spent at a higher percentage of peak oxygen consumption (VO2). This has the effect of eliciting a higher rate of energy production, requiring different metabolic pathways to produce energy and different muscle fiber recruitment patterns from those elicited by continuous training. Studies have suggested that when compared to moderate-intensity-aerobic continuous training (MI-ACT), HI-AIT results in greater skeletal muscle fat oxidation and improved glucose tolerance [13, 14]. It should be noted that improvements in fat oxidation and glucose tolerance have been demonstrated in apparently healthy cohorts as opposed to patient diagnosed with HF. Because lactate can accumulate rapidly during HI-AIT, the body’s ability to remove lactate (through bicarbonate buffering and lactate utilization in tissues other than those from which it was produced) is enhanced. Given that lactate accumulation is a key factor underlying the hyperventilatory response to exercise in HF, this has the effect of increasing the aerobic or ventilatory threshold. An increase in the ventilatory threshold is an important adaptation to ET in patients with HF because it permits the patient to perform more work at a submaximal level before excessive fatigue or dyspnea [15, 16]. At least some of the available studies suggest that these factors combine to elicit more robust CV and muscular adaptations when compared to continuous ET [10, 12, 17].
Protocols for interval training in HF
Specific approaches to interval ET in HF have varied considerably, but all have involved the principle that interspersing periods of high-intensity exercise results in a superior response to ET. Some examples of HI-AIT protocols are presented in Table 1. Approaches to interval ET have typically involved 2–4-min periods at an intensity equivalent to at least 80–90 % of peak VO2, followed by a similar duration of lower-intensity ET such as 40–50 % of peak VO2 or a passive recovery. Some investigators have employed high-intensity intervals for durations as little as 30 s, followed by 30 or 60 s of low-intensity exercise or passive recovery, and several approaches in between these have been described. Meyer et al. [18] recently compared four HI-AIT protocols using “on-phase” and recovery intervals of 30 or 90 s. The recovery phase was either active or passive. The results of this study suggest shorter “on-phase” and recovery intervals, the latter of which is passive, may be optimal among the four protocols compared with respect to patient tolerance and the amount of work performed. To the best of our knowledge, there have been no studies of this nature that have included HI-AIT protocols with longer “on-phase” and recovery periods for comparative purposes. Lastly, warm-up and cool-down periods, prior to and following the training session, should be included for 5–10 min, at or below the lower-intensity recovery interval. Notably, all of these approaches have been demonstrated to be effective in achieving favorable ET responses, albeit to varying degrees.
Table 1.
Recent studies comparing high-intensity-aerobic interval training to moderate-intensity-aerobic continuous training
| Author and year of study | Cohort characteristics | High-intensity-aerobic interval training parameters | Moderate-intensity-aerobic continuous training parameters | Main outcomes |
|---|---|---|---|---|
| Dimopoulos et al. [23] | 24 (23 male) patients with HF (38 % ischemic etiology) 20/24 patients prescribed a beta-blocker and 23/24 prescribed an ACE inhibitor NYHA class I/II/III: 7/15/2 Mean age: 60.5 ± 9.4 years LVEF: 32.1 ± 10.6 % 10 subjects randomized to HI-AIT group, 14 to MI-ACT training Baseline peak VO2: HI-AIT = 15.4 ± 4.7 mlO2 kg−1 min−1; MI-ACT = 15.5 ± 3.7 mlO2 kg−1 min−1 |
3 supervised sessions per week for 12 weeks Baseline cardiopulmonary exercise test to determine peak VO2 Cycle ergometer was exercise mode; 100 % of work rate achieved on baseline cardiopulmonary exercise test for 30 s followed by 30 s of rest; exercise intensity increased 10 % each month of the program; total exercise time = 40 min |
3 supervised sessions per week for 12 weeks Baseline cardiopulmonary exercise test to determine peak VO2 and work rate Cycle ergometer was exercise mode; 50 % of work rate achieved on baseline cardiopulmonary exercise test; exercise intensity increased 5 % each month of the program; total exercise time = 40 min |
No adverse events reported Peak VO2 significantly increased by 8 % in the HI-AIT and 6 % in the MI-ACT group; difference between groups not significantly different No significant change in the VE/VCO2 slope in either the HI-AIT or MI-ACT group Significant increase in HRR in the MI-ACT group only |
| Wislof etf al. [10] | 27 (20 male) patients with ischemic HF All patients prescribed a beta- blocker and ACE inhibitor Mean age: 75.5 ± 11.1 years LVEF <40 % in all subjects 9 subjects randomized to HI-AIT group, 9 to MI-ACT training and 9 to control Baseline peak VO2: HI-AIT = 13.0 ± 1.6 mlO2 kg−1 min−1; MI-ACT = 13.0 ± 1.1 mlO2 kg−1 min−1 |
2 supervised sessions and 1 unsupervised session per week for 12 weeks Baseline cardiopulmonary exercise test to determine peak VO2 Treadmill was exercise mode; 10-min warm-up at 50–60 % of peak VO2; followed by four 4-min intervals at 90–95 % peak HR; each interval separated by 3-min active pause at 50–75 % of peak HR; session ended with 3-min cool-down at 50–70 % of peak HR; total exercise time = 38 min |
2 supervised sessions and 1 unsupervised session per week for 12 weeks Baseline cardiopulmonary exercise test to determine peak VO2 and peak HR Treadmill was exercise mode; 70–75 % of peak HR; total exercise time = 47 min |
1 death in the MI-ACT group; no other adverse events reported Peak VO2 increased 46 % in the HI-AIT group and 14 % in the MI-ACT group MacNew global quality of life score significantly improved in both HI-AIT and MI-ACT groups; significantly greater improvement in HI-AIT vs. MI-ACT Significant LV remodeling, systolic function and diastolic function found in the HI-AIT group only Markers of aerobic metabolism and BNP only improved in the HI-AIT group Endothelial function improved in both the MI-ACT and HI-AIT groups; greater improvements in the HI-AIT group |
| Fu et al. [24] | 45 patients (29 male) with HF (64 % ischemic etiology) 43/45 patients prescribed a beta-blocker and 37/45 prescribed an ACE inhibitor All subjects in NYHA class II or III Mean age: ≈ 67.2 ±2.1 years LVEF ≤40 % in all subjects 15 subjects randomized to HI-AIT group, 15 to MI-ACT training and 15 to control Baseline peak VO2: HI-AIT = 16.0 ± 1.0 mlO2 kg−1 min−1; MI-ACT = 15.9 ± 0.7 mlO2 kg−1 min−1 |
3 supervised sessions per week for 12 weeks Baseline cardiopulmonary exercise test to determine peak VO2 and HR Cycle ergometer was exercise mode; 3 min warm-up at 30 % of HR reserve; followed by five 3-min intervals at 80 % HR reserve; each interval separated by 3-min active pause at 40 % of HR reserve; session ended with 3 min cool-down at 30 % of HR reserve; total exercise time 33 min |
3 supervised sessions per week for 12 weeks Baseline cardiopulmonary exercise test to determine peak VO2 and HR Cycle ergometer was exercise mode; 3 min warm-up at 30 % of HR reserve; followed by 30 min at 60 % HR reserve; session ended with 3 min cool-down at 30 % of HR reserve; total exercise time 36 min |
No adverse events reported Peak VO2 (20 %) and the VE/VCO2 slope significantly (~15 %) improved in the HI-AIT group only Significant improvement in the MLWHFQ in both the HI-AIT and MI-ACT groups Significant improvement in SF-36 in HI-AIT group only Resting LVEF significantly increased in the HI-AIT group only BNP and IL-6 significantly reduced in the HI-AIT group only |
HF heart failure, LVEF left ventricular ejection fraction, ACE angiotensin-converting enzyme, HI-AIT high-intensity-aerobic interval training, MI-ACT moderate intensity-aerobic continuous training, VO2 oxygen consumption, NYHA New York Heart Association, HR heart rate, VE/VCO2 min ventilation/carbon dioxide production, BNP b-type natriuretic peptide, IL-6 interleukin-6, MLWHFQ Minnesota living with heart failure questionnaire, SF-36 short form 36
General recommendations for HI-AIT parameters
Generally, HI-AIT programs in patients with HF have been shown to be safe to this point, although the number of investigations assessing this important issue is currently limited. Moreover, the currently limited number of studies addressing this area each assessed relatively small cohorts. Thus, these investigations were not sufficiently powered to address the safety of HI-AIT in an appropriate way. Plans for a larger investigation that will more adequately address the issue of HI-AIT safety in patients with HF are under-way [19]. Given the current insufficient assessment of the safety of HI-AIT in patients with HF, we would like to stress the point that the training parameters described in this section should not be viewed as clinical practice recommendations at this point in time. Rather, these recommendations should be viewed as HI-AIT parameters that are acceptable for future research.
It should further be recognized that HI-AIT, even if deemed clinically acceptable in the future, is likely not appropriate for all patients with HF, as ET intensities beyond 60–70 % of maximum will not be tolerated by some patients even for a very short period of time. Importantly, while the traditional goal of HI-AIT has been improved athletic performance, this goal does not apply to patients with HF; rather, the goal in these patients should be to improve exercise capacity and enhance the ability to perform activities of daily living. In addition, patients who are highly deconditioned or susceptible to rhythm abnormalities are not candidates for interval ET. Such individuals should participate in more conventional continuous programs. Other patients may need to begin with a MI-ACT program and gradually evolve to HI-AIT program as their functional capabilities improve and they are able to tolerate higher levels of ET.
With the above caveats in mind, the following protocol has been used successfully by several groups and in at least one study has resulted in superior ET responses when compared to a MI-ACT program [10]. While the specifics of the work/rest intervals have varied widely, this approach is used as an illustration because it represents a reasonably conservative method that is likely to be tolerable for most HF patients appropriate for HI-AIT. Moreover, as will be discussed subsequently (see “Directions for future research” section and Table 1), initial evidence indicates the approach illustrated below may produce optimal training benefits compared to other approached to HI-AIT (i.e., shorter “on-phase” and recovery). Many aerobic exercise modes are suitable for this form of training, but studies have typically involved walking or cycling:
10-min warm-up at 40–50 % of peak VO2 (≈ 60–70 % of peak heart rate)
3-min interval at 80–90 % of peak VO2 (≈ 85–95 % of peak heart rate)
3-min active recovery at & 40–50 % of peak VO2 (≈ 60–70 % of peak heart rate)
Repeat intervals 4–6 times
5 min cool-down at 30–40 % of peak VO2 (50–60 % of peak heart rate)
Again, it should be noted that the ET intensities are relative to the individual; the absolute energy expended by two individuals may be quite different, although both are exercising at the same relative intensity of 80–90 % of their peak capacity. During the early phase of the rehabilitation program, it would be appropriate for patients to perform 3–4 repeat intervals and increase to 4–6 intervals after adapting to the ET regimen. Some studies have used a 1:2 ET/rest interval ratio for patients who are beginning a program and have progressed toward a 1:1 ET/rest interval ratio as ET tolerance improves.
It should be emphasized that the HI-AIT model proposed in this section has demonstrated efficacy in a limited number of HF patients to this point and should thus not be regarded as a “gold-standard”, but rather one of several potentially appropriate approaches. As a specific example, while the HI-AIT model proposed in this section recommends the exercise intensity be set according to percent of peak VO2 or peak HR, other approaches, such as the determination of critical power [20, 21] or critical velocity [22], have been proposed as alternate methods to set exercise training intensity, a crucial parameter consideration for HI-AIT. Evaluation of the value of these alternate exercise intensity prescription techniques are important considerations when performing HI-AIT in HF cohorts in the future.
Functional and clinical benefits: HI-AIT versus MI-ACT
There have been a number of previous investigations that have assessed the effects of interval aerobic ET in patients with HF. This body of research was eloquently summarized in a recent meta-analysis by Smart et al. [17]. Collectively, these investigations indicate the improvements in aerobic capacity and ventilatory efficiency are greater with interval ET when compared to a continuous training program in patients with HF. However, the majority of these investigations compared interval and continuous aerobic ET programs that utilized similar ET intensities. Therefore, a number of the studies included in this meta-analysis did not employ interval training programs that were high intensity, which is the primary thrust of this review.
An early study by Meyer et al. [25] in 1997 assessed the effect of HI-AIT in a HF cohort without including a MI-ACT group for comparison. The cohort consisted of 18 male patients (age: 52 ± 2 years) with severe systolic HF [left ventricular ejection fraction (LVEF) (21 ± 1 %)], half of whom were on a transplant list at the time of the study. This investigation employed a training intensity set at 50 % of the workload achieved during a steep ramp test on a cycle ergometer (increase workload 25 Watts every 10 s until patient could not maintain 60 rotations per min). The steep ramp test was conducted each week of the 3-week training program to adjust training intensity. Subjects exercised on a cycle ergometer at this intensity for 30 s, followed by a 60 active recovery period (pedaling at 15 Watts) for a total of 15 min, 5 times per week over 3 weeks. In addition, subjects walked on a treadmill for 10 min three times a week. The treadmill sessions also utilized a HI-AIT training model with a 60 s “on-phase” (mean speed 2.4 mph) and a 60 s recovery phase (mean speed 0.9 mph). There were no adverse events reported in this investigation. By the end of the program, the training work rate for the cycle ergometer portion of the program was more than double the work rate obtained at 75 % of peak VO2 during a standard exercise testing (100 ± 7 vs. 47 ± 5 Watts). Using a standard ramp cycle ergometry protocol, peak VO2 was also significantly increased by ~20 % following the three-week program (12.2 ± 0.7–14.6 ± 0.7 ml kg−1 min−1, p<0.001).
Two more recent investigations have compared HI-AIT to HI-AIT in combination with resistance training. Tasoulis et al. [26] randomized 46 patients with systolic HF to either perform 12 weeks of HI-AIT alone (19 males and 2 females; age: 53 ± 11 years; LVEF: 34.1 ± 11.0 %) or a combination of HI-AIT and resistance training (19 males and 6 females; age: 53 ± 12 years; mean LVEF: 35.6 ± 10.3 %). Both groups trained 3 times per week for a total of 36 sessions. The HI-AIT program followed the guidelines proposed by Meyer et al. [25], and the training intensity was adjusted every six sessions according to a new steep ramp test. For subjects in the HI-AIT only group, sessions were 40 min in duration. For subjects in the combined HI-AIT and resistance training group, the aerobic training portion was 20 min with an additional 20 min for strength training. The strength training component of the program consisted of both upper (with loads allowing for 10 repetitions) and lower (loads at 55–65 % of 2 repetition max) extremity maneuvers. No adverse events were reported in this study. Peak VO2 was significantly increased by ~8.5 % (16.4 ± 4.1–17.8 ± 4.6 ml kg−1 min−1, p < 0.01) in the HI-AIT only group and by ~18 % (16.2 ± 5.3–19.1 ± 5.8 ml kg−1 min−1, p < 0.001) in the combined HI-AIT and resistance training group. Anagnostakou et al. [27] randomized 28 patients with systolic HF to either perform 12 weeks of HI-AIT only (12 males and 2 females; age: 52 ± 11 years; LVEF: 36 ± 13 %) or a combination of HI-AIT and resistance training (11 males and 3 females; age: 54 ± 10 years; mean LVEF: 39 ± 11 %). The training protocol employed was identical to the study by Tasoulos et al. [26]. No adverse events were reported in this study. Peak VO2 was significantly increased by ~9.5 % (15.7 ± 4.0–17.2 ± 3.7 ml kg−1 min−1, p < 0.05) in the HI-AIT only group and by ~16.5 % (15.7 ± 6.0–18.3 ± 6.3 ml kg−1 min−1, p < 0.01) in the combined HI-AIT and resistance training group. While additional work is needed, these two studies indicate the combination of HI-AIT and strength training may have a synergistic beneficial effect on functional outcomes.
In another recent investigation, Freyssin et al. [28] randomized 26 patients with systolic HF to either perform 8 weeks of HI-AIT (6 males and 6 females; age: 54 ± 9 years; LVEF: 27.8 ± 4.7 %) or MI-ACT (7 males and 7 females; age: 55 ± 12 years; mean LVEF: 30.7 ± 7.8 %). Both aerobic training programs were one component of a multidisciplinary cardiac rehabilitation program, which consisted of 8 weeks of “physical activity and education sessions”. The physical activity component consisted of 13 h of exercise each week (2–3 h per day, 5 days per week). Strengthening, stretching and relaxation exercise were performed by both groups. For the HI-AIT group, the steep ramp test [25] was again used to establish exercise intensity. Each HI-AIT session consisted of 3 bouts of exercise, each separated by 5 min of rest. Each bout included 12, 30 s repetitions at either 50 % (first four weeks of training) or 80 % (last 4 weeks of training) of maximal power as determined by the steep ramp test. The MI-ACT group performed 45 min of exercise, either on a treadmill or cycle ergometer, at an intensity corresponding to the first ventilatory threshold, which was determined by cardiopulmonary exercise testing on a treadmill. No adverse events were reported in this study. Peak VO2 was significantly increased by 27.1 % (10.7 ± 2.9–13.6 ± 3.2 ml kg−1 min−1, p < 0.001) in the HI-AIT group and by only 2 % (10.6 ± 4.1–10.8 ± 4.1 ml kg−1 min−1, p: NS) in the MI-ACT group.
Upon a detailed analysis of the current body of literature, there appears to be a limited number of recent investigations that have compared HI-AIT to MI-ACT, in isolation (i.e., no additional rehabilitation interventions utilized), in patients with HF. These investigations are summarized in Table 1. All three investigations trained both the HI-AIT and MI-ACT groups for 36 sessions over 12 weeks. These studies included cohorts that were exclusively diagnosed with systolic HF and were pre-dominantly male. The majority of patients in all three studies were prescribed both a beta-blocking agent and angiotensin-converting enzyme inhibitor, indicated the cohorts were managed according to present-day standards of care. The parameters for the MI-ACT program were similar among the three investigations, but differences did exist in the HI-AIT arms. Specifically, the ET intensity for the “on-phase” of the HI-AIT program in the study by Dimopoulos et al. [23] was significantly shorter compared to the investigations by Wisloff et al. [10] and Fu et al. [24]. Moreover, Dimopoulos et al. [23] employed a passive recovery phase while Wisloff et al. [10] and Fu et al. [24] both employed an active recovery. None of the investigations, however, reported any adverse events during these various ET protocols. The investigation by Dimopoulos et al. [23] reported a significant but comparable improvement in aerobic capacity in the HI-AIT and MI-ACT groups. Conversely, ventilatory efficiency was unchanged in both ET groups. Wisloff et al. [10] also reported a significant improvement in aerobic capacity following both HI-AIT and MI-ACT. However, the improvement in aerobic capacity was significantly greater in the HI-AIT group. Similarly, quality of life was significantly improved in both the HI-AIT and MI-ACT groups with greater benefits realized with the former ET program. Fu et al. [24] found significant improvements in aerobic capacity and ventilatory efficiency only in the HI-AIT group. In this latter study, while one quality of life questionnaire demonstrated significant improvements in both the HI-AIT and MI-ACT groups, a second questionnaire only found significant improvements in subjects participating in the HI-AIT program. Therefore, to this point, the overall benefits of HI-AIT appear to be at least comparable and potentially somewhat superior to that of MI-ACT.
Physiologic benefits: HI-AIT versus MI-ACT
The physiologic benefits of MI-ACT are well documented in patients with HF [3, 5, 29]. Using this traditional approach to ET, favorable physiologic adaptations have been demonstrated in skeletal muscle, the vasculature, neurohormonal activation and systemic inflammation. While MI-ACT does not appear to improve left ventricular (LV) systolic function (i.e., increased stroke volume and cardiac output), a significant improvement in LV diastolic function has been demonstrated [3].
Although analysis of the physiologic benefits of HI-AIT in HF is in its initial phases, intriguing and clinically relevant findings have emerged, particularly in relation to acute and chronic effects on LV function. Tomczak et al. [30] assessed the acute effects of a single HI-AIT session on LV function in a group of nine patients (age 49 ± 16 years; 6 males) with mild (New York Heart Association class I–II) nonischemic systolic HF (LVEF \50 %). The ET session was identical to the protocol utilized by Wisloff et al. [10] (see Table 1 for ET parameters), and LV function was assessed by magnetic resonance imaging. Immediately following the ET session, LV end systolic volume significantly decreased (6 %) while LV systolic annular velocity significantly increased (21 %). Thirty minutes following the ET session, LVEF was significantly increased (2.4 %). Measures of LV diastolic function were also significantly improved following the HI-AIT session, as reflected by a 24 and 18 % increase in left ventricular untwisting rate immediately following and 30 min post exercise, respectively. The authors theorized improvements in LV systolic function immediately following and up to 30 min post a single HI-AIT session may be due to a decrease in systemic vascular resistance and/or enhanced myocardial contractility. Improvements in LV diastolic function may have been associated with an increase in LV suction facilitated by an increase in peak LV untwisting rate following systole, the latter of which is also augmented by enhanced myocardial contractility [31]. Wisloff et al. [10] (see Table 1 for ET parameters and key physiologic findings) performed chronic HI-AIT and compared it with a MI-ACT program in a cohort of systolic HF patients. Comparing the effects of HI-AIT and MI-ACT programs, this investigation found significant improvements in LV systolic and diastolic function were only apparent following the HI-AIT program. Most notable was the significant improvement in LV systolic function following HI-AIT (i.e., LVEF = 35 % increase, stroke vvolume = 17 % increase and cardiac output = 11 % increase), a finding that has generally not been shown with MI-ACT. Fu et al. [24] (see Table 1 for ET parameters and key physiologic findings), assessing cardiac hemodynamics via bioreactance [32], also found measures of LV systolic function were significantly improved following a chronic HI-AIT program (31 % increase in peak cardiac output) while no changes were demonstrated in the MI-ACT group. Resting LVEF, measured by echocardiography, was also significantly improved in the HI-AIT group only (27 % increase). Both Wisloff et al. [10] and Fu et al. [24] demonstrated a significant reduction in b-type natriuretic peptide in groups participating in HI-AIT (40 and 50 % decrease, respectively), whereas no change was found in subjects participating in MI-ACT. Improvements in this important neurohormonal marker are an indirect indication of CV remodeling and improved CV function. The physiologic mechanism(s) for improvements in LVEF following chronic HI-AIT is not entirely clear. There is, however, initial evidence from animal studies to suggest cardiomyocyte adaptations, for example, both size and contractile characteristics (i.e., fractional shortening and calcium handling [33–35]), are more responsive to the stimulus afforded by HI-AIT compared to MI-ACT [12].
Initial evidence also indicates positive physiologic adaptations to the vasculature and skeletal muscle are realized following HI-AIT. Wisloff et al. [10] (see Table 1 for key physiologic findings) found endothelial function was significantly improved following both HI-AIT and MI-ACT, although improvements were greater in the former group. The authors theorized this finding may be due to the fact that a higher amount of shear stress, which facilitates positive physiologic adaptations in the vasculature, was induced by HI-AIT compared to MI-ACT. Moreover, mitochondrial function in the vastus lateralis was only significantly improved in the HI-AIT group. This latter finding is also likely due to the fact that the higher ET stimulus provided by HI-AIT results in a magnified physiologic adaptation in the skeletal musculature.
It has long been recognized that physiologic adaptations precipitated by an aerobic ET program are significantly influenced by ET intensity. In patients with HF, initial evidence indicates HI-AIT surpasses the threshold needed to elicit improvements in LV systolic function, an adaptation that has generally not been demonstrated with MI-ACT programs. Moreover, initial evidence indicates other physiologic adaptations in the vasculature and skeletal muscle are enhanced by HI-AIT in HF. The combination of central and peripheral physiologic adaptations uniquely brought about by HI-AIT, the former of which strongly influences aerobic capacity [36], is a plausible hypothesis as to why this training approach has been shown to elicit significantly greater improvements in various parameters of LV, skeletal muscle and endothelial function, as well as overall aerobic capacity, compared to MI-ACT [10, 24].
Directions for future research
Given the positive results from investigations that are currently available, it is the opinion of this writing group that future research should undoubtedly continue to examine HI-AIT in patients with HF, as a number of issues require further exploration. The currently available studies have assessed rather small cohorts that were primarily male. Thus, future investigations should increase their sample size and include a more balanced distribution according to sex. Other key baseline characteristics, including HF etiology and disease severity, should be considered in future study designs to ensure more heterogeneous samples representative of broader HF populations are analyzed. Along the lines of HF etiology, both patients with reduced and preserved ejection fraction should be included in these future investigations. Investigations including more subjects with a greater degree of heterogeneous characteristics should continue to assess the functional, quality of life and physiologic benefits of HI-AIT with hopes of further bolstering the positive results that are currently available. Given the increased exertional demands required for HI-AIT, it is also important to determine long-term patient compliance with this type of rehabilitation program. Demonstration of high patient compliance is needed to determine whether the benefits of HI-AIT from a well-controlled research environment are transferrable to a less-controlled clinical environment. This appears particularly important considering the relatively poor real-life compliance recently noted in the major HF-ACTION study [2]. From a technical standpoint, future studies should compare different HI-AIT regimens to determine whether there is an optimal “on-phase”/recovery cycle with respect to time, intensity and frequency. The summary of ET studies provided in Table 1 indicates functional improvements elicited by HI-AIT may differ according to these ET parameters. Specifically, the limited amount of evidence indicates a HI-AIT program that utilizes a longer “on-phase” (i.e., ~3 min vs. 30 s) and an active recovery phase may be preferable. More research is needed to solidify the initial observations related to differences in outcomes according to HI-AIT parameters in order to provide definitive recommendations for clinical practice. In addition, the use of HI-AIT in conjunction with other established rehabilitation techniques, specifically resistance training [37], has demonstrated initial promise [26, 27] and is therefore a viable research pursuit moving forward. Once a greater number of subjects with HF have participated in studies assessing HI-AIT, meaningful information regarding adverse event rates and the safety of this ET approach will become available. If present and future studies, taken together, include a sufficient sample (i.e., several hundred to several thousand subjects) and collectively represent the heterogeneous characteristics of the HF population as a whole (i.e., age, sex, HF etiology, disease severity, baseline fitness level), it will be possible to determine the type of HF characteristics that allow for implementation of a HI-AIT program as well as those characteristics that would preclude participation in this type of ET program. Lastly, with MI-AIT programs, there appears to be certain HF patients who do not respond to the exercise program, which is a prognostically ominous finding [38]. Determining whether this pattern occurs in certain HF patients who participate in HI-AIT training would be of value.
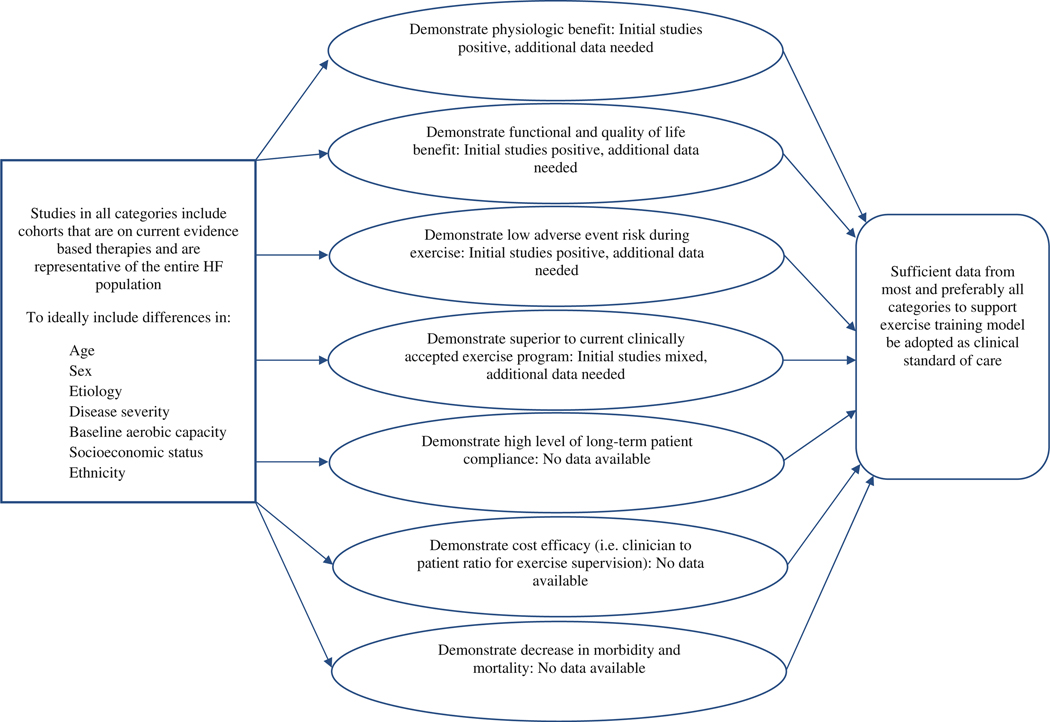
Information on patient safety demonstrating the adverse event risk is low and comparable to MI-ACT [39] is essential for HI-AIT to be accepted as a clinical standard of care in this patient population. Along these same lines, the implementation of HI-AIT will likely require a higher level of professional supervision (i.e., a lower patient to clinician ratio), at least in the early stages of implementation of an HI-AIT program. This potential change in supervision requirements in order to ensure patient safety has implications for the cost efficacy of supervised rehabilitation and therefore must be analyzed. Lastly, although it would be a substantial undertaking to perform with adequate statistical power, large randomized trials investigating the impact HI-AIT has on morbidity and mortality in patients with HF would be highly informative. This latter endeavor would be particularly relevant if future investigations continued to demonstrate positive outcomes (i.e., functional capacity, quality of life, physiology and low adverse event risk). Moreover, demonstration that HI-AIT improves prognosis is essential if this form of training is going to be recommended as a replacement for MI-ACT in any situation, given the latter training approach has demonstrated an ability to improve outcomes in patients with HF [7]. Figure 1 illustrates a forward-looking research paradigm for HI-AIT; highlighting factors to consider for cohort characteristics, areas of investigation, both new and continuing, and findings needed to support this form of aerobic ET as a clinical standard of care in the HF population. The writing group acknowledges it would be a daunting task to fulfill all of the research recommendations proposed in Fig. 1. Even so, the breadth of the body of evidence supporting HI-AIT is only in its initial stages and would at a minimum have to approach what is currently available in support of MI-ACT, the latter of which has >25 years of studies to underscore its benefits, addressing the majority of areas illustrated in Fig. 1 to some level.
Fig. 1.
Characteristics of heart failure cohorts and areas of research needed to support the clinical application of high-intensity-aerobic interval training in heart failure
Fortunately, a new randomized multicenter European trial, entitled SMARTEX-HF, is designed to address many of the aforementioned issues [19]. This trial will recruit 200 subjects with systolic HF (NYHA class II-III, LVEF B ≤ 35 %) and randomize them to either a: (1) 12-week supervised HI-AIT program, (2) 12-week supervised MI-ACT program or (3) education on performing an independent exercise program. The HI-AIT program will be identical to that proposed by Wisloff et al. [10] (see Table 1 for ET parameters). In all groups, subjects will be advised to exercise regularly following the initial 12-week program (either supervised ET or encouragement to perform ET regularly). Subjects will also undergo assessments at 12 weeks and 1-year follow-up to assess changes in cardiopulmonary exercise testing performance, echocardiography, b-type natriuretic peptide and quality of life. The primary outcome measure will be LV remodeling. Subjects will also be tracked for ET compliance, ET-related adverse events, and overall morbidity and mortality. The SMAR-TEX-HF trial will address a majority of research areas requiring further inquiry illustrated in Fig. 1 and will therefore greatly assist in determining the clinical utility of HI-AIT in patients with HF.
Conclusions
It is clear that patients with HF benefit from MI-ACT, which is currently the clinical standard of care in this chronic disease population. While initial evidence demonstrating the benefits of HI-AIT in the HF population is compelling, we feel there is currently insufficient evidence to supplant a continuous MI-ACT approach with this new ET model. This recommendation is not based on the findings of any one study, which are all positive to this point, but rather the relatively small body of collective evidence demonstrating the value of HI-AIT that is currently available. Collectively, these studies, summarized in this review, have included just over 100 subjects with HF who participated in the HI-AIT arms. As discussed in the Directions for Future Research Section, numerous clinically relevant questions (e.g., patient safety, impact on prognosis, training compliance) need a significant amount of additional supporting evidence before HI-AIT can be advocated as a clinical standard of care in the clinical rehabilitation setting. Given HI-AIT clearly shows promise in the HF population, continued research is strongly encouraged to further bolster support for its clinical application. The proposed SMARTEX-HF study [19] will be a major advance and once completed, it is likely more definitive recommendations can begin to be made with regard to determining the clinical role of HI-AIT in patients with HF.
Footnotes
Conflict of interest None.
Contributor Information
Ross Arena, Division of Cardiology, Department of Internal Medicine, University of New Mexico School of Medicine, Albuquerque, NM, USA.
Jonathan Myers, Division of Cardiology, VA Palo Alto Healthcare System, Stanford University, Palo Alto, CA, USA.
Daniel E. Forman, Division of Cardiovascular Medicine, Brigham and Women’s Hospital, Boston, MA, USA
Carl J. Lavie, Department of Cardiovascular Diseases, John Ochsner Heart and Vascular Institute, Ochsner Clinical School, The University of Queensland School of Medicine, New Orleans, LA, USA Pennington Biomedical Research Center, Louisiana State, University System, Baton Rouge, LA, USA.
Marco Guazzi, Department of Cardiology, I.R.C.C.S. Policlinico San Donato, Milan, Italy.
References
- 1.Lee AP, Ice R, Blessey R, Sanmarco ME (1979) Long-term effects of physical training on coronary patients with impaired ventricular function. Circulation 60:1519–1526 [DOI] [PubMed] [Google Scholar]
- 2.O’Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, Rendall DS, Miller NH, Fleg JL, Schulman KA, McKelvie RS, Zannad F, Piña IL (2009) Efficacy and safety of exercise training in patients with chronic heart failure. JAMA 301:1439–1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Downing J, Balady GJ (2011) The role of exercise training in heart failure. J Am Coll Cardiol 58:561–569 [DOI] [PubMed] [Google Scholar]
- 4.Keteyian SJ (2010) Exercise in the management of patients with chronic heart failure. Curr Heart Fail Rep 7:35–41 [DOI] [PubMed] [Google Scholar]
- 5.Keteyian SJ, Fleg JL, Brawner CA, Pina IL (2010) Role and benefits of exercise in the management of patients with heart failure. Heart Fail Rev 15:523–530 [DOI] [PubMed] [Google Scholar]
- 6.Davies EJ, Moxham T, Rees K, Singh S, Coats AJS, Ebrahim S, Lough F, Taylor RS (2010) Exercise training for systolic heart failure: cochrane systematic review and meta-analysis. Eur J Heart Fail 12:706–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Exercise training meta-analysis of trials in patients with chronic heart failure (ExTraMATCH) (2004) BMJ 328:189–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balady GJ, Williams MA, Ades PA, Bittner V, Comoss P, Foody JM, Franklin B, Sanderson B, Southard D (2007) Core components of cardiac rehabilitation/secondary prevention programs: 2007 Update: a scientific statement from the American heart association exercise, cardiac rehabilitation, and prevention Committee, the Council on Clinical cardiology; the councils on cardiovascular nursing, epidemiology and prevention, and nutrition, physical activity, and metabolism; and the American association of Cardiovascular and pulmonary rehabilitation. Circulation 115:2675–2682 [DOI] [PubMed] [Google Scholar]
- 9.Jessup M, Abraham WT, Casey DE, Feldman AM, Francis GS, Ganiats TG, Konstam MA, Mancini DM, Rahko PS, Silver MA, Stevenson LW, Yancy CW (2009) Focused Update: ACCF/AHA guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology foundation/American Heart Association task force on practice guide-lines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation 119:1977–2016 [DOI] [PubMed] [Google Scholar]
- 10.Wisloff U, Stoylen A, Loennechen JP, Bruvold M, Rognmo O, Haram PM, Tjonna AE, Helgerud J, Slordahl SA, Lee SJ, Videm V, Bye A, Smith GL, Najjar SM, Ellingsen O, Skjaerpe T (2007) Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation 115:3086–3094 [DOI] [PubMed] [Google Scholar]
- 11.Thompson PD, Franklin BA, Balady GJ, Blair SN, Corrado D, Estes NA III, Fulton JE, Gordon NF, Haskell WL, Link MS, Maron BJ, Mittleman MA, Pelliccia A, Wenger NK, Willich SN, Costa F (2007) Exercise and acute cardiovascular events placing the risks into perspective: a scientific statement from the American Heart Association Council on nutrition, physical activity, and metabolism and the council on clinical cardiology. Circulation 115:2358–2368 [DOI] [PubMed] [Google Scholar]
- 12.Kemi OJ, Wisloff U (2010) High-intensity aerobic exercise training improves the heart in health and disease. J Cardiopulm Rehabil Prev 30:2–11 [DOI] [PubMed] [Google Scholar]
- 13.Boutcher SH (2011) High-intensity intermittent exercise and fat loss. J Obes 2011:868305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trapp EG, Chisholm DJ, Freund J, Boutcher SH (2008) The effects of high-intensity intermittent exercise training on fat loss and fasting insulin levels of young women. Int J Obes (Lond) 32:684–691 [DOI] [PubMed] [Google Scholar]
- 15.Myers J, Ashley E (1997) Dangerous curves. A perspective on exercise, lactate, and the anaerobic threshold. Chest 111:787–795 [DOI] [PubMed] [Google Scholar]
- 16.Sullivan MJ, Higginbotham MB, Cobb FR (1989) Exercise training in patients with chronic heart failure delays ventilatory anaerobic threshold and improves submaximal exercise performance. Circulation 79:324–329 [DOI] [PubMed] [Google Scholar]
- 17.Smart NA, Dieberg G, Giallauria F (2011). Intermittent versus continuous exercise training in chronic heart failure: a meta-analysis. Int J Cardiol (in press) [DOI] [PubMed] [Google Scholar]
- 18.Meyer P, Normandin E, Gayda M, Billon G, Guiraud T, Bosquet L, Fortier A, Juneau M, White M, Nigam A (2012) High-intensity interval exercise in chronic heart failure: protocol optimization. J Card Fail 18:126–133 [DOI] [PubMed] [Google Scholar]
- 19.Stoylen A, Conraads V, Halle M, Linke A, Prescott E, Ellingsen O (2011) Controlled study of myocardial recovery after interval training in heart failure: SMARTEX-HF—rationale and design. Eur J Cardiovasc Prev Rehabil (in press) [DOI] [PubMed] [Google Scholar]
- 20.Chidnok W, Dimenna FJ, Bailey SJ, Vanhatalo A, Morton RH, Wilkerson DP, Jones AM (2012) Exercise tolerance in intermittent cycling: application of the critical power concept. Med Sci Sports Exerc 44:966–976 [DOI] [PubMed] [Google Scholar]
- 21.Jones AM, Vanhatalo A, Burnley M, Morton RH, Poole DC (2010) Critical power: implications for determination of V O2max and exercise tolerance. Med Sci Sports Exerc 42: 1876–1890 [DOI] [PubMed] [Google Scholar]
- 22.Midgley AW, Mc Naughton LR (2006) Time at or near VO2max during continuous and intermittent running. A review with special reference to considerations for the optimisation of training protocols to elicit the longest time at or near VO2max. J Sports Med Phys Fitness 46:1–14 [PubMed] [Google Scholar]
- 23.Dimopoulos S, Anastasiou-Nana M, Sakellariou D, Drakos S, Kapsimalakou S, Maroulidis G, Roditis P, Papazachou O, Vogiatzis I, Roussos C, Nanas S (2006) Effects of exercise rehabilitation program on heart rate recovery in patients with chronic heart failure. Eur J Cardiovasc Prev Rehabil 13:67–73 [DOI] [PubMed] [Google Scholar]
- 24.Fu TC, Wang CH, Lin PS, Hsu CC, Cherng WJl (2011). Aerobic interval training improves oxygen uptake efficiency by enhancing cerebral and muscular hemodynamics in patients with heart failure. Int J Cardiol (in press) [DOI] [PubMed] [Google Scholar]
- 25.Meyer K, Samek L, Schwaibold M, Westbrook S, Hajric R, Beneke R, Lehmann M, Roskamm H (1997) Interval training in patients with severe chronic heart failure: analysis and recommendations for exercise procedures. Med Sci Sports Exerc 29: 306–312 [DOI] [PubMed] [Google Scholar]
- 26.Tasoulis A, Papazachou O, Dimopoulos S, Gerovasili V, Karatzanos E, Kyprianou T, Drakos S, Anastasiou-Nana M, Roussos C, Nanas S (2010) Effects of interval exercise training on respiratory drive in patients with chronic heart failure. Respir Med 104:1557–1565 [DOI] [PubMed] [Google Scholar]
- 27.Anagnostakou V, Chatzimichail K, Dimopoulos S, Karatzanos E, Papazachou O, Tasoulis A, Anastasiou-Nana M, Roussos C, Nanas S (2011) Effects of interval cycle training with or without strength training on vascular reactivity in heart failure patients. J Card Fail 17:585–591 [DOI] [PubMed] [Google Scholar]
- 28.Freyssin C, Verkindt C, Prieur F, Benaich P, Maunier S, Blanc P (2012). Cardiac rehabilitation in chronic heart failure: effect of an 8-week, high-intensity interval training versus continuous training. Archives of Physical Medicine and Rehabilitation (in press) [DOI] [PubMed] [Google Scholar]
- 29.Pina IL, Apstein CS, Balady GJ, Belardinelli R, Chaitman BR, Duscha BD, Fletcher BJ, Fleg JL, Myers JN, Sullivan MJ (2003) Exercise and heart failure: a statement from the american heart association committee on exercise, rehabilitation, and prevention. Circulation 107:1210–1225 [DOI] [PubMed] [Google Scholar]
- 30.Tomczak CR, Thompson RB, Paterson I, Schulte F, Cheng-Baron J, Haennel RG, Haykowsky MJ (2011) Effect of acute high-intensity interval exercise on postexercise biventricular function in mild heart failure. J Appl Physiol 110:398–406 [DOI] [PubMed] [Google Scholar]
- 31.Sengupta PP, Tajik AJ, Chandrasekaran K, Khandheria BK (2008) Twist mechanics of the left ventricle: principles and application. JACC Cardiovasc Imaging 1:366–376 [DOI] [PubMed] [Google Scholar]
- 32.Keren H, Burkhoff D, Squara P (2007) Evaluation of a noninvasive continuous cardiac output monitoring system based on thoracic bioreactance. Am J Physiol Heart Circul Physiol 293:H583–H589 [DOI] [PubMed] [Google Scholar]
- 33.Kemi OJ, Loennechen JP, Wisloff U, Ellingsen O (2002) Intensity-controlled treadmill running in mice: cardiac and skeletal muscle hypertrophy. J Appl Physiol 93:1301–1309 [DOI] [PubMed] [Google Scholar]
- 34.Kemi OJ, Haram PM, Loennechen JP, Osnes JB, Skomedal T, Wisloff U, Ellingsen O (2005) Moderate vs. high exercise intensity: differential effects on aerobic fitness, cardiomyocyte contractility, and endothelial function. Cardiovasc Res 67: 161–172 [DOI] [PubMed] [Google Scholar]
- 35.Wisloff U, Helgerud J, Kemi OJ, Ellingsen O (2001) Intensity-controlled treadmill running in rats: VO(2 max) and cardiac hypertrophy. Am J Physiol Heart Circ Physiol 280:H1301–H1310 [DOI] [PubMed] [Google Scholar]
- 36.Kemi OJ, Haram PM, Wisloff U, Ellingsen O (2004) Aerobic fitness is associated with cardiomyocyte contractile capacity and endothelial function in exercise training and detraining. Circulation 109:2897–2904 [DOI] [PubMed] [Google Scholar]
- 37.Braith R, Beck D (2008) Resistance exercise: training adaptations and developing a safe exercise prescription. Heart Fail Rev 13:69–79 [DOI] [PubMed] [Google Scholar]
- 38.Tabet JY, Meurin P, Beauvais F, Weber H, Renaud N, Thabut G, Cohen-Solal A, Logeart D, Driss AB (2008) Absence of exercise capacity improvement after exercise training program/clinical perspective. Circ Heart Fail 1:220–226 [DOI] [PubMed] [Google Scholar]
- 39.Keteyian SJ (2011) Exercise training in congestive heart failure: risks and benefits. Prog Cardiovasc Dis 53:419–428 [DOI] [PubMed] [Google Scholar]