Abstract
Dermal invasion is a hallmark of malignant melanoma. Although the molecular alterations that drive the progression of primary melanoma to metastatic disease have been studied extensively, the early progression of noninvasive primary melanoma to an invasive state is poorly understood. To elucidate the mechanisms underlying the transition from radial to vertical growth, the first step in melanoma invasion, we developed a zebrafish melanoma model in which constitutive activation of ribosomal protein S6 kinase A1 drives tumor invasion. Transcriptomic analysis of ribosomal protein S6 kinase A1–activated tumors identified metabolic changes, including up-regulation of genes associated with oxidative phosphorylation. Vertical growth phase human melanoma cells show higher oxygen consumption and preferential utilization of glutamine compared to radial growth phase melanoma cells. Peroxisome proliferator activated receptor γ coactivator (PGC)-1α, has been proposed as a master regulator of tumor oxidative phosphorylation. In human primary melanoma specimens, PGC1α protein expression was found to be positively associated with increased tumor thickness and expression of the proliferative marker Ki-67 and the reactive oxygen species scavenger receptor class A member 3. PGC1α depletion modulated cellular processes associated with primary melanoma growth and invasion, including oxidative stress. These results support a role for PGC1α in mediating glutamine-driven oxidative phosphorylation to facilitate the invasive growth of primary melanoma.
The molecular mechanisms underlying melanoma metastasis have been studied extensively; however, the processes that control the early progression of primary melanoma are not well understood. During the radial growth phase (RGP), primary melanoma cells are confined to the epidermis. As the earliest step of progression, a melanoma transitions to a vertical growth phase (VGP), in which tumor cells invade the dermis and develop the capacity for metastasis.1 This invasive behavior is a hallmark of melanoma and is thought to reflect its neural crest origin.2 Some studies have identified molecular alterations that contribute to primary melanoma invasion. For example, deregulation of transcription factors, such as cyclic AMP–responsive element binding protein, leads to changes in expression or function of adhesion molecules, matrix-degrading enzymes, and survival factors that promote melanoma cell invasion.3 Oncogenic activation of BRAF V600E in human melanocytes results in up-regulation of genes associated with growth, motility, inflammation, and extracellular matrix degradation.4 Multiple miRNAs, including miR-382 and miR-516b, have been shown to alter the expression of genes that are crucial for melanoma invasion and progression.5 However, to date no studies have reported a role for altered metabolism in the early progression of primary melanoma.
The importance of metabolic reprogramming in cancer initiation and progression is recognized,6,7 and melanoma cells are able to utilize and regulate multiple metabolic pathways to sustain their proliferation, invasion, and metastasis, including glycolysis, oxidative phosphorylation (OXPHOS), tricarboxylic acid cycle, and amino acid and lipid metabolism.8 Induction of the transcription coactivator peroxisome proliferator–activated receptor γ coactivator (PGC)-1α (PPARGC1A) in a subset of metastatic melanomas by the melanocyte lineage factor microphthalmia-associated transcription factor (MITF) has been shown to promote mitochondrial capacity, OXPHOS, and resistance to oxidative stress and mitogen-activated protein kinase pathway inhibitors,9, 10, 11 but its relevance in primary melanoma remains to be established. Here, we tested the hypothesis that an altered metabolic state promotes invasive growth of primary melanoma, using an integrated approach that utilized a genetic zebrafish model of invasive melanoma, as well as human melanoma cell lines and patient tumor specimens.
Materials and Methods
Zebrafish Melanoma Model
To develop a zebrafish model of invasive melanoma, MiniCoopR vectors were generated as described.12 An N-terminal myristoylation sequence (Myr-RSK1) was added to generate constitutively active human ribosomal protein S6 kinase A1 (RSK1; RPS6KA1).13 For melanoma assays, microinjection of zebrafish embryos was performed from an in-cross of Tg[mitfa:BRAF(V600E);p53(M214K/M214K);nacre]. Melanocyte-rescued animals were raised to adulthood and observed weekly for melanoma onset as previously described.14 Tumor invasion was assessed by histology, and melanoma tissue was used for RNA extraction for RNA sequencing (RNA-Seq).
Melanoma Cell Culture and RNA Interference
Primary melanoma cell lines (WM35, WM278, WM115, and WM3248) were obtained from the Wistar Institute (Philadelphia, PA), used within 20 passages after resuscitation, and cultured in MCDB153/L15 medium (4:1 v/v) supplemented with 2% fetal bovine serum, 5 mg/mL insulin, 15 mg/mL bovine pituitary extract, 1.68 mmol/L calcium chloride, 5 ng/mL epidermal growth factor, and 1% penicillin/streptomycin.
To knock down PGC1α expression, siRNAs were purchased from Qiagen (Germantown, MD): PPARGC1A (PGC1α; catalog number SI02639833; AllStars negative control: catalog number 102780). WM3248 melanoma cells were transfected with siRNA against PGC1α or with AllStars negative control siRNA at a final concentration of 30 nmol/L, using Lipofectamine 2000 reagent (Life Technologies, Grand Island, NY), according to the manufacturer's instructions.
RNA-Seq Analysis
Samples were sequenced at the Cornell University Transcriptional Regulation and Expression Facility (Ithaca, NY; zebrafish melanoma model) and the New York University Genome Technology Core (New York, NY) WM3248 human melanoma cells) using Hi-Seq. Per-read per-sample FASTQ files were generated using bcl2fastq2 Conversion software version 2.20 (Illumina, San Diego, CA) to convert per-cycle BCL base call file output by the sequencing instrument into the FASTQ format. Alignment software, STAR version 2.6.1d,15 was used for mapping reads to the hg19 human and Zv9 zebrafish reference genomes, respectively, and Fastq Screen software version 0.13.0 (Barbraham Institute, Cambridge, UK) was utilized to check for sample contamination. FeatureCounts software16 Subread package version 1.6.3 was used to generate matrices of read counts for annotated genomic features of each human and zebrafish data set. For differential gene statistical comparisons between groups of samples, the DESeq2 package17 Bioconductor version 3.10 in the R statistical programming environment was utilized. Genes with a false-discovery rate of <0.05 were considered significantly different between groups. Heatmaps were generated using the pheatmap R software package version 1.0.12 (Raivo Kolde, University of Tartu, Estonia). Pathway analysis was performed using ClusterProfiler version 3.14.318 to identify enriched Kyoto Encyclopedia of Genes and Genomes signaling pathways based on top significantly expressed genes. Gene Set Enrichment Analysis in Python version 3.8 was used to identify genes that were enriched in a specific phenotype of interest. Additional gene list enrichment analysis of PGC1α-regulated transcripts was performed using the EnrichR web server (https://amp.pharm.mssm.edu/Enrichr, last accessed December 17, 2019).19 RNA-Seq data were deposited at the NCBI Gene Expression Omnibus public repository (https://www.ncbi.nlm.nih.gov/geo, accession numbers GSE144117 and GSE145101).
Oxygen Consumption Rate Assay
Oxygen consumption rate (OCR) of human primary melanoma cell lines was assessed using the OCR Assay Kit (Cayman Chemicals, Ann Arbor, MI), according to the manufacturer's instructions. Antimycin A, a mitochondrial electron transport chain inhibitor, was used as a negative control for zero oxygen consumption. Fluorescence was measured using a SpectraMax M3 plate reader (Molecular Devices, LLC, San Jose, CA) at 380 nm (excitation) and 650 nm (emission). Oxygen consumption was calculated using linear regression to determine the slope for each signal profile.
Metabolic Profiling
Metabolic phenotype profiling of 367 carbon energy and nitrogen substrates was performed in WM35 and WM3248 primary melanoma cell lines using Phenotype MicroArrays M1 to M4 (Biolog, Hayward, CA), according to the manufacturer's instructions. Briefly, 2 × 104 cells were seeded in each well of four 96-well plates. After 48 hours of incubation at 37°C in 5% CO2, Biolog Redox Dye MA (Biolog) was added to wells. Tetrazolium dye reduction for each well was determined using a SpectraMax M3 plate reader (Molecular Devices) at an absorbance of 590 nm.
Melanoma Patients and Tumor Tissue Specimens
Patients with primary melanoma (n = 46) were enrolled in the New York University Langone Medical Center Interdisciplinary Melanoma Cooperative Group biospecimen database, with prospectively recorded demographic, clinical, and pathologic data.20 The study protocol was approved by the New York University School of Medicine institutional review board. All patients gave written, informed consent before the study.
PGC1α, Ki-67, and SCARA3 IHC
Immunohistochemistry (IHC) was performed on formalin-fixed paraffin-embedded primary melanoma specimens, as described.21 Antibodies used were anti-PGC1α rabbit monoclonal antibody (catalog number ab84139, 1:200 dilution; Abcam, Cambridge, MA), anti-Ki-67 rabbit monoclonal antibody D2H10 (catalog number 9207, 1:800 dilution; Cell Signaling Technology, Danvers, MA), or anti–scavenger receptor class A, member 3 (SCARA3) rabbit polyclonal N2C2 (catalog number GTX100595, 1:500 dilution; GeneTex, Irvine, CA). PGC1α expression was scored based on staining intensity and distribution in tumors22 by an attending melanoma pathologist (F.D.) who was blinded to the clinical data. A score of 0 (absent), 1 (weak), or 2 (strong) was assigned to each sample based on PGC1α staining intensity, compared to positive and negative control tissues. Next, the distribution of PGC1α staining was assessed, in which focal staining (1% to 50% of the tumor) was a score of 1 and diffuse staining (>50% of the tumor) was a score of 2. The final PGC1α expression score was the sum of intensity and distribution scores, resulting in three discrete PGC1α expression groups: 0, absent; 2, weak; and 3, 4, strong. Ki-67 expression was scored as a percentage of positive tumor cells, as described.23
RNA Extraction and Quantitative RT-PCR
Total RNA was isolated from cells using a QIAshredder and RNeasy Plus Mini Kit (Qiagen), according to the manufacturer's instructions. For cDNA synthesis, 0.5 μg of RNA was reverse-transcribed using a QuantiTect Reverse Transcription kit (Qiagen). Quantitative RT-PCR was performed using TaqMan gene expression assays (PPARGC1A, Hs00173304_m1; 18S, Hs03003631_g1), TaqMan Fast Advanced Master Mix, and the Applied Biosystems StepOnePlus Real-Time PCR System (Life Technologies). All data were normalized to expression of the 18S housekeeping gene. Relative PGC1α expression levels between samples were calculated using the 2−ΔΔCt method.24
Results
Up-Regulation of Oxidative Phosphorylation in Invasive Melanoma
To identify mediators of primary melanoma invasion, we developed a zebrafish tumor model [Tg(mitfa:BRAF(V600E));p53(lf);mitfa(lf)] that allowed expression of constitutively active Myr-RSK1 in rescued melanocytes from a MiniCoopR tol2-based transposon vector.14 Previously, this model was used to show that activation of RSK1, a key downstream effector of ERK1/2 signaling,25 promotes vertical invasion of melanoma.26 To gain a more detailed understanding of the mechanisms underlying the invasive growth of primary melanoma, this investigation identified cellular processes that are altered between Myr-RSK1 and Green fluorescent protein (control) tumors in this in vivo model using RNA-Seq (Figure 1A). The analysis revealed 1249 transcripts whose expression was significantly different (adjusted P < 0.05) between Myr-RSK1 tumors and Green fluorescent protein control tumors (Figure 1B and Supplemental Table S1). Notably, analysis of differentially expressed genes between control and RSK1-activated tumors identified significant enrichment for gene ontology terms associated with metabolism, including electron transport chain, oxidative phosphorylation, and fatty acid biosynthesis (Figure 1C and Supplemental Tables S2 and S3). The analysis also identified enrichment of gene ontology terms that have been associated with melanoma development and progression (Figure 1C), including extracellular matrix reorganization, cell cycle progression, regulation of growth, calcium signaling, histone modifications, and RNA processing.27, 28, 29, 30 Further analysis using Kyoto Encyclopedia of Genes and Genomes gene sets identified 51 genes belonging to the OXPHOS pathway that were up-regulated in Myr-RSK1 tumors relative to Green fluorescent protein control tumors (Figure 1D).
Figure 1.
A zebrafish model identification of increased oxidative phosphorylation in invasive melanoma. A: Zebrafish melanoma model used for RNA-Seq comparison of low- and high-invasive melanoma. Six independent tumors from each experimental group [green fluorescent protein (GFP) control or constitutively active/myristoylated ribosomal protein S6 kinase (Myr-RSK)-1] were used for RNA-Seq comparative analysis. B: Heatmap showing 1249 genes differentially expressed between zebrafish melanomas expressing GFP (control) or Myr-RSK1. Red and green indicate up- and down-regulated genes, respectively. C: Gene ontology analysis showing enrichment of biological processes among genes differentially expressed between Myr-RSK1 and GFP data sets. D: Heatmap showing 51 genes from the oxidative phosphorylation Kyoto Encyclopedia of Genes and Genomes (KEGG) gene set that are significantly up-regulated in RSK1 tumors. Red and blue indicate up- and down-regulation, respectively. E: Oxygen consumption rate for radial growth phase (RGP) (WM35) and vertical growth phase (VGP) (WM278, WM115, and WM3248) melanoma cell lines of increasing invasive potential. F: Metabolic profiling assay comparing utilization of glutamine and arginine as carbon sources in RGP (WM35) versus VGP (WM3248) melanoma cells. Data are expressed as means ± SD. n = 6 per group (B). ∗∗P < 0.01 versus all other groups (one-way analysis of variance). ECM, extracellular matrix; GO, gene ontology; nc, noncoding.
To establish a role for increased OXPHOS in invasive primary human melanoma, this study used a cell-based assay to assess the OCR in a panel of RGP and VGP human melanoma cell lines (WM35, WM278, WM115, and WM3248). In this assay, the signal from a phosphorescent oxygen probe was quenched by oxygen, resulting in a signal that was inversely proportional to the amount of oxygen present in the sample. The results showed that the OCR was significantly higher in more invasive WM3248 VGP melanoma cells compared to RGP and less invasive VGP melanoma cell lines (WM35, WM278, and WM115) (Figure 1E). Finally, to understand the utilization of carbon sources to fuel this increased OXPHOS, a metabolic phenotype microarray was used to compare RGP (WM35) and high invasive VGP (WM3248) melanoma cell lines. WM3248 cells showed increased utilization of glutamine and arginine carbon sources relative to WM35 cells (Figure 1F), suggesting that glutamine and arginine metabolism might support OXPHOS and an invasive VGP melanoma phenotype. Taken together, these results suggest a role for increased OXPHOS in primary melanoma invasion.
Association between Expression of PGC1α and Primary Melanoma Invasion and Proliferation
The transcriptional coactivator PGC1α promotes mitochondrial biogenesis in various tissues to increase energy production through OXPHOS.31 More recently, PGC1α was shown to promote OXPHOS in metastatic melanoma.10,11 Based on the results of the OCR assays in RGP and VGP melanoma cell lines, which showed that OXPHOS was elevated in highly invasive cells in this study, it was hypothesized that PGC1α expression would be positively associated with tumor thickness in primary melanoma specimens. To test this hypothesis, IHC was used to assess PGC1α protein expression in a cohort of primary melanoma specimens (n = 46) (Supplemental Table S4). Nuclear expression of PGC1α was determined using a composite scoring method that reflected staining intensity and distribution (Materials and Methods), and samples were stratified into groups using the following IHC scale: 0, absent PGC1α expression; 2, weak PGC1α expression; or 3 or 4, strong PGC1α expression. The results showed that 18 of 46 tumors (39.1%) had absent nuclear expression of PGC1α, 12 of 46 tumors (26.1%) had weak nuclear expression of PGC1α, and 16 of 46 tumors (34.8%) had strong nuclear expression of PGC1α. Nuclear expression of PGC1α was significantly higher in thick, more invasive primary melanomas compared to thin, less invasive primary melanomas (Figure 2), and a significant positive correlation was found to exist between PGC1α expression and tumor thickness (Pearson r = 0.437, P = 0.0024). In parallel, the expression of the cellular proliferation marker Ki-67 was assessed by IHC in the same primary melanoma tissue cohort. A significant positive association between PGC1α and Ki-67 expression was found (Figure 2). All tumors shown in Figure 2 belonged to the nodular melanoma histologic subtype (Supplemental Figure S1). These results are consistent with a role for PGC1α in promoting the vertical growth and invasion that are a hallmark of early melanoma progression, at least in part through the up-regulation of OXPHOS.
Figure 2.
Associations between peroxisome proliferator–activated receptor γ coactivator (PGC)-1α and primary melanoma thickness and Ki-67 expression. A: Top panel: PGC1α expression in primary melanomas of 4 mm thickness. Bottom panel: Ki-67 expression according to PGC1α expression (absent, weak, or strong). B: Representative immunohistochemistry photomicrographs showing nuclear expression of PGC1α (left column) and Ki-67 (right column) in primary melanoma specimens, each of which is of the nodular melanoma histologic subtype. Insets show the main image at higher magnification. Data are expressed as means ± SD. n = 46 (A). ∗P < 0.05 (two-tailed unpaired t-test). Scale bars: 0.2 mm (main images); 0.1 mm (insets). Original magnification: ×10 (main images); ×40 (insets).
Associations between PGC1α Regulation of Expression of Genes and Oxidative Stress, Growth, and Invasion of Primary Melanoma Cells
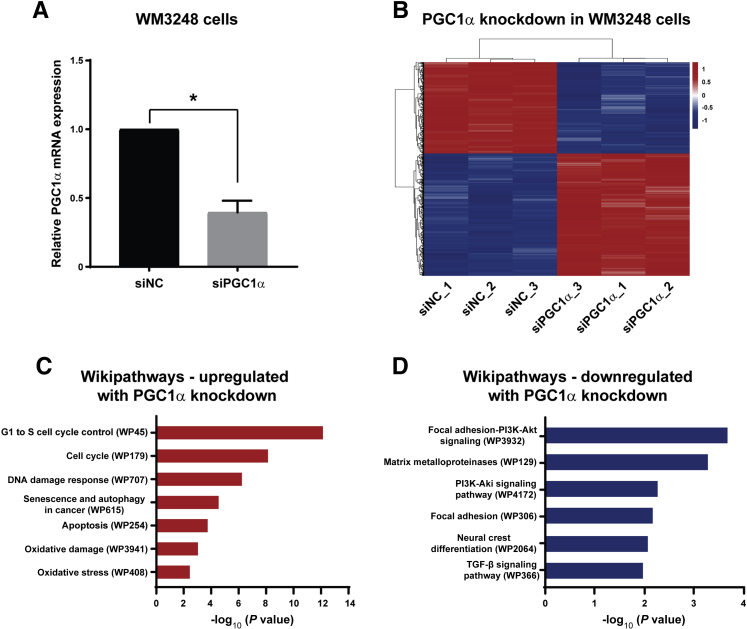
This study next sought to elucidate how PGC1α promotes primary melanoma progression. RNA interference was used to deplete PGC1α levels in invasive WM3248 primary melanoma cells, and transfection with siRNA targeting PGC1α produced a >60% reduction in PGC1α mRNA levels (Figure 3A). To identify transcripts regulated by PGC1α in primary melanoma, RNA-Seq was performed using RNA extracted from WM3248 cells that were transfected with siRNA against PGC1α or negative control siRNA (Figure 3B). Genes whose expression was significantly altered after PGC1α knockdown (adjusted P < 0.05 and log2 fold-change ≥0.5 or ≤−0.5) (Supplemental Table S5) were analyzed for functional enrichment among canonical pathways (Wikipathways). In PGC1α-depleted WM3248 cells, this study identified up-regulation of oxidative stress, cell cycle control, DNA damage response, senescence and autophagy, and apoptosis; and down-regulation of focal adhesion/phosphatidylinositol 3-kinase–Akt signaling, matrix metalloproteinases, focal adhesion, neural crest differentiation, and transforming growth factor β signaling (Figure 3, C and D, and Supplemental Tables S6 and S7). The observation that PGC1α-regulated transcripts were enriched for genes involved in cell cycle control was interesting, given the positive association between PGC1α and Ki-67 identified earlier in primary melanoma specimens (Figure 2).
Figure 3.
Peroxisome proliferator-activated receptor γ coactivator (PGC)-1α knockdown induction of transcriptional changes associated with oxidative stress, growth, and invasion in WM3248 primary melanoma cells. A: Knockdown of PGC1α mRNA in WM3248 primary melanoma cells after transfection with siRNA against PGC1α (siPGC1α) compared with negative control siRNA (siNC). B: Heatmap for comparison of gene expression (by RNA-Seq) between WM3248 cells transfected with siPGC1α or siNC. Red and blue indicate up- and down-regulated genes, respectively. C and D: Analyses of functional pathways (Wikipathways) enriched for genes that are up-regulated (C) or down-regulated (D) by PGC1α knockdown in WM3248 melanoma cells. Data are expressed as means ± SD. ∗P < 0.05 (two-tailed unpaired t-test). PI3K, phosphatidylinositol 3-kinase; TGF, transforming growth factor.
To further substantiate the link between PGC1α, OXPHOS, and oxidative stress in invasive primary melanoma, this study assessed the expression of SCARA3, a protein that depletes reactive oxygen species (ROS) to protect cells from oxidative stress.32 First, significantly higher expression of scara3 in invasive, RSK1-activated zebrafish melanomas compared to control tumors was observed (Figure 4A). Next, SCARA3 protein expression by IHC was assessed in a subset of primary melanoma tissues (n = 22) that we had also stained for PGC1α. The results showed co-localization and a positive association between PGC1α and SCARA3 levels in serial tissue sections of primary melanoma (Figure 4B). Together, these findings suggest that the activation of SCARA3 expression could represent an antioxidant response in some tumors that helps to mitigate ROS production and support the invasive growth of PGC1α high/OXPHOS high primary melanoma.
Figure 4.
Co-expression of the reactive oxygen species scavenger, scavenger receptor class A member 3 (SCARA3), with peroxisome proliferator-activated receptor γ coactivator (PGC)-1α in human primary melanoma specimens. A: Increased scara3 mRNA expression in tumors from myristoylated ribosomal protein S6 kinase (Myr-RSK1) zebrafish melanomas relative to green fluorescent protein (GFP) (control) zebrafish tumors. B: Representative immunohistochemistry (IHC) photomicrographs showing focal co-localization of PGC1α (left panel) and SCARA3 (right panel) expression in serial tissue sections from human primary melanoma. Arrows denote areas of PGC1α and SCARA3 positive staining. Data are expressed as means ± SD. n = 22 cases analyzed (B). ∗P < 0.05. Scale bars = 0.2 mm. Original magnification, ×10.
Discussion
Although the effects of somatic mutations and aberrant cell signaling pathways on the transition of primary melanoma from radial to vertical growth have been previously studied,1, 2, 3, 4, 5 there has been a limited understanding of the contribution of altered tumor metabolism to the invasive growth of primary melanoma. The current analyses using a zebrafish model of invasive melanoma and human primary melanoma cell lines and patient tissues support an important role for OXPHOS in primary melanoma invasive growth. These metabolic analyses identified increased OCR and utilization of glutamine by invasive primary melanoma cells. This study shows a role for PGC1α in mediating this invasive phenotype, and that its protein expression is associated with increased primary melanoma thickness and Ki-67 expression. The suppression of PGC1α expression in primary melanoma cells led to alterations in oxidative damage/stress, cell cycle control, focal adhesion, activity of matrix metalloproteinases, and transforming growth factor β signaling, among other pathways. The links between PGC1α, OXPHOS, oxidative stress, and the progression of primary melanoma were further substantiated by an increased expression of the ROS scavenger scara3 in invasive zebrafish tumors and its colocalization with PGC1α in human primary melanoma specimens, which is consistent with an antioxidant response in these tumors.
This study used a zebrafish model with a MiniCoopR expression system that produced melanocyte-specific activation of RSK1 and BRAF V600E, in addition to loss of function of p53 and MITF, to investigate the processes underlying invasive melanoma growth.14 The biological relevance of this model relates to the observation that RSK1 is hyperactivated in highly invasive VGP melanomas compared to less invasive RGP melanomas, and that RSK1 inhibition suppresses melanoma cell migration, invasion, and proliferation.26 Zebrafish melanoma models have been shown to consistently recapitulate the human disease, as zebrafish melanocytes are distributed throughout the basal layer of the epidermis in a fashion similar to that in human melanocytes, whereas melanocytes localize primarily to hair follicles in mice.33 Zebrafish models have been used to identify novel melanoma drivers and suppressors in the context of tumor initiation and progression, and to visualize interactions between melanoma and the tumor microenvironment.33 This study highlights the utility of this zebrafish model as a tool to study regulators of melanoma invasion. To substantiate these findings, parallel analyses of human primary melanoma cell lines and tumor specimens from melanoma patients were performed. Interestingly, the increased OXPHOS we observed in invasive RSK1-driven zebrafish melanomas was not accompanied by up-regulated PGC1α expression, which likely reflects underlying biological differences between humans and zebrafish. Rather than identifying homologous genes, the current zebrafish model revealed aberrant OXPHOS as a pathway that is associated with invasive growth of primary melanoma, and which could be interrogated in more detail in the context of human melanoma.
This study identified a role for altered tumor cell metabolism, and particularly for OXPHOS, in primary melanoma invasion. Previous studies have shown that some melanomas demonstrate the Warburg effect and are reliant on glycolysis for energy production,8 such as that induced by BRAF activation,34 whereas other melanomas rely on oxidative phosphorylation associated with increased PGC1α expression.11 Tumor cells can also utilize multiple energy sources, including pyruvate or fatty acids.35 Glutamine is an alternative carbon source for melanoma cells, and feeds the tricarboxylic acid cycle as well as driving fatty acid biosynthesis.36 In this context, glutamine-fueled mitochondrial OXPHOS is decoupled from glycolysis and promotes metabolic plasticity of tumor cells that is associated with metastasis and drug resistance. For example, the metabolic switch of some melanomas to OXPHOS has been linked to resistance to mitogen-activated protein kinase pathway inhibitors.9,37 Furthermore, a recent study showed that OXPHOS is enriched in melanoma brain metastases compared to extracranial metastases, and that growth of melanoma brain metastases could be suppressed by an OXPHOS inhibitor, IACS-010759.38 The potential utility of this strategy in melanoma patients has been questioned, as such inhibitors might be expected to block OXPHOS not only in tumor cells but also in anti-tumor T cells. However, inhibition of OXPHOS does not appear to block effector T-cell function, and instead has been shown to sensitize melanoma xenografts to immune checkpoint inhibitors, possibly in part by blocking the activity of myeloid-derived suppressor cells.39
A central finding of this study was that PGC1α protein levels were associated with increased primary melanoma thickness, invasion, and proliferation, suggesting that PGC1α is involved in metabolic rewiring to increase OXPHOS and ATP production and drive an invasive primary melanoma phenotype. This finding is supported by reports that high PGC1α expression is associated with a worse prognosis in metastatic melanoma.10,11 In contrast, Luo et al40 found that PGC1α knockdown promoted invasion of metastatic melanoma cell lines and that PGC1α levels were inversely correlated with vertical growth in specimens from patients with primary melanoma. That finding suggests that the impact of PGC1α knockdown may be context specific. Additionally, Luo et al40 identified reduced levels of PGC1A mRNA in VGP versus RGP melanoma microarray data sets, but did not verify this finding with PGC1α protein. In contrast, the present study assessed, by IHC, PGC1α protein levels in tissues from patients with primary melanoma, and PGC1α protein levels are more likely to reflect the true functional impact of PGC1α in early melanoma progression, particularly given reports that have highlighted discrepancies between mRNA and protein expression in vivo.41,42
A consequence of a metabolic switch to OXPHOS in some melanomas is the increased production of ROS, which promotes oxidative stress, mitochondrial release of cytochrome C, and apoptosis.43 Tumor cells with high levels of PGC1α may thus be protected from ROS in a PGC1α-dependent manner,11 since PGC1α inhibition has been correlated with elevated ROS generation and a hypoxia-inducible factor 1α–mediated switch to glycolysis.44 Moreover, increased apoptosis of PGC1α-inhibited A375P melanoma cells can be reversed by treatment with antioxidants.11 These findings also suggest that SCARA3 may be an important component of the antioxidant response in melanoma cells as they adapt to OXPHOS to sustain their growth and invasion during early melanoma progression. Further studies are needed to fully define the mechanisms by which PGC1α mitigates the oxidative stress associated with ROS generation in the context of primary melanoma growth and invasion.
In conclusion, the current study data using a zebrafish melanoma model, as well as human primary melanoma cell lines and tumor tissues, support a role for PGC1α in mediating a metabolic switch to glutamine-driven OXPHOS during early melanoma progression. PGC1α is up-regulated in thick primary melanomas and drives melanoma cell invasion and proliferation by modulating the expression of genes involved in oxidative stress, cell cycle control, DNA damage response, apoptosis, focal adhesion, matrix metalloproteinases, phosphatidylinositol 3-kinase–Akt, and transforming growth factor β signaling. This observation that highly invasive primary melanoma cells utilize glutamine to a greater extent than less invasive primary melanoma cells suggests that they might also be vulnerable to targeting glutamine metabolism or uptake. Several different therapeutic strategies have been proposed to exploit this preference.45,46 Ultimately, an understanding of the role of metabolic rewiring in primary melanoma invasion and growth is likely to provide insight into the biology underlying melanoma progression, and might highlight novel vulnerabilities that could be targeted to treat advanced disease.
Acknowledgments
We thank Chloe Goldman for technical assistance; the New York University Genome Technology Center for expert library preparation, sequencing, and data analysis; and the High Performance Computing Core, Medical Center Information Technology group (New York University Langone Medical Center) for computing resources.
Footnotes
Supported by NIH National Cancer Institute, New York University (NYU) Cancer Institute Cancer Center Support grant P30CA016087, NYU Melanoma Specialized Program of Research Excellence (SPORE) grant P50CA016087 (I.O.), Goldberg Charitable Trust, and the Wings for Things Foundation.
Disclosures: None declared.
Supplemental material for this article can be found at http://doi.org/10.1016/j.ajpath.2020.01.012.
Contributor Information
Keith M. Giles, Email: keith.giles@nyumc.org.
Iman Osman, Email: iman.osman@nyumc.org.
Author Contributions
A.S. identified human melanoma samples for analysis, contributed significantly to writing manuscript; A.J. performed data analysis and literature review; I.B. performed IHC and assisted with RNA extraction; A.I. performed manuscript preparation; F.D. reviewed IHC and scored tissues; Y.H. performed zebrafish model development; K.G. was the primary contributor to intellectual and written components of the manuscript, performed statistical analyses, and designed and conducted experiments; I.O., oversaw the melanoma biobank and performed manuscript preparation.
Supplemental Data
Supplemental Figure S1.
Low-magnification digital hematoxylin and eosin (H&E)-stained images of human primary melanoma cases for analysis of peroxisome proliferator-activated receptor γ coactivator (PGC)-1α and Ki-67 expression. A–C: Digital H&E-stained images of primary melanoma cases (A: 09–43, B: 14–76, and C: 13–97) used for analysis of PGC1α and Ki-67 expression in Figure 2B. Each of the three representative cases were of the nodular melanoma histologic subtype. Blue and green circling represents the outline of the tumor regions, as assessed by an attending melanoma pathologist (F.D.). Scale bars: 4 mm (A); 3 mm (B); 6 mm (C).
References
- 1.Kibbi N., Kluger H., Choi J.N. Melanoma: clinical presentations. Cancer Treat Res. 2016;167:107–129. doi: 10.1007/978-3-319-22539-5_4. [DOI] [PubMed] [Google Scholar]
- 2.Thomas A.J., Erickson C.A. The making of a melanocyte: the specification of melanoblasts from the neural crest. Pigment Cell Melanoma Res. 2008;21:598–610. doi: 10.1111/j.1755-148X.2008.00506.x. [DOI] [PubMed] [Google Scholar]
- 3.Mobley A.K., Braeuer R.R., Kamiya T., Shoshan E., Bar-Eli M. Driving transcriptional regulators in melanoma metastasis. Cancer Metastasis Rev. 2012;31:621–632. doi: 10.1007/s10555-012-9358-8. [DOI] [PubMed] [Google Scholar]
- 4.Ryu B., Moriarty W.F., Stine M.J., DeLuca A., Kim D.S., Meeker A.K., Grills L.D., Switzer R.A., Eller M.S., Alani R.M. Global analysis of BRAFV600E target genes in human melanocytes identifies matrix metalloproteinase-1 as a critical mediator of melanoma growth. J Invest Dermatol. 2011;131:1579–1583. doi: 10.1038/jid.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanniford D., Segura M.F., Zhong J., Philips E., Jirau-Serrano X., Darvishian F., Berman R.S., Shapiro R.L., Pavlick A.C., Brown B., Osman I., Hernando E. Identification of metastasis-suppressive microRNAs in primary melanoma. J Natl Cancer Inst. 2015;107:dju494. doi: 10.1093/jnci/dju494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Pavlova N.N., Thompson C.B. The emerging hallmarks of cancer metabolism. Cell Metab. 2016;23:27–47. doi: 10.1016/j.cmet.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischer G.M., Vashisht Gopal Y.N., McQuade J.L., Peng W., DeBerardinis R.J., Davies M.A. Metabolic strategies of melanoma cells: mechanisms, interactions with the tumor microenvironment, and therapeutic implications. Pigment Cell Melanoma Res. 2018;31:11–30. doi: 10.1111/pcmr.12661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gopal Y.N., Rizos H., Chen G., Deng W., Frederick D.T., Cooper Z.A., Scolyer R.A., Pupo G., Komurov K., Sehgal V., Zhang J., Patel L., Pereira C.G., Broom B.M., Mills G.B., Ram P., Smith P.D., Wargo J.A., Long G.V., Davies M.A. Inhibition of mTORC1/2 overcomes resistance to MAPK pathway inhibitors mediated by PGC1alpha and oxidative phosphorylation in melanoma. Cancer Res. 2014;74:7037–7047. doi: 10.1158/0008-5472.CAN-14-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haq R., Shoag J., Andreu-Perez P., Yokoyama S., Edelman H., Rowe G.C., Frederick D.T., Hurley A.D., Nellore A., Kung A.L., Wargo J.A., Song J.S., Fisher D.E., Arany Z., Widlund H.R. Oncogenic BRAF regulates oxidative metabolism via PGC1alpha and MITF. Cancer Cell. 2013;23:302–315. doi: 10.1016/j.ccr.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vazquez F., Lim J.H., Chim H., Bhalla K., Girnun G., Pierce K., Clish C.B., Granter S.R., Widlund H.R., Spiegelman B.M., Puigserver P. PGC1alpha expression defines a subset of human melanoma tumors with increased mitochondrial capacity and resistance to oxidative stress. Cancer Cell. 2013;23:287–301. doi: 10.1016/j.ccr.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ceol C.J., Houvras Y., Jane-Valbuena J., Bilodeau S., Orlando D.A., Battisti V., Fritsch L., Lin W.M., Hollmann T.J., Ferre F., Bourque C., Burke C.J., Turner L., Uong A., Johnson L.A., Beroukhim R., Mermel C.H., Loda M., Ait-Si-Ali S., Garraway L.A., Young R.A., Zon L.I. The histone methyltransferase SETDB1 is recurrently amplified in melanoma and accelerates its onset. Nature. 2011;471:513–517. doi: 10.1038/nature09806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimamura A., Ballif B.A., Richards S.A., Blenis J. Rsk1 mediates a MEK-MAP kinase cell survival signal. Curr Biol. 2000;10:127–135. doi: 10.1016/s0960-9822(00)00310-9. [DOI] [PubMed] [Google Scholar]
- 14.Iyengar S., Houvras Y., Ceol C.J. Screening for melanoma modifiers using a zebrafish autochthonous tumor model. J Vis Exp. 2012:e50086. doi: 10.3791/50086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dobin A., Davis C.A., Schlesinger F., Drenkow J., Zaleski C., Jha S., Batut P., Chaisson M., Gingeras T.R. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liao Y., Smyth G.K., Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30:923–930. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 17.Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu G., Wang L.G., Han Y., He Q.Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16:284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen E.Y., Tan C.M., Kou Y., Duan Q., Wang Z., Meirelles G.V., Clark N.R., Ma'ayan A. EnrichR: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinformatics. 2013;14:128. doi: 10.1186/1471-2105-14-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wich L.G., Hamilton H.K., Shapiro R.L., Pavlick A., Berman R.S., Polsky D., Goldberg J.D., Hernando E., Manga P., Krogsgaard M., Kamino H., Darvishian F., Lee P., Orlow S.J., Ostrer H., Bhardwaj N., Osman I. Developing a multidisciplinary prospective melanoma biospecimen repository to advance translational research. Am J Transl Res. 2009;1:35–43. [PMC free article] [PubMed] [Google Scholar]
- 21.Scanlon P., Tian J., Zhong J., Silva I., Shapiro R., Pavlick A., Berman R., Osman I., Darvishian F. Enhanced immunohistochemical detection of neural infiltration in primary melanoma: is there a clinical value? Hum Pathol. 2014;45:1656–1663. doi: 10.1016/j.humpath.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCarty K.S., Jr., Szabo E., Flowers J.L., Cox E.B., Leight G.S., Miller L., Konrath J., Soper J.T., Budwit D.A., Creasman W.T., Seigler H.F., McCarty K.S. Use of a monoclonal anti-estrogen receptor antibody in the immunohistochemical evaluation of human tumors. Cancer Res. 1986;46:4244s–4248s. [PubMed] [Google Scholar]
- 23.Robinson E.M., Rosenbaum B.E., Zhang Y., Rogers R., Tchack J., Berman R.S., Darvishian F., Osman I., Shapiro R.L., Shao Y., Polsky D. Association between Ki-67 expression and clinical outcomes among patients with clinically node-negative, thick primary melanoma who underwent nodal staging. J Surg Oncol. 2018;118:150–156. doi: 10.1002/jso.25111. [DOI] [PubMed] [Google Scholar]
- 24.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Anjum R., Blenis J. The RSK family of kinases: emerging roles in cellular signalling. Nat Rev Mol Cell Biol. 2008;9:747–758. doi: 10.1038/nrm2509. [DOI] [PubMed] [Google Scholar]
- 26.Salhi A., Farhadian J.A., Giles K.M., Vega-Saenz de Miera E., Silva I.P., Bourque C., Yeh K., Chhangawala S., Wang J., Ye F., Zhang D.Y., Hernando-Monge E., Houvras Y., Osman I. RSK1 activation promotes invasion in nodular melanoma. Am J Pathol. 2015;185:704–716. doi: 10.1016/j.ajpath.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alonso S.R., Ortiz P., Pollan M., Perez-Gomez B., Sanchez L., Acuna M.J., Pajares R., Martinez-Tello F.J., Hortelano C.M., Piris M.A., Rodriguez-Peralto J.L. Progression in cutaneous malignant melanoma is associated with distinct expression profiles: a tissue microarray-based study. Am J Pathol. 2004;164:193–203. doi: 10.1016/s0002-9440(10)63110-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hofmann U.B., Houben R., Brocker E.B., Becker J.C. Role of matrix metalloproteinases in melanoma cell invasion. Biochimie. 2005;87:307–314. doi: 10.1016/j.biochi.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 29.Sarkar D., Leung E.Y., Baguley B.C., Finlay G.J., Askarian-Amiri M.E. Epigenetic regulation in human melanoma: past and future. Epigenetics. 2015;10:103–121. doi: 10.1080/15592294.2014.1003746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun J., Lin S., Keeley T., Yang S. Disseminating melanoma cells surf on calcium waves. Mol Cell Oncol. 2015;2:e1002714. doi: 10.1080/23723556.2014.1002714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scarpulla R.C. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim Biophys Acta. 2011;1813:1269–1278. doi: 10.1016/j.bbamcr.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han H.J., Tokino T., Nakamura Y. CSR, a scavenger receptor-like protein with a protective role against cellular damage caused by UV irradiation and oxidative stress. Hum Mol Genet. 1998;7:1039–1046. doi: 10.1093/hmg/7.6.1039. [DOI] [PubMed] [Google Scholar]
- 33.van Rooijen E., Fazio M., Zon L.I. From fish bowl to bedside: the power of zebrafish to unravel melanoma pathogenesis and discover new therapeutics. Pigment Cell Melanoma Res. 2017;30:402–412. doi: 10.1111/pcmr.12592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ratnikov B.I., Scott D.A., Osterman A.L., Smith J.W., Ronai Z.A. Metabolic rewiring in melanoma. Oncogene. 2017;36:147–157. doi: 10.1038/onc.2016.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakajima E.C., Van Houten B. Metabolic symbiosis in cancer: refocusing the Warburg lens. Mol Carcinog. 2013;52:329–337. doi: 10.1002/mc.21863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Filipp F.V., Ratnikov B., De Ingeniis J., Smith J.W., Osterman A.L., Scott D.A. Glutamine-fueled mitochondrial metabolism is decoupled from glycolysis in melanoma. Pigment Cell Melanoma Res. 2012;25:732–739. doi: 10.1111/pcmr.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang G., Frederick D.T., Wu L., Wei Z., Krepler C., Srinivasan S. Targeting mitochondrial biogenesis to overcome drug resistance to MAPK inhibitors. J Clin Invest. 2016;126:1834–1856. doi: 10.1172/JCI82661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fischer G.M., Jalali A., Kircher D.A., Lee W.C., McQuade J.L., Haydu L.E., Joon A.Y., Reuben A., de Macedo M.P., Carapeto F.C.L., Yang C., Srivastava A., Ambati C.R., Sreekumar A., Hudgens C.W., Knighton B., Deng W., Ferguson S.D., Tawbi H.A., Glitza I.C., Gershenwald J.E., Vashisht Gopal Y.N., Hwu P., Huse J.T., Wargo J.A., Futreal P.A., Putluri N., Lazar A.J., DeBerardinis R.J., Marszalek J.R., Zhang J., Holmen S.L., Tetzlaff M.T., Davies M.A. Molecular profiling reveals unique immune and metabolic features of melanoma brain metastases. Cancer Discov. 2019;9:628–645. doi: 10.1158/2159-8290.CD-18-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim S.H., Li M., Trousil S., Zhang Y., Pasca di Magliano M., Swanson K.D., Zheng B. Phenformin inhibits myeloid-derived suppressor cells and enhances the anti-tumor activity of PD-1 blockade in melanoma. J Invest Dermatol. 2017;137:1740–1748. doi: 10.1016/j.jid.2017.03.033. [DOI] [PubMed] [Google Scholar]
- 40.Luo C., Lim J.H., Lee Y., Granter S.R., Thomas A., Vazquez F., Widlund H.R., Puigserver P. A PGC1alpha-mediated transcriptional axis suppresses melanoma metastasis. Nature. 2016;537:422–426. doi: 10.1038/nature19347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tian Q., Stepaniants S.B., Mao M., Weng L., Feetham M.C., Doyle M.J., Yi E.C., Dai H., Thorsson V., Eng J., Goodlett D., Berger J.P., Gunter B., Linseley P.S., Stoughton R.B., Aebersold R., Collins S.J., Hanlon W.A., Hood L.E. Integrated genomic and proteomic analyses of gene expression in mammalian cells. Mol Cell Proteomics. 2004;3:960–969. doi: 10.1074/mcp.M400055-MCP200. [DOI] [PubMed] [Google Scholar]
- 42.Vogel C., Abreu Rde S., Ko D., Le S.Y., Shapiro B.A., Burns S.C., Sandhu D., Boutz D.R., Marcotte E.M., Penalva L.O. Sequence signatures and mRNA concentration can explain two-thirds of protein abundance variation in a human cell line. Mol Syst Biol. 2010;6:400. doi: 10.1038/msb.2010.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wen S., Zhu D., Huang P. Targeting cancer cell mitochondria as a therapeutic approach. Future Med Chem. 2013;5:53–67. doi: 10.4155/fmc.12.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lim J.H., Luo C., Vazquez F., Puigserver P. Targeting mitochondrial oxidative metabolism in melanoma causes metabolic compensation through glucose and glutamine utilization. Cancer Res. 2014;74:3535–3545. doi: 10.1158/0008-5472.CAN-13-2893-T. [DOI] [PubMed] [Google Scholar]
- 45.Qin J.Z., Xin H., Nickoloff B.J. Targeting glutamine metabolism sensitizes melanoma cells to TRAIL-induced death. Biochem Biophys Res Commun. 2010;398:146–152. doi: 10.1016/j.bbrc.2010.06.057. [DOI] [PubMed] [Google Scholar]
- 46.Wang Q., Beaumont K.A., Otte N.J., Font J., Bailey C.G., van Geldermalsen M., Sharp D.M., Tiffen J.C., Ryan R.M., Jormakka M., Haass N.K., Rasko J.E., Holst J. Targeting glutamine transport to suppress melanoma cell growth. Int J Cancer. 2014;135:1060–1071. doi: 10.1002/ijc.28749. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.