Abstract
To survive, cancer cells must resist numerous internal and environmental insults associated with neoplasia that jeopardize proteostasis within the endoplasmic reticulum (ER). Solid and hematopoietic tumors often experience genomic instability, oncogene activation, increased protein secretion demands, and somatic mutations in proteins handled by the secretory pathway that impede their folding. Invasion or metastasis into foreign environments can expose tumor cells to hypoxia, oxidative stress, lack of growth signals, inadequate amino acid supplies, glucose deprivation, and lactic acidosis, all of which pose challenges for protein processing in the ER. Together, these conditions can promote the buildup of misfolded proteins in the ER to cause ER stress, which then activates the unfolded protein response (UPR). An intracellular signaling network largely initiated by three ER transmembrane proteins, the UPR constantly surveils protein folding conditions within the ER lumen and when necessary initiates counteractive measures to maintain ER homeostasis. Under mild or moderate levels of ER stress, the homeostatic UPR sets in motion transcriptional and translational changes that promote cell adaption and survival. However, if these processes are unsuccessful at resolving ER stress, a terminal UPR program dominates and actively signals cell suicide. This article summarizes the mounting evidence that cancer cells are predisposed to ER stress and vulnerable to targeted interventions against ongoing UPR signaling.
The ER Is the Gateway to the Secretory Pathway
The endoplasmic reticulum (ER) is the foremost intracellular compartment of the secretory pathway in eukaryotic cells. In addition to its essential roles in calcium handling and lipid biosynthesis, the ER is a specialized organelle where the synthesis, folding, and maturation begin for approximately a third of the proteome, including nearly all proteins destined for secretion or residence in the plasma membrane. After cotranslational translocation into the lumen of the ER, these polypeptides are actively folded and modified by an assembly line of ER-resident enzymes, including chaperones, foldases, glycosylating enzymes, and oxidoreductases. Despite the efficiency of these protein-folding machines, a third of all polypeptides translocated into the ER fail to fold correctly.1 Misfolded proteins are targeted for elimination by quality control systems, including the ER-associated degradation pathway, which directs them to the cytoplasm for ubiquitinylation and 26S proteasomal degradation, as well as autophagy. Numerous physiological and pathologic insults can perturb protein folding and cause accumulation of misfolded forms in the ER, including nutrient fluctuations, increased demands on protein secretion, hypoxia, mutations in client proteins of the secretory pathway that stabilize or promote aggregation of intermediate folding forms, and reduction in calcium levels with inhibitory effects on calcium-dependent chaperones. If its capacity to either properly fold proteins or dispose of those that fail quality control is overwhelmed, the ER lumen will begin to accumulate misfolded proteins, a harmful situation called ER stress that threatens the function and survival of the cell.
The Unfolded Protein Response
Homeostatic versus Terminal Responses
Proteostasis, a portmanteau constructed by combining the words proteome and homeostasis, is a state of overall protein health maintained through the coordinated control of the biogenesis, folding, transport, and degradation of polypeptides in the cell. When misfolded proteins pile up in the ER above an acceptable level, this loss in ER proteostasis triggers an intracellular signal transduction pathway known as the unfolded protein response (UPR).2 In mammalian cells, the UPR is initiated by three single-pass ER transmembrane proteins that possess an ER lumenal domain capable of detecting misfolded proteins: inositol-requiring enzyme 1α (IRE1α; officially known as endoplasmic reticulum to nucleus signaling), protein kinase R (PRK)-like ER kinase (PERK; also officially known as eukaryotic translation initiation factor 2 α kinase 3), and activating transcription factor 6 (ATF6).3 When the ER is operating under homeostatic conditions, the lumenal domains of IRE1α, PERK, and ATF6 are held in a monomeric and inactive state through interaction with an abundant ER chaperone called binding Ig protein [BiP; alias glucose-regulated protein, 78kD (GRP78) and heat shock 70 kDa protein 5 (HSPA5)]. However, given its higher affinity for the exposed hydrophobic polypeptide domains contained within misfolded proteins, BiP/GRP78 is titrated off the ER stress sensors and onto misfolded proteins as they begin to accumulate within the ER lumen, thereby priming the UPR stress sensors to be ready to signal. Misfolded proteins are then capable of serving as direct ligands for the ER lumenal domains of the unrestrained UPR sensors, which leads to their oligomerization and activation.4 The initial outputs of the UPR work to restore ER homeostasis by decreasing demand and increasing capacity on the protein-folding machinery in an effort to preserve cell function, a process termed homeostatic UPR.5 However, if these corrective measures prove inadequate to restore homeostasis, then the ER sensors initiate an alternative program called the terminal UPR, which actively signals cell destruction.6,7
The Three Branches of the UPR
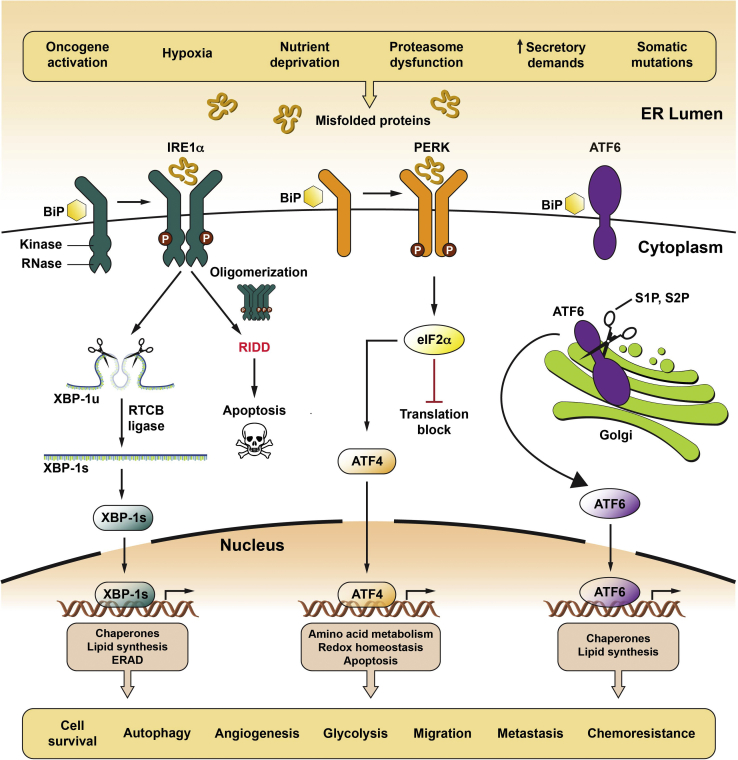
IRE1α is a bifunctional kinase/endoribonuclease (RNase) that employs autophosphorylation as a readout of ER stress severity to govern cell outcome (Figure 1).8, 9, 10 Rectifiable ER stress causes low-level, transient kinase autophosphorylation and dimerization/tetramerization that confines IRE1α′s RNase activity to excising a 26-nucleotide nonconventional intron from the mRNA encoding X-box binding protein 1 (XBP1). Religation of spliced XBP1 by a cytoplasmic ligase called RNA 2′,3′-cyclic phosphate and 5′-OH ligase changes the open reading frame such that its translation produces the homeostatic transcription factor spliced XBP1 (XBP1s),11,12 which up-regulates dozens of genes that code for ER biogenesis, protein folding, and quality control constituents.13 In contrast, persistent and unresolved ER stress leads to sustained, high-level IRE1α autophosphorylation, which drives higher-order oligomerization of IRE1α. When oligomerized, IRE1α′s RNase further increases its XBP1 splicing activity, but, more important, it lowers its specificity to endonucleolytically degrade hundreds of mRNA species as they attempt translation at the ER membrane8,9,14—a process known as regulated IRE1α-dependent decay (RIDD). Although depletion of secretory pathway transcripts through RIDD may initially limit ER stress by reducing the protein-folding burden on the ER, the unselective destruction of mRNAs at the ER membrane eventually results in the depletion of critical enzymatic and structural components of the ER. The net consequence of RIDD is that it actively destroys ER function. Moreover, RIDD reduces select miRNAs, such as miR-17, that are repressing proapoptotic targets, like the pro-oxidant protein thioredoxin-interacting protein, which leads to their up-regulation and consequent prodeath effects.15,16 Thus, hyperactivated in response to severe ER stress, IRE1α′s RNase promotes a terminal over homeostatic UPR in which RIDD-mediated ER destruction and proapoptotic signals overwhelm the adaptive signaling through XBP1 mRNA splicing.
Figure 1.
Role of the unfolded protein response (UPR) in cancer. Cancer cells frequently encounter extrinsic stresses that challenge protein folding in the endoplasmic reticulum (ER), including hypoxia, nutrient deprivation, and growth factor withdrawal. Intrinsic stresses, such as oncogene activation, proteasome dysfunction, increased glycolysis, and increased protein secretion, can lead to additional demands on the secretory pathway. Furthermore, genomic instability and somatic mutations that cripple protein folding can lead to ER stress. On detecting an accumulation of ER misfolded proteins, the UPR is initiated by three transmembrane ER proteins: inositol-requiring enzyme 1α (IRE1α; alias endoplasmic reticulum to nucleus signaling), PRK-like ER kinase (PERK; alias eukaryotic translation initiation factor 2 α kinase 3), and activating transcription factor 6α (ATF6α; see text for signaling details). The combined outputs of the UPR can regulate tumor growth at many levels, including cell survival, autophagy, angiogenesis, glycolysis, migration, metastases, and chemoresistance. Modified from Oakes10 with permission from APS Publications. BiP, binding Ig protein; eIF2α, eukaryotic translation initiation factor 2α; ERAD, ER-associated degradation; Redox, oxidation-reduction; RIDD, regulated IRE1α-dependent decay; RTCB, RNA 2′,3′-cyclic phosphate and 5′-OH ligase; S1P, site-1 protease; S2P, site-2 protease; XBP1, X-box binding protein 1-unspliced; XBP-1s, spliced X-box binding protein 1.
On activation by ER stress, PERK has a cytosolic kinase that trans-autophosphorylates and then phosphorylates eukaryotic translation initiation factor 2α (eIF2α), which prevents association of the eIF2-GTP-Met-tRNA ternary complex to reduce protein translation, thereby lessening protein-folding burden in the ER.17 A second target phosphorylated by PERK is nuclear factor erythroid 2–related factor 2,18 which then transcriptionally up-regulates antioxidants and other factors that protect against oxidative stress. Moreover, PERK's inhibitory efforts on Cap-dependent protein translation promote ER homeostasis because translation machinery becomes biased for mRNAs containing an internal ribosome entry site, such as activating transcription factor 4 [ATF4; alias cAMP-response element binding protein 2 (CREB2)], a major adaptive factor that induces genes involved in protein folding, resistance to oxidative stress, autophagy, and amino acid metabolism.19 Although a hiatus in global protein translation can be advantageous for ER stressed cells by giving them extra time to process a stockpile of proteins, a prolonged block in translation from persistent PERK signaling is incompatible with cell viability. Therefore, ATF4 transcriptionally up-regulates growth arrest and DNA damage-inducible protein, a key regulatory subunit of protein phosphatase 1, which then dephosphorylates eIF2α to restore mRNA translation.20 However, if ER stress remains unresolved, sustained PERK activation will result in up-regulation of the C/EBP homologous protein [alias growth arrest and DNA damage 153 (GADD153)] transcription factor, which rapidly reduces expression of short-lived antiapoptotic proteins B-cell lymphoma–extra large (normal half-life 4 to 18 hours) and myeloid cell leukemia 1 (normal half-life 0.5 hours) and increases expression of proapoptotic BCL2 family proteins to promote cell death.21,22 Hence, like IRE1α, prolonged PERK activation is detrimental toward cell survival (Figure 1).
ATF6 is a type II transmembrane protein that contains a basic leucine zipper transcription factor within its cytoplasmic domain. On detection of ER misfolded proteins, ATF6 shuttles to the Golgi apparatus, where it undergoes intramembrane cleavage by the site-1 and site-2 proteases to release the p50 ATF6(N) transcription factor, which then translocates to the nucleus.23 In cooperation with XBP1s, ATF6(N) up-regulates many genes (eg, BiP and DnaJ homolog subfamily C member 3, alias P58IPK) that enlarge ER size and protein-folding capacity as well as up-regulate ER-associated degradation pathway components.24 Under high ER stress, ATF6 has been reported to reduce antiapoptotic proteins, such as Mcl-1,25 but, in general, the contributions of ATF6 to ER stress-induced cell death are not yet well understood (Figure 1).
ER Stress in Cancer
Does the UPR Prevent or Promote Cancer?
One of the most common features of cancer cells is their ability to locally spread or metastasize into other tissues, where hostile environmental conditions, such as hypoxia, low glucose, growth factor deficiency, lactic acidosis, oxidative stress, and amino acid starvation, jeopardize the fidelity of protein folding in the ER.26 Moreover, intrinsic stresses shared by many tumor cells, including oncogene activation, aneuploidy, and increased glycolysis, often lead to a higher steady-state level in protein translation and protein secretion, adding pressure on the secretory pathway.27 Furthermore, genomic instability and somatic mutations in secretory pathway proteins can disrupt their folding and cause ER stress. As such, numerous studies have documented high-level activation of all three branches of the UPR (IRE1α, PERK, and ATF6) in a wide range of human hematopoietic (leukemia, lymphoma, and myeloma) and solid tumors, including glioblastoma and carcinomas of the breast, stomach, colon, esophagus, lung, prostate, pancreas, and liver.26 Somatic mutations in or near one of the enzymatic domains (kinase or RNase) of IRE1α have been found rarely (<1%) in glioblastoma, lung carcinoma, and ovarian cancer.28 Previous studies have found that most of these cancer-associated mutations in IRE1α disable its apoptotic outputs,9,29 suggesting that some cancer cells may shut down the terminal UPR to survive. Likewise, a loss-of-function mutation (c.499C>A) in XBP1 that prevents its proper mRNA splicing has been reported in rare myeloma cases and may be associated with resistance to proteasome inhibitors.30 Furthermore, the ER chaperones BiP/GRP78 and GRP94, which promote adaptation to ER stress, are overexpressed in a variety of cancer types.31 Both GRP78 and GRP94 are also up-regulated by a variety of oncogenic viruses, including hepatitis C virus and human papillomavirus.32,33 Taken together, these studies demonstrate UPR activation in many neoplasms.
Despite the vast evidence of ongoing ER stress in multiple cancer types, whether the UPR restrains or promotes tumor growth in patients has been hotly debated. For example, pharmacologically inhibiting IRE1α in >300 cancer cell lines in culture failed to impair tumor cell viability.34 Moreover, PERK haploinsufficiency has been reported to promote melanoma development.35 Conceptionally, targeting the UPR might block ER stress-induced apoptosis and inadvertently promote cancer progression, raising concerns about the safety of therapeutics against this pathway. However, over the past few years, multiple preclinical studies have provided strong evidence that in vivo the UPR supports various aspects of tumor growth, including angiogenesis, metabolism, metastasis, and chemoresistance. For example, studies have found that IRE1α′s homeostatic target XBP1 promotes tumor progression in multiple preclinical models of triple-negative breast cancer.36 Genetic deletion of IRE1α in a human glioma cell line inhibited angiogenesis and decreased tumor growth when these cells were subsequently injected into mice.37 The IRE1α-XBP1s pathway has also been shown to support prostate cancer growth through activating c-MYC signaling,38 raising the possibility that other MYC-driven tumors will have a similar dependency on the UPR. PERK has been documented to support tumor growth in murine models of mammary cancer by limiting oxidative damage,39 and PERK inhibition reduces breast cancer metastasis to the lung after tail vein injection into immunocompromised mice.40 Moreover, PERK signaling in HT29 colon cancer cell lines promotes chemoresistance.41 Fewer studies have examined the role of ATF6 in cancer. However, a recent report suggests that high ATF6 expression is a marker of precancerous lesions in the setting of colorectal carcinoma.42 Together, these data strongly suggest that the UPR has tumor-promoting functions in vivo.
The Role of the UPR in Neoplasms of Professional Secretory Cells
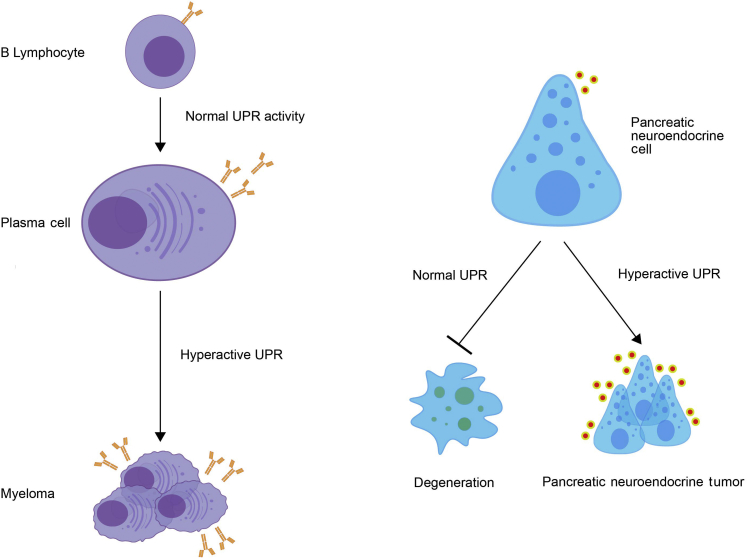
Recently, the UPR has emerged as an attractive target in two neoplasms that derive from professional secretory cells and retain or even exceed their normally high protein secretion burden into malignancy. The first such neoplasm is myeloma, a tumor of malignant plasma cells that can often secrete their own weight in Ig each day. The earliest hint that the UPR might be particularly important to plasma cells came from developmental studies. In mice, deletion of Ire1α or Xbp1 prevents the differentiation of B lymphocytes into plasma cells,43 a process that requires a massive expansion of the ER to accommodate the enormous Ig production levels of this cell type. It was later reported that at least 50% of primary human myelomas have elevated levels of XBP1s.44 This set up the prediction that if IRE1α/XBP1 signaling is required for normal plasma cell differentiation, then perhaps constitutive signaling through XBP1s could lead to extended plasma cell survival and a permissive environment for the accumulation of additional oncogenic events. Further supporting this idea, transgenic expression of Xbp1s in B lymphocytes of mice leads to a plasma cell malignancy with many features of myeloma.44 There is also evidence to suggest that proteasome inhibitors that the Food and Drug Administration approved as first-line therapy for myeloma work in part by halting the disposal of misfolded proteins through the ER-associated degradation pathway and thus triggering ER stress–induced apoptosis,45 again implicating the UPR as a potential target in this disease. The strongest evidence comes from a recent report showing that genetic disruption of IRE1α or XBP1s, or pharmacologic inhibition of IRE1α′s kinase activity, significantly reduced s.c. or orthometastatic myeloma tumor growth in mice.46 Mechanistically, blockade of IRE1α/XBP1 signaling leads to a reduction in cytokines and chemokines known to promote myeloma growth.
Pancreatic neuroendocrine tumors (PanNETs) are the second secretory neoplasm for which the UPR has been shown to play a critical role on the basis of recent work from our laboratory. PanNETs originate from professional secretory cells, which are critically dependent on a functional UPR for proper development and maintenance. Homozygous deletion of Perk in mice causes rapid pancreatic β-cell apoptosis, leading to infantile diabetes, pancreatic exocrine insufficiency, and early growth defects.47,48 This phenocopies Wolcott-Rallison syndrome, a rare human diabetic syndrome caused by homozygous loss-of-function mutations in PERK.48 Similarly, the genetic removal of Ire1α in adult murine β cells results in their severe dysfunction and defective insulin secretion,49 whereas deleting Xbp1 specifically in the β-cell compartment leads to upstream IRE1α hyperactivation, degeneration of β cells, and hyperglycemia.50 Although healthy endocrine cells can secrete large amounts of protein in response to appropriate signals, the vast majority of PanNETs constitutively hypersecrete one or more hormones. For example, although each normal pancreatic β cell is capable of releasing an estimated 1 million molecules of insulin per minute,51 some insulinoma PanNET cells secrete >10-fold higher amounts of this hormone, even under normoglycemic conditions.52 On the basis of these observations, we predicted that PanNETs would be particularly dependent on the UPR to manage ER stress.
Consistent with this notion, human PanNETs were found to have elevated ER stress markers (eg, BiP) and up-regulation of homeostatic outputs of the IRE1α and PERK arms of the UPR.53 More important, rat insulinoma INS-1 cells (a well-established PanNET model) grown in culture have low levels of ER stress and UPR activation; however, the same cells grown in mice as xenografts show marked up-regulation of the UPR, demonstrating that these tumor cells face unique challenges to ER proteostasis in vivo (eg, hypoxia and nutrient deprivation). As such, although loss of IRE1α or PERK had little effect on the growth of INS-1 cells in culture, it markedly halted their ability to form tumors in vivo. Pharmacologic modulation of IRE1α and PERK in INS-1 xenografts or in the spontaneous genetic (RIP-Tag2) mouse PanNET model showed that UPR signaling was optimized for adaptation in vivo, and that inhibiting either IRE1α or PERK led to hyperactivation and apoptotic signaling through the reciprocal arm, thereby halting the growth and survival of the tumor cells.
Taken together, these preclinical studies argue that the homeostatic UPR is frequently activated in cancer, especially highly secretory neoplasms, and necessary for the survival and/or growth of the tumor cells under conditions that stress the ER (Figure 2). However, if the UPR is to be targeted effectively to control cancer, the mechanisms by which UPR activation is promoting tumor growth must be understood. Although much work remains, recent studies have started to uncover how a deregulated UPR contributes to cancer.
Figure 2.
Normal and malignant professional secretory cells are critically dependent on the unfolded protein response (UPR). Plasma cells and pancreatic neuroendocrine cells, two cell types with a high secretory burden, require the UPR for their development and/or function. However, elevated UPR signaling is commonly seen in neoplasms derived from these cells (eg, myeloma and pancreatic neuroendocrine tumors) and appears critical for their growth and survival.
Protumorigenic Outputs of the UPR
The UPR-Autophagy Connection
Autophagy is an evolutionarily conserved pathway that regulates lysosomal degradation of long-lived proteins and damaged organelles, including the ER. In mammalian cells, >30 autophagy-related genes (ATGs) orchestrate each step in the autophagy process, including induction, vesicle initiation, autophagosome maturation, fusion with the lysosome, and lysosomal digestion. Through recycling damaged or surplus cell components, autophagy can provide energy or macromolecular building blocks to maintain cellular homeostasis and promote survival. The role of autophagy in cancer is complex and context dependent.54 The cellular homeostasis functions of autophagy, especially those of limiting the accumulation of reactive oxygen species and preventing DNA damage, seem to play an important role in preventing tumorigenesis. For example, monoallelic deletion of Beclin-1 (mammalian ortholog of yeast Atg6 encoded by the BECN1 gene) occurs in approximately 50% of breast, ovarian, and prostate cancers55; and mice heterozygous for one copy of Becn1 show a high incidence of lymphoma, hepatocellular carcinoma, and lung cancer.56,57 However, autophagy induction in tumors exposed to hypoxia and/or low nutrient conditions can provide energy sources that promote cancer cell survival and resistance to chemotherapy.58 Furthermore, autophagy activity in nearby tumor stromal fibroblasts can lead to the delivery of metabolites, such as lactate, ketone bodies, and amino acids, to serve as fuel to promote tumor growth.59 As such, blocking autophagy has antitumor activity in many murine cancer models, with the evidence strongest for tumors driven by oncogenic RAS.60
Over the past several years, a great deal of cross talk has been found between the UPR and autophagy pathways. Although the molecular mechanisms linking these two ancient survival pathways are still being worked out, it has been reported that each arm of the UPR is capable of regulating autophagy in distinct ways.54 For example, the IRE1α/XBP1 pathway has been shown to induce autophagy in multiple cell types. This is in part because of the ability of XBP1s to bind directly to the BECN1 promoter and enhance expression of Beclin-1.61 However, despite its proautophagy effects when activated, XBP1 deficiency has been reported to result in enhanced autophagy in cells from amyotrophic lateral sclerosis patients62 and in neurons through a mechanism that involves elevated expression of the transcription factor forkhead box protein 01.63 Hence, it appears that either insufficient or excess IRE1α/XBP1 signaling can promote autophagy. The PERK pathway has been shown to be required for autophagy triggered by polyglutamine aggregates.64 The PERK output ATF4 is critical for the up-regulation of ATG12,65 a key component of the complex responsible for elongation of autophagosomes. In addition, C/EBP homologous protein can increase the expression of ATG5.66 Finally, there have been multiple reports that ATF6 is required for autophagy induction through a mechanism that involves transcriptionally up-regulating death-associated kinase 1, which promotes autophagosome formation by phosphorylating Beclin-1.67 Finally, the UPR and autophagy pathways have both been shown to coordinately regulate several histone deacetylases implicated in cancer, such as the cytoplasmic deacetylase histone deacetylase 6, which acts on α-tubulin, heat shock protein 90, and stress granules to promote tumor cell survival and migration.68 Hence, although there is much to learn about the molecular interplay between these pathways, the UPR-autophagy connection is likely to be important in the context of neoplasia.
UPR Signals Tumor Angiogenesis in Response to Hypoxia
Solid tumor growth is limited by the ability to provide sufficient oxygen, glucose, and other nutrients to the expanding cell mass, especially those cells in the center of the tumor. When challenged with hypoxia and glucose deprivation, tumor cells up-regulate expression of vascular endothelial growth factor (VEGF), fibroblast growth factor 2, and other proangiogenic signals to promote neoangiogenesis. These same conditions cause ER stress and activate the UPR, which regulates the transcriptional and post-translational control of several proangiogenic factors. All three arms of the UPR have been shown to strongly up-regulate VEGF-A,69 which stimulates angiogenesis to protect rapidly growing tumor cells from hypoxia. The UPR transcription factor ATF4 directly binds the VEGF-A promoter,70 and XBP1 transcriptionally up-regulates VEGF-A and IL-6 expression, possibly through a hypoxia-inducible factor-1α (HIF1α)–dependent mechanism.36,37 In several human cancer lines, hypoxia increases the transcription of two essential autophagy genes, microtubule-associated protein 1 light chain 3β and ATG5, through the PERK outputs ATF4 and C/EBP homologous protein, respectively.66 By up-regulating autophagy in this way, PERK can promote tumor cell survival under hypoxic conditions. The ER chaperone BiP/GRP78 also promotes cancer growth and chemoresistance, in part through up-regulating tumor angiogenesis.71 Another UPR-regulated ER chaperone, called oxygen-regulated protein 150, promotes the VEGF secretion.72 Interestingly, VEGF has also been reported to activate the UPR in endothelial cells in the absence of ER stress through a noncanonical mechanism involving phospholipase C and mammalian target of rapamycin signaling.73 In such a way, VEGF seems capable of maintaining its own expression through triggering the UPR in endothelial cells, a feed-forward loop with the potential to strongly promote angiogenesis.74
The UPR, Cell Migration, and Metastasis
Evidence has emerged that the UPR can regulate cytoskeleton remodeling and cell migration. Independent of its canonical role as a UPR signal transducer, IRE1α was reported to control cytoskeleton dynamics and cell motility through directly binding filamin A.75 Consistent with this notion, there have been studies suggesting that the UPR may govern cancer cell metastasis, at least in preclinical models. Tumor cells often undergo epithelial-to-mesenchymal transition before metastasis, which involves acquisition of a highly secretory phenotype and increased UPR signaling. In breast tumor models, PERK inhibition attenuates metastasis to the lung after tail-vein injection into an immunocompromised mouse.40 ATF4 has been reported as critical for metastatic potential of breast cancer cells subjected to hypoxia through a mechanism involving activation of lysosome-associated membrane protein 3.76,77 On the other hand, expressing a dominant negative mutant of IRE1α in glioma tumor cells led to reduced growth and angiogenesis in vivo, but increased invasiveness.37,78 Hence, although blocking IRE1α signaling may inhibit primary tumor progression, it has the potential to promote a more invasive phenotype. In this model, IRE1α regulates glioma cell migration through RIDD-mediated decay of the mRNA for secreted protein acidic and rich in cysteine (alias osteonectin), a matrix-associated protein important for the interaction between glioma cells and the extracellular matrix. The inhibition of IRE1α′s RNase leads to increased secreted protein acidic and rich in cysteine and promotes tumor cell invasion.79 The proinvasive phenotype these cells acquire in response to IRE1α inhibition is akin to that of some cancer types treated with direct angiogenesis inhibitors (eg, those targeting VEGF).80
The UPR Regulates Metabolism
When faced with low nutrient conditions, cancer cells are capable of modifying their metabolism through UPR activation.81 In response to IRE1α-mediated splicing, XBP1s transcriptionally up-regulates components of the hexosamine biosynthetic pathway that catalyze the conversion of glucose to UDP-acetylglucosamine, which serves as substrates for O- and N-linked glycosylation to promote proteostasis.82,83 Moreover, another known target of XBP1 is HIF1α, which up-regulates glucose transporters to facilitate glycolysis.84
The UPR and Resistance to Treatment
Despite major advances in the effectiveness of cytotoxic chemotherapy and targeted therapies, most ultimately fail because of the emergence of resistant clones. The mechanisms involved in chemotherapy resistance vary widely, depending on the therapy employed and cancer type. However, common mechanisms include drug inactivation through detoxification pathways, down-regulation of drug transporters, up-regulation of efflux pumps, up-regulation of prosurvival proteins, and enhanced DNA repair.85
Mounting evidence over the past decade suggests that the UPR plays a key role in resistance to chemotherapy, hormone therapy, and targeted therapies for cancer.81 The initial link was noted when UPR activation was found to correlate with drug resistance in solid tumors, in particular breast cancer and colorectal carcinoma.86, 87, 88, 89 Increased expression of the UPR sensors and their downstream outputs (including XBP1s) is correlated with tamoxifen resistance, decreased time to recurrence, and shortened survival.90 Whether and how the UPR is directly contributing to chemoresistance in vivo remain poorly understood. However, in vitro studies on cultured cancer cell lines have demonstrated that UPR activation can promote survival against chemotherapy agents. Many studies have shown that GRP78 overexpression can prevent drug-induced apoptosis, in part through inhibition of proapoptotic Bcl-2–associated protein expression and caspase-7 activation.91 In HT29 colon cancer cells, PERK was reported to promote chemoresistance through the regulation of nuclear factor erythroid 2–related factor 2,41 and genetic or pharmacologic inhibition of PERK sensitized human colon cancer cells to 5-fluorouracil chemotherapy.92 Genetic deletion of IRE1α sensitized KRAS-mutant colon cancer cells to mitogen-activated protein kinase kinase (MEK) inhibitors.93 However, more studies are needed to determine the mechanisms through which each arm of the UPR can contribute to chemoresistance and whether inhibiting the UPR is a beneficial strategy for increasing response to chemotherapy.
Cross Talk between UPR and Other Oncogenic Signaling Pathways
In addition to its distinctive signaling outputs, the UPR communicates in complex ways with other major signaling pathways that impact tumor development. For example, the UPR can have a major impact on the NF-κB and HIF1α pathways, two signaling pathways widely known to promote tumorigenesis in some settings. PERK-mediated inhibition of Cap-dependent protein translation results in a relative reduction in IκB, leading to nuclear translocation of NF-κB and transcription of its target genes.94 Through its association with tumor necrosis factor receptor–associated factor 2, IRE1α activation can lead to IκB phosphorylation and degradation, again resulting in NF-κB signaling.95 In triple-negative breast cancer cell lines, IRE1α′s adaptive output XBP1 was found to form a transcriptional complex with HIF1α and regulate expression of HIF1α targets through the recruitment of RNA polymerase II.36 These examples exemplify the elaborate wiring between the UPR and other signaling pathways that together will determine tumor cell behavior and the challenges in trying to isolate the UPR-specific roles in cancer.
The Impact of the UPR on Tumor Immunity
As a point of reference, this review is focused on how tumor cell intrinsic ER stress and UPR signaling impact cancer growth. However, it is now well established that the UPR plays an essential role in the differentiation and function of many immune cell types, and therefore has consequences on antigen presentation, antibody production, inflammation, and tumor immunity. In particular, signaling through the IRE1α/XBP1 pathway in tumor-associated dendritic cells blunts antitumor immunity. As such, genetic or pharmacologic inhibition of this pathway has been shown to enhance T-cell antitumor immunity and increase the survival of cancer-bearing mice.96 Moreover, several approved chemotherapeutics work in part through inducing immunogenic cell death and antitumor immunity. The process of immunogenic cell death involves the translocation of calreticulin from the ER lumen to the plasma membrane, where it enhances the transfer of tumor antigens to dendritic cells.97,98 Recently, PERK activation and eIF2α phosphorylation have been found to be a marker for immunogenic cell death and tumors susceptible to therapies that induce this process.99,100 Hence, it appears that targeting the UPR can have anticancer benefits at both the level of the tumor cells themselves and the antitumor capacity of T cells. Readers interested in learning more about the UPR and tumor immunosurveillance can find several recent reviews on the topic.101,102
Targeting the UPR in Cancer
Considering the evidence of aberrant UPR signaling in cancer, it should come as no surprise that there is strong interest in pharmacologically controlling its outputs as a strategy to limit tumor growth. As the UPR first responders, IRE1α, PERK, and ATF6 are obvious targets through which to control this pathway. In particular, there has been much effort in developing small-molecule modulators of the enzyme active sites of IRE1α and PERK. Various small molecules have been identified that directly bind and inhibit IRE1α′s RNase.103, 104, 105, 106 Most direct inhibitors identified contain a reactive electrophile that covalently binds the RNase active site of IRE1α, with the most common pharmacophore being a salicylaldehyde, including SFT-083010, MKC-3946, and 4μ8c. These salicylaldehyde-based inhibitors form a Schiff base with K907 in the active site of the RNase.106,107 These compounds inhibit IRE1α′s RNase activity in cell culture, and several have shown antitumor activity in xenograft models.108 For example, IRE1α RNase inhibitors were found to decrease the tumor cell secretome and enhance the response to chemotherapy in xenograft models of triple-negative breast cancer.109 However, the selectivity of these compounds is not well understood given their likely reactivity against many intracellular proteins.
As a strategy to generate more specific IRE1α inhibitors, the first-in-class ATP-competitive IRE1α kinase-inhibiting RNase attenuators that bind into the kinase domain and allosterically inhibit IRE1α′s RNase were developed.110 Subsequently, second-generation, more advanced kinase-inhibiting RNase attenuators capable of dose dependently inhibiting endogenous IRE1α′s kinase activity, oligomerization, ER-localized mRNA decay, and Xbp1 mRNA cleavage in vivo were developed.9 A group at Amgen (Thousand Oaks, CA) subsequently published a series of potent and selective IRE1α kinase inhibitors, which did not impair the growth of >300 tumor cell lines when administered for 24 hours in vitro.34 The i.p. delivery of the best compound from this series (compound 18 or kinase-inhibiting RNase attenuator 8) showed efficacy in preclinical models of myeloma and pancreatic neuroendocrine tumors.46,53
A team at GlaxoSmithKline (Brentford, UK) developed highly potent and selective inhibitors of PERK's kinase domain, including GSK2606414 and GSK2656157.111 Oral administration of GSK2606414 leads to therapeutic doses in the central nervous system and protects against preclinical models of neurodegeneration.112 Moreover, GSK2656157 showed antitumor effects in xenograft models of multiple myeloma and pancreatic adenocarcinoma in immunocompromised mice.111 However, most of the PERK inhibitors tested to date quickly cause pancreatic β-cell loss and diabetes, which represent a major barrier for clinical development. The β-cell toxicity seen with PERK inhibition phenocopies Wolcott-Rallison syndrome, the previously mentioned rare diabetic syndrome in humans caused by PERK mutations.
Small molecules have also been identified that attenuate eIF2α phosphorylation without inhibiting PERK per se. Recently, a symmetric bis-glycolamide, named integrated stress response inhibitor, was discovered that binds to and activates elongation initiation factor 2 B, a guanine nucleotide exchange factor that then releases eIF2α phosphorylation-mediated inhibition of protein translation.113 Integrated stress response inhibitor administration is generally well tolerated by rodents, and it has been described to enhance their memory formation through mechanisms that are still not completely understood.114 Recently, integrated stress response inhibitor was shown to cause tumor regression and prolong survival in patient-derived models of advanced prostate cancer.115
Although by no means exhaustive, these examples of small-molecule inhibitors against IRE1α and PERK highlight the therapeutic potential and risks of targeting the UPR in cancer. For a more complete discussion of therapeutics against the UPR, readers should refer to several recent reviews on the topic.116,117
Conclusions
The UPR is a signal transduction pathway that becomes activated when the cell is unable to maintain proteostasis within the ER. In response to a buildup of misfolded proteins in the ER lumen, a condition called ER stress, the UPR sets in motion adaptive outputs that decrease protein-folding load and increase the capacity of the ER secretory pathway to restore homeostasis. However, if these corrective actions are unsuccessful at restoring proteostasis within the ER, the UPR triggers prodeath signals to cause cell demise. Cancer cells face many threats to ER proteostasis, including hypoxia, nutrient deprivation, proteasome dysfunction, increased demands on the secretory pathway, and somatic mutations in its client proteins. UPR hyperactivation is well documented in many types of solid and hematopoietic malignancies. Xenograft studies in mice suggest that the UPR supports tumor growth. Depending on the tumor model, UPR activation has been shown to regulate cell survival, angiogenesis, inflammation, invasion, metastasis, and chemoresistance. As such, the UPR is emerging as an attractive therapeutic target in cancer, but many more studies are needed to understand the benefits and risks of modulating the UPR in any particular tumor type. Fortunately, the recent development of selective small-molecule inhibitors of upstream UPR components has finally made these experiments feasible.
Acknowledgments
I thank Elena Schifirnet and Diane Silva for careful editing, and Christine Lin and H. Divya Gala for help with figure design.
Footnotes
Supported by NIH grants R01CA219815 and R01EY027810.
Disclosures: S.A.O. is a founder, equity holder, and consultant for OptiKIRA, LLC (Cleveland, OH) and a consultant for Kezar Life Sciences (South San Francisco, CA).
S.A.O. is the 2019 recipient of the American Society for Investigative Pathology Outstanding Investigator Award, which is presented annually to a midcareer investigator with demonstrated excellence in research in experimental pathology. Portions of this work were presented at the 2018 Pathobiology for Investigators, Students, and Academicians scientific meeting, held at the University of Michigan, Ann Arbor, MI, on October 20–22, 2018, where S.A.O. delivered the Outstanding Investigator Award lecture.
References
- 1.Schubert U., Anton L.C., Gibbs J., Norbury C.C., Yewdell J.W., Bennink J.R. Rapid degradation of a large fraction of newly synthesized proteins by proteasomes. Nature. 2000;404:770–774. doi: 10.1038/35008096. [DOI] [PubMed] [Google Scholar]
- 2.Hetz C., Chevet E., Oakes S.A. Proteostasis control by the unfolded protein response. Nat Cell Biol. 2015;17:829–838. doi: 10.1038/ncb3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang S., Kaufman R.J. The impact of the unfolded protein response on human disease. J Cell Biol. 2012;197:857–867. doi: 10.1083/jcb.201110131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gardner B.M., Walter P. Unfolded proteins are Ire1-activating ligands that directly induce the unfolded protein response. Science. 2011;333:1891–1894. doi: 10.1126/science.1209126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walter P., Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 6.Shore G.C., Papa F.R., Oakes S.A. Signaling cell death from the endoplasmic reticulum stress response. Curr Opin Cell Biol. 2011;23:143–149. doi: 10.1016/j.ceb.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oakes S.A., Papa F.R. The role of endoplasmic reticulum stress in human pathology. Annu Rev Pathol. 2015;10:173–194. doi: 10.1146/annurev-pathol-012513-104649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han D., Lerner A.G., Vande Walle L., Upton J.P., Xu W., Hagen A., Backes B.J., Oakes S.A., Papa F.R. IRE1alpha kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell. 2009;138:562–575. doi: 10.1016/j.cell.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghosh R., Wang L., Wang E.S., Perera B.G., Igbaria A., Morita S., Prado K., Thamsen M., Caswell D., Macias H., Weiberth K.F., Gliedt M.J., Alavi M.V., Hari S.B., Mitra A.K., Bhhatarai B., Schurer S.C., Snapp E.L., Gould D.B., German M.S., Backes B.J., Maly D.J., Oakes S.A., Papa F.R. Allosteric inhibition of the IRE1alpha RNase preserves cell viability and function during endoplasmic reticulum stress. Cell. 2014;158:534–548. doi: 10.1016/j.cell.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oakes S.A. Endoplasmic reticulum proteostasis: a key checkpoint in cancer. Am J Physiol Cell Physiol. 2017;312:C93–C102. doi: 10.1152/ajpcell.00266.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calfon M., Zeng H., Urano F., Till J.H., Hubbard S.R., Harding H.P., Clark S.G., Ron D. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415:92–96. doi: 10.1038/415092a. [DOI] [PubMed] [Google Scholar]
- 12.Yoshida H., Matsui T., Yamamoto A., Okada T., Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–891. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- 13.Lee A.H., Iwakoshi N.N., Glimcher L.H. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol. 2003;23:7448–7459. doi: 10.1128/MCB.23.21.7448-7459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hollien J., Lin J.H., Li H., Stevens N., Walter P., Weissman J.S. Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J Cell Biol. 2009;186:323–331. doi: 10.1083/jcb.200903014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Upton J.P., Wang L., Han D., Wang E.S., Huskey N.E., Lim L., Truitt M., McManus M.T., Ruggero D., Goga A., Papa F.R., Oakes S.A. IRE1alpha cleaves select microRNAs during ER stress to derepress translation of proapoptotic caspase-2. Science. 2012;338:818–822. doi: 10.1126/science.1226191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lerner A.G., Upton J.P., Praveen P.V., Ghosh R., Nakagawa Y., Igbaria A., Shen S., Nguyen V., Backes B.J., Heiman M., Heintz N., Greengard P., Hui S., Tang Q., Trusina A., Oakes S.A., Papa F.R. IRE1alpha induces thioredoxin-interacting protein to activate the NLRP3 inflammasome and promote programmed cell death under irremediable ER stress. Cell Metab. 2012;16:250–264. doi: 10.1016/j.cmet.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harding H.P., Zhang Y., Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- 18.Cullinan S.B., Zhang D., Hannink M., Arvisais E., Kaufman R.J., Diehl J.A. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol Cell Biol. 2003;23:7198–7209. doi: 10.1128/MCB.23.20.7198-7209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harding H.P., Novoa I., Zhang Y., Zeng H., Wek R., Schapira M., Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6:1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 20.Connor J.H., Weiser D.C., Li S., Hallenbeck J.M., Shenolikar S. Growth arrest and DNA damage-inducible protein GADD34 assembles a novel signaling complex containing protein phosphatase 1 and inhibitor 1. Mol Cell Biol. 2001;21:6841–6850. doi: 10.1128/MCB.21.20.6841-6850.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCullough K.D., Martindale J.L., Klotz L.O., Aw T.Y., Holbrook N.J. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001;21:1249–1259. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marciniak S.J., Yun C.Y., Oyadomari S., Novoa I., Zhang Y., Jungreis R., Nagata K., Harding H.P., Ron D. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18:3066–3077. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ye J., Rawson R.B., Komuro R., Chen X., Dave U.P., Prywes R., Brown M.S., Goldstein J.L. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol Cell. 2000;6:1355–1364. doi: 10.1016/s1097-2765(00)00133-7. [DOI] [PubMed] [Google Scholar]
- 24.Okada T., Yoshida H., Akazawa R., Negishi M., Mori K. Distinct roles of activating transcription factor 6 (ATF6) and double-stranded RNA-activated protein kinase-like endoplasmic reticulum kinase (PERK) in transcription during the mammalian unfolded protein response. Biochem J. 2002;366:585–594. doi: 10.1042/BJ20020391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morishima N., Nakanishi K., Nakano A. Activating transcription factor-6 (ATF6) mediates apoptosis with reduction of myeloid cell leukemia sequence 1 (Mcl-1) protein via induction of WW domain binding protein 1. J Biol Chem. 2011;286:35227–35235. doi: 10.1074/jbc.M111.233502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moenner M., Pluquet O., Bouchecareilh M., Chevet E. Integrated endoplasmic reticulum stress responses in cancer. Cancer Res. 2007;67:10631–10634. doi: 10.1158/0008-5472.CAN-07-1705. [DOI] [PubMed] [Google Scholar]
- 27.Dejeans N., Manie S., Hetz C., Bard F., Hupp T., Agostinis P., Samali A., Chevet E. Addicted to secrete: novel concepts and targets in cancer therapy. Trends Mol Med. 2014;20:242–250. doi: 10.1016/j.molmed.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 28.Greenman C., Stephens P., Smith R., Dalgliesh G.L., Hunter C., Bignell G. Patterns of somatic mutation in human cancer genomes. Nature. 2007;446:153–158. doi: 10.1038/nature05610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xue Z., He Y., Ye K., Gu Z., Mao Y., Qi L. A conserved structural determinant located at the interdomain region of mammalian inositol-requiring enzyme 1alpha. J Biol Chem. 2011;286:30859–30866. doi: 10.1074/jbc.M111.273714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hong S.Y., Hagen T. Multiple myeloma Leu167Ile (c.499C>A) mutation prevents XBP1 mRNA splicing. Br J Haematol. 2013;161:898–901. doi: 10.1111/bjh.12310. [DOI] [PubMed] [Google Scholar]
- 31.Fernandez P.M., Tabbara S.O., Jacobs L.K., Manning F.C., Tsangaris T.N., Schwartz A.M., Kennedy K.A., Patierno S.R. Overexpression of the glucose-regulated stress gene GRP78 in malignant but not benign human breast lesions. Breast Cancer Res Treat. 2000;59:15–26. doi: 10.1023/a:1006332011207. [DOI] [PubMed] [Google Scholar]
- 32.Liberman E., Fong Y.L., Selby M.J., Choo Q.L., Cousens L., Houghton M., Yen T.S. Activation of the grp78 and grp94 promoters by hepatitis C virus E2 envelope protein. J Virol. 1999;73:3718–3722. doi: 10.1128/jvi.73.5.3718-3722.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ajiro M., Zheng Z.M. E6ˆE7, a novel splice isoform protein of human papillomavirus 16, stabilizes viral E6 and E7 oncoproteins via HSP90 and GRP78. mBio. 2015;6 doi: 10.1128/mBio.02068-14. e02068-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harrington P.E., Biswas K., Malwitz D., Tasker A.S., Mohr C., Andrews K.L., Dellamaggiore K., Kendall R., Beckmann H., Jaeckel P., Materna-Reichelt S., Allen J.R., Lipford J.R. Unfolded protein response in cancer: IRE1alpha inhibition by selective kinase ligands does not impair tumor cell viability. ACS Med Chem Lett. 2015;6:68–72. doi: 10.1021/ml500315b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pytel D., Gao Y., Mackiewicz K., Katlinskaya Y.V., Staschke K.A., Paredes M.C., Yoshida A., Qie S., Zhang G., Chajewski O.S., Wu L., Majsterek I., Herlyn M., Fuchs S.Y., Diehl J.A. PERK is a haploinsufficient tumor suppressor: gene dose determines tumor-suppressive versus tumor promoting properties of PERK in melanoma. PLoS Genet. 2016;12:e1006518. doi: 10.1371/journal.pgen.1006518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen X., Iliopoulos D., Zhang Q., Tang Q., Greenblatt M.B., Hatziapostolou M., Lim E., Tam W.L., Ni M., Chen Y., Mai J., Shen H., Hu D.Z., Adoro S., Hu B., Song M., Tan C., Landis M.D., Ferrari M., Shin S.J., Brown M., Chang J.C., Liu X.S., Glimcher L.H. XBP1 promotes triple-negative breast cancer by controlling the HIF1alpha pathway. Nature. 2014;508:103–107. doi: 10.1038/nature13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Auf G., Jabouille A., Guerit S., Pineau R., Delugin M., Bouchecareilh M., Magnin N., Favereaux A., Maitre M., Gaiser T., von Deimling A., Czabanka M., Vajkoczy P., Chevet E., Bikfalvi A., Moenner M. Inositol-requiring enzyme 1alpha is a key regulator of angiogenesis and invasion in malignant glioma. Proc Natl Acad Sci U S A. 2010;107:15553–15558. doi: 10.1073/pnas.0914072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sheng X., Nenseth H.Z., Qu S., Kuzu O.F., Frahnow T., Simon L., Greene S., Zeng Q., Fazli L., Rennie P.S., Mills I.G., Danielsen H., Theis F., Patterson J.B., Jin Y., Saatcioglu F. IRE1alpha-XBP1s pathway promotes prostate cancer by activating c-MYC signaling. Nat Commun. 2019;10:323. doi: 10.1038/s41467-018-08152-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bobrovnikova-Marjon E., Grigoriadou C., Pytel D., Zhang F., Ye J., Koumenis C., Cavener D., Diehl J.A. PERK promotes cancer cell proliferation and tumor growth by limiting oxidative DNA damage. Oncogene. 2010;29:3881–3895. doi: 10.1038/onc.2010.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feng Y.X., Sokol E.S., Del Vecchio C.A., Sanduja S., Claessen J.H., Proia T.A., Jin D.X., Reinhardt F., Ploegh H.L., Wang Q., Gupta P.B. Epithelial-to-mesenchymal transition activates PERK-eIF2alpha and sensitizes cells to endoplasmic reticulum stress. Cancer Discov. 2014;4:702–715. doi: 10.1158/2159-8290.CD-13-0945. [DOI] [PubMed] [Google Scholar]
- 41.Salaroglio I.C., Panada E., Moiso E., Buondonno I., Provero P., Rubinstein M., Kopecka J., Riganti C. PERK induces resistance to cell death elicited by endoplasmic reticulum stress and chemotherapy. Mol Cancer. 2017;16:91. doi: 10.1186/s12943-017-0657-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hanaoka M., Ishikawa T., Ishiguro M., Tokura M., Yamauchi S., Kikuchi A., Uetake H., Yasuno M., Kawano T. Expression of ATF6 as a marker of pre-cancerous atypical change in ulcerative colitis-associated colorectal cancer: a potential role in the management of dysplasia. J Gastroenterol. 2018;53:631–641. doi: 10.1007/s00535-017-1387-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang K., Wong H.N., Song B., Miller C.N., Scheuner D., Kaufman R.J. The unfolded protein response sensor IRE1alpha is required at 2 distinct steps in B cell lymphopoiesis. J Clin Invest. 2005;115:268–281. doi: 10.1172/JCI21848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carrasco D.R., Sukhdeo K., Protopopova M., Sinha R., Enos M., Carrasco D.E., Zheng M., Mani M., Henderson J., Pinkus G.S., Munshi N., Horner J., Ivanova E.V., Protopopov A., Anderson K.C., Tonon G., DePinho R.A. The differentiation and stress response factor XBP-1 drives multiple myeloma pathogenesis. Cancer Cell. 2007;11:349–360. doi: 10.1016/j.ccr.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ri M. Endoplasmic-reticulum stress pathway-associated mechanisms of action of proteasome inhibitors in multiple myeloma. Int J Hematol. 2016;104:273–280. doi: 10.1007/s12185-016-2016-0. [DOI] [PubMed] [Google Scholar]
- 46.Harnoss J.M., Le Thomas A., Shemorry A., Marsters S.A., Lawrence D.A., Lu M. Disruption of IRE1alpha through its kinase domain attenuates multiple myeloma. Proc Natl Acad Sci U S A. 2019;116:16420–16429. doi: 10.1073/pnas.1906999116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harding H.P., Zhang Y., Bertolotti A., Zeng H., Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell. 2000;5:897–904. doi: 10.1016/s1097-2765(00)80330-5. [DOI] [PubMed] [Google Scholar]
- 48.Delepine M., Nicolino M., Barrett T., Golamaully M., Lathrop G.M., Julier C. EIF2AK3, encoding translation initiation factor 2-alpha kinase 3, is mutated in patients with Wolcott-Rallison syndrome. Nat Genet. 2000;25:406–409. doi: 10.1038/78085. [DOI] [PubMed] [Google Scholar]
- 49.Hassler J.R., Scheuner D.L., Wang S., Han J., Kodali V.K., Li P., Nguyen J., George J.S., Davis C., Wu S.P., Bai Y., Sartor M., Cavalcoli J., Malhi H., Baudouin G., Zhang Y., Yates J.R., III, Itkin-Ansari P., Volkmann N., Kaufman R.J. The IRE1alpha/XBP1s pathway is essential for the glucose response and protection of beta cells. PLoS Biol. 2015;13:e1002277. doi: 10.1371/journal.pbio.1002277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee A.H., Heidtman K., Hotamisligil G.S., Glimcher L.H. Dual and opposing roles of the unfolded protein response regulated by IRE1alpha and XBP1 in proinsulin processing and insulin secretion. Proc Natl Acad Sci U S A. 2011;108:8885–8890. doi: 10.1073/pnas.1105564108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scheuner D., Kaufman R.J. The unfolded protein response: a pathway that links insulin demand with beta-cell failure and diabetes. Endocr Rev. 2008;29:317–333. doi: 10.1210/er.2007-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Minn A.H., Lan H., Rabaglia M.E., Harlan D.M., Peculis B.A., Attie A.D., Shalev A. Increased insulin translation from an insulin splice-variant overexpressed in diabetes, obesity, and insulin resistance. Mol Endocrinol. 2005;19:794–803. doi: 10.1210/me.2004-0119. [DOI] [PubMed] [Google Scholar]
- 53.Moore P.C., Qi J.Y., Thamsen M., Ghosh R., Peng J., Gliedt M.J., Meza-Acevedo R., Warren R.E., Hiniker A., Kim G.E., Maly D.J., Backes B.J., Papa F.R., Oakes S.A. Parallel signaling through IRE1alpha and PERK regulates pancreatic neuroendocrine tumor growth and survival. Cancer Res. 2019;79:6190–6203. doi: 10.1158/0008-5472.CAN-19-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yan M.M., Ni J.D., Song D., Ding M., Huang J. Interplay between unfolded protein response and autophagy promotes tumor drug resistance. Oncol Lett. 2015;10:1959–1969. doi: 10.3892/ol.2015.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liang X.H., Jackson S., Seaman M., Brown K., Kempkes B., Hibshoosh H., Levine B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402:672–676. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- 56.Qu X., Yu J., Bhagat G., Furuya N., Hibshoosh H., Troxel A., Rosen J., Eskelinen E.L., Mizushima N., Ohsumi Y., Cattoretti G., Levine B. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J Clin Invest. 2003;112:1809–1820. doi: 10.1172/JCI20039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yue Z., Jin S., Yang C., Levine A.J., Heintz N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc Natl Acad Sci U S A. 2003;100:15077–15082. doi: 10.1073/pnas.2436255100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jiang X., Overholtzer M., Thompson C.B. Autophagy in cellular metabolism and cancer. J Clin Invest. 2015;125:47–54. doi: 10.1172/JCI73942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Capparelli C., Guido C., Whitaker-Menezes D., Bonuccelli G., Balliet R., Pestell T.G., Goldberg A.F., Pestell R.G., Howell A., Sneddon S., Birbe R., Tsirigos A., Martinez-Outschoorn U., Sotgia F., Lisanti M.P. Autophagy and senescence in cancer-associated fibroblasts metabolically supports tumor growth and metastasis via glycolysis and ketone production. Cell Cycle. 2012;11:2285–2302. doi: 10.4161/cc.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guo J.Y., Chen H.Y., Mathew R., Fan J., Strohecker A.M., Karsli-Uzunbas G., Kamphorst J.J., Chen G., Lemons J.M., Karantza V., Coller H.A., Dipaola R.S., Gelinas C., Rabinowitz J.D., White E. Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis. Genes Dev. 2011;25:460–470. doi: 10.1101/gad.2016311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Margariti A., Li H., Chen T., Martin D., Vizcay-Barrena G., Alam S., Karamariti E., Xiao Q., Zampetaki A., Zhang Z., Wang W., Jiang Z., Gao C., Ma B., Chen Y.G., Cockerill G., Hu Y., Xu Q., Zeng L. XBP1 mRNA splicing triggers an autophagic response in endothelial cells through BECLIN-1 transcriptional activation. J Biol Chem. 2013;288:859–872. doi: 10.1074/jbc.M112.412783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Suzuki H., Kanekura K., Levine T.P., Kohno K., Olkkonen V.M., Aiso S., Matsuoka M. ALS-linked P56S-VAPB, an aggregated loss-of-function mutant of VAPB, predisposes motor neurons to ER stress-related death by inducing aggregation of co-expressed wild-type VAPB. J Neurochem. 2009;108:973–985. doi: 10.1111/j.1471-4159.2008.05857.x. [DOI] [PubMed] [Google Scholar]
- 63.Vidal R.L., Figueroa A., Court F.A., Thielen P., Molina C., Wirth C., Caballero B., Kiffin R., Segura-Aguilar J., Cuervo A.M., Glimcher L.H., Hetz C. Targeting the UPR transcription factor XBP1 protects against Huntington's disease through the regulation of FoxO1 and autophagy. Hum Mol Genet. 2012;21:2245–2262. doi: 10.1093/hmg/dds040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kouroku Y., Fujita E., Tanida I., Ueno T., Isoai A., Kumagai H., Ogawa S., Kaufman R.J., Kominami E., Momoi T. ER stress (PERK/eIF2alpha phosphorylation) mediates the polyglutamine-induced LC3 conversion, an essential step for autophagy formation. Cell Death Differ. 2007;14:230–239. doi: 10.1038/sj.cdd.4401984. [DOI] [PubMed] [Google Scholar]
- 65.Wang J., Kang R., Huang H., Xi X., Wang B., Wang J., Zhao Z. Hepatitis C virus core protein activates autophagy through EIF2AK3 and ATF6 UPR pathway-mediated MAP1LC3B and ATG12 expression. Autophagy. 2014;10:766–784. doi: 10.4161/auto.27954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rouschop K.M., van den Beucken T., Dubois L., Niessen H., Bussink J., Savelkouls K., Keulers T., Mujcic H., Landuyt W., Voncken J.W., Lambin P., van der Kogel A.J., Koritzinsky M., Wouters B.G. The unfolded protein response protects human tumor cells during hypoxia through regulation of the autophagy genes MAP1LC3B and ATG5. J Clin Invest. 2010;120:127–141. doi: 10.1172/JCI40027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gade P., Ramachandran G., Maachani U.B., Rizzo M.A., Okada T., Prywes R., Cross A.S., Mori K., Kalvakolanu D.V. An IFN-gamma-stimulated ATF6-C/EBP-beta-signaling pathway critical for the expression of death associated protein kinase 1 and induction of autophagy. Proc Natl Acad Sci U S A. 2012;109:10316–10321. doi: 10.1073/pnas.1119273109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Koeneke E., Witt O., Oehme I. HDAC family members intertwined in the regulation of autophagy: a druggable vulnerability in aggressive tumor entities. Cells. 2015;4:135–168. doi: 10.3390/cells4020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ghosh R., Lipson K.L., Sargent K.E., Mercurio A.M., Hunt J.S., Ron D., Urano F. Transcriptional regulation of VEGF-A by the unfolded protein response pathway. PLoS One. 2010;5:e9575. doi: 10.1371/journal.pone.0009575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang Y., Alam G.N., Ning Y., Visioli F., Dong Z., Nor J.E., Polverini P.J. The unfolded protein response induces the angiogenic switch in human tumor cells through the PERK/ATF4 pathway. Cancer Res. 2012;72:5396–5406. doi: 10.1158/0008-5472.CAN-12-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dong D., Ni M., Li J., Xiong S., Ye W., Virrey J.J., Mao C., Ye R., Wang M., Pen L., Dubeau L., Groshen S., Hofman F.M., Lee A.S. Critical role of the stress chaperone GRP78/BiP in tumor proliferation, survival, and tumor angiogenesis in transgene-induced mammary tumor development. Cancer Res. 2008;68:498–505. doi: 10.1158/0008-5472.CAN-07-2950. [DOI] [PubMed] [Google Scholar]
- 72.Ozawa K., Tsukamoto Y., Hori O., Kitao Y., Yanagi H., Stern D.M., Ogawa S. Regulation of tumor angiogenesis by oxygen-regulated protein 150, an inducible endoplasmic reticulum chaperone. Cancer Res. 2001;61:4206–4213. [PubMed] [Google Scholar]
- 73.Karali E., Bellou S., Stellas D., Klinakis A., Murphy C., Fotsis T. VEGF signals through ATF6 and PERK to promote endothelial cell survival and angiogenesis in the absence of ER stress. Mol Cell. 2014;54:559–572. doi: 10.1016/j.molcel.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 74.Urra H., Hetz C. A novel ER stress-independent function of the UPR in angiogenesis. Mol Cell. 2014;54:542–544. doi: 10.1016/j.molcel.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 75.Urra H., Henriquez D.R., Canovas J., Villarroel-Campos D., Carreras-Sureda A., Pulgar E., Molina E., Hazari Y.M., Limia C.M., Alvarez-Rojas S., Figueroa R., Vidal R.L., Rodriguez D.A., Rivera C.A., Court F.A., Couve A., Qi L., Chevet E., Akai R., Iwawaki T., Concha M.L., Glavic A., Gonzalez-Billault C., Hetz C. IRE1alpha governs cytoskeleton remodelling and cell migration through a direct interaction with filamin A. Nat Cell Biol. 2018;20:942–953. doi: 10.1038/s41556-018-0141-0. [DOI] [PubMed] [Google Scholar]
- 76.Mujcic H., Nagelkerke A., Rouschop K.M., Chung S., Chaudary N., Span P.N., Clarke B., Milosevic M., Sykes J., Hill R.P., Koritzinsky M., Wouters B.G. Hypoxic activation of the PERK/eIF2alpha arm of the unfolded protein response promotes metastasis through induction of LAMP3. Clin Cancer Res. 2013;19:6126–6137. doi: 10.1158/1078-0432.CCR-13-0526. [DOI] [PubMed] [Google Scholar]
- 77.Nagelkerke A., Bussink J., Mujcic H., Wouters B.G., Lehmann S., Sweep F.C., Span P.N. Hypoxia stimulates migration of breast cancer cells via the PERK/ATF4/LAMP3-arm of the unfolded protein response. Breast Cancer Res. 2013;15:R2. doi: 10.1186/bcr3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jabouille A., Delugin M., Pineau R., Dubrac A., Soulet F., Lhomond S., Pallares-Lupon N., Prats H., Bikfalvi A., Chevet E., Touriol C., Moenner M. Glioblastoma invasion and cooption depend on IRE1alpha endoribonuclease activity. Oncotarget. 2015;6:24922–24934. doi: 10.18632/oncotarget.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dejeans N., Pluquet O., Lhomond S., Grise F., Bouchecareilh M., Juin A., Meynard-Cadars M., Bidaud-Meynard A., Gentil C., Moreau V., Saltel F., Chevet E. Autocrine control of glioma cells adhesion and migration through IRE1alpha-mediated cleavage of SPARC mRNA. J Cell Sci. 2012;125:4278–4287. doi: 10.1242/jcs.099291. [DOI] [PubMed] [Google Scholar]
- 80.Sennino B., McDonald D.M. Controlling escape from angiogenesis inhibitors. Nat Rev Cancer. 2012;12:699–709. doi: 10.1038/nrc3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Avril T., Vauleon E., Chevet E. Endoplasmic reticulum stress signaling and chemotherapy resistance in solid cancers. Oncogenesis. 2017;6:e373. doi: 10.1038/oncsis.2017.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang Z.V., Deng Y., Gao N., Pedrozo Z., Li D.L., Morales C.R., Criollo A., Luo X., Tan W., Jiang N., Lehrman M.A., Rothermel B.A., Lee A.H., Lavandero S., Mammen P.P.A., Ferdous A., Gillette T.G., Scherer P.E., Hill J.A. Spliced X-box binding protein 1 couples the unfolded protein response to hexosamine biosynthetic pathway. Cell. 2014;156:1179–1192. doi: 10.1016/j.cell.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Denzel M.S., Storm N.J., Gutschmidt A., Baddi R., Hinze Y., Jarosch E., Sommer T., Hoppe T., Antebi A. Hexosamine pathway metabolites enhance protein quality control and prolong life. Cell. 2014;156:1167–1178. doi: 10.1016/j.cell.2014.01.061. [DOI] [PubMed] [Google Scholar]
- 84.Ferrer C.M., Lynch T.P., Sodi V.L., Falcone J.N., Schwab L.P., Peacock D.L., Vocadlo D.J., Seagroves T.N., Reginato M.J. O-GlcNAcylation regulates cancer metabolism and survival stress signaling via regulation of the HIF-1 pathway. Mol Cell. 2014;54:820–831. doi: 10.1016/j.molcel.2014.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Holohan C., Van Schaeybroeck S., Longley D.B., Johnston P.G. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer. 2013;13:714–726. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- 86.Davies M.P., Barraclough D.L., Stewart C., Joyce K.A., Eccles R.M., Barraclough R., Rudland P.S., Sibson D.R. Expression and splicing of the unfolded protein response gene XBP-1 are significantly associated with clinical outcome of endocrine-treated breast cancer. Int J Cancer. 2008;123:85–88. doi: 10.1002/ijc.23479. [DOI] [PubMed] [Google Scholar]
- 87.Lee E., Nichols P., Groshen S., Spicer D., Lee A.S. GRP78 as potential predictor for breast cancer response to adjuvant taxane therapy. Int J Cancer. 2011;128:726–731. doi: 10.1002/ijc.25370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gallerne C., Prola A., Lemaire C. Hsp90 inhibition by PU-H71 induces apoptosis through endoplasmic reticulum stress and mitochondrial pathway in cancer cells and overcomes the resistance conferred by Bcl-2. Biochim Biophys Acta. 2013;1833:1356–1366. doi: 10.1016/j.bbamcr.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 89.Ryan D., Carberry S., Murphy A.C., Lindner A.U., Fay J., Hector S., McCawley N., Bacon O., Concannon C.G., Kay E.W., McNamara D.A., Prehn J.H. Calnexin, an ER stress-induced protein, is a prognostic marker and potential therapeutic target in colorectal cancer. J Transl Med. 2016;14:196. doi: 10.1186/s12967-016-0948-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Andruska N.D., Zheng X., Yang X., Mao C., Cherian M.M., Mahapatra L., Helferich W.G., Shapiro D.J. Estrogen receptor alpha inhibitor activates the unfolded protein response, blocks protein synthesis, and induces tumor regression. Proc Natl Acad Sci U S A. 2015;112:4737–4742. doi: 10.1073/pnas.1403685112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee E., Nichols P., Spicer D., Groshen S., Yu M.C., Lee A.S. GRP78 as a novel predictor of responsiveness to chemotherapy in breast cancer. Cancer Res. 2006;66:7849–7853. doi: 10.1158/0008-5472.CAN-06-1660. [DOI] [PubMed] [Google Scholar]
- 92.Shi Z., Yu X., Yuan M., Lv W., Feng T., Bai R., Zhong H. Activation of the PERK-ATF4 pathway promotes chemo-resistance in colon cancer cells. Sci Rep. 2019;9:3210. doi: 10.1038/s41598-019-39547-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sustic T., van Wageningen S., Bosdriesz E., Reid R.J.D., Dittmar J., Lieftink C., Beijersbergen R.L., Wessels L.F.A., Rothstein R., Bernards R. A role for the unfolded protein response stress sensor ERN1 in regulating the response to MEK inhibitors in KRAS mutant colon cancers. Genome Med. 2018;10:90. doi: 10.1186/s13073-018-0600-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jiang H.Y., Wek S.A., McGrath B.C., Scheuner D., Kaufman R.J., Cavener D.R., Wek R.C. Phosphorylation of the alpha subunit of eukaryotic initiation factor 2 is required for activation of NF-kappaB in response to diverse cellular stresses. Mol Cell Biol. 2003;23:5651–5663. doi: 10.1128/MCB.23.16.5651-5663.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hu P., Han Z., Couvillon A.D., Kaufman R.J., Exton J.H. Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1alpha-mediated NF-kappaB activation and down-regulation of TRAF2 expression. Mol Cell Biol. 2006;26:3071–3084. doi: 10.1128/MCB.26.8.3071-3084.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cubillos-Ruiz J.R., Silberman P.C., Rutkowski M.R., Chopra S., Perales-Puchalt A., Song M., Zhang S., Bettigole S.E., Gupta D., Holcomb K., Ellenson L.H., Caputo T., Lee A.H., Conejo-Garcia J.R., Glimcher L.H. ER stress sensor XBP1 controls anti-tumor immunity by disrupting dendritic cell homeostasis. Cell. 2015;161:1527–1538. doi: 10.1016/j.cell.2015.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Obeid M., Panaretakis T., Joza N., Tufi R., Tesniere A., van Endert P., Zitvogel L., Kroemer G. Calreticulin exposure is required for the immunogenicity of gamma-irradiation and UVC light-induced apoptosis. Cell Death Differ. 2007;14:1848–1850. doi: 10.1038/sj.cdd.4402201. [DOI] [PubMed] [Google Scholar]
- 98.Gardai S.J., McPhillips K.A., Frasch S.C., Janssen W.J., Starefeldt A., Murphy-Ullrich J.E., Bratton D.L., Oldenborg P.A., Michalak M., Henson P.M. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123:321–334. doi: 10.1016/j.cell.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 99.Bezu L., Sauvat A., Humeau J., Gomes-da-Silva L.C., Iribarren K., Forveille S., Garcia P., Zhao L., Liu P., Zitvogel L., Senovilla L., Kepp O., Kroemer G. eIF2alpha phosphorylation is pathognomonic for immunogenic cell death. Cell Death Differ. 2018;25:1375–1393. doi: 10.1038/s41418-017-0044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kepp O., Semeraro M., Bravo-San Pedro J.M., Bloy N., Buque A., Huang X., Zhou H., Senovilla L., Kroemer G., Galluzzi L. eIF2alpha phosphorylation as a biomarker of immunogenic cell death. Semin Cancer Biol. 2015;33:86–92. doi: 10.1016/j.semcancer.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 101.Grootjans J., Kaser A., Kaufman R.J., Blumberg R.S. The unfolded protein response in immunity and inflammation. Nat Rev Immunol. 2016;16:469–484. doi: 10.1038/nri.2016.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Osorio F., Lambrecht B.N., Janssens S. Antigen presentation unfolded: identifying convergence points between the UPR and antigen presentation pathways. Curr Opin Immunol. 2018;52:100–107. doi: 10.1016/j.coi.2018.04.020. [DOI] [PubMed] [Google Scholar]
- 103.Papandreou I., Denko N.C., Olson M., Van Melckebeke H., Lust S., Tam A., Solow-Cordero D.E., Bouley D.M., Offner F., Niwa M., Koong A.C. Identification of an Ire1alpha endonuclease specific inhibitor with cytotoxic activity against human multiple myeloma. Blood. 2011;117:1311–1314. doi: 10.1182/blood-2010-08-303099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mimura N., Fulciniti M., Gorgun G., Tai Y.T., Cirstea D., Santo L., Hu Y., Fabre C., Minami J., Ohguchi H., Kiziltepe T., Ikeda H., Kawano Y., French M., Blumenthal M., Tam V., Kertesz N.L., Malyankar U.M., Hokenson M., Pham T., Zeng Q., Patterson J.B., Richardson P.G., Munshi N.C., Anderson K.C. Blockade of XBP1 splicing by inhibition of IRE1alpha is a promising therapeutic option in multiple myeloma. Blood. 2012;119:5772–5781. doi: 10.1182/blood-2011-07-366633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Volkmann K., Lucas J.L., Vuga D., Wang X., Brumm D., Stiles C., Kriebel D., Der-Sarkissian A., Krishnan K., Schweitzer C., Liu Z., Malyankar U.M., Chiovitti D., Canny M., Durocher D., Sicheri F., Patterson J.B. Potent and selective inhibitors of the inositol-requiring enzyme 1 endoribonuclease. J Biol Chem. 2011;286:12743–12755. doi: 10.1074/jbc.M110.199737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cross B.C., Bond P.J., Sadowski P.G., Jha B.K., Zak J., Goodman J.M., Silverman R.H., Neubert T.A., Baxendale I.R., Ron D., Harding H.P. The molecular basis for selective inhibition of unconventional mRNA splicing by an IRE1-binding small molecule. Proc Natl Acad Sci U S A. 2012;109:E869–E878. doi: 10.1073/pnas.1115623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sanches M., Duffy N.M., Talukdar M., Thevakumaran N., Chiovitti D., Canny M.D., Lee K., Kurinov I., Uehling D., Al-awar R., Poda G., Prakesch M., Wilson B., Tam V., Schweitzer C., Toro A., Lucas J.L., Vuga D., Lehmann L., Durocher D., Zeng Q., Patterson J.B., Sicheri F. Structure and mechanism of action of the hydroxy-aryl-aldehyde class of IRE1 endoribonuclease inhibitors. Nat Commun. 2014;5:4202. doi: 10.1038/ncomms5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhao N., Cao J., Xu L., Tang Q., Dobrolecki L.E., Lv X. Pharmacological targeting of MYC-regulated IRE1/XBP1 pathway suppresses MYC-driven breast cancer. J Clin Invest. 2018;128:1283–1299. doi: 10.1172/JCI95873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Logue S.E., McGrath E.P., Cleary P., Greene S., Mnich K., Almanza A., Chevet E., Dwyer R.M., Oommen A., Legembre P., Godey F., Madden E.C., Leuzzi B., Obacz J., Zeng Q., Patterson J.B., Jager R., Gorman A.M., Samali A. Inhibition of IRE1 RNase activity modulates the tumor cell secretome and enhances response to chemotherapy. Nat Commun. 2018;9:3267. doi: 10.1038/s41467-018-05763-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang L., Perera B.G., Hari S.B., Bhhatarai B., Backes B.J., Seeliger M.A., Schurer S.C., Oakes S.A., Papa F.R., Maly D.J. Divergent allosteric control of the IRE1alpha endoribonuclease using kinase inhibitors. Nat Chem Biol. 2012;8:982–989. doi: 10.1038/nchembio.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Atkins C., Liu Q., Minthorn E., Zhang S.Y., Figueroa D.J., Moss K., Stanley T.B., Sanders B., Goetz A., Gaul N., Choudhry A.E., Alsaid H., Jucker B.M., Axten J.M., Kumar R. Characterization of a novel PERK kinase inhibitor with antitumor and antiangiogenic activity. Cancer Res. 2013;73:1993–2002. doi: 10.1158/0008-5472.CAN-12-3109. [DOI] [PubMed] [Google Scholar]
- 112.Moreno J.A., Halliday M., Molloy C., Radford H., Verity N., Axten J.M., Ortori C.A., Willis A.E., Fischer P.M., Barrett D.A., Mallucci G.R. Oral treatment targeting the unfolded protein response prevents neurodegeneration and clinical disease in prion-infected mice. Sci Transl Med. 2013;5:206ra138. doi: 10.1126/scitranslmed.3006767. [DOI] [PubMed] [Google Scholar]
- 113.Sidrauski C., McGeachy A.M., Ingolia N.T., Walter P. The small molecule ISRIB reverses the effects of eIF2alpha phosphorylation on translation and stress granule assembly. Elife. 2015;4:e05033. doi: 10.7554/eLife.05033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sidrauski C., Acosta-Alvear D., Khoutorsky A., Vedantham P., Hearn B.R., Li H., Gamache K., Gallagher C.M., Ang K.K., Wilson C., Okreglak V., Ashkenazi A., Hann B., Nader K., Arkin M.R., Renslo A.R., Sonenberg N., Walter P. Pharmacological brake-release of mRNA translation enhances cognitive memory. Elife. 2013;2:e00498. doi: 10.7554/eLife.00498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nguyen H.G., Conn C.S., Kye Y., Xue L., Forester C.M., Cowan J.E., Hsieh A.C., Cunningham J.T., Truillet C., Tameire F., Evans M.J., Evans C.P., Yang J.C., Hann B., Koumenis C., Walter P., Carroll P.R., Ruggero D. Development of a stress response therapy targeting aggressive prostate cancer. Sci Transl Med. 2018;10:eaar2036. doi: 10.1126/scitranslmed.aar2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hetz C., Axten J.M., Patterson J.B. Pharmacological targeting of the unfolded protein response for disease intervention. Nat Chem Biol. 2019;15:764–775. doi: 10.1038/s41589-019-0326-2. [DOI] [PubMed] [Google Scholar]
- 117.Maly D.J., Papa F.R. Druggable sensors of the unfolded protein response. Nat Chem Biol. 2014;10:892–901. doi: 10.1038/nchembio.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]