Abstract
Spontaneous preterm labor is frequently caused by an inflammatory response in the gestational tissues elicited by either infectious or sterile agents. In sterile preterm labor, the key regulators of inflammation are not identified, but platelet-activating factor (PAF) is implicated as a potential rate-limiting effector agent. Since Toll-like receptor (TLR)-4 can amplify PAF signaling, we evaluated whether TLR4 contributes to inflammation and fetal loss in a mouse model of PAF-induced sterile preterm labor, and whether a small-molecule TLR4 inhibitor, (+)-naltrexone, can mitigate adverse PAF-induced effects. The administration of carbamyl (c)-PAF caused preterm labor and fetal loss in wild-type mice but not in TLR4-deficient mice. Treatment with (+)-naltrexone prevented preterm delivery and alleviated fetal demise in utero elicited after cPAF administered by i.p. or intrauterine routes. Pups born after cPAF and (+)-naltrexone treatment exhibited comparable rates of postnatal survival and growth to carrier-treated controls. (+)-Naltrexone suppressed the cPAF-induced expression of inflammatory cytokine genes Il1b, Il6, and Il10 in the decidua; Il6, Il12b, and Il10 in the myometrium; and Il1b and Il6 in the placenta. These data demonstrate that the TLR4 antagonist (+)-naltrexone inhibits the inflammatory cascade induced by cPAF, preventing preterm birth and perinatal death. The inhibition of TLR4 signaling warrants further investigation as a candidate strategy for fetal protection and delay of preterm birth elicited by sterile stimuli.
Preterm delivery, defined as birth at <37 weeks of gestation,1 occurs in 5% to 18% of pregnancies, depending on geographic location and socioeconomic status.2 Globally, approximately 15 million preterm births result in >1 million neonatal deaths every year.3 Infants born preterm often experience serious lifelong health problems, including cerebral palsy, brain injury, respiratory dysfunction, and developmental delay.4 The majority of preterm births follow spontaneous preterm labor.5 There are urgent needs for defining the common pathophysiological mechanisms by which various factors and exposures interact to trigger preterm labor,6,7 and for identifying key rate-limiting mechanisms that can be targeted for effective pharmacologic interventions.8,9
Inflammatory signaling is a central mechanism of parturition, driving both preterm and physiological term labor.10, 11, 12 Toll-like receptor (TLR)-4 is a pivotal upstream driver of inflammation provoked by microbial triggers13, 14, 15, 16 that are implicated in up to 40% of preterm births.17, 18, 19 Recent studies have identified TLR4 as a tractable target for pharmacologic intervention in infection-associated preterm birth,20 using neutralizing antibodies15; lipid A mimetic CXR-52614; or, most promisingly, a small-molecule antagonist, (+)-naloxone, the (+)-isomer of the opioid antagonist (−)-naloxone.21 In addition to infection, sterile inflammation associated with multiple gestations, cervical insufficiency, psychosocial stress, and environmental toxin exposure can also trigger spontaneous preterm labor. In these conditions, meta-inflammation is elicited by sterile proinflammatory mediators including oxidized lipids and damage-associated molecular patterns released by stressed and dying cells,22,23 but how these triggers converge to elicit inflammation and promote parturition is not clear.
One key mediator of sterile inflammation that is implicated in both sterile and infection-associated preterm labor is the glycophospholipid platelet-activating factor (PAF; 1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine).24 PAF is synthesized by alveolar type II cells in the fetal lung,25 in response to up-regulated expression of lysophosphatidylcholine acetyltransferase-1 activity in late gestation,26,27 and progressively accumulates in amniotic fluid prior to labor in mice27 and humans.28,29 Amniotic fluid PAF signaling then acts through its cognate G-protein coupled receptor (PAFr), to activate downstream cellular and transcriptional responses via GTPase, protein kinase C, and tyrosine kinase signaling pathways.30 PAFr expression becomes progressively elevated in late gestation in the uterus and cervix in mice31 and in the myometrium, cervix, placenta, and fetal membrane in humans.32, 33, 34, 35, 36 In mice, PAF promotes uterine activation and transition to a contractile state37 by targeting uterine cells to stimulate the activation of NF-κB and inflammatory cytokine synthesis.24,27 The addition of PAF to human choriodecidua elicits up-regulation of the uterine activation gene Ptgs2,34 and stimulates contractile activity in human myometrial strips.32 PAF also induces the secretion of proinflammatory cytokines in a PAFr-dependent manner in human cervical fibroblasts.32,33
PAF becomes elevated in inflammatory conditions after altered expression of enzymes controlling PAF synthesis and catabolism.38, 39, 40 Rodent models indicate a dynamic mechanism by which regulators of PAF homeostasis modulate PAF accumulation in late gestation, and indicate a role for PAF as a crucial fetal mediator of the timing of labor.27 In mice, preterm delivery can be induced by intrauterine (i.u.) or intra-amniotic administration of carbamyl (c)-PAF, a PAF homologue rendered resistant to degradation by the addition of the carbamyl group.27,31,41,42 Conversely, mice genetically deficient in upstream regulators of PAF synthesis or steroid receptor coactivator 1 or 2 show delayed parturition.24,27 In rats, treatment with a PAF receptor antagonist caused extended duration of labor,43 and i.v. infusion of cPAF for 7 days in late gestation elicited decreased fetal and placental weight.44,45 In women with preterm labor, PAF accumulates in amniotic fluid prematurely.46,47 Certain conditions shift the balance of PAF synthesis and catabolism and alter the rate of its accumulation. For example, smoking increases the synthesis of PAF in the fetal lung and contributes to amniotic fluid PAF accumulation46,47 associated with fetal hypoxia.48
The mechanism by which PAF induces inflammation to drive sterile preterm labor is not clear. It is biologically plausible that TLR4 contributes to PAF-induced inflammation and preterm birth given that PAF-induced mediators of sterile inflammation are ligands for TLR4, or interact with TLR4 signaling.22,49 Experiments in intestinal epithelial cells indicate that in addition to PAFr, PAF activates TLR4, driving robust proinflammatory signaling.50 Peritoneal macrophages from Tlr4−/− mice secrete less tumor necrosis factor and C-C motif chemokine ligand 5 after in vitro culture with cPAF, compared with wild-type (WT) controls.41 This finding raises the question of whether TLR4 may also be an effective target for pharmacologic intervention in preterm birth elicited by PAF. Using BALB/c mice, this study investigated whether PAF-induced preterm birth requires TLR4 signaling, and whether (+)-naltrexone, a small-molecule TLR4 antagonist closely related to (+)-naloxone,51,52 is effective in suppressing the PAF-induced inflammatory cascade leading to preterm birth.
Materials and Methods
Mice
BALB/c mice were obtained from the Animal Resource Centre (Perth, WA, Australia). Mice with a null mutation in Tlr4 (Tlr4−/−) backcrossed onto BALB/c for >10 generations were from Professor Shizuo Akira (Osaka University, Osaka, Japan), a gift from Professor Paul Foster (University of Newcastle, Newcastle, NSW, Australia). Mice were housed and maintained in the specific pathogen-free University of Adelaide Medical School Animal House with a 12-hour light/12-hour dark cycle. Breeder chow food and water were provided ad libitum, and animals were used according to the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes with approval from the University of Adelaide Animal Ethics Committee. One to three virgin females of 8 to 14 weeks of age were housed with a proven fertile male of the same genotype and checked daily between 8 and 10 am for vaginal plugs. The morning of plug detection was designated as gestational day (gd) 0.5. Mated females were removed from the male and housed individually.
Treatments and Pregnancy Outcomes
For i.p. cPAF, pregnant Tlr4−/− and WT BALB/c female mice were administered 2 μg/mouse of cPAF [1-O-palmitol-2-(N-methylcarbamyl)-sn-glycero-3-phosphocholine] or phosphate-buffered saline (PBS) vehicle i.p. at 10:00 AM to 12:00 PM on gd 16.5. For i.u. cPAF, pregnant BALB/c mice were anesthetized with isoflurane between 10:00 AM and 12:00 PM on gd 16.5, and a 1.5-cm midline incision was made on the lower abdomen. cPAF (35 μg in 100 μL) or PBS vehicle was injected in the right uterine horn at a site between two adjacent fetuses most proximal to the cervix. The abdominal incision was closed in two layers, using sutures through the peritoneal wall and the skin.
Additional groups of pregnant WT females given cPAF or vehicle either i.p. or i.u. (as described in the previous paragraph) were immediately administered TLR4 antagonist (+)-naltrexone (60 mg/kg in PBS i.p.) or PBS control within 5 minutes of cPAF administration, plus a further three equivalent doses at 12-hour intervals on gd 17.0, 17.5, and 18.0.
Mice were monitored by video recording. Preterm delivery was defined as delivery of at least one pup within 48 hours of cPAF treatment. On gd 18.5, undelivered pregnant females were sacrificed by cervical dislocation, and the intact uterus was removed. Total implantation sites were counted and classified as viable (presence of live fetus and placenta) or not viable (anemic, malformed, or severely growth-retarded fetus). Mice with at least one viable fetus were classified as having ongoing viable pregnancy. Each viable fetus was dissected from the amniotic sac and umbilical cord, then fetuses and placentas were weighed, and the fetal–placental weight ratio was calculated. A second cohort of females given cPAF or vehicle i.p. was monitored until birth, and the time of delivery and number of viable pups born were recorded. Pups were weighed at 12 to 24 hours after delivery, at 8 days of age, and at weaning at 21 days of age.
Cytokine and Uterine Activation Gene Expression
Pregnant Tlr4−/− and WT females treated with cPAF, and/or (+)-naltrexone or PBS, were sacrificed by cervical dislocation 4 hours after treatment, and the intact uterus was removed. Two implantation sites per dam were harvested, and the uterine myometrium (from implantation sites), entire uterine decidua (at placental attachment site), placenta, and fetal membranes were dissected and snap-frozen in liquid N2, then stored at −80°C. Uterine, placental, decidual, and fetal membrane tissues were homogenized using ceramic beads (Missouri Biotechnology Association, Jefferson City, MO) in TRIzol (Ambion RNA, Carlsbad, CA), and RNA was precipitated using isopropanol and ethanol. RNA purity and concentration were determined by measuring A260 and A280 in a NanoDrop 1000 Spectrophotometer (Thermo Fisher Scientific, Wilmington, DE) and then stored at −80°C. RNA was treated with RNase-free DNAse using a Turbo DNA-Free Kit (Ambion RNA) per the manufacturer's instructions. RNA integrity was verified by agarose gel electrophoresis to visualize 28S and 18S bands on gel images captured using the Gel Doc-EZ imager (Bio-Rad Laboratories, Hercules, CA).
Total RNA was reverse-transcribed into complementary DNA using Superscript II Reverse Transcriptase (InvitroGen, Carlsbad, CA) per the manufacturer's instructions. Primer sequences for genes encoding uterine activation regulators, proinflammatory cytokines, anti-inflammatory cytokines, and receptors PAFr and TLR4 (Table 1) were designed, optimized, and validated in-house. Quantitative PCR reactions containing 2 μL of complementary DNA (10 ng/μL), and 18 μL of master mix consisting of 1× Power SYBR Green PCR Master Mix (Life Technologies, Carlsbad, CA), forward and reverse primer (Table 1), were set up using a QIAgility benchtop liquid handling system (Qiagen, Valencia, CA). Nontemplate control samples containing water in place of cDNA were included. Quantitative PCR was performed in a C1000 Touch Thermal Cycler (Bio-Rad Laboratories) under the following conditions: 2 minutes at 50°C, 10 minutes at 95°C, followed by 40 cycles of 20 seconds at 95°C and 1 minute at 60°C. Melt curve analysis ensured a lack of amplification of nonspecific products for all primer sets. Previous studies have confirmed that Actb is stable in uterus, placenta, fetal membrane, and fetus of control mice or mice given microbial agents.52, 53, 54 Each gene of interest was expressed relative to Actb levels using the formula: mRNA level = Log2 − (CtActb − CtTarget gene).55
Table 1.
Primers for mRNA Expression Analysis by Quantitative PCR
| Gene | Primer sequence | GenBank accession no. |
|---|---|---|
| Actb | F: 5′-CGTGGGCCGCCCTAGGCACCA-3′ R: 5′-ACACGCAGCTCATTGTA-3′ |
NM_007393.3 |
| Il1b | F: 5′-CCAAAGCAATACCCAAAGAAA-3′ R: 5′-GCTTGTGCTCTGCTTGTGAG-3′ |
NM_008361.3 |
| Il10 | F: 5′-AGGCGCTGTCATCGATTTCT-3′ R: 5′-TGGCCTTGTAGACACCTTGGT-3′ |
NM_010548.2 |
| Il12b | F: 5′-TGACACGCCTGAAGAAGA-3′ R: 5′-AGAGACGCCATTCCACAT-3′ |
NM_001303244.1 |
| Il6 | F: 5′-ACAACCACGGCCTTCCCTAC-3′ R: 5′-TCCACGATTTCCCAGAGAACA-3′ |
NM_031168.1 |
| Ptafr | F: 5′-TATGGCTGACCTGCTCTTCCTGAT-3′ R: 5′-TATTGGGCACTAGGTTGGTGGAGT-3′ |
NM_001081211.2 |
| Ptgs2 | F: 5′-GTTTGCATTCTTTGCCCAGC-3′ R: 5′-AGTCCACTCCATGGCCCAGT-3′ |
NM_008969.3 |
| Tlr4 | F: 5′-CAAGGGATAAGAACGCTGAGA-3′ R: 5′-GCAATGTCTCTGGCAGGTGTA-3′ |
NM_021297.3 |
| Tnf | F: 5′-GTAGCCCACGTCGTAGCAAAC-3′ R: 5′-CTGGCACCACTAGTTGGTTGTC-3′ |
NM_013693.3 |
GenBank accession numbers available at https://www.ncbi.nlm.nih.gov/genbank.
F, forward; R, reverse.
Bacterial Endotoxin
cPAF reconstituted in endotoxin-free water was confirmed to contain <0.001 EU/μg of bacterial endotoxin using a QCL-100 limulus amebocyte lysate assay (Lonza, Basel, Switzerland), according to the manufacturer's instructions. The assay lower limit of detection was 0.1 EU/mL, with intra-assay precision of <4% and interassay precision of <10%.
Statistical Analysis
Statistical analysis was conducted using SPSS software version 20.0 (SPSS Inc., Chicago, IL). Data were tested for normality using the Shapiro-Wilk test. Analysis of variance and post hoc Sidak t-tests were used when data were normally distributed. The Kruskal-Wallis and U-tests were used when data were not normally distributed. Categorical data were compared by χ2 analysis. Fetal weight, placental weight, and fetal–placental weight ratio data were analyzed using mixed-model analysis of variance, and data are expressed as estimated marginal means ± SEM. Differences between groups were considered significant when P < 0.05.
Results
Dependence of cPAF-Induced Preterm Delivery on TLR4
To investigate the role of TLR4 in PAF-induced preterm delivery, pregnant WT and Tlr4−/− females were administered cPAF or PBS control i.p. on gd 16.5. Mice were observed for preterm delivery for the next 48 hours. In the absence of preterm birth, mice were sacrificed on gd 18.5, and implantation sites, fetal viability, and fetal and placental weights were then determined.
cPAF administration was associated with a preterm birth rate of 64% (9 of 14), compared with zero in the PBS control group (Figure 1A), such that 36% of pregnancies yielded viable pups compared with 100% with vehicle control (both, P < 0.001, χ2 test) (Figure 1B). Among the dams not delivered by gd 18.5, despite no difference in total implantation sites per pregnant dam (Figure 1C), there were 73% and 64% declines in the number (Figure 1D) and percentage (Figure 1E) of viable fetuses per dam, respectively, after cPAF treatment compared with those in the PBS control group (48 dams) (both, P < 0.001, one-way analysis of variance).
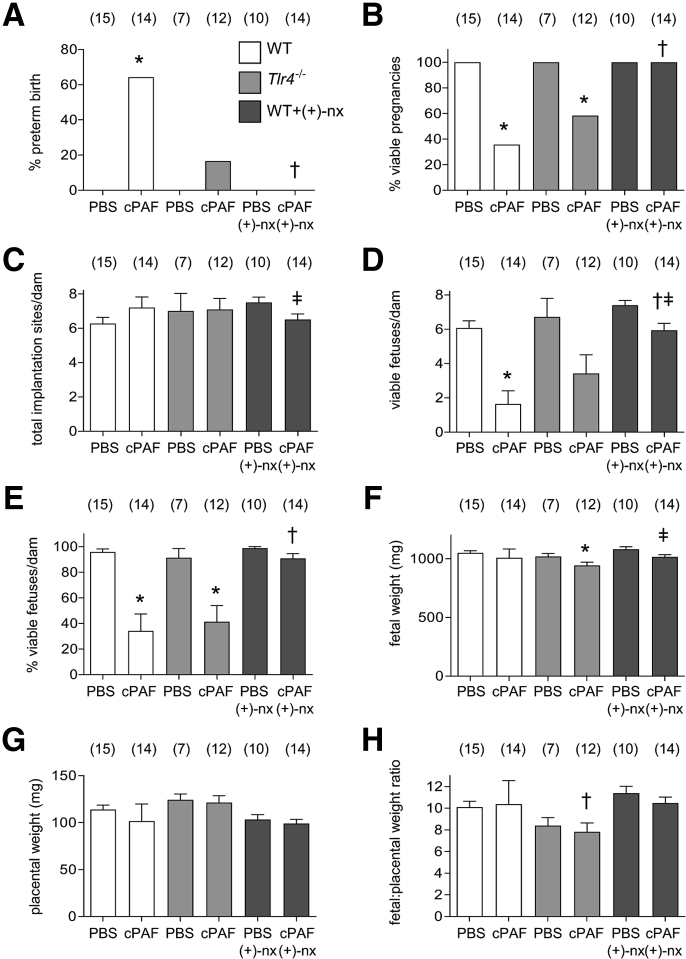
Figure 1.
Effects of genetic Toll-like receptor (TLR)-4 deficiency and TLR4 antagonist (+)-naltrexone [(+)-nx] on i.p. carbamyl–platelet activating factor (cPAF)-induced fetal death and preterm birth. Wild-type (WT) females or Tlr4−/− females were mated to males of the same genotype. On gestational day (gd) 16.5, pregnant females were administered 2 μg of cPAF or phosphate-buffered saline (PBS) vehicle control i.p. Additional groups of WT mice were given cPAF or PBS and (+)-nx (60 mg/kg) i.p. on gd 16.5, 17.0, 17.5, and 18.0. A: Mice were monitored for preterm delivery within 48 hours of cPAF administration to determine the percentage of preterm births. B–E: In the absence of preterm birth, mice were sacrificed on gd 18.5, and pregnancy outcomes were measured to record the percentage of viable pregnancies (at least one viable fetus) (B), total implantation sites per dam (C), the number of viable fetuses per dam (D), and the percentage of viable fetuses per dam (E). F–H: Effects of cPAF, genotype, and (+)-nx on the weights of fetuses and placentas, and fetal:placental weight ratio. Categorical data were compared by χ2 analysis (A and B); other data are expressed as means ± SEM and were analyzed by one-way analysis of variance and post-hoc Sidak t-test, or two-way analysis of variance (C–H). The numbers of dams are shown in parentheses. ∗P < 0.05 versus within-genotype PBS control; †P < 0.05 versus cPAF in WT mice; ‡P < 0.05 versus (+)-nx alone.
Tlr4−/− mice appeared to be relatively protected from preterm delivery, with 17% (2 of 12) of mice delivering preterm (Figure 1A). However, increased fetal death was evident in Tlr4−/− mice given cPAF, resulting in 42% fewer viable pregnancies than in Tlr4−/− mice given PBS (P < 0.001) (Figure 1B). The mean number of viable fetuses per dam was reduced by 42% compared with that in PBS-treated Tlr4−/− dams (P = 0.064) (Figure 1D), and the percentage viable fetuses was reduced by 55% (P = 0.002) (Figure 1E).
The fetal weight (Figure 1F), placental weight (Figure 1G), and fetal–placental weight ratio (Figure 1H) of viable fetuses from WT dams were not altered with cPAF administration. Fetuses from Tlr4−/− dams given cPAF exhibited a 7% smaller mean fetal weight compared with that in the Tlr4−/− females given PBS (P = 0.031) (Figure 1F), whereas placental weight was not affected with cPAF (Figure 1G). Placental weight was increased, and the fetal–placental weight ratio was reduced, in Tlr4−/− dams compared with those in WT dams (both, P < 0.001) (Figure 1, G and H), suggesting a reduced placental efficiency independent of cPAF treatment.
(+)-Naltrexone Prevention of Intraperitoneal cPAF-Induced Preterm Delivery
The TLR4 antagonist (+)-naltrexone was evaluated for its ability to suppress cPAF-induced preterm birth. Pregnant WT females were administered cPAF or PBS i.p. on gd 16.5, then four doses of (+)-naltrexone on gd 16.5, 17.0, 17.5, and 18.0. (+)-Naltrexone apparently protected mice from cPAF-induced preterm delivery (Figure 1A), and reversed the cPAF-induced reduction in viable pregnancy rate (Figure 1B). The number of viable fetuses per undelivered dam was comparable to those in the PBS- or (+)-naltrexone only–treated control groups when measured as a percentage of total implants (53 dams, one-way analysis of variance) (Figure 1E), notwithstanding small reductions in total and viable implantation sites in the cPAF and (+)-naltrexone group (both, P < 0.050) (Figure 1, C and D). Mean fetal weight was 6% smaller in dams given cPAF and (+)-naltrexone, compared with that in (+)-naltrexone only–treated controls (P = 0.024) (Figure 1F). Placental weight and fetal–placental weight ratio in the dams given cPAF and (+)-naltrexone were not different from those in either the PBS- or (+)-naltrexone only–treated controls (Figure 1, G and H).
(+)-Naltrexone Prevention of Intrauterine cPAF-Induced Preterm Delivery
(+)-Naltrexone was next investigated for its ability to suppress preterm birth induced by i.u. cPAF. This route of delivery more closely approximates the physiological site of accumulation in the amniotic fluid. Pregnant WT females were administered cPAF or PBS i.u. on gd 16.5, and outcomes were recorded on gd 18.5. Preterm birth occurred in 4 of 13 pregnant WT mice (31%) given cPAF (Figure 2A), associated with a 38% reduction in the number of viable pregnancies compared with that in PBS controls (P < 0.001) (Figure 2B). cPAF also was associated with substantial fetal death in dams that did not progress to preterm labor. The number of total implantation sites per dam was unchanged (Figure 2C), but the number and percentage of viable fetuses per dam declined by 64% and 60%, respectively (50 dams; both, P < 0.001, one-way analysis of variance) (Figure 2, D and E). The fetal weight (Figure 2F) and fetal–placental weight ratio (Figure 2H) were reduced after cPAF administration compared with those in controls (both, P < 0.010), but placental weights were unchanged (Figure 2G).
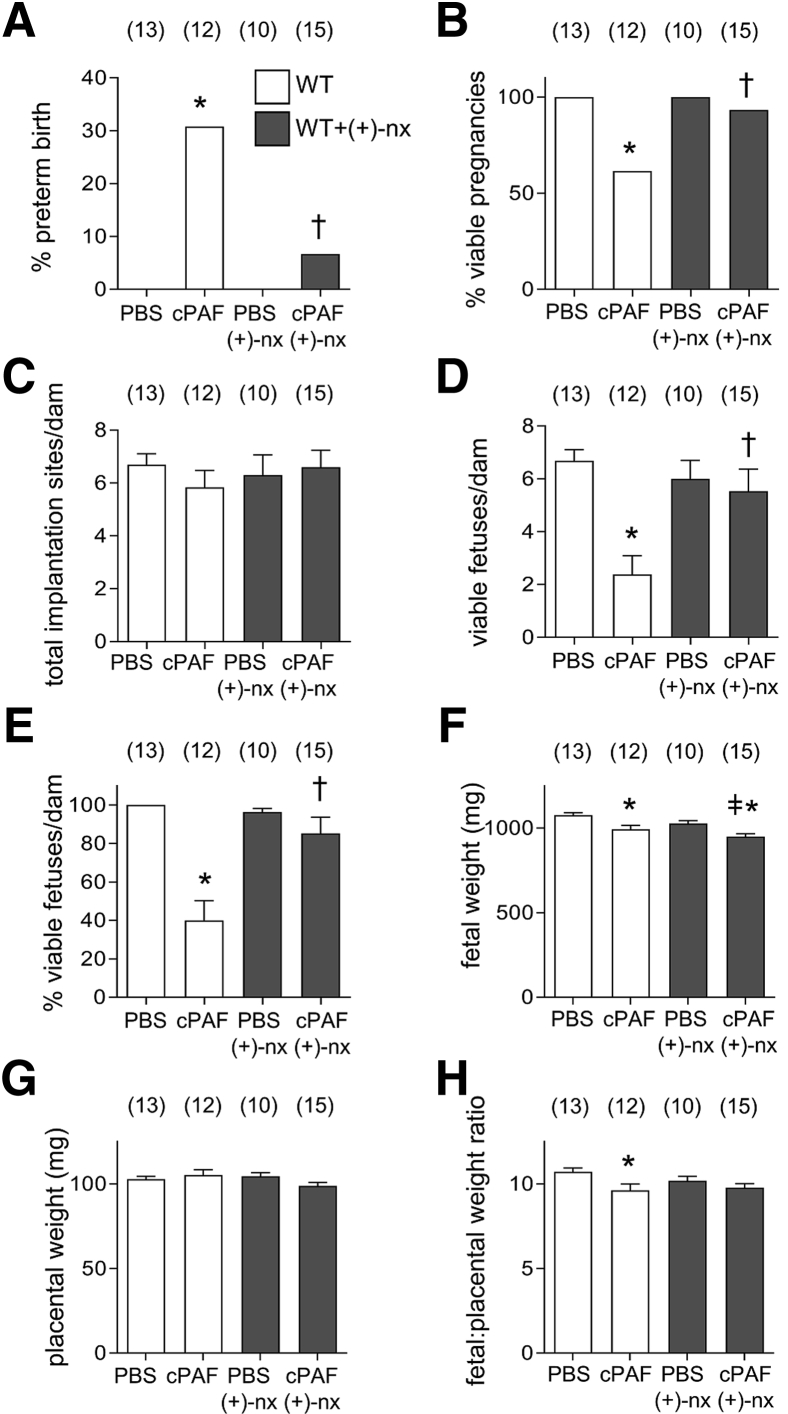
Figure 2.
Effects of TLR4 antagonist (+)-naltrexone [(+)-nx] on intrauterine (i.u.) carbamyl–platelet activating factor (cPAF)-induced preterm birth. Wild-type (WT) females were mated to males of the same genotype. On gestational day (gd) 16.5, pregnant females were administered 35 μg of cPAF or phosphate-buffered saline (PBS) vehicle control i.u. Additional groups of WT mice were given cPAF or PBS and (+)-nx (60 mg/kg) i.p. on gd 16.5, 17.0, 17.5, and 18.0. A: Mice were monitored for preterm delivery within 48 hours of cPAF administration to determine the percentage of preterm births. B–E: In the absence of preterm birth, mice were sacrificed on gd 18.5 and pregnancy outcomes were measured to record the percentage of viable pregnancies (at least one viable fetus) (B), total implantation sites per dam (C), the number of viable fetuses per dam (D), and the percentage of viable fetuses per dam (E). F–H: Effects of cPAF and (+)-nx on the weights of fetuses and placentas and fetal:placental weight ratio. Categorical data were compared by χ2 analysis (A and B); other data are expressed as means ± SEM and were analyzed by analysis of variance and post-hoc Sidak t-test (C–H). The numbers of dams are shown in parentheses. ∗P < 0.05 versus PBS control; †P < 0.05 versus cPAF in WT mice; ‡P < 0.05 versus (+)-nx alone.
Dams administered cPAF followed by (+)-naltrexone showed improved outcomes, with fewer preterm births, than with cPAF alone (Figure 2A), and no reduction in the percentage of viable pregnancies compared with that in PBS controls (Figure 2B). Both the number (Figure 2D) and percentage (Figure 2, C and E) of viable fetuses per dam were comparable to those in the PBS-treated or (+)-naltrexone only–treated control groups. (+)-Naltrexone was not associated with improved fetal weight (Figure 2F), but was associated with mitigation of the reduced fetal–placental weight ratio seen after cPAF administration (Figure 2, G and H).
Dependence of cPAF-Induced Postnatal Loss on TLR4
To investigate the interaction between TLR4, cPAF, and postnatal outcomes, pregnant WT and Tlr4−/− females were given cPAF i.p. on gd 16.5 and then were monitored for preterm delivery and pup survival after birth. In dams given cPAF, 68% (13 of 19) delivered prematurely (Figure 3A), associated with a significant reduction in the mean time of delivery (Figure 3B) and the majority of delivered pups failed to survive (all P < 0.001) (Figure 3C). The weight of surviving pups at 24 hours after birth was not different from that in the dams given cPAF (Figure 3D). A total of 30% of pups born to dams given cPAF survived to 3 weeks, compared with 92% of pups from control dams (P < 0.001) (Figure 3E). Surviving female pups from dams given cPAF were smaller than controls at 3 weeks (P = 0.007) (Figure 3G), although a smaller weight in males was not evident (P = 0.083) (Figure 3F).
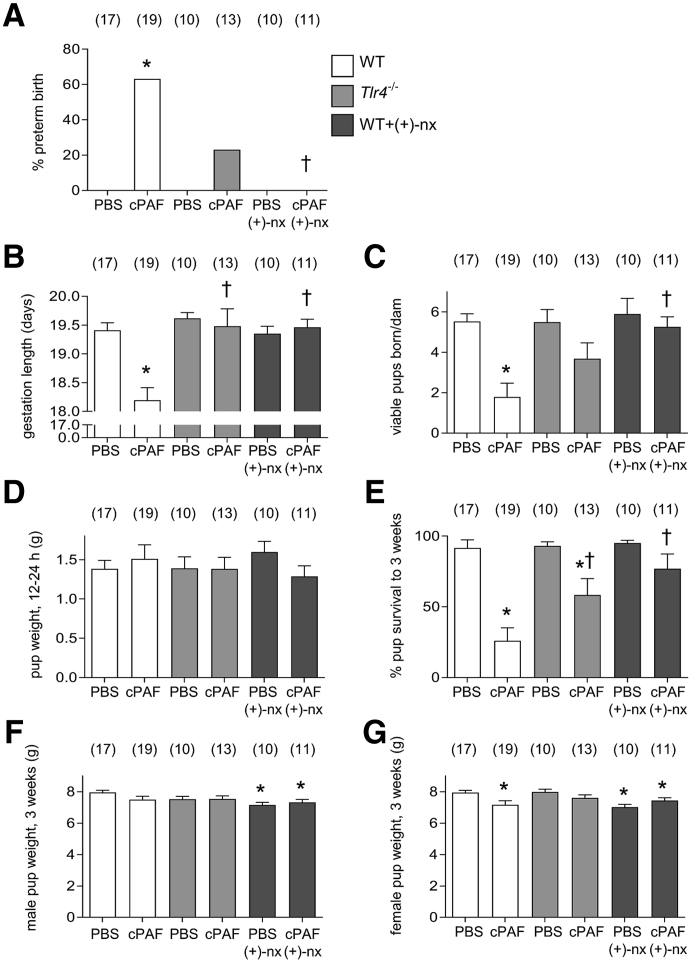
Figure 3.
Effects of genetic Toll-like receptor (TLR)-4 deficiency and TLR4 antagonist (+)-naltrexone [(+)-nx] on i.p. carbamyl–platelet activating factor (cPAF)-induced perinatal outcomes. Wild-type (WT) females or Tlr4−/− females were mated to males of the same genotype. On gestational day (gd) 16.5, pregnant females were administered 2 μg of cPAF or phosphate-buffered saline (PBS) vehicle control i.p. Additional groups of WT mice were given cPAF or PBS and (+)-nx (60 mg/kg) i.p. on gd 16.5, 17.0, 17.5, and 18.0. All mice were then monitored for timing of birth. The percentages of mice delivering preterm (A), length of gestation (B), number of viable pups born per dam (C), pup weight at 24 hours (D), pup survival to 3 weeks (E), and weight of surviving male (F), and female (G) pups at 3 weeks were recorded. Categorical data were compared by χ2 analysis (A); other data are expressed as means ± SEM and were analyzed by analysis of variance and post-hoc Sidak t-test (B–F). The numbers of dams are shown in parentheses. ∗P < 0.05 versus within-genotype PBS control; †P < 0.05 versus cPAF in WT mice.
TLR4 deficiency again apparently protected mice from cPAF-induced preterm delivery, with 23% (3 of 13) of Tlr4−/− dams given cPAF delivering preterm (Figure 3A), and with a significant increase in gestation length compared with that with cPAF alone (P < 0.001), comparable to gestation length in PBS controls (Figure 3B). The number of viable pups delivered by Tlr4−/− dams given cPAF was not significantly different compared with those in WT and Tlr4−/− females given PBS (P = 0.080) (Figure 3C), and the weight of surviving pups at 24 hours was not different after cPAF administration (Figure 3D). In litters from Tlr4−/− dams, the percentage of pups surviving to 3 weeks was improved compared with that from WT dams given cPAF (P = 0.035) but less than that from Tlr4−/− dams given PBS (P = 0.012) (Figure 3E), and the weights of male and female pups at 3 weeks were similar to those in pups from Tlr4−/− females given PBS (Figure 3, F and G).
(+)-Naltrexone Prevention of cPAF-Induced Postnatal Loss
To determine whether (+)-naltrexone improves cPAF-induced adverse neonatal outcomes, pregnant WT females were administered cPAF or vehicle i.p. on gd 16.5, then four doses of (+)-naltrexone, and they were monitored for time of delivery and survival of pups. None of 11 dams given cPAF and (+)-naltrexone delivered preterm, and gestation length was no different from that in controls given PBS (Figure 3, A and B). All dams delivered a mean number of viable pups similar to those in the PBS-treated controls and the (+)-naltrexone only–treated controls (Figure 3C). The weight of surviving pups at 24 hours was not different after maternal cPAF and (+)-naltrexone treatment, compared with those with PBS or (+)-naltrexone alone (Figure 3D). Mean pup survival at 3 weeks after maternal cPAF and (+)-naltrexone was 77%, a substantial improvement compared with the 30% survival in the cPAF group (P < 0.001). All delivered pups survived in 6 of 11 litters, and ranged from 0% to 75% in the other 5 litters. Thus, survival in this group was not statistically different from those in the PBS control and (+)-naltrexone–only groups (Figure 3E). However, the modest growth impairment evident at 3 weeks in pups from dams given cPAF was not recovered with (+)-naltrexone, and both male and female pups from dams given cPAF and (+)-naltrexone were smaller at 3 weeks than pups from dams given PBS (P < 0.050). A similar reduction in pup weight at 3 weeks was seen in pups from dams given (+)-naltrexone alone (P < 0.050) (Figure 3, F and G).
Dependence of cPAF-Induced Inflammatory Cytokine Expression on TLR4
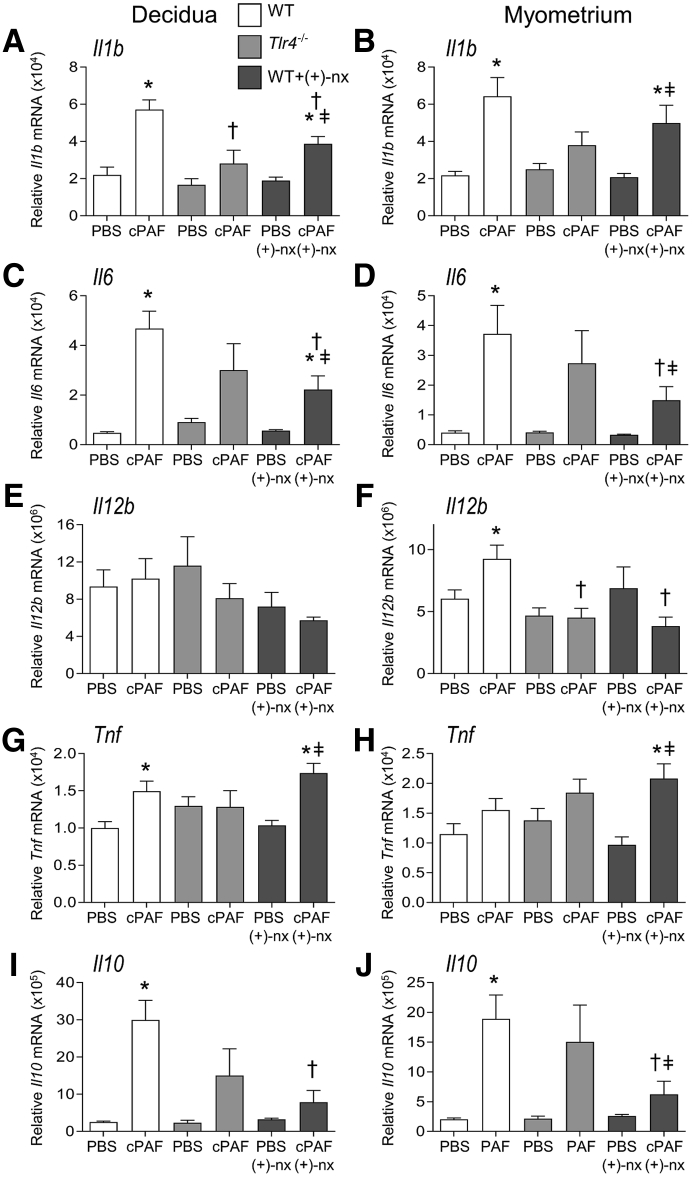
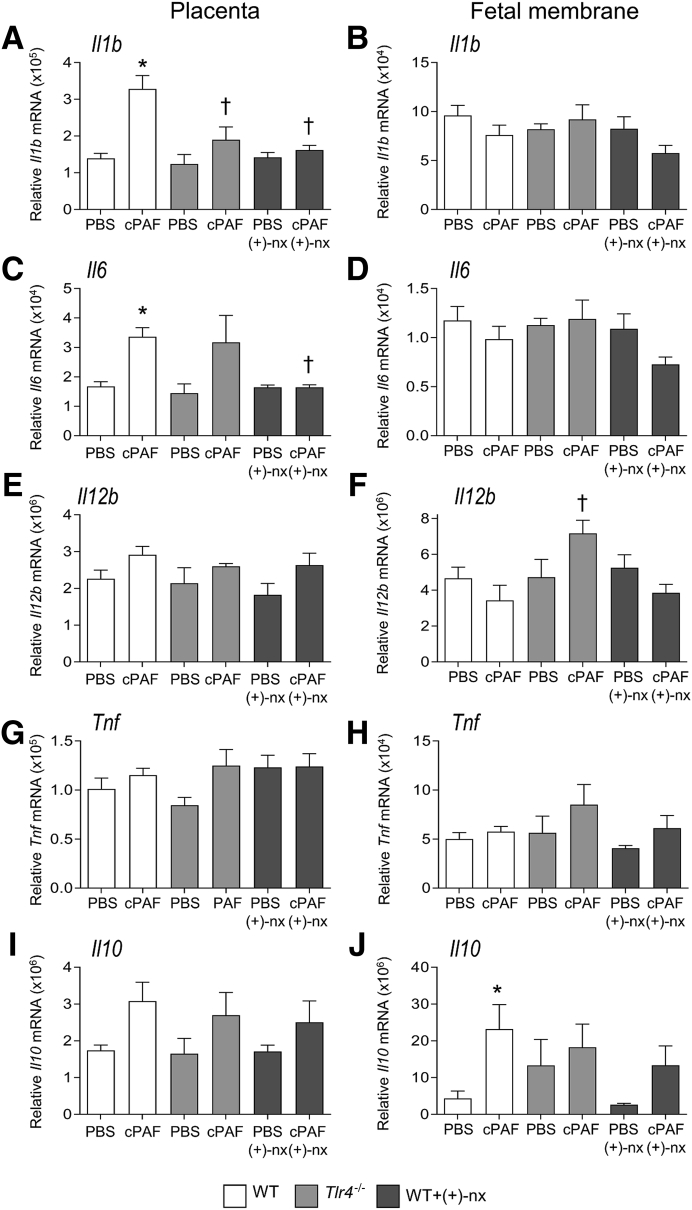
To examine the mechanism by which TLR4 may facilitate cPAF-mediated progression to preterm birth, inflammatory cytokine induction by cPAF in WT and Tlr4−/− dams was quantified. In the decidua and myometrium of WT dams, cPAF was associated with 2.6- and 2.9-fold increases, respectively, in Il1b expression (Figure 4, A and B), 9.4- and 9.3-fold increases in Il6 (Figure 4, C and D), and 12.0- and 9.4-fold increases in Il10 (all, P < 0.020) (Figure 4, I and J), but no change in Il12b (Figure 4E), compared with those in tissues from control mice given PBS. In tissues from Tlr4−/− dams, cPAF was not associated with consistent alterations in cytokine expression other than trends towards increased decidual and myometrial Il6 and myometrial Il10 (all, P < 0.100) (Figure 4, C, D, and J). Tlr4−/− dams given cPAF showed reduced decidual Il1b and myometrial Il12b expression relative to those in WT females given cPAF (both, P < 0.010) (Figure 4, A and F).
Figure 4.
Effects of genetic Toll-like receptor (TLR)-4 deficiency and TLR4 antagonist (+)-naltrexone [(+)-nx] on carbamyl–platelet activating factor (cPAF)-induced inflammatory cytokine gene expression in the decidua and myometrium. Wild-type (WT) females or Tlr4−/− females were mated to males of the same genotype. On gestational day (gd) 16.5, pregnant females were administered 2 μg of cPAF or phosphate-buffered saline (PBS) vehicle control i.p. Additional groups of WT mice were given cPAF or PBS and (+)-nx (60 mg/kg) i.p. on gd 16.5, 17.0, 17.5, and 18.0. Mice were allowed to progress to birth, with monitoring for preterm delivery within 48 hours of cPAF administration. At 4 hours post-treatment, decidua (A, C, E, G, and I) and myometrium (B, D, F, H, and J) were harvested and relative expression of Il1b (A and B), Il6 (C and D), Il12b (E and F), Tnf (G and H), and Il10 (I and J) were measured by quantitative PCR normalized to Actb. Data were tested for normality by Shapiro-Wilk test. Analysis of variance and post hoc Sidak t-test were used when data were normally distributed, or Kruskal-Wallis and U-tests when data were not normally distributed. Data are expressed as means ± SEM relative gene expression in tissue pooled from two implantation sites per dam. n = 6 to 10 dams/group. ∗P < 0.05 versus within-genotype PBS control; †P < 0.05 versus cPAF in WT mice; ‡P < 0.05 versus (+)-nx alone.
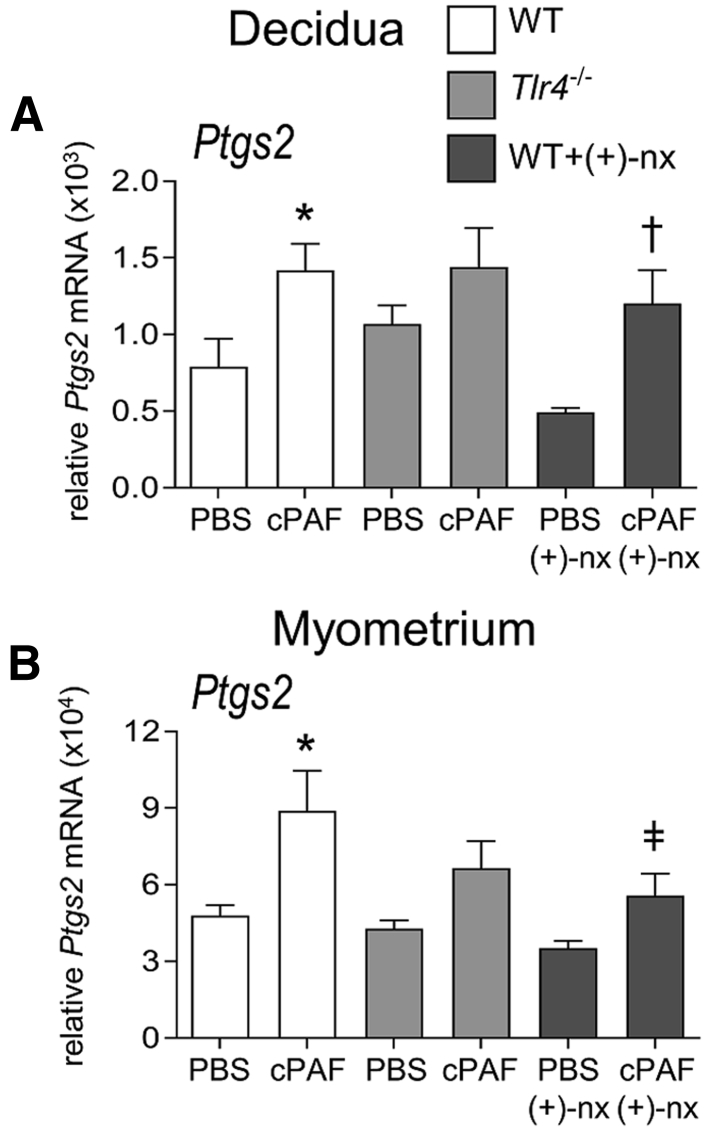
Uterine activation genes were also evaluated. Ptgs2 encoding prostaglandin-endoperoxide synthase 2 (cyclooxygenase-2) was elevated by 1.8 and 1.9-fold in the decidua and myometrium of WT mice given cPAF (both, P < 0.030), but Ptgs2 expression was variable and not consistently elevated in Tlr4−/− mice given cPAF (Figure 5). Other uterine activation genes (Oxtr, Gja1, Ptgs1, and Ptgfr) were not elevated with cPAF treatment, likely because of the short 4-hour time window between cPAF treatment and tissue analysis (data not shown).
Figure 5.
Effects of genetic Toll-like receptor (TLR)-4 deficiency and TLR4 antagonist (+)-naltrexone [(+)-nx] on carbamyl–platelet activating factor (cPAF)-induced uterine activation gene Ptgs2 expression in the decidua and myometrium. Wild-type (WT) females or Tlr4−/− females were mated to males of the same genotype. On gestational day 16.5, pregnant females were administered 2 μg of cPAF or phosphate-buffered saline (PBS) vehicle control i.p. Additional groups of WT mice were given cPAF or PBS and (+)-naltrexone (60 mg/kg) i.p. At 4 hours post-treatment, decidua (A) and myometrium (B) were harvested, and the relative expression of Ptgs2 was measured by quantitative PCR normalized to Actb. Data were tested for normality by Shapiro-Wilk test. Analysis of variance and post hoc Sidak t-test were used when data were normally distributed, or Kruskal-Wallis and U-tests when data were not normally distributed. Data are expressed as means ± SEM relative gene expression in tissue pooled from two implantation sites per dam. n = 6 to 10 dams/group. ∗P < 0.05 versus within-genotype PBS control; †P < 0.05 versus cPAF in WT mice; or ‡P < 0.05 versus (+)-nx alone.
In the placenta, cPAF was associated with 2.4- and 2.0-fold increases in Il1b and Il6 expression (Figure 6, A and C). Placental Il12b, Tnf and Il10 were not induced by cPAF (Figure 6, E, G and I). In Tlr4−/− females, cPAF was not associated with induced placental Il1b expression (Figure 6A), with Il6 expression being variable and not consistently induced (P = 0.076) (Figure 6C). In the fetal membrane, cPAF was associated with a 5.3-fold increase in Il10 expression in WT mice (P = 0.032) (Figure 6J), whereas Il1b, Il6, Il12b and Tnf were not induced (Figure 6, B, D, F and H). Tlr4−/− females did not respond consistently with cPAF, other than a 1.8-fold increase in Il12b expression (P = 0.007) (Figure 6F).
Figure 6.
Effects of genetic Toll-like receptor (TLR)-4 deficiency and TLR4 antagonist (+)-naltrexone [(+)-nx] on carbamyl–platelet activating factor (cPAF)-induced inflammatory cytokine gene expression in the placenta and fetal membrane. Wild-type (WT) females or Tlr4−/− females were mated to males of the same genotype. On gestational day 16.5, pregnant females were administered 2 μg of cPAF or phosphate-buffered saline (PBS) vehicle control i.p. Additional groups of WT mice were given cPAF or PBS and (+)-nx (60 mg/kg) i.p. At 4 hours post-treatment, placenta (A, C, E, G, and I) and fetal membrane (B, D, F, H, and J) were harvested, and the relative expression of Il1b (A and B), Il6 (C and D), Il12b (E and F), Tnf (G and H), and Il10 (I and J) were measured by quantitative PCR normalized to Actb. Data were tested for normality by Shapiro-Wilk test. Analysis of variance and post hoc Sidak t-test were used when data were normally distributed, or Kruskal-Wallis and U-tests when data were not normally distributed. Data are expressed as means ± SEM relative gene expression in tissue pooled from two implantation sites per dam. n = 6 to 10 dams/group. ∗P < 0.05 versus within-genotype PBS control; †P < 0.05 versus cPAF in WT mice.
(+)-Naltrexone Suppression of cPAF-Induced Inflammatory Cytokine Expression
To examine the mechanisms underlying the protective actions of (+)-naltrexone, this study evaluated whether inflammatory cytokines induced by cPAF are influenced by (+)-naltrexone administration. The administration of (+)-naltrexone was associated with substantially reduced cPAF-driven Il1b, Il6, and Il10 expression in the decidua, by 32%, 53%, and 74%, respectively (all, P < 0.01) (Figure 4, A, C, and I), and Il6 and Il10 in the myometrium, by 59% and 67% (both, P < 0.05) (Figure 4, D and J). Il12b and Tnf in the placenta (Figure 4, E and G), and Il1b, Il6 and Tnf in the myometrium (Figure 4, B, D, and H) were not changed by (+)-naltrexone. cPAF-induced Il12b expression in the myometrium was reduced by 59% with (+)-naltrexone (P = 0.001) (Figure 4F). Tnf expression with cPAF administration in the decidua and myometrium was not decreased with (+)-naltrexone (P > 0.100) (Figure 4, G and H). Only modest, nonsignificant attenuation of Ptgs2 expression in the myometrium, but not the decidua, occurred after (+)-naltrexone administration (P = 0.083) (Figure 5).
In the placenta, co-administration of (+)-naltrexone was associated with dampened Il1b and Il6 induction, by 52% and 53%, respectively (both, P < 0.001), to levels comparable to those in the PBS and (+)-naltrexone–only control groups (Figure 6, A and C). In the fetal membrane, the induction of Il10 with cPAF was not affected by co-administration of (+)-naltrexone (Figure 6J). (+)-Naltrexone administration without cPAF had no effect on cytokine expression in any maternal or fetal tissues (Figures 4 and 6).
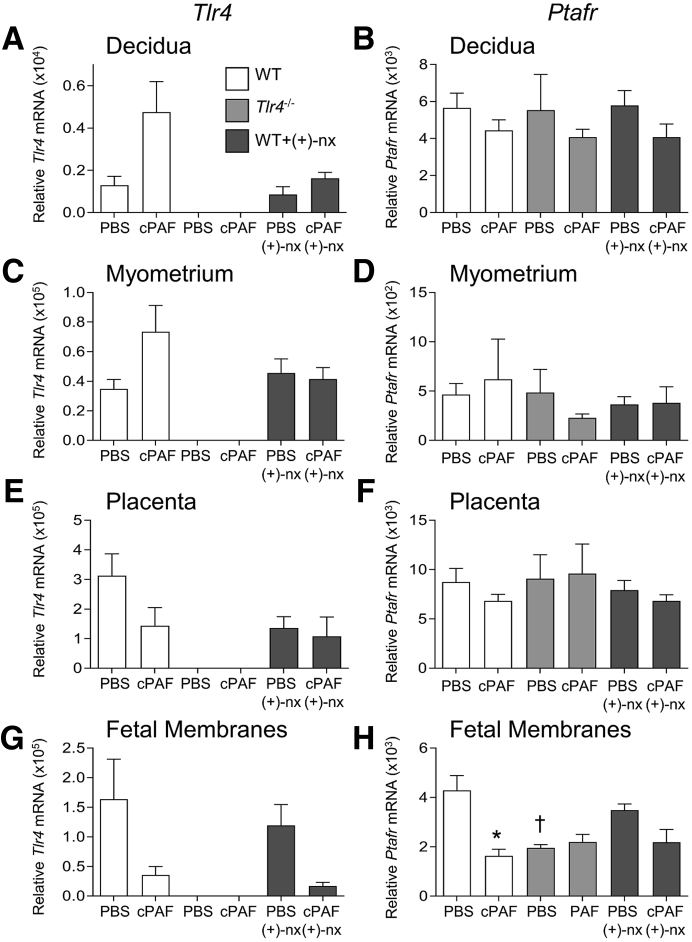
cPAF Modulation of Tlr4 and Ptafr Expression
To investigate the mechanism by which cPAF may induce inflammatory cytokine expression, this study quantified Tlr4 and Ptafr expression after cPAF and (+)-naltrexone administration. Gestational tissue expression of both genes was highly variable between dams. In the decidua of WT dams, Tlr4 expression after cPAF administration was variable but not consistently higher than in tissues from control mice given PBS (P = 0.062) (Figure 7A). Likewise, no consistent changes attributable to cPAF or (+)-naltrexone were seen in the myometrium, placenta, or fetal membranes (Figure 7, C, E, and G). In the fetal membranes, cPAF was associated with suppressed Ptafr expression (P = 0.001), and Tlr4−/− mice had lower Ptafr compared with that in WT mice (P = 0.010) (Figure 7H), but Ptafr was not regulated by cPAF or (+)-naltrexone in other tissues (Figure 7, B, D, and F).
Figure 7.
Effects of carbamyl–platelet activating factor (cPAF), genetic Toll-like receptor (TLR)-4 deficiency, and TLR4 antagonist (+)-naltrexone [(+)-nx] on Tlr4 and Ptafr gene expression in the decidua, myometrium, placenta, and fetal membrane. Wild-type (WT) females or Tlr4−/− females were mated to males of the same genotype. On gestational day 16.5, pregnant females were administered 2 μg of cPAF or phosphate-buffered saline (PBS) vehicle control i.p. Additional groups of WT mice were given cPAF or PBS and (+)-nx (60 mg/kg) i.p. At 4 hours post-treatment, decidua (A and B), myometrium (C and D), placenta (E and F), and fetal membrane (G and H) were harvested, and the relative expression of Tlr4 (A, C, E, and G) and Ptafr (B, D, F, and H) were quantified by quantitative PCR normalized to Actb. Data were tested for normality by Shapiro–Wilk test. Analysis of variance and post hoc Sidak t-test were used when data were normally distributed, or Kruskal-Wallis and U-tests when data were not normally distributed. Data are expressed as means ± SEM relative gene expression in tissue pooled from two implantation sites per dam. n = 6 to 10 dams/group. ∗P < 0.05 versus within-genotype PBS control; †P < 0.05 versus PBS in WT mice.
Discussion
Inflammation is a central mechanism in the pathophysiology of spontaneous preterm labor, and TLR4 is a crucial upstream mediator of proinflammatory signals that initiate and amplify inflammatory activation, to reverse uterine quiescence leading to myometrial contractile activity.14,15,21 The extent to which there is overlap in sterile and microbial mechanisms of preterm parturition, and the point at which these pathways converge, have not been clear. The current experiments indicate that TLR4 is a crucial upstream driver not just for preterm birth induced by infection, but also for sterile preterm labor induced by PAF, a key fetal signal implicated in spontaneous preterm labor in women.46,47 This study found that genetic deficiency in TLR4 was associated with reduced susceptibility to cPAF-induced preterm delivery and poor neonatal outcomes, and pharmacologic inhibition of TLR4 signaling with (+)-naltrexone appeared to be effective in blocking PAF-induced preterm birth. TLR4 is evidently required for amplification of the proinflammatory effects of cPAF through local induction of IL1β and IL6, two important rate-limiting regulators of progression to preterm birth,56,57 since the expression levels of Il1b and Il6 were reduced in gestational tissues with genetic Tlr4 deficiency, or inhibition of Tlr4 signaling.
Several lines of evidence point to PAF as a key effector of the inflammatory cascade underpinning labor,25,41 a role not unexpected given its potent ability to induce and amplify pathogenesis in a range of acute and chronic inflammatory conditions, including cardiovascular disease, asthma, endotoxin shock, diabetes, acute allergic reactions, thrombosis, and ischemic bowel necrosis.23,58 PAF elicits its effects through binding PAFr59,60 expressed mainly by platelets, monocytes, and neutrophils.61 Once synthesized, PAF is rapidly degraded by PAF-acetylhydrolase, but reduced PAF-acetylhydrolase synthesis under inflammatory conditions can retard PAF hydrolysis and contribute to the proinflammatory effects of PAF.40
In vivo experiments in mice have demonstrated that endogenous PAF production is crucial in enhancing infection-induced inflammation in maternal and fetal tissues, to initiate the activation of cervical ripening and preterm delivery.31,41,62 The administration of a PAF antagonist, CV-6209, reduced the incidence of preterm birth and fetal death after i.u. lipopolysaccharide (LPS) administration,31 whereas preterm birth induced by i.u. heat-killed E. coli also depends on intact PAFr signaling.41 This requirement for PAFr signaling likely reflects PAF-induced activation of macrophages and granulocytes that accumulate in the choriodecidua and amnion in late gestation.10,63
To develop a readily accessible model of sterile preterm birth that allowed the investigation of candidate pharmacologic interventions, this study utilized i.p. administration of cPAF at 2 μg/mouse, a dose identified in preliminary experiments to reliably generate a 40% to 60% preterm birth rate (H.H.W., unpublished data). A similar rate of preterm birth was achieved by the i.u. administration of cPAF, but a higher dose (35 μg/uterus) was required, comparable to doses previously reported with this route.31 Uterine tissues and fetal membranes exhibit endogenous resistance to proinflammatory triggers, through local effects of progesterone, T-regulatory cells, and repressors of NF-κB activation.12 Whether the effects of cPAF are amplified through inflammation-induced luteolysis and progesterone decline as occurs after LPS administration64 remains to be investigated. A limitation of this model is the use of synthetic cPAF to mimic elevated endogenous PAF. cPAF is chemically modified to prevent rapid degradation by PAF-acetylhydrolase and to ensure continued bioavailability, and this retarded catabolism presumably amplifies relevant downstream effector mechanisms.
Using WT and Tlr4−/− mice, this study reports that intact TLR4 signaling is essential for progression to cPAF-induced preterm delivery. In the absence of Tlr4, cPAF fails to induce the crucial proinflammatory cytokine genes Il1b and Il6 in the decidua and placenta, and less Il6 expression is also seen in the myometrium, compared with expression in WT females. In in vivo experiments in mice, exogenous IL1β is sufficient to elicit preterm birth65 and causes fetal inflammatory injury associated with brain, lung, and gastrointestinal tract pathology.57,66 In human myometrial cells, IL1β is a potent inducer of genes controlling uterine activation and contractile activity, cooperating with prostaglandin F2α to induce IL6 and cyclooxygenase-2.67 However TLR4-independent mechanisms of cPAF-induced uterine activation must also exist, as Ptgs2 expression was similarly induced in the myometrium of WT and Tlr4-null mutant mice administered cPAF. This study also quantified several other uterine activation genes, but given the short 4-hour interval between giving cPAF and gene expression analysis, no induction by cPAF or attenuation by Tlr4 deficiency was observed for Oxtr, Gja1, Ptgs1, and Ptgfr.
Also consistent with both Tlr4-dependent and -independent effects of cPAF on fetal viability, decidual, myometrial, and placental Il6 expression were only partially attenuated in the absence of Tlr4. Like IL1β, IL6 is a major upstream driver of preterm birth and mediator of fetal and placental inflammatory injury.56,68 Similarly, induction in mice of the key anti-inflammatory protective cytokine Il10 was not impacted by Tlr4 deficiency.54,69 A Tlr4-independent element of the PAFr signaling pathway potentially invokes Tlr2, or other pattern recognition receptors or amplifying signals,35,41 to induce proinflammatory cytokines in response to cPAF in Tlr4−/− mice. Il12b was induced in the fetal membrane of Tlr4−/− mice but not WT mice, suggesting attenuation of non–Tlr4-mediated PAF signaling when Tlr4 is absent. Elevated IL12 indicates a phenotype shift in macrophages toward an immunogenic M1 phenotype that has been reported to be associated with the labor-associated inflammatory response in decidual and placental membranes in women.70,71
Interpreting data from the Tlr4−/− mice is complicated by Tlr4 having both beneficial and detrimental effects on reproductive outcomes.21,72,73 Tlr4−/− mice produce moderately smaller litter sizes even without inflammatory challenge,21 implying a role for TLR4 signaling in normal pregnancy through regulating the uterine immune response at conception,73 or other pathways. This result resonates with the findings from a recent report that TLR4 signaling modulated fetal developmental programming in utero.72 It is therefore possible that cPAF interacts with TLR4 deficiency to adversely impact fetal outcomes through mechanisms independent of inflammatory cytokines. Tlr4−/− females had a larger placenta and a lower fetal–placental weight ratio compared with WT BALB/c females, independent of cPAF treatment. Reduced placental efficiency may reflect an underlying developmental aberration that increases susceptibility to alternate pathways of cPAF-induced fetal loss, such as via PAFr and TLR2.
In addition to preterm birth, cPAF administration was associated with significant fetal loss regardless of the route of administration. Although a substantial proportion of fetuses from cPAF-treated dams appeared viable in late gestation and survived birth and the early postnatal period, there was considerable loss in the preweaning phase, accompanied by growth impairment in surviving pups. This postnatal loss is consistent with the cPAF-mediated induction of fetal inflammatory injury that manifests as reduced viability in early life. Postnatal growth impairment often accompanies fetal inflammatory injury, and there are likely shared mechanisms originating in utero74 that can be exacerbated by effects of perinatal inflammation on lactation and infant gastrointestinal function.75 Although i.p. cPAF did not impact fetal weight in late gestation or at 24 hours after birth, there was a modest fetal growth restriction when cPAF was administered i.u. This finding is consistent with a previous result in rats showing that intravenous infusion of cPAF from gd 14 to 21 was associated with decreases in fetal and placental weights.44,45 This apparent impact of prebirth exposure to cPAF on postnatal outcomes in surviving pups was not examined in previous studies.44,45
Importantly, the small-molecule TLR4 antagonist (+)-naltrexone was associated with the prevention of cPAF-induced preterm birth, and fetal and neonatal death, regardless of whether cPAF was delivered i.p. or i.u. The preventative action of (+)-naltrexone was achieved through the suppression of Il1β and Il6 synthesis, which act to accelerate uterine maturation and contractility, and to cause fetal inflammatory injury associated with late gestation and early postnatal demise.56,57,65,67 These results support earlier data indicating that TLR4 is important for sensing endogenous signals that initiate labor.21 Interestingly, (+)-naltrexone was associated with only moderate suppression of the cPAF-mediated induction of Ptgs2 expression in the myometrium, and did not alter its expression in the decidua. This finding is consistent with Tlr4 null mutation not affecting Ptgs2 expression, and further implicates signaling pathways other than TLR435,41 in cPAF-mediated up-regulation of this uterine activation gene. Importantly, however, the induction of Ptgs2 was insufficient to result in preterm birth, implying that Tlr4-driven factors other than cyclooxygenase-2 are required for uterine activation and contractile activity.
(+)-Naltrexone is the (+)-isomer of the opioid receptor antagonist (−)-naltrexone. Like the structurally related opioid receptor antagonist (−)-naloxone, (−)-naltrexone exerts a specific TLR4 antagonist activity76 and is used clinically for the treatment of drug and alcohol abuse. Both drugs are orally active and readily cross the blood–brain barrier.51 Most importantly, the chiral isomers (+)-naltrexone and (+)-naloxone do not exert opioid receptor antagonism, and thus do not inhibit the analgesic effects of opioids. Both (+)-naltrexone and (+)-naloxone bind to the LPS binding pocket of lymphocyte antigen 96 (MD2) to inhibit the TLR4–TIR-domain-containing adapter-inducing interferon-β–interferon-regulatory factor 3 signaling pathway, but not LPS-induced mitogen-activated protein kinase and NF-κB activation in vitro.51,52
The administration of (+)-naltrexone suppressed both i.p. and i.u. cPAF-induced preterm delivery, and substantially reduced the degree of fetal, neonatal, and postnatal death. These findings demonstrate that (+)-naltrexone protection of fetuses from precocious cPAF-induced inflammation in late gestation can result in normal development of offspring at least to weaning age. Indeed (+)-naloxone was more effective than Tlr4 null mutation in protecting uterine quiescence and fetal survival from cPAF-induced inflammation, although as mentioned four paragraphs above there may be confounding effects in the Tlr4−/− model.
cPAF-mediated induction of Il1b, Il6, and Il10 was reduced in the placenta, decidua, and myometrium even after just one dose of (+)-naltrexone. Suppression of Il1b was most pronounced, with only partial suppression of decidual and myometrial Il6 expression. Given the key role of Il1β in driving uterine activation and fetal injury,57 it is reasonable to infer that suppression of Il1β synthesis in the fetal and maternal tissues is the principal mechanism by which (+)-naltrexone inhibits the effects of cPAF, and protects mice from preterm delivery as well as fetal and neonatal death.
(+)-Naltrexone did not prevent all consequences of cPAF-induced fetal injury—notably, growth restriction induced by cPAF administration was not restored, suggesting that mechanisms independent of Tlr4 perpetuate cPAF-induced constraints on fetal growth. Growth impairment persisted after birth, where (+)-naltrexone failed to reverse the reduced weight at weaning in pups exposed to cPAF in utero. There was variability in the degree to which (+)-naltrexone protected against perinatal and postnatal loss after cPAF exposure, with postnatal death of pups in some litters despite mitigation of preterm birth. Additionally, (+)-naltrexone given on its own was associated with a small reduction in weaning weight, consistent with our observations that appropriate levels of endogenous Tlr4 signaling are required for optimal modulation of fetal growth.72
Previous studies utilized the small-molecule TLR4 antagonist (+)-naloxone to inhibit infection-induced preterm birth, and found a similar mechanism mediated through the suppression of Il1b, Il6, Tnf, and Il10 in gestational tissues.21 Preliminary experiments showed that in WT BALB/c mice, (+)-naltrexone was more efficient than was (+)-naloxone in preventing LPS-induced preterm birth (P.Y.C. and Camilla Dorian, unpublished data). On the other hand, treatment with (+)-naloxone did not affect the weaning weight of C57BL/6 pups.21,77 Further studies are required for understanding the strain-dependent effects of these drugs, and to identify the genetic factors that influence responsiveness. It will be also important to determine whether (+)-naltrexone is effective in preventing preterm delivery if administered with a delay after cPAF, as would more closely mimic the human clinical scenario.
The mechanisms by which TLR4 mediates responsiveness to cPAF are not clear, but likely involve TLR4 sensing of endogenous damage-associated molecular patterns released in response to PAF-induced injury in gestational tissues.22 In women and in mice, several endogenous TLR4 ligands are released during preterm labor, including surfactant proteins,78, 79, 80 high-mobility group box protein 1,81 and hyaluronan.82 In addition, there is evidence in other tissues linking PAF and TLR4 in a cross-regulatory network. Previous studies have shown that LPS signaling via TLR4 can alter PAF responsiveness in macrophages and epithelial cells,41 and conversely that PAFr signaling modulates the effects of LPS in macrophages, by attenuating the activation of NF-κB.50,83 The possibility of Tlr4-mediated induction of PAFr seemed likely based on the observation that peritoneal macrophages from Tlr4−/− mice have lower expression of Ptafr mRNA, either with or without treatment with cPAF.41 However, these data do not support Tlr4- or cPAF-mediated induction of Ptafr expression. In contrast, the finding of up-regulated Tlr4 in decidual tissue after cPAF administration is consistent with an effect of PAF inducing Tlr4 expression on decidual cells, or alternatively cPAF causing infiltration of leukocytes expressing high levels of Tlr4.
Overall, this investigation has demonstrated that cPAF administration induces preterm delivery in mice and affects the viability of fetuses and pups, and that these effects can be abrogated by treatment with the Tlr4 antagonist (+)-naltrexone to suppress Il1β and Il6 production. These results indicate that (+)-naltrexone, (+)-naloxone, or related small-molecule TLR inhibitors should be investigated as candidate pharmacologic interventions in human preterm labor. TLR4 inhibitors could have value as prophylactic agents, for example in high-risk women with PAF-inducing conditions such as smoking, drug use, and/or multiple pregnancy,46,47 or when TLR4 single-nucleotide polymorphisms associated with premature rupture of membranes are identified.84,85 TLR4 inhibitors have the advantage of targeting the upstream causes of the labor cascade, unlike agents such as prostaglandin inhibitors that do not suppress proinflammatory activity.86,87
Key considerations are the possible impact on the neonate of in utero exposure to TLR4 inhibitors, and their effects on fetal inflammatory injury,7 which may cause neurodevelopmental disability and other chronic health conditions,4 even when inflammation in utero does not progress to preterm labor.88 Clinical application of TLR inhibitors would require extensive investigation of the benefits and risks of pharmacologic delay of preterm birth to offspring, particularly effects on neurodevelopment, to ensure that exposure did not exacerbate inflammatory injury in utero. Recent studies on the long-term health and developmental outcomes of pups exposed to the TLR4 inhibitor (+)-naloxone are encouraging,72 but similar studies of (+)-naltrexone are required. Preclinical studies to explore the effects of these drugs on PAF-induced activation of inflammatory mediators in human uterus and placenta, and to define the relevant signaling pathways by which PAF signals in these tissues, are now warranted.
Footnotes
Supported by National Health and Medical Research Council of Australia project grant APP1140916, and the Intramural Research Programs of the National Institute on Drug Abuse and National Institute of Alcohol Abuse and Alcoholism.
Disclosures: None declared.
References
- 1.Slattery M.M., Morrison J.J. Preterm delivery. Lancet. 2002;360:1489–1497. doi: 10.1016/S0140-6736(02)11476-0. [DOI] [PubMed] [Google Scholar]
- 2.Blencowe H., Cousens S., Oestergaard M.Z., Chou D., Moller A.B., Narwal R., Adler A., Vera Garcia C., Rohde S., Say L., Lawn J.E. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162–2172. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 3.Blencowe H., Cousens S., Chou D., Oestergaard M., Say L., Moller A.B., Kinney M., Lawn J., Born Too Soon Preterm Birth Action Group Born too soon: the global epidemiology of 15 million preterm births. Reprod Health. 2013;10 Suppl 1:S2. doi: 10.1186/1742-4755-10-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saigal S., Doyle L.W. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371:261–269. doi: 10.1016/S0140-6736(08)60136-1. [DOI] [PubMed] [Google Scholar]
- 5.Goldenberg R.L., Culhane J.F., Iams J.D., Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lackritz E.M., Wilson C.B., Guttmacher A.E., Howse J.L., Engmann C.M., Rubens C.E., Mason E.M., Muglia L.J., Gravett M.G., Goldenberg R.L., Murray J.C., Spong C.Y., Simpson J.L., Preterm Birth Research Priority Setting Group A solution pathway for preterm birth: accelerating a priority research agenda. Lancet Glob Health. 2013;1:e328–e330. doi: 10.1016/S2214-109X(13)70120-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Romero R., Dey S.K., Fisher S.J. Preterm labor: one syndrome, many causes. Science. 2014;345:760–765. doi: 10.1126/science.1251816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keelan J.A. Pharmacological inhibition of inflammatory pathways for the prevention of preterm birth. J Reprod Immunol. 2011;88:176–184. doi: 10.1016/j.jri.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Elovitz M.A. Anti-inflammatory interventions in pregnancy: now and the future. Semin Fetal Neonatal Med. 2006;11:327–332. doi: 10.1016/j.siny.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Christiaens I., Zaragoza D.B., Guilbert L., Robertson S.A., Mitchell B.F., Olson D.M. Inflammatory processes in preterm and term parturition. J Reprod Immunol. 2008;79:50–57. doi: 10.1016/j.jri.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Romero R., Espinoza J., Goncalves L.F., Kusanovic J.P., Friel L.A., Nien J.K. Inflammation in preterm and term labor and delivery. Sem Fetal Neonatal Med. 2006;11:317–326. doi: 10.1016/j.siny.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lappas M., Rice G.E. Transcriptional regulation of the processes of human labor and delivery. Placenta. 2009;30 Suppl A:S90–S95. doi: 10.1016/j.placenta.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Wang H., Hirsch E. Bacterially-induced preterm labor and regulation of prostaglandin-metabolizing enzyme expression in mice: the role of Toll-like receptor 4. Biol Reprod. 2003;69:1957–1963. doi: 10.1095/biolreprod.103.019620. [DOI] [PubMed] [Google Scholar]
- 14.Liu H., Redline R.W., Han Y.W. Fusobacterium nucleatum induces fetal death in mice via stimulation of TLR4-mediated placental inflammatory response. J Immunol. 2007;179:2501–2508. doi: 10.4049/jimmunol.179.4.2501. [DOI] [PubMed] [Google Scholar]
- 15.Li L., Kang J., Lei W. Role of Toll-like receptor 4 in inflammation-induced preterm delivery. Mol Hum Reprod. 2010;16:267–272. doi: 10.1093/molehr/gap106. [DOI] [PubMed] [Google Scholar]
- 16.Kim Y.M., Romero R., Chaiworapongsa T., Kim G.J., Kim M.R., Kuivaniemi H., Tromp G., Espinoza J., Bujold E., Abrahams V.M., Mor G. Toll-like receptor-2 and -4 in the chorioamniotic membranes in spontaneous labor at term and in preterm parturition that are associated with chorioamnionitis. Am J Obstet Gynecol. 2004;191:1346–1355. doi: 10.1016/j.ajog.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 17.Romero R., Salafia C.M., Athanassiadis A.P., Hanaoka S., Mazor M., Sepulveda W., Bracken M.B. The relationship between acute inflammatory lesions of the preterm placenta and amniotic fluid microbiology. Am J Obstet Gynecol. 1992;166:1382–1388. doi: 10.1016/0002-9378(92)91609-e. [DOI] [PubMed] [Google Scholar]
- 18.Goldenberg R.L., Hauth J.C., Andrews W.W. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342:1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 19.Goncalves L.F., Chaiworapongsa T., Romero R. Intrauterine infection and prematurity. Ment Retard Dev Disabil Res Rev. 2002;8:3–13. doi: 10.1002/mrdd.10008. [DOI] [PubMed] [Google Scholar]
- 20.Robertson S.A., Wahid H.H., Chin P.Y., Hutchinson M.R., Moldenhauer L.M., Keelan J.A. Toll-like receptor-4: a new target for preterm labor pharmacotherapies? Curr Pharm Des. 2018;24:960–973. doi: 10.2174/1381612824666180130122450. [DOI] [PubMed] [Google Scholar]
- 21.Wahid H.H., Dorian C., Chin P.Y., Hutchinson M.R., Rice K.C., Olson D.M., Moldenhauer L.M., Robertson S.A. Toll-like receptor 4 is an essential upstream regulator of on-time parturition and perinatal viability in mice. Endocrinology. 2015;156:3828–3841. doi: 10.1210/en.2015-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piccinini A.M., Midwood K.S. DAMPening inflammation by modulating TLR signalling. Mediators Inflamm. 2010;2010:672395. doi: 10.1155/2010/672395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tselepis A.D., John Chapman M. Inflammation, bioactive lipids and atherosclerosis: potential roles of a lipoprotein-associated phospholipase A2, platelet activating factor-acetylhydrolase. Atheroscler Suppl. 2002;3:57–68. doi: 10.1016/s1567-5688(02)00045-4. [DOI] [PubMed] [Google Scholar]
- 24.Mendelson C.R., Montalbano A.P., Gao L. Fetal-to-maternal signaling in the timing of birth. J Steroid Biochem Mol Biol. 2017;170:19–27. doi: 10.1016/j.jsbmb.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toyoshima K., Narahara H., Furukawa M., Frenkel R.A., Johnston J.M. Platelet-activating factor. Role in fetal lung development and relationship to normal and premature labor. Clin Perinatol. 1995;22:263–280. [PubMed] [Google Scholar]
- 26.Chen X., Hyatt B.A., Mucenski M.L., Mason R.J., Shannon J.M. Identification and characterization of a lysophosphatidylcholine acyltransferase in alveolar type II cells. Proc Natl Acad Sci U S A. 2006;103:11724–11729. doi: 10.1073/pnas.0604946103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao L., Rabbitt E.H., Condon J.C., Renthal N.E., Johnston J.M., Mitsche M.A., Chambon P., Xu J., O'Malley B.W., Mendelson C.R. Steroid receptor coactivators 1 and 2 mediate fetal-to-maternal signaling that initiates parturition. J Clin Invest. 2015;125:2808–2824. doi: 10.1172/JCI78544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Billah M.M., Johnston J.M. Identification of phospholipid platelet-activating factor (1-0-alkyl-2-acetyl-sn-glycero-3-phosphocholine) in human amniotic fluid and urine. Biochem Biophys Res Commun. 1983;113:51–58. doi: 10.1016/0006-291x(83)90430-8. [DOI] [PubMed] [Google Scholar]
- 29.Hoffman D.R., Truong C.T., Johnston J.M. The role of platelet-activating factor in human fetal lung maturation. Am J Obstet Gynecol. 1986;155:70–75. doi: 10.1016/0002-9378(86)90081-5. [DOI] [PubMed] [Google Scholar]
- 30.Shukla S.D. Platelet-activating factor receptor and signal transduction mechanisms. FASEB J. 1992;6:2296–2301. doi: 10.1096/fasebj.6.6.1312046. [DOI] [PubMed] [Google Scholar]
- 31.Elovitz M.A., Wang Z., Chien E.K., Rychlik D.F., Phillippe M. A new model for inflammation-induced preterm birth: the role of platelet-activating factor and Toll-like receptor-4. Am J Pathol. 2003;163:2103–2111. doi: 10.1016/S0002-9440(10)63567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tetta C., Montrucchio G., Alloatti G., Roffinello C., Emanuelli G., Benedetto C., Camussi G., Massobrio M. Platelet-activating factor contracts human myometrium in vitro. Proc Soc Exp Biol Med. 1986;183:376–381. doi: 10.3181/00379727-183-42435. [DOI] [PubMed] [Google Scholar]
- 33.Sugano T., Narahara H., Nasu K., Arima K., Fujisawa K., Miyakawa I. Effects of platelet-activating factor on cytokine production by human uterine cervical fibroblasts. Mol Hum Reprod. 2001;7:475–481. doi: 10.1093/molehr/7.5.475. [DOI] [PubMed] [Google Scholar]
- 34.Alvi S.A., Brown N.L., Bennett P.R., Elder M.G., Sullivan M.H. Corticotrophin-releasing hormone and platelet-activating factor induce transcription of the type-2 cyclo-oxygenase gene in human fetal membranes. Mol Hum Reprod. 1999;5:476–480. doi: 10.1093/molehr/5.5.476. [DOI] [PubMed] [Google Scholar]
- 35.Sugano T., Nasu K., Narahara H., Kawano Y., Nishida Y., Miyakawa I. Platelet-activating factor induces an imbalance between matrix metalloproteinase-1 and tissue inhibitor of metalloproteinases-1 expression in human uterine cervical fibroblasts. Biol Reprod. 2000;62:540–546. doi: 10.1095/biolreprod62.3.540. [DOI] [PubMed] [Google Scholar]
- 36.Bjorkhem I., Diczfalusy U. Oxysterols: friends, foes, or just fellow passengers? Arterioscler Thromb Vasc Biol. 2002;22:734–742. doi: 10.1161/01.atv.0000013312.32196.49. [DOI] [PubMed] [Google Scholar]
- 37.Kim B.K., Ozaki H., Lee S.M., Karaki H. Increased sensitivity of rat myometrium to the contractile effect of platelet activating factor before delivery. Br J Pharmacol. 1995;115:1211–1214. doi: 10.1111/j.1476-5381.1995.tb15027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qiu Z.H., Leslie C.C. Protein kinase C-dependent and -independent pathways of mitogen-activated protein kinase activation in macrophages by stimuli that activate phospholipase A2. J Biol Chem. 1994;269:19480–19487. [PubMed] [Google Scholar]
- 39.Oka S., Arita H. Inflammatory factors stimulate expression of group II phospholipase A2 in rat cultured astrocytes. Two distinct pathways of the gene expression. J Biol Chem. 1991;266:9956–9960. [PubMed] [Google Scholar]
- 40.Cao Y., Stafforini D.M., Zimmerman G.A., McIntyre T.M., Prescott S.M. Expression of plasma platelet-activating factor acetylhydrolase is transcriptionally regulated by mediators of inflammation. J Biol Chem. 1998;273:4012–4020. doi: 10.1074/jbc.273.7.4012. [DOI] [PubMed] [Google Scholar]
- 41.Agrawal V., Jaiswal M.K., Ilievski V., Beaman K.D., Jilling T., Hirsch E. Platelet-activating factor: a role in preterm delivery and an essential interaction with Toll-like receptor signaling in mice. Biol Reprod. 2014;91:119. doi: 10.1095/biolreprod.113.116012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maul H., Shi L., Marx S.G., Garfield R.E., Saade G.R. Local application of platelet-activating factor induces cervical ripening accompanied by infiltration of polymorphonuclear leukocytes in rats. Am J Obstet Gynecol. 2002;187:829–833. doi: 10.1067/mob.2002.126983. [DOI] [PubMed] [Google Scholar]
- 43.Zhu Y.P., Hoffman D.R., Hwang S.B., Miyaura S., Johnston J.M. Prolongation of parturition in the pregnant rat following treatment with a platelet activating factor receptor antagonist. Biol Reprod. 1991;44:39–42. doi: 10.1095/biolreprod44.1.39. [DOI] [PubMed] [Google Scholar]
- 44.Thaete L.G., Neerhof M.G., Jilling T., Caplan M.S. Infusion of exogenous platelet-activating factor produces intrauterine growth restriction in the rat. J Soc Gynecol Investig. 2003;10:145–150. doi: 10.1016/s1071-5576(03)00005-4. [DOI] [PubMed] [Google Scholar]
- 45.Neerhof M.G., Khan S., Synowiec S., Qu X.W., Thaete L.G. The significance of endothelin in platelet-activating factor-induced fetal growth restriction. Reprod Sci. 2012;19:1175–1180. doi: 10.1177/1933719112443875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoffman D.R., Romero R., Johnston J.M. Detection of platelet-activating factor in amniotic fluid of complicated pregnancies. Am J Obstet Gynecol. 1990;162:525–528. doi: 10.1016/0002-9378(90)90423-5. [DOI] [PubMed] [Google Scholar]
- 47.Silver R.K., Caplan M.S., Kelly A.M. Amniotic fluid platelet-activating factor (PAF) is elevated in patients with tocolytic failure and preterm delivery. Prostaglandins. 1992;43:181–187. doi: 10.1016/0090-6980(92)90085-8. [DOI] [PubMed] [Google Scholar]
- 48.Longo L.D. The biological effects of carbon monoxide on the pregnant woman, fetus, and newborn infant. Am J Obstet Gynecol. 1977;129:69–103. doi: 10.1016/0002-9378(77)90824-9. [DOI] [PubMed] [Google Scholar]
- 49.Chen G.Y., Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soliman A., Michelsen K.S., Karahashi H., Lu J., Meng F.J., Qu X., Crother T.R., Rabizadeh S., Chen S., Caplan M.S., Arditi M., Jilling T. Platelet-activating factor induces TLR4 expression in intestinal epithelial cells: implication for the pathogenesis of necrotizing enterocolitis. PLoS One. 2010;5:e15044. doi: 10.1371/journal.pone.0015044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hutchinson M.R., Zhang Y., Brown K., Coats B.D., Shridhar M., Sholar P.W., Patel S.J., Crysdale N.Y., Harrison J.A., Maier S.F., Rice K.C., Watkins L.R. Non-stereoselective reversal of neuropathic pain by naloxone and naltrexone: involvement of Toll-like receptor 4 (TLR4) Eur J Neurosci. 2008;28:20–29. doi: 10.1111/j.1460-9568.2008.06321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang X., Zhang Y., Peng Y., Hutchinson M.R., Rice K.C., Yin H., Watkins L.R. Pharmacological characterization of the opioid inactive isomers (+)-naltrexone and (+)-naloxone as antagonists of Toll-like receptor 4. Br J Pharmacol. 2016;173:856–869. doi: 10.1111/bph.13394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Robertson S.A., Care A.S., Skinner R.J. Interleukin 10 regulates inflammatory cytokine synthesis to protect against lipopolysaccharide-induced abortion and fetal growth restriction in mice. Biol Reprod. 2007;76:738–748. doi: 10.1095/biolreprod.106.056143. [DOI] [PubMed] [Google Scholar]
- 54.Robertson S.A., Skinner R.J., Care A.S. Essential role for IL-10 in resistance to lipopolysaccharide-induced preterm labor in mice. J Immunol. 2006;177:4888–4896. doi: 10.4049/jimmunol.177.7.4888. [DOI] [PubMed] [Google Scholar]
- 55.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 56.Robertson S.A., Christiaens I., Dorian C.L., Zaragoza D.B., Care A.S., Banks A.M., Olson D.M. Interleukin-6 is an essential determinant of on-time parturition in the mouse. Endocrinology. 2010;151:3996–4006. doi: 10.1210/en.2010-0063. [DOI] [PubMed] [Google Scholar]
- 57.Nadeau-Vallee M., Quiniou C., Palacios J., Hou X., Erfani A., Madaan A., Sanchez M., Leimert K., Boudreault A., Duhamel F., Rivera J.C., Zhu T., Noueihed B., Robertson S.A., Ni X., Olson D.M., Lubell W., Girard S., Chemtob S. Novel noncompetitive IL-1 receptor-biased ligand prevents infection- and inflammation-induced preterm birth. J Immunol. 2015;195:3402–3415. doi: 10.4049/jimmunol.1500758. [DOI] [PubMed] [Google Scholar]
- 58.Frenkel R.A., Muguruma K., Johnston J.M. The biochemical role of platelet-activating factor in reproduction. Prog Lipid Res. 1996;35:155–168. doi: 10.1016/0163-7827(96)00002-1. [DOI] [PubMed] [Google Scholar]
- 59.Shimizu T. Lipid mediators in health and disease: enzymes and receptors as therapeutic targets for the regulation of immunity and inflammation. Annu Rev Pharmacol Toxicol. 2009;49:123–150. doi: 10.1146/annurev.pharmtox.011008.145616. [DOI] [PubMed] [Google Scholar]
- 60.Prescott S.M., Zimmerman G.A., McIntyre T.M. Platelet-activating factor. J Biol Chem. 1990;265:17381–17384. [PubMed] [Google Scholar]
- 61.Zimmerman G.A., McIntyre T.M., Prescott S.M., Stafforini D.M. The platelet-activating factor signaling system and its regulators in syndromes of inflammation and thrombosis. Crit Care Med. 2002;30:S294–S301. doi: 10.1097/00003246-200205001-00020. [DOI] [PubMed] [Google Scholar]
- 62.Maul H., Shi L., Marx S.G., Garfield R.E., Saade G.R. Platelet-activating factor antagonist WEB-2170 inhibits lipopolysaccharide-induced, but not antiprogestin-induced, preterm cervical ripening in timed-pregnant rats. Am J Obstet Gynecol. 2003;189:963–967. doi: 10.1067/s0002-9378(03)00722-1. [DOI] [PubMed] [Google Scholar]
- 63.Gomez-Lopez N., Vadillo-Perez L., Nessim S., Olson D.M., Vadillo-Ortega F. Choriodecidua and amnion exhibit selective leukocyte chemotaxis during term human labor. Am J Obstet Gynecol. 2011;204:364.e9–364.e16. doi: 10.1016/j.ajog.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 64.Erlebacher A., Zhang D., Parlow A.F., Glimcher L.H. Ovarian insufficiency and early pregnancy loss induced by activation of the innate immune system. J Clin Invest. 2004;114:39–48. doi: 10.1172/JCI20645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nadeau-Vallee M., Chin P.Y., Belarbi L., Brien M.E., Pundir S., Berryer M.H., Beaudry-Richard A., Madaan A., Sharkey D.J., Lupien-Meilleur A., Hou X., Quiniou C., Beaulac A., Boufaied I., Boudreault A., Carbonaro A., Doan N.D., Joyal J.S., Lubell W.D., Olson D.M., Robertson S.A., Girard S., Chemtob S. Antenatal suppression of IL-1 protects against inflammation-induced fetal injury and improves neonatal and developmental outcomes in mice. J Immunol. 2017;198:2047–2062. doi: 10.4049/jimmunol.1601600. [DOI] [PubMed] [Google Scholar]
- 66.Beaudry-Richard A., Nadeau-Vallee M., Prairie E., Maurice N., Heckel E., Nezhady M., Pundir S., Madaan A., Boudreault A., Hou X., Quiniou C., Sierra E.M., Beaulac A., Lodygensky G., Robertson S.A., Keelan J., Adams-Waldorf K., Olson D.M., Rivera J.C., Lubell W.D., Joyal J.S., Bouchard J.F., Chemtob S. Antenatal IL-1-dependent inflammation persists postnatally and causes retinal and sub-retinal vasculopathy in progeny. Sci Rep. 2018;8:11875. doi: 10.1038/s41598-018-30087-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Leimert K.B., Verstraeten B.S., Messer A., Nemati R., Blackadar K., Fang X., Robertson S.A., Chemtob S., Olson D.M. Cooperative effects of sequential PGF2alpha and IL-1beta on IL-6 and COX-2 expression in human myometrial cells. Biol Reprod. 2019;100:1370–1385. doi: 10.1093/biolre/ioz029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gomez-Lopez N., Olson D.M., Robertson S.A. Interleukin-6 controls uterine Th9 cells and CD8 T regulatory cells to accelerate parturition in mice. Immunol Cell Biol. 2016;94:79–89. doi: 10.1038/icb.2015.63. [DOI] [PubMed] [Google Scholar]
- 69.Murphy S.P., Hanna N.N., Fast L.D., Shaw S.K., Berg G., Padbury J.F., Romero R., Sharma S. Evidence for participation of uterine natural killer cells in the mechanisms responsible for spontaneous preterm labor and delivery. Am J Obstet Gynecol. 2009;200:308.e1–308.e9. doi: 10.1016/j.ajog.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Derricott H., Jones R.L., Greenwood S.L., Batra G., Evans M.J., Heazell A.E. Characterizing villitis of unknown etiology and inflammation in stillbirth. Am J Pathol. 2016;186:952–961. doi: 10.1016/j.ajpath.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 71.Xu Y., Romero R., Miller D., Kadam L., Mial T.N., Plazyo O., Garcia-Flores V., Hassan S.S., Xu Z., Tarca A.L., Drewlo S., Gomez-Lopez N. An M1-like macrophage polarization in decidual tissue during spontaneous preterm labor that is attenuated by rosiglitazone treatment. J Immunol. 2016;196:2476–2491. doi: 10.4049/jimmunol.1502055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chin P.Y., Dorian C., Sharkey D.J., Hutchinson M.R., Rice K.C., Moldenhauer L.M., Robertson S.A. Toll-like receptor-4 antagonist (+)-naloxone confers sexually dimorphic protection from inflammation-induced fetal programming in mice. Endocrinology. 2019;160:2646–2662. doi: 10.1210/en.2019-00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schjenken J.E., Glynn D.J., Sharkey D.J., Robertson S.A. TLR4 signaling is a major mediator of the female tract response to seminal fluid in mice. Biol Reprod. 2015;93:68. doi: 10.1095/biolreprod.114.125740. [DOI] [PubMed] [Google Scholar]
- 74.Boyle A.K., Rinaldi S.F., Norman J.E., Stock S.J. Preterm birth: inflammation, fetal injury and treatment strategies. J Reprod Immunol. 2017;119:62–66. doi: 10.1016/j.jri.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 75.Ley D., Desseyn J.L., Mischke M., Knol J., Turck D., Gottrand F. Early-life origin of intestinal inflammatory disorders. Nutr Rev. 2017;75:175–187. doi: 10.1093/nutrit/nuw061. [DOI] [PubMed] [Google Scholar]
- 76.Sziebert L., Thomson P.D., Jinkins J., Rice K., Adams T., Jr., Henriksen N., Traber L.D., Traber D.L. Effect of naloxone treatment on the cardiopulmonary response to endotoxin in sheep. Adv Shock Res. 1983;10:121–128. [PubMed] [Google Scholar]
- 77.Chin P.Y., Dorian C.L., Hutchinson M.R., Olson D.M., Rice K.C., Moldenhauer L.M., Robertson S.A. Novel Toll-like receptor-4 antagonist (+)-naloxone protects mice from inflammation-induced preterm birth. Sci Rep. 2016;6:36112. doi: 10.1038/srep36112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Condon J.C., Jeyasuria P., Faust J.M., Mendelson C.R. Surfactant protein secreted by the maturing mouse fetal lung acts as a hormone that signals the initiation of parturition. Proc Natl Acad Sci U S A. 2004;101:4978–4983. doi: 10.1073/pnas.0401124101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Montalbano A.P., Hawgood S., Mendelson C.R. Mice deficient in surfactant protein A (SP-A) and SP-D or in TLR2 manifest delayed parturition and decreased expression of inflammatory and contractile genes. Endocrinology. 2013;154:483–498. doi: 10.1210/en.2012-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ohya M., Nishitani C., Sano H., Yamada C., Mitsuzawa H., Shimizu T., Saito T., Smith K., Crouch E., Kuroki Y. Human pulmonary surfactant protein D binds the extracellular domains of Toll-like receptors 2 and 4 through the carbohydrate recognition domain by a mechanism different from its binding to phosphatidylinositol and lipopolysaccharide. Biochemistry. 2006;45:8657–8664. doi: 10.1021/bi060176z. [DOI] [PubMed] [Google Scholar]
- 81.Dubicke A., Andersson P., Fransson E., Andersson E., Sioutas A., Malmstrom A., Sverremark-Ekstrom E., Ekman-Ordeberg G. High-mobility group box protein 1 and its signalling receptors in human preterm and term cervix. J Reprod Immunol. 2010;84:86–94. doi: 10.1016/j.jri.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 82.Akgul Y., Holt R., Mummert M., Word A., Mahendroo M. Dynamic changes in cervical glycosaminoglycan composition during normal pregnancy and preterm birth. Endocrinology. 2012;153:3493–3503. doi: 10.1210/en.2011-1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ishizuka E.K., Filgueiras L.R., Rios F.J., Serezani C.H., Jancar S. PAFR activation of NF-kappaB p65 or p105 precursor dictates pro- and anti-inflammatory responses during TLR activation in murine macrophages. Sci Rep. 2016;6:32092. doi: 10.1038/srep32092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lorenz E., Hallman M., Marttila R., Haataja R., Schwartz D.A. Association between the Asp299Gly polymorphisms in the Toll-like receptor 4 and premature births in the Finnish population. Pediatr Res. 2002;52:373–376. doi: 10.1203/00006450-200209000-00011. [DOI] [PubMed] [Google Scholar]
- 85.Rey G., Skowronek F., Alciaturi J., Alonso J., Bertoni B., Sapiro R. Toll receptor 4 Asp299Gly polymorphism and its association with preterm birth and premature rupture of membranes in a South American population. Mol Hum Reprod. 2008;14:555–559. doi: 10.1093/molehr/gan049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Challis J.R., Sloboda D.M., Alfaidy N., Lye S.J., Gibb W., Patel F.A., Whittle W.L., Newnham J.P. Prostaglandins and mechanisms of preterm birth. Reproduction. 2002;124:1–17. doi: 10.1530/rep.0.1240001. [DOI] [PubMed] [Google Scholar]
- 87.Iams J.D. Prevention of preterm parturition. N Engl J Med. 2014;370:1861. doi: 10.1056/NEJMc1402822. [DOI] [PubMed] [Google Scholar]
- 88.Elovitz M.A., Brown A.G., Breen K., Anton L., Maubert M., Burd I. Intrauterine inflammation, insufficient to induce parturition, still evokes fetal and neonatal brain injury. Int J Dev Neurosci. 2011;29:663–671. doi: 10.1016/j.ijdevneu.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]