In response to the coronavirus 2019 (COVID-19) pandemic, an overwhelming number of clinical trials have been registered to test a variety of preventive and therapeutic strategies, as comprehensively summarized by Lythgoe and Middleton [1]. Under such an urgent circumstance, the quality of these clinical studies is inevitably of serious concern. Here, we propose applying prospective meta-analysis approaches to maximize their values, and to minimize research waste and bias of the ongoing and future COVID-19 trials.
Inadequate Preclinical Investigation Prior to Clinical Trials
In drug development, extensive preclinical studies are universally required to generate sufficient data regarding feasibility, safety, and efficacy, before launching clinical trials. However, this classical approach is not applicable for combating COVID-19 [1], because it is a new disease that only emerged in December 2019 and has already evolved into a global pandemic with an urgent and unmet clinical need. Current studies are mainly based on repurposing existing approved drugs or pipeline compounds. These agents have been shown to be effective in other disease indications that appear to share some similar pathophysiological pathways with COVID-19, and a few may have some evidence from cell culture models showing inhibition of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection [2], the causative agent of COVID-19.
Inadequate preclinical studies impose a high risk of failure in clinical trials. For example, sofosbuvir, targeting hepatitis C virus RNA-dependent RNA polymerase (RdRp), was shown to inhibit hepatitis E virus (HEV) in cell culture model by one experimental study [3]. This immediately triggered clinical application for treating HEV cases, generating inconclusive and controversial results [4]. A follow-up clinical trial to test efficacy demonstrated that sofosbuvir was unable to cure any patients with chronic HEV infection [5]. Recent in silico molecular docking has indicated that sofosbuvir may inhibit RdRp of SARS-CoV-2 [6]. Without further extensive preclinical studies, sofosbuvir has already entered clinical trials for treating COVID-19 [1]. Widening testing of experimental therapies in patients with COVID-19 which have not been concretely evaluated will likely produce contradictory results.
Challenges for Conducting High Quality COVID-19 Clinical Trials
The COVID-19 pandemic has put great pressure on healthcare workers and regulatory authorities to swiftly make treatment available [7]. Conducting clinical trials during this crisis is a heroic but difficult task, because clinicians have to provide patient care while they themselves are at risk of encountering infection. A substantial proportion of the registered COVID-19 interventional clinical trials are nonrandomized with small patient sizes, and many are observational studies [1]. Furthermore, the local epidemics associated with the pandemic are highly dynamic. Once the outbreak is under control locally, there might not be sufficient patients to be enrolled for ongoing studies in the region. For instance, two remdesivir trials in China (Clinical Trial Numberi: NCT04252664 and NCT04257656) were halted due to lack of patients with COVID-19ii , iii.
While currently it is probably not feasible to prevent the cutting of corners in these expedited clinical trials in the middle of the pandemic, the key question is how the results from these studies of suboptimal quality can be best utilized to generate reliable conclusions.
Unique Opportunities and Advantages of Prospective Meta-Analysis
For evidence-based healthcare, systematic reviews and meta-analyses comprehensively summarize data from multiple resources, and are positioned at the top of the evidence hierarchy [8]. However, traditional systematic reviews and meta-analyses only retrospectively include published studies. Given that positive results are more likely to be published, this process then bears the high risk of selection and publication biases. With respect to COVID-19, most of the registered trials are still ongoing and few have been published [1]. Even after completion of these trials, there will be time lags for the peer-review and publication processes, even though preprint servers have greatly facilitated the speed at which COVID-19 research data are being shared.
By contrast, prospective meta-analyses, as a newly developed methodology, predefine eligible studies for inclusion before the results of those studies became known, to objectively address the planned research questions [9]. This process restricts its application to only high priority research questions for which little or no previous evidence exists, but where new studies are rapidly emerging. This perfectly fits the context of ongoing and upcoming COVID-19 clinical trials. There are a large number of ongoing studies in parallel evaluating, for example, antiviral drugs, such as remdesivir, lopinavir/ritonavir, favipiravir, or interferon alpha, and the antimalarial drugs chloroquine and hydroxychloroquine [1]. Given that many studies have small patient sizes or have difficulties in recruiting the targeted number, these individual studies will be underpowered to address the main clinical questions, especially when the effects are moderate. We propose that when several trials are investigating the same treatment or intervention for patients with COVID-19 with compatible study designs and outcome measures, these studies should be pooled to form a collaboration or consortium of prospective meta-analysis (Figure 1 ). For example, there are five randomized trials comparing remdesivir with standard treatment (Clinical Trial Number: NCT04292899, NCT04292730, and NCT04315948; Eudra CT Numberiv: 2020-000841-15 and 2020-000842-32) [1]. These studies can be considered to form a prospective meta-analysis consortium. Similarly, this approach can also be applicable to the trials comparing hydroxychloroquine with standard treatment (Clinical Trial Number: NCT04315948, NCT04261517, and NCT04316377 and Chinese Clinical Trail Registryv: ChiCTR2000030054, ChiCTR2000029868, and ChiCTR2000029740) [1]. This will likely enhance the statistical power to reliably detect the targeted effects or other clinical outcomes, and to avoid unnecessary biases.
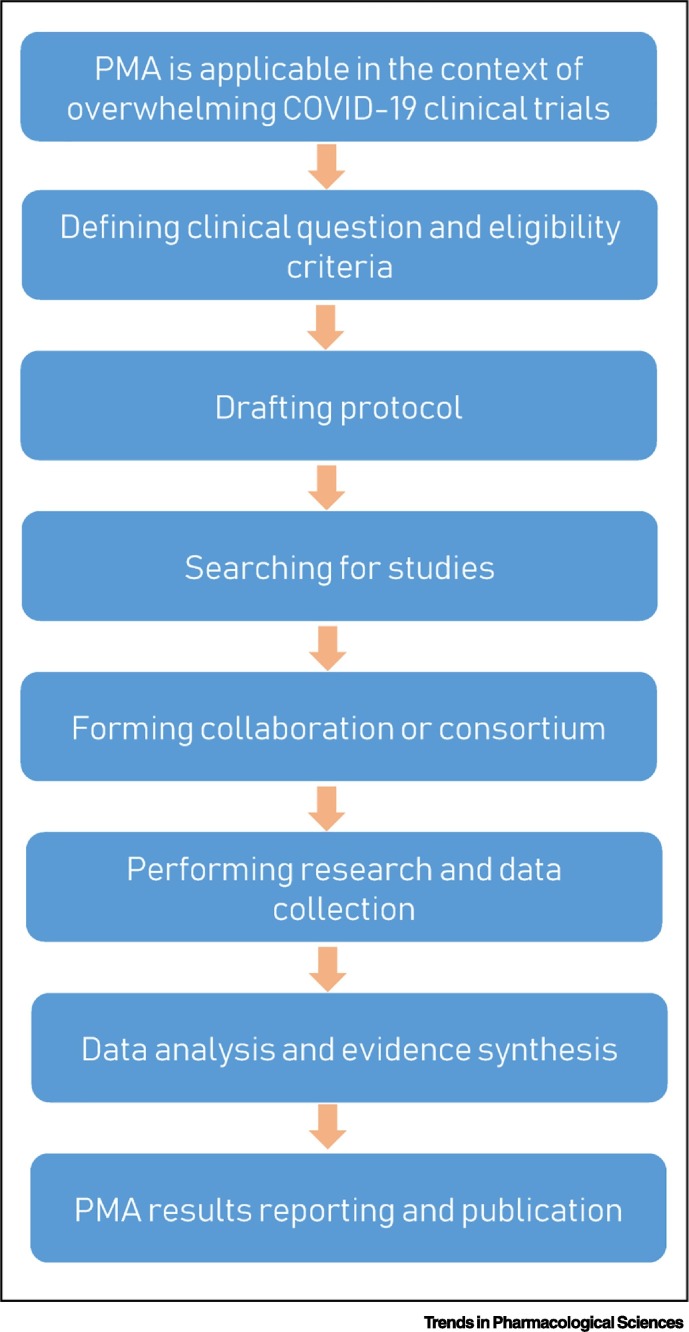
Figure 1.
A Workflow for Conducting Prospective Meta-Analyses (PMAs) in the Context of Overwhelming Coronavirus 2019 (COVID-19) Clinical Trials.
Prospectively forming a collaboration or consortium of PMA to pool multiple eligible studies that aim to address the same clinical question will likely generate reliable data for guiding clinical management and regulatory decision-making. This will not affect the individual studies and will not prevent the publication of the results of those individual studies.
In summary, because of the nature of COVID-19 and the global emergency, many ongoing clinical studies are of suboptimal quality [1]. Prospective meta-analysis can serve as an innovative solution to generate reliable data for guiding clinical management and regulatory decision-making [9]. However, the success of this approach requires deep understanding of the principle and methodology of the prospective meta-analysis, significant efforts to organize the consortium, and the solidarity that individual investigators will be willing to share their own data.
Acknowledgements
The work was supported by the Ministry of Education of China for an Innovative Research Team in University grant (No. IRT_17R88; to Z.M.).
Resources
ihttps://clinicaltrials.gov/iiwww.globaltimes.cn/content/1185827.shtmliiihttps://medcitynews.com/2020/04/two-chinese-studies-of-gileads-covid-19-drug-halted-for-lack-of-patients/ivwww.clinicaltrialsregister.eu/vwww.chictr.org.cn/References
- 1.Lythgoe M.P., Middleton P. Ongoing clinical trials for the management of the COVID-19 pandemic. Trends Pharmacol. Sci. 2020;41:363–382. doi: 10.1016/j.tips.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang M. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dao Thi V.L. Sofosbuvir inhibits hepatitis E virus replication in vitro and results in an additive effect when combined with ribavirin. Gastroenterology. 2016;150:82–85. doi: 10.1053/j.gastro.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Kamar N. Direct-acting antiviral therapy for hepatitis E virus? Lancet Gastroenterol. Hepatol. 2017;2:154–155. doi: 10.1016/S2468-1253(16)30242-4. [DOI] [PubMed] [Google Scholar]
- 5.Cornberg M. Efficacy and safety of sofosbuvir monotherapy in patients with chronic hepatitis E-The HepNet SofE pilot study. J. Hepatol. 2019;70:E129–E130. doi: 10.1016/j.jhep.2020.05.020. [DOI] [PubMed] [Google Scholar]
- 6.Elfiky A.A. Anti-HCV, nucleotide inhibitors, repurposing against COVID-19. Life Sci. 2020;248:117477. doi: 10.1016/j.lfs.2020.117477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ji Y. Potential association between COVID-19 mortality and health-care resource availability. Lancet Glob. Health. 2020;8 doi: 10.1016/S2214-109X(20)30068-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paul M., Leibovici L. Systematic review or meta-analysis? Their place in the evidence hierarchy. Clin. Microbiol. Infect. 2014;20:97–100. doi: 10.1111/1469-0691.12489. [DOI] [PubMed] [Google Scholar]
- 9.Seidler A.L. A guide to prospective meta-analysis. BMJ. 2019;367:l5342. doi: 10.1136/bmj.l5342. [DOI] [PubMed] [Google Scholar]