Abstract
SARS-Coronavirus-2 (SARS-CoV-2) causes Coronavirus disease 2019 (COVID-19), an infectious respiratory disease causing thousands of deaths and overwhelming public health systems. The international spread of SARS-CoV-2 is associated with the ease of global travel, and societal dynamics, immunologic naiveté of the host population, and muted innate immune responses. Based on these factors and the expanding geographic scale of the disease, the World Health Organization (WHO) declared the COVID-19 outbreak a pandemic–the first caused by a coronavirus. In this review, we summarize the current epidemiological status of COVID-19 and consider the virological and immunological lessons, animal models, and tools developed in response to prior SARS-CoV and MERS-CoV outbreaks that can serve as resources for development of SARS-CoV-2 therapeutics and vaccines. In particular, we discuss structural insights into the SARS-CoV-2 spike protein, a major determinant of transmissibility, and discuss key molecular aspects that will aid in understanding and fighting this new global threat.
Keywords: coronavirus, COVID-19, SARS-CoV-2, pandemic, spike, host immune response
Highlights
A substantial body of scientific knowledge has been established from studies on the related SARS- and MERS-CoVs, and their respective diseases. These lessons have started to guide SARS-CoV-2 studies, therapeutics, and vaccinology.
The Spike (S) protein is key to CoV infection and pathogenesis. SARS-CoV-2 S protein shows a stronger binding affinity for the host ACE2 receptor and is uniquely cleaved by furin. Some existing monoclonal antibodies against SARS-CoV S protein show cross-reactivity to SARS-CoV-2, raising their therapeutic potential against COVID-19.
Immunodominant epitopes identified in SARS-CoV are highly conserved in SARS-CoV-2 and have a high potential to elicit functional T cell responses.
Viral inactivation of type I interferon responses contributes to imbalanced host cytokine/chemokine responses that lead to SARS- and MERS-CoV infections and immunopathogenesis.
The COVID-19 Pandemic to Date
From Emergence in Wuhan to Global Pandemic
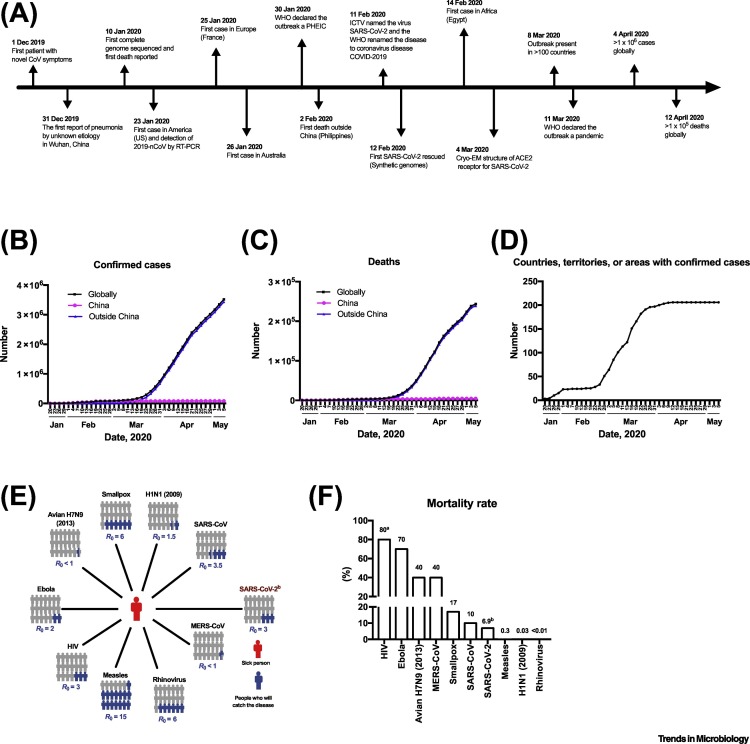
In December 2019, a novel human coronavirus (HCoV) was identified as the causative agent of clusters of pneumonia in China. The virus was named as SARS-CoV-2, based on its phylogenetic and taxonomic similarity to SARS-CoV [1], which caused an outbreak of severe acute respiratory syndrome (SARS) in 2002. The WHO named the disease caused by SARS-CoV-2 as coronavirus disease 2019 (COVID-19). The first confirmed SARS-CoV-2 case was found in Wuhan, China (Figure 1A). Subsequently, medical workers and family clusters who had not visited Wuhan tested positive for SARS-CoV-2, thus confirming human-to-human transmission [2]. SARS-CoV-2 rapidly spread to most countries and has resulted in thousands of fatalities (Figure 1B–D). WHO declared a pandemic on 11 March 2020. As of 5 May 2020, over 3 500 000 cases have been confirmed in over 185 countries, with over 243 000 deaths, suggesting a case fatality rate (CFR) of 6.9% (WHO report 106) (Figure 1B–D). However, nominal CFR are strongly influenced by the extent of testing of suspected cases and under-testing can result in higher apparent CFR. In this review, we summarize current progress in epidemiology and detection methods for SARS-CoV-2 and discuss findings concerning general characteristics of pathogenic coronaviruses. These studies form the foundation for future efforts to develop vaccines and pre- and postexposure therapeutics.
Figure 1.
Timeline of Major Events for Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2), Total Numbers of Coronavirus Disease 2019 (COVID-19) Cases, and Basic Reproduction Number (R0) and Mortality Rate of Select Viruses.
(A) Timeline of events, (B) cumulative confirmed cases, (C) reported deaths, and (D) countries with reported cases. Squares indicates the total number of COVID-19 cases reported worldwide. Circles and triangles indicate the number of COVID-19 cases reported in China and in other countries, respectively. Data from WHO as of May 5 2020. (E) R0 value indicates the number of people (colored in blue) infected from one contagious person (colored in red). (F) Case fatality rate (CFR) of select viruses. aCFR is based on patients not receiving therapy. bData on SARS-CoV-2 are derived from WHO. Abbreviations: Cryo-EM, Cryogenic electron microscopy; ICTV, International Committee on Taxonomy of Viruses; PHEIC, public health emergency of international concern; WHO, World Health Organization.
Transmissibility of SARS-CoV-2
The spread of an infectious disease is dependent on the transmissibility of the causative pathogen. The basic reproduction number, R 0, is used to measure the potential transmission of a disease and is defined as the average number of people who will catch a disease from one contagious person [3]. A higher CFR is generally associated with lower transmissibility (Figure 1E,F) [4]. Although COVID-19 has a lower mortality risk than SARS and Middle East respiratory syndrome (MERS), its rapid spread and higher R0 of ~3 contributed to the WHO designation of COVID-19 as a pandemic. The transmission of SARS-CoV-2 from infected, yet asymptomatic, carriers has been reported [5]. A metapopulation model simulation estimated that the transmission rate by asymptomatic persons or persons with mild symptoms is 55% [6], which adds to the difficulties in preventing transmission of SARS-CoV-2.
Clinical Manifestations of COVID-19
The most common symptoms of COVID-19 are fever (89.5%), cough (73.4%), dyspnea (38.5%), and myalgia (31.3%), similar to SARS and MERS (Table 1 ). The median time from disease onset to dyspnea in COVID-19 is 8 days (range, 5–13 days) [7]. Another study of 291 patients with COVID-19 for whom the exposure date was known showed that the median incubation period was 4 days, with an interquartile range of 2–7 [8].
Table 1.
Clinical Features of COVID-19, SARS, and MERS
| Symptom | COVID-19 |
SARS |
MERS |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Studya |
|||||||||
| 1 (n = 62) [91] |
2 (n = 1099) [8] |
3 (n = 138) [92] |
4 (n = 140) [9] |
5 (n = 41) [7] |
6 (n = 99) [10] |
Range (average) | 7 Range [93] |
8 Range [94] |
|
| Fever | 77.4 | 88.7 | 98.6 | 91.7 | 97.6 | 82.8 | 77.4–98.6 (89.5) | 99–100 | 98 |
| Cough | 80.6 | 67.8 | 59.4 | 75.0 | 75.6 | 81.8 | 67.8–86.2 (73.4) | 62–100 | 83 |
| Dyspnea | – | – | 31.2 | 36.7 | 55.0 | 31.3 | 31.2–55.0 (38.5) | – | – |
| Myalgia | 51.6 | 14.9 | 34.8 | – | 43.9 | 11.1 | 11.1–51.6 (31.3) | 45–61 | 32 |
| Panting | 3.2 | – | – | – | 29.3 | – | 3.2–29.3 (16.2) | – | – |
| Headache | 34.4 | 13.6 | 6.5 | – | 7.9 | 8.1 | 6.5–34.4 (14.1) | 20–56 | 11 |
| Sore throat | – | 13.9 | 17.4 | – | – | 5.1 | 5.1–17.4 (12.1) | 13–25 | 14 |
| Nausea/vomiting | – | 5.0 | 6.3-10 | 22.3 | – | 1.0 | 2.0–22.3 (9.4) | 20–35 | 21 |
| Diarrhea | 4.8 | 3.8 | 10.1 | 12.9 | 2.6 | 2.0 | 2.0–12.9 (6.1) | 20–25 | 26 |
| Rhinorrhea | – | – | – | – | – | 4.0 | 4.0 (4.0) | 2–24 | 6 |
| Abdominal pain | – | – | 2.2 | 5.8 | – | – | 2.2–5.8 (4.0) | – | – |
Percentage of study subjects that experienced the indicated symptom; for fields without values, this symptom was not evaluated.
In patients having confirmed SARS-CoV-2 infection, 15–42% have severe symptoms and some progress to acute respiratory distress syndrome (ARDS), which can be fatal [8,9]. COVID-19 is suggested to cause more severe illness in older people and patients with underlying diseases, such as hypertension, cardiovascular disease, or diabetes [7,10]. The CFR increased considerably among patients aged between 60 and 80 years (30%) and reached 36% in patients older than 80 years of age [11].
SARS-CoV-2 can also infect younger individuals, including children. Among 731 individuals younger than 15 years of age with confirmed SARS-CoV-2 infection, 12.9% were asymptomatic, 84% had mild to moderate disease symptoms, and <3% of cases had severe or life-threatening symptoms [12]. However, SARS-CoV-2 infection in children can still be fatal. [13].
Interspecies Transmission of CoVs
CoVs have been found to infect humans and a wide variety of domestic and wild vertebrates [14]. Most CoV infections in animals cause mild to severe gastrointestinal or respiratory infections [14]. In humans, CoV infections also cause respiratory illnesses. Four human CoVs, HCoV 229E, OC43, NL63, and HKU1 are associated with the common cold and have low morbidity [14].
In 2003, the SARS outbreak drew extensive attention to HCoVs. Fever is the most common clinical sign for SARS-CoV infection [14] and lower respiratory tract symptoms develop several days after disease onset. Of patients infected with SARS-CoV, 10–20% progressed to respiratory failure after initial symptoms failed to resolve [14]. During the SARS outbreak there were 8096 reported cases worldwide and the CFR was 10% (Figure 1F). The human-to-human transmission of SARS-CoV was controlled by public health measures shortly after the virus emerged and no infections in humans have been reported since 2004. Another HCoV-associated respiratory disease caused by MERS-CoV was identified in 2012. Similar to SARS-CoV, the clinical signs of MERS-CoV infection include fever, cough, and/or shortness of breath. To date, there have been 2494 laboratory-confirmed MERS-CoV cases, with a 40% CFR (Figure 1F).
Evolution in Host Species and Genetics of Virus–Host Shifts
RNA viruses accumulate substitutions in their genomes due to the low fidelity of viral RNA polymerases [15]. A typical mutation rate of 1 in 104 results in quasispecies diversity that can promote viral adaptation and potentially virulence [16]. CoVs undergo substitutions/mutations that drive CoV evolution [17]. Recombination events also provide another opportunity for the acquisition or modification of genes by CoVs. Thus, genomic RNA of CoVs can be modified via several pathways to promote rapid evolution and the ability to spill over into new host species [17].
Genetic studies revealed molecular evidence indicating that SARS-CoV likely originated from bats, with civet cats as an intermediate host [17]. MERS-CoV, however, was found to be transmitted to humans via camels [18]. Genetic studies indicate that SARS-CoV-2 likely originated from a bat CoV [19]. Current reports demonstrated that CoVs from pangolin showed the highest homology with SARS-CoV-2 in the receptor binding domain in S protein [20,21] and suggest that the pangolin could be an intermediate host.
Genomic Comparison of SARS-CoV-2 with SARS-CoV and MERS-CoV
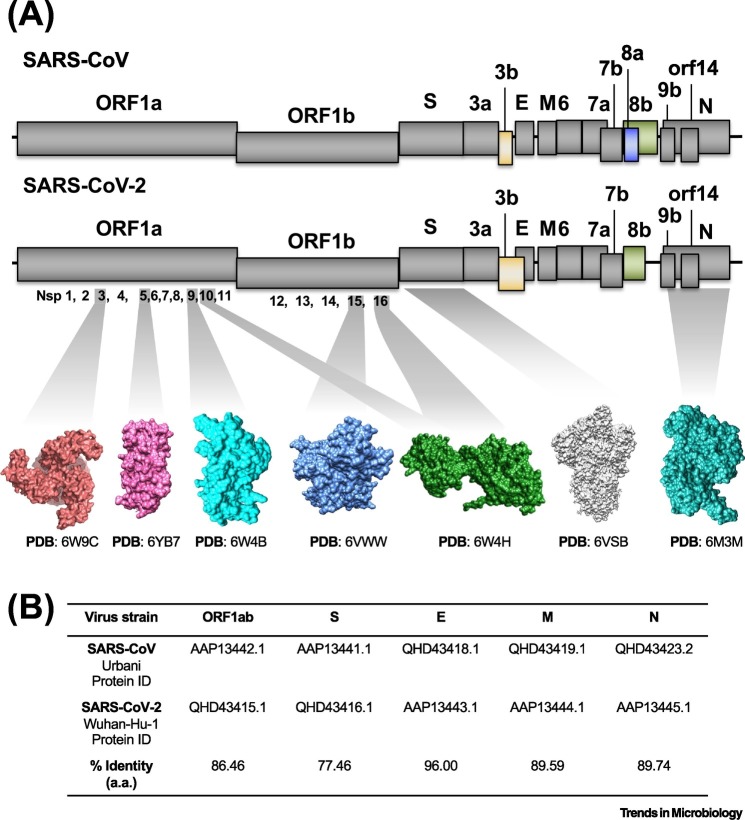
The SARS-CoV-2 genome is 29 903 nucleotides, which contains 14 open reading frames (ORFs) [22] (Figure 2A). ORF1a and 1b encode the polyproteins pp1a and pp1ab, the latter through a ribosomal frameshifting mechanism at the 1a-1b gene boundary. These polyproteins are cleaved by viral proteases into 16 nonstructural proteins (nsp). Four ORFs encode structural proteins such as the spike (S), envelope (E), membrane (M), and nucleocapsid (N) genes. Between these structural genes, a series of accessory genes encode accessory proteins, which regulate infection but do not incorporate into the virion (ORFs 3a, 3b, 6, 7a, 7b, 8b, 9b, and 14) (Figure 2A).
Figure 2.
Genomic Distribution of Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2) and SARS-CoV.
(A) The genomes of SARS-CoV (upper panel) and SARS-CoV-2 (lower panel) are shown as lines and the open reading frames (ORFs) are represented by gray and colored boxes to indicate those that have similar and different lengths, respectively. The atomic structure for some of the SARS-CoV-2 proteins is shown in surface representation; main protease 3CLpro or nonstructural protein 5 (nsp5) with unliganded active site in pink, nsp9 RNA binding protein in cyan, nsp15 endoribonuclease in marine blue, nsp16–nsp10 complex in green, prefusion spike glycoprotein in gray, and nucleocapsid protein N terminal RNA binding domain in deep turquoise. For visual clarity, the length of the boxes is not proportional to the real sequence length and the atomic structures are not proportional to their molecular weight. (B) Percentage identity matrix for the alignment of SARS-CoV and SARS-CoV-2 amino acids. Abbreviations: PDB, Protein Data Bank.
The SARS-CoV-2 genome shares 79% and 50% sequence identity with SARS-CoV and MERS-CoV genome, respectively [19]. The gene arrangement of SARS-CoV-2 is similar to SARS-CoV, with some variations (Figure 2A). The viroporin 8a protein is present in SARS-CoV but absent in SARS-CoV-2. This protein was also lost during the SARS pandemic in 2003, demonstrating that it is not essential for the virulence [23]. Meanwhile, the 8b protein is 17 amino acids longer in SARS-CoV-2 than in SARS-CoV and the 3b protein of SARS-CoV-2 is only 22 amino acids as compared with 154 amino acids for SARS-CoV.
Among the structural proteins E, M, N, and S, the E protein has the highest similarity between SARS-CoV-2 and SARS-CoV (96% identity) (Figure 2B). The sequence conservation of E could be due to its critical role in the virus life cycle and, as a transmembrane protein, E is relatively protected from immune surveillance [24]. The S glycoprotein of SARS-CoV-2 has the largest sequence divergence (76% identity with SARS-CoV) [25], which likely reflects increased immune pressure. Indeed, the SARS-CoV-2 S protein has 380 amino acid sequence substitutions compared with other SARS-like viruses [26].
CoV Life Cycle
The CoV life cycle (Figure 3 ) begins with interactions between S on the virion surface and specific virus receptors. HCoV-NL63 [27], SARS-CoV [28], and SARS-CoV-2 use angiotensin converting enzyme 2 (ACE2) as a receptor [29]. The entry of the virus is through receptor-mediated endocytosis, followed by fusion of viral and host cell membranes [30]. A fusion event also occurs between viral particles and the plasma membrane on the cell surface [31]. Exposure to low pH in the endosome activates host proteases, such as cathepsin L and TMPRSS2, that cleave the S protein [30]. This cleavage induces a conformational change in the S protein that promotes fusion between virus and cell membranes and subsequent release of viral genomic RNA into the cytoplasm. Viral genomic RNA serves as an mRNA for translation [32]. Two viral proteases, nsp3 (PLpro) and nsp5 (3CLpro), cleave the polyproteins that comprise mature nsps [32]. nsp3, nsp4, and nsp6 also modify the endoplasmic reticulum (ER) membrane to yield unique membrane structures termed double-membrane vesicles (DMVs) [33., 34., 35.]. Viral RNA transcription is carried out in the DMV, where viral RNAs are protected from host pattern recognition receptors [33]. The N protein interacts with genomic RNA to form the ribonucleoprotein complex [36], which is recruited to the viral assembly site, the ER–Golgi intermediate compartment where viral particles are formed [37]. Following viral assembly, the newly formed viral particles are transported to the cell surface in vesicles and released by exocytosis [30].
Figure 3.
Coronavirus (CoV) Life Cycle and Host Immune Response.
The CoV life cycle initiates with the binding of spike proteins on the virion surface to their specific receptor [e.g., ACE2 for severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2)] on target cells. Following receptor binding and endocytosis, S protein undergoes a conformational change and viral genomic RNA is released into the cytoplasm. Viral genomic RNA functions as mRNA, which is translated into polyproteins (pp) pp1a and pp1ab, which are then proteolytically cleaved into mature nonstructural proteins (nsps). Many nsps synergize to modify the endoplasmic reticulum (ER) membrane to form double membrane vesicles (DMVs) where transcription of genomic and subgenomic RNA occurs. The truncated subgenomic RNA of the 5′-ends are used as templates to translate structural (S, E, M, and N) and accessory proteins. S, E, and M assemble together with nucleocapsid (one copy of viral genome encapsulated by N proteins) at the ER–Golgi intermediate compartment (ERGIC) and viral progeny are released by exocytosis. Host immune responses are triggered by danger signals from infected cells or free virions, which are recognized by innate immune cells. Elevated proinflammatory cytokines and chemokines can be seen in asymptomatic to mild cases. Exuberated cytokine production resulting in cytokine storm exacerbates the severity of coronavirus disease 2019 (COVID-19). Lymphopenia (T and B cells) and neutrophil infiltration into infected sites contribute to the pathogenesis of COVID-19. Several CoV proteins have been reported to be capable of inhibiting the type I IFN signaling pathway. Figure created with BioRender (biorender.com).
Structural Insights into SARS-CoV-2 S-Receptor and -Antibody Interactions
Unique Furin Cleavage Site in the SARS-CoV-2 S Protein
The SARS-CoV-2 S protein has 1273 amino acids and can be divided in two domains, S1 and S2 (Figure 4A). S1 contains the receptor-binding domain (RBD) and facilitates attachment of the virus to host cells. S2 drives fusion of viral and host membrane and contains the fusion peptide [38], two heptad repeat regions, and the transmembrane domain that anchors S in the viral membrane [39]. The SARS-CoV-2 S protein trimerizes to form a metastable prefusion spike [Protein Data Bank (PDB): 6vsb] [40]; Figure 4A) in which S1 stabilizes S2, including the fusion peptide. Under this conformation, SARS-CoV-2 S1 and S2 domains are separated by a flexible loop containing a cleavage site that is exposed and accessible to host proteases (Figure 4A). Cleavage triggers irreversible conformational changes that are required for membrane fusion to occur [31,41,42].
Figure 4.
Atomic Structure of Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2) Receptor-Binding Domain (RBD).
(A) Schematic architecture of the SARS-CoV-2 spike glycoprotein. Degree of protein surface conservation between trimeric SARS-CoV-2 and SARS-CoV spike protein. A color range is shown with green and magenta representing not conserved and highly conserved, respectively. The atomic position of the cleavage site is indicated by an arrow. (B) Cartoon representation of a structural alignment of the SARS-CoV (gray) and SARS-CoV-2 (green) RBDs interacting with ACE2 receptor (orange) shown in surface representation. Corresponding footprints of SARS-CoV RBD and SARS-CoV-2 RBD overlaid on the ACE2 receptor are colored in gray and green, respectively, to illustrate overlap between both interaction sites. A differential loop between SARS-CoV-2 and SARS-CoV is indicated with a dashed circle. (C) Closed and opened conformation of the SARS-CoV-2 S with one of the RBD domains buried or exposed, respectively (yellow). Abbreviations: FP, Fusion peptide; HR, heptad repeat regions; RRAR, unique furin cleavage site; SP, signal peptide; TM, transmembrane domain.
Both SARS-CoV-2 S and SARS-CoV S are activated by enzymatic cleavage at two sites: S1/S2 and S2′. The S2′ cleavage sites are similar, but the S1/S2 cleavage sites differ. The earlier SARS-CoV encodes a single arginine [43] while SARS-CoV-2 encodes PRRAR [44]. Both viral S proteins are primed by the serine protease TMPRSS2 in human cells [29], while SARS-CoV-2 can additionally be cleaved by furin at its unique RRAR site. It has been hypothesized that furin-mediated precleavage at the S1/S2 site is important for subsequent S activation by TMPRSS2, as demonstrated previously for other CoVs [29,31]. The proline inserted before the RRAR cleavage site could promote addition of O-linked glycans [45]. However, the importance of this proline and any associated glycans are not yet understood. The S2′ cleavage site for SARS-CoV and SARS-CoV-2 (Arg 797 and Arg 815, respectively) is further required to mediate membrane fusion and entry [41]. Replication-defective vesicular stomatitis virus particles displaying either SARS-CoV-2 or SARS-CoV S proteins can infect the same range of cell lines, suggesting that the addition of a new cleavage site and proline do not change virus tropism [29].
SARS-CoV-2-S and ACE2 Interaction
The ability of SARS-CoV to infect a variety of species is linked to changes in the RBDs that affect ACE2 binding activity [46]. The RBDs of SARS-CoV-2 and SARS-CoV are 76% identical [47] and structurally very similar (Figure 4B), with similar binding interfaces between ACE2 and SARS-CoV (PDB: 2AJF) [46] and SARS-CoV-2 (Figure 4B) (PDB: 6M17) [48], however, the binding affinity of SARS-CoV-2 for ACE2 is 10–20-fold higher than SARS-CoV [40]. Structural studies captured the SARS-CoV-2 RBD in two different conformations: ‘opened’ when the RBDs are exposed and ready for the interaction with the receptor and ‘closed’ when the RBDs are buried in the interacting interface of the protomers and not accessible to the receptor [49] (Figure 4C).
Neutralizing Antibodies Compete for Binding at the RBD
The S glycoprotein is the main target for the protective humoral immune response (Figure 3). Antibodies against S are predicted to neutralize infection by blocking ACE2 binding to the RBD [50]. The crystal structure of SARS-CoV S RBD in complex with the human monoclonal antibody 396 (m396) illustrated that the antibody footprint overlaps with that of the receptor on the RBD [51]. m396 does not crossreact to SARS-CoV-2 since two critical residues, Ile 489 and Tyr 491, that are key to m396 binding, are not conserved. However, crossreactivity is noted for antibody CR3022 [52] and antibody 47D11 that target highly conserved epitopes in the RBD [53].
Host Immune Responses to SARS-CoV and MERS-CoV
Innate Immunity
Innate immunity serves as the first line of virus clearance and initiates adaptive immunity via cytokine/chemokine secretion. Dysregulation of cytokine production resulting in a cytokine storm is believed to be associated with disease severity. Elevated secretion of cytokines, particularly interleukin (IL)-2, IL-6, IL-10, IP-10, G-CSF, MCP-1, MIP1α, and TNFα were found in severe COVID-19 patient (Figure 3) [7,54,55]. Similarly, increased level of serum cytokines was found in SARS [56,57] and MERS patients [58]. Meanwhile, both signal transduction and production of type I interferon (IFN), the cytokine that limits viral spread by elevating neighboring cells to antiviral status, were delayed in SARS [57] and MERS patients [58]. Delayed IFN responses permit robust viral replication, accumulation of cytokine/chemokine-producing monocytes/macrophages, and increase in disease severity in SARS-CoV [59] and MERS-CoV [60] infected mice. Similarly, MERS-CoV M, ORF 4a, ORF 4b, and ORF 5 antagonized IFN pathways and, in turn, diminished type I IFN production [61]. Impaired type I IFN response and uncontrolled inflammatory response subsequently contribute to adverse disease outcomes. In fact, different type I IFNs have been shown to have antiviral effects against both SARS-CoV [62] and MERS-CoV [63] in vitro. However, there is no clear evidence that type I IFN therapies had direct benefit for SARS and MERS patients [62,64].
Adaptive Immunity
The second arm of host immunity against viral infection is adaptive immunity that involves T cell and B cell responses. CD4 T cells promote development of antibody responses, whereas CD8 T cells can directly kill virus-infected cells. Immunogenic CD4 and CD8 T cell epitopes in SARS and MERS patients were found to localize mainly to structural proteins, particularly the S protein [65,66]. Several T cell epitopes for SARS-CoV-2 have been predicted by computational analysis [25,67], although these predictions require additional validation in terms of their immunodominance in human populations. A summary of the experimentally confirmed immunodominant epitopes and their HLA-restrictions identified for SARS-CoV that could be used for eliciting crossreactive T cell responses against SARS-CoV-2 is shown in Table 2 . These epitopes are highly conserved between SARS-CoV-2 isolates. We aligned 93 S protein sequences and 103 N protein sequences from SARS-CoV-2 isolates. Among the 20 epitopes (Table 2), only two viral isolates contained a single amino acid substitution at two different epitopes (see the supplemental information online). Polyfunctional virus-specific CD8 T cells can be sustained in SARS patients for more than 1 year after recovery [68]. However, in terms of clinical features, COVID-19 (63%), SARS (80%), and MERS (34%) patients often exhibit lymphopenia with reduced numbers of CD4 and CD8 T cells (Figure 3) [7,69]. MERS-CoV-infected T cells undergo apoptosis mediated by both intrinsic and extrinsic pathways [70]. Further investigation is required to determine whether lymphopenia seen in severe COVID-19 patients is correlated with lymphocyte apoptosis. Moreover, MERS-CoV, but not SARS-CoV, can infect both CD4 and CD8 T cells from human blood and lymphoid organs via DPP4 receptor binding [71].
Table 2.
Immunodominant T Cell Epitopes Identified in SARS-CoV
| T cell epitopes | Protein | Peptide position | Sequencea | HLA-restriction | Refs |
|---|---|---|---|---|---|
| CD4 T cell immunodominant epitopes | |||||
| Spike | 159–171 | CTFEYISDAFSLD | HLA-DRB1*0401 and HLA-DRB1*0701 | [95] | |
| Spike | 166–178 | DAFSLDVSEKSGN | HLA-DRB1*0401 | [95] | |
| Spike | 358–374 | STFFSTFKCYGVSATKL | HLA-DR | [96] | |
| Spike | 427–444 | NIDATSTGNYNYKYRYLR | HLA-DR | [96] | |
| Spike | 449–461 | RPFERDISNVPFS | HLA-DRB1*0401 | [95] | |
| Spike | 729–745 | TECANLLLQYGSFCTQL | HLA-DR | [96] | |
| Spike | 1083–1097 | SWFITQRNFFSPQII | HLA-DRB1*0401 | [95] | |
| Nucleocapsid | 346–362 | N.A.b | N.A. | [97] | |
| CD8 T cell immunodominant epitopes | |||||
| Spike | 411–420 | KLPDDFMGCV | HLA-A*02:01 | [98] | |
| Spike | 787–795 | ILPDPLKPT | HLA-A*02:01 | [99] | |
| Spike | 940–948 | ALNTLVKQL | HLA-A*02:01 | [100] | |
| Spike | 958–966 | VLNDILSRL | HLA-A*02:01 | [101] | |
| Spike | 978–986 | LITGRLQSL | HLA-A*02:01 | [102] | |
| Spike | 1042–1050 | VVFLHVTYV | HLA-A*02:01 | [99] | |
| Spike | 1167–1175 | RLNEVAKNL | HLA-A*02:01 | [103] | |
| Spike | 1174–1182 | NLNESLIDL | HLA-A*02:01 | [100] | |
| Spike | 1203–1211 | FIAGLIAIV | HLA-A*02:01 | [102] | |
| Nucleocapsid | 216–225 | GETALALLLL | HLA-B*40:01 | [104] | |
| Nucleocapsid | 223–231 | LLLDRLNQL | HLA-A*02:01 | [99] | |
| Nucleocapsid | 227–235 | RLNQLESKV | HLA-A*02:01 | [99] | |
| Nucleocapsid | 317–325 | GMSRIGMEV | HLA-A*02:01 | [99] | |
| Nucleocapsid | 331–347 | N.A. | N.A. | [97] | |
| Nucleocapsid | 346–362 | N.A. | N.A. | [97] | |
Underlined sequences indicate identical amino acids between SARS-CoV (GenBank accession number: NC_004718.3) and SARS-CoV-2 (GenBank accession number: MN908947.3).
Peptide sequence was not included in the original article.
Neutralizing antibodies against SARS-CoV S can prevent viral entry and protect against SARS-CoV challenge [72]. In addition, passive transfer of convalescent sera from recovered SARS-CoV patients decreased viral burden in recipient SARS patients [73] and is being advanced as a potential treatment for COVID-19 [74,75]. The mean time for seroconversion in SARS patients was around 2 weeks after disease onset [76]. Whether neutralizing antibodies can offer protection from or limit the spread of SARS-CoV-2 infection is currently unclear. In rhesus macaques reinfected with SARS-CoV-2 28 days after prior challenge, no viral replication was observed and the animals exhibited no clinical signs, but the animals showed increasing titers of neutralizing antibodies after rechallenge [77]. These results suggest that prior SARS-CoV-2 infection could elicit protective immunity against subsequent virus exposure, although the long-term protection offered by neutralizing antibodies requires further study.
Vaccine Strategies
Vaccination with adenovirus-delivered SARS-CoV proteins, including N and the S protein S1 fragment can induce virus-specific neutralizing antibodies and nucleocapsid-specific T cell responses in rhesus macaques [78]. Immunization with vaccinia virus carrying full-length SARS-CoV-S reduced viral titer in BALB/c mice after SARS-CoV challenge [79]. For MERS-CoV, immunization with full-length MERS-CoV S protein delivered using recombinant vaccinia virus induced high levels of neutralizing antibodies and virus-specific IFNγ-producing CD8+ T cell responses [80]. The MERS-CoV RBD protein, specifically the S358-588 fragment, induced high immunogenicity and elicited a strong neutralizing antibody response in vaccinated mice and rabbits [81,82]. A recent review summarizes current vaccine studies for SARS-CoV and MERS-CoV [83]. Future vaccine efforts could be focused on enhancing mucosal immunity in the respiratory tract using optimized administration routes, antigens, and adjuvants to evaluate how vaccine-induced immune responses in the lungs correlate with protection.
Animal Models for Antiviral Discovery and Vaccine Development
To date, the most commonly used animal models for SARS are older (i.e., 12–14-month-old) BALB/c mice [84] and transgenic mice (K18-hACE2) that express the SARS-CoV receptor human ACE2 under the control of an epithelial cell-specific promotor on a C57BL/6 background. SARS infections in these mice are lethal [85]. Several other mouse strains, including C57BL/6, 129S Sv/Ev, and STAT1–/– mice have been reported to be susceptible to SARS-CoV infection [86]. Additionally, the use of a virus adapted to mice (SARS-MA15, SARS-CoV passaged 15 in BALB/c mice) produces clinical disease in young (6–8-week-old) BALB/c mice [87] that is similar to ARDS observed in humans [88]. For MERS, transgenic mice encoding human DPP4 showed viral replication with interstitial pneumonia [89]. Thus, older BALB/c mice, hACE2 transgenic mice, mice lacking one or more components of the IFN system, and mouse-adapted viruses will likely be important tools for developing mouse models of SARS-CoV-2 infection and disease. In fact, a recent study has already shown that hACE2 transgenic mice with SARS-CoV-2 infection reproduce the clinical symptoms of disease, supporting virus replication in lung tissue [90].
Concluding Remarks
SARS-CoV-2 represents the third HCoV, after MERS-CoV and SARS-CoV, to emerge in the 21st century. Although these viruses have had a marked impact on public health and the economy, no effective vaccine or treatment is available. The virological and immunological lessons from prior CoV outbreaks can guide us in understanding, treating, and eventually preventing COVID-19. In particular, evaluation of S protein molecular structures, neutralizing antibody responses, and immunodominant epitopes that elicit strong T cell responses will all be critical for the development of comprehensive vaccine strategies to fight emerging CoVs (see Outstanding Questions).
Outstanding Questions.
Which viral and host factors contribute to SARS-CoV-2 transmissibility and infectivity?
Which cells are permissive to SARS-CoV-2 infection?
Does prior SARS-CoV-2 infection protect against subsequent infection?
What are the magnitude and quality of innate immune responses to SARS-CoV-2?
What are the magnitude and quality of adaptive immune responses to SARS-CoV-2?
Which host factors allow for durable, protective immunity following SARS-CoV-2 infection?
Is there antibody-dependent enhancement of SARS-CoV-2 infection?
Which viral and host factors contribute to disease severity?
What are the immune response profiles in COVID-19 patients and do these profiles correlate to disease severity?
What are the mechanisms and sources of SARS-CoV-2 cytokine storm?
What are the most immunocompetent and relevant mouse models of COVID-19?
Can a peptide vaccine for SARS-CoV-2 be designed based on identification of immunodominant epitopes?
How do the differences in amino acid sequence, such as furin cleavage site, make differences between SARS-CoV-2 and other human coronaviruses?
Alt-text: Outstanding Questions
Acknowledgments
We thank Sharon Schendel for critical reading of the manuscript and numerous helpful suggestions. This research was funded by Bill and Melinda Gates Foundation grant # INV-006133.
Footnotes
Supplemental information associated with this article can be found online at https://doi.org/10.1016/j.tim.2020.05.012.
Supplemental Information
Supplementary material
References
- 1.Gorbalenya A.E. The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan J.F. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heffernan J.M. Perspectives on the basic reproductive ratio. J. R. Soc. Interface. 2005;2:281–293. doi: 10.1098/rsif.2005.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen J. Pathogenicity and transmissibility of 2019-nCoV-A quick overview and comparison with other emerging viruses. Microbes Infect. 2020;22:69–71. doi: 10.1016/j.micinf.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bai Y. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020 doi: 10.1001/jama.2020.2565. Published online February 21, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li R. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV2) Science. 2020;368:489–493. doi: 10.1126/science.abb3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guan W.J. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang J.J. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020 doi: 10.1111/all.14238. Published online February 19, 2020. [DOI] [PubMed] [Google Scholar]
- 10.Chen N. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riou J. Adjusted age-specific case fatality ratio during the COVID-19 epidemic in Hubei, China, January and February 2020. medRxiv. 2020 doi: 10.1101/2020.03.04.20031104. Published online March 6, 2020. [DOI] [Google Scholar]
- 12.Dong Y. Epidemiological characteristics of 2143 pediatric patients with 2019 coronavirus disease in China. Pediatrics. 2020 doi: 10.1542/peds.2020-0702. Published online March 16, 2020. [DOI] [Google Scholar]
- 13.Lu X. SARS-CoV-2 infection in children. N. Engl. J. Med. 2020;382:1663–1665. doi: 10.1056/NEJMc2005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brownlie J., Whittaker G. Coronaviridae. In: MacLachlan N.J., Dubovi E.J., editors. Fenner’s Vetertinary Virology. Elsevier; 2017. pp. 435–461. [Google Scholar]
- 15.Drake J.W., Holland J.J. Mutation rates among RNA viruses. Proc. Natl. Acad. Sci. U. S. A. 1999;96:13910–13913. doi: 10.1073/pnas.96.24.13910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vignuzzi M. Quasispecies diversity determines pathogenesis through cooperative interactions in a viral population. Nature. 2006;439:344–348. doi: 10.1038/nature04388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bolles M. SARS-CoV and emergent coronaviruses: viral determinants of interspecies transmission. Curr. Opin. Virol. 2011;1:624–634. doi: 10.1016/j.coviro.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haagmans B.L. Middle East respiratory syndrome coronavirus in dromedary camels: an outbreak investigation. Lancet Infect. Dis. 2014;14:140–145. doi: 10.1016/S1473-3099(13)70690-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu R. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lam T.T. Identifying SARS-CoV-2 related coronaviruses in Malayan pangolins. Nature. 2020 doi: 10.1038/s41586-020-2169-0. Published online March 26, 2020. [DOI] [PubMed] [Google Scholar]
- 21.Zhang T. Probable pangolin origin of SARS-CoV-2 associated with the COVID-19 outbreak. Curr. Biol. 2020;30:1578. doi: 10.1016/j.cub.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim D. The architecture of SARS-CoV-2 transcriptome. Cell. 2020;181:914–921. doi: 10.1016/j.cell.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castano-Rodriguez C. Role of severe acute respiratory syndrome coronavirus viroporins E, 3a, and 8a in replication and pathogenesis. mBio. 2018;9 doi: 10.1128/mBio.02325-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeDiego M.L. Coronavirus virulence genes with main focus on SARS-CoV envelope gene. Virus Res. 2014;194:124–137. doi: 10.1016/j.virusres.2014.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahmed S.F. Preliminary identification of potential vaccine targets for the COVID-19 coronavirus (SARS-CoV-2) based on SARS-CoV immunological studies. Viruses. 2020;12 doi: 10.3390/v12030254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu A. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020;27:325–328. doi: 10.1016/j.chom.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hofmann H. Human coronavirus NL63 employs the severe acute respiratory syndrome coronavirus receptor for cellular entry. Proc. Natl. Acad. Sci. U. S. A. 2005;102:7988–7993. doi: 10.1073/pnas.0409465102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li W. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoffmann M. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fehr A.R., Perlman S. Coronaviruses: an overview of their replication and pathogenesis. In: Maier H., editor. Coronaviruses: Methods and Protocols. Springer; 2015. pp. 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park J.-E. Proteolytic processing of Middle East respiratory syndrome coronavirus spikes expands virus tropism. Proc. Natl. Acad. Sci. U. S. A. 2016;113:12262–12267. doi: 10.1073/pnas.1608147113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thiel V. Mechanisms and enzymes involved in SARS coronavirus genome expression. J. Gen. Virol. 2003;84:2305–2315. doi: 10.1099/vir.0.19424-0. [DOI] [PubMed] [Google Scholar]
- 33.den Boon J.A., Ahlquist P. Organelle-like membrane compartmentalization of positive-strand RNA virus replication factories. Annu. Rev. Microbiol. 2010;64:241–256. doi: 10.1146/annurev.micro.112408.134012. [DOI] [PubMed] [Google Scholar]
- 34.Gosert R. RNA replication of mouse hepatitis virus takes place at double-membrane vesicles. J. Virol. 2002;76:3697–3708. doi: 10.1128/JVI.76.8.3697-3708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knoops K. SARS-coronavirus replication is supported by a reticulovesicular network of modified endoplasmic reticulum. PLoS Biol. 2008;6 doi: 10.1371/journal.pbio.0060226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Haan C.A., Rottier P.J. Molecular interactions in the assembly of coronaviruses. Adv. Virus Res. 2005;64:165–230. doi: 10.1016/S0065-3527(05)64006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klumperman J. Coronavirus M proteins accumulate in the Golgi complex beyond the site of virion budding. J. Virol. 1994;68:6523–6534. doi: 10.1128/jvi.68.10.6523-6534.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Masters P.S. The molecular biology of coronaviruses. Adv. Virus Res. 2006;66:193–292. doi: 10.1016/S0065-3527(06)66005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu Y. Crystal structure of severe acute respiratory syndrome coronavirus spike protein fusion core. J. Biol. Chem. 2004;279:49414–49419. doi: 10.1074/jbc.M408782200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wrapp D. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Belouzard S. Activation of the SARS coronavirus spike protein via sequential proteolytic cleavage at two distinct sites. Proc. Natl. Acad. Sci. U. S. A. 2009;106:5871–5876. doi: 10.1073/pnas.0809524106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walls A.C. Tectonic conformational changes of a coronavirus spike glycoprotein promote membrane fusion. Proc. Natl. Acad. Sci. U. S. A. 2017;114:11157–11162. doi: 10.1073/pnas.1708727114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yao Y.X. Cleavage and serum reactivity of the severe acute respiratory syndrome coronavirus spike protein. J. Infect. Dis. 2004;190:91–98. doi: 10.1086/421280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coutard B. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antivir. Res. 2020;176:104742. doi: 10.1016/j.antiviral.2020.104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andersen K.G. The proximal origin of SARS-CoV-2. Nat. Med. 2020;26:450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li F. Structural biology: structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science. 2005;309:1864–1868. doi: 10.1126/science.1116480. [DOI] [PubMed] [Google Scholar]
- 47.Zhou P. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yan R. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367:1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walls A.C. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–292. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu Z. Potent cross-reactive neutralization of SARS coronavirus isolates by human monoclonal antibodies. Proc. Natl. Acad. Sci. U. S. A. 2007;104:12123–12128. doi: 10.1073/pnas.0701000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prabakaran P. Structure of severe acute respiratory syndrome coronavirus receptor-binding domain complexed with neutralizing antibody. J. Biol. Chem. 2006;281:15829–15836. doi: 10.1074/jbc.M600697200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yuan M. A highly conserved cryptic epitope in the receptor-binding domains of SARS-CoV-2 and SARS-CoV. Science. 2020;368:630–633. doi: 10.1126/science.abb7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang C. A human monoclonal antibody blocking SARS-CoV-2 infection. Nat. Commun. 2020;11:2251. doi: 10.1038/s41467-020-16256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qin C. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa248. Published online March 12, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shi Y. Immunopathological characteristics of coronavirus disease 2019 cases in Guangzhou, China. medRxiv. 2020 doi: 10.1101/2020.03.12.20034736. Published online March 16, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen J., Subbarao K. The Immunobiology of SARS*. Annu. Rev. Immunol. 2007;25:443–472. doi: 10.1146/annurev.immunol.25.022106.141706. [DOI] [PubMed] [Google Scholar]
- 57.Perlman S., Netland J. Coronaviruses post-SARS: update on replication and pathogenesis. Nat. Rev. Microbiol. 2009;7:439–450. doi: 10.1038/nrmicro2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mubarak A. Middle East respiratory syndrome coronavirus (MERS-CoV): infection, immunological response, and vaccine development. J. Immunol. Res. 2019;2019:6491738. doi: 10.1155/2019/6491738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Channappanavar R. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe. 2016;19:181–193. doi: 10.1016/j.chom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Channappanavar R. IFN-I response timing relative to virus replication determines MERS coronavirus infection outcomes. J. Clin. Invest. 2019;130:3625–3639. doi: 10.1172/JCI126363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang Y. The structural and accessory proteins M, ORF 4a, ORF 4b, and ORF 5 of Middle East respiratory syndrome coronavirus (MERS-CoV) are potent interferon antagonists. Protein Cell. 2013;4:951–961. doi: 10.1007/s13238-013-3096-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stockman L.J. SARS: systematic review of treatment effects. PLoS Med. 2006;3 doi: 10.1371/journal.pmed.0030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou J. Middle East respiratory syndrome coronavirus infection: virus-host cell interactions and implications on pathogenesis. Virol. J. 2015;12:218. doi: 10.1186/s12985-015-0446-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arabi Y.M. Ribavirin and interferon therapy for critically ill patients with Middle East respiratory syndrome: a multicenter observational study. Clin. Infect. Dis. 2019;70:1837–1844. doi: 10.1093/cid/ciz544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li C.K. T cell responses to whole SARS coronavirus in humans. J. Immunol. 2008;181:5490–5500. doi: 10.4049/jimmunol.181.8.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shin H.S. Immune responses to Middle East respiratory syndrome coronavirus during the acute and convalescent phases of human infection. Clin. Infect. Dis. 2019;68:984–992. doi: 10.1093/cid/ciy595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grifoni A. A sequence homology and bioinformatic approach can predict candidate targets for immune responses to SARS-CoV-2. Cell Host Microbe. 2020;27:671–680. doi: 10.1016/j.chom.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen H. Response of memory CD8+ T cells to severe acute respiratory syndrome (SARS) coronavirus in recovered SARS patients and healthy individuals. J. Immunol. 2005;175:591–598. doi: 10.4049/jimmunol.175.1.591. [DOI] [PubMed] [Google Scholar]
- 69.Channappanavar R. T cell-mediated immune response to respiratory coronaviruses. Immunol. Res. 2014;59:118–128. doi: 10.1007/s12026-014-8534-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chu H. Middle East respiratory syndrome coronavirus efficiently infects human primary T lymphocytes and activates the extrinsic and intrinsic apoptosis pathways. J. Infect. Dis. 2016;213:904–914. doi: 10.1093/infdis/jiv380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ying T. Discovery of T-cell infection and apoptosis by Middle East respiratory syndrome coronavirus. J. Infect. Dis. 2016;213:877–879. doi: 10.1093/infdis/jiv381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Buchholz U.J. Contributions of the structural proteins of severe acute respiratory syndrome coronavirus to protective immunity. Proc. Natl. Acad. Sci. U. S. A. 2004;101:9804–9809. doi: 10.1073/pnas.0403492101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cheng Y. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur. J. Clin. Microbiol. Infect. Dis. 2005;24:44–46. doi: 10.1007/s10096-004-1271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Duan K. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl. Acad. Sci. U. S. A. 2020;117:9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bloch E.M. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J. Clin. Invest. 2020 doi: 10.1172/JCI138745. Published online April 7, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Woo P.C. Longitudinal profile of immunoglobulin G (IgG), IgM, and IgA antibodies against the severe acute respiratory syndrome (SARS) coronavirus nucleocapsid protein in patients with pneumonia due to the SARS coronavirus. Clin. Diagn. Lab. Immunol. 2004;11:665–668. doi: 10.1128/CDLI.11.4.665-668.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bao L. Reinfection could not occur in SARS-CoV-2 infected rhesus macaques. bioRxiv. 2020 doi: 10.1101/2020.03.13.990226. Published online March 14, 2020. [DOI] [Google Scholar]
- 78.Gao W. Effects of a SARS-associated coronavirus vaccine in monkeys. Lancet. 2003;362:1895–1896. doi: 10.1016/S0140-6736(03)14962-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bisht H. Severe acute respiratory syndrome coronavirus spike protein expressed by attenuated vaccinia virus protectively immunizes mice. Proc. Natl. Acad. Sci. U. S. A. 2004;101:6641–6646. doi: 10.1073/pnas.0401939101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Volz A. Protective efficacy of recombinant modified vaccinia virus Ankara delivering Middle East respiratory syndrome coronavirus spike glycoprotein. J. Virol. 2015;89:8651–8656. doi: 10.1128/JVI.00614-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ma C. Searching for an ideal vaccine candidate among different MERS coronavirus receptor-binding fragments--the importance of immunofocusing in subunit vaccine design. Vaccine. 2014;32:6170–6176. doi: 10.1016/j.vaccine.2014.08.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mou H. The receptor binding domain of the new Middle East respiratory syndrome coronavirus maps to a 231-residue region in the spike protein that efficiently elicits neutralizing antibodies. J. Virol. 2013;87:9379–9383. doi: 10.1128/JVI.01277-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tse L.V. The current and future state of vaccines, antivirals and gene therapies against emerging coronaviruses. Front. Microbiol. 2020;11:658. doi: 10.3389/fmicb.2020.00658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Roberts A. Aged BALB/c mice as a model for increased severity of severe acute respiratory syndrome in elderly humans. J. Virol. 2005;79:5833–5838. doi: 10.1128/JVI.79.9.5833-5838.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McCray P.B., Jr. Lethal infection of K18-hACE2 mice infected with severe acute respiratory syndrome coronavirus. J. Virol. 2007;81:813–821. doi: 10.1128/JVI.02012-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gretebeck L.M., Subbarao K. Animal models for SARS and MERS coronaviruses. Curr. Opin. Virol. 2015;13:123–129. doi: 10.1016/j.coviro.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Roberts A. A mouse-adapted SARS-coronavirus causes disease and mortality in BALB/c mice. PLoS Pathog. 2007;3 doi: 10.1371/journal.ppat.0030005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Frieman M. Molecular determinants of severe acute respiratory syndrome coronavirus pathogenesis and virulence in young and aged mouse models of human disease. J. Virol. 2012;86:884–897. doi: 10.1128/JVI.05957-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhao J. Rapid generation of a mouse model for Middle East respiratory syndrome. Proc. Natl. Acad. Sci. U. S. A. 2014;111:4970–4975. doi: 10.1073/pnas.1323279111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bao L. The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Nature. 2020 doi: 10.1038/s41586-020-2312-y. Published online May 7, 2020. [DOI] [PubMed] [Google Scholar]
- 91.Xu X.W. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Han Q. Recent insights into 2019-nCoV: a brief but comprehensive review. J. Infect. 2020;80:373–377. doi: 10.1016/j.jinf.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Assiri A. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect. Dis. 2013;13:752–761. doi: 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zumla A. Middle East respiratory syndrome. Lancet. 2015;386:995–1007. doi: 10.1016/S0140-6736(15)60454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang J. Searching immunodominant epitopes prior to epidemic: HLA class II-restricted SARS-CoV spike protein epitopes in unexposed individuals. Int. Immunol. 2009;21:63–71. doi: 10.1093/intimm/dxn124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Libraty D.H. Human CD4(+) memory T-lymphocyte responses to SARS coronavirus infection. Virology. 2007;368:317–321. doi: 10.1016/j.virol.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Peng H. Long-lived memory T lymphocyte responses against SARS coronavirus nucleocapsid protein in SARS-recovered patients. Virology. 2006;351:466–475. doi: 10.1016/j.virol.2006.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhou M. Screening and identification of severe acute respiratory syndrome-associated coronavirus-specific CTL epitopes. J. Immunol. 2006;177:2138–2145. doi: 10.4049/jimmunol.177.4.2138. [DOI] [PubMed] [Google Scholar]
- 99.Tsao Y.P. HLA-A*0201 T-cell epitopes in severe acute respiratory syndrome (SARS) coronavirus nucleocapsid and spike proteins. Biochem. Biophys. Res. Commun. 2006;344:63–71. doi: 10.1016/j.bbrc.2006.03.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen Y.Z. Identification of SARS-COV spike protein-derived and HLA-A2-restricted human CTL epitopes by using a new muramyl dipeptide derivative adjuvant. Int. J. Immunopathol. Pharmacol. 2010;23:165–177. doi: 10.1177/039463201002300115. [DOI] [PubMed] [Google Scholar]
- 101.Lv Y. Identification of a novel conserved HLA-A*0201-restricted epitope from the spike protein of SARS-CoV. BMC Immunol. 2009;10:61. doi: 10.1186/1471-2172-10-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang Y.D. T-cell epitopes in severe acute respiratory syndrome (SARS) coronavirus spike protein elicit a specific T-cell immune response in patients who recover from SARS. J. Virol. 2004;78:5612–5618. doi: 10.1128/JVI.78.11.5612-5618.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang B. Identification of an HLA-A*0201-restricted CD8+ T-cell epitope SSp-1 of SARS-CoV spike protein. Blood. 2004;104:200–206. doi: 10.1182/blood-2003-11-4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Oh H.L. Engineering T cells specific for a dominant severe acute respiratory syndrome coronavirus CD8 T cell epitope. J. Virol. 2011;85:10464–10471. doi: 10.1128/JVI.05039-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material