Abstract
As the novel coronavirus severe acute respiratory syndrome coronavirus 2 caused coronavirus disease 2019 cases in the United States, the initial test was developed and performed at the Centers for Disease Control and Prevention. As the number of cases increased, the demand for tests multiplied, leading the Centers for Disease Control and Prevention to use the Emergency Utilization Authorization to allow clinical and commercial laboratories to develop tests to detect the presence of the virus. Many nucleic acid tests based on RT-PCR were developed, each with different techniques, specifications, and turnaround time. As the illnesses turned into a pandemic, testing became more crucial. The test supply became inadequate to meet the need and so it had to be prioritized according to guidance. For surveillance, the need for serologic tests emerged. Here, we review the timeline of test development, the turnaround times, and the various approved tests, and compare them as regards the genes they detect. We concentrate on the point-of-care tests and discuss the basis for new serologic tests. We discuss the testing guidance for prioritization and their application in a hospital setting.
Key words: COVID-19, coronavirus, SARS-CoV-2, viral genes, S protein, N protein, E protein, M protein, RT-PCR, test, point-of-care, prioritization, guidance, Centers for Disease Control and Prevention, Food and Drug Administration, World Health Organization, nucleic acid test, serologic test
Abbreviations used: CCHMC, Cincinnati Children’s Hospital Medical Center; CDC, Centers for Disease Control and Prevention; COVID-19, Coronavirus disease 2019; EUA, Emergency Use Authorization; FDA, Food and Drug Administration; IFU, Instructions for use; LOD, Limit of detection; N, Nucleocapsid; NAT, Nucleic acid test; POC, Point of care; S, Spike; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; WHO, World Health Organization
As severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) arrived in the United States causing coronavirus disease 2019 (COVID-19), one of the most talked about issues in the management of the disease and the resulting pandemic has been clinical testing. A unique situation arose of a communicable and highly contagious disease necessitating the rapid diagnosis of patients and the identification of nonsymptomatic infected persons. Unfortunately, the United States did not have a Food and Drug Administration (FDA)-approved laboratory test for the illness. The FDA ultimately used its Emergency Use Authorizations (EUAs) on February 4, 2020, to allow for more rapid and widespread development and implementation of in vitro testing.1 Indeed, companies and organizations used the EUA to file applications for new tests based on different methodologies, amounting to 48 applications in the span of 3 months from the beginning of February to the end of April 2020. In addition, multiple other tests were put in place under a separate authorization by a Presidential memorandum in early March, allowing laboratories that carry Clinical Laboratory Improvement Amendment certification to put tests in place without an EUA from the FDA. This created an unprecedented situation where the medical community and the public may not be familiar with the various new tests for COVID-19 that are offered to patients and hospitals.
The purpose of this review was to provide information, up-to-date as of the date of submission of the manuscript to the journal, on the various tests that have been developed, their scientific basis, and their interpretation. We give a real-world example demonstrating the time lag in the return of test results and review testing prioritization guidance because the supply of tests remains below the perceived need.
Methods
Viral tests
A search of the FDA Web site was conducted to retrieve all instructions for use (IFU) filed by the various laboratory testing companies. The search included the date of the first approval of an EUA on February 4, 2020, to the date of submitting this manuscript to the journal on April 27, 2020. Of these, the type of test, the test characteristics, and methodology were extracted and tabulated.2
Tracking of turnaround time
Cincinnati Children’s Hospital Medical Center (CCHMC) is a large, quaternary care pediatric center with more than 700 beds spread over 2 inpatient facilities and 16 outpatient facilities. Records of all SARS-CoV-2 testing collected from individuals at CCHMC starting on March 16, 2020, and up to April 24, 2020, were included. Data were extracted from a clinical decision support system (Vigilanz Corp, Chicago, Ill). Each record extracted included a timestamp for test collection and report into the electronic medical record. The turnaround time was calculated as the difference in time between each collection and reporting timestamp. These records were then grouped by date of collection, and turnaround time was evaluated using a statistical process control chart and the Western Electric rules for determining special cause variation were used.3 , 4 Turnaround time was assessed using X-bar and S control charts.4
Testing prioritization
A review of the testing prioritization guidance by the World Health Organization (WHO) and the Centers for Disease Control and Prevention (CDC) was performed. In addition, an example of a local application is provided. The guidance for prioritization of testing was generated by a multidisciplinary committee of the CCHMC, which included faculty from Infection Diseases, Infection Control, Hematology and Oncology, Allergy and Immunology, Rheumatology, Pulmonary Medicine, Gastroenterology, Hospital Medicine, Surgery, and Medical Ethics.
Testing for COVID-19
Timeline of development and approval of tests for COVID-19
EUAs
EUAs are supported by the Secretary of Health and Human Services declaration that circumstances exist to justify emergency use of testing for detection and diagnosis of COVID-19. The process to obtain EUA is as follows. After developing a test and within 15 days of starting to use the test, the company, laboratory, or organization submits an IFU to the FDA. The IFU has basic information on the methodology used, the source of samples, the collection methods, and the reagents and instruments used. The IFU has data on the performance of the tests as regards sensitivity and specificity. The FDA issues a letter authorizing the use of the test under the conditions specified in the application and the IFU. The FDA waves the current good manufacturing practice requirements, including the quality system requirements with respect to the design, manufacture, packaging, labeling, storage, and distribution of the product. Tests available through an EUA have not undergone a thorough FDA review; however, approved tests are considered effective on the basis of presented scientific evidence.
The first EUA was issued on February 4, 2020, by the Secretary of Health and Human Services for emergency use of the CDC 2019-Novel Coronavirus Real-Time RT-PCR Diagnostic Panel considering the current public health emergency. Subsequently, on February 29, 2020, an immediately in effect guidance policy was released by the FDA to assist with the expansion of available tests and testing facilities throughout the United States. Since this time and up to April 27, 2020, 48 unique tests were approved for use through the EUA, of which 41 tests are nucleic acid–based tests and 7 are antibody-based tests (Table I, Table II, and III ).
Table I.
Companies and laboratories that have received EUA for RT-PCR–based tests
| Date EUA1 issued | Manufacturer | Primers | Positive human gene control |
|---|---|---|---|
| April 23, 2020 | SD Biosensor, Inc | E, ORF1ab (RdRp) | None |
| April 22, 2020 | Altona Diagnostics GmbH | E, S | None |
| April 21, 2020 | Seegene, Inc | E, N, RdRp | None |
| April 20, 2020 | Trax Management Services Inc | E, RdRp | RNase P |
| April 18, 2020 | Osang Healthcare | ORF1ab | RNase P |
| April 17, 2020 | Fosun Pharma USA Inc | E, N, ORF1ab | None |
| April 16, 2020 | GenoSensor, LLC | E, N, ORF1ab | GUSB |
| April 16, 2020 | Korval Labs Inc | NA | RNase P |
| April 15, 2020 | Maccura Biotechnology (USA) LLC | E/N, ORF1ab | None |
| April 10, 2020 | Atila BioSystems | N/ORF1ab | GAPDH |
| April 8, 2020 | DiCarta, Inc | N, ORF1ab, E | RNase P |
| April 7, 2020 | InBios International, Inc | E, N, ORF1b of the RdRp gene | RNase P |
| April 6, 2020 | Gnomegen LLC | N1, N2 | RNase P |
| April 3, 2020 | Co-Diagnostics, Inc | NA | RNase P |
| April 3, 2020 | ScienCell Research Laboratories | NA | RNase P |
| April 3, 2020 | Luminex Corporation | N1, N2 | RNase P |
| April 2, 2020 and April 8, 2020 | Becton, Dickinson & Company (BD) | N1, N2 | RNase P |
| April 1, 2020 | Ipsum Diagnostics, LLC | N | RNase P |
| March 30, 2020 | QIAGEN GmbH | ORF1b, E | None |
| March 30, 2020 | NeuMoDx Molecular, Inc | N, nonstructural protein gene | None |
| March 27, 2020 | Luminex Molecular Diagnostics, Inc | ORF1ab, N, E | None |
| March 26, 2020 | BGI Genomics Co. Ltd | ORF1ab | β-Actin |
| March 25, 2020 | Avellino Lab USA, Inc | NA | RNase P |
| March 24, 2020 | PerkinElmer, Inc | N, ORF1ab | None |
| March 23, 2020 | Mesa Biotech Inc | N | None |
| March 23, 2020 | BioFire Defense, LLC | ORF1ab, ORF 8 | None |
| March 20, 2020 | Cepheid | N2, E | None |
| March 20, 2020 | Primerdesign Ltd | ORF1ab | None |
| March 19, 2020 | GenMark Diagnostics, Inc | NA | None |
| March 19, 2020 | DiaSorin Molecular LLC | ORF1ab, S | None |
| March 18, 2020 | Abbott Molecular | NA | None |
| March 17, 2020 | Quest Diagnostics Infectious Disease, Inc | N1, N3 | None |
| March 17, 2020 | Quidel Corporation | NA | None |
| March 16, 2020 | Laboratory Corporation of America (LabCorp) | N1, N2, N3 | Hs RPP30 |
| March 16, 2020 | Hologic, Inc | NA | None |
| March 13, 2020 | Thermo Fisher Scientific, Inc | ORF1ab, N, S | None |
| March 12, 2020 | Roche Molecular Systems, Inc | N1, E | None |
| February 29, 2020 | Wadsworth Center, New York State Department of Public Health (CDC) | N1, N2 | RNase P |
| February 4, 2020 | CDC | N1, N2 | RNase P |
Table shows the date of approval and genes detected by the assay where indicated in the IFU application.
BGI, Beijing Genomics Institute; E, envelope; GAPDH, gluceraldehyde 3-phasphate dehydrogenase; GUSB, glucuronidase beta; ORF, Open Reading Frame; NA, not available; RdRp, RNA-dependent RNA polymerase.
Table II.
Examples of companies offering tests that are packaged in cartridges and POC tests
| Date EUA issued | Manufacturer | Test name | Primers | Comments |
|---|---|---|---|---|
| March 23, 2020 | Mesa Biotech Inc | Accula SARS-Cov-2 Test | N | Assay has cross-reactivity with SARS-CoV |
| March 23, 2020 | BioFire Defense, LLC | BioFire COVID-19 Test | ORF1ab, ORF 8 | Assay has cross-reactivity of 80% or greater homology to bat coronavirus RaTG13 and to pangolin coronavirus isolate MP789 |
| March 20, 2020 | Cepheid | Xpert Xpress SARS-CoV-2 test | N2, E | |
| March 19, 2020 | GenMark Diagnostics, Inc | The True Sample-to-Answer Solution | NA | Assay has cross-reactivity with SARS-CoV-1 |
| March 18, 2020 | Abbott Molecular | ID Now | ORF1b poly gene (RdRp gene), E |
E, Envelope; NA, not available; ORF, Open Reading Frame; RdRp, RNA-dependent RNA polymerase.
Table III.
EUAs for RT-PCR tests
| Date EUA issued | Laboratory | Primers | Positive human gene control |
|---|---|---|---|
| April 24, 2020 | AIT Laboratories | ORF1ab, N, S | None |
| April 24, 2020 | Ultimate Dx Laboratory | ORF1ab | β-actin |
| April 23, 2020 | Southwest Regional PCR Laboratory LLC. dba MicroGen DX | N1, N2, N3 | RNaseP gene |
| April 22, 2020 | Diatherix Eurofins Laboratory | S | None |
| April 20, 2020 | Mayo Clinic Laboratories, Rochester, Minn | None | |
| April 15, 2020 | CirrusDx Laboratories | E, N, RdRp | None |
| April 15, 2020 | Hackensack University Medical Center Molecular Pathology Laboratory | N2, E | RNaseP gene |
| April 14, 2020 | Exact Sciences Laboratories | E, N, RdRp | NA |
| April 14, 2020 | Infectious Diseases Diagnostics Laboratory, Boston Children’s Hospital | S | None |
| April 13, 2020 | Pathology/Laboratory Medicine Lab of Baptist Hospital Miami | N2 | RNase P |
| April 13, 2020 | Integrity Laboratories | N1, N2 | RNase P |
| April 10, 2020 | Specialty Diagnostic (SDI) Laboratories | ORF1ab | β-actin |
| April 10, 2020 | Rutgers Clinical Genomics Laboratory-Rutgers University | N, S, ORF1ab | None |
| April 10, 2020 | Orig3n, Inc | N1, N2, N3 | RNase P |
| April 10, 2020 | University of North Carolina Medical Center | E | None |
| April 8, 2020 | Stanford Health Care Clinical Virology Laboratory | E | RNase P |
| April 6, 2020 | Viracor Eurofins Clinical Diagnostics | N1, N2 | None |
| April 3, 2020 | Massachusetts General Hospital | N1, N2 | RNase P |
| April 2, 2020 | Diagnostic Molecular Laboratory – Northwestern Medicine | N1 | RNase P |
| April 2, 2020 | Infectious Disease Diagnostics Laboratory, Children’s Hospital of Philadelphia | N2 | β-actin |
| March 31, 2020 | Yale New Haven Hospital, Clinical Virology Laboratory | N1, N2 | RNase P |
Tests are authorized for use in the single laboratory that developed the single authorized test and that is certified under Clinical Laboratory Improvement Amendments, to perform high-complexity tests.
E, Envelope; NA, not available; ORF, Open Reading Frame; RdRp, RNA-dependent RNA polymerase.
Additional authorizations by states
To expand testing availability, states approved authorization of Clinical Laboratory Improvement Amendment–certified laboratories to develop and perform testing. The first such approval was given to the Wadsworth Center in New York on March 12, 2020. A Presidential memorandum followed on March 13, 2020, allowing other states to authorize laboratories to develop and perform testing without EUA approval by the FDA. State authorization requires laboratories to follow state law, and state-specific processes. The states of Connecticut, Maryland, Mississippi, Nevada, New York, and Washington have used this pathway to allow laboratories in the state to implement testing through a state-specific process.
In addition, on March 31, 2020, the FDA issued another EUA allowing the use of molecular-based laboratory developed tests by a Clinical Laboratory Improvement Amendment–approved developing laboratory. This directive broadened the scope of which entities could develop and use testing. This authorization restricts the use of the test to the laboratory where it was developed. Since this time, 21 academic hospital and commercial laboratories have been approved to use their diagnostic testing. Each of these are nucleic-acid–based tests (Table III).
Finally, the FDA has issued a policy allowing serologic testing to be developed and marketed by commercial manufacturers without application for an EUA. In these cases, the test must be validated, and the FDA notified that the test is in use. Many commercial companies are currently using serologic testing. Since the FDA issued the policy, more than 70 test developers have notified the agency that they have serological tests available for use. However, the FDA has warned that some firms are falsely claiming that their serological tests are FDA approved or authorized, or falsely claiming that they can diagnose COVID-19.5
PCR-based tests
PCR tests are widely used for the detection of viruses in human disease and are currently the most commonly used nucleic acid tests (NATs) performed in clinical laboratories. PCR instruments and techniques are in widespread use in both clinical and research laboratories and the basis of the tests is well known. The tests consist of nucleic acid extraction and purification from the human specimen using authorized extraction methods/instruments followed by real-time RT-PCR, where the RNA is reverse transcribed into cDNA and then amplified using the primer sets and detected using specific probes.
PCR-based tests were the initial tests used, starting with the CDC 2019-Novel Coronavirus Real-Time RT-PCR Diagnostic Panel. These tests detect nucleic acid from SARS-CoV-2 in patients suspected to have COVID-19 infection.
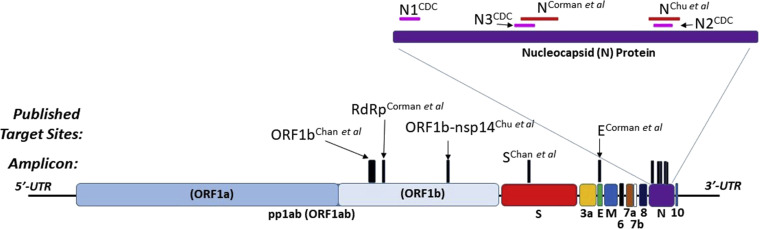
SARS-CoV-2 is a positive-sense, single-stranded RNA virus. The various PCR-based tests developed amplify different segments of the genome (Fig 1 ).6, 7, 8, 9 These include the RNA genome segments that code for the spike (S) protein, the nucleocapsid (N) proteins 1 and 2, the membrane protein, and the envelope (E) protein, in addition to different Open Reading Frame segments, 1a and 1b. Open Reading Frame 1b is the RNA-dependent RNA polymerase. SARS-CoV-2 is part of the larger class of coronaviruses, which include endemic coronaviruses, Middle East respiratory syndrome, and SARS-CoV viruses. Virus-specific PCR-based tests required generating primers and probes unique to SARS-CoV-2 but not to the other closely related coronaviruses.6, 7, 8, 9
Fig 1.
SARS-CoV-2 genome and RT-PCR primer/amplicon sites: Schematic of SARS-CoV-2 genome with localization of various published RT-PCR amplicons in ORF1ab/b, S, E, and N genes. Primer/amplicon sequences aligned with 2 highly similar viral consensus sequences (EPI_ISL_412026 [BetaCoV/Hefei/2/2020] and MT106052.1 [2019-nCoV/USA-CA7/2020]) using NCBI BLAST program. E, Envelope; ORF, Open Reading Frame; RdRp, RNA-dependent RNA polymerase; UTR, untranslated region. Primer sequences from referenced publications.6, 7, 8 US CDC primers (N1, N2, N3) as reported in Udugama et al.9
The CDC Real-Time RT-PCR Diagnostic Panel was the first to receive approval and used the N1 and N2 genome segment. The CDC generated the primers and probes and made the materials available for other laboratories to use with the same test. Laboratories have used different combinations of primers and probes, while some laboratories do not disclose the targets or sequences of their primers/probes (Table I, Table II, and III).
As part of the quality assessment of each assay, the FDA requires demonstration of specificity and exclusivity. Exclusivity means that no other viruses or bacteria from a specified list are detected by the test. These are infectious organisms that may be close genetically to SARS-CoV-2, or would be expected to be present in patients presenting with the same symptoms of COVID-19 (Table IV ).
Table IV.
To show exclusivity, an RT-PCR–based test needs to demonstrate that other relevant infectious agents were not detected by the test
| Virus | Strain |
|---|---|
| Human coronavirus | 229E |
| Human coronavirus | OC43 |
| Human coronavirus | NL63 |
| Human coronavirus | HKU1 |
| MERS-coronavirus | |
| SARS-coronavirus | |
| Bocavirus | |
| Mycoplasma pneumoniae | |
| Streptococcus | |
| Influenza A (H1N1) | |
| Influenza A (H3N2) | |
| Influenza B | |
| Human adenovirus, type 1 | Ad71 |
| Human metapneumovirus | |
| Respiratory syncytial virus | Long A |
| Rhinovirus | |
| Parainfluenza 1 | C35 |
| Parainfluenza 2 | Greer |
| Parainfluenza 3 | C-43 |
| Parainfluenza 4 | M-25 |
This table presents a list of the initial CDC RT-PCR–based test used to demonstrate exclusivity.
MERS, Middle East respiratory syndrome.
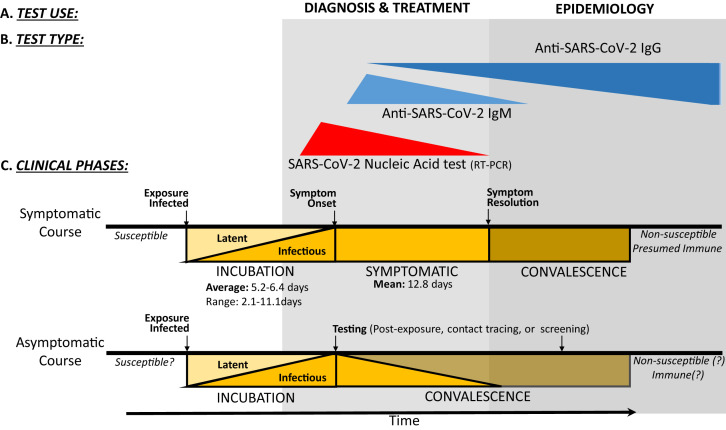
Positive results indicate the presence of SARS-CoV-2 nucleic acid; however, patient infection status should be determined from testing in combination with clinical history and additional diagnostic tools. Negative results do not rule out SARS-CoV-2 infection and should be used in combination with other clinical features and testing to determine patient management. Repeat testing, using various biospecimens (respiratory secretions, sputum, stool, rectal swabs, and serum), should be considered in patients with a high clinical suspicion of COVID-19 with a negative nucleic acid–based test result for the virus.10, 11, 12, 13 Depending on the performance of the internal controls of the assays, the test results are reported as positive, negative, inconclusive, or invalid. Tests that detect 2 or more viral genes are interpreted differently in various assays. Some assays require that both viral genes be detected for the test result to be interpreted as positive, whereas others rely on the detection of 1 of 2 viral genes for a positive interpretation. These test results are positive during the incubation period, which is several days before the onset of symptoms of the disease and remain positive for the duration of symptoms. The tests detect parts of the viral RNA that can be present after the virus fragments. Therefore, the results can continue to be positive after the resolution of symptoms, even though a complete infectious virus may no longer be present (Fig 2 ).13, 14, 15, 16 A few tests report cross-reactivity with other coronaviruses, specifically viruses that cause infections in animals, such as bats. Because of the relatively infrequent occurrence of these zoonotic coronaviruses “jumping species” into humans (once a decade over the last 20 years), this form of false-positive results is unlikely to occur again during the current pandemic (Table II).
Fig 2.
COVID-19 testing uses and modalities by clinical phase: Diagnosis, treatment, infection control, and epidemiologic monitoring (A) are reliant on the thoughtful deployment and use of various clinical testing modalities (B), including NAT (primarily implemented via RT-PCR) and anti–SARS-CoV-2 serology (IgM and IgG). Each testing modality has maximal utility at a given clinical phase (C), with NATs being most useful during late incubation and symptomatic illness and serology being useful during resolution of illness/convalescence. Classically, antiviral IgM antibodies develop early in an acute infection before IgG antibodies, but recent reports suggest that IgM and IgG seroconversion occurs simultaneously in many subjects during the second week of infection. The persistence of protective humoral IgG-mediated immunity is not yet established for COVID-19 survivors. The natural history of asymptomatic carriers of SARS-CoV-2 is also not yet clear.13, 14, 15, 16
RT-PCR–based NATs are inherently quantitative, but current clinical COVID-19 tests are generally being promoted by test manufacturers as qualitative tests (positive/negative). Multiple research groups have published RT-PCR methods for the quantification of SARS-CoV-2 from various clinical specimens (respiratory secretions, stool, serum),10, 11, 12 demonstrating that viral shedding generally peaks in the airway during the first 1 to 2 weeks of infection. Viral titers were significantly higher in patients older than 60 years and positively correlated with age, but results varied as to whether viral load at presentation was higher in severe versus mild cases.10 , 11 Clinical laboratories using commercial kits may elect to report a quantitative measure as well as a qualitative categorical result (positive vs negative). Given the variability in the amount of biospecimen recovered per specimen, the more important readout is whether a given test kit uses amplification/detection of a human gene as an RNA extraction/RNA quality control measure (Tables I and II). Viral quantification is also highly dependent on sampling technique and the accurate measure/estimation of the amount of input material. Beyond sampling issues, the clinical utility of a quantitative SARS-CoV-2 test is unclear at this time, although a threshold of less than 100,000 viral copies/mL (sputum) has been suggested as a putative criteria for clearing patients postinfection.12
NATs with automation and rapid turnaround time
Several laboratories have recently offered improved automation of NATs by packaging all the reagents on a cartridge. Some of the proprietary technologies are highlighted here. Complete information on each test can be obtained from the manufacturer’s Web sites or package inserts.
GenMark DX has an EUA for The True Sample-to-Answer Solution ePlex instrument, which automates all aspects of nucleic acid testing including extraction, amplification, and detection, combining electrowetting and GenMark’s eSensor technology in a single-use cartridge. The system can provide results in under 2 hours, with the capacity to process 96 tests in an 8-hour shift.
Cepheid has an EUA for its point-of-care (POC) Xpert Xpress SARS-CoV-2 test. This system requires the use of single-use disposable cartridges that hold the RT-PCR reagents and host the RT-PCR process.
Biofire Defense has an EUA for the BioFire COVID-19 Test, which is a disposable, closed system that stores all the necessary reagents for sample preparation for RT-PCR and detection to isolate, amplify, and detect nucleic acid from SARS-CoV-2 within a single nasopharygeal swab specimen. After sample collection, the user injects hydration solution and sample combined with sample buffer into the pouch, places the pouch into a Film Array instrument, and starts a run that takes about 50 minutes.
Mesa has an EUA for its Accula SARS-CoV-2 Test, which detects the viral RNA in approximately 30 minutes. It uses a test cassette that contains internal process positive and negative controls, enzymes, OscAR reagents, a detection strip, and the Accula Dock, which controls reaction temperatures, timing, and fluid movements within the test cassette. The test results are interpreted by the visualization of blue test lines on the detection strip in the test cassette.
Abbott has an EUA for its ID NOW, which is a rapid, 13 minutes or less, instrument-based isothermal test with a graphical user interface. The kit contains all components required to detect 2 genes with the same fluorescence channel. The 2 gene targets are not differentiated and amplification of either or both gene targets leads to a fluorescence signal. The sample is added to a sample receiver where it is transferred via a transfer cartridge to the test base, initiating target amplification. Heating, mixing, and detection are provided by the ID NOW Instrument.
Comparison of NAT techniques and effect on sensitivity and specificity
In contrast to traditional RT-PCR, which generally requires an RNA isolation step as well as use of a thermocycler, various rapid isothermic nucleic acid application systems have been commercialized as infectious disease POC tests. As an example, the COVID-19 ID Now system, discussed above, uses nicking enzyme amplification reaction isothermic amplification chemistry to reverse transcribe and exponentially amplify a small region of the SARS-CoV-2 genome. The system uses 2 sequence-specific templates (primers) and 3 enzymes (a thermostable DNA polymerase, a reverse transcriptase, and a thermostable nicking endonuclease) as well as fluorescently labeled molecular beacons to detect the virus-derived amplicon.17
Unlike a traditional RT-PCR test, which uses a positive control human gene (extraction control), the ID Now system does not report out whether a given sample contained sufficient sample-derived RNA to be valid. As a POC test, it has a low throughput (1 sample every 13 minutes vs 96-384 samples per run for RT-PCR in ∼90 minutes), but it does not require transport of the sample to a clinical laboratory, RNA extraction, or batching with other samples. The ID Now system does, however, have an impressive reported limit of detection (LOD), claimed by the manufacturer to be 125 genome equivalents per milliliter. For comparison, the commercially available Beijing Genomics Institute (BGI) RT-PCR kit has an LOD of 100 copies/mL. The Beijing Genomics Institute kit uses the Chinese CDC Orf1ab primers/probe with an RNA extraction control targeting the human β-actin gene (Fig 1). Of note, the Chinese CDC protocol provided to the WHO targets 2 genes (Orf1ab and N), but the Beijing Genomics Institute kit targets only 1 viral gene. Surprisingly, the US CDC kit has a significantly higher LOD at 1000 copies/mL, but this is partially because of the kit’s more stringent criteria for generating a positive result. The US CDC kit requires both SARS-CoV-2 N gene targeting amplicons (N1 & N2) to be positive/detected (Fig 1). If only 1 of the amplicons is detected, the test protocol suggests that the test be read out as “inconclusive” and repeated. If the US CDC kit used 1 gene/amplicon (N2) with the most efficient RNA extraction system tested, the kit’s LOD would be 316 copies/mL. Of note, the US CDC kit also uses an extraction positive control that detects the human RNase P gene.
In summary, a test that does not use a concurrent sample-derived positive control would have a higher false-negative rate than a test with a human gene positive control. If a sample contained an amplification inhibitor, both ID Now and an RT-PCR assay with an extraction control would report that the test was invalid. If a sample had insufficient sample (ie, not enough RNA isolated from human epithelial cells), ID Now would report a negative result whereas an RT-PCR test with an extraction control would report invalid results (insufficient sample vs inhibitor).
Antibody-based tests
The SARS-CoV-2 particle is composed of multiple virally encoded proteins. The S protein is the largest structural protein and makes the distinct spikes on the surface of the virus. For most coronaviruses, S protein is cleaved by a host cell furin-like protease into 2 separate polypeptides S1 and S2. The N protein binds the viral genome in a beads-on-a-string–type conformation. E protein is found in small quantities embedded within the host-derived viral envelope membrane. It is not necessary for viral replication but it is for pathogenesis. Membrane (M) protein is the most abundant structural protein. It may have 2 different conformations to enable it to promote membrane curvature as well as bind to the N protein. Hemagglutinin-esterase dimer protein is present in a subset of betacoronaviruses but is absent from the SARS-CoV-2 genome.18 , 19
In some cases, positive serology may reflect previous or current infection with other non–SARS-CoV-2 strains. Utilization of a SARS-CoV-2–specific protein for immunoassays is ideal to limit possible false-positive test results resulting from cross-reactivity with other viruses. The N and S2 proteins share a highly conserved structure with SARS-CoV, with a 90% homology in both protein sequences between the 2 viruses. However, other coronavirus protein sequences are much less conserved in the N and S2 proteins. The S1 subunit is less conserved and more highly specific to SARS-CoV-2. In addition, the receptor-binding domain of the S protein shows much less cross-reactivity between SARS-Cov-2 and other coronavirus strains.20 These are more ideal protein targets for serologic testing. Currently available tests and previous serology research studies have used antibodies against the N protein, receptor-binding domain, or S protein. Still, all serology test methodologies may lead to false-positive results from acute infection with a different coronavirus due to some sequence similarities. Antibody-based testing evaluates for the presence of IgM and IgG specific to SARS-CoV-2 in whole blood, plasma, or serum.21 , 22 Antiviral IgA antibodies were studied in patients diagnosed with COVID-19. Studies showed that IgA antibodies targeting the S protein are detected from day 6 to 8 and up to 42 days, with a peak at 20 to 22 days.21 , 23 , 24 However, at this time there are no commercial tests currently developed to measure anti–SARS-CoV-2 IgAs.
The timing of the emergence of IgM and IgG specific to SARS-CoV-2 during COVID-19 has been described in few publications.14 , 20 These indicate that IgM and IgG appear almost simultaneously and with an onset occurring approximately 1 week after the onset of first symptoms, similar to reports of antibody kinetics from SARS-CoV-1 infections.25 Antibody titers vary by severity of symptoms, with levels being highest in the most severe infections. Thus, it is not recommended to use serologic tests to confirm COVID-19 when the nucleic acid–based test results are negative. Serologic tests have shown cross-reactivity with other coronaviruses and can be seen in persons who were negative to SARS-CoV-2 by RT-PCR–based tests and have had no symptoms of COVID-19. Although the serologic tests approved measure antibodies to the S and N viral proteins, not all such antibodies are neutralizing antibodies; hence, they may not be reliable in identifying persons who are protected from the SARS-CoV-2 infection. Compared with RT-PCR–based tests, antibody-based tests have lower sensitivity and specificity. Negative results should be interpreted with caution, because negative serology does not definitively rule out an acute infection.
Rapidity of development and length of persistence of specific antibodies are not fully described, and therefore the presence of IgM or IgG may not indicate an active infection. Current infection is suspected with positive IgM and negative IgG; however, current or recent infection may also be indicated with positive results for both IgM and IgG. Positive testing to IgG alone indicates recent or past infection. Results from serologic testing should not be used in isolation. They should be combined with results from other laboratory tests including PCR-based tests and clinical history and presentation. It is still not known which antibodies will confer immunity and the duration of the immunity conferred.
Previous studies have demonstrated a range of rapidity of seroconversion of patients infected with SARS-CoV-2. IgM is typically present first; however, IgG often emerges quickly thereafter. A recent study demonstrated seroconversion of IgM and IgG in 94.3% and 79.8% of patients 15 days after symptom development, respectively.14 Other studies demonstrated seroconversion between less than 1 week to more than 6 weeks after symptom development.10 , 13 , 20 Patients with more severe disease presentations were more likely to develop antibodies earlier than those with milder disease and have higher peaks of antibody levels.13
Seven laboratories have received EUA for an antibody-based test.
Reported performance characteristics of the tests listed below were generated by the manufacturer and as of this writing have not been independently validated or corroborated by the FDA, the US CDC, or other independent researchers.
The Cellex qSARS-CoV-2 IgG/IgM Rapid Test was the first to be approved on April 1, 2020. It is a lateral flow chromatographic immunoassay that can detect IgM and IgG antibodies against SARS-CoV-2. Of note, the information provided on the testing procedure does not specify on which epitopes of the 4 structural proteins of the virus (S, N, E, and M) the detected patient-derived IgM and IgG bind. Per the manufacturer, the test performance as regards positive percent agreement and negative percent agreement is120/128 (93.8%) (95% CI, 88.2%-96.8%) and 240/250 (96.0%) (95% CI, 92.8%-97.8%], respectively.
The DPP COVID-19 IgM/IgG System manufactured by Chembio Diagnostic Systems, Inc, is a rapid immunochromatographic test for recognition of IgM and IgG for SARS-Cov-2 using the targeted N protein. False-positive results may occur secondary to the presence of antibodies to other coronaviruses. Samples used for validation included patient plasma or venous whole blood obtained from less than 1 to 21 days postsymptom onset. Per the manufacturer, the test performance as regards to positive percent agreement was 24/31 (77.4%) (95% CI, 60.2%-88.6%) for IgM and 27/31 (87.1%) (95% CI, 71.1%-94.9%) for IgG. Test performance as regards to negative percent agreement was 40/41 (97.6%) (95% CI, 87.4%-99.6%) for IgM and 38/41 (92.74%) (95% CI, 80.6%-97.5%). Cross-reactivity was found during testing for other coronaviruses, but not for other noncoronaviruses.
The VITROS Immunodiagnostic Products Ant-SARS-CoV2 Total Reagent Pack manufactured by Ortho-Clinical Diagnostics, Inc, is an ELISA for total SARS-Cov-2 IgM and IgG. The target protein is the S protein. Samples used for validation included serum or plasma samples obtained between 1 and 9 days after symptom development for samples with known symptom onset data; however, the date of symptom onset was not disclosed for all used samples. Per the manufacturer, test performance as regards to the positive percent agreement was 30/36 (83.3%) (95% CI, 67.2%-93.6%) and as regards to negative percent agreement was 400/400. There was no demonstrated cross-reactivity with other upper respiratory tract viral infections, although cross-reactivity with alternative coronaviruses was not evaluated.
The COVID-19 ELISA IgG Antibody Test manufactured at Mount Sinai is an ELISA-based test evaluating the presence of SARS-Cov-2 IgG using an initial target protein of the receptor-binding domain, followed by a confirmatory ELISA against the full S protein. Samples used for validation included those obtained 7 to 14 days after symptom development. Sensitivity is unknown, and no testing to evaluate for possible cross-reactivity with other coronaviruses was completed.
In addition to the 7 FDA-approved tests, multiple other serologic tests are being conducted under the Presidential authorization dated March 13, 2020. According to a recent publication evaluating the test performance of 12 serologic tests, the tests vary in sensitivity, specificity, and their ability to measure IgM or IgG or both simultaneously. Given the large number of these tests being designed and used in public, commercial, and academic institution laboratories, it will continue to be very difficult to assess and compare the tests’ performances and their place in the testing algorithms.
Antigen-based tests
On May 8, 2020, the FDA gave an EUA for an antigen-based test to Quidel Corporation. An antigen-based test detects the viral protein, and in this case the test detects the N protein antigen from SARS-CoV-2. The test is qualitative and uses a nasopharyngeal or nasal sample, which can be used directly or can be placed in viral transport media to transport to a laboratory. It is a POC test that uses lateral flow technology. Antigen tests are very specific but are not sensitive, so a negative test result in circumstances that suggest active infection need to be confirmed with an NAT.
Sample acquisition for tests
Each test’s EUA specifies the sample on which the test can be run. Because SARS-CoV-2 resides in the upper and lower airways, the preferred location for obtaining these specimens is from the nasopharynx, which will represent the upper and lower airways. However, other specimens can be analyzed in symptomatic patients if obtaining a nasopharyngeal specimen is not possible including oropharyngeal specimen, or a bilateral midturbinate or anterior nares specimen in symptomatic patients as well as throat swabs. Sputum from symptomatic patients with a productive cough or a lower respiratory aspirate in mechanically ventilated patients and bronchoalveolar lavage fluid can be tested. Recent data suggest that the virus replicates in the throat; hence, throat swabs can be used for testing.12 In a publication that states that saliva can be collected for test, a review of the Methods section suggests that the sample collected is sputum and that the use of the word saliva is a misnomer.10 A study under review and accessed online tested nasopharyngeal and saliva samples from confirmed patients with COVID-19 and self-collected samples from health care workers on COVID-19 wards. They compared SARS-CoV-2 detection from patient-matched nasopharyngeal and saliva samples. They found that saliva yielded greater detection sensitivity and consistency throughout the course of infection, and self-sample collection of saliva has less variability than nasoparyngeal samples.26 The FDA has approved 1 test for saliva samples to be run only in the Rutgers Clinical Genomics Laboratory (Table III). No definitive studies have been published delineating differences in sensitivity or specificity of PCR-based testing results between different sampling locations for SARS-CoV-2. However, the CDC recommends the nasopharyngeal sampling location over other sampling locations at this time. Anterior nare and midturbinate sampling locations are recommended by the CDC as possible alternative sites in actively symptomatic patients, and only if nasopharyngeal sampling is not possible. Samples should always be obtained with a sterile swab. The FDA recommends flocked swabs and cautions against the use of calcium alginate swabs or wooden-handled swabs, because these may affect testing by inhibiting PCR testing or by inactivating the virus. Flocked swabs are often more effective for sample collection than cotton, rayon, or Dacron swabs, and cotton-tipped swabs may also contain ingredients that inhibit PCR testing.
Using appropriate technique during sample acquisition is paramount to ensure accurate results. Inappropriate sampling may lead to insufficient sample volume or type and may result in false-negative results.27 The CDC recommends obtaining nasopharyngeal specimens by inserting the sterile swab through the nares, confirming the swab is parallel to the palate. Appropriate depth of insertion equals the distance from the nostrils to the outer opening of the ear. Encountering resistance after insertion can also indicate suitable contact with the nasopharynx. The swab is then rolled and kept in place for several seconds to allow absorption of secretions.28 These samples should only be obtained by medical professionals, with proper personal protection equipment in place. In addition, false-negative results may be received if the sample is improperly handled, or if the patient is no longer actively shedding viral particles.
For most RT-PCR–based tests, a transport media is required. For these, the CDC has designated viral transport media/universal transport media as the preferred transport media; however, other transport media or laboratory-created media are likely also acceptable. Specimens should be evaluated rapidly; however, storage for up to 72 hours at 4°C is approved. Limited data exist regarding freezing specimens, and stability should be assessed before use if the specimen was frozen. Recent RT-PCR POC tests, for example, Abbott’s ID NOW test discussed above, requires that sample swab be placed directly on the sample receiver. Antibody-based tests require a serum sample obtained from a finger prick or a venipuncture.
Test specimens are to be obtained by a health care worker, and no self-testing is available. This creates an interdependence of testing with health care workers where increasing the number of tests uses scarce resources of health care workers and the required personal protection equipment. However, one of the main benefits of testing is to protect health care workers from exposure to undiagnosed infected persons. On April 21, 2020, the FDA reissued the EUA for the Laboratory Corporation of America (LabCorp) COVID-19 RT-PCR Test to permit testing of self-collected nasal swab samples by patients at home using LabCorp’s Pixel by LabCorp COVID-19 Test home collection kit. This kit is different in that the swab used for sample collection is a cotton swab.
Timing of tests
A limited number of studies have compared the timing of detection of the viral DNA, and the timing of seroconversion, with the course of the illness starting with the incubation period and during convalescence.12 Figure 2 depicts the relationship among the 3 factors. It is important to note that the yield of the tests compared with the stage of the illness also depends on how mild or severe the illness is, which introduces a fourth factor in these analyses. As of the time of submission of this manuscript, the RT-PCR–based tests continue to be used for diagnosis of infection after onset of symptoms and in evaluating contacts of confirmed patients in the process of contact tracing. Repeat testing by RT-PCR–based tests after resolution of symptoms may be used to determine when a patient is no longer contagious. Several issues need to be taken into consideration. The duration of viral shedding can be prolonged and is also related to the severity of the illness. Detection of viral fragments by the test may give a false-positive test result. The use of RT-PCR–based tests for epidemiologic surveillance requires well- designed prospective studies.
One study looked at PCR-based tests for surveillance of nonsymptomatic persons. Two New York hospitals implemented universal SARS-CoV-2 screening using PCR-based testing from nasopharyngeal specimens from pregnant women upon admission for delivery of their infant. In these asymptomatic obstetric patients, 13.7% were positive for SARS-CoV-2. The authors suggest that universal screening may allow for improved isolation and personal protection equipment practices.29
Serologic tests are not recommended to be used for diagnosis of the infection because the timing of the appearance of IgM and IgG is variable and may be delayed. They are also related to the severity of the infection, with higher IgG levels in more severe infections.14 Serologic tests can be used in epidemiologic studies and surveillance as well as identifying patients who can become convalescent serum donors for experimental treatment protocols.
Roadblocks to testing
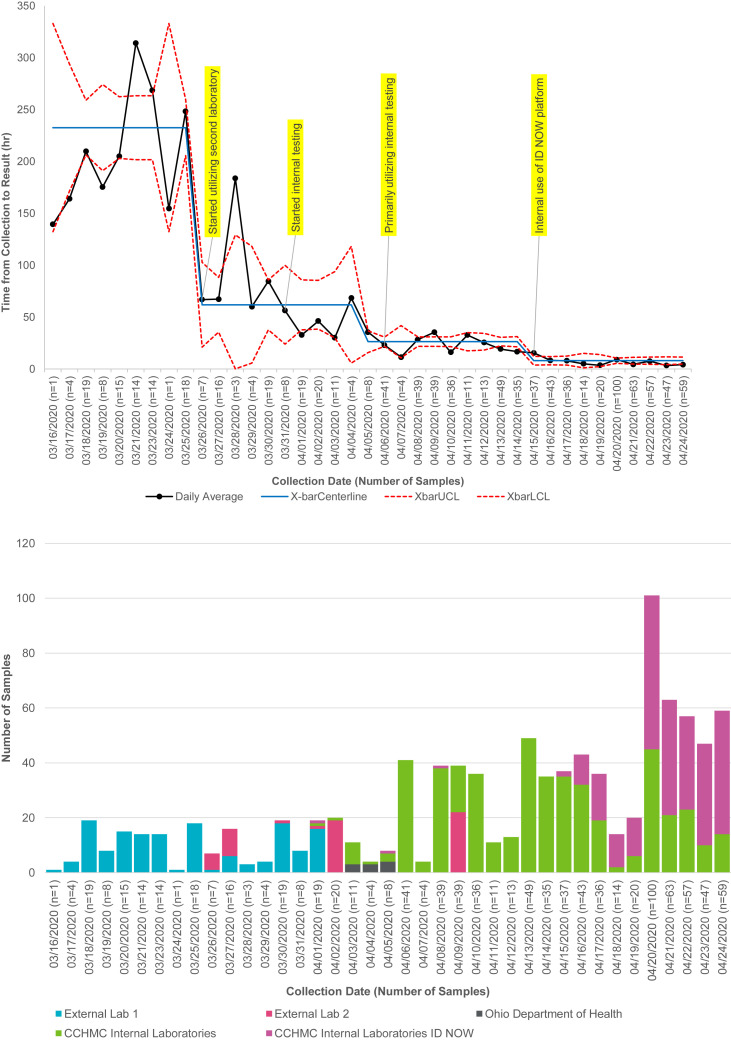
Although all these tests and others have received EUAs, the consensus is that testing has not been as available as needed for the rapid diagnosis of symptomatic patients and for the detection of the infection in mildly symptomatic people or the asymptomatic contacts of patients diagnosed with COVID-19. This has resulted from multifactorial issues ranging from overwhelming the commercial laboratories to exhausting limited supplies of swabs, transport media, reagents, and cartridges/kits for platform instruments needed for the assays. A potential strategy is to move the testing to “in-house” laboratories residing within hospitals and medical institutions. Currently, there are no national data on the turnaround time of testing, so we present here our experience at CCHMC, Cincinnati, Ohio. We tracked the time it took for COVID-19 test results to return since March 16, 2020. The longest turnaround was for samples collected on March 21, 202. A total of 14 samples that day had an average turnaround of 13.1 days. The 25th, 50th, and 75th percentiles were 12.2 days, 13.6 days, and 13.7 days, respectively. This meant that a patient suspected of COVID would have been infectious for a long duration before confirmation of the infection, resulting in difficulty in managing contacts. The first reduction in turnaround time was seen for samples collected on March 31, 2020, when a second external laboratory was contracted to perform the test. The second reduction in turnaround time was seen for samples collected on April 5, 2020. The remarkable decrease in the turnaround time occurred on April 13, 2020, after testing started in-house, with a turnaround time of a few hours to a maximum of 24 hours, and further decrease noted thereafter when the Abbott ID NOW was used to test patients immediately before surgical procedures (Fig 3 ).30
Fig 3.
Tracking of time to receipt of COVID-19 RT-PCR test results by date of test and change by testing site. For the X-bar portion of the X-bar and S control chart, each data point represents the average turnaround time of all tests collected that day. The center line is the average of the daily data points, subgrouped by preintervention and following system changes, when special cause is detected according to Western Electric methodology. Control limits on the X-bar are calculated, on the basis of average SD of the subgrouped points, multiplied by a factor for group size for each data point.30
Prioritization of testing
Guidance for prioritization of testing in the face of limited resources has been offered by the WHO (Table V ).31 Highest priority is recommended for vulnerable populations who may require hospital-level care for COVID-19, heath care workers including nonclinical staff, and the first symptomatic patients in a closed setting such as long-term living facilities. Prioritizing these specific populations allows for more rapid interventions for the most vulnerable patients, decreases the risk of transmission between and from health care providers, and helps to more rapidly identify and limit outbreaks in contained settings. The CDC has updated its guidance on testing on March 24, 2020 (Table VI ).32 The highest priority groups for testing include hospitalized patients and symptomatic health care workers, to reduce the spread of infection in the health care system and optimize care for those patients requiring hospital care. Lower priority test groups include symptomatic patients who reside in a long-term care facility, are aged 65 years or older, and patients with underlying medical conditions or first responders, because these groups may be at a higher risk of complications if infected. Lowest priority is given to groups such as other critical workers or other individuals who are symptomatic, asymptomatic health care workers or first responders, or those living in communities with high hospitalization rates secondary to SARS-CoV-2 in an effort to protect health care workers and reduce the spread of the virus through communities. The CDC recommends that asymptomatic persons not be given priority for testing. The difficulties in obtaining an adequate number of tests has led hospitals and institutions to create algorithms to prioritize which patients need to be tested. The CCHMC has assembled a committee with representation from clinical divisions and has generated 3 tiers of testing included as an example of the local application of the testing guidance (Table VII ). The principle behind the guidance is to suit the availability of tests to the areas of highest need on the basis of experience gained to date from the behavior and course of the pandemic in other countries. The WHO guidelines prioritize the vulnerable populations, health care workers, and those in closed environments who are most likely to be infected. The CDC guidance prioritizes symptomatic individuals in similar tiers as the WHO and guides against testing of asymptomatic individuals. The CCHMC guidance prioritizes testing for patients who are at the highest risk of getting the infection, patients who are at risk of complications and hence may benefit from available therapies, which are mostly experimental at this time, and patients in whom a negative test result is necessary before proceeding with critical procedures. The tiers reflect the testing priorities of the CDC and the WHO, to assist clinicians with testing decision making. Priority is given to symptomatic inpatients who are immunocompromised, reside in a long-term care facility, depend on respiratory technology, are younger than 1 year, or those who develop symptoms after hospital admission. Testing is also recommended for inpatients with 2 or more SARS-CoV-2–specific symptoms without an alternative unifying diagnosis, symptomatic oncology patients evaluated in the emergency department, or other asymptomatic higher risk populations. These high-risk asymptomatic groups include those pretransplant patients with an organ offer, patients receiving high-risk surgical procedures, chemotherapy, or bone marrow transplant, solid-organ transplant patients with acute rejection treated by biologic therapy, or neonates from a SARS-CoV-2–positive mother.
Table V.
Guidance from the WHO around testing in the face of limited resources31
In the setting of limited resources in areas with community transmission, prioritization for testing should be given to:
|
Table VI.
CDC guidance32 for prioritizing testing as of March 22, 2020
|
Priority 1 Ensure optimal care options for all hospitalized patients, lessen the risk of nosocomial infections, and maintain the integrity of the health care system
|
|
Priority 2 Ensure that those who are at the highest risk of complication of infection are rapidly identified and appropriately triaged
|
|
Priority 3 As resources allow, test individuals in the surrounding community of rapidly increasing hospital cases to decrease community spread, and ensure health of essential workers
|
Nonpriority
|
Table VII.
Prioritization of use of testing for COVID-19 at the CCHMC∗
Inpatients or those being admitted with fever OR respiratory distress
|
Other inpatients or those being admitted
|
Symptomatic outpatients
|
Asymptomatic patients
|
ATG, Anti-thymocyte globulin; BMT, bone marrow transplantation; ED, emergency department.
This is a recitation of general scientific principles, intended for broad and general physician understanding and knowledge, and is offered solely for educational and informational purposes as an academic service of the CCHMC. This should in no way be considered as an establishment of any type of standard of care, nor is it offering medical advice for a particular patient or as constituting medical consultation services, either formal or informal. Although this may be consulted for guidance, it is not intended for use as a substitute for independent professional medical judgment, advice, diagnosis, or treatment.
Numbers of tests, locations, and outcomes
According to the COVID-19 tracking project, and as of May 13, 2020, the number of tests performed was approximately 10 million (9,974,831), with close to 1.4 million (1,382,304) positive at a cumulative rate of 13.6% positivity.33 Tests are conducted at public health, clinical, and commercial laboratories. According to data on the CDC Web site, up to May 1, 2020, of the 676,488 tests done at public health laboratories, 396,585 at clinical laboratories, and 3,809,190 at commercial laboratories, the percent positive is 15.7%, 10.2%, and 18.0%, respectively.32
Discussion
Despite the surge in the number of laboratories and institutions offering testing, there remains a need for increased testing capacity. There is yet no available data on what the indications were for the already performed tests in order to relate the pretest probability with the outcome of the test, whether positive or negative. Per guidance, only symptomatic persons were tested; however, the degree of adherence to the guidance is not known.
There are multiple unresolved clinical issues surrounding the use of the COVID-19 diagnostics tests outlined above, ranging from technical limitations of each testing modality to their utility for individual patient management and population-wide infection control. NATs remain the most useful test for the diagnosis of acute COVID-19, but have some clear limitation. NATs currently available for clinical use do not distinguish between functional/infectious (“live”) and nonfunctional/noninfectious viral RNA. These tests primarily quantify genomic viral RNA. Genomic viral RNA not properly enveloped and packaged into an intact viral particle, however, is generally not infectious, but can still be detected via NATs. This limitation makes clear demarcation of when a specific patient is no longer infectious more difficult to determine. A recent clinical report showed that patients with COVID-19 who were serially tested for SARS-CoV-2 via RT-PCR could remain positive for 3 to 4 weeks or longer, well past the time when they were symptomatic.13 Whether these subjects remain contagious for as long as they are SARS-CoV-2 PCR positive is not entirely clear. Reassuringly, some emerging lines of evidence (viral culture, viral gene transcription studies) suggest that active viral shedding ceases after approximately 10 to 14 days of infection. Functional and nonfunctional SARS-Cov-2 viral particles can be distinguished from each other via viral culture techniques. In a recent study, “live” SARS-CoV-2 could not be cultured from patient sputum after day 8 of symptoms.12 Unfortunately, SARS-CoV-2 viral culture is labor/time intensive, not easily scalable, and requires significant biosafety measures be in place (Biosafety level 3–equipped laboratory per US CDC guidance). An alternative method to distinguish between live and inactive viral RNA relies on quantification of viral subgenomic RNAs (sgRNAs), which are transcribed only within infected cells actively shedding viral particles. SARS-CoV-2 sgRNAs could be detected in patient sputum out to the 10th symptomatic day, whereas this signal disappeared at day 5 of symptoms in throat swab specimens, suggesting that viral replication continues in the lower airway throughout the second week of infection.12 Taken together, current evidence would support quarantining SARS-CoV-2 subjects, whether symptomatic or asymptomatic, for 2 weeks after onset of symptoms.
As the pandemic progresses and more people are presumed recovered, the population immunity and true surveillance can be measured only with antibody-based tests. As of today only 7 such tests have received EUA; however, multiple other tests are in development in commercial laboratories and academic institutions. With the large numbers of people needing antibody-based tests, the same hurdles may develop, in addition to the lower sensitivity and specificity of these tests. The virus has several different immune-dominant proteins (S and N proteins), and different antigens will likely play different roles in the development of immunity; therefore, knowledge of the antibodies that will predict protection will have to be established.
Conclusions
Tests for COVID-19 have been instrumental in the management of the pandemic. The utility of COVID-19 testing has been diminished by limited resources, reducing the number of tests that could be offered in the face of a rapidly increasing epidemic. There has been difficulty in extending the tests to the populations most in need and possibly a lack of stringent application of the testing guidelines. It is the hope that as the pandemic subsides, antibody-based tests would have a larger contribution to the surveillance of the population and that the widespread use of NATs would provide much needed data on the spread of the pandemic particularly in vulnerable populations. It is also the hope that testing devices would move toward POC tests and possibly self-tests, which would allow the continued monitoring of the disease spread as well as facilitate preparedness for future recurrences of the disease that are predicted.
Footnotes
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
References
- 1.Food and Drug Administration https://www.fda.gov/media/135659/download Available at:
- 2.Food and Drug Administration https://www.fda.gov/medical-devices/emergency-situations-medical-devices/emergency-use-authorizations#covid19ivd Available at:
- 3.Brady P.W., Tchou M.J., Ambroggio L., Schondelmeyer A.C., Shaughnessy E.E. Quality improvement feature series article 2: displaying and analyzing quality improvement data. J Pediatric Infect Dis Soc. 2018;7:100–103. doi: 10.1093/jpids/pix077. [DOI] [PubMed] [Google Scholar]
- 4.Benneyan J.C., Lloyd R.C., Plsek P.E. Statistical process control as a tool for research and healthcare improvement. Qual Saf Health Care. 2003;12:458–464. doi: 10.1136/qhc.12.6.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Food and Drug Administration https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-serological-tests Available at:
- 6.Chu D.K.W., Pan Y., Cheng S.M.S., Hui K.P.Y., Krishnan P., Liu Y. Molecular diagnosis of a novel coronavirus (2019-nCoV) causing an outbreak of pneumonia. Clin Chem. 2020;66:549–555. doi: 10.1093/clinchem/hvaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan J.F., Yuan S., Kok K.H., To K.K., Chu H., Yang J. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020:25. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Udugama B., Kadhiresan P., Kozlowski H.N., Malekjahani A., Osborne M., Li V.Y.C. Diagnosing COVID-19: the disease and tools for detection. ACS Nano. 2020;14:3822–3835. doi: 10.1021/acsnano.0c02624. [DOI] [PubMed] [Google Scholar]
- 10.To K.K., Tsang O.T., Leung W.S., Tam A.R., Wu T.C., Lung D.C. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20:565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng S., Fan J., Yu F., Feng B., Lou B., Zou Q. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ. 2020;369:m1443. doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, et al. Virological assessment of hospitalized patients with COVID-2019 [published online ahead of print April 1, 2020]. Nature. https://doi.org/10.1038/s41586-020-2196-x. [DOI] [PubMed]
- 13.Yongchen Z., Shen H., Wang X., Shi X., Li Y., Yan J. Different longitudinal patterns of nucleic acid and serology testing results based on disease severity of COVID-19 patients. Emerg Microbes Infect. 2020;9:833–836. doi: 10.1080/22221751.2020.1756699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao J, Yuan Q, Wang H, Liu W, Liao X, Su Y, et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019 [published online ahead of print March 28, 2020]. Clin Infect Dis. https://doi.org/10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed]
- 15.Backer J.A., Klinkenberg D., Wallinga J. Incubation period of 2019 novel coronavirus (2019-nCoV) infections among travellers from Wuhan, China, 20-28 January 2020. Euro Surveill. 2020:25. doi: 10.2807/1560-7917.ES.2020.25.5.2000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guan W.J., Ni Z.Y., Hu Y., Liang H.R., Chen Z.S., Li Y.M. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nie S., Roth R.B., Stiles J., Mikhlina A., Lu X., Tang Y.W. Evaluation of Alere I Influenza A&B for rapid detection of influenza viruses A and B. J Clin Microbiol. 2014;52:3339–3344. doi: 10.1128/JCM.01132-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mousavizadeh L, Ghasemi S. Genotype and phenotype of COVID-19: their roles in pathogenesis [published online ahead of print March 31, 2020]. J Microbiol Immunol Infect. https://doi.org/10.1016/j.jmii.2020.03.022. [DOI] [PMC free article] [PubMed]
- 19.Chan J.F., Kok K.H., Zhu Z., Chu H., To K.K., Yuan S. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect. 2020;9:221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okba N.M.A., Muller M.A., Li W., Wang C., GeurtsvanKessel C.H., Corman V.M. Severe acute respiratory syndrome coronavirus 2-specific antibody responses in coronavirus disease 2019 patients. Emerg Infect Dis. 2020;26 doi: 10.3201/eid2607.200841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo L, Ren L, Yang S, Xiao M, Chang D, Yang F, et al. Profiling early humoral response to diagnose novel coronavirus disease (COVID-19) [published online ahead of print March 21, 2020]. Clin Infect Dis. https://doi.org/10.1093/cid/ciaa310. [DOI] [PMC free article] [PubMed]
- 22.Li Z, Yi Y, Luo X, Xiong N, Liu Y, Li S, et al. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis [published online ahead of print February 27, 2020]. J Med Virol. https://doi.org/10.1002/jmv.25727. [DOI] [PMC free article] [PubMed]
- 23.Matricardi PM, Dal Negro RW, Nisini R. The first, holistic immunological model of COVID-19: implications for prevention, diagnosis, and public health measures [published online ahead of print May 2, 2020]. Pediatr Allergy Immunol. 10.1111/pai.13271. [DOI] [PMC free article] [PubMed]
- 24.Padoan A., Sciacovelli L., Basso D., Negrini D., Zuin S., Cosma C. IgA-Ab response to spike glycoprotein of SARS-CoV-2 in patients with COVID-19: a longitudinal study. Clin Chim Acta. 2020;507:164–166. doi: 10.1016/j.cca.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsueh P.R., Huang L.M., Chen P.J., Kao C.L., Yang P.C. Chronological evolution of IgM, IgA, IgG and neutralisation antibodies after infection with SARS-associated coronavirus. Clin Microbiol Infect. 2004;10:1062–1066. doi: 10.1111/j.1469-0691.2004.01009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wyllie A.L., Fournier J., Casanovas-Massana A., Campbell M., Tokuyama M., Vijayakumara P. Saliva is more sensitive for SARS-CoV-2 detection in COVID-19 patients than nasopharyngeal swabs. https://www.medrxiv.org/content/10.1101/2020.04.16.20067835v1 Available at: Published 2020. Accessed May 12, 2020.
- 27.Miller J.M., Binnicker M.J., Campbell S., Carroll K.C., Chapin K.C., Gilligan P.H. A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2018 update by the Infectious Diseases Society of America and the American Society for Microbiology. Clin Infect Dis. 2018;67:813–816. doi: 10.1093/cid/ciy584. [DOI] [PubMed] [Google Scholar]
- 28.https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html Available at:
- 29.Sutton D, Fuchs K, D’Alton M, Goffman D. Universal screening for SARS-CoV-2 in women admitted for delivery [published online ahead of print April 13, 2020]. N Engl J Med. https://doi.org/10.1056/NEJMc2009316. [DOI] [PMC free article] [PubMed]
- 30.Provost L.P., Murray S.K. Jossey-Bass; San Francisco, CA: 2011. The health care data guide: learning from data for improvement. [Google Scholar]
- 31.Laboratory testing for coronavirus disease 2019 (COVID-19) in suspected human cases. Interim guidance 2 March 2020. https://apps.who.int/iris/bitstream/handle/10665/331329/WHO-COVID-19-laboratory-2020.4-eng.pdf?sequence=1&isAllowed=y Available at:
- 32.Centers for Disease Control and Prevention https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covidview.html Available at:
- 33.https://covidtracking.com/data/us-daily Available at: