Abstract
First humanoid coronavirus was discovered in the middle of 1960s, the class of viruses are considered to be a huge threat. The first onset of human coronavirus, SARS (SARS-CoV) appeared in 2003 which spanned five continents having lethal effects on human population accompanied by The Middle East Respiratory Syndrome Coronavirus in 2012 with a death rate of 35%. The viruses remain a threat till date and are of serious concern since no vaccine or specified drug therapy has been approbated for treating human coronaviruses. The viruses became a pandemic worldwide with the emergence of Wuhan coronavirus (2019-nCoV). SARS-CoV2 viral manifestation poses a serious human life risk by causing acute lung injury and various respiratory outcomes and has become a global concern. High pathogenicity and transmission rate of the viral strain has become the spotlight of research community throughout the world. With the ongoing studies on viral structure and host interactions, the intricacy of the viral proteome structure and replication cycle proposes a need to explore our understanding of host factors playing role in viral multiplication cycle. This review provides insight into our prevalent perception of coronavirus-host interactions, structure of SARS-CoV2, receptor mediated entry of virus inside the human cells, ongoing clinical trials, drug therapies and treatments that are being used to combat COVID-19 targeting viral fusion, replication and its multiplication.
Key Words: Human coronavirus, SARS-CoV2, Virus, Structure, Therapy, Treatments
Introduction
Named for their crown like spikes coronaviruses are categorized under Coronaviridae family, subfamily coronavirinae, order Nidovirales. A positive sense single stranded RNA with 5′–cap and 3′ polyA tail ranging from 26–32 Kbs forms the genetic material of coronaviruses (1).Virion envelope is formed of spike (S) protein, membrane (M) protein and the envelope (E) protein, a helical nucleocapsid surrounds the viral genome. Adding to the three envelope proteins some coronaviruses also contain a hemagglutinin esterase (HE) protein (2). The family of virus can infect different animal species along with humans and have been known for over 50 years, with the discovery of first coronavirus in 1930 (3,4) Four major subgroupings of the coronavirus family include alpha, β-infecting mammals; γ and δ-infecting birds predominantly. Human coronaviruses were first spotted amid-1960s. Amongst, seven different coronaviruses that can infect humans, the serotypes that can infect humans are of OC43-like and 229E type. Most common human coronaviruses belong to serotypes OC43 and 229E, both sharing common ancestor with bat coronavirus, (5). With the divergence of closely associated bat coronaviruses and SARS-CoV in 1986, major outbreak of human coronaviruses causing fatal pneumonia started from the beginning of the 21st century. With the spurt of SARS-CoV in 2003 spreading to five different continents with a 10% lethal rate and the incurrence of (MERS-CoV) with a fatality rate of 35% in 2012 (6), outbreak of human coronavirus is considered to be a huge threat due to non-affirmation of any vaccine or specific drug therapy for treating human coronaviruses. With the newly evolved Wuhan coronavirus (COVID-19), the situation becomes alarming all over the world. The viruses became a pandemic worldwide due to their potential to grow and high transmission rate posing a serious public health risk. On February 28th, 2020 WHO raised the threat caused by this epidemic to the very high level (7). Based on sequence analysis reports, the 2019-nCoV belongs to the group of β-coronaviruses possessing a characteristic coronavirus genome structure, the family have Bat-SARS-like (SL)-ZC45, Bat-SL ZXC21, SARS-CoV and MERS-CoV (8). Ancestral linkages based on phylogenetic analysis of CoVs shows close relation between bat-SL-CoV ZC45 and bat-SL-CoV ZXC21 and COVID-19 as compared to SARS virus (8). Coronavirus access host cells via its spike proteins, attached on viral envelope. The spike protein initiates receptor mediated binding between the virus and host cells, receptor binding is succeeded by the fusion of viral and host membranes (9). To confirm the viral mode of entrance inside the human cell, the receptor binding domain of 2019-nCoV spike protein was compared with those of SARS-CoV and SARS-like CoVs.
Structural analysis of 2019-nCov revealed vital analogies in amino acid sequence and envisaged 3D structure between 2019-nCoV and SARS-CoV, the findings further supports ACE2 receptor mediated entry of 2019-nCoV (10). Soon after the depiction and characterization of COVID-19, molecular assays to detect variant virus in the clinical samples were developed by American Centers for Disease Control and Prevention. A diagnostic tool based on RT-PCR has also been described, the method intended to target various combinations of viral genome including Orf region, envelope, nucleocapsid, and RNA-dependent RNA polymerase (RdRp) genes (11). For attachment to its host cell coronaviruses use homotrimers of the spike (S) glycoprotein, the S protein acts as a precursor of about 1300 amino acids and is synthesized as single polypeptide chain. S represents the main antigenic determinant at viral surface hence during viral infection S protein remains the target of neutralizing antibodies. Antigen-antibody interaction between viral and human cell proteins units makes it a prime focus towards vaccine designing (12).
Structural Analysis of SARS-C0V-2
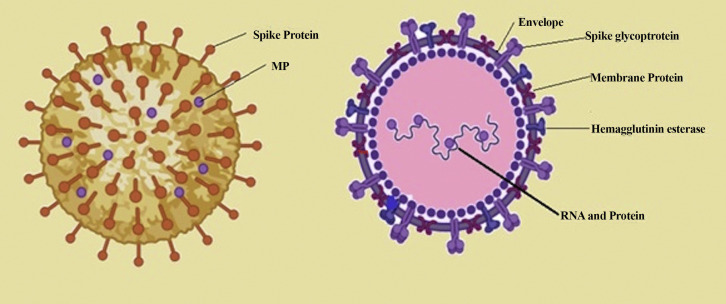
Based on sequencing and identification data SARS-CoV-2 was determined as a novel CoV having 88% sequence uniqueness with two bat-derived SARS-like CoV, 79.5% resemblance with SARS-CoV and 50% sequence homology to MERS-CoV (13). Structural analysis of coronavirus genome encodes four major structural proteins (Figure 1 ): S,N,M, E: S protein, N (nucleocapsid), M (membrane) and the E (envelope) protein (1). Different proteins encoded by vial genome regulate different functions, N protein is a multifunction protein regulating transcription processes and virion assembly efficiently. Trimeric S protein (each monomer 180 kDa) initiates viral entry into the target cell. COVID-19 spike (S) protein strongly determines viral transmission capacity and host tropism. S protein has a core domain and an external subdomain. Spike (S) protein consists of S1 and S2 subunit both involved in receptor binding and membrane fusion respectively (14). The S1 subunit of spike protein folds as two independent domains both formed by the N and C terminal of S1 protein. Either of the two domains can serve as the receptor binding domain depending on the viral strain (15). A serine and arginine rich domain involved in cell signalling known as SR-domain or linker region (LKR) separates the CTD from NTD. The LKR region has been proposed to bind with viral replicase-transcriptase complex (nsp3) and binding induced conformational changes in the LKR region are known to regulate intracellular localization of nucleocapsid (N) protein to the replication site (16). ORF1ab, ORF3a, ORF6, ORF7a, ORF10 and ORF8 forms the non-structural protein part of the virus that are transcribed. ORF1a and ORF1b cleavage produces a set of non-structural proteins that assemble together to carry out viral replication and transcription processes (17).
Figure 1.
Structure of virion: coronavirus genome has RNA bound nucleocapsid protein including four major structural proteins: S, N, M, E: spike glycoprotein, nucleocapsid (N) protein, membrane(M) protein and the envelope(E) protein.
Viral protein nsp12 (RNA-dependent RNA polymerase) mediates viral replication inside the host cell. The palm domain of RdRp consists of conserved polymerase motifs A-G forming the SARS CoV-2 RdRp active site. The configuration of COVID 19 RdRp active site resembles other RNA polymerases (18). Main proteases present in the viral genome governs coronavirus replication, proteases dissever proteins in to functional proteins which are then packaged into the virion. The proteases found in SARS-CoV-2 are highly homologous to the proteases present in SARS-CoV genome (19). Based on phylogenetic analysis by (20) indicated the origination of 2019-nCoV from bat with an unknown intermediate transmitter. Despite the positioning of the receptor binding domains in their respective down conformations structural analysis reports depicts a high resemblance between SARS-coronavirus and SARS-CoV2 structures. There is a high degree of homology between N-terminal domains (NTDs) and receptor binding domain (RBD) subdomains–SD1and SD2 along with S2 subunit of RBDs of SARS-CoV and SARS CoV-2 viral genome (21). Based on recent structural studies and homoly patterns SARS CoV-2 is found to exhibit a high affinity binding potential (10–20 fold) to human ACE2 than that of SARS-CoV owing to its high spread rate among human populations (14).
Receptor Mediated Viral Entry Inside the Cell
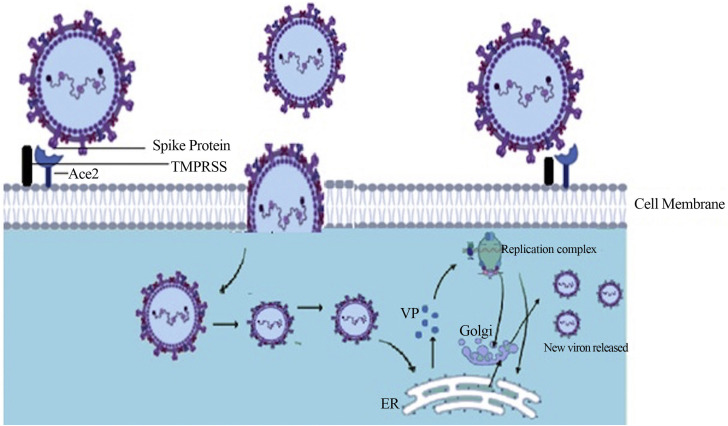
Human coronaviruses access the host cells through a receptor mediated entry felicitated by specific receptors present on host cells. Aminopeptidase-N (CD13) and sialic acid as a receptor determinant have been identified as the most common routes for viral entry. The synergy between the spike region of virus and its receptor initiates the attachment of viral particles to the host cell. There are variations present in RBD sites within the S1 region of spike protein among different coronavirus strains. The interaction between the S protein and its receptor strongly determine the pathogenesis of a coronavirus. Host receptor binding is mediated by the N terminal domain-NTD of spike protein S1 subunit. The S1-NTD interacts with the glycans present on host cell receptor surface. A galectin fold mediating binding to sialic acids are thought to be commonly shared among all coronavirus S1-NTD (22). Zoonotic β-coronaviruses (SARS-CoV) NTD shows a high binding affinity towards Angiotensin converting enzyme 2(ACE2) which serves as an operative receptor for viral entry (14). Cellular peptidase dipeptidyl peptidae 4 (DPP4) along with ACE2 has also been reported as cellular receptors used by β-coronaviruses MERS-CoV and bat coronavirus HKU4 (23). After receptor recognition and viral entry, host cell's machinery is then required for the production of viral proteins and viral progenies. The virus enters the host cell, uncoats followed by transcription and translation of viral genome. An exclusive feature of replication is a 3′ conterminal nested set formed by all the mRNAs; translating only particular portions of the 5′ ends producing 7 mRNAs. The shortest of the 7 mRNAs produced encodes the N protein, and the others designated to the synthesis of a further segment of the genome individually. Proteins formed are then massed together at the cell membrane with the incorporation of genomic RNA as a mature particle budded from internal membranes of the cell (24). Viral replication in the host cells induces ER stress as various stages of viral replication are closely associated with the endoplasmic reticulum (ER). Glycosylated S protein induced ER stress has also been descried in coronavirus-infected cells (25) (Figure 2 ). Subsequently, an adaptive immune response is induced by the host cells as a response to virus infection to limit viral pathogenesis. Various receptor classes trigger virus recognition in host cells-cytosolic sensors like MDA-5, RIG-I, toll like receptors (TLRs) expressed on the cell surface or different classes of TLRs- present in the immune cell endosomes, TLR3 and TLR4. A TLR-like signalling pathway non-dependent on canonical TRAF3-mediated signal pathway has also been observed as recognition receptor to SARS-CoV M protein (22).
Figure 2.
A Model of coronavirus entry and replication inside the host cell: After receptor recognition virus binds with host cell Ace2 receptor. TMPRSS (Trans-membrane Serine Protease) facilitated viral entry, uncoating followed by transcription and translation of viral genome (Replication complex) with the release of viral protein. Viral replication in the host cells induces ER stress due to close association of several stages of the coronavirus replication cycle with the endoplasmic reticulum (ER) producing viral proteins (VP) later forms new virion.
On analysis, the receptor binding motif (RBM) in S protein revealed conserved amino acid residues between SARS-viral genome and SARS-CoV-2 genome showing 70% similarity, having nine common residues essential for receptor recognition suggesting same host cell receptor used by CoV-2 strain for cell entry (9). A high propensity associated with ACE2 receptor and S1 domain of spike protein has been shown by (26), 293T cells were used and transfected with ACE2 encoding plasmid, specific recognition was shown by cells transfected with plasmid expressing ACE2 towards S1-Ig. A similar study was conducted by (27, 28) to evaluate whether ACE2 is the common receptor used by 2019-nCoV for cellular entry. In the study lung cell lines (HeLA) from humans, Chinese horse shoe bats, civets, pigs and mice were used. HeLa cells used for the study both expressed as well as did not show expression towards ACE2 proteins from humans. The study revealed that 2019-nCoV could use all the ACE2 receptors to the cells expressing ACE2, with mouse ACE2 being an exception, which indicates the probability of ACE2 being the entry receptor used by 2019-nCoV. A close relation exists between the binding energy and RBD interface sequence of COVID-19 and SARS-CoV-2 suggests the possibility of a recombination event involving viral S protein between SARS-CoV and COVID-19 of ancestral lineages (26,28,29). Two main residues N479 and T487 have been associated in SARS-CoV to the recognition of the human ACE2 receptor. Intraspecies infection was possible due to Y442, L472, N479, D480, and T487 residues present in SARS-CoV. Some residues upon slight modification results in a stronger interaction with the receptors on human cell: L455, F486, Q493, and N501. In SARS-CoV-2, the N479 residues coincide to Q493 human cell receptor and T487 shows receptor recognition to N501 which are supported by the local environment present in the ACE2 receptor producing highly favourable electrostatic interactions for receptor binding (19).
Protein-protein docking experiments performed by Micholas Smith and Jeremy C. Smith, 2020 indicates several differences in the sequences of SARS-CoV and COVID-19/SARS-CoV-2 but having similar binding affinities for the human hACE2 receptor. Based on early genome data of SARS-CoV-2, a close association has been linked between the spike protein of viral genome and specific receptors present on human cells. Therefore, ligand receptor binding upholds spike(S) glycoprotein mediated viral entry into the cell in SARS-C0V2 (30, 31, 32). The RBD-spike protein acts as receptor recognition site that recognizes the receptors present on hACE2 (human angiotensin converting enzyme 2) regulated by TMPRSS2 proteases. Cryo-electron microscopy structures shows that two trimers of S protein can bind to n ACE2 homodimer simultaneously each through a monomer (33). On successful binding of viral S protein RBD to its receptor (ACE2) there is significant reduction in ACE2 mRNA expression, the virus uses ACE2 receptors for entering their target cells, where it proliferates causing the upper respiratory tract infection and damages lung tissue triggering acute lung injury (34).
Ongoing Clinical Trials Data
Till date, there are no specific medications or vaccines available to treat COVID-19. The clinical trials are in process on several potential antiviral drugs and vaccines. This review summarizes recent documentations regarding suggested treatments either therapeutic drugs or vaccines and provides recent clinical experiments and treatments that may lead to combat COVID-19. Below is some available literature of pre-clinical and clinical trials of several therapeutic drugs and vaccines used against COVID-19 treatment.
Fusion Inhibitors
These are the antiretroviral drugs that can interfere in binding, fusion and virus entry to a human cell. Some of drugs that are being used in case of COVID-19 are summarized here.
Recombinant human angiotensin-converting enzyme 2 (APN01)
APN01 mimic the human ACE2, the main receptor used for virus protein S to enter host cell. The virus binds to soluble recombinant rhACE2 (APN01) instead of cellular ACE2 on surface of cell which then inhibits the virus action. APN01 also act as anti-inflammatory against acute respiratory distress syndrome (35,36). Due to dual action of mechanism, APN01 could be a potential drug against COVID-19.
Chloroquine/hydroxychloroquine as fusion inhibitors
Chloroquine and hydroxychloroquine, antimalarial drugs mainly used for treating rheumatoid arthritis and systemic lupus erythematosus(37). Both chloroquine and hydroxychloroquine have shown in vitro activity against COVID-19 (18). The mode of action for both drugs are same. It involves ACE2 cellular receptor inhibition by viral protein glycosylation (37). It also increases pH of multivesicular bodies thus inhibits fusion of the virus. It also modulates immune system through attenuation of cytokines release (38). Others mechanism of action may include: viral enzymes (DNA/RNA Polymerases) inhibition, and inhibition of transport of virus particle and its assembly. Chloroquine alone has potential benefit against COVID-19 pneumonia patients. The data has been evaluated for the same effect and it has shown significant results. Despite this, hydroxychloroquine has also shown significant response against COVID-19. An in vitro study from china also stated that hydroxychloroquine shows more efficacy than Chloroquine (20,39). Hydroxychloroquine also showed significant activity against COVID-19 given in combination with azithromycin (40).
Arbidol Hydrochloride as Fusion Inhibitor
Arbidol Hydrochloride named Umifenovir, a broad-spectrum antiviral drug used for the treatment of human influenza infection and Arboviruses. Due to its hydrophobicity, it can form aromatic stacking interaction with some amino acids rendering it to act against viruses. It can also form stable interaction with plasma membrane of host cell which prevent viral entery (41,42). In a study it is stated that this drug is being used as a potential drug against COVID-19 combined with other HIV drugs in recent trials (13,43).
Drugs Used to Inhibit Viral RNA-dependent RNA Polymerase
The replication of Covid-19 depends on the mechanism of viral RNA-dependent RNA polymerase (RdRp) which can be a direct target for various antiviral drugs such as Remdesivir (GS-5734), Ribavirin and Favipiravir (T-705).
Remdesivir (GS-5734)
Remdesivir, an antiviral drug, has been a high potential antiviral treatment till date suited against varied range of RNA viruses. The USFDA has approved its use in Covid-19 (www.usfda.gov/corona). Remdesivir is a monophosphate prodrug, an inhibitor of RNA polymerases which is an analogue to adenosine. Remdesivir incorporates into developing viral RNA, thereby terminates viral RNA chain and consequently put an end to replication of the viral genome (43,44). Remdesivir has been administered to severe covid-19 patients in Japan, United States and Europe. Clinical trials conducted in patients of COVID-19 against efficiency of Remdesivir were evaluated and data shows its efficiency against coronavirus (clinicaltrials.gov/NCT04323761). It has also shown a significant activity against coronavirus in pre-clinical trials Successful case reporting Remdesivir as an potent antiviral drug has been reported (45).
Ribavirin
Ribavirin is an analogue to guanosine, a broad-spectrum antiviral drug which suppress viral RNA-dependent RNA polymerase activity. It involves several mechanism of action to inhibit replication of several virus (46). It is analogue to guanine, preventing the binding of the correct nucleotides which leads to inhibition of viral RNA synthesis and mRNA capping (47). It also acts a mutagen which leads to viral RNA termination and produces defective replicated viruses. It is earlier stated in vitro study that it also stimulates immunity through its immunomodulatory action and enhances the action of INF-α receptor and down-regulate the genes that are involved in interferon inhibition (48). Ribavirin has been certified against the treatment of COVID-19 (49). The activity of Ribavirin against other CoVs like SARS and MERS support its potential against COVID-19 treatment (50).
Favipiravir
Favipiravir- has also shown great deal of anti-viral effects against a wide array of human-infecting RNA viruses (51). As Similar to Remdesivir, it targets the viral RNA polymerase, making it a plausible treatment for Covid-19. It is an analogue to guanine which incorporates into host cell and converts to Favipiravir ribofuranosyl-5′-triphosphate (favipiravir-RTP) (52) thus acting as an inhibitor of RdRp. It can also undergo fatal RNA transversion mutations producing a nonviable viral phenotype causing alteration of viral replication (53). The clinical efficacy of Favipiravir against COVID-19 shown a significant activity (clinicaltrials.gov/NCT04310228). The NMPA of China approved this drug as first anti-COVID-19 drug.
Viral Protease Inhibitors
Protease inhibitors are antiviral drugs which after binding to viral proteases inhibits proteolytic cleavage of precursors protein required for production of infectious viral particles. Some Protease inhibitors are currently used in clinical and preclinical practices against COVID-19.
Ivermectin
Ivermectin, an anti-parasitic drug has antiviral activity against different viruses including HIV, dengue, Zika and influenza (54). It inhibits the interaction between viral protein and the importin (IMP) α/ß1 heterodimer, responsible for nuclear transport of viral protein (55). In Monash Biomedicine Discovery Institute Scientist Dr. Kylie Wagstaff conducted a study and found a significant reduction in viral RNAs with a single dose of Ivermectin within 48 h.
Lopinavir/Ritonavir
Lopinavir and Ritonavir, a combination drug commonly name–HAART (highly active antiretroviral therapy) are used against HIV/AIDS (56). Lopinavir and ritonavir suppress coronavirus infection by binding to 3CL pro, a key enzyme needed for replication of corona virus thereby inhibit viral assembly (FDA Approved Drug Information). This combination is also used in clinical studies for treatment of COVID-19 (43,57). Yet it has showed a negative result against COVID-19 but it is still possible that if this combination is combined with other drugs having high affinity for COVID-19 3CL pro, it may be useful for less severe cases of COVID-19.
Darunavir
Darunavir, HIV protease inhibitor which is synthetic nonpeptidic has been used in clinical trials against COVID-19. It competes with the active site of viral proteases via hydrogen bonds forms a inhibitor-enzyme complex thereby preventing cleavage of the polyproteins produces immature, non-infectious viral particles (58). It could have significant virological and immunological outcomes. Darunavir with Cobicistat is being given to patients under clinical trials against COVID-19 (ChiCTR; https://clinicaltrials.gov/ct2/show/NCT04252274). This drug might have a great advantage in coronavirus.
Velpatasvir/Ledipasvir
Along with sofosbuvir, an HCV NS5B polymerase inhibitor is used against the infection of chronic hepatitis C virus. These are NS5A inhibitors preventing HCV viral RNA replication and virion assembly (59). The COVID-19 and hepatitis C virus shares similar replication mechanism therefore sofosbuvir has been tested in clinical trials against COVID-19 recently. In one of the in vitro study, the virtual screening showed that velpatasvir in combination with sofosbuvir and ledipasvir in combination sofosbuvir may work against COVID-19 efficiently (8).
Baricitinib
An antiviral and an anti-inflammatory drug used to treat autoimmune disease called rheumatoid arthritis, is a selectively reversible Janus Kinase (JAK) inhibitor which ultimately modulates gene expression in immunological cells (60). This drug is being tested in clinical trials Phase 3 against COVID-19 and it showed efficacy for COVID-19.
Drugs in the reduction of inflammatory response
These drugs categorised under immunosuppressive drug which inhibits various immunological effects. There are various drugs under clinical trials. Some of them are mentioned here within.
Humanized Monoclonal Antibody
Tocilizumab
Tocilizumab, an immune suppressant, used against rheumatoid arthritis (61) which binds to interleukin-6 receptors (soluble and membrane bound) competitively thereby inhibiting its immunological effects (Talluri www.preprints.org). It is suggested drug to treat COVID-19 patients in China shown its remarkable effectiveness and safety in clinical practice (www.reuters.com.). It is also seen Tocilizumab has efficacy over COVID-19 in severe cases in Italy and Naples (National Library of Medicine). Siltuximab, another humanized Monoclonal Antibody with same mechanism of action as Tocilizumab, this drug has been used in various clinical studies and found it as a potential drug against COVID-19 (clinicaltrials.gov/ct2/show/NCT04329650).
TZLS-501
TZLS-501, the humanized monoclonal antibody developed by Tiziana Life Sciences, Biotechnology Company. It works as an inhibitor of IL-6 receptor in humans thereby reduces there by reduces chronic inflammation of lungs, it is under preclinical trials (Tiziana Life Sciences).
Use of Monoclonal Antibodies Against COVID-19 Treatment.
Anti-C5a
The humanized anti-human C5 monoclonal antibody, a landmark in the anti-complement drug discovery act as complement-specific inhibitor works as inhibitor of C5 protein of the cascade complement system of the immune system thereby attenuating the inflammatory Response. The suppression of complement represents a common therapeutic approach against virus induced infections such as pneumonia caused by COVID-19. MDA by National Medical Product Administration (NMDA) of China approved it for phase II clinical trial against COVID-19 treatment. Monoclonal Antibody named BDB-1 thought to have the action of Attenuating Inflammatory Response due COVID-19 infection (62). This drug has showed an efficacy against COVID-19. U.S National Library of Medicines suggested Eculizumab as a one of the drugs for Attenuating Inflammatory Response (clinicaltrials.gov/ct2/show/record/NCT04346797). Eculizumab as the first complement-specific inhibitor also works as inhibitor of C5 protein of the complement system of the immune system (63).
COVID-19 Vaccines Under Trial
Safe and effective development of vaccines is urgently required to prevent this pandemic COVID-19 infection in the future. It requires a long time to prepare a vaccine as it is highly complex procedure involving various steps. Several vaccines which are in process, are summarized here.
Vaccines Using Molecular Clamp’ Approach
The vaccine developed by this approach adds a gene to viral proteins to stabilize them which can trigger the body's immune system thereby creating antibodies against it. The scientists from University of Queensland in Australia supported by Coalition for Epidemic Preparedness Innovations (CEPI) are developing this vaccine. This vaccine is also under pre-clinical trial (University of Queensland Australia).
NO-4800-DNA Based Vaccine
The Inovio Pharmaceuticals, supported by Coalition for Epidemic Preparedness Innovations (CEPI) synthesized DNA based vaccine. This vaccine administered to human body can be then translated into proteins thereby induces immune response. This vaccine is presently under Phase 1/2 Clinical Trial in Korea (Inovio Pharmaceuticals Inc.).
NVX-CoV2373
NVX-CoV2373 is a nanoparticles-based vaccine developed by Novavax and funded by CEPI (Coalition for Epidemic Preparedness Innovations). This vaccine has surpassed the preclinical trials and about to undergo testing on human in coming weeks (Novavax).
mRNA-1273, mRNa Vaccine
mRNA-1273, developed by NIAID scientists in collaboration with other scientists at the US National Institute of Allergy and Infectious Diseases. This research is funded by CEPI (https://www.wsj.com).The mRNA-1273 encodes a perfusion-stabilized viral spike protein of COVID-19 act against COVID-19. The mRNA vaccine is under Phase 2 of clinical trial in US.
An Adenovirus Vector Vaccine
An Institute of Military Medicine under the Academy of Military Sciences has developed an adenovirus vector vaccine. The adenovirus-based vector contains the gene from spike protein of coronavirus which act as a vaccine against COVID-19. This vaccine has entered in Phase 2 clinical trials (Xinhua).
Inactivated Vaccine
Another vaccine developed by Sinovac Research and Development Ltd. a biopharmaceutical company in Beijing. The inactivated vaccines have been extracted from the live viruses grown on large scale. The pathogenicity is maintained while destroying the infectious part of virus so that it can be recognized by immune system thus induces adaptive immune response. This vaccine is under clinical trial in China (Xinhua).
COVID-19 Convalescent Plasma Therapy
Amongst the few emergency treatments to treat viral infection, doctors may consider the century old technique of supplying antibodies to critically ill patients from blood donors who have recovered. In this technique, the plasma is collected from the recovered person as it contains antibodies against COVID-19 developed while fighting with the virus. It is then used to treat affected COVID-19 patients. Once the COVID-19 patient has recovered, the blood sample is collected and the plasma is extracted out of it after proper checked for any other diseases. The extracted plasma containing antibodies against COVID-19 is then injected to a patient under treatment which further generates an immune response and protects against COVID-19 infections. This therapy has already been explored in some countries and showed improvement in some cases (64).
Conclusion
The study of coronavirus structure and virus-host interactions using systematic screening approaches can help in the development of antiviral strategies that involves the use of specific inhibitors. Insights into the similarities between SARS-CoV2 and other coronaviruses along with the study of host directed approach could help in developing broad spectrum therapeutic strategies to treat viral infection. Therapeutics drugs that are presently taken into consideration as potential medicament for COVID-19 includes antiviral drugs, anti-parasitic drugs, anti-inflammatory drugs, immunosuppressants drugs and inhibitors. The repurposing of Drugs can be another promising option to treat COVID-19 infections. However, use of convalescent plasma therapy as emergency treatments till the development of a potential drug therapy and vaccine can be considered to treat critically ill patients. A combination of drugs targeting viral replication and its spread inside human body could be of great help. Binding of viral particles to the host cell receptors is the key to viral spread and pathogenesis, using receptor analogues, inhibitors, creating alkaline environments inside the body to prevent receptor binding and further spread of virus is of great importance. Developing strategies to control host receptor binding to the viral particles thus disabling the virus to enter the cells could be of great help towards combating the disease. The treatment of COVID-19 infection is constantly changing with time which will enumerate further perception into the role of above-mentioned treatments against COVID-19 infection.
Avenues for COVID-19 Therapies based on ligand receptor binding:
-
a).
Use of Sheddase proteins like ADAM10, TACE for cleaving the extracellular domain of ACE2
-
b).
Increasing levels of angiotensin 7 which is an inhibitor of ACE2, leading to downregulation of protein
-
c).
Introducing anti ACE2 antibodies
-
d).
Antibodies developed during SARS-CoV infection
-
e).
Role of apelin, which is a substrate for ACE2.Introduction of apelin may lead to interaction between apelin and ACE2 receptors, making it less available for viral RBD. Moreover, viral target is cardioprotective protein and apelin shows cardioprotective effects and inhibition in lipid peroxidation and reduced ROS formation.
(ARCMED_2020_650)
References
- 1.Schoeman D., Fielding B.C. Coronavirus envelope protein: current knowledge. Virol J. 2019;16:69. doi: 10.1186/s12985-019-1182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perlman S., Netland J. Coronaviruses post-SARS: update on replication and pathogenesis. Nat Rev Microbiol. 2009;7:439–450. doi: 10.1038/nrmicro2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiss S.R., Navas-Martin S. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol Mol Biol Rev. 2005;69:635–664. doi: 10.1128/MMBR.69.4.635-664.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Estola T. Coronaviruses, a new group of animal RNA viruses. Avian Dis. 1970;14:330–336. [PubMed] [Google Scholar]
- 5.Belouzard S., Millet J.K., Licitra B.N. Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses. 2012;4:1011–1033. doi: 10.3390/v4061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cui J., Li F., Shi Z.-L. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17:181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cascella M., Rajnik M., Cuomo A. StatPearls Publishing; Treasure Island (FL): 2020. Features, evaluation and treatment coronavirus (COVID-19). StatPearls [Internet] [PubMed] [Google Scholar]
- 8.Chen Y.W., Yiu C.-P.B., Wong K.-Y. Prediction of the SARS-CoV-2 (2019-nCoV) 3C-like protease (3CL pro) structure: virtual screening reveals velpatasvir, ledipasvir, and other drug repurposing candidates. Version 2. F1000Res. 2020;9:129. doi: 10.12688/f1000research.22457.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wan Y., Shang J., Graham R. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020;94 doi: 10.1128/JVI.00127-20. e00127–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.York A. Novel coronavirus takes flight from bats? Nat Rev Microbiol. 2020;18:191. doi: 10.1038/s41579-020-0336-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Binnicker M.J. Emergence of a novel coronavirus disease (covid-19) and the importance of diagnostic testing: Why partnership between clinical laboratories, public health agencies, and industry is essential to control the outbreak. Clin Chem. 2020;66:664–666. doi: 10.1093/clinchem/hvaa071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tortorici M.A., Walls A.C., Lang Y. Structural basis for human coronavirus attachment to sialic acid receptors. Nat Struct Mol Biol. 2019;26:481–489. doi: 10.1038/s41594-019-0233-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV) Biosci Trends. 2020;14:69–71. doi: 10.5582/bst.2020.01020. [DOI] [PubMed] [Google Scholar]
- 14.Li F. Structure, function, and evolution of coronavirus spike proteins. Annu Rev Virol. 2016;3:237–261. doi: 10.1146/annurev-virology-110615-042301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kubo H., Yamada Y.K., Taguchi F. Localization of neutralizing epitopes and the receptor-binding site within the amino-terminal 330 amino acids of the murine coronavirus spike protein. J Virol. 1994;68:5403–5410. doi: 10.1128/jvi.68.9.5403-5410.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McBride R., Van Zyl M., Fielding B.C. The coronavirus nucleocapsid is a multifunctional protein. Viruses. 2014;6:2991–3018. doi: 10.3390/v6082991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wenzhong L., Hualan L. COVID-19: Attacks the 1-Beta Chain of Hemoglobin and Captures the Porphyrin to Inhibit Human Heme Metabolism. ChemRxiv. 2020 doi: 10.26434/chemrxiv.11938173.v8. Preprint. [DOI] [Google Scholar]
- 18.Gao J., Tian Z., Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14:72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 19.Ortega J.T., Serrano M.L., Pujol F.H. Role of changes in SARS-CoV-2 spike protein in the interaction with the human ACE2 receptor: An in silico analysis. EXCLI J. 2020;19:410–417. doi: 10.17179/excli2020-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dong L., Hu S., Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discov Ther. 2020;14:58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- 21.Wrapp D., Wang N., Corbett K.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Wilde A.H., Snijder E.J., Kikkert M. Host factors in coronavirus replication. Curr Top Microbiol Immunol. 2018;419:1–42. doi: 10.1007/82_2017_25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raj V., Mou H., Smits S. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature. 2013;495:251–254. doi: 10.1038/nature12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tyrrell D.A., Myint S.H. 4th edition. University of Texas Medical Branch; Galveston: 1996. Coronaviruses. Medical Microbiology. Chapter 60, Coronaviruses. [PubMed] [Google Scholar]
- 25.Fung T.S., Liao Y., Liu D.X. Regulation of stress responses and translational control by coronavirus. Viruses. 2016;8:184. doi: 10.3390/v8070184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li W., Moore M.J., Vasilieva N. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou P., Yang X.-L., Wang X.-G. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Othman H., Bouslama Z., Brandenburg J.-T. Interaction of the spike protein RBD from SARS-CoV-2 with ACE2: Similarity with SARS-CoV, hot-spot analysis and effect of the receptor polymorphism. Biochem Biophys Res Commu. 2020;527:702–708. doi: 10.1016/j.bbrc.2020.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith M., Smith J.C. Repurposing therapeutics for covid-19: Supercomputer-based docking to the sars-cov-2 viral spike protein and viral spike protein-human ace2 interface. ChemRxiv. 2020 [Google Scholar]
- 31.Mir M., Mansoor S., Bhat A. A Review on Probable Lysosomotropic Properties of Sodium Bicarbonate to Restrain Viral Entry of Coronavirus 2 (SARS-CoV-2) SSRN. 2020 [Google Scholar]
- 32.Tortorici M.A., Veesler D. Structural insights into coronavirus entry. Adv Virus Res. 2019;105:93–116. doi: 10.1016/bs.aivir.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao Y., Yan L., Huang Y. Structure of RNA-dependent RNA polymerase from 2019-nCoV, a major antiviral drug target. BioRxiv. 2020 [Google Scholar]
- 34.Zhang C., Shi L., Wang F.-S. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monteil V., Kwon H., Prado P. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020;181:905–913.e7. doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khan A., Benthin C., Zeno B. A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Crit Care. 2017;21:234. doi: 10.1186/s13054-017-1823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Savarino A., Di Trani L., Donatelli I. New insights into the antiviral effects of chloroquine. Lancet Infect Dis. 2006;6:67–69. doi: 10.1016/S1473-3099(06)70361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cortegiani A., Ingoglia G., Ippolito M. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care. 2020;57:279-283. doi: 10.1016/j.jcrc.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yao X., Ye F., Zhang M. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa237. ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gautret P., Lagier J.-C., Parola P. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;105949 doi: 10.1016/j.ijantimicag.2020.105949. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Boriskin Y., Leneva I., Pecheur E.-I. Arbidol: a broad-spectrum antiviral compound that blocks viral fusion. Curr Med Chem. 2008;15:997–1005. doi: 10.2174/092986708784049658. [DOI] [PubMed] [Google Scholar]
- 42.Blaising J., Polyak S.J., Pécheur E.-I. Arbidol as a broad-spectrum antiviral: an update. Antiviral Res. 2014;107:84–94. doi: 10.1016/j.antiviral.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Z., Chen X., Lu Y. Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment. Biosci Trends. 2020;14:64–68. doi: 10.5582/bst.2020.01030. [DOI] [PubMed] [Google Scholar]
- 44.Warren T.K., Jordan R., Lo M.K. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. 2016;531:381–385. doi: 10.1038/nature17180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Agostini M.L., Andres E.L., Sims A.C. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. MBio. 2018;9 doi: 10.1128/mBio.00221-18. e00221–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Te H.S., Randall G., Jensen D.M. Mechanism of action of ribavirin in the treatment of chronic hepatitis C. Gastroenterol Hepatol (N Y) 2007;3(3):218. [PMC free article] [PubMed] [Google Scholar]
- 47.Graci J.D., Cameron C.E. Mechanisms of action of ribavirin against distinct viruses. Rev Med Virol. 2006;16:37–48. doi: 10.1002/rmv.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martin P., Jensen D.M. Ribavirin in the treatment of chronic hepatitis C. J Gastroenterol Hepatol. 2008;23:844–855. doi: 10.1111/j.1440-1746.2008.05398.x. [DOI] [PubMed] [Google Scholar]
- 49.Li G., De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) Nat Rev Drug Discov. 2020;19:149–150. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- 50.Habib A.M.G., Ali M.A.E., Zouaoui B.R. Clinical outcomes among hospital patients with Middle East respiratory syndrome coronavirus (MERS-CoV) infection. BMC Infect Dis. 2019;19:1–6. doi: 10.1186/s12879-019-4555-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Du Y.X., Chen X.P. Favipiravir: pharmacokinetics and concerns about clinical trials for 2019-nCoV infection. Clin Pharmacol Ther. 2020 doi: 10.1002/cpt.1844. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 52.Furuta Y., Komeno T., Nakamura T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc Jpn Acad Ser B Phys Biol Sci. 2017;93:449–463. doi: 10.2183/pjab.93.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baranovich T., Wong S.-S., Armstrong J. T-705 (favipiravir) induces lethal mutagenesis in influenza A H1N1 viruses In vitro. J Virol. 2013;87:3741–3751. doi: 10.1128/JVI.02346-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wagstaff K.M., Sivakumaran H., Heaton S.M. Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus. Biochem J. 2012;443:851–856. doi: 10.1042/BJ20120150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang S.N., Atkinson S.C., Wang C. The broad spectrum antiviral ivermectin targets the host nuclear transport importin α/β1 heterodimer. Antiviral Res. 2020;177:104760. doi: 10.1016/j.antiviral.2020.104760. [DOI] [PubMed] [Google Scholar]
- 56.Liu X., Wang X.-J. Potential inhibitors against 2019-nCoV coronavirus M protease from clinically approved medicines. J Genet Genomics. 2020;47:119–121. doi: 10.1016/j.jgg.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cao B., Wang Y., Wen D. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leonis G., Czyżnikowska Z.A., Megariotis G. Computational studies of darunavir into HIV-1 protease and DMPC bilayer: necessary conditions for effective binding and the role of the flaps. J Chem Inf Model. 2012;52:1542–1558. doi: 10.1021/ci300014z. [DOI] [PubMed] [Google Scholar]
- 59.Ju J., Li X., Kumar S. Nucleotide Analogues as Inhibitors of SARS-CoV Polymerase. BioRxiv. 2020 doi: 10.1101/2020.03.12.989186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stebbing J., Phelan A., Griffin I. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis. 2020;20:400–402. doi: 10.1016/S1473-3099(20)30132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jones G., Sebba A., Gu J. Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: the AMBITION study. Ann Rheum Dis. 2010;69:88–96. doi: 10.1136/ard.2008.105197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tu Y.-F., Chien C.-S., Yarmishyn A.A. A Review of SARS-CoV-2 and the Ongoing Clinical Trials. Int J Mol Sci. 2020;21:2657. doi: 10.3390/ijms21072657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ricklin D., Mastellos D.C., Reis E.S. The renaissance of complement therapeutics. Nat Rev Nephrol. 2018;14:26. doi: 10.1038/nrneph.2017.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shen C., Wang Z., Zhao F. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323:1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]