Abstract
Recent narrative reviews have described the potential efficacy of providing individuals infected with coronavirus disease 2019 (COVID-19) with additional micronutrients to reduce disease severity. Although there are compelling reasons why providing additional micronutrients or conditional amino acids may affect COVID-19-related outcomes, evidence is lacking. The objective of this scoping review is to explore and describe the literature examining the effect of providing additional micronutrients or conditional amino acids (glutamine, arginine) in adults with conditions or infections similar to COVID-19 infection on COVID-19-related health outcomes. A literature search of the MEDLINE database and hand search of Cochrane Database of systematic reviews retrieved 1,423 unique studies, and 8 studies were included in this scoping review. Four studies examined a target population with ventilator-related pneumonia and acute respiratory distress syndrome, and the other 4 studies included patients who were at risk for ventilator-associated pneumonia. Interventions included intravenous ascorbic acid, intramuscular cholecalciferol, enteral and intramuscular vitamin E, enteral zinc sulfate, and oral and parenteral glutamine. In 6 of the 8 included studies, baseline status of the nutrient of interest was not reported and, thus, it is uncertain how outcomes may vary in the context of nutrient deficiency or insufficiency compared with sufficiency. In the absence of direct evidence examining efficacy of providing additional micronutrients or conditional amino acids to standard care, registered dietitian nutritionists must rely on clinical expertise and indirect evidence to guide medical nutrition therapy for patients infected with COVID-19.
The coronavirus disease 2019 (COVID-19) pandemic has resulted in immeasurable adverse health effects across the world. Recent narrative reviews have described the potential efficacy of providing additional micronutrients to reduce disease severity in individuals infected with COVID-19.1, 2, 3 Suspected efficacy of providing additional micronutrients to patients to reduce disease severity is based on known mechanisms of micronutrients, including optimizing the immune system and reducing inflammation, as well as results from trials with humans infected with other viruses and in animal models of coronavirus. These narratives describe compelling reasons why providing micronutrients, particularly ascorbic acid (vitamin C) and cholecalciferol (vitamin D), may treat underlying insufficiencies or deficiencies to benefit immune function prior to and after contracting COVID-19 infection.
In addition to optimizing immune function, another key consideration when providing medical nutrition therapy (MNT) to critically ill patients infected with the COVID-19 virus is the increased risk for malnutrition. Nutrition support is in high demand for critically ill patients being treated in intensive care units during the current pandemic.4, 5, 6 Although conditional amino acids are not essential in healthy individuals, needs are increased during critical illness, and these amino acids become essential. Although nutrition support provides essential nutrients, it is possible that providing conditional amino acids, which become essential in the context of critical care, above those provided in standard nutrition support may allow for increased capacity for recovery and maintenance or improvement of nutritional status.7 However, evidence is lacking regarding the effect of providing additional conditional amino acids in the context of critical illness due to COVID-19 infection.
To provide evidence-based practice, it is important to determine the evidence available to support nutrition interventions. In the absence of direct evidence to support nutrition interventions for the population of interest, practitioners must depend upon their clinical expertise and indirect evidence. Although there is ample evidence to suggest that supplementing or fortifying nutrition support with micronutrients or conditional amino acids may be beneficial for COVID-19 patients, there is little evidence directly testing these interventions in this population. Therefore, the objective of this scoping review is to explore and describe the literature examining the effect of providing additional micronutrients or conditional amino acids (glutamine, arginine) on COVID-19-related health outcomes in adults with conditions or infections similar to COVID-19 infection.
Methods
This scoping review followed the protocols developed by Arksey and O’Malley8 and refined by Levac9 et al and the Joanna Briggs Institute.10 The protocol for this scoping review adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses scoping review checklist11 and was registered on Open Science Framework (https://osf.io/9rm6u/).12
Eligibility Criteria
This scoping review defined the research question and eligibility criteria according to the Population-Concept-Context approach.10 The populations of interest were humans infected with a type of coronavirus (COVID-19, severe acute respiratory syndrome, Middle East respiratory syndrome), with acute respiratory distress syndrome (ARDS), or those at risk of contacting or with ventilator-associated pneumonia, because findings in these populations may inform individuals currently infected with the COVID-19 coronavirus. The concept of this scoping review is provision of additional micronutrients or the conditional amino acids glutamine or arginine. The context was left open to capture all potential articles examining populations of interest. There were no limits on publication dates. Only articles published in English were included in this scoping review due to resource constraints. Additional eligibility criteria can be found in Figure 1 .
Figure 1.
Eligibility criteria for a scoping review examining the effect of providing micronutrients or conditional amino acids in COVID-19a-related conditions on COVID-19-related outcomes.
| Category | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Study type | Peer-reviewed literature | Gray literature |
| Population | Individuals with suspected or confirmed viral infections related to the coronavirus (COVID-19a, SARSb, MERSc) or ARDSd or who are at risk for or who have ventilator-associated pneumonia Human |
Individuals with no suspected or confirmed viral infections related to the coronavirus (COVID-19, SARS, MERS) or ARDS or who are not at risk for or who have ventilator-associated pneumonia Newborn or preterm human infants Animal studies; cell or in vitro studies |
| Intervention | Vitamins and mineral supplements, including thiamine, ascorbic acid, cholecalciferol, and vitamins A and E; zinc; colloidal silver; multivitamin Single amino acids: glutamine, arginine |
Does not examine the effect of specified nutrient Herbal supplements |
| Comparison | No limits | No limits |
| Outcomes | Mortality Quality of life Development of COVID-19 or ventilator-associated pneumonia Hospital admission Intubation Days on ventilator Length of stay Symptom severity Nutrition or BMIe status Other coronavirus- and nutrition-related outcomes |
Outcomes that are not coronavirus- or nutrition-related |
| Setting | No limits | No limits |
| Sample size | No limits | No limits |
| Study designs | Intervention and observational primary studies Systematic review and meta-analyses |
Narrative reviews, commentary, editorials, letters to the editor, conference abstracts |
| Year range | No limits | No limits |
| Language | English | Non-English |
COVID-19 = coronavirus disease 2019.
SARS = severe acute respiratory syndrome.
MERS = Middle East respiratory syndrome.
ARDS = acute respiratory disease.
BMI = body mass index.
Search Plan
MEDLINE (EBSCO) database was searched on April 21, 2020, to identify titles and abstracts with both the population and concept of interest. Search terms for the coronavirus were adapted from a recent search strategy developed by the National Institute for Health and Care Excellence for this project.13 Additional search terms included “micronutrient,” “ascorbic acid,” “vitamin D,” “zinc,” “multivitamin,” “glutamine,” and “arginine.” The only filter used was for the English language. A detailed search plan can be found in Figure 2 . Relevant systematic and narrative reviews as well as the Cochrane Database of Systematic Reviews were hand searched for potentially included studies not identified in the MEDLINE search.
Figure 2.
MEDLINE search plan for scoping review examining efficacy of providing additional micronutrients and conditional amino acids on coronavirus disease 2019–related outcomes. Date searched: April 21, 2020; limits: English language.
| Search no. | Query |
|---|---|
| S21 | S10 AND S20 |
| S20 | S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 |
| S19 | (MH “Glutamine+”) OR (MH “Alanine+”) OR (MH “Arginine+”) |
| S18 | colloidal silver |
| S17 | (MH “Zinc+”) |
| S16 | (MH “Vitamin E+”) |
| S15 | (MH “Vitamin D+”) |
| S14 | (MH “Ascorbic Acid+”) |
| S13 | (MH “Vitamin B 12+”) OR (MH “Vitamin B 6+”) OR (MH “Thiamine+”) OR (MH “Vitamin B Complex”) |
| S12 | (MH “Vitamin A+”) OR (MH “beta Carotene”) |
| S11 | (MH “Micronutrients+”) |
| S10 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 |
| S9 | (MH “Respiratory Distress Syndrome, Adult”) |
| S8 | (MH “Pneumonia, Ventilator-Associated”) |
| S7 | (MH “Middle East Respiratory Syndrome Coronavirus”) |
| S6 | (MH “Severe Acute Respiratory Syndrome”) OR (MH “SARS Virus”) |
| S5 | ((outbreak∗ or wildlife∗ or pandemic∗ or epidemic∗) adj1 (China∗ or Chinese∗ or Huanan∗)) |
| S4 | (((respiratory∗ adj2 (symptom∗ or disease∗ or illness∗ or condition∗)) or “seafood market∗” or “food market∗”) adj10 (Wuhan∗ or Hubei∗ or China∗ or Chinese∗ or Huanan∗)) |
| S3 | ((corona∗ or corono∗) adj1 (virus∗ or viral∗ or virinae∗)) |
| S2 | (coronavirus∗ or coronovirus∗ or coronavirinae∗ or Coronavirus∗ or Coronovirus∗ or Wuhan∗ or Hubei∗ or Huanan or “2019-nCoV” or 2019nCoV or nCoV2019 or “nCoV-2019” or “COVID-19” or COVID19 or “CORVID-19” or CORVID19 or “WN-CoV” or WNCoV or “HCoV-19” or HCoV19 or CoV or “2019 novel∗” or Ncov or “n-cov” or “SARS-CoV-2” or “SARSCoV-2” or “SARSCoV2” or “SARS-CoV2” or SARSCov19 or “SARS-Cov19” or “SARSCov-19” or “SARS-Cov-19” or Ncovor or Ncorona∗ or Ncorono∗ or NcovWuhan∗ or NcovHubei∗ or NcovChina∗ or NcovChinese∗) |
| S1 | (MH “Coronavirus Infections+”) OR (MH “Coronavirus+”) |
Study Selection and Data Extraction
Title and abstract screening were conducted in 2 phases using Rayyan, an online software program.14 In the first phase, a reviewer (M.R.) excluded studies with animals or cells as the population of interest as well as studies that were not primary research studies or systematic reviews. Any remaining abstracts were reviewed by 2 independent reviewers. Full texts of potentially included articles were reviewed for eligibility by 2 reviewers (M.R. and F.W.C.), and discrepancies were settled through consensus. Each stage of the review process was documented in a Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart.15
The following data were extracted from included studies: bibliographic information; details on the target population including disease or illness status, ventilator status, and age; details on the intervention including the nutrient, dose, mode, and duration; outcomes of interest reported; and summary of study results. These data were extracted onto a standardized study characteristics table. Studies with similar populations or interventions were grouped and described narratively. As is customary for scoping reviews, no critical appraisal of study quality was conducted.
Results
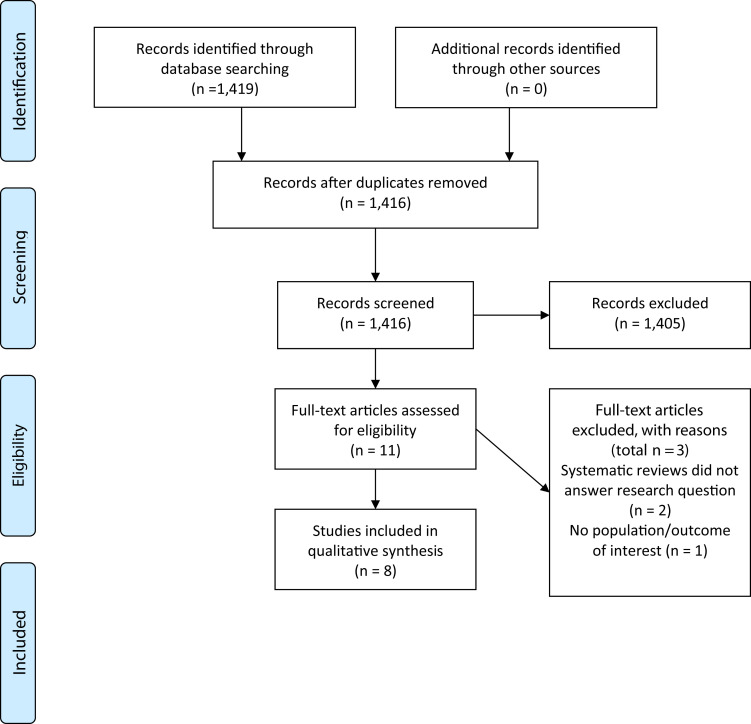
There were 1,423 unique studies identified with the database search; full texts of 11 studies were examined for inclusion, and 8 studies were included in this scoping review (Figure 3 ).16, 17, 18, 19, 20, 21, 22, 23, 24 The majority of the studies identified in the initial search were excluded during title and abstract screening. Primary reasons for exclusion included the following: studies in animals or cells; human studies not examining a population of interest; and studies not being primary research studies (eg, narrative reviews and commentaries).
Figure 3.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses Flow diagram for scoping review examining the effect of micronutrients and conditional amino acids in coronavirus disease 2019–related conditions on coronavirus disease 2019–related outcomes.
Characteristics of the 8 included studies are shown in the Table . There were 5 randomized controlled trials represented in 6 publications16 , 17 , 19 , 21, 22, 23 and 3 nonrandomized controlled studies18 , 20 , 24 published between 198720 and 2019.16 The number of participants ranged from 1420 to 186.24 Although all studies included critically ill patients, the sample characteristics differed slightly. For example, Lin et al included patients in burn shock resuscitation,18 and Kaya et al included ventilated patients in neurosurgical intensive care units.17
Table.
Study characteristics and major results for studies included in a scoping review examining efficacy of providing additional micronutrients or conditional amino acids on coronavirus-related outcomes
| Study | Population | Intervention | Comparison group | Outcomes reported | Major results |
|---|---|---|---|---|---|
| Ascorbic acid | |||||
| Fowler et al 201916 RCTa PMIDb 31573637 |
N = 167 ICUc patients with sepsis and acute respiratory distress syndrome Plasma ascorbate levels at baseline were marginally deficient in both groups Mean ± SDd age: 54.8 ± 16.7 |
Nutrient: ascorbic acid Dose: 50 mg/kg in dextrose 5% in water Mode: intravenous infusion Duration: every 6 h for 96 h |
Placebo (dextrose 5% in water only) | Organ failure (modified SOFAe score), C-reactive protein levels, thrombomodulin levels | Compared with placebo, ascorbic acid did not significantly improve reported outcomes. |
| Lin et al 201818 Retrospective case-control PMID 29931212 |
N = 80 Patients in burn shock resuscitation Baseline ascorbic acid status not reported Mean ± SD age: 41±15 (intervention group) and 42.4 ± 17 (comparison group) |
Nutrient: high-dose ascorbic acid Dose: started at a dose of 66 mg/kg/h Mode: intravenous infusion Duration: mean time 4:01 ± 15 h |
No treatment | Ventilator-associated pneumonia, mortality | There were no significant differences in the incidence of ventilator-associated pneumonia or mortality between the 2 groups. |
| Cholecalciferol | |||||
| Miroliaee et al 201721, 201819 RCT PMID 29248753 29201115 |
N = 49 Patients with ventilator-related pneumonia and cholecalciferol deficiency Mean ± SD age: 57.83 ± 18.84 (intervention group) and 56.45 ± 20.70 (comparison group) |
Nutrient: cholecalciferol Dose: 300,000 U Mode: intramuscular Duration: N/Af |
Placebo | IL-6g, CRPh, CPISi score (pneumonia score), SOFA score, mortality | Compared with placebo, cholecalciferol group had significantly lower IL-6 levels and mortality, but not CRP level and SOFA or CPIS score. |
| Vitamin E | |||||
| Hajimahmoodi et al 200923 RCT No PMID |
N = 20 ICU patients with acute respiratory distress syndrome Vitamin E status at baseline not reported Mean ± SD age: 51.2 ± 6.41 |
Nutrient: vitamin E (600 IU/d) Mode: intramuscular Duration: 3 d |
Placebo (normal saline) | APACHEj II score | Vitamin E appeared to be beneficial in decreasing APACHE II score (significant changes in APACHE II in the intervention group). |
| Seeger et al 198720 Before-after study PMID 3117857 |
N = 14 Ventilated and intubated acute respiratory failure patients in ICU Vitamin E status at baseline was not reported Participant ages not reported |
Nutrient: vitamin E (d,1-alpha-tocopherylacertate) Dose: 3 g/d Mode: enteral (liquid oil directly in gastric tube in 6 doses) Duration: 10 d or if mechanical ventilation is not needed before 10 d |
No comparison group | Mortality | No difference seen in mortality according to the increase in plasma tocopherols from the intervention. |
| Zinc | |||||
| Hasanzadeh et al 201724 Prospective cohort study PMID 28197049 |
N = 186 Adult mechanically ventilated trauma patients in the ICU Zinc status at baseline not reported Zinc 24.4% <30 y 51.2% 30-65 y 24.4% >65 y No zinc 21.2% <30 y 50% 30-65 y 28.8% >65 y |
Nutrient: zinc sulfate Dosage: 60-90 mg/d Mode: nasogastric tube Duration: 1 y |
No zinc sulfate | Occurrence of ventilator-associated pneumonia measured with CPIS | Patients receiving zinc sulfate had a smaller hazard of progression to ventilator-associated pneumonia. |
| Glutamine | |||||
| Aydoğmuş et al 201222 RCT PMID 25207045 |
N = 40 in glutamine and comparison groups Patients on mechanical ventilator support for at least 7 d in the ICU Mean ± SD age: Nonglutamine group: 45 ± 18.2 y Glutamine group: 36.35 ± 16.37 y |
Nutrient: glutamine Dose: 40 g/d Mode: TPNk Duration: 7 d |
TPN without glutamine | Development of ventilator-associated pneumonia, CRP | There was no difference development of ventilator-associated pneumonia or CRP levels between groups. |
| Kaya et al 201717 RCT PMID 28096000 |
N = 88 Ventilated patients in neurosurgical ICU; expected to be ventilated at least 5 d Mean ± SD age: 48.57 ± 17.36 |
Nutrient: glutamine Concentration: 5% Mode: oral care Duration: 5 d |
Oral care with 2% chlorhexidine gluconate solution | Ventilator-related pneumonia measured with Clinical Infection Score (chest x-rays; endotracheal aspirate cultures), acute APACHE II score |
No difference between groups at day 1, 3, or 5 (P > .05) |
RCT = randomized controlled trail.
PMID = PubMed ID.
ICU = intensive care unit.
SD = standard deviation.
SOFA = sequential organ failure assessment.
N/A = not available.
IL-6 = interleukin-6.
CRP = C-reactive protein.
CPIS = Clinical Pulmonary Infection Score.
APACHE = Acute Physiology and Chronic Health Evaluation.
TPN = total parenteral nutrition.
Four studies focused on patients who had ventilator-related pneumonia or ARDS,16 , 19 , 20 , 23 and the remaining 4 studies included patients who were at risk for ventilator-associated pneumonia.17 , 18 , 22 , 24
Study interventions were heterogeneous. Fowler et al16 and Lin et al18 examined the effect of vitamin C or ascorbic acid via intravenous infusion, and the remaining included studies investigated other single nutrients delivered through various modes: glutamine orally17 or parenterally,22 vitamin D or cholecalciferol intramuscularly,19 vitamin E (d,1-alpha-tocopherylacertate) enterally20 or intramuscularly,23 and zinc sulfate enterally.24 The duration of the intervention also varied (Table).
Except for Lin et al18 and Seeger et al,20 all other 6 studies had a comparison group.16 , 17 , 19 , 22, 23, 24 Among the 8 included studies, reported outcomes included organ failure, inflammatory and vascular injury markers, pneumonia score, ventilator-related pneumonia, and mortality.
Five studies did not find any improvement in their reported outcomes.16, 17, 18 , 20 , 22 In 3 studies, the authors reported a potential benefit of the intervention on outcomes: intramuscular cholecalciferol on mortality,19 intramuscular vitamin E on Acute Physiology and Chronic Health Evaluation score in patients with ARDS,23 and zinc sulfate on the incidence of ventilator-associated pneumonia in ventilated patients in intensive care units.24 In 2 studies, authors indicated deficiency of the nutrient of interest at baseline,16 , 19 but baseline status of the intervention nutrient was not described in the remaining studies.
Discussion
This scoping review included 8 unique studies examining the effect of providing additional micronutrients or conditional amino acids on COVID-19-related health outcomes in individuals with ARDS and in individuals with or at risk for ventilator-associated pneumonia. Although the search plan included individuals infected with a form of coronavirus (COVID-19, severe acute respiratory syndrome, Middle East respiratory syndrome), there were no studies identified with these target populations. Overall, sparse evidence of heterogeneous interventions described some benefit of intramuscular cholecalciferol and vitamin E and zinc via a nasogastric tube on coronavirus-related outcomes, but findings should be interpreted with caution because this scoping review did not critically analyze risk of bias or certainty of evidence. In addition, most studies did not report the baseline status of the nutrients being supplemented. Thus, it is unclear if results would have been different if participants were exclusively insufficient or deficient vs sufficient. It is possible that treating baseline deficiency may result in improved outcomes,25 although providing additional nutrients to a sufficient individual would result in no effect. Hence, further investigation is warranted.
There has been considerable interest in the efficacy of micronutrient therapy to reduce the severity and symptoms of COVID-19 infection, particularly in the context of critical illness.1, 2, 3 , 26 , 27 Recent reviews include comprehensive discussion of the potential effects of providing additional micronutrients to individuals with COVID-19,1 particularly ascorbic acid3 , 28 and cholecalciferol.2 , 26 , 27 In these reviews, the authors provide compelling logic that patients infected with COVID-19 or with comparable conditions could benefit from addition of these nutrients. Authors describe biological functions of these micronutrients and discuss how supplementation has been effective in treating other viruses such as the common cold or influenza, particularly in the context of insufficiency or deficiency. Authors also provide evidence describing efficacy of providing micronutrients in the context of animal models of coronavirus. However, human trials examining efficacy of providing micronutrients and conditional amino acids were lacking, which was supported by the dearth of evidence discovered in this scoping review.
There is minimal available evidence to guide nutrition care for registered dietitian nutritionist (RDNs) working with patients infected with COVID-19. Indeed, there is little evidence to guide practice for individuals with similar conditions, including alternative versions of the coronavirus, ARDS, or ventilator-associated pneumonia. In these circumstances, it is critical for RDNs to rely on their scientific training and clinical expertise and the nutrition care process to determine if a patient is deficient in an essential nutrient and if treating the respective deficiency is a priority. RDNs can also extrapolate evidence from populations presenting with similar signs and symptoms, such as those with critical illness or on mechanical ventilation, to inform practice for individuals with COVID-19 infections. RDNs should consider how baseline nutrient status may affect outcomes, because treating a deficiency or insufficiency may result in improved outcomes, although providing nutrients above meeting needs may have no effect.
In the current COVID-19 crisis, it is not possible to wait until clinical trials are published on each intervention delivered before implementing the intervention with a patient, as would ideally be the case in standard care. Instead, RDNs must use ingenuity and innovation and work as part of a multidisciplinary team to determine priorities and risk-benefit ratio of interventions when collaborating to manage health condition in adults infected with COVID-19.
COVID-19 Research Moving Forward
In a recent consensus report, the Expert Group on Clinical Treatment of New Corona Virus Disease in Shanghai described that high-dose intravenous ascorbic acid treatment is recommended for patients with light or general symptoms29 to prevent and control cytokine storms. Several trials have been registered examining the effect of providing antioxidants,30 ascorbic acid,31, 32, 33, 34, 35 and cholecalciferol.36, 37, 38 Thus, although there is no research to support evidence-based recommendations at this time, evidence to inform provision of additional micronutrients for individuals with COVID-19 infections may be available moving forward. There were no registered trials found directly examining the effects of glutamine or arginine.
To provide evidence-based practice for RDNs, it is crucial that RDNs participate in COVID-19-related research when possible. In addition to participating in formal research studies, RDNs can contribute their experiences in delivering MNT to this population by documenting care and outcomes in the Academy of Nutrition and Dietetics Health Informatics Infrastructure.39 RDNs working with COVID-19 patients are essential workers and are likely stressed for time. However, any documentation of current practices can help contribute to a growing pool of evidence supporting the efficacy of MNT in COVID-19-affected patients.
Strengths and Limitations
This scoping review followed a rigorous process and examined the availability of interventions of potential utility in populations that may be comparable and applicable to the COVID-19-infected population. A limitation of this scoping review included the lack of evidence available in target populations and lack of documentation of baseline nutrient status of participants in included articles. Moving forward, authors of scoping and systematic reviews examining potential efficacy of interventions in patients with COVID-19 infection should consider including a broader population base, including those with critical illness or respiratory infections, to identify evidence that can be extrapolated to the population of interest. Another limitation of this scoping review was searching the MEDLINE database and Cochrane Database of Systematic Reviews only in the interest of providing information to practitioners in a rapid manner. However, studies cited in the included articles or in any relevant narrative reviews were evaluated for inclusion. This scoping review did not cover all nutrients that may be beneficial to COVID-19 patients, including probiotics or oral nutrition supplements.
Conclusion
Individuals infected with COVID-19 may have baseline nutrient deficiencies or increased nutrient needs due to COVID-19 pathology. Current reviews and registered trials discuss the potential utility of providing additional micronutrients and glutamine in contexts that may apply to those infected with COVID-19. However, evidence in human subjects is very limited, and it is unclear if results may vary according to baseline nutrient status. RDNs must work with the multidisciplinary team and rely on clinical expertise and indirect evidence to guide MNT for patients infected with COVID-19 to reduce adverse effects from COVID-19 infection.
Biographies
M. Rozga is a nutrition researcher, Academy of Nutrition and Dietetics Evidence Analysis Center, Chicago, IL.
F. W. Cheng is a nutrition researcher, Academy of Nutrition and Dietetics Evidence Analysis Center, Chicago, IL.
L. Moloney is a nutrition researcher, Academy of Nutrition and Dietetics Evidence Analysis Center, Chicago, IL.
D. Handu is senior scientific director, Academy of Nutrition and Dietetics Evidence Analysis Center, Chicago, IL.
Footnotes
STATEMENT OF POTENTIAL CONFLICT OF INTEREST No potential conflict of interest was reported by the authors.
FUNDING/SUPPORT This work was supported by the Academy of Nutrition and Dietetics.
AUTHOR CONTRIBUTIONS All authors wrote sections of the first draft, thoroughly edited the manuscript, and approved the final draft.
References
- 1.Zhang L., Liu Y. Potential interventions for novel coronavirus in China: A systematic review. J Med Virol. 2020;92(5):479–490. doi: 10.1002/jmv.25707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grant W.B., Lahore H., McDonnell S.L., et al. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12(4):988. doi: 10.3390/nu12040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng R.Z. Can early and high intravenous dose of vitamin C prevent and treat coronavirus disease 2019 (COVID-19)? Med Drug Discov. 2020;5:100028. doi: 10.1016/j.medidd.2020.100028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laviano A., Koverech A., Zanetti M. Nutrition support in the time of SARS-CoV-2 (COVID-19) Nutrition. 2020:110834. doi: 10.1016/j.nut.2020.110834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martindale R.P.J., Taylor B., Warren M., McClave S. Nutrition therapy in the patient with COVID-19 disease requiring ICU care. American Society for Parenteral and Enteral Nutrition, Society of Critical Care Medicine. https://www.sccm.org/getattachment/Disaster/Nutrition-Therapy-COVID-19-SCCM-ASPEN.pdf?lang=en-US Available at: Accessed April 1, 2020.
- 6.Iyer R., Bansal A. What do we know about optimal nutritional strategies in children with pediatric acute respiratory distress syndrome? Ann Transl Med. 2019;7(19):510. doi: 10.21037/atm.2019.08.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morris C.R., Hamilton-Reeves J., Martindale R.G., Sarav M., Ochoa Gautier J.B. Acquired amino acid deficiencies: A focus on arginine and glutamine. Nutr Clin Pract. 2017;32(1 suppl):30s–47s. doi: 10.1177/0884533617691250. [DOI] [PubMed] [Google Scholar]
- 8.Arksey H., O’Malley L. Scoping studies: Towards a methodological framework. Int J Soc Res Methodol Theory Pract. 2005;8(1):19–32. [Google Scholar]
- 9.Levac D., Colquhoun H., O’Brien K.K. Scoping studies: Advancing the methodology. Implement Sci. 2010;5:69. doi: 10.1186/1748-5908-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peters MDJ G.C.M.P., Baldini Soares C., Khalil H., Parker D. Joanna Briggs Institute Reviewer’s Manual, JBI, 2020. Joanna Briggs Institute; Adelaide, Australia: 2020. Chapter 11: Scoping reviews (2020 version) [Google Scholar]
- 11.Tricco A.C., Lillie E., Zarin W., et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann Intern Med. 2018;169(7):467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 12.Rozga M. The effect of micronutrient and single amino acid supplementation on coronavirus-related outcomes: A scoping review. Open Science Framework. osf.io/9rm6u Published 2020. Accessed April 24, 2020.
- 13.The National Institute for Health and Care Excellence (NICE) Interim process and methods for developing rapid guidelines on COVID-19, 7 Appendix: Search strategy for Medline (Ovid Platform) https://www.nice.org.uk/process/pmg35/chapter/appendix-search-strategy-for-medline-ovid-platform Published 2020. Accessed April 25, 2020.
- 14.Ouzzani M., Hammady H., Fedorowicz Z., Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Fowler A.A., 3rd, Truwit J.D., Hite R.D., et al. Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: The CITRIS-ALI randomized clinical trial. JAMA. 2019;322(13):1261–1270. doi: 10.1001/jama.2019.11825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaya H., Turan Y., Tunalı Y., et al. Effects of oral care with glutamine in preventing ventilator-associated pneumonia in neurosurgical intensive care unit patients. Appl Nurse Res. 2017;33:10–14. doi: 10.1016/j.apnr.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Lin J., Falwell S., Greenhalgh D., Palmieri T., Sen S. High-dose ascorbic acid for burn shock resuscitation may not improve outcomes. Burn Care Res. 2018;39(5):708–712. doi: 10.1093/jbcr/irx030. [DOI] [PubMed] [Google Scholar]
- 19.Miroliaee A.E., Salamzadeh J., Shokouhi S., Sahraei Z. The study of vitamin D administration effect on CRP and interleukin-6 as prognostic biomarkers of ventilator associated pneumonia. J Crit Care. 2018;44:300–305. doi: 10.1016/j.jcrc.2017.08.040. [DOI] [PubMed] [Google Scholar]
- 20.Seeger W., Ziegler A., Wolf H.R. Serum alpha-tocopherol levels after high-dose enteral vitamin E administration in patients with acute respiratory failure. Intensive Care Med. 1987;13(6):395–400. doi: 10.1007/BF00257683. [DOI] [PubMed] [Google Scholar]
- 21.Miroliaee A.E., Salamzadeh J., Shokouhi S., et al. Effect of vitamin D supplementation on procalcitonin as prognostic biomarker in patients with ventilator associated pneumonia complicated with vitamin D deficiency. Iran J Pharm Res. 2017;16(3):1254–1263. [PMC free article] [PubMed] [Google Scholar]
- 22.Aydoğmuş M.T., Tomak Y., Tekin M., Kati I., Huseyinoglu U. Glutamine supplemented parenteral nutrition to prevent ventilator-associated pneumonia in the intensive care unit. Balkan Med J. 2012;29(4):414–418. doi: 10.5152/balkanmedj.2012.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hajimahmoodi M., Mojtahedzadeh M., GhaffarNatanzi N., et al. Effects of vitamin E administration on APACHE II Score in ARDS patients. DARU: J Pharmaceutical Sci. 2009;17(1):24–28. [Google Scholar]
- 24.Hasanzadeh Kiabi F., Alipour A., Darvishi-Khezri H., Aliasgharian A., Emami Zeydi A. Zinc supplementation in adult mechanically ventilated trauma patients is associated with decreased occurrence of ventilator-associated pneumonia: A secondary analysis of a prospective, observational study. Indian J Crit Care Med. 2017;21(1):34–39. doi: 10.4103/0972-5229.198324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marik P.E., Kory P., Varon J. Does vitamin D status impact mortality from SARS-CoV-2 infection? Med Drug Discov. 2020:100041. doi: 10.1016/j.medidd.2020.100041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weng H., Li J.-G., Mao Z., Zeng X.-T. Randomised trials of vitamin D 3 for critically ill patients in adults: systematic review and meta-analysis with trial sequential analysis. Intensive Care Med. 2017;43(2):277–278. doi: 10.1007/s00134-016-4591-1. [DOI] [PubMed] [Google Scholar]
- 27.Molloy E.J., Murphy N. Vitamin D, Covid-19 and children. Ir Med J. 2020;113(4):64. [PubMed] [Google Scholar]
- 28.Boretti A., Banik B.K. Intravenous vitamin C for reduction of cytokines storm in acute respiratory distress syndrome. PharmaNutrition. 2020:100190. doi: 10.1016/j.phanu.2020.100190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shanghai Expert Group on Clinical Treatment of New Coronavirus Diseases Expert consensus on comprehensive treatment of cornoavirus diseases in Shanghai in 2019 comprehensive treatment of coronavirus disease expert consensus. China Journal of Infectious Diseases. 2020:38. doi: 10.3760/cma.j.issn.1000-6680.2020.0016. [DOI] [Google Scholar]
- 30.ClinicalTrials.gov Anti-inflammatory/antioxidant oral nutrition supplementation in COVID-19 (ONSCOVID19); NCT04323228. National Library of Medicine (US) https://clinicaltrials.gov/ct2/show/NCT04323228 Published 2020. Accessed April 28, 2020.
- 31.ClinicalTrials.gov Early infusion of vitamin C for treatment of novel COVID-19 acute lung injury (EVICT-CORONA-ALI); NCT04344184. National Library of Medicine (US) https://clinicaltrials.gov/ct2/show/NCT04344184 Published 2020. Accessed April 28, 2020.
- 32.ClinicalTrials.gov Administration of intravenous vitamin C in novel coronavirus infection (COVID-19) and decreased oxygenation (AVoCaDO); NCT04357782. National Library of Medicine (US) https://clinicaltrials.gov/ct2/show/NCT04357782 Published 2020. Accessed April 28, 2020.
- 33.ClinicalTrials.gov Pharmacologic ascorbic acid as an activator of lymphocyte signaling for COVID-19 treatment; NCT04363216. National Library of Medicine (US) https://clinicaltrials.gov/ct2/show/NCT04363216 Published 2020. Accessed April 28, 2020.
- 34.ClinicalTrials.gov Use of ascorbic acid in patients with COVID 19; NCT04323514. National Library of Medicine (US) https://clinicaltrials.gov/ct2/show/NCT04323514 Published 2020. Accessed April 28, 2020.
- 35.ClinicalTrials.gov Vitamin C infusion for the treatment of severe 2019-nCoV infected pneumonia; NCT04264533. National Library of Medicine (US) https://clinicaltrials.gov/ct2/show/NCT04264533 Published 2020. Accessed April 28, 2020.
- 36.ClinicalTrials.gov Vitamin D on prevention and treatment of COVID-19 (COVITD-19); NCT04334005. National Library of Medicine (US) https://clinicaltrials.gov/ct2/show/NCT04334005 Published 2020. Accessed April 28, 2020.
- 37.ClinicalTrials.gov Impact of zinc and vitamin D3 supplementation on the survival of aged patients infected with COVID-19 (ZnD3-CoVici); NCT04351490. National Library of Medicine (US) https://clinicaltrials.gov/ct2/show/NCT04351490 Published 2020. Accessed April 28, 2020.
- 38.ClinicalTrials.gov COvid-19 and vitamin D supplementation: A multicenter randomized controlled trial of high dose versus standard dose vitamin D3 in high-risk COVID-19 patients (CoVitTrial); NCT04344041. National Library of Medicine (US) https://clinicaltrials.gov/ct2/show/NCT04344041 Published 2020. Accessed April 28, 2020.
- 39.Academy of Nutrition and Dietetics ANDHII. https://www.eatrightpro.org/research/projects-tools-and-initiatives/andhii Accessed April 28, 2020. [DOI] [PubMed]