Abstract
Background:
The cardiovascular system exhibits strong circadian rhythms to maintain normal functioning. Irregular sleep schedules, characterized by high day-to-day variability in sleep duration or timing, represent possibly milder but much more common/chronic disruption of circadian rhythms in the general population than shift work.
Objectives:
We aimed to prospectively examine the association between sleep regularity and risk of cardiovascular disease (CVD).
Methods:
In the Multi-Ethnic Study of Atherosclerosis, 1,992 participants free of CVD completed 7-day wrist actigraphy for sleep assessment in 2010–2013, and were prospectively followed through 2016. We assessed sleep regularity using the standard deviation (SD) of actigraphy-measured sleep duration and sleep onset timing across 7 days. Incident CVD included non-fatal and fatal cardiovascular events. Cox proportional hazards model was used to estimate hazard ratios (HR) for incident CVD according to SD of sleep duration and timing, adjusted for traditional CVD risk factors (including CVD biomarkers) and other sleep-related factors (including average sleep duration).
Results:
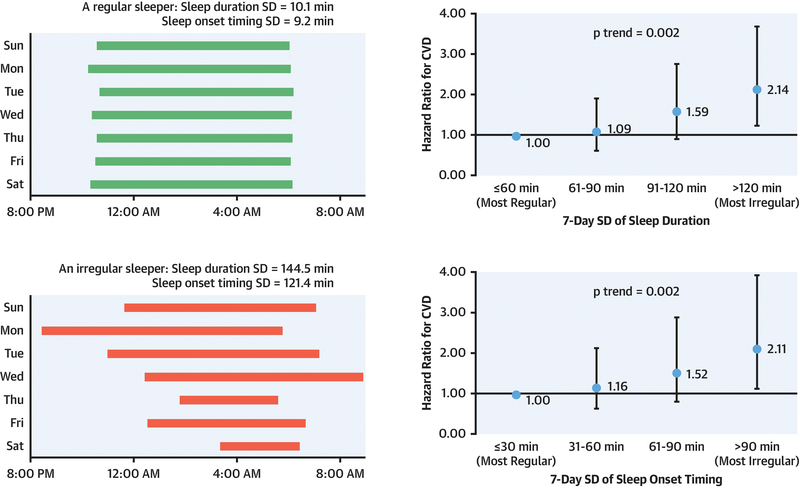
During a median follow-up of 4.9 years, 111 participants developed CVD events. The multivariable-adjusted HR (95% CI) for CVD across categories of sleep duration SD was 1.00 (reference) for ≤60 min, 1.09 (0.62, 1.92) for 61–90 min, 1.59 (0.91, 2.76) for 91–120 min, and 2.14 (1.24, 3.68) for >120 min (p-trend=0.002). Similarly, compared to participants with a sleep timing SD ≤30 min, the HR (95% CI) for CVD was 1.16 (0.64, 2.13) for 31–60 min, 1.52 (0.81, 2.88) for 61–90 min, and 2.11 (1.13, 3.91) for >90 min (p-trend=0.002). Exclusion of current shift workers yielded similar results.
Conclusion:
Irregular sleep duration and timing may be novel risk factors for CVD, independent of traditional CVD risk factors and sleep quantity/quality.
Keywords: Cardiovascular disease, Circadian rhythms, Cohort study, Risk factor, Sleep patterns
Condensed abstract:
In a diverse, community-based sample, participants with most irregular sleep duration or timing had more than doubled risk of developing cardiovascular disease over 5 years of follow-up compared with those with most regular sleep patterns. These associations remained robust after considering known cardiovascular risk factors and other sleep variables such as sleep duration and sleep disorders. Our results suggest that irregular sleep patterns may be a novel and independent cardiovascular risk factor, and may add to current cardiovascular prevention recommendations that emphasize physical activity, healthy diet and sufficient sleep.
Introduction
The longstanding clinical observations that adverse cardiovascular events occur at a higher frequency in the morning suggest the existence of circadian mechanisms in the pathogenesis of cardiovascular disease (CVD) (1–3). Almost all major cardiovascular functions, including heart rate, blood pressure, vascular tone and endothelia functions, display tightly regulated circadian patterns (4–8). Prior studies have linked certain forms of disrupted circadian rhythms to increased CVD risk. For example, shift work was associated with modestly increased risk of coronary heart disease (CHD) and stroke after accounting for CVD risk factors (9,10); several studies reported 4% to 29% higher incidence of myocardial infarction following transition to daylight saving time (11), which represents a combination of circadian phase shift and sleep loss by one hour.
It is now increasingly recognized that circadian disruption may be a ubiquitous phenomenon that is not restricted to specific settings (e.g., in shift workers) and may accumulate over time (12,13), particularly considering increased day-to-day exposures to factors that can disrupt circadian rhythms (e.g., light exposure at night, media device use in the bedroom, etc.). However, the impact of such ubiquitous exposures to chronic circadian disruption on cardiovascular health has not been addressed at the population level. Irregular sleep schedules, characterized by high day-to-day variability in sleep duration or timing, may represent milder but chronic disruption of the circadian clock that is broadly relevant across the population. Specifically, individuals who frequently alter their sleep duration or sleep timing on a night-to-night basis may have higher cardiometabolic risk due to disrupted circadian functions. Assessment of sleep regularity requires assessment of sleep timing and duration across multiple nights, and is often quantified by use of actigraphy over several days (14–19).
A large amount of experimental and epidemiological evidence also supports the importance of adequate sleep duration for cardiovascular health (20,21). While adequacy of sleep duration is often evaluated using measures of average, or habitual, sleep duration, this measure may not capture individuals with high sleep duration variability, in whom intermittent periods of sleep deprivation may not be fully compensated for by sleeping longer on other nights. In support of a role of sleep variability in cardiometabolic health, recent literature demonstrates that higher variability in sleep duration or timing is associated with unfavorable metabolic profiles such as higher blood pressure, dysregulated blood lipids and insulin resistance (14–19). Given that these adverse metabolic factors are precursors and strong predictors for CVD (22), irregular sleep may increase CVD risk through influencing metabolic health, in addition to its direct impact on the inherent rhythmicity of the cardiovascular system. Using 7-day actigraphic measurements of sleep collected under habitual settings, we hypothesize that both irregular sleep duration and timing at baseline are associated with increased incidence of CVD over 5 years and that these associations are partly explained by worse baseline metabolic profiles among irregular sleepers.
Methods
Study population.
We used the data from the Multi-Ethnic Study of Atherosclerosis (MESA), a prospective study of clinical and subclinical risk factors for progression of atherosclerosis (23). In 2000–2002, 6,814 white (38%), African American (28%), Hispanic (22%) and Chinese American (12%) participants aged 45–84 years who were free of clinical CVD were enrolled from six field centers across the U.S. (Baltimore City and Baltimore County, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles County, California; Northern Manhattan and the Bronx, New York; and St. Paul, Minnesota), and completed a baseline examination. Five follow-up examinations were performed throughout 2018 to update information on disease diagnoses, medication use and lifestyle factors. Of 3,789 eligible participants who were invited to the MESA Sleep Ancillary Study at MESA Exam 5 (2010–2013), 2,261 (59.7%) participated in and completed the sleep exam which included 7-day wrist actigraphy, 1-night at-home polysomnography and questionnaire-based sleep assessment (24). Compared with sleep study participants, those who did not participate were slightly older and more likely to be white, have ever smoked and have history of hypertension or chronic obstructive pulmonary disease (24). We further excluded participants with pre-existing CVD at the sleep study, missing CVD event follow-up after the sleep study, or had <5 days of actigraphic recordings, leaving 1,992 available for analysis. The institutional review board at each study site approved the study, and all participants provided written informed consent.
Sleep assessment.
Participants wore the Actiwatch Spectrum wrist actigraph (Philips Respironics, Murrysville, PA) on their nondominant wrist for 7 consecutive days, while also indicating their sleep and wake times in the accompanying sleep diary. The actigraphic signals were scored as wake or sleep for each 30-second epoch according to increases or decreases in activity count, in combination with the event marker input by the participants, self-recorded sleep diary and environmental light changes. The data were processed by a certified technician at the Sleep Reading Center of Brigham and Women’s Hospital using Actiware-Sleep version 5.59 analysis software (Mini Mitter Co., Inc. Bend, OR). The inter-scorer reliability (based on subsamples of rescored data) was 0.96 for sleep onset timing and 0.89 for wake timing. We considered two standard deviation (SD) measures to quantify sleep regularity: (1) 7-day SD in sleep duration and (2) 7-day SD in sleep onset timing. Given their moderate correlation (r=0.48), we examined their associations with CVD risk separately in all analyses.
At-home polysomnography was performed for one night using the Compumedics Somte system (Compumedics, Abbottsvile, Australia) according to the protocol developed previously (25), which measures sleep-disordered breathing, sleep stages (including rapid eye movement [REM] sleep and stage N3 sleep), wake after sleep onset (WASO) and heart rate. Sleep-disordered breathing was measured by the Apnea-Hypopnea Index (AHI) which included all obstructive apneas plus hypopneas associated with ≥4% oxygen desaturation. There was a moderate agreement between actigraphy-measured and polysomnography-measured sleep duration on the same night (weighted kappa statistic=0.3), which was mainly due to underestimation of WASO by actigraphy (26).
Participants also completed a sleep questionnaire to report their sleep habits and sleep-related traits, including insomnia symptoms measured by the Women’s Health Initiative Insomnia Rating Scale (WHIIRS>10 for elevated insomnia symptoms (27)), daytime sleepiness measured by the Epworth Sleepiness Scale (ESS>10 for excessive daytime sleepiness (28)), and chronotype measured by the modified Horne-Ostberg Morningness-Eveningness Questionnaire (MEQ≥18 for morning type and MEQ≤11 for evening type (29)). Usual work schedule was grouped as ‘do not work’, ‘day shift’ and ‘other shift’ that included responses of ‘afternoon shift’, ‘night shift’, ‘split shift’, ‘irregular shift/on-call’ and ‘rotating shifts’.
Assessment of cardiovascular endpoints.
Incident cardiovascular events were identified at each MESA follow-up examination, as well as by telephone interviews conducted every 9 to 12 months. An estimated 98% of self-reported hospitalized cardiovascular events were adjudicated by expert review of medical records or death certificates, and 95% of out-of-hospital cardiovascular deaths were confirmed by next-of-kin (30). The primary endpoint was predefined as incident total CVD events, which included myocardial infarction, CHD death, resuscitated cardiac arrest, angina followed by revascularization, stroke, stroke death, and other atherosclerotic or CVD deaths, consistent with the prior study (31). The secondary endpoint was incidence of a hard CVD outcome comprising fatal and non-fatal CHD and stroke.
Statistical analysis.
Participants contributed person-time of follow-up from the date of sleep study until occurrence of a cardiovascular event (including cardiovascular death), other death, or the date of last follow-up. Cox proportional hazards regression was used to estimate hazard ratios (HR) and 95% confidence intervals (CI) for incident CVD in relation to measures of sleep regularity. The primary sleep regularity measure was 7-day SD of sleep duration or sleep onset timing, divided into four groups in 30-min increments to allow sufficient numbers of CVD cases in each group. Due to the different data distribution, the categories were ≤60, 61–90, 91–120 and >120 min for sleep duration SD and ≤30, 31–60, 61–90 and >90 min for sleep onset timing SD. The secondary sleep regularity measure included the continuous SD measures (per hour) as well as the binary measures of >90 versus ≤90 min (for both SD measures), a predefined cutoff that has been associated with metabolic abnormalities in the prior investigation (14). The proportional hazards assumption was tested by examining the interactions between the continuous sleep regularity measures and the follow-up time and found not to be violated (p-interaction=0.58 for sleep duration SD and 0.29 for sleep onset timing SD).
All analyses were stratified by study site and adjusted for age at sleep study, sex, race/ethnicity, education and work schedules (Model 1). To evaluate whether the associations with sleep regularity were independent of known CVD risk factors, the multivariable model (Model 2) adjusted for cigarette smoking, physical activity, depressive symptoms (measured by Center for Epidemiologic Studies Depression scale), BMI, diabetes status, systolic blood pressure, HDL cholesterol and total cholesterol, all collected at MESA Exam 5. As multiple sleep traits (e.g., sleep duration (20), sleep apnea (32), insomnia symptoms (33), etc.) have also been shown to predict CVD risk, we further controlled for several sleep-related factors in the final fully-adjusted model (Model 3), including average sleep duration (from actigraphy), insomnia symptoms, self-reported chronotype, excessive daytime sleepiness and sleep apnea (by the AHI).
We performed subgroup analyses to explore whether associations with sleep regularity differed by age, sex, race/ethnicity, average sleep duration and work schedules. Stratum-specific associations were estimated for the continuous sleep regularity measures (per hour), and a likelihood ratio test comparing the models with versus without the multiplicative interaction terms was used to assess statistical significance. We also conducted several sensitivity analyses to test the robustness of the results. First, to address the potential reverse causation that certain preclinical symptoms of CVD may disturb sleep patterns, we conducted a series of lagged analyses by excluding incident CVD cases diagnosed within the first 6 months, 12 months and 18 months after the sleep study. Second, we repeated the analysis evaluating associations with hard CVD outcomes, the secondary endpoint. Third, we examined the associations between sleep regularity measures on weekdays and incident total CVD. All analyses were conducted in SAS 9.4 (SAS Institute, Cary, NC), and two-sided p-values <0.05 were considered statistically significant.
Results
Of 1,992 MESA participants free of known CVD at the time of the sleep study, 786 (39.5%) had sleep duration SD >90 min and 510 (25.6%) had sleep onset timing SD >90 min (Table 1). Metabolic and sleep-related parameters were in general worse comparing participants with higher (>90 min) versus lower (≤90 min) sleep duration SD, including higher levels of BMI, blood pressure, diabetes prevalence, AHI, insomnia symptoms, daytime sleepiness, as well as longer WASO, shorter average sleep duration, less REM sleep and higher heart rate during sleep. Compared to those with regular sleep, participants with more irregular sleep were more likely to be African-Americans, have non-day shift work, currently smoke, report higher depressive symptoms, and identify themselves as evening or intermediate chronotype and less likely as morning chronotype. We observed similar differences by sleep onset timing SD.
Table 1.
Age-standardized characteristics of the study sample by sleep regularity measures at MESA Sleep Ancillary Study
| Sleep duration SD | Sleep onset timing SD | |||||
|---|---|---|---|---|---|---|
| <90 min (n = 1,206) | ≥90 min (n = 786) | p Value* | <90 min (n = 1,482) | ≥90 min (n = 510) | p Value* | |
| Age, years | 69.1 (9.0) | 69.5 (9.5) | 0.31 | 69.4 (9.1) | 68.8 (9.6) | 0.19 |
| Male, % | 45 | 46 | 0.60 | 45 | 47 | 0.50 |
| Race/ethnicity | <0.001 | <0.001 | ||||
| White, % | 43 | 29 | 41 | 28 | ||
| Chinese American, % | 11 | 13 | 11 | 12 | ||
| African American, % | 23 | 35 | 24 | 38 | ||
| Hispanic, % | 24 | 23 | 24 | 22 | ||
| Work schedules | 0.01 | <0.001 | ||||
| Day shift, % | 33 | 27 | 32 | 27 | ||
| Other shift, % | 11 | 15 | 11 | 18 | ||
| Do not work, % | 56 | 58 | 57 | 55 | ||
| Education | 0.80 | 0.23 | ||||
| HS or less, % | 31 | 31 | 31 | 31 | ||
| Some college, % | 29 | 30 | 29 | 31 | ||
| Bachelor, % | 20 | 19 | 21 | 17 | ||
| Graduate school, % | 20 | 20 | 19 | 22 | ||
| Body mass index, kg/m2 | 28.5 ± 5.4 | 29.1 ± 6.0 | 0.02 | 28.5 ± 5.5 | 29.4 ± 6.1 | 0.005 |
| Current smoker, % | 5 | 10 | <0.001 | 6 | 12 | <0.001 |
| Physical activity, MET-hrs/week | 41.4 ± 37.4 | 42.6 ± 40.0 | 0.51 | 42.1 ± 38.2 | 41.4 ± 39.2 | 0.71 |
| Depressive symptoms | 7.6 ± 7.0 | 9.0 ± 8.0 | <0.001 | 7.6 ± 7.0 | 9.6 ± 8.3 | <0.001 |
| Diastolic blood pressure, mmHg | 68.0 ± 9.7 | 69.2 ± 10.3 | 0.007 | 67.9 ± 9.6 | 70.0 ± 10.9 | <0.001 |
| Systolic blood pressure, mmHg | 121.9 ± 19.8 | 124.1 ± 20.9 | 0.02 | 122.2 ± 19.2 | 124.5 ± 23.0 | 0.04 |
| HDL cholesterol, mg/dL | 56.4 ± 16.2 | 55.0 ± 16.7 | 0.05 | 56.2 ± 16.3 | 54.9 ± 16.7 | 0.11 |
| Total cholesterol, mg/dL | 185.4 ± 35.2 | 184.4 ± 37.8 | 0.57 | 185.7 ± 36.0 | 182.9 ± 36.8 | 0.13 |
| Diabetes, % | 15 | 24 | <0.001 | 16 | 25 | <0.001 |
| Average sleep duration, h† | 7.4 ± 1.3 | 6.7 ± 1.5 | <0.001 | 7.4 ± 1.2 | 6.3 ± 1.6 | <0.001 |
| Insomnia, %‡ | 21 | 26 | 0.01 | 23 | 24 | 0.67 |
| Excessive daytime sleepiness, %‡ | 12 | 18 | <0.001 | 13 | 18 | 0.01 |
| Chronotype‡ | <0.001 | <0.001 | ||||
| Evening type, % | 6 | 9 | 6 | 10 | ||
| Intermediate type, % | 38 | 44 | 38 | 47 | ||
| Morning type, % | 55 | 47 | 55 | 43 | ||
| Apnea-Hypopnea Index§ | 19.0 ± 16.9 | 21.4 ± 19.1 | 0.001 | 19.4 ± 17.0 | 21.5 ± 19.9 | 0.005 |
| WASO, min§ | 86.0 ± 58.7 | 105.0 ± 71.6 | <0.001 | 89.7 ± 60.8 | 104.5 ± 74.1 | <0.001 |
| %REM sleep§ | 18.5 ± 6.3 | 17.4 ± 6.9 | 0.001 | 18.1 ± 6.3 | 18.0 ± 7.4 | 0.78 |
| %Stage N3 sleep§ | 10.4 ± 9.0 | 10.0 ± 9.2 | 0.32 | 10.4 ± 9.0 | 9.9 ± 9.2 | 0.29 |
| Average heart rate in sleep, bpm§ | 63.6 ± 8.9 | 65.0 ± 9.8 | 0.002 | 63.6 ± 9.0 | 65.5 ± 10.0 | 0.005 |
Values are % or mean ± SD.
P-value was calculated using t-test for continuous variables and Chi-square test for categorical variables.
Based on 7-day actigraphy.
Based on sleep questionnaire.
Based on one-night polysomnography. AHI = Apnea-Hypopnea Index; MESA = the Multi-Ethnic Study of Atherosclerosis; REM = rapid eye movement; SD = standard deviation; WASO = wake after sleep onset
A total of 111 incident total CVD events occurred after a median follow-up of 4.9 years (including 35 myocardial infarctions, 16 CHD deaths, 30 strokes, 17 other coronary events and 13 other atherosclerotic or CVD deaths; Supplemental Table 1), yielding an overall incidence rate of 11.8 per 1,000 person-years. In unadjusted analysis, there was a trend toward increased incidence of CVD with more irregular sleep duration, with the CVD incidence per 1,000 person-years being 8.8, 9.1, 14.1 and 19.3 for sleep duration SD categories of ≤60, 61–90, 91–120 and >120 min, respectively (Table 2). After adjustment for CVD risk factors (Model 2), the risk for incident CVD increased progressively with increasing sleep duration variability. Compared with sleep duration SD ≤60 min, the HR (95% CI) was 1.07 (0.61, 1.88) for sleep duration SD 61–90 min, 1.54 (0.89, 2.65) for 91–120 min, and 2.02 (1.20, 3.39) for >120 min. When evaluating sleep duration SD as a continuous variable, every 1-hour increase in sleep duration SD was associated with 36% higher CVD risk (95% CI: 1.07, 1.73; p-trend=0.02). The association became somewhat stronger after further adjusting for sleep-related factors (Model 3).
Table 2.
Associations of 7-day variability in sleep duration and sleep onset timing with risk of total cardiovascular disease
| Cases/Person-years | Model 1 | Model 2 HR (95% CI) | Model 3 | |
|---|---|---|---|---|
| SD of sleep duration | ||||
| Categorical | ||||
| ≤60 min | 29/3,278 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 61–90 min | 23/2,541 | 1.06 (0.61, 1.85) | 1.07 (0.61, 1.88) | 1.09 (0.62, 1.92) |
| 91–120 min | 26/1,842 | 1.61 (0.93, 2.76) | 1.54 (0.89, 2.65) | 1.59 (0.91, 2.76) |
| >120 min | 33/1,711 | 2.18 (1.31, 3.63) | 2.02 (1.20, 3.39) | 2.14 (1.24, 3.68) |
| Continuous | ||||
| Per 1 hour | 111/9,372 | 1.41 (1.12, 1.79) | 1.36 (1.07, 1.73) | 1.39 (1.08, 1.78) |
| P-trend | 0.006 | 0.02 | 0.002 | |
| Binary | ||||
| ≤90 min | 52/5,818 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| >90 min | 59/3,554 | 1.83 (1.25, 2.68) | 1.72 (1.17, 2.53) | 1.76 (1.18, 2.63) |
| P-value | 0.004 | 0.01 | 0.009 | |
| SD of sleep onset timing | ||||
| Categorical | ||||
| ≤30 min | 17/1,846 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 31–60 min | 31/3,235 | 1.19 (0.66, 2.16) | 1.21 (0.66, 2.22) | 1.16 (0.64, 2.13) |
| 61–90 min | 25/2,022 | 1.51 (0.81, 2.82) | 1.48 (0.79, 2.78) | 1.52 (0.81, 2.88) |
| >90 min | 38/2,269 | 2.05 (1.14, 3.69) | 1.95 (1.08, 3.54) | 2.11 (1.13, 3.91) |
| Continuous | ||||
| Per 1 hour | 111/9,372 | 1.17 (1.06, 1.30) | 1.16 (1.05, 1.29) | 1.18 (1.06, 1.31) |
| P-trend | 0.002 | 0.005 | 0.002 | |
| Binary | ||||
| ≤90 min | 73/7,103 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| >90 min | 38/2,269 | 1.67 (1.12, 2.48) | 1.58 (1.05, 2.37) | 1.69 (1.10, 2.61) |
| P-value | 0.01 | 0.03 | 0.02 | |
Model 1: Stratified by study site, and adjusted for age, sex, race/ethnicity, education and work schedules Model 2: Model 1 + adjusted for smoking status, physical activity, depressive symptoms, BMI, diabetes status, systolic blood pressure, HDL cholesterol and total cholesterol
Model 3: Model 2 + adjusted for average sleep duration, insomnia symptom scores, chronotype, Epworth Sleepiness Scale and AHI
AHI = Apnea-Hypopnea Index; BMI = body mass index; CI = confidence interval; HR = hazard ratio; SD = standard deviation
A similar positive association between sleep onset timing SD and incident CVD risk was observed (Table 2). Compared with sleep onset timing SD ≤30 min, the HR (95% CI) for CVD after adjusting for CVD risk factors and sleep-related factors (Model 3) was 1.16 (0.64, 2.13) for 31–60 min, 1.52 (0.81, 2.88) for 61–90 min, and 2.11 (1.13, 3.91) for >90 min, with 18% higher risk (95% CI: 1.06, 1.31) for every 1-hour increase in sleep onset timing SD (p-trend=0.002). When sleep duration SD and sleep onset timing SD were simultaneously included in the model, the association was attenuated for both sleep regularity measures; the fully-adjusted HR (95% CI) associated with 1-hour increase in sleep variability was 1.23 (0.94, 1.63) for sleep duration and 1.14 (1.01, 1.28) for sleep timing.
Tests for effect modification by age, sex, race/ethnicity, average sleep duration, or work schedules were not statistically significant (p-interaction>0.43; Table 3), although a suggestion of positive associations between irregular sleep and CVD risk was observed in racial/ethnic minorities (African-Americans, Hispanics and Chinese-Americans) and not in whites. The results did not alter considerably when we excluded CVD cases diagnosed in the first 6 months (n=8), 12 months (n=24), or 18 months (n=36) after sleep study (Supplemental Table 2) or when only hard CVD outcomes (n=81) were considered (Supplemental Table 3). Despite a tendency towards lower sleep variability during weekdays (e.g., 34.1% had sleep duration SD >90 min and 39.8% had sleep onset timing SD >60 min during weekdays, compared to 7-day SD of 39.5% and 47.2%, respectively), there were similar positive associations between weekday sleep irregularity measures and CVD risk (Supplemental Table 4).
Table 3.
Subgroup analysis of the association between sleep variability (per hour) and incident cardiovascular disease
| Sleep duration SD | Sleep onset timing SD | |||
|---|---|---|---|---|
| HR (95% CI)1 | p for interaction2 | HR (95% CI)1 | p for interaction2 | |
| Age | 0.63 | 0.96 | ||
| <70 years | 1.50 (0.99, 2.27) | 1.17 (0.96, 1.42) | ||
| ≥70 years | 1.34 (0.97, 1.87) | 1.19 (1.04, 1.36) | ||
| Sex | 0.57 | 0.44 | ||
| Male | 1.54 (1.11, 2.14) | 1.25 (1.09, 1.44) | ||
| Female | 1.40 (0.90, 2.19) | 1.17 (0.96, 1.42) | ||
| Race/ethnicity | 0.43 | 0.61 | ||
| White | 0.91 (0.57, 1.46) | 1.04 (0.84, 1.29) | ||
| African American | 1.66 (0.93, 2.95) | 1.34 (1.05, 1.71) | ||
| Hispanic | 2.19 (1.35, 3.57) | 1.38 (1.13, 1.69) | ||
| Chinese | 2.41 (0.77, 7.59) | 1.55 (0.96, 2.51) | ||
| Average sleep duration | 0.92 | 0.76 | ||
| <7 hours | 1.46 (0.99, 2.15) | 1.22 (1.04, 1.42) | ||
| 7–8 hours | 1.40 (0.85, 2.33) | 1.30 (1.06, 1.59) | ||
| >8 hours | 1.16 (0.66, 2.04) | 1.03 (0.74, 1.45) | ||
| Work schedules | 0.84 | 0.76 | ||
| Day shift/do not work | 1.42 (1.08, 1.87) | 1.20 (1.07, 1.35) | ||
| Other shift | 1.66 (0.65, 4.21) | 1.43 (0.93, 2.20) | ||
Stratified by study site, and adjusted for age, sex, race/ethnicity, education, work schedules, smoking status, physical activity, depressive symptoms, BMI, diabetes status, systolic blood pressure, HDL cholesterol, total cholesterol, average sleep duration, insomnia symptom scores, chronotype, Epworth Sleepiness Scale and AHI
P for interaction was calculated by the likelihood ratio test comparing the models with versus without the cross-product interaction term(s)
AHI = Apnea-Hypopnea Index; BMI = body mass index; CI = confidence interval; HR = hazard ratio; SD = standard deviation
Discussion
In this first prospective investigation of sleep regularity, a marker for chronic circadian disruption and intermittent sleep deprivation, with CVD incidence, individuals with the most irregular sleep duration or timing had >2-fold risk of developing CVD over a median follow-up of 4.9 years compared with individuals with the most regular sleep patterns. This association was consistently observed in different strata of the study sample, including subgroups defined by age, sex, race/ethnicity (except white participants), sleep duration and work schedules. Most importantly, the results remained robust after considering multiple established CVD risk factors and conventional measures of sleep quantity and quality, suggesting that irregular sleep duration and timing may be novel and independent risk factors for CVD.
No prior studies have examined the ubiquitous exposure to chronic circadian disruption, as measured by 7-day variations in sleep duration and sleep onset timing, in relation to CVD risk. However, multiple lines of evidence suggest that circadian disruption in specific settings could confer increased CVD risk. For example, studies from the U.S. and several European countries found a significant rise in heart attack and stroke in the first few days after shift to daylight saving time in the spring (11). Elevated CVD risk has been observed among rotating night shift workers or frequent intercontinental travelers, including flight crews (9,10,34,35). Social jetlag, defined as the difference in sleep timing between workdays and free days, was associated with unfavorable cardiometabolic risk factors (36,37). Although social jetlag is a major contributor to day-to-day sleep irregularity, our results indicate that irregular sleep restricted to weekdays was also associated with increased CVD risk.
Our findings are plausible given prior research linking actigraphy-assessed sleep regularity with metabolic risk factors that predispose to atherosclerosis (14–19). While we also observed that participants with irregular sleep tended to have worse cardiometabolic risk profiles at baseline, adjustment for established CVD risk factors (e.g., blood pressure, lipids, diabetes, etc.) only explained a small portion of the associations between sleep irregularity and CVD risk. This suggests that there may be other mechanisms through which irregular sleep influences CVD risk. Circadian clock genes, such as clock, per2 and bmal1, have been shown experimentally to control a broad range of cardiovascular rhythms and functions, from blood pressure and endothelial functions to vascular thrombosis and cardiac remodeling (38–41). Circadian disruption and poor sleep also influence the rhythms of the autonomic nervous system (42,43), which directly govern normal cardiac functioning. Irregular sleep may further disturb behavioral rhythms with regard to timing/amount of eating or exercise, which poses even higher CVD risk to irregular sleepers, such as nocturnal food intake and breakfast skipping (44–46). Few studies, however, have examined the biologic consequences of irregular sleep per se under a long-term habitual setting, and more studies are needed to understand the exact mechanisms linking irregular sleep and CVD risk.
While rotating night shift work is considered as a more severe form of circadian disruption than sleep irregularity, the increased CVD risk associated with sleep irregularity in this study appears stronger compared to prior reports of shift work and CVD (12). There are multiple potential explanations, including a healthy worker effect in published studies and the ubiquitous exposure to circadian disruption (e.g., irregular sleep schedules) among non-shift workers or short-term shift workers (12), which may mask the contrast between shift workers and non-shift workers. Our findings underscore the importance of considering sources of circadian disruption other than shift work, such as that resulting from large night-to-night variability in sleep duration and timing, and highlight that irregular sleep is highly prevalent in this racial/ethnically diverse community sample.
Our findings highlight the importance of sleep variability as a healthy sleep target apart from sleep duration, consistent with emerging concepts of multi-dimensional components of sleep health (47). Most prior large epidemiological studies focused on average sleep duration, with both short and long sleep durations associated with adverse cardiometabolic profiles (20,48). Our findings showing increased CVD incidence with variable sleep duration or timing persisted after adjusting for average sleep duration. Further research is needed to determine the extent to which increased sleep variability influences CVD via effects on circadian disruption versus exposing individuals to intermittent short sleep that is inadequately compensated for by over-sleeping.
Our results suggest initial sleep consistency targets that can be tested for improving cardiometabolic health. A 7-day SD greater than 90 minutes for either sleep duration variability or sleep onset timing variability was consistently associated with higher CVD risk. Whether this cutoff can be generalized as a threshold target for promoting cardiometabolically healthy sleep requires studies in other populations. If confirmed, use of wearable devices or specialized mobile apps may provide opportunities to incorporate sleep consistency targets into future interventions designed to reduce CVD incidence.
Study strengths
The strengths of the study included the prospective study design, objectively assessed sleep variability over 7 days, expert adjudication of incident CVD outcomes, and availability of many important factors that we could control for in the analysis. The results remain robust in multiple sensitivity analyses that address potential reverse causality and subgroup heterogeneity, lending additional credibility to an association of irregular sleep patterns with CVD risk.
Study limitations
First, the study sample is modest (but large for an actigraphy study) with relatively short follow-up. Therefore, we cannot fully address potential reverse causation by introducing a longer disease induction period (e.g., 2 years), and interpretation of the subgroup analysis should be cautious given the sample size. The modest sample also precluded our ability to examine associations with individual types of CVD endpoints, such as CHD versus stroke, or mortality. Second, residual and unmeasured confounding could not be excluded. Finally, there are limitations for actigraphy data. For example, as actigraphy estimates sleep-wake times based on activity, it only modestly correlates with polysomnography-measured sleep parameters and may not perform well in individuals with abnormal activity patterns during sleep (26,49). Nonetheless, it is a recommended tool for assessing chronic insomnia and circadian rhythm disorders in clinical settings (50).
In conclusion, higher day-to-day variations in sleep duration and timing are both associated with increased risk of CVD, independent of a wide range of factors including CVD risk factors, sleep duration, and sleep apnea. Given the strong biological plausibility of our associations and prior research implicating sleep variability with CVD risk factors, our results support considering inconsistent sleep patterns as a novel CVD risk factor and suggest the need to evaluate the role of healthy sleep practice interventions as a strategy for cardiovascular risk reduction.
Supplementary Material
Central illustration: Regulare and irregular sleep patterns in relation to cardiovascular disease risk.
The left panel shows 2 real examples of 7-day sleep patterns measured by actigraphy: a highly regular sleeper with approximately 10 min SD for both sleep duration and sleep onset timing and a highly irregular sleeper with >120 min for both SD measures. These 2 examples correspond to the ‘most regular’ and ‘most irregular’ categories in the figures on the right panel. Compared with those with the most regular sleep patterns, participants with the most irregular sleep patterns had >2-fold increased risk of developing cardiovascular events over a median follow-up of 4.9 years (p-trend=0.002 for both sleep duration SD and sleep onset timing SD). Abbreviations: CVD = cardiovascular disease; HR = hazard ratio; SD = standard deviation
CLINICAL PERSPECTIVES.
Competency in Medical Knowledge: Irregular sleep patterns in older people without known cardiovascular disease are associated with an elevated risk of ischemic events.
Translational Outlook: Given the high prevalence of irregular sleep patterns in the population, future studies should evaluate the impact on cardiovascular health of interventions that enhance sleep pattern consistency.
Acknowledgements:
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. T.H. and S.R. are the guarantors of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding: This research was supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences. The MESA Sleep Study was supported by NHLBI grant HL56984. T.H. was supported by K01HL143034, and S.R. was supported by R35HL135818. The funders had no role of in the design and conduct of the study, in the collection, analysis, and interpretation of the data, and in the preparation, review, or approval of the manuscript
Abbreviations:
- AHI
Apnea-Hypopnea Index
- BMI
body mass index
- CHD
coronary heart disease
- CI
confidence interval
- CVD
cardiovascular disease
- HR
hazard ratio
- MESA
the Multi-Ethnic Study of Atherosclerosis
- REM
rapid eye movement
- SD
standard deviation
- WASO
wake after sleep onset
Footnotes
Disclosures
S.R. reports consulting fees from Jazz Pharmaceutical and Respircardia Inc., unrelated to this work, and grant support from Jazz Pharmaceutical unrelated to this work. No other potential conflicts of interest relevant to this article were reported.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Muller JE, Stone PH, Turi ZG et al. Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med 1985;313:1315–22. [DOI] [PubMed] [Google Scholar]
- 2.Muller JE, Ludmer PL, Willich SN et al. Circadian variation in the frequency of sudden cardiac death. Circulation 1987;75:131–8. [DOI] [PubMed] [Google Scholar]
- 3.Wroe SJ, Sandercock P, Bamford J, Dennis M, Slattery J, Warlow C. Diurnal variation in incidence of stroke: Oxfordshire community stroke project. Bmj 1992;304:155–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Laake LW, Luscher TF, Young ME. The circadian clock in cardiovascular regulation and disease: Lessons from the Nobel Prize in Physiology or Medicine 2017. Eur Heart J 2018;39:2326–2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Panza JA, Epstein SE, Quyyumi AA. Circadian variation in vascular tone and its relation to alpha-sympathetic vasoconstrictor activity. N Engl J Med 1991;325:986–90. [DOI] [PubMed] [Google Scholar]
- 6.Thosar SS, Butler MP, Shea SA. Role of the circadian system in cardiovascular disease. J Clin Invest 2018;128:2157–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paschos GK, FitzGerald GA. Circadian clocks and vascular function. Circ Res 2010;106:833–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Degaute JP, van de Borne P, Linkowski P, Van Cauter E. Quantitative analysis of the 24-hour blood pressure and heart rate patterns in young men. Hypertension 1991;18:199–210. [DOI] [PubMed] [Google Scholar]
- 9.Vetter C, Devore EE, Wegrzyn LR et al. Association Between Rotating Night Shift Work and Risk of Coronary Heart Disease Among Women. Jama 2016;315:1726–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vyas MV, Garg AX, Iansavichus AV et al. Shift work and vascular events: systematic review and meta-analysis. Bmj 2012;345:e4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manfredini R, Fabbian F, De Giorgi A et al. Daylight saving time and myocardial infarction: should we be worried? A review of the evidence. Eur Rev Med Pharmacol Sci 2018;22:750–755. [DOI] [PubMed] [Google Scholar]
- 12.Erren TC, Lewis P. Hypothesis: ubiquitous circadian disruption can cause cancer. Eur J Epidemiol 2019;34:1–4. [DOI] [PubMed] [Google Scholar]
- 13.Erren TC, Lewis P. Can yesterday’s smoking research inform today’s shiftwork research? Epistemological consequences for exposures and doses due to circadian disruption at and off work. J Occup Med Toxicol 2017;12:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang T, Redline S. Cross-sectional and Prospective Associations of Actigraphy-Assessed Sleep Regularity With Metabolic Abnormalities: The Multi-Ethnic Study of Atherosclerosis. Diabetes Care 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel SR, Hayes AL, Blackwell T et al. The association between sleep patterns and obesity in older adults. Int J Obes (Lond) 2014;38:1159–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sohail S, Yu L, Bennett DA, Buchman AS, Lim AS. Irregular 24-hour activity rhythms and the metabolic syndrome in older adults. Chronobiol Int 2015;32:802–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor BJ, Matthews KA, Hasler BP et al. Bedtime Variability and Metabolic Health in Midlife Women: The SWAN Sleep Study. Sleep 2016;39:457–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spruyt K, Molfese DL, Gozal D. Sleep duration, sleep regularity, body weight, and metabolic homeostasis in school-aged children. Pediatrics 2011;127:e345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lunsford-Avery JR, Engelhard MM, Navar AM, Kollins SH. Validation of the Sleep Regularity Index in Older Adults and Associations with Cardiometabolic Risk. Sci Rep 2018;8:14158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cappuccio FP, Cooper D, D’Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J 2011;32:1484–92. [DOI] [PubMed] [Google Scholar]
- 21.Covassin N, Singh P. Sleep Duration and Cardiovascular Disease Risk: Epidemiologic and Experimental Evidence. Sleep Med Clin 2016;11:81–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson PW, D’Agostino RB, Parise H, Sullivan L, Meigs JB. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation 2005;112:3066–72. [DOI] [PubMed] [Google Scholar]
- 23.Bild DE, Bluemke DA, Burke GL et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol 2002;156:871–81. [DOI] [PubMed] [Google Scholar]
- 24.Chen X, Wang R, Zee P et al. Racial/Ethnic Differences in Sleep Disturbances: The Multi-Ethnic Study of Atherosclerosis (MESA). Sleep 2015;38:877–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Redline S, Sanders MH, Lind BK et al. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep Heart Health Research Group. Sleep 1998;21:759–67. [PubMed] [Google Scholar]
- 26.Jackson CL, Patel SR, Jackson WB 2nd,, Lutsey PL, Redline S. Agreement between self-reported and objectively measured sleep duration among white, black, Hispanic, and Chinese adults in the United States: Multi-Ethnic Study of Atherosclerosis. Sleep 2018;41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levine DW, Kripke DF, Kaplan RM et al. Reliability and validity of the Women’s Health Initiative Insomnia Rating Scale. Psychol Assess 2003;15:137–48. [DOI] [PubMed] [Google Scholar]
- 28.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991;14:540–5. [DOI] [PubMed] [Google Scholar]
- 29.Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol 1976;4:97–110. [PubMed] [Google Scholar]
- 30.Bluemke DA, Kronmal RA, Lima JA et al. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol 2008;52:2148–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mackey RH, Greenland P, Goff DC Jr.,, Lloyd-Jones D, Sibley CT, Mora S. High-density lipoprotein cholesterol and particle concentrations, carotid atherosclerosis, and coronary events: MESA (multi-ethnic study of atherosclerosis). J Am Coll Cardiol 2012;60:508–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Somers VK, White DP, Amin R et al. Sleep apnea and cardiovascular disease: an American Heart Association/american College Of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council On Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation 2008;118:1080–111. [DOI] [PubMed] [Google Scholar]
- 33.Huang T, Zeleznik OA, Poole EM et al. Habitual sleep quality, plasma metabolites and risk of coronary heart disease in post-menopausal women. Int J Epidemiol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McNeely E, Gale S, Tager I et al. The self-reported health of U.S. flight attendants compared to the general population. Environ Health 2014;13:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim JN, Lee BM. Risk factors, health risks, and risk management for aircraft personnel and frequent flyers. J Toxicol Environ Health B Crit Rev 2007;10:223–34. [DOI] [PubMed] [Google Scholar]
- 36.Wong PM, Hasler BP, Kamarck TW, Muldoon MF, Manuck SB. Social Jetlag, Chronotype, and Cardiometabolic Risk. J Clin Endocrinol Metab 2015;100:4612–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parsons MJ, Moffitt TE, Gregory AM et al. Social jetlag, obesity and metabolic disorder: investigation in a cohort study. Int J Obes (Lond) 2015;39:842–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Viswambharan H, Carvas JM, Antic V et al. Mutation of the circadian clock gene Per2 alters vascular endothelial function. Circulation 2007;115:2188–95. [DOI] [PubMed] [Google Scholar]
- 39.Young ME, Brewer RA, Peliciari-Garcia RA et al. Cardiomyocyte-specific BMAL1 plays critical roles in metabolism, signaling, and maintenance of contractile function of the heart. J Biol Rhythms 2014;29:257–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anea CB, Zhang M, Stepp DW et al. Vascular disease in mice with a dysfunctional circadian clock. Circulation 2009;119:1510–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Curtis AM, Seo SB, Westgate EJ et al. Histone acetyltransferase-dependent chromatin remodeling and the vascular clock. J Biol Chem 2004;279:7091–7. [DOI] [PubMed] [Google Scholar]
- 42.Burgess HJ, Trinder J, Kim Y, Luke D. Sleep and circadian influences on cardiac autonomic nervous system activity. Am J Physiol 1997;273:H1761–8. [DOI] [PubMed] [Google Scholar]
- 43.Huang T, Poole EM, Vetter C et al. Habitual sleep quality and diurnal rhythms of salivary cortisol and dehydroepiandrosterone in postmenopausal women. Psychoneuroendocrinology 2017;84:172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. Circadian timing of food intake contributes to weight gain. Obesity (Silver Spring) 2009;17:2100–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garaulet M, Gomez-Abellan P, Alburquerque-Bejar JJ, Lee YC, Ordovas JM, Scheer FA. Timing of food intake predicts weight loss effectiveness. Int J Obes (Lond) 2013;37:604–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rong S, Snetselaar LG, Xu G et al. Association of Skipping Breakfast With Cardiovascular and All-Cause Mortality. J Am Coll Cardiol 2019;73:2025–2032. [DOI] [PubMed] [Google Scholar]
- 47.Buysse DJ. Sleep health: can we define it? Does it matter? Sleep 2014;37:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shan Z, Ma H, Xie M et al. Sleep duration and risk of type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care 2015;38:529–37. [DOI] [PubMed] [Google Scholar]
- 49.Sadeh A. The role and validity of actigraphy in sleep medicine: an update. Sleep Med Rev 2011;15:259–67. [DOI] [PubMed] [Google Scholar]
- 50.Smith MT, McCrae CS, Cheung J et al. Use of Actigraphy for the Evaluation of Sleep Disorders and Circadian Rhythm Sleep-Wake Disorders: An American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med 2018;14:1231–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.