Abstract
Osmanthus fragrans is a well-known native plant in China, and carotenoids are the main group of pigments in the petals. Abscisic acid (ABA) is one of the products of the metabolic pathway of carotenoids. Application of ABA could affect pigmentation of flower petals by changing the carotenoid content. However, little is known about the effects of ABA treatment on carotenoid accumulation in O. fragrans. In this study, different concentrations of ABA (0, 150 and 200 mg/L) were spread on the petals of O. fragrans ‘Yanhonggui’. The petal color of ‘Yanhonggui’ receiving every ABA treatment was deeper than that of the control. The content of total carotenoids in the petals significantly increased with 200 mg/L ABA treatment. In the petals, α-carotene and β-carotene were the predominant carotenoids. The expression of several genes involved in the metabolism of carotenoids increased with 200 mg/L ABA treatment, including PSY1, PDS1, Z-ISO1, ZDS1, CRTISO, NCED3 and CCD4. However, the transcription levels of the latter two carotenoid degradation-related genes were much lower than of the five former carotenoid biosynthesis-related genes; the finding would explain the significant increase in total carotenoids in ‘Yanhonggui’ petals receiving the 200 mg/L ABA treatment.
Keywords: Osmanthus fragrans, carotenoid, abscisic acid
1. Introduction
Osmanthus fragrans is a favorite traditional flower in China because of its delightful flower color and scent. Carotenoids are the major pigments in the petals of O. fragrans [1]. Carotenoids produce many yellow, orange and red pigments in nature, including many fruits [2], vegetables, flowers [3], butterflies and crayfish [4]. In the petals, which mainly contain carotenoids, the kind and content of the carotenoids determine the color. In Marigold (Calendula officinalis), for example, the carotenoid content of the most pigmented varieties is approximately 100-fold that of white flowers [5]. In addition, carotenoid metabolism-related genes contribute to the color formation in these flowers, including biosynthesis- and degradation-related genes. In plants, carotenoids are derived from the 2-C-methyl-D-erythritol 4-phosphate (MEP) pathway in plastids [4]. Phytoene, formed by phytoene synthase (PSY), is the first committed step in the metabolic pathway of carotenoids. Then, the colorless phytoene is catalyzed by four enzymes, including phytoenedesaturase (PDS), ζ-carotene isomerase (Z-ISO), ζ-carotene desaturase (ZDS) and carotenoid isomerase (CRTISO), to produce lycopene. Lycopene is catalyzed by lycopene β-cyclase (LCYB) and lycopene ε-cyclase (LCYE) to form α-carotene; in contrast, β-carotene is catalyzed only by LCYB. Furthermore, α-carotene is converted to lutein by β-ring carotene hydroxylase (CHYB) and ε-ring carotene hydroxylase (CHYE), which is the end-product of the α-carotene branch. β-caroteneis catalyzed by CHYB twice to form zeaxanthin. Then, zeaxanthin is converted to neoxanthin by neoxanthin synthase (NSY). The isomers of violaxanthin and neoxanthinare cleaved by nine-cis-epoxy-carotenoid dioxygenases (NCEDs) to produce ABA [6,7,8].
Plant hormones play important roles in plant growth, development and secondary metabolism. Previous studies have shown that ethylene treatment can increase the content of carotenoids in different species, such as Citrus sinensis Osbeck [9], Satsuma mandarin (Citrus unshiu Marc.) [10] and papaya (Carica papaya cv. ‘Golden’) [11], because genes involved in biosynthesis of carotenoids, such as PSY, LCYB and CHYB, are upregulated by ethylene treatment [9]. In contrast, IAA treatment delays carotenoid accumulation in tomato [12]. For methyl jasmonate (MeJA), carotenoid accumulation was enhanced in Coriander (Coriandrum sativum) receiving MeJA treatment because of the increased transcripts level of CsCHYE, CsPDS, CsZDS and CsLCYE [13]. Other plant hormones, such as salicylic acid (SA), can also enhance the accumulation of carotenoids [14]. ABA regulates a wide range of plant growth and developmental processes [15,16] and mediates plant responses to environmental stresses [17,18]. In addition, the application of exogenous ABA has been shown to affect carotenoid content in Lycopersicon esculentum, as ABA treatment decreased the content of lutein but significantly increased the content of β-carotene. In two additional species of tomato, the application of ABA increased lutein, β-carotene and zeaxanthin in their leaves [19,20]. Correlated with the change in carotenoids, the expression of genes involved in the metabolic pathways of carotenoids was also influenced by ABA treatment. Recently, it has been shown that the expression of other carotenoid biosynthesis-related genes, such as PSY, could also be increased by ABA treatment [21]. In O. fragrans, the research are mainly about its flavor and color. Wang et al. found that O. fragrans could be divided into two clusters, an orange-red cluster and a yellowish-white cluster, respectively, in terms of the content of carotenoids and flavonoids in petals. The petals of the yellowish-white cultivars exhibited high contents of β-carotene, lutein and α-carotene, whereas the petals of the orange-red cultivars mainly contained β-carotene and α-carotene. The profound diversity in the total carotenoid concentrations of the two clusters was determined by the transcript levels of OfCCD4 [22]. The main volatile organic compounds in petals of O. fragrans are linalool, α-ionone, β-ionone, and γ-decalactone, and 19 °C would promote the emission of a floral scent [23]. Studies have shown that the biosynthesis of volatile compounds in O. fragrans is caused by the OfCCD4 instead of OfCCD1 [24,25]. However, there is no report on ABA regulation of carotenoids in O. fragrans. Our purpose was to determine the effects of different ABA concentrations on carotenoids accumulation and the expression patterns of genes involved in carotenoids metabolism in the petals of O. fragrans ‘Yanhonggui’. This will help us have a better understanding of carotenoids accumulation in petals of O. fragrans under stress, because the production of ABA would increase when plants receive abiotic stress [26,27]. Furthermore, it would provide us with basic knowledge on breeding new cultivars of O. fragrans with deeper or lighter color petals.
2. Results
2.1. Effect of ABA Treatment on the Petal Color
In order to understand the petals’ color change after ABA treatment, we used Color Reader to measure the phenotype of the petals’ a* (redness), b* (yellowness), L* (lightness), C* (chroma) and h* (hue-angle). The a* and b* values of ‘Yanhonggui’ petals receiving ABA treatment at S2 showed no difference compared to the control, and the L* values of petals receiving 150 mg/L ABA were higher than the other two treatments (0 and 200 mg/L ABA) (Table 1). At S3, the a* values were similar among treatments, while the L* value of the control was highest, followed by the 150 mg/L and 200 mg/L ABA treatments. There were no differences in h* values among every treatment. The L*, h*, C* and b* values were similar to the control, and the a* values for plants receiving the 150 mg/L ABA treatment were higher than the control but were similar to those receiving the 200 mg/L ABA treatment.
Table 1.
Phenotype parameters of ‘Yanhonggui’ receiving different concentrations of ABA treatment.
| Stage | Treatment | a* | b* | L* | C* | h* |
|---|---|---|---|---|---|---|
| S2 | 0 mg/L | 0 | 35.60 ± 0.98a | 52.35 ± 0.03a | 63.00 ± 0.23b | 63.32 ± 0.58a |
| 150 mg/L | 150 | 33.50 ± 0.60a | 54.77 ± 1.21a | 67.33 ± 0.60a | 64.20 ± 1.31a | |
| 200 mg/L | 200 | 35.57 ± 0.45a | 51.93 ± 2.52a | 63.13 ± 1.27b | 62.96 ± 2.30a | |
| S3 | 0 mg/L | 0 | 38.70 ± 0.69a | 47.95 ± 2.45a | 61.75 ± 0.43a | 61.64 ± 2.34a |
| 150 mg/L | 150 | 38.80 ± 0.06a | 50.55 ± 0.89a | 60.00 ± 0.52a | 63.73 ± 0.67a | |
| 200 mg/L | 200 | 41.20 ± 1.31a | 49.20 ± 2.02a | 58.67 ± 1.10b | 64.17 ± 2.38a | |
| S4 | 0 mg/L | 0 | 42.35 ± 0.03b | 48.80 ± 1.73a | 59.15 ± 0.55ab | 64.63 ± 1.29b |
| 150 mg/L | 150 | 44.15 ± 0.55a | 49.40 ± 1.33a | 56.55 ± 0.61b | 66.26 ± 1.36ab | |
| 200 mg/L | 200 | 44.20 ± 0.17a | 55.43 ± 1.79a | 60.83 ± 1.39a | 70.91 ± 1.48a |
S2: initial flowering stage. S3: full flowering stage. S4: late flowering stage. Tukey’s multiple-range test was used, and least significant range analysis at 5% significance is shown by lowercase letters for the same stage for the control and treatments. All experiments were performed in triplicate. Data are shown as means ± SEM. Means followed by the same letter do not differ significantly.
2.2. Effect of ABA Treatment on Carotenoid Composition of the Petals
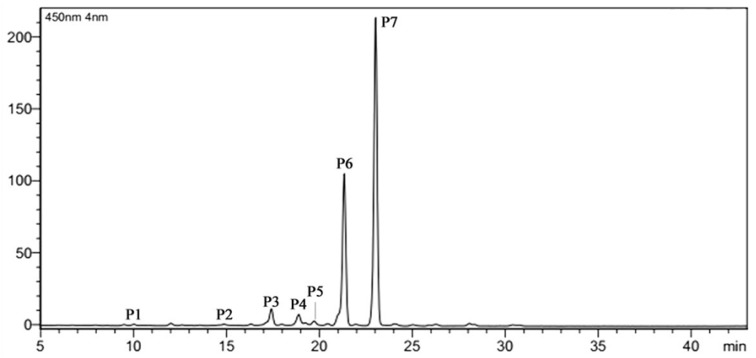
The carotenoids content in petals of ‘Yanhonggui’ were analyzed using the HPLC system. Carotenoids detected in ‘Yanhonggui’ petals included the internal standard β-apo-8′-carotenol (Figure 1, P3), phytoene (P1), lutein (P2), β-cryptoxanthin (P5), α-carotene (P6), β-carotene (P7), and an unidentified carotenoid (P4) based on their retention at S4 [22]. The total carotenoid content (TC) obtained with 200 mg/L ABA treatment was the highest, and the content of carotenoids in petals receiving the 150 mg/L ABA treatment was similar to that of the control, with 14,047.00 μg/g, 14,188.34 μg/g, and 15,328.64 μg/g dry weight (DW) content obtained for the tested treatments (control, 150 mg/L and 200 mg/L ABA treatment) (Table 2). The major carotenoids detected were α-and β-carotene, which accounted for more than 90% of the total carotenoids. Furthermore, with the 200 mg/L ABA treatment, β-carotene had the highest content (8887.31 μg/g DW); however, there was no significant difference between the 150 mg/L ABA treatment and the control of β-carotene. Similarly, the amount of α-carotene was highest for the 200 mg/L ABA treatment (5740.65 μg/g DW), followed by the 150 mg/L ABA treatment and the control (5089.04 μg/g and 4779.50 μg/g DW, respectively). Furthermore, the amounts of the other carotenoids were much lower than α- and β-carotene. Among these carotenoids, phytoene content showed no remarkable difference among all three treatments. For lutein, the content with ABA treatment was higher than that of the control. In contrast, the detected content of β-cryptoxanthin with the 150 mg/L ABA treatment was lower than that of the other two treatments.
Figure 1.
HPLC chromatograms of carotenoid pigments extracted from petals of ‘Yanhonggui’. P1: phytoene. P2: lutein. P3: internal standard β-apo-8′-carotenol. P4: unidentified carotenoid. P5: β-cryptoxanthin. P6: α-carotene. P7: β-carotene.
Table 2.
Carotenoid content in the petals of ‘Yanhonggui’ receiving ABA treatments.
| Content | 0 mg/L ABA | 150 mg/L ABA | 200 mg/L ABA |
|---|---|---|---|
| P1 | 44.81 ± 0.91b | 53.90 ± 2.53a | 59.17 ± 1.15a |
| P2 | 33.53 ± 0.55c | 53.92 ± 0.29a | 43.96 ± 0.64b |
| P4 | 376.97 ± 0.36b | 364.53 ± 0.38b | 404.89 ± 7.80a |
| P5 | 185.05 ± 2.56a | 150.90 ± 1.03b | 192.67 ± 2.90a |
| P6 | 4779.50 ± 15.97c | 5089.04 ± 64.07b | 5740.65 ± 72.36a |
| P7 | 8627.15 ± 1.05b | 8476.13 ± 98.27b | 8887.31 ± 27.09a |
| TC | 14,047 ± 13.07b | 14,188.43 ± 165.24b | 15,328.64 ± 95.05a |
Tukey’s multiple-range test was used, and least significant range analysis at 5% significance is shown by lowercase letters for the same stage for the control and treatments. All experiments were performed in triplicate. Data are shown as means ± SEM. Means followed by the same letter do not differ significantly.
2.3. Effect of ABA Treatment on the Expression of Genes Involved in Carotenoid Metabolism in the Petals
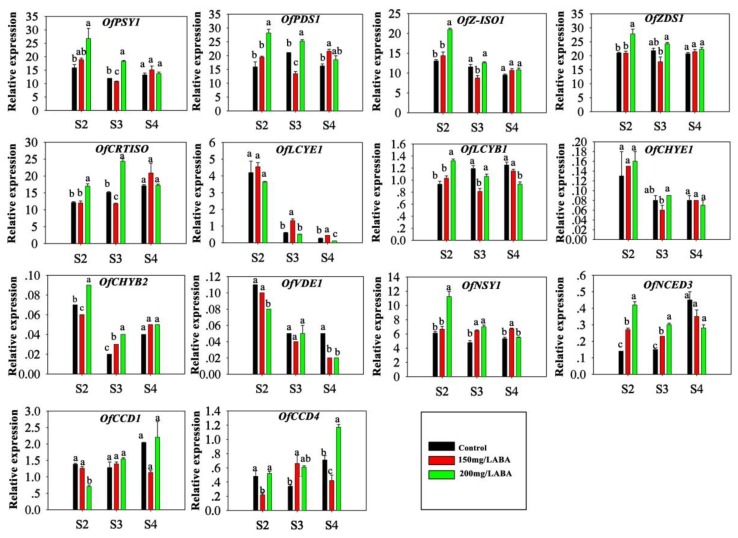
Quantitative real-time PCR analysis of fourteen genes involved in carotenoid matabolism was performed. The OfACT gene was an internal control. At S2 and S3, high ABA treatment (200 mg/L ABA) increased the expression of the upstream genes OfPSY1, OfPDS1, OfZDS1, OfCRTISO, OfCHYB2, OfNSY1 and OfNCED3, which are involved in the biosynthetic pathway of carotenoids, and the transcription levels of OfZ-ISO and OfLCYB1 were only upregulated at S2 with the 200 mg/L ABA treatment. The expression of OfCCD4 was upregulated at S3 and S4. Moreover, not all of the genes mentioned above were affected at other stages by ABA treatment. In contrast, expression of OfCCD1 and OfVDE decreased with the 200 mg/L ABA treatment at S2. Unlike the gene expression patterns found with the 200 mg/L ABA treatment, the application of 150 mg/L ABA only increased the expression of several genes, such as OfCHYB2 and OfCCD4 at S3, OfLCYE1 and OfNSY1 at S3 and S4, and OfNCED3 at S2 and S3. However, the transcript levels of other genes detected with the 150 mg/L ABA treatment were not affected and even decreased in some stages (Figure 2).
Figure 2.
Expression levels of genes involved in carotenoid metabolism in ‘Yanhonggui’ petals receiving ABA treatments. Tukey’s multiple-range test was used, and least significant range analysis at 5% significance is shown by lowercase letters for the same stage for the control and treatments. All experiments were performed in triplicate. Data are shown as means ± SEM. Means followed by the same letter do not differ significantly.
3. Discussion
Previous studies showed that the a* value was positively affected by TC [22]; in other words, the petals of cultivar which have deeper color contain more TC and the a* value was higher. In the present study, the a* value showed a significant difference at S4 but not S2 or S3 with ABA treatment. These results indicated that TC only changed at S4 significantly with ABA treatment in petals of ‘Yanhonggui’. So we only detected the carotenoid content at S4. The pattern of TC was consistent with the a* value pattern in petals receiving ABA treatment. The application of ABA increased the content of α-carotene, β-carotene and lutein in petals of ‘Yanhonggui’ (Table 2). Furthermore, α- and β-carotene were the main carotenoids, which confirms the petal color of ‘Yanhonggui’ [28]. In a previous study, it was reported that ABA treatment mainly increased the β-carotene and lutein content in the leaves of tomato [20]. In the fruit tissue of tomato, the β-carotene content was also increased by ABA treatment [19]. Lutein and β-carotene contents were also higher in ABA-treated plants than in control plants in Chinese cabbage and turnip [29,30]. Interestingly, different concentrations of ABA have different effects on the content of carotenoids. For pepper fruits, the main carotenoid, capsanthin, was increased by treatment with 150 mg/L ABA, while higher concentrations of ABA treatment reduced the content of capsanthin [31]. These results indicate that ABA treatments have positive effects on carotenoid accumulation. However, ABA treatment may have negative effects on carotenoids accumulation. The content of total carotenoids in tea plant (Camellia sinensis) flowers were reduced by ABA treatment [32]. Similar results were found in the callus of Scutellaria baicalensis after ABA treatment [33]. The effect of ABA treatment on carotenoids accumulation may vary among plant species. In pepper fruits, 150 mg/L ABA treatment promoted the accumulation of capsanthin, and it was 200 mg/L ABA treatment that significantly promoted carotenoid accumulation in the petals of ‘Yanhonggui’.
The genes involved in carotenoid metabolism—OfPSY1, OfPDS1, OfZ-ISO1, OfZDS1 and OfCRTISO, OfCCD4 and OfNCED3—were upregulated by ABA treatment in the present study. Consistent results were found in other species. In S. baicalensis [33] and Brassica oleracea [34], CCD4 and NCED were also upregulated by ABA treatment; the expression of OsPSY and OsNCED in rice (Oryza sativa) [21] was higher with ABA treatment than in the control. Additionally, in Brassica rapa, B. oleracea [34], Suaeda salsa [35] and Glycine max [36], ABA upregulated transcript levels of CCD1 and CCD4. PSY are the key enzymes indirectly regulating the biosynthesis of carotenoids that also contribute to phytoene—the first carotenoid produced in plants [6]. It has been indicated that abiotic stresses, including ABA, could regulate carotenoid accumulation via regulation of the expression of PSY [21,37]. Furthermore, ABA responsive elements (ABREs) have been found in the promoter of OsPSY [21] and AhNCED1 in peanut (Arachis hypogaea) [38], OfCCD1, OfCCD4 [39] and AtNCED [40,41]—indicating that the expression of CCD1, CCD4, NCED and PSY could be induced by ABA because it has been reported that genes whose promoters contained ABREs could respond to ABA treatment in Arabidopsis thaliana [42,43]. The relative higher transcription of OfPSY1, OfPDS1, OfZ-ISO1, OfZDS1 and OfCRTISO led to the higher content of precursor carotenoids. Then the higher transcripts level of OfLCYB1, which is the key gene involved in production of α- and β-carotene, caused a higher content of α- and β-carotene. While the precursor carotenoid detected was only phytoene and no lycopene was detected. These results were consistent with our previous study [22]. The little amount of precursor carotenoids could be caused by the downstream gene, OfLCYB1, in present study, because ABA treatment promotes the expression of it. Although the expression levels of OfPSY1, OfPDS1, OfZ-ISO1, OfZDS1, OfCRTISO, OfCCD4 and OfNCED were upregulated, the transcription level of OfCCD4 and OfNCED was much lower than that of OfPSY, OfPDS1, OfZ-ISO1, OfZDS1 and OfCRTISO, which indicates that the content of produced carotenoids was much more than that of degraded carotenoids. Previous studies on carotenoids accumulation in different O. fragrans showed that, consistent with the higher carotenoids contents, OfPSY1, OfPDS1, OfZ-ISO1, OfZDS1, and OfCRTISO in the upstream pathways were highly expressed in the petals, and the expression profiles of OfLCYB1 in petals were higher than those of OfLCYE1. Regarding the genes of the degradation pathway, the expression level of OfCCD1 was negatively correlated with the concentration of carotenoids, whereas the expression levels of OfNCED3 and OfCCD4, which were highly expressed in petals, showed no correlation with the TC content [22]. In the present study, the same genes mentioned above have similar expression patterns in petals. The expression of these genes was promoted with ABA treatment, especially the genes involved in carotenoids biosynthesis such as OfPSY1 and OfLCYB1. Additionally, there is evidence of positive feedback regulation between OsPSY3, OsNCEDs and ABA treatment in rice. OsPSY3 transcripts are up-regulated during increased abscisic acid (ABA) formation upon salt treatment and drought. The simultaneous induction of genes encoding 9-cis-epoxycarotenoid dioxygenases (NCEDs) is involved in the initial steps of ABA biosynthesis. Then, OsPSY3 and the OsNCEDs are induced by ABA to promote ABA production [21]. Similarly, the application of ABA could indirectly regulate the metabolic pathway of carotenoids in A. thaliana [37]. According to the above conclusion and the results of the present study, we propose that there is a similar mechanism in the petals of ‘Yanhonggui’ treated with ABA. The application of ABA promoted the expression of genes whose promoters contained ABREs including OfPSY and OfNCED—the two key genes involved in ABA biosynthesis [21,44,45], and other genes like OfPDS1, OfZ-ISO1, OfZDS1 OfCRTISO and OfLCYB1. The higher expression of biosynthetic genes promotes the production of carotenoids, α-carotene and β-carotene, especially; on the other hand, the higher expression of OfNCED3 could promote the biosynthesis of ABA. The newly biosynthesized ABA, cooperating with absorbed exogenous ABA, would promote the expression of OfPSY1, OfPDS1, OfZ-ISO1, OfZDS1, OfCRTISO and OfNCED3. Finally, the application of ABA significantly increased the total carotenoids in petals of ‘Yanhonggui’. ABA, genes including OfPSY1, OfPDS1, OfZ-ISO1, OfZDS1, OfCRTISO and OfNCED3, and carotenoids all formed a positive feedback loop in ‘Yanhonggui’ treated with ABA. As more carotenoids are contained in the petals, a deeper color is produced. It has been proven that pale flowers have fewer carotenoids than more deeply colored flowers [7]. Furthermore, the ABA-signaling pathway is a complicated network, which includes ABA receptors (ABARs) [46], type 2C phosphatases (PP2Cs) [47], ABRE-binding factors (ABFs) [48], and other proteins. Further investigation would be the measurement of ABA and the contribution of endogenous and absorbed exogenous ABA on the carotenoids metabolic pathway, and the mechanism of the ABA-signaling pathway on carotenoid metabolism to elucidate the mechanism of petal color change in O. fragrans treated with ABA.
4. Materials and Methods
4.1. Plant Materials and Growth Condition
O. fragrans ‘Yanhonggui’ potted plants were grown under greenhouse conditions at Zhejiang A&F University and then were incubated at a constant temperature (25 °C) and relative humidity (70%) before the Linggeng stage (S1: flower buds are similar to round bells). The growth medium was a sterile mixture of peat: vermiculite: perlite (1:1:1). Samples were subjected to 12/12 hour light/dark periods until the treatment was completed. The light intensity inside the incubator was set to 5000 Lux by white fluorescent tubes. Then, 0 mg/L, 150 mg/L and 200 mg/L (±) ABA (Sigma-Aldrich, Shanghai, China) was spread onto the flowers every 24 h from the Linggeng stage (S1). Nine of O. fragrans ‘Yanhonggui’ potted plants with similar heights, and similar amounts of branches and buds were used in this present study. Each treatment contained three O. fragrans ‘Yanhonggui’ potted plants. Flower petals from different branches of same plant at the initial flowering stage (S2: flower petals start to expand), full flowering stage (S3: flower petals are fully open, and the anthers are yellow), and the late flowering stage (S4: flower petals are fully open, and the anthers are brown) [1] (Figure 3) were collected and mixed. At S2 and S3, mixed petals were randomly divided into two groups, part of the petals immediately frozen with liquid nitrogen, and then stored at −80 °C until they were used for gene expression analysis, and the rest was used for the measurement of flower color. At S4, mixed flower petals were randomly divided into three groups, and the first group of petals were immediately frozen with liquid nitrogen, and then stored at −80 °C until they were used for gene expression analysis; the second group of petals was directly frozen after collection by lyophilization in a freeze dryer and stored at −20 °C for carotenoids analysis. The third group of flower petals was used for the measurement of flower color. All nine plants were sampled at every stage.
Figure 3.
Flowering stages of ‘Yanhonggui’.
4.2. Measurement of Flower Color
About 15 flowers at different branches from same plant were collected and mixed, and their petals were used for the measurement of flower color. Petals from every plant (all nine plants and three in each treatment), were measured respectively. The color of fresh petals was measured using lightness (L*), hue-angle (h*), and two chromatic components, a* and b*, of the CIEL*a*b*color coordinates using a Color Reader (CR-10; Konica Minolta Sensing Inc., Tokyo, Japan). L* values indicated lightness, and the h* values were calculated from tan−1(b*/a*) [49].
4.3. Carotenoids Extraction and Analysis
About 60 flowers from different branches of the same plants were mixed and their petals were used for carotenoid extraction and analysis. Petals from every plant (all nine plants and three in each treatment) were collected respectively. The extraction of carotenoids was performed following the method described by Delpinorius et al. (2014) [50], with some procedural improvements. After grinding with liquid nitrogen and drying by lyophilization in a dark room, 0.05 g dry sample was placed in a 10-mL vessel with 4 mL methanol. Next, 2.3 mL of a NaCl solution (10%, w/v) was added. The mixture was shaken for 15 min and cooled at 4 °C. The mixture was centrifuged at 2700× g for 3 min at 4 °C, and the organic layer was recovered. The aqueous phase was re-extracted with 2 mL of hexane: diethyl ether (3:1, v/v) for 10 min and centrifuged in the same conditions. The organic solutions were combined in a 5 mL glass tube and dried under a nitrogen stream at room temperature. The dry residue was saponified at room temperature for 24 h using 1.5 mL of a 6% KOH solution in methanol (w/v). After the addition of 1.5 mL of sodium chloride solution (10%, w/v), the mixture was placed in a freezer for 15 min. Then, 2.5 mL of hexane: diethyl ether (3:1, v/v) was added, and the mixture was vortexed and centrifuged at 433× g for 3 min (this step was repeated until the aqueous phase was colorless). The organic layers were combined, and the solvent was removed under a nitrogen stream. The residue was stored at −80 °C under nitrogen until HPLC analysis.
4.4. Analysis of Carotenoids
The residue of carotenoids was dissolved in 1.5 mL methyl tert-butyl ether (MTBE) that contained β-apo-8′-carotenal as an internal standard (5 μg/mL) [51]. The carotenoid content analysis was performed by using a high-performance liquid chromatography (HPLC) system (Shimadzu Corporation, Kyoto, Japan). This system consists of an LC-20AT infusion pump, CTO-10AS VP column oven and SPD-M20A spectrophotometric detector. A C30-column (YMC Co. Ltd. Japan, 4.6 × 250 mm, 5 μm) was used at 25 °C, with a flow rate of 0.8 mL/min, and the data were recorded from 200 to 800 nm and measured at 450 nm. An amount of 10 μL analytes was injected. The elution solvents were solvent A (methanol) and solvent B (MTBE), and elution was performed as follows: 0 min, 0% B; 18min, 46% B; 35 min, 70% B; 37min, 70% B; 40min, 0% B; and 47min, 0% B.
4.5. RNA Extraction and cDNA Synthesis
About 60 flowers from different branches of the same tree were mixed and their petals were used for RNA extraction and analysis. Petals from every plant (all nine plants and three in each treatment), were collected and stored respectively. Samples from every plant were used for RNA extraction and cDNA synthesis. Total RNA was extracted using a NucleoSpin RNA Plant kit (TaKaRa, Dalian, China), and the cDNA was synthesized using a PrimeScript II Hi Fidelity RT-PCR kit (TaKaRa, Dalian, China).
4.6. Expression Analysis by Real-Time PCR
The primers used in RT-PCR reactions were developed by Zhang [28]. The expression level of OfACT was used as the reference [52]. The transcript levels of genes were analyzed by quantitative real-time PCR (RT-qPCR) using SYBRPremix Ex Taq II polymerase (TaKaRa, Dalian, China)and the Light Cycler 480 II Real Time System (Roche, Switzerland) according to the manufacturers’ instructions. Each 20 µL qRT-PCR reaction contained 10 µL SYBR R Premix Ex TaqTM II (Takara), 5 µL cDNA (100 ng/µL), 1.0 µL of 10 µM forward and reverse primer (designed using Primer Premier 5.0 software). The thermal cycling conditions were as follows: an initial denaturation of 30 s at 95 °C, followed by 40 cycles at 95 °C for 5 s and 60 °C for 30 s; then 95 °C for 5 s, 60 °C for 1 min and 95 °C for 15 s was used for dissociation curve analysis. The relative expression levels were calculated using 2-ΔCt [53]. Each statistical analysis was performed using IBM SPSS Statistics 19.0 (IBM, Armonk, NY, USA). The data were analyzed using a Tukey’s multiple-range test (P < 0.05) with three replicates.
5. Conclusion
In this research, O. fragrans ‘Yanhonggui’ were sprayed with different concentrations of ABA. The increased TC, α- and β-carotenes were due to the higher expression of OfLCYB1 upon receiving 200 mg/L ABA treatment. The higher transcription levels of OfPSY1, OfPDS1, Z-ISO1, OfCRTISO were upregulated by ABA treatment in O. fragrans petals, providing the precursors to produce the main carotenes, α- and β-carotene. While the much lower expression levels of OfNCED3 and OfCCD4 caused resulted in the degraded carotenoids content being much lower than produced carotenoids content. As a result, the increased carotenoids content, especially α- and β-carotenes, was greater than that of the control, and led to the deeper color in O. fragrans petals.
Author Contributions
Conceptualization, Y.L. and H.Z.; methodology, Y.L., H.Z., and Y.W.; investigation, Y.L.; writing—original draft preparation, Y.L.; writing—review and editing, B.D., C.Z., L.Y. and H.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (Grant No. 31501790), the Zhejiang Provincial Natural Science Foundation of China (Grant No. LQ19C160002 and LQ15C160004), and the Zhejiang Provincial Major Program of New Cultivar Breeding (Grant No. 2016C02056-12).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Han Y., Wang X., Chen W., Dong M., Yuan W., Liu X., Shang F. Differential expression of carotenoid-related genes determines diversified carotenoid coloration in flower petal of Osmanthus fragrans. Tree Genet. Genomes. 2014;10:329–338. doi: 10.1007/s11295-013-0687-8. [DOI] [Google Scholar]
- 2.Naznin M., Lefsrud M., Gravel V., Azad M. Blue Light added with Red LEDs Enhance Growth Characteristics, Pigments Content, and Antioxidant Capacity in Lettuce, Spinach, Kale, Basil, and Sweet Pepper in a Controlled Environment. Plants. 2019;8:93. doi: 10.3390/plants8040093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodríguez-deLeón E., Jiménez-Halla J.O., Báez J.E., Bah M.M. A Simple and Efficient Method for the Partial Synthesis of Pure (3R,3′S)-Astaxanthin from (3R,3′R,6′R)-Lutein and Lutein Esters via (3R,3′S)-Zeaxanthin and Theoretical Study of Their Formation Mechanisms. Molecules. 2019;24:1386. doi: 10.3390/molecules24071386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cazzonelli C.I., Pogson B.J. Source to sink: Regulation of carotenoid biosynthesis in plants. Trends Plant Sci. 2010;15:266–274. doi: 10.1016/j.tplants.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Moehs C.P., Tian L., Osteryoung K.W., Dellapenna D. Analysis of carotenoid biosynthetic gene expression during marigold petal development. Plant Mol. Biol. 2001;45:281–293. doi: 10.1023/A:1006417009203. [DOI] [PubMed] [Google Scholar]
- 6.Nisar N., Li L., Lu S., Khin N.C., Pogson B.J. Carotenoid metabolism in plants. Mol. Plant. 2015;8:68–82. doi: 10.1016/j.molp.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Ohmiya A. Qualitative and quantitative control of carotenoid accumulation in flower petals. Sci. Hortic. 2013;163:10–19. doi: 10.1016/j.scienta.2013.06.018. [DOI] [Google Scholar]
- 8.Zhu C., Bai C., Sanahuja G., Yuan D., Farre G., Naqvi S., Shi L., Capell T., Christou P. The regulation of carotenoid pigmentation in flowers. Arch. Biochem. Biophys. 2010;504:132–141. doi: 10.1016/j.abb.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 9.Rodrigo M.J., Zacarias L. Effect of postharvest ethylene treatment on carotenoid accumulation and the expression of carotenoid biosynthetic genes in the flavedo of orange (Citrus sinensis L. Osbeck) fruit. Postharvest Biol. Technol. 2007;43:14–22. doi: 10.1016/j.postharvbio.2006.07.008. [DOI] [Google Scholar]
- 10.Ma G., Zhang L., Kato M., Yamawaki K., Kiriiwa Y., Yahata M., Ikoma Y., Matsumoto H. Effect of the combination of ethylene and red LED light irradiation on carotenoid accumulation and carotenogenic gene expression in the flavedo of citrus fruit. Postharvest Biol. Technol. 2015;99:99–104. doi: 10.1016/j.postharvbio.2014.08.002. [DOI] [Google Scholar]
- 11.Barreto G.P.M., Fabi J.P., Rosso V.V.D., Cordenunsi B.R., Lajolo F.M., Nascimento J.R.O.D., Mercadante A.Z. Influence of ethylene on carotenoid biosynthesis during papaya postharvesting ripening. J. Food Compos. Anal. 2011;24:620–624. doi: 10.1016/j.jfca.2011.02.006. [DOI] [Google Scholar]
- 12.Su L., Diretto G., Purgatto E., Danoun S., Zouine M., Li Z., Roustan J.P., Bouzayen M., Giuliano G., Chervin C. Carotenoid accumulation during tomato fruit ripening is modulated by the auxin-ethylene balance. BMC Plant Biol. 2015;15:114. doi: 10.1186/s12870-015-0495-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Divya P., Puthusseri B., Savanur M.A., Lokesh V., Neelwarne B. Effects of methyl jasmonate and carotenogenic inhibitors on gene expression and carotenoid accumulation in coriander (Coriandrum sativum L.) foliage. Food Res. Int. 2018;111:11–19. doi: 10.1016/j.foodres.2018.04.040. [DOI] [PubMed] [Google Scholar]
- 14.Saini R.K., Prashanth K.V.H., Shetty N.P., Giridhar P. Elicitors, SA and MJ enhance carotenoids and tocopherol biosynthesis and expression of antioxidant related genes in Moringa oleifera Lam. leaves. Acta Physiol. Plant. 2014;36:2695–2704. doi: 10.1007/s11738-014-1640-7. [DOI] [Google Scholar]
- 15.Karppinen K., Hirvelä E., Nevala T., Sipari N., Suokas M., Jaakola L. Changes in the abscisic acid levels and related gene expression during fruit development and ripening in bilberry (Vaccinium myrtillus L.) Phytochemistry. 2013;95:127–134. doi: 10.1016/j.phytochem.2013.06.023. [DOI] [PubMed] [Google Scholar]
- 16.Nambara E., Okamoto M., Tatematsu K., Yano R., Seo M., Kamiya Y. Abscisic acid and the control of seed dormancy and germination. Seed Sci. Res. 2010;20:55–67. doi: 10.1017/S0960258510000012. [DOI] [Google Scholar]
- 17.Neves D.M., Filho M.A., Bellete B.S., Silva M.F., Souza D.T., Dos S.S.F.W., Costa M.G., Gesteira A.S. Comparative study of putative 9-cis-epoxycarotenoid dioxygenase and abscisic acid accumulation in the responses of Sunki mandarin and Rangpur lime to water deficit. Mol. Biol. Rep. 2013;40:5339–5349. doi: 10.1007/s11033-013-2634-z. [DOI] [PubMed] [Google Scholar]
- 18.Xia H., Wu S., Ma F. Cloning and expression of two 9-cis-epoxycarotenoid dioxygenase genes during fruit development and under stress conditions from Malus. Mol. Biol. Rep. 2014;41:6795–6802. doi: 10.1007/s11033-014-3565-z. [DOI] [PubMed] [Google Scholar]
- 19.Barickman T.C., Kopsell D.A., Sams C.E. Abscisic acid impacts tomato carotenoids, soluble sugars, and organic acids. HortScience. 2016;51:370–376. doi: 10.21273/HORTSCI.51.4.370. [DOI] [Google Scholar]
- 20.Barickman T.C., Kopsell D.A., Sams C.E. Abscisic Acid Increases Carotenoid and Chlorophyll Concentrations in Leaves and Fruit of Two Tomato Genotypes. J. Am. Soc. Hortic. Sci. 2014;139:261–266. doi: 10.21273/JASHS.139.3.261. [DOI] [Google Scholar]
- 21.Welsch R., Wüst F., Bär C., Al-Babili S., Beyer P. A Third Phytoene Synthase Is Devoted to Abiotic Stress-Induced Abscisic Acid Formation in Rice and Defines Functional Diversification of Phytoene Synthase Genes. Plant Physiol. 2008;147:367–380. doi: 10.1104/pp.108.117028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y., Zhang C., Dong B., Fu J., Hu S., Zhao H. Carotenoid Accumulation and Its Contribution to Flower Coloration of Osmanthus fragrans. Front. Plant Sci. 2018;9:1499. doi: 10.3389/fpls.2018.01499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu J., Hou D., Zhang C., Bao Z., Zhao H., Hu S. The Emission of the Floral Scent of Four Osmanthus fragrans Cultivars in Response to Different Temperatures. Molecules. 2017;22:430. doi: 10.3390/molecules22030430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baldermann S., Kato M., Fleischmann P., Watanabe N. Biosynthesis of α- and β-ionone, prominent scent compounds, in flowers of Osmanthus fragrans. Acta Biochim. Pol. 2012;59:79–81. doi: 10.18388/abp.2012_2176. [DOI] [PubMed] [Google Scholar]
- 25.Huang F.C., Molnar P., Schwab W. Cloning and functional characterization of carotenoid cleavage dioxygenase 4 genes. J. Exp. Bot. 2009;60:3011–3022. doi: 10.1093/jxb/erp137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hatmi S. Drought stress tolerance in grapevine involves activation of polyamine oxidation contributing to improved immune response and low susceptibility to Botrytis cinerea. J. Exp. Biol. 2015;66:775. doi: 10.1093/jxb/eru436. [DOI] [PubMed] [Google Scholar]
- 27.Zhang W., Yang H., You S., Xu Y., Ran K., Fan S. Cloning, characterization and functional analysis of the role MhNCED3, a gene encoding 9-cis-epoxycarotenoid dioxygenase in Malus hupehensis Rehd., plays in plant tolerance to osmotic and Cd2+ stresses. Plant Soil. 2014;381:143–160. doi: 10.1007/s11104-014-2120-y. [DOI] [Google Scholar]
- 28.Zhang C., Wang Y., Fu J., Bao Z., Zhao H. Transcriptomic analysis and carotenogenic gene expression related to petal coloration in Osmanthus fragrans ‘Yanhong Gui’. Trees. 2016;30:1207–1223. doi: 10.1007/s00468-016-1359-8. [DOI] [Google Scholar]
- 29.Thiruvengadam M., Baskar V., Kim S.H., Chung I.M. Effects of abscisic acid, jasmonic acid and salicylic acid on the content of phytochemicals and their gene expression profiles and biological activity in turnip (Brassica rapa ssp. rapa) Plant Growth Regul. 2016;80:1–14. doi: 10.1007/s10725-016-0178-7. [DOI] [Google Scholar]
- 30.Thiruvengadam M., Kim S.H., Chung I.M. Exogenous phytohormones increase the accumulation of health-promoting metabolites, and influence the expression patterns of biosynthesis related genes and biological activity in Chinese cabbage (Brassica rapa spp. pekinensis) Sci. Hortic. 2015;193:136–146. doi: 10.1016/j.scienta.2015.07.007. [DOI] [Google Scholar]
- 31.Tian S.L., Li L., Tian Y.Q., Shah S.N.M., Gong Z.H. Effects of Abscisic Acid on Capsanthin Levels in Pepper Fruit. J. Am. Soc. Hortic. Sci. 2016;141:609–616. doi: 10.21273/JASHS03898-16. [DOI] [Google Scholar]
- 32.Baldermann S., Yang Z., Sakai M., Fleischmann P., Morita A., Todoroki Y., Watanabe N. Influence of exogenously applied abscisic acid on carotenoid content and water uptake in flowers of the tea plant (Camellia sinensis) J. Sci. Food Agric. 2013;93:1660–1664. doi: 10.1002/jsfa.5944. [DOI] [PubMed] [Google Scholar]
- 33.Tuan P.A., Kim J.K., Lee S., Chae S.C., Park S.U. Molecular characterization of carotenoid cleavage dioxygenases and the effect of gibberellin, abscisic acid, and sodium chloride on the expression of genes involved in the carotenoid biosynthetic pathway and carotenoid accumulation in the callus of Scutellaria baicalensis Georgi. J. Agric. Food Chem. 2013;61:5565–5572. doi: 10.1021/jf401401w. [DOI] [PubMed] [Google Scholar]
- 34.Kim Y., Hwang I., Jung H.J., Park J.I., Kang J.G., Nou I.S. Genome-Wide Classification and Abiotic Stress-Responsive Expression Profiling of Carotenoid Oxygenase Genes in Brassica rapa and Brassica oleracea. J. Plant Growth Regul. 2016;35:202–214. doi: 10.1007/s00344-015-9520-y. [DOI] [Google Scholar]
- 35.Cao Y., Guo X.-L., Zhang Q., Cao Z.-Y., Zhao Y.-X., Zhang H. Isolation and characterization of carotenoid cleavage dioxygenase genein halophyte Suaeda salsa. Plant Growth Regul. 2005;46:61–67. doi: 10.1007/s10725-005-5667-z. [DOI] [Google Scholar]
- 36.Wang R.K., Wang C.E., Fei Y.Y., Gai J.Y., Zhao T.J. Genome-wide identification and transcription analysis of soybean carotenoid oxygenase genes during abiotic stress treatments. Mol. Biol. Rep. 2013;40:4737–4745. doi: 10.1007/s11033-013-2570-y. [DOI] [PubMed] [Google Scholar]
- 37.Meier S., Tzfadia O., Vallabhaneni R., Gehring C., Wurtzel E.T. A transcriptional analysis of carotenoid, chlorophyll and plastidial isoprenoid biosynthesis genes during development and osmotic stress responses in Arabidopsis thaliana. BMC Syst. Biol. 2011;5:77. doi: 10.1186/1752-0509-5-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wan X.R., Mo A.Q., Liu S., Liang J.H., Li L., Yu S.Y., Zheng Y.X. Molecular cloning and Gus-aided activity assaying of promoter sequence of AhNCED1 gene from Arachis hypogaea L. cv. Yueyou 7. J. Nucl. Agric. Sci. 2011;25:692–699. [Google Scholar]
- 39.Liu Y.C., Wang Y.G., Zhang C., Dong B., Fu J.X., Hu S.Q., Zhao H.B. Cloning and transient expression assay of OfCCD1 gene promotersfrom Osmanthus fragrans. J. Zhejiang A F Univ. 2018;35:596–603. [Google Scholar]
- 40.Wan X.R., Mo A.Q., Guo X.J., Yang M.X., Yu S.Y., Cao J.P. Osmotic Stress Tolerance Improvement of Arabidopsis Plants Ectopically Expressing Peanut AhNCED1 Gene. Acta Agron. Sin. 2010;36:1440–1449. doi: 10.1016/S1875-2780(09)60070-5. [DOI] [Google Scholar]
- 41.Wan X.R., Mo A.Q., Pan J.H., Liang C.Y. Molecular cloning and Gus-aided activity analysis of promoter region sequence of AtNCED2 gene from Arabidopsis thaliana L. Biotechnology. 2010;20:18–23. [Google Scholar]
- 42.Bhalothia P., Sangwan C., Alok A., Mehrotra S., Mehrotra R. PP2C-like Promoter and Its Deletion Variants Are Induced by ABA but Not by MeJA and SA in Arabidopsis thaliana. Front. Plant Sci. 2016;7:547. doi: 10.3389/fpls.2016.00547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mehrotra R., Mehrotra S. Promoter activation by ACGT in response to salicylic and abscisic acids is differentially regulated by the spacing between two copies of the motif. J. Plant Physiol. 2010;167:1214–1218. doi: 10.1016/j.jplph.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 44.Argyris J., Truco M.J., Ochoa O., McHale L., Dahal P., Deynze A.V., Michelmore R.W., Bradford K.J. A gene encoding an abscisic acid biosynthetic enzyme (LsNCED4) collocates with the high temperature germination locus Htg6.1 in lettuce (Lactuca sp.) Theor. Appl. Genet. 2011;122:95–108. doi: 10.1007/s00122-010-1425-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lindgren LO S.K. Höglund AS, Seed-specific overexpression of an endogenous Arabidopsis phytoene synthase gene results indelayed germination and increased levels of carotenoids, chlorophyll, and abscisic acid. Plant Physiol. 2003;132:779–785. doi: 10.1104/pp.102.017053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prital O., Shaarmoshe L., Wiseglass G., Peleg Z., Mosquna A. Non-redundant functions of the dimeric ABA receptor BdPYL1 in the grass Brachypodium. Plant J. 2017;92:774–786. doi: 10.1111/tpj.13714. [DOI] [PubMed] [Google Scholar]
- 47.Melcher K., Ng L.M., Zhou X.E., Soon F.F., Xu Y., Suinopowell K.M., Park S.Y., Weiner J.J., Fujii H., Chinnusamy V. A Gate-Latch-Lock Mechanism for Hormone Signaling by Abscisic Acid Receptors. Nature. 2009;462:602–608. doi: 10.1038/nature08613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choi H., Hong J., Ha J., Kang J., Kim S.Y. ABFs, a family of ABA-responsive element binding factors. J. Biol. Chem. 2000;275:1723–1730. doi: 10.1074/jbc.275.3.1723. [DOI] [PubMed] [Google Scholar]
- 49.Ureshino K., Nakayama M., Miyajima I. Contribution made by the carotenoid cleavage dioxygenase 4 gene to yellow colour fade in azalea petals. Euphytica. 2016;207:401–417. doi: 10.1007/s10681-015-1557-2. [DOI] [Google Scholar]
- 50.Delpinorius A., Eras J., Marsolvall A., Vilaró F., Balcells M., Canelagarayoa R. Ultra performance liquid chromatography analysis to study the changes in the carotenoid profile of commercial monovarietal fruit juices. J. Chromatogr. A. 2014;1331:90–99. doi: 10.1016/j.chroma.2014.01.044. [DOI] [PubMed] [Google Scholar]
- 51.Baldermann S., Kato M., Kurosawa M., Kurobayashi Y., Fujita A., Fleischmann P., Watanabe N. Functional characterization of a carotenoid cleavage dioxygenase 1 and its relation to the carotenoid accumulation and volatile emission during the floral development of Osmanthus fragrans Lour. J. Exp. Bot. 2010;61:2967–2977. doi: 10.1093/jxb/erq123. [DOI] [PubMed] [Google Scholar]
- 52.Zhang C., Fu J., Wang Y., Bao Z., Zhao H. Identification of Suitable Reference Genes for Gene Expression Normalization in the Quantitative Real-Time PCR Analysis of Sweet Osmanthus (Osmanthus fragrans Lour) PLoS ONE. 2015;10:e0136355. doi: 10.1371/journal.pone.0136355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schmittgen T.D., Livak K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]