Abstract
Inflammasomes are an essential part of the innate immune system. They are necessary for the development of a healthy immune response against infectious diseases. Inflammasome activation leads to the secretion of pro-inflammatory cytokines such as IL-1β and IL-18, which stimulate the adaptive immune system. Inflammasomes activators can be used as adjuvants to provide and maintain the strength of the immune response. This review is focused on the mechanisms of action and the effects of adjuvants on inflammasomes. The therapeutic and prophylaxis significance of inflammasomes in infectious diseases is also discussed.
Keywords: vaccines, adjuvants, inflammasomes, innate immunity, cytokines
1. Introduction
Vaccination is the most effective way to prevent infectious diseases [1]. There are several different types of vaccines available, including live attenuated and inactivated vaccines, subunit and toxoid vaccines, virus-like particle (VLP) based vaccines, as well as polysaccharide and polysaccharide conjugate vaccines [2]. Vaccines containing live or whole killed pathogens can induce strong protective immune responses without adjuvants. However, on rare occasions these vaccines can revert to a virulent strain through back-mutation, compensatory mutations, recombination or reassortment [3]. This can cause disease and side effects as a result of immunization, which is not characteristic of other vaccines [4]. In contrast, subunit, recombinant, polysaccharide, and conjugated vaccines are generally safe, but they are less effective without adjuvants. Adjuvants can be an organic or non-organic derivative [5]. Some of them are naturally occurring, for instance, mineral salts of aluminum or bacterial components that are used in complete Freund’s adjuvant (CFA) [6]. In contrast, subunit vaccines have limited immunogenicity, mostly because they lack natural adjuvants [7]. Therefore, currently, almost every vaccine contains adjuvants, which enhance and prolong the immune response [8].
There are several adjuvants that have been approved for human application, including aluminum salts, emulsions such as MF-59, and AS03 [9]. For most of these adjuvants, the mechanism of action remains unclear [10]. Among adjuvants, aluminum salts are the most studied. It is believed that aluminum salts might act as a matrix for antigen adsorption [11]. Adsorbed antigens appear to be more stable, which is crucial for them to retain immunogenicity [12]. A similar mechanism of action was suggested for incomplete Freund’s adjuvant (IFA), which can establish a depot at the site of injection for slow antigen release [13]. Some adjuvants also act through activation of toll-like receptors‘ (TLRs) [14]. For example, CFA, monophosphoryl Lipid A (MPL), flagellin and adjuvants based on CpG are ligands for TLR 2, 4, 5 and 9, respectively [15,16,17,18]. TLR agonists were shown to be effective adjuvants [19]. CFA appears to have the highest adjuvant activity compared to other adjuvants [20,21]. However, CFA has been shown to be associated with numerous side effects such as granulomas, ulcerative necrosis and sterile abscesses at the injection site [22].
Most of these adjuvants are still undergoing clinical trials, while aluminum salts remain a gold standard [23]. An ideal adjuvant should meet specific requirements such as safety, bio-degradability, and long-term stability. Most of the approved adjuvants do not fully comply with these criteria [9]. Therefore, the search for and development of new adjuvants is urgently needed to improve vaccines. The discovery of inflammasome structure and function initiated a new age in the development of vaccines and adjuvants. It has been demonstrated that inflammasomes, which can recognize damage-associated molecular patterns (DAMPs), are also involved in the mechanisms of adjuvant action [24].
2. Innate Immunity
The immune response can be divided into innate and adaptive [25]. For innate immunity, all receptors expressed by cells are encoded in the genome and passed to the next generation, unlike receptors of the adaptive immune system, which are formed as a result of V(D)J rearrangement and somatic hypermutation [26]. The innate immune system is characterized by its capacity to recognize a wide range of pathogens including viruses, fungi, and bacteria through the number of pattern recognition receptors (PRRs) [18]. PRRs recognize conservative microbial signals, the so-called pathogen-associated molecular patterns (PAMPs) [27]. The activated innate immune system elicits an inflammatory response followed by production of cytokines and chemokines to attract immune cells to the place of infection and create an adaptive immune response [28]. PRRs include TLRs, RIG-1-like receptors (RLRs) and pattern recognizing NOD-like receptors (NLRs) [27]. NLRs are the key sensors in the innate immune response and are one of the main inflammasome components involved in sensing pathogens [29].
3. Inflammasomes
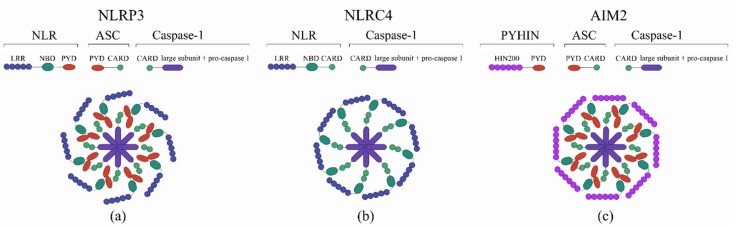
Inflammasomes represent a group of cytosolic multiprotein complexes composed of a sensor protein belonging to the NLR or Pyrin and HIN domain families (PYHIN), a caspase-1 effector and an adaptor protein ASC [30]. The structure of inflammasome is shown in Figure 1.
Figure 1.
The structure of the inflammasome. (a) NOD-like receptor protein 3 (NLRP3) inflammasome, (b) NLR-family CARD domain-containing protein 4 (NLRC4) inflammasome, (c) Absent in melanoma 2 (AIM2) inflammasome. The inflammasome structure is composed of functional units such as a leucine-rich repeat (LRR) C-terminal or DNA-binding domain (HIN200), a nucleotide binding domain (NBD), a pyrin domain (PYD), and a caspase recruitment domain (CARD).
Inflammasomes are expressed in various cells, including granulocytes, Т- and В-cells, monocytes, hepatocytes, neurons, microglia, and Langerhans cells [31,32,33]. The functions of inflammasomes include sensing the pathogen and launching a subsequent innate immune response [34,35]. Inflammasomes are formed when PAMP and DAMP are sensed in the cytoplasm [36]. This induces conformational changes in the pre-assembled NLRP3-inactive inflammasome [37]. Finally, activated inflammasome proteolytically cleaves pro-caspase 1, releasing the active caspase 1 [38]. Next, the caspase-1 digests pro-interleukin-1-beta (IL-1β) and pro-interleukin-18 (IL-18) to their active forms, which are secreted by a cell [39,40]. These interleukins are involved in establishing the inflammation and immune response that protect against microorganisms.
IL-18 can activate lymphocytes [41,42]. Also, it can increase Т- and B-cell proliferation, enhance the activity of natural killers (NKs), stimulate secretion of interferon-gamma (IFNγ), tumor necrosis factor-alpha (TNFα), interleukin-1 (IL-1) and interleukin-2 (IL-2) [43,44]. IL-18 induced release of TNFα and IL-1 appears to depend on inflammasome activation [45]. Another inflammasome product, IL-1β, seems to stimulate the proliferation of Т-cells [46,47,48]. Also, IL-1β may increase the number of CD103+CD69+ tissue-resident memory Т-cells (TRM) and dendritic cells [49]. It appears that IL-18 and IL-1β, which are products of activated inflammasomes, have a similar effect on leukocytes, that is, they stimulate proliferation and promote immune competence [50,51,52]. Moreover, some cytokines can be administered as adjuvants. IL-6, IL-1 and IL-12 promote either Th2- or Th1-type responses, thereby enhancing systemic immunity to co-administered antigens. IL-18 enhances the effects of IL-12 in inducing an antigen-specific Th1 type CD4+ T cell response as well as high titers of IgG antibodies [53,54].
4. Adjuvants as Inflammasome Activators
Most adjuvants have similar mechanisms of inflammasome activation, including lysosome degradation and cathepsine release and formation of reactive oxygen species, which subsequently lead to IL-1β and IL-18 secretion (Figure 2 and Figure 3).
Figure 2.
Molecular pathways of inflammasome activation by natural and artificial (synthetic) adjuvants.
Figure 3.
Molecular pathways of inflammasome activation by pathogen component-based adjuvants.
4.1. Aluminum Adjuvants
Aluminum-containing adjuvants were first used in human vaccines in 1932 [55]. Then, they were applied in numerous studies, where their efficiency as adjuvants was confirmed. Currently, aluminum adjuvants such as aluminium potassium sulphate, aluminium hydroxide, aluminium phosphate, and amorphous aluminium hydroxyphosphate sulfate are considered to be the gold standard for newly developing adjuvants [56]. Interestingly, the most commonly used adjuvants, such as aluminum salts, are capable of activation of NLRP3 inflammasome [57]. It has been shown that aluminum hydroxide-based adjuvants can stimulate secretion of pro-inflammatory cytokines IL-1β and IL-18 by activating NLRP3 inflammasome [58,59]. A mechanism of inflammasome activation was explained by the lysosome damage induced by aluminum hydroxide, which triggers the release of cathepsin. Free cathepsin B can initiate the inflammasome assembly, which activates caspase-1 and releases active cytokines [60,61]. Initial activation of a nuclear transcription factor (NF-κB) via lipopolysaccharide (LPS) appears to be required for NLRP3 activation by aluminum hydroxide [62]. Also, aluminum hydroxide can increase the uric acid levels at the site of vaccination, which could promote pro-IL-1b and pro-IL-18 conversion into their active forms by activated NLRP3 inflammasome [63].
4.2. Chitosan
Natural substances have also been shown to possess adjuvant activity. For example, chitosan, a highly biodegradable chitin derivative can enhance both humoral and cell-mediated immune response, which is equipotent to IFA and superior to aluminum hydroxide [64]. Chitosan can enter the cell by phagocytosis and activate NLRP3 inflammasome [65]. Beuter et al. have demonstrated that the mechanism of inflammasome activation is associated with potassium ion efflux and lysosome destabilization, as well as the formation of reactive oxygen species (ROS) [66]. Also, chitosan, used as an influenza vaccine adjuvant, was shown to increase the production of IL-2, IL-4, IL-6, TNF, IL-17A, and IL-10. Increased IFNγ production and IgG titers indicate the enhancement of the cell-mediated and humoral immune responses by chitosan adjuvant [67].
4.3. Saponins
Saponins isolated from the soapwort plant are also known to enhance an immune response [68]. Kensil et al. were the first to isolate saponin fractions from the soapwort plant with various degrees of adjuvant properties [69]. Adjuvants based on the QS-21 portion of saponin had low toxicity combined with excellent adjuvant effects. They were used in more than 100 human clinical trials [70,71] where they demonstrated the ability to induce humoral and cell-mediated immune response against a wide range of antigens [72]. Also, the QS-21 fraction was superior to aluminum salts because they induced a Th1-type immune response, which is essential for controlling intracellular pathogens [73].
The mechanism of the QS-21 adjuvant activity is being investigated. Recent studies have confirmed that QS-21 activates NLPR3 inflammasome [74]. An arrangement of NLPR3 inflammasome activation by QS-21 was linked to lysosome destabilization and cathepsin B release [75]. Interestingly, QS-21 can correctly activate NLRP3 inflammasome in CD169+ macrophages localized in lymph nodes [76] and increase serum levels of IL-2 and IFNγ [77].
4.4. Synthetic Cation Polymeric Adjuvants
Progress in organic synthesis methods has contributed to the generation of artificial molecules with adjuvant activity. One of these is synthetic cation polymeric adjuvants containing biodegradable particles, which form nanoparticles [78]. Nanoparticles based on poly-d,l-lactide-co-glycolide, cationic lipids possess adjuvant properties that could be mediated by activation of the NLRP3 inflammasome [79]. The mechanism of inflammasome activation appears to be related to lysosome disruption and cathepsin B release as well as ROS formation [80].
These nanoparticle adjuvants were tested in animal models where they increased IgG titer and the level of TNF-α, INFγ, IL-17A, and IL-1β cytokines [81,82].
4.5. Cholera Toxin B
Cell wall components, some endotoxins, as well as nucleic acids are also capable of inflammasome activation. Therefore, they might have potential utility in adjuvant applications [83]. Cholera toxin, a protein of Vibrio cholerae, consists of six subunits, including one toxin A and five toxin B [84]. Cholera toxin B is a non-toxic protein with proven adjuvant efficacy, especially for mucosal vaccines [85,86]. It can also bind to GM1 ganglioside receptor and enter the cell, thereby activating NLRP3 and other inflammasomes [87]. The mechanism of activation appears to be related to the cholera toxin B enhancement of the small Rho GTPases (RhoA) activity via protein kinase A [88]. As a result, a pyrin receptor interacts with modified RhoA and triggers inflammasome self-assembly [89]. The efficacy of cholera toxin B as an adjuvant was demonstrated in a mouse model [90]. This adjuvant was able to increase the circulating IgG titers as well as mucosal IgA levels. Cholera toxin B also enhanced Т-cell proliferation and increased IL-17A and IFNγ production [91].
4.6. Flagellin
Flagellin, which forms the hollow filaments in bacterial flagellum, represents bacterial pathogenicity and virulence factor [92]. Several studies have demonstrated flagellin adjuvant activity in the mucosal vaccination [93,94,95]. Like LPS, it is a natural TLR agonist and can also trigger inflammasome assembly [96,97,98]. However, in contrast to LPS, flagellin induces formation of both NLRP3 and NLRC4 inflammasomes [99,100]. The efficacy of the flagellin as an adjuvant was demonstrated in experiments with mice and primates in a flu model [101,102]. Flagellin was shown to induce the production of various pro-inflammatory cytokines such as IL-6, IL-8, and CXCL2, which can facilitate a productive immune response [103,104,105]. It has been demonstrated that flagellin activates inflammasomes through TLR5 or NLRC4 receptors [106]. According to Dos Reis et al., the NLRP3 receptor function is not absolutely required for the flagellin adjuvant activity. For example, it was demonstrated that flagellin can stimulate the assembly of another inflammasome, NLRC4 in cells with defective NLRP3 receptors [100]. While flagellin activates NLRC4 inflammasome directly via the NAIP5-receptor, the formation of NLRP3 inflammasome appears to be indirectly regulated via cathepsin B by destabilizing lysosomes [107,108].
4.7. Nucleic Acids
Nucleic acids are traditionally accepted as a genetic vaccine; however, they can potentially be used as adjuvants. Viral single and double-stranded RNAs are recognized by the intracellular receptor RIG-1 (retinoic acid-inducible gene I), which can subsequently activate NLRP3 inflammasome [109]. Therefore, RNA appears to be useful as both a genetic vaccine epitope and an adjuvant [110]. These adjuvants have been shown to cause low toxicity and adverse effects [111]. Also, non-translational RNA can enhance an immune response [112]. For example, Doener et al. demonstrated that both cell-based and humoral immune responses were significantly increased in healthy volunteers who received a licensed rabies vaccine containing non-translational RNA adjuvant, CV8102 [113].
Double-stranded DNA could be detected by the AIM2 inflammasome [114], triggering inflammasome assembly and increasing IL-1β and IL-18 secretion [115,116]. Therefore, plasmids which are utilized as genetic vaccines can activate AIM2 inflammasome and enhance cell-based and humoral immune responses, even if they do not code pathogenic proteins [117,118].
5. Conclusions
Inflammation is an important component of the immune response, as it plays a role in stimulating leukocyte proliferation and differentiation, releases regulatory cytokines and activates non-specific immunity [119]. Inflammation could be regulated by many pathways, including inflammasomes [120]. As inflammasome senses PAMPs and DAMPs, it starts forming multiprotein complexes, and releasing active caspase 1, which, in turn, cleaves IL-1β and IL-18 [36]. Both cytokines play essential roles in establishing and maintaining inflammation [39,43,44,46,48,49,50,51,52]. Also, these cytokines directly stimulate immune cells like T- and B-lymphocytes and dendritic cells [41,42,47]. Inflammation-caused leukocyte activation and migration to the site of infection contributes to antigen recognition and presentation [121].
Therefore, activation of inflammasomes as part of immune recognition during vaccination could help facilitate antigen recognition and presentation. Inflammasomes could be targeted by adjuvants [122]. As adjuvants help to induce a strong and long-lasting immune response, targeting inflammasomes could be a potential mechanism of their action. Adjuvants are widely utilized in vaccines and their effect can directly depend on their capacity to activate the inflammasome. In this review we summarized information on adjuvants currently used for vaccination and their effect on inflammasome activation. Several adjuvants, aluminum salts, MF-59, and AS03 have been shown to activate inflammasome as part of their mechanism of action. It appears that inflammasome activation facilitates the development of a strong and long-lasting immune response [34,35]. This was shown for aluminum salts, MF-59, and AS03 adjuvants, which are currently used as part of influenza, polio, hepatitis A and B vaccines [123,124,125,126,127]. Using inflammasomes as a target for adjuvants mimics the innate immune response to an infectious agent and increases the effectiveness of the vaccine. Therefore, targeting molecular mechanisms involved in inflammasome activation could be beneficial for the development of new approaches for adjuvant‘ design and to further advance the development of effective vaccine formulations.
Acknowledgments
This study was supported by the Russian Government Program of Competitive Growth of Kazan Federal University.
Author Contributions
Authors contributed to the manuscript equally. All authors have read and agreed to the published version of the manuscript.
Funding
The reviewed study was funded by grant МК-2393.2019.4. Albert Rizvanov was supported by the subsidy allocated to Kazan Federal University for the state assignment in the sphere of scientific activities.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.WHO Team Vaccines. [(accessed on 29 March 2020)]; Available online: https://www.who.int/topics/vaccines/en/
- 2.Vetter V., Denizer G., Friedland L.R., Krishnan J., Shapiro M. Understanding modern-day vaccines: What you need to know. Ann. Med. 2018 doi: 10.1080/07853890.2017.1407035. [DOI] [PubMed] [Google Scholar]
- 3.Frey J. Biological safety concepts of genetically modified live bacterial vaccines. Vaccine. 2007 doi: 10.1016/j.vaccine.2006.11.058. [DOI] [PubMed] [Google Scholar]
- 4.Kirtland K.A., Lin X., Kroger A.T., Myerburg S., Rodgers L. Frequency and cost of live vaccines administered too soon after prior live vaccine in children aged 12 months through 6 years, 2014–2017. Vaccine. 2019 doi: 10.1016/j.vaccine.2019.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKee A.S., Marrack P. Old and new adjuvants. Curr. Opin. Immunol. 2017 doi: 10.1016/j.coi.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coffman R.L., Sher A., Seder R.A. Vaccine adjuvants: Putting innate immunity to work. Immunity. 2010 doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vartak A., Sucheck S.J. Recent advances in subunit vaccine carriers. Vaccines. 2016;4:12. doi: 10.3390/vaccines4020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Djurisic S., Jakobsen J.C., Petersen S.B., Kenfelt M., Klingenberg S.L., Gluud C. Aluminium adjuvants used in vaccines. Cochrane Database Syst. Rev. 2018 doi: 10.1002/14651858.CD013086. [DOI] [Google Scholar]
- 9.Del Giudice G., Rappuoli R., Didierlaurent A.M. Correlates of adjuvanticity: A review on adjuvants in licensed vaccines. Semin. Immunol. 2018 doi: 10.1016/j.smim.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Tritto E., Mosca F., De Gregorio E. Mechanism of action of licensed vaccine adjuvants. Vaccine. 2009 doi: 10.1016/j.vaccine.2009.01.084. [DOI] [PubMed] [Google Scholar]
- 11.Glenny A.T., Buttle G.A.H., Stevens M.F. Rate of disappearance of diphtheria toxoid injected into rabbits and guinea - pigs: Toxoid precipitated with alum. J. Pathol. Bacteriol. 1931 doi: 10.1002/path.1700340214. [DOI] [Google Scholar]
- 12.Harrison W.T. Some Observations on the Use of Alum Precipitated Diphtheria Toxoid. Am. J. Public Heal. Nations Heal. 1935 doi: 10.2105/AJPH.25.3.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang J.C.C., Diveley J.P., Savary J.R., Jensen F.C. Adjuvant activity of incomplete Freund’s adjuvant. Adv. Drug Deliv. Rev. 1998 doi: 10.1016/S0169-409X(98)00009-X. [DOI] [PubMed] [Google Scholar]
- 14.Van Duin D., Medzhitov R., Shaw A.C. Triggering TLR signaling in vaccination. Trends Immunol. 2006 doi: 10.1016/j.it.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Reed S.G., Hsu F.C., Carter D., Orr M.T. The science of vaccine adjuvants: Advances in TLR4 ligand adjuvants. Curr. Opin. Immunol. 2016 doi: 10.1016/j.coi.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 16.Tallant T., Deb A., Kar N., Lupica J., De Veer M.J., DiDonato J.A. Flagellin acting via TLR5 is the major activator of key signaling pathways leading to NF-κB and proinflammatory gene program activation in intestinal epithelial cells. BMC Microbiol. 2004 doi: 10.1186/1471-2180-4-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krug A., Rothenfusser S., Selinger S., Bock C., Kerkmann M., Battiany J., Sarris A., Giese T., Speiser D., Endres S., et al. CpG-A Oligonucleotides Induce a Monocyte-Derived Dendritic Cell-Like Phenotype That Preferentially Activates CD8 T Cells. J. Immunol. 2003 doi: 10.4049/jimmunol.170.7.3468. [DOI] [PubMed] [Google Scholar]
- 18.Lim S.K. Freund adjuvant induces TLR2 but not TLR4 expression in the liver of mice. Int. Immunopharmacol. 2003 doi: 10.1016/S1567-5769(02)00256-4. [DOI] [PubMed] [Google Scholar]
- 19.Steinhagen F., Kinjo T., Bode C., Klinman D.M. TLR-based immune adjuvants. Vaccine. 2011;29:3341–3355. doi: 10.1016/j.vaccine.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stils H.F. Adjuvants and Antibody Production: Dispelling the Myths Associated with Freund’s Complete and Other Adjuvants. ILAR J. 2005 doi: 10.1093/ilar.46.3.280. [DOI] [PubMed] [Google Scholar]
- 21.Tal Y., Souan L., Cohen I.R., Meiner Z., Taraboulos A., Mor F. Complete Freund’s adjuvant immunization prolongs survival in experimental prion disease in mice. J. Neurosci. Res. 2003 doi: 10.1002/jnr.10474. [DOI] [PubMed] [Google Scholar]
- 22.Fontes J.A., Barin J.G., Talor M.V., Stickel N., Schaub J., Rose N.R., Č Iháková D. Complete Freund’s adjuvant induces experimental autoimmune myocarditis by enhancing IL-6 production during initiation of the immune response. Immun. Inflamm. Dis. 2017 doi: 10.1002/iid3.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.HogenEsch H., O’Hagan D.T., Fox C.B. Optimizing the utilization of aluminum adjuvants in vaccines: you might just get what you want. NPJ Vaccines. 2018 doi: 10.1038/s41541-018-0089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyaji E.N., Carvalho E., Oliveira M.L.S., Raw I., Ho P.L. Trends in adjuvant development for vaccines: DAMPs and PAMPs as potential new adjuvants. Brazilian J. Med. Biol. Res. 2011 doi: 10.1590/S0100-879X2011000600003. [DOI] [PubMed] [Google Scholar]
- 25.Medzhitov R., Preston-Hurlburt P., Janeway C.A. A human homologue of the Drosophila toll protein signals activation of adaptive immunity. Nature. 1997 doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 26.Hoebe K., Janssen E., Beutler B. The interface between innate and adaptive immunity. Nat. Immunol. 2004 doi: 10.1038/ni1004-971. [DOI] [PubMed] [Google Scholar]
- 27.Takeuchi O., Akira S. Pattern Recognition Receptors and Inflammation. Cell. 2010 doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 28.Turner M.D., Nedjai B., Hurst T., Pennington D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta Mol. Cell Res. 2014 doi: 10.1016/j.bbamcr.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 29.Medzhitov R., Janeway C.A. Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296:298–300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- 30.Strowig T., Henao-Mejia J., Elinav E., Flavell R. Inflammasomes in health and disease. Nature. 2012 doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- 31.Boaru S.G., Borkham-Kamphorst E., Tihaa L., Haas U., Weiskirchen R. Expression analysis of inflammasomes in experimental models of inflammatory and fibrotic liver disease. J. Inflamm. (UK) 2012 doi: 10.1186/1476-9255-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bruey J.M., Bruey-Sedano N., Luciano F., Zhai D., Balpai R., Xu C., Kress C.L., Bailly-Maitre B., Li X., Osterman A., et al. Bcl-2 and Bcl-X L Regulate Proinflammatory Caspase-1 Activation by Interaction with NALP1. Cell. 2007 doi: 10.1016/j.cell.2007.01.045. [DOI] [PubMed] [Google Scholar]
- 33.Kummer J.A., Broekhuizen R., Everett H., Agostini L., Kuijk L., Martinon F., Van Bruggen R., Tschopp J. Inflammasome components NALP 1 and 3 show distinct but separate expression profiles in human tissues suggesting a site-specific role in the inflammatory response. J. Histochem. Cytochem. 2007 doi: 10.1369/jhc.6A7101.2006. [DOI] [PubMed] [Google Scholar]
- 34.Ghiringhelli F., Apetoh L., Tesniere A., Aymeric L., Ma Y., Ortiz C., Vermaelen K., Panaretakis T., Mignot G., Ullrich E., et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1Β-dependent adaptive immunity against tumors. Nat. Med. 2009 doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- 35.Ichinohe T., Lee H.K., Ogura Y., Flavell R., Iwasaki A. Inflammasome recognition of influenza virus is essential for adaptive immune responses. J. Exp. Med. 2009 doi: 10.1084/jem.20081667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schroder K., Tschopp J. The Inflammasomes. Cell. 2010 doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 37.Compan V., Baroja-Mazo A., López-Castejón G., Gomez A.I., Martínez C.M., Angosto D., Montero M.T., Herranz A.S., Bazán E., Reimers D., et al. Cell Volume Regulation Modulates NLRP3 Inflammasome Activation. Immunity. 2012 doi: 10.1016/j.immuni.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 38.Kawai T., Akira S. The role of pattern-recognition receptors in innate immunity: Update on toll-like receptors. Nat. Immunol. 2010 doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 39.Fantuzzi G., Dinarello C.A. Interleukin-18 and interleukin-1β: Two cytokine substrates for ICE (caspase-1) J. Clin. Immunol. 1999 doi: 10.1023/A:1020506300324. [DOI] [PubMed] [Google Scholar]
- 40.Schumann R.R., Belka C., Reuter D., Lamping N., Kirschning C.J., Weber J.R., Pfeil D. Lipopolysaccharide activates caspase-1 (interleukin-1-converting enzyme) in cultured monocytic and endothelial cells. Blood. 1998;91:577–584. doi: 10.1182/blood.V91.2.577. [DOI] [PubMed] [Google Scholar]
- 41.Leite-de-Moraes M.C., Arnould A., Machavoine F., Schneider E., Dy M., Hameg A., Herbelin A., Koezuka Y. A distinct IL-18-induced pathway to fully activate NK T lymphocytes independently from TCR engagement. J. Immunol. 1999;163:5871–5876. [PubMed] [Google Scholar]
- 42.Barbulescu K., Becker C., Schlaak J.F., Schmitt E., Meyer zum Büschenfelde K.H., Neurath M.F. IL-12 and IL-18 differentially regulate the transcriptional activity of the human IFN-gamma promoter in primary CD4+ T lymphocytes. J. Immunol. 1998;160:3642–3647. [PubMed] [Google Scholar]
- 43.Dinarello C.A. IL-18: AtH1-inducing, proinflammatory cytokine and new member of the IL-1 family. J. Allergy Clin. Immunol. 1999 doi: 10.1016/S0091-6749(99)70518-X. [DOI] [PubMed] [Google Scholar]
- 44.Nakanishi K., Yoshimoto T., Tsutsui H., Okamura H. Interleukin-18 is a unique cytokine that stimulates both Th1 and Th2 responses depending on its cytokine milieu. Cytokine Growth Factor Rev. 2001 doi: 10.1016/S1359-6101(00)00015-0. [DOI] [PubMed] [Google Scholar]
- 45.Tsuji N.M., Tsutsui H., Seki E., Kuida K., Okamura H., Nakanishi K., Flavell R.A. Roles of caspase-1 in Listeria infection in mice. Int. Immunol. 2004 doi: 10.1093/intimm/dxh041. [DOI] [PubMed] [Google Scholar]
- 46.Ben-Sasson S.Z., Hogg A., Hu-Li J., Wingfield P., Chen X., Crank M., Caucheteux S., Ratner-Hurevich M., Berzofsky J.A., Nir-Paz R., et al. IL-1 enhances expansion, effector function, tissue localization, and memory response of antigen-specific CD8 T cells. J. Exp. Med. 2013 doi: 10.1084/jem.20122006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ben-Sasson S.Z., Hu-Li J., Quiel J., Cauchetaux S., Ratner M., Shapira I., Dinarello C.A., Paul W.E. IL-1 acts directly on CD4 T cells to enhance their antigen-driven expansion and differentiation. Proc. Natl. Acad. Sci. USA. 2009 doi: 10.1073/pnas.0902745106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ben-Sasson S.Z., Wang K., Cohen J., Paul W.E. IL-1β strikingly enhances antigen-driven CD4 and CD8 T-cell responses. Cold Spring Harb. Symp. Quant. Biol. 2013 doi: 10.1101/sqb.2013.78.021246. [DOI] [PubMed] [Google Scholar]
- 49.Lapuente D., Storcksdieck Genannt Bonsmann M., Maaske A., Stab V., Heinecke V., Watzstedt K., Heß R., Westendorf A.M., Bayer W., Ehrhardt C., et al. IL-1β as mucosal vaccine adjuvant: The specific induction of tissue-resident memory T cells improves the heterosubtypic immunity against influenza A viruses article. Mucosal Immunol. 2018 doi: 10.1038/s41385-018-0017-4. [DOI] [PubMed] [Google Scholar]
- 50.Xu D., Chan W.L., Leung B.P., Hunter D., Schulz K., Carter R.W., McInnes I.B., Robinson J.H., Liew F.Y. Selective expression and functions of interleukin 18 receptor on T helper (Th) type 1 but not Th2 cells. J. Exp. Med. 1998 doi: 10.1084/jem.188.8.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guo L., Wei G., Zhu J., Liao W., Leonard W.J., Zhao K., Paul W. IL-1 family members and STAT activators induce cytokine production by Th2, Th17, and Th1 cells. Proc. Natl. Acad. Sci. USA. 2009 doi: 10.1073/pnas.0906988106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blom L., Poulsen L.K. IL-1 Family Members IL-18 and IL-33 Upregulate the Inflammatory Potential of Differentiated Human Th1 and Th2 Cultures. J. Immunol. 2012 doi: 10.4049/jimmunol.1103685. [DOI] [PubMed] [Google Scholar]
- 53.Boyaka P.N., McGhee J.R. Cytokines as adjuvants for the induction of mucosal immunity. Adv. Drug Deliv. Rev. 2001 doi: 10.1016/S0169-409X(01)00170-3. [DOI] [PubMed] [Google Scholar]
- 54.Eberl M., Beck E., Coulson P.S., Okamura H., Wilson R.A., Mountford A.P. IL-18 potentiates the adjuvant properties of IL-12 in the induction of a strong Th1 type immune response against a recombinant antigen. Vaccine. 2000 doi: 10.1016/S0264-410X(99)00532-0. [DOI] [PubMed] [Google Scholar]
- 55.Di Pasquale A., Preiss S., Da Silva F.T., Garçon N. Vaccine adjuvants: From 1920 to 2015 and beyond. Vaccines. 2015;3:320–343. doi: 10.3390/vaccines3020320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marrack P., McKee A.S., Munks M.W. Towards an understanding of the adjuvant action of aluminium. Nat. Rev. Immunol. 2009 doi: 10.1038/nri2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li H., Nookala S., Re F. Aluminum Hydroxide Adjuvants Activate Caspase-1 and Induce IL-1 and IL-18 Release. J. Immunol. 2007 doi: 10.4049/jimmunol.178.8.5271. [DOI] [PubMed] [Google Scholar]
- 58.Eisenbarth S.C., Colegio O.R., O’Connor W., Sutterwala F.S., Flavell R.A. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008 doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li H., Willingham S.B., Ting J.P.-Y., Re F. Cutting Edge: Inflammasome Activation by Alum and Alum’s Adjuvant Effect Are Mediated by NLRP3. J. Immunol. 2008 doi: 10.4049/jimmunol.181.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schotte P., Van Criekinge W., Van De Craen M., Van Loo G., Desmedt M., Grooten J., Cornelissen M., De Ridder L., Vandekerckhove J., Fiers W., et al. Cathepsin B-mediated activation of the proinflammatory caspase-11. Biochem. Biophys. Res. Commun. 1998 doi: 10.1006/bbrc.1998.9425. [DOI] [PubMed] [Google Scholar]
- 61.Vancompernolle K., Van Herreweghe F., Pynaert G., Van De Craen M., De Vos K., Totty N., Sterling A., Fiers W., Vandenabeele P., Grooten J. Atractyloside-induced release of cathepsin B, a protease with caspase-processing activity. FEBS Lett. 1998 doi: 10.1016/S0014-5793(98)01275-7. [DOI] [PubMed] [Google Scholar]
- 62.Franchi L., Núñez G. The Nlrp3 inflammasome is critical for aluminum hydroxide-mediated IL-1β secretion but dispensable for adjuvant activity. Eur. J. Immunol. 2008 doi: 10.1002/eji.200838549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kool M., Petrilli V., De Smedt T., Rolaz A., Hammad H., van Nimwegen M., Bergen I.M., Castillo R., Lambrecht B.N., Tschopp J. Cutting Edge: Alum Adjuvant Stimulates Inflammatory Dendritic Cells through Activation of the NALP3 Inflammasome. J. Immunol. 2014 doi: 10.4049/jimmunol.181.6.3755. [DOI] [PubMed] [Google Scholar]
- 64.Zaharoff D.A., Rogers C.J., Hance K.W., Schlom J., Greiner J.W. Chitosan solution enhances both humoral and cell-mediated immune responses to subcutaneous vaccination. Vaccine. 2007 doi: 10.1016/j.vaccine.2006.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bueter C.L., Lee C.K., Rathinam V.A.K., Healy G.J., Taron C.H., Specht C.A., Levitz S.M. Chitosan but not chitin activates the inflammasome by a mechanism dependent upon phagocytosis. J. Biol. Chem. 2011 doi: 10.1074/jbc.M111.274936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bueter C.L., Lee C.K., Wang J.P., Ostroff G.R., Specht C.A., Levitz S.M. Spectrum and Mechanisms of Inflammasome Activation by Chitosan. J. Immunol. 2014 doi: 10.4049/jimmunol.1301695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sui Z., Chen Q., Fang F., Zheng M., Chen Z. Cross-protection against influenza virus infection by intranasal administration of M1-based vaccine with chitosan as an adjuvant. Vaccine. 2010 doi: 10.1016/j.vaccine.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 68.Rönnberg B., Fekadu M., Morein B. Adjuvant activity of non-toxic Quillaja saponaria Molina components for use in ISCOM matrix. Vaccine. 1995 doi: 10.1016/0264-410X(95)00105-A. [DOI] [PubMed] [Google Scholar]
- 69.Kensil C.R., Patel U., Lennick M., Marciani D. Separation and characterization of saponins with adjuvant activity from Quillaja saponaria molina cortex. J. Immunol. 1991;146:431–437. [PubMed] [Google Scholar]
- 70.Garçon N., Di Pasquale A. From discovery to licensure, the Adjuvant System story. Hum. Vaccines Immunother. 2017 doi: 10.1080/21645515.2016.1225635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Didierlaurent A.M., Laupèze B., Di Pasquale A., Hergli N., Collignon C., Garçon N. Adjuvant system AS01: Helping to overcome the challenges of modern vaccines. Expert Rev. Vaccines. 2017 doi: 10.1080/14760584.2016.1213632. [DOI] [PubMed] [Google Scholar]
- 72.Coccia M., Collignon C., Hervé C., Chalon A., Welsby I., Detienne S., Van Helden M.J., Dutta S., Genito C.J., Waters N.C., et al. Cellular and molecular synergy in AS01-adjuvanted vaccines results in an early IFNγ response promoting vaccine immunogenicity. NPJ Vaccines. 2017 doi: 10.1038/s41541-017-0027-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lacaille-Dubois M.A. Updated insights into the mechanism of action and clinical profile of the immunoadjuvant QS-21: A review. Phytomedicine. 2019 doi: 10.1016/j.phymed.2019.152905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marty-Roix R., Vladimer G.I., Pouliot K., Weng D., Buglione-Corbett R., West K., MacMicking J.D., Chee J.D., Wang S., Lu S., et al. Identification of QS-21 as an inflammasome-activating molecular component of saponin adjuvants. J. Biol. Chem. 2016 doi: 10.1074/jbc.M115.683011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Welsby I., Detienne S., N’Kuli F., Thomas S., Wouters S., Bechtold V., De Wit D., Gineste R., Reinheckel T., Elouahabi A., et al. Lysosome-dependent activation of human dendritic cells by the vaccine adjuvant QS-21. Front. Immunol. 2017 doi: 10.3389/fimmu.2016.00663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Detienne S., Welsby I., Collignon C., Wouters S., Coccia M., Delhaye S., Van Maele L., Thomas S., Swertvaegher M., Detavernier A., et al. Central role of CD169+ lymph node resident macrophages in the adjuvanticity of the QS-21 component of AS01. Sci. Rep. 2016 doi: 10.1038/srep39475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vandepapelière P., Horsmans Y., Moris P., Van Mechelen M., Janssens M., Koutsoukos M., Van Belle P., Clement F., Hanon E., Wettendorff M., et al. Vaccine Adjuvant Systems containing monophosphoryl lipid A and QS21 induce strong and persistent humoral and T cell responses against hepatitis B surface antigen in healthy adult volunteers. Vaccine. 2008 doi: 10.1016/j.vaccine.2007.12.038. [DOI] [PubMed] [Google Scholar]
- 78.Karandikar S., Mirani A., Waybhase V., Patravale V.B., Patankar S. Nanovaccines for oral delivery-formulation strategies and challenges. Nanostructures Oral Med. :2017. doi: 10.1016/B978-0-323-47720-8.00011-0. [DOI] [Google Scholar]
- 79.Hornung V., Latz E. Critical functions of priming and lysosomal damage for NLRP3 activation. Eur. J. Immunol. 2010 doi: 10.1002/eji.200940185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhong Z., Zhai Y., Liang S., Mori Y., Han R., Sutterwala F.S., Qiao L. TRPM2 links oxidative stress to NLRP3 inflammasome activation. Nat. Commun. 2013 doi: 10.1038/ncomms2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li T., He J., Horvath G., Próchnicki T., Latz E., Takeoka S. Lysine-containing cationic liposomes activate the NLRP3 inflammasome: Effect of a spacer between the head group and the hydrophobic moieties of the lipids. Nanomedicine. 2018 doi: 10.1016/j.nano.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 82.Safari Zanjani L., Shapouri R., Dezfulian M., Mahdavi M., Shafiee Ardestani M. Exotoxin A-PLGA nanoconjugate vaccine against Pseudomonas aeruginosa infection: Protectivity in murine model. World J. Microbiol. Biotechnol. 2019 doi: 10.1007/s11274-019-2669-y. [DOI] [PubMed] [Google Scholar]
- 83.Franchi L., Muñoz-Planillo R., Núñez G. Sensing and reacting to microbes through the inflammasomes. Nat. Immunol. 2012 doi: 10.1038/ni.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Spangler B.D. Structure and function of cholera toxin and the related Escherichia coli heat-labile enterotoxin. Microbiol. Rev. 1992;56:622–647. doi: 10.1128/MMBR.56.4.622-647.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lycke N., Holmgren J. Strong adjuvant properties of cholera toxin on gut mucosal immune responses to orally presented antigens. Immunology. 1986;59:301–308. [PMC free article] [PubMed] [Google Scholar]
- 86.Elson C.O., Ealding W. Generalized systemic and mucosal immunity in mice after mucosal stimulation with cholera toxin. J. Immunol. 1984;132:2736–2741. [PubMed] [Google Scholar]
- 87.Orimo T., Sasaki I., Hemmi H., Ozasa T., Fukuda-Ohta Y., Ohta T., Morinaka M., Kitauchi M., Yamaguchi T., Sato Y., et al. Cholera toxin B induces interleukin-1β production from resident peritoneal macrophages through the pyrin inflammasome as well as the NLRP3 inflammasome. Int. Immunol. 2019 doi: 10.1093/intimm/dxz004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Terrinoni M., Holmgren J., Lebens M., Larena M. Proteomic analysis of cholera toxin adjuvant-stimulated human monocytes identifies Thrombospondin-1 and Integrin-β1 as strongly upregulated molecules involved in adjuvant activity. Sci. Rep. 2019 doi: 10.1038/s41598-019-38726-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xu H., Yang J., Gao W., Li L., Li P., Zhang L., Gong Y.N., Peng X., Xi J.J., Chen S., et al. Innate immune sensing of bacterial modifications of Rho GTPases by the Pyrin inflammasome. Nature. 2014 doi: 10.1038/nature13449. [DOI] [PubMed] [Google Scholar]
- 90.Wakabayashi A., Shimizu M., Shinya E., Takahashi H. HMGB1 released from intestinal epithelia damaged by cholera toxin adjuvant contributes to activation of mucosal dendritic cells and induction of intestinal cytotoxic T lymphocytes and IgA. Cell Death Dis. 2018 doi: 10.1038/s41419-018-0665-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Holmgren J., Nordqvist S., Blomquist M., Jeverstam F., Lebens M., Raghavan S. Preclinical immunogenicity and protective efficacy of an oral Helicobacter pylori inactivated whole cell vaccine and multiple mutant cholera toxin: A novel and non-toxic mucosal adjuvant. Vaccine. 2018 doi: 10.1016/j.vaccine.2018.07.073. [DOI] [PubMed] [Google Scholar]
- 92.Hayashi F., Smith K.D., Ozinsky A., Hawn T.R., Yi E.C., Goodlett D.R., Eng J.K., Akira S., Underhill D.M., Aderem A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001 doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 93.Levi R., Arnon R. Synthetic recombinant influenza vaccine induces efficient long-term immunity and cross-strain protection. Vaccine. 1996 doi: 10.1016/0264-410X(95)00088-I. [DOI] [PubMed] [Google Scholar]
- 94.Ben-Yedidia T., Arnon R. Effect of pre-existing carrier immunity on the efficacy of synthetic influenza vaccine. Immunol. Lett. 1998 doi: 10.1016/S0165-2478(98)00073-X. [DOI] [PubMed] [Google Scholar]
- 95.Ben-Yedidia T., Marcus H., Reisner Y., Arnon R. Intranasal administration of peptide vaccine protects human/mouse radiation chimera from influenza infection. Int. Immunol. 1999;11:1043–1051. doi: 10.1093/intimm/11.7.1043. [DOI] [PubMed] [Google Scholar]
- 96.Murthy K.G.K., Deb A., Goonesekera S., Szabó C., Salzman A.L. Identification of Conserved Domains in Salmonella muenchen Flagellin That Are Essential for Its Ability to Activate TLR5 and to Induce an Inflammatory Response in Vitro. J. Biol. Chem. 2004 doi: 10.1074/jbc.M307759200. [DOI] [PubMed] [Google Scholar]
- 97.Gewirtz A.T., Navas T.A., Lyons S., Godowski P.J., Madara J.L. Cutting Edge: Bacterial Flagellin Activates Basolaterally Expressed TLR5 to Induce Epithelial Proinflammatory Gene Expression. J. Immunol. 2001 doi: 10.4049/jimmunol.167.4.1882. [DOI] [PubMed] [Google Scholar]
- 98.Lu J., Sun P.D. Structural biology - The structure of the TLR5-flagellin complex: A new mode of pathogen detection, conserved receptor dimerization for signaling. Sci. Signal. 2012 doi: 10.1126/scisignal.2002963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Halff E.F., Diebolder C.A., Versteeg M., Schouten A., Brondijk T.H.C., Huizinga E.G. Formation and structure of a NAIP5-NLRC4 inflammasome induced by direct interactions with conserved N- and C-terminal regions of flagellin. J. Biol. Chem. 2012 doi: 10.1074/jbc.M112.393512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dos Reis E.C., Leal V.N.C., Soares J.L.D.S., Fernandes F.P., de Lima D.S., Pontillo A. Flagellin/NLRC4 Pathway Rescues NLRP3-Inflammasome Defect in Dendritic Cells From HIV-Infected Patients: Perspective for New Adjuvant in Immunocompromised Individuals. Front. Immunol. 2019;10:1291. doi: 10.3389/fimmu.2019.01291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim J.R., Holbrook B.C., Hayward S.L., Blevins L.K., Jorgensen M.J., Kock N.D., De Paris K., D’Agostino R.B., Aycock S.T., Mizel S.B., et al. Inclusion of Flagellin during Vaccination against Influenza Enhances Recall Responses in Nonhuman Primate Neonates. J. Virol. 2015 doi: 10.1128/JVI.00549-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bruxelle J.F., Mizrahi A., Hoÿs S., Collignon A., Janoir C., Péchiné S. Clostridium difficile flagellin FliC: Evaluation as adjuvant and use in a mucosal vaccine against Clostridium difficile. PLoS ONE. 2017 doi: 10.1371/journal.pone.0187212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sierro F., Dubois B., Coste A., Kaiserlian D., Kraehenbuhl J.-P., Sirard J.-C. Flagellin stimulation of intestinal epithelial cells triggers CCL20-mediated migration of dendritic cells. Proc. Natl. Acad. Sci. USA. 2002 doi: 10.1073/pnas.241308598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zeng H., Carlson A.Q., Guo Y., Yu Y., Collier-Hyams L.S., Madara J.L., Gewirtz A.T., Neish A.S. Flagellin Is the Major Proinflammatory Determinant of Enteropathogenic Salmonella. J. Immunol. 2003 doi: 10.4049/jimmunol.171.7.3668. [DOI] [PubMed] [Google Scholar]
- 105.Steiner T.S., Nataro J.P., Poteet-Smith C.E., Smith J.A., Guerrant R.L. Enteroaggregative Escherichia coli expresses a novel flagellin that causes IL-8 release from intestinal epithelial cells. J. Clin. Investig. 2000 doi: 10.1172/JCI8892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vijay-Kumar M., Carvalho F.A., Aitken J.D., Fifadara N.H., Gewirtz A.T. TLR5 or NLRC4 is necessary and sufficient for promotion of humoral immunity by flagellin. Eur. J. Immunol. 2010 doi: 10.1002/eji.201040421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lage S.L., Buzzo C.L., Amaral E.P., Matteucci K.C., Massis L.M., Icimoto M.Y., Carmona A.K., D’Imperio Lima M.R., Rodrigues M.M., Ferreira L.C.S., et al. Cytosolic flagellin-induced lysosomal pathway regulates inflammasome-dependent and -independent macrophage responses. Proc. Natl. Acad. Sci. USA. 2013 doi: 10.1073/pnas.1305316110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhao Y., Yang J., Shi J., Gong Y.N., Lu Q., Xu H., Liu L., Shao F. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature. 2011 doi: 10.1038/nature10510. [DOI] [PubMed] [Google Scholar]
- 109.Poeck H., Bscheider M., Gross O., Finger K., Roth S., Rebsamen M., Hannesschläger N., Schlee M., Rothenfusser S., Barchet W., et al. Recognition of RNA virus by RIG-I results in activation of CARD9 and inflammasome signaling for interleukin 1Β production. Nat. Immunol. 2010 doi: 10.1038/ni.1824. [DOI] [PubMed] [Google Scholar]
- 110.Martínez-Gil L., Goff P.H., Hai R., García-Sastre A., Shaw M.L., Palese P. A Sendai Virus-Derived RNA Agonist of RIG-I as a Virus Vaccine Adjuvant. J. Virol. 2013 doi: 10.1128/JVI.02338-12. [DOI] [Google Scholar]
- 111.Heidenreich R., Jasny E., Kowalczyk A., Lutz J., Probst J., Baumhof P., Scheel B., Voss S., Kallen K.J., Fotin-Mleczek M. A novel RNA-based adjuvant combines strong immunostimulatory capacities with a favorable safety profile. Int. J. Cancer. 2015 doi: 10.1002/ijc.29402. [DOI] [PubMed] [Google Scholar]
- 112.Ziegler A., Soldner C., Lienenklaus S., Spanier J., Trittel S., Riese P., Kramps T., Weiss S., Heidenreich R., Jasny E., et al. A New RNA-Based Adjuvant Enhances Virus-Specific Vaccine Responses by Locally Triggering TLR- and RLH-Dependent Effects. J. Immunol. 2017 doi: 10.4049/jimmunol.1601129. [DOI] [PubMed] [Google Scholar]
- 113.Doener F., Hong H.S., Meyer I., Tadjalli-Mehr K., Daehling A., Heidenreich R., Koch S.D., Fotin-Mleczek M., Gnad-Vogt U. RNA-based adjuvant CV8102 enhances the immunogenicity of a licensed rabies vaccine in a first-in-human trial. Vaccine. 2019 doi: 10.1016/j.vaccine.2019.02.024. [DOI] [PubMed] [Google Scholar]
- 114.Hornung V., Ablasser A., Charrel-Dennis M., Bauernfeind F., Horvath G., Caffrey D.R., Latz E., Fitzgerald K.A. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009 doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dombrowski Y., Peric M., Koglin S., Kammerbauer C., Göß C., Anz D., Simanski M., Gläser R., Harder J., Hornung V., et al. Cytosolic DNA triggers inflammasome activation in keratinocytes in psoriatic lesions. Sci. Transl. Med. 2011 doi: 10.1126/scitranslmed.3002001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kimkong I., Avihingsanon Y., Hirankarn N. Expression profile of HIN200 in leukocytes and renal biopsy of SLE patients by real-time RT-PCR. Lupus. 2009 doi: 10.1177/0961203309106699. [DOI] [PubMed] [Google Scholar]
- 117.Suschak J.J., Wang S., Fitzgerald K.A., Lu S. Identification of Aim2 as a Sensor for DNA Vaccines. J. Immunol. 2014 doi: 10.4049/jimmunol.1402530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mann C.J., Anguela X.M., Montané J., Obach M., Roca C., Ruzo A., Otaegui P., Mir L.M., Bosch F. Molecular signature of the immune and tissue response to non-coding plasmid DNA in skeletal muscle after electrotransfer. Gene Ther. 2012 doi: 10.1038/gt.2011.198. [DOI] [PubMed] [Google Scholar]
- 119.Barton G.M. A calculated response: Control of inflammation by the innate immune system. J. Clin. Investig. 2008 doi: 10.1172/JCI34431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mariathasan S., Monack D.M. Inflammasome adaptors and sensors: Intracellular regulators of infection and inflammation. Nat. Rev. Immunol. 2007 doi: 10.1038/nri1997. [DOI] [PubMed] [Google Scholar]
- 121.Wedmore C.V., Williams T.J. Control of vascular permeability by polymorphonuclear leukocytes in inflammation. Nature. 1981 doi: 10.1038/289646a0. [DOI] [PubMed] [Google Scholar]
- 122.Harris J., Sharp F.A., Lavelle E.C. The role of inflammasomes in the immunostimulatory effects of particulate vaccine adjuvants. Eur. J. Immunol. 2010 doi: 10.1002/eji.200940172. [DOI] [PubMed] [Google Scholar]
- 123.Butler N.R., Wilson B.D.R., Benson P.F., Dudgeon J.A., Ungar J., Beale A.J. Effect of aluminium phosphate on antibody response to killed poliomyelitis vaccine. Lancet. 1962 doi: 10.1016/S0140-6736(62)90003-X. [DOI] [PubMed] [Google Scholar]
- 124.Murray K., Bruce S.A., Hinnen A., Wingfield P., van Erd P.M., de Reus A., Schellekens H. Hepatitis B virus antigens made in microbial cells immunise against viral infection. EMBO J. 1984 doi: 10.1002/j.1460-2075.1984.tb01861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Peetermans J. Production, quality control and characterization of an inactivated hepatitis A vaccine. Vaccine. 1992 doi: 10.1016/0264-410X(92)90557-Z. [DOI] [PubMed] [Google Scholar]
- 126.Podda A. The adjuvanted influenza vaccines with novel adjuvants: Experience with the MF59-adjuvanted vaccine. Vaccine. 2001 doi: 10.1016/S0264-410X(00)00499-0. [DOI] [PubMed] [Google Scholar]
- 127.Tregoning J.S., Russell R.F., Kinnear E. Adjuvanted influenza vaccines. Hum. Vaccines Immunother. 2018 doi: 10.1080/21645515.2017.1415684. [DOI] [PMC free article] [PubMed] [Google Scholar]