Abstract
Salinity is a major abiotic stress which limits crop production, especially under rainfed conditions. Selenium (Se), as an important micronutrient, plays a vital role in mitigating detrimental effects of different abiotic stresses. The objective of this research was to examine the effect of Se fertilization on black gram (Vigna mungo) under salt stress. Our results showed that salt stress (100 mM NaCl) in leaves significantly induced oxidative damage and caused a decline in relative water content, chlorophyll (Chl), stomatal conductance (gs), photochemical efficiency (Fv/Fm), sucrose, and reducing sugars. A low dose of Se (1.5 ppm) significantly reduced hydrogen peroxide content, malondialdehyde formation, cell membrane damage, and also improved antioxidative enzyme activities, including superoxide dismutase, catalase, ascorbate peroxidase, glutathione reductase, and glutathione peroxidase under salt stress. Se-treated plants exhibited higher Chl, gs, Fv/Fm, sucrose, and reducing sugars than untreated plants in response to salt stress. In addition, Se application enhanced Se uptake and reduced Na+ uptake, but Cl− remained unaffected. Our results indicated that a low dose of Se effectively alleviated salt damage via inhibition of Na+ uptake and enhanced antioxidant defense resulting in a significant decrease in oxidative damage, and maintained gaseous exchange and PS II function for sucrose and reducing sugars accumulation in black gram.
Keywords: ascorbate-glutathione cycle, micronutrient, fertilization, oxidative damage, chlorophyll, sugar
1. Introduction
Agricultural productivity is adversely affected by soil salinity worldwide. Presently, twenty percent of the world’s arable land and almost half of all irrigated land areas are severely influenced by enormous salt stress. More and more areas are becoming barren because of salt accumulation [1]. The improvement of crop resistance to salinity would increase the food production area of the world tremendously. Adverse effects of salt toxicity on plant physiology result in a reduction of osmotic potential of soil solution and ion toxicity, leading to the disintegration of ionic distribution and water potential in plants [1,2]. A disturbance in the metabolic equilibrium induced by salinity also accelerates oxidative stress resulting in an excessive production of reactive oxygen species (ROS) [3]. The ROS can impart severe damage to numerous biomolecules (e.g., lipid, protein, and DNA) that are crucial for plant existence [4]. Osmotic stress triggered by salinity results in a substantial decrease in stomatal aperture, which reduces photosynthetic capacity [5]. Thus, the antioxidant defense system and photosynthesis are extremely important for plants’ survival under salt toxicity [5,6]. In many plants, reduced growth is closely associated with a decline in photosynthesis under saline conditions [6,7,8]. High salinity inhibits photosynthetic activity of plants associated with stomatal restriction such as stomatal closure [5,9] and non-stomatal restrictions, including denaturation of membrane and enzymatic proteins in photosynthetic apparatus [7,10], chlorophyll (Chl) degradation [8,11], and chloroplast ultrastructure [12]. Plants have a well-organized system of numerous processes involved in the regulation of salt tolerance, such as different types of compatible solutes, antioxidant defense, polyamines, ions uptake, and compartmentalization of injurious ions and ionic transport [13].
Nutrient amelioration has been proposed to mitigate the harmful impacts of salt toxicity in plants [14]. However, these studies have mainly targeted potassium [15,16], calcium [17,18], and phosphorus [16]. Se is an important micronutrient for human beings and livestock [19,20]. A deficiency of Se is responsible for asthma, hypothyroidism, and weakened immune systems in humans [21,22]. The positive effects of Se on plants are concentration or species dependent. Plants are very sensitive to Se (the trace concentration), and higher amounts (>400 µg Sed−1) have proven to be drastic and even lethal for plants [23,24]. Due to similar properties, Se and sulphur (S) are absorbed by plants through sulphate transporters and assimilated by the sulfur assimilating pathway [25,26]. Some studies have reported that Se induced beneficial effects on different physiological processes of plants such as improvement in stress tolerance [27] and photosynthesis [28], and alleviation of senescence [29,30]. Previously, if has been shown that Se considerably improved plant growth and physiochemical attributes under salt stress. However, the putative role of Se was dose dependent and varied with its concentration, duration of application, and plant species at different growth stages [31]. The application of Se has enhanced carbohydrate content in the leaves of beans (Phaseolus vulgaris) [32] and promoted plant growth and soluble sugar content in coffee (Coffea arabica) leaves [33]. Foliar application of Se has increased the quantity and quality of green tea (Camellia sinensis) leaves [34]. Se fertilization as selenite to potato tubers has increased protein content and reduced glycoalkaloids, and NO3− accumulation [35]. The accumulation of more starch grains and growth improvement have been observed in lettuce plants treated with Se [36]. Se-treated mung bean have also been shown to accumulate more sucrose and starch [37]. For stress tolerance, minute quantities of Se have improved antioxidant enzyme activities and growth during senescence and ultraviolet radiations in lettuce (Lactuca sativa) and ryegrass (Lolium perenne) [29,38,39]. The application of Se could mitigate the light-induced oxidative damage in potato (Solanum tuberosum) [40].
Black gram (Vigna mungo L.) belongs to the family of Fabaceae and is one of the most significant economical pulse crops used as a food, green manure, and fodder. Black gram is also a rich and easy source of proteins (20% to 24%), fats (1% to 2%), oil (2.1%), vitamins (A and B), and carbohydrates that are essential for human health [41]. Due to its excellent ability to fix atmospheric nitrogen and convert it to plant useable forms such as nitrates and ammonium ions, black gram plays a vital role in the improvement of soil fertility worldwide [42]. In this study, we focused on the role of different concentrations of Se fertilization in salt-stressed black gram plants on ion uptake, oxidative damage, antioxidative metabolism, photosynthetic functions, and sucrose accumulation. It is beneficial to understand the effects of Se on salt tolerance in crops and investigate farming practices to enhance Se concentration in food commodities.
2. Materials and Methods
2.1. Planting Material and Treatments
A pot experiment was conducted at the research area of the Agronomy Department PMAS Arid Agriculture University, Rawalpindi from March to June 2018. The research area was located at 33.6492° N latitude and 73.0815° E longitude. The experiment was laid out in plastic pots with 18 cm diameter and 22 cm depth. Each pot contained 5 kg loam, 10 milligram Ca3(PO4)2 kg−1, and 0.5 Kg farmyard manure, respectively. All pots were arranged under a completely randomized design (CRD). After filling, each pot was treated with a salt solution (100 mM NaCl) until its saturation and dried out for two days. Later, the sodium selenate (Na2SeO4) was dissolved in distilled water (1.5, 3, and 4.5 ppm) and irrigated in fully dried soil before sowing. Seeds of black gram cultivar “Chakwal Mash” were obtained from the National Agriculture Research Centre and used as plant material. Before sowing, seeds were washed with 75% ethanol and deionized water for 5 min. Ten seeds were sown in each pot, and after thinning, three plants were maintained in each pot. Plants were well irrigated daily to prevent a risk of water stress in a greenhouse (average temperatures of 25/20 °C (day/night) and 800 μmoL m−2 s−1 photosynthetically active radiation). At the flowering stage (35 days after sowing), the fully expanded and mature leaves from each treatment were selected for assessment of physiological parameters. Each treatment was thrice replicated (non-saline control, salt stress, salt + 1.5 ppm Se, salt + 3 ppm Se, and salt + 4.5 ppm Se), and a total of 15 pots (each pot contained three plants) was used for the above treatments.
2.2. Determination of Se, Na, and Cl Concentrations
Leaf samples were washed with deionized water, blotted to eliminate extra water, and then dried in an oven. Dried samples were thoroughly homogenized with mortar and pestle, shifted to a digestion flask consisting of ten milliliters of 4 M HNO3, and placed at room temperature overnight. After being heated at 125 °C for 4 hrs, solutions were diluted (50 mL) and cooled for further measurements. The Se concentration (µg g−1 DW) was measured in triplicate using atomic absorption spectrometry [43]. For Na and Cl determination, samples were dried and ground to powder form, and then shifted to an Erlenmeyer flask containing 6 mL of HClO4 and HNO3 solution. The flasks were placed for 30 min at 40 °C in a water bath until condensed to 1 milliliter extract by heating at 150 to 180 °C. Distilled water was used to dissolve this residue to the final volume of 100 mL. The Na concentration in the leaf samples was estimated by using flame photometry, whereas Cl- was determined by precipitation titration with Ag2NO3 following Mohr’s method [44].
2.3. Determination of Leaf Water Status
Leaf samples were detached to calculate the relative water content (RWC) following the procedure described by [45]. Fresh weight (FW) was noted instantly and dipped in deionized water overnight to obtain the turgid weight (TW). Samples were placed in an electric oven for one day at 75 °C to obtain dry weight (DW). The RWC was estimated using the following equation: RWC (%) = 100 × [(FW – DW)/(TW – DW)].
2.4. Measurement of Chlorophyll Content, Stomatal Conductance, and Photochemical Efficiency
In order to examine the Chl content, 1 g fresh leaf samples were ground in 80 percent CH3COCH3, and centrifuged at 1816g for 10 min. The supernatant was obtained, and an absorbance value at 645 and 663 nm was noted to measure the total Chl content [46] against 80 percent acetone as a blank. Stomatal conductance (gs) was estimated from fully expanded, mature, and healthy leaves using a portable leaf porometer (model SC1, Decagon Devices, Pullman, WA, USA) at 11.00 a.m. and given as mmol m−2 s−1.
Leaves were obtained from the top second or third branch, and photochemical efficiency was estimated as Chl fluorescence following a dark-adapted test of modulated Chl fluorometer (OS1-FL, Opti-Sciences, Tyngsboro, MA, USA) at 11:00 h. The fluorometer provided a solid-state light source of 660 nm and had filters to block radiations above 690 nm. The mean intensity of this modulated light was set from 0 to 1 mE. A PIN silicon photodiode was used for detection in the range 700–750 nm with adequate filtering to eliminate extraneous light. Leaves were placed in the instrument clamps under darkness to cease photosynthesis (light reaction) for 45 min. Clamps were connected with the optic fiber of the device, and clamps valves were opened. After the onset of the device, the modulated light of 695 nm was radiated towards the leaf through the optic fiber. Photosystem II (PSII) activity was given as the Fv/Fm ratio.
2.5. Determination of Oxidative Damage and Cell Membrane Stability
Lipid peroxidation was estimated as the malondialdehyde (MDA) content according to the procedure of [47]. Then, 0.5 g of leaf tissue samples were homogenized in 5 milliliters of 5% TCA. Homogenate was centrifuged for 10 min at 25 °C, heated at a high temperature (98 °C) for 10 min, and allowed to cool in ice immediately. The absorbance value was estimated at a wavelength of 532 nm. For the estimation of hydrogen peroxide (H2O2) concentration, protocols involving potassium iodide (KI) were used. Absorbance values were noticed at 390 nm. The quantity of hydrogen peroxide produced was obtained by following the standard curve drawn with previously known readings of hydrogen peroxide [48]. In order to measure cell membrane stability, leaf samples (0.2 g) were collected, rinsed with distilled water to eliminate attached electrolytes, and kept in vials that contained ten milliliters of distilled water. Closed vials were placed at 25 °C for six h, and electrical conductivity (C1) of the solution was determined. After this, samples were kept in an electric oven at 90 °C for two h, and the electrical conductivity (C2) of solution was estimated. Electrolyte leakage (EL) was measured in percent as follows: EL% = 100 × EC1/EC2.
2.6. Determination of Antioxidant Enzyme Activities
Leaf tissue was frozen and subsequently homogenized in 4 mL of a solution having 1% (w/v) polyvinylpolypyrrolidone (PVP), 50 mM phosphate buffer (pH 7.0), and 0.2 mM ascorbic acid. Then, the homogenate was centrifuged for 30 min at 15,000g. The supernatant was rapidly desalted at 4 ºC by passing it through four milliliters Sephadex G-25 columns pre-equilibrated with a buffer consisting of 0.05% BSA, 0.25 mM MgCl2, 1 mM EDTA, 20 mM HEPES-NaOH (pH 7.5), and 0.01% 2-mercaptoethanol. All events were completed at 0 to 4 °C. The SOD activity was measured according to the method of [49]. The reaction mixture comprised of 13 mM methionine, 2 mM riboflavin, 5 mL enzyme extract, 50 mM phosphate buffer (pH 7.8), 75 mM p-nitroblue tetrazolium chloride (NBT), and 0.1 mM EDTA. One unit of SOD activity was defined as the concentration of the SOD enzyme needed for 50% inhibition of nitro blue tetrazolium reduction. The CAT activity was measured as a decline in absorbance value at 240 nm for 1 min resulting in the disintegration of H2O2 following the procedure of [50]. The assay medium consisted of 15 mM H2O2 and 50 mM phosphate buffer (pH 7.0).
The APX activity was estimated as a reduction in absorbance value at 290 nm for 1 min according to the procedure of [51]. The estimation mixture included 0.1 mM H2O2, 50 mM sodium phosphate buffer (pH 7.0), 0.5 mM ASA, 0.1 mM EDTA, and 0.15 mL enzyme extract. The GR activity was examined, as explained by [52]. Then, oxidized GSH (GSSG)-dependent NADPH oxidation was followed at 340 nm in 1 mL of reaction mixture including 0.1 mM NADPH, 100 mM sodium phosphate buffer (pH 7.8), 50 µL extract, and 0.5 mM GSSG. The GPX activity was determined using the protocol of [53] with H2O2 as a substrate. The reaction mixture included 1 mM NaN3, 1 mM EDTA, 1 U glutathione reductase, 25 µL sample solution, 100 mM Na-phosphate buffer (pH 7.5), 0.12 mM NADPH, 2 mM GSH, and 0.6 mM H2O2. The reaction was initiated by adding H2O2. The NADPH oxidation was measured at 340 nm for 1 min.
2.7. Determination of Sucrose and Reducing Sugars
The sucrose content was estimated by following the procedure described by [54]. Leaf tissue was extracted using 80 percent ethanol and repeated three times at 80 °C for 1.5 hours for each extraction. The extract was collected and vaporized in an oven at 40 °C. Then, 200 mL of aliquots from samples and standard sucrose were mixed with the reaction solution (1 mL) which consisted of 0.4 mM NADP+, 5 mM MgCl2, 0.02% (weight/volume) BSA, 2 mg/mL yeast hexokinase (EC 2.7.1.1), 100 mM imidazole buffer (pH 6.9, 60 mM imidazole HCl, 40 mM imidazole base), 1 mM ATP, 0.5 mM dithiothreitol, 20 mg/mL yeast invertase (EC3.2.1.26), and 1 mg/mL yeast phosphoglucoisomerase (EC 5.3.1.9) and incubated at 25 °C for 30 min to allow conversion of fructose and glucose to glucose-6-phosphate, and readings were noted at 340 nm. After 85 milliliters of C6H12O6-6-PO4−2 dehydrogenase (70 units/mL) was added, the mixture was blended thoroughly. Absorbance was again recorded when the readings became stable after 5 min. Blanks were run using 1 milliliter of the reaction mixture and 200 milliliters of the extract without invertase. A standard curve was used to convert values obtained from the samples to sucrose concentrations and given as µmol/g (DW). To assess the reducing sugars, the DNSA reagent (1 mL 3,5-dinitrosalicylic acid) was mixed with ethanol extract (1 mL). The reaction mixture was heated for 12 min, and then 2 mL of distilled water was added. The absorbance was noted against a blank consisting of 80% C2H5OH instead of C2H5OH extract. The content of the reducing sugars was estimated using a standard curve.
2.8. Statistical Analysis
Statistical analysis was conducted using statistix 8.1 (version, 8.1. Statistix, USA). Significant differences among all treatments were measured by using ANOVA (one way) in combination with LSD (least significant difference) test. The significance of differences was evaluated at the 5 percent probability level (p < 0.05).
3. Results
3.1. Effects of Exogenous Se on Se, Na, and Cl Uptake under Salt Stress
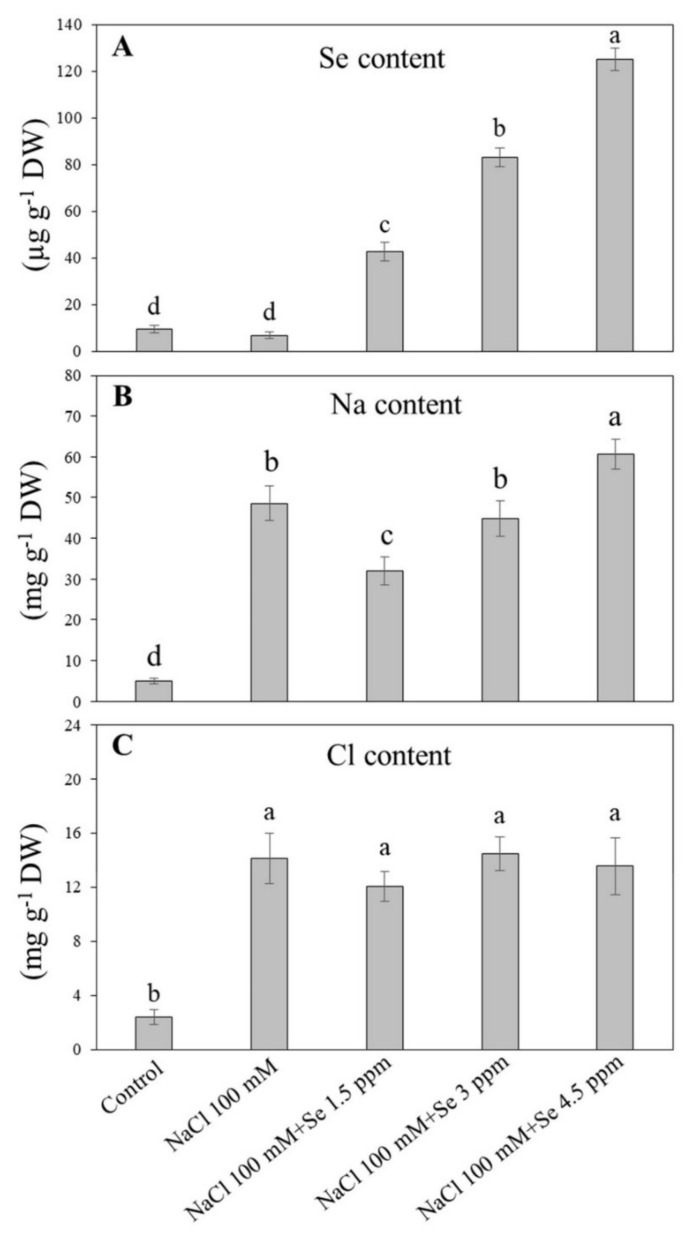
Salt stress did not affect Se accumulation, but exogenous Se fertilization significantly enhanced Se uptake in leaves of black gram (Figure 1A). For all exogenous Se treatments, the highest Se accumulation (125.133 µg g−1 DW) was observed in treatment (NaCl 100 mM + Se 4.5 ppm), second (83.233 µg g−1 DW) in treatment (NaCl 100 mM + Se 3 ppm), and lowest (42.667 µg g−1 DW) in treatment (NaCl 100 mM + Se 1.5 ppm), which showed a progressive trend in Se accumulation with an increase in its applied concentrations (Figure 1A). The application of NaCl significantly increased Na uptake in leaves (Figure 1B). In combined applications of Se and NaCl, only a lower concentration of Se (1.5 ppm) significantly restricted Na accumulation in leaves; the three ppm of Se had no significant effect on Na uptake. In comparison, the higher level (4.5 ppm) significantly increased Na accumulation in leaves of black gram. As compared to the control, salt stress and salt stress combined with exogenous Se (1.5, 3, and 4.5 ppm) application induced significant increases in Cl accumulation, but no significance in Cl uptake were observed among these four treatments (NaCl, NaCl + 1.5 ppm Se, NaCl +3 ppm Se, and NaCl+4.5 ppm Se) (Figure 1C).
Figure 1.
Effect of selenium (Se) fertilization on (A) Se uptake; (B) sodium (Na) uptake; and (C) chloride (Cl) uptake in leaves if black gram plants under salt stress. Values are mean ± standard error (n = 3). Different letters in vertical columns show a significant difference. A comparison of mean was confirmed by LSD at p < 0.05.
3.2. Effects of Exogenous Se on Oxidative Damage and Cell Membrane Stability under Salt Stress
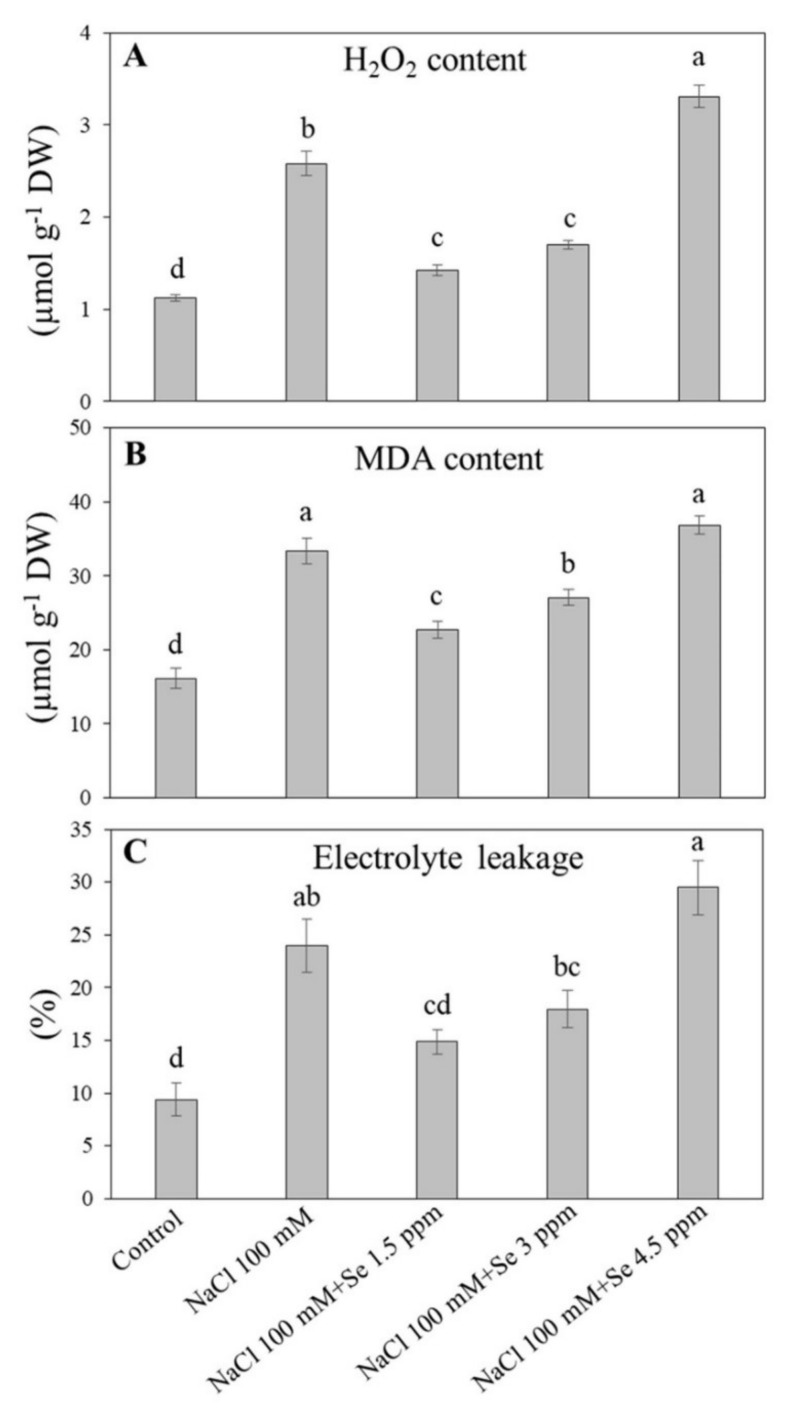
Salt stress (NaCl 100 mM) significantly influenced H2O2 concentration in leaves of black gram plants. However, low and intermediate Se application significantly reduced H2O2 with respect to Se-free saline treatment, whereas it was even increased at the high dose (Figure 2A). Lipid peroxidation, as reflected by MDA content, was greatly enhanced by salt stress in black gram plants (Figure 2B). Among all treatments, higher MDA content was noticed in the treatment (NaCl 100 mM + Se 4.5 ppm) and salt stress, while the lowest (16.100 µmol g−1 DW) in the control, which suggested that Se at high concentrations was toxic for black gram plants under saline conditions. Salt treatment (NaCl 100 mM) significantly elevated MDA content in black gram plants as compared with the control. The MDA content was significantly reduced by Se treatment (NaCl 100 mM + Se 1.5 or 3 ppm) as compared with salt stress (NaCl 100 mM) (Figure 2B). Salt stress significantly increased the level of EL up to (24%) in contrast to the control. The low concentration of Se (NaCl 100 mM + Se 1.5 ppm) reduced EL in relative to salt treatment (NaCl 100 mM). EL exhibited an increasing trend from lower (1.5 ppm) to higher doses of Se (4.5 ppm) application under salt stress (Figure 2C).
Figure 2.
Effect of selenium (Se) fertilization on (A) hydrogen peroxide (H2O2); (B) malondialdehyde (MDA) content; and (C) electrolyte leakage (EL) in leaves of black gram plants under salt stress. Values are mean ± standard error (n = 3). Different letters in vertical columns show a significant difference. A comparison of mean was confirmed by LSD at p < 0.05.
3.3. Effects of Exogenous Se on Antioxidant Defense under Salt Stress
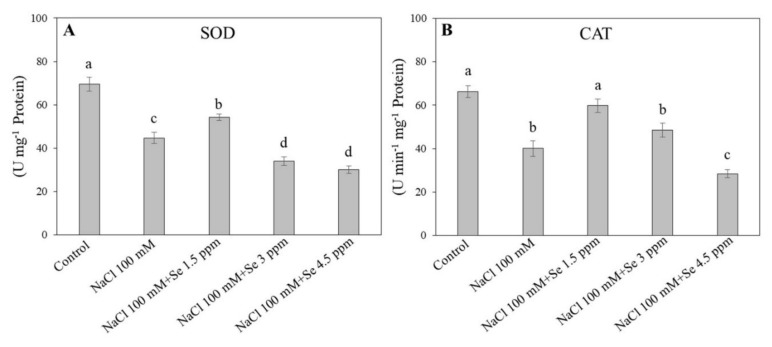
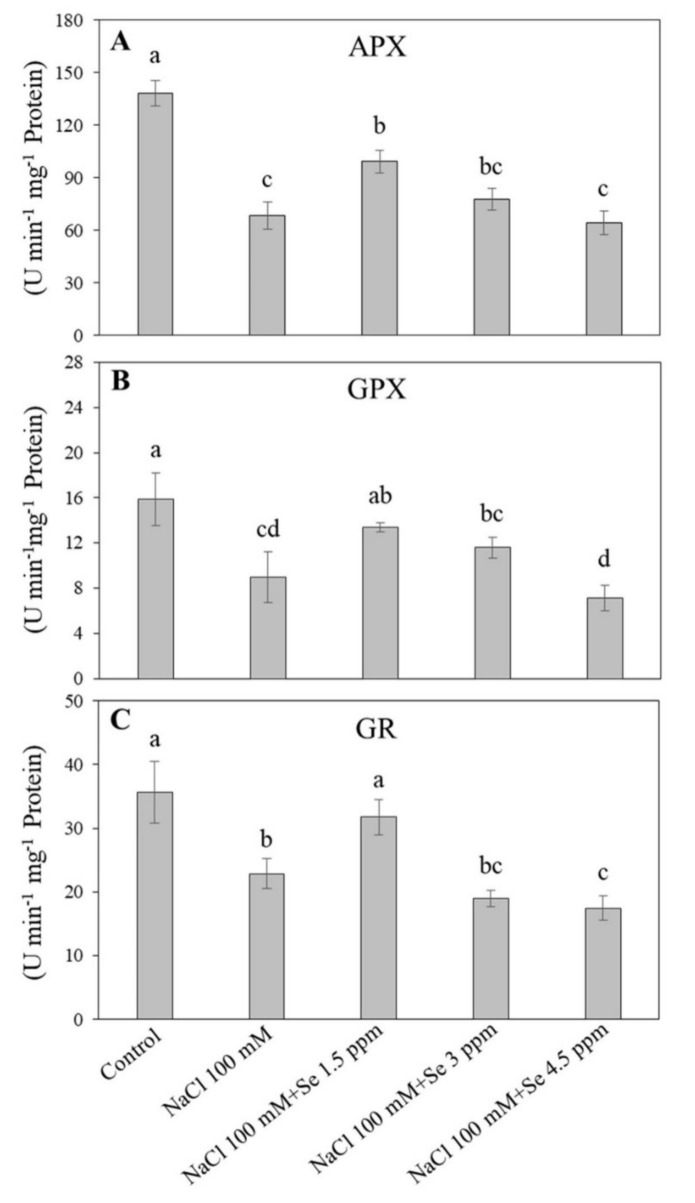
Salt stress negatively influenced the defensive system of black gram plants by impairing the antioxidant enzyme activities (Figure 3 and Figure 4). The SOD and CAT activities in the control were found to be maximum, whereas salt stress reduced their activities (Figure 3A). An improvement in SOD and CAT activities was observed in the treatment (NaCl 100 mM + Se 1.5 ppm) as compared with the salt treatment (NaCl 100 mM) in leaves. The higher dose of Se (3 and 4.5 ppm) reduced SOD and CAT activities along with the salt application (Figure 3). Similar trends were noticed in changes of APX, GPX, and GR activities (Figure 4). The Se (1.5 ppm) application improved the APX activity in leaves by 30.89% under salt stress as compared with the salt treatment (NaCl 100 mM). Significant declines in GPX and GR activities were found under the 100 mM NaCl treatment as compared with the control (Figure 4B,C). The maximum GPX (15.867 U min−1 mg−1 protein) and GR (35.600 U min−1 mg−1 protein) activities were recorded in the control, while the minimum GPX ( 8.967 µ guaiacol min−1 mg−1 protein) and GR (22.867 µmoL NADPH min−1 mg−1 protein) activities were measured in the treatment (NaCl 100 mM + Se 4.5 ppm), respectively. The addition of Se at the 1.5 ppm dose maintained GPX and GR activities at normal levels (Figure 4B,C).
Figure 3.
Effect of selenium (Se) fertilization on (A) superoxide dismutase (SOD) and (B) catalase (CAT) activities in leaves of black gram plants under salt stress. Values are mean ± standard error (n = 3). Different letters in vertical columns show a significant difference between each treatment. A comparison of mean was confirmed by LSD at p < 0.05.
Figure 4.
Effect of selenium (Se) fertilization on (A) ascorbate peroxidase (APX); (B) glutathione peroxidase (GPX); and (C) glutathione reductase (GR) in leaves of black gram plants under salt stress. Values are mean ± standard error (n = 3). Different letters in vertical columns show a significant difference. A comparison of mean was confirmed by LSD at p < 0.05.
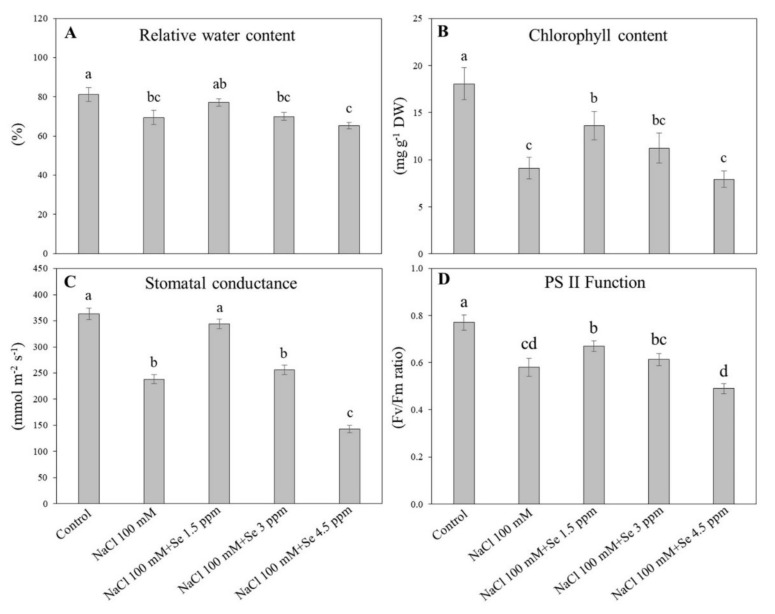
3.4. Effects of Exogenous Se on Leaf Water Status and Photochemical Efficiency under Salt Stress
The highest RWC (81.167%) was noted in the control and the lowest RWC (65.333%) was detected in the treatment (NaCl 100mM + Se 4.5 ppm) (Figure 5A). The RWC in the salt treatment (NaCl 100 mM) was significantly decreased as compared with the control. The Se at a lower dose (1.5 ppm) with salt application significantly improved the leaf RWC as compared with the salt only treatment (100 mM); however, the higher doses of Se with salt decreased the leaf RWC drastically (Figure 5A). Different applications of Se and NaCl significantly affected total Chl content in black gram leaves (Figure 5B). Salt stress significantly decreased the total Chl content, but Se application at a lower level (1.5 ppm) alleviated the salt-induced decline in total Chl content in black gram plant leaves (Figure 5B). In contrast, a significant reduction in total Chl content was noticed when the Se concentration was increased beyond 1.5 ppm under salt stress (Figure 5B). NaCl (100 mM) treatment significantly reduced gs by 34.35% as compared with the control. The treatment (NaCl 100 mM + Se 1.5 ppm) showed a 30.76% increase in gs as compared with the salt treatment (NaCl 100 mM) (Figure 5C). The higher dose of Se (4.5 ppm) under salt stress significantly inhibited gs as compared with the salt treatment (Figure 5C). The maximum Fv/Fm (0.770) for photosystem II was observed in the control, whereas the minimum Fv/Fm (0.490) was detected in the treatment (NaCl 100 mM + Se 4.5 ppm). The salt only treatment (NaCl 100 mM) significantly reduced Fv/Fm by 24.67% as compared with the control. A low dose of Se (1.5 ppm) significantly alleviated the harmful effects of salinity in leaves of black gram plants (Figure 5D).
Figure 5.
Effect of selenium (Se) fertilization on (A) relative water content (RWC); (B) chlorophyll (Chl); (C) stomatal conductance (gs); and (D) photochemical efficiency (Fv/Fm) in leaves of black gram plants under salt stress. Values are mean ± standard error (n = 3). Different letters in vertical columns show a significant difference. A comparison of mean was confirmed by LSD at p < 0.05.
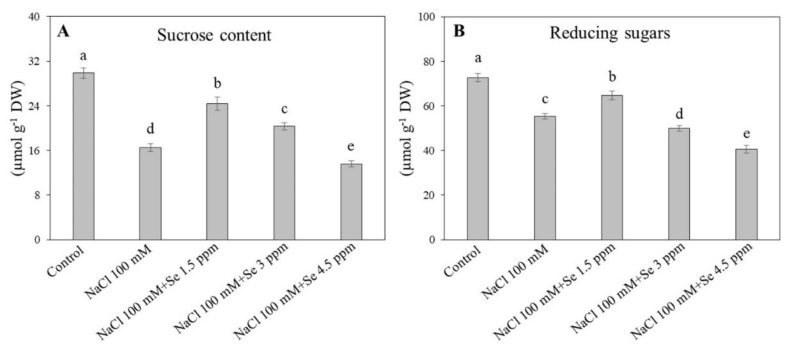
3.5. Effects of Exogenous Se on Sugar Accumulation under Salt Stress
Salt stress and Se application significantly affected sucrose concentration in leaves of black gram plants (Figure 6A). In contrast to the control plants, sucrose concentration in leaves significantly decreased by 54.57% in plants growing in salt stress (NaCl 100 mM) (Figure 6A). The combined treatment (NaCl 100 mM + Se 1.5 ppm) significantly alleviated the harmful impact of salinity and successfully improved sucrose concentration by 32.28% as compared with the salt treatment (NaCl 100 mM). Moreover, the treatment (NaCl 100 mM + Se 3 ppm) maintained significantly higher sucrose content than the salt treatment. The treatment (NaCl 100 mM + Se 4.5 ppm) significantly reduced sucrose content as compared with the salt only treatment (Figure 6A). Reducing sugars significantly declined in leaves under salt stress in spite of the Se application (Figure 6B). In contrast to the control, the salt treatment significantly decreased the reducing sugar level by 23.78%, but the addition of Se (NaCl 100 mM + Se 1.5 ppm) significantly mitigated the adverse effects of salt stress on reducing sugars. The concentration of Se beyond 1.5 ppm (3 and 4.5 ppm) exhibited toxic effects and adversely influenced reducing sugars accumulation in the leaves of black gram plants under toxic salt conditions (Figure 6B). Figure 7 presented the protective role of Se at an optimum concentration (1.5 ppm) in leaves of black gram under salt stress.
Figure 6.
Effect of selenium (Se) fertilization on (A) sucrose content and (B) reducing sugars in leaves of black gram plants under salt stress. Values are mean ± standard error (n = 3). Different letters in vertical columns show a significant difference. A comparison of mean was confirmed by LSD at p < 0.05.
Figure 7.
Comprehensive schematic diagram presenting the protective role of selenium (Se) at an optimum concentration (1.5 ppm) in leaves of black gram under salt stress.
4. Discussion
Salinity is extremely harmful to crops belonging to the family of Fabaceae such as mungbean (Vigna radiata), soybean (Glycine max), and many other pulse crops, resulting in reduced growth and yield [5]. On the basis of previous studies about the advantageous roles of Se on plants under stressed conditions [55], we examined the role of Se application in black gram plants under salt-stressed soil. Interestingly, the results showed that only 1.5 ppm of Se supplements under salt-stressed soil improved the performance of various physiological and biochemical parameters in black gram plants, whereas 3 and 4.5 ppm dose amplified damage under salt stress. In addition, the plants uptake of Se was not affected due to salt stress indicating that Se was probably not a salt-responsive element in black gram. As expected, salt stress significantly increased Na and Cl ions in leaves of black gram with more Na than Cl ions. Similar findings were reported in some grass species [56] and Avicennia germinans [57]. To date, it is unknown which element (Na or Cl) is more responsible for NaCl-induced ionic stress in black gram. It has been reported that Se application at low doses (5 and 10 μM) decreased Cl content in cucumber plants under salt stress [58]. Exogenous Se significantly reduced Na accumulation in roots when maize (Zea mays) suffered from salt stress [59]. To date, limited information is available about Na and Se antagonism in legumes grown under salt-stressed soil. In the present study, the application of Se (1.5 ppm) significantly increased Se accumulation in leavs and decreased Na uptake with no significant effect on Cl uptake under salt stress. These results indicated that an appropriate dose of Se (1.5 ppm) effectively alleviated salt damage due to the inhibition of Na uptake in black gram.
Under salt stress, plants accumulate high levels of reactive oxygen species that are extremely dangerous for various cellular components, including lipids, nucleic acids, proteins, and pigments leading to membrane lipid peroxidation and decline in membrane stability [60,61]. Membrane integrity and stability could be estimated by MDA content and EL level [62]. Salt-tolerant wheat (Triticum aestivum) and tomato (Solanum lycopersicum) demonstrated lower H2O2 and MDA content in contrast to susceptible ones [63,64]. In addition, Se application could reduce the MDA content in soybean during senescence [30]. Our study demonstrated that salt stress significantly increased H2O2 and MDA content and EL level. However, membrane damage in black gram plants affected by salt stress was observed to be significantly decreased in leaves fertilized with 1.5 ppm dose of Se, which is in accordance with previous reports [14,58]. It has been reported that Se application improved antioxidant enzyme activities, including SOD, POD, and GR, and alleviated oxidative damage in various plants under saline conditions [6,14,59]. In our study, significant promotion in activities of SOD, CAT, APX, GR, and GPX were detected in salt-Se-treated black gram plants (NaCl 100 mM + Se 1.5 ppm) in contrast to salt-affected plants (NaCl 100 mM). These findings were in agreement with previous results where Se improved antioxidant defense in sorrel seedlings under salt stress [14]. Different antioxidants such as SOD, CAT, APX, GPX, and GR are extremely important for ROS scavenging in plants under salt-stressed conditions. SOD is the integral and front line in antioxidant defense against oxidative damage by converting superoxide radical (O2−) into hydrogen peroxide and molecular oxygen [65]. CAT is principally involved in the removal of surplus H2O2 [55,66]. When Se is present, GPX and GR are more efficient in eliminating H2O2 as compared with APX and CAT [6]. GPX, APX, and GR are crucial globular proteins of the ascorbate-glutathione cycle playing important roles in tandem with ascorbate peroxidase to scavenge H2O2 [66], which have been described to be stimulated markedly in plants growing under stressed abiotic conditions [43,67,68]. In the present study, a low dose of Se (1.5 ppm) improved salt tolerance in black gram through enhancement of the antioxidant defense, thereby alleviating the salt-induced membrane lipid peroxidation and enhancing membrane stability. In addition to the advantageous roles of Se application at a lower concentration (1.5 ppm), higher levels (3 and 4.5 ppm) exacerbated harmful effects caused by salinity, which could be associated with the considerable accumulation of Se and Na ions in leaves. The present reports are in accordance with earlier findings with respect to the interactive roles of Se and arsenic and higher concentrations of Se assisted arsenic uptake [69].
Salt stress induces physiological drought resulting in a decline in leaf water potential, RWC, stomatal conductance, and ultimately transpiration. The current study found that black gram plants exposed to salt treatment exhibited a significant reduction in RWC, which probably was related to a hindrance in water uptake. Salt induced reduction in RWC has been observed in triticale [70], safflower [71], and sorghum [72]. An inhibition in gs was also observed in this study, which is similar to results reported in salt-affected mustard (Brassica spp.) [73] and barley (Hordeum vulgare) [74]. Interestingly, the application of Se alleviated declines in the gs of black gram leaves caused by salt. Similar results of Se on RWC and gs have been found in potato and olive (Olea europaea) under drought stress [75,76]. Water status and gaseous exchange through stoma are directly related to photosynthesis. A decline in Chl content and fluorescence causes a significant reduction in photosynthetic function when plants respond to salt stress. The photosynthetic rate has been significantly improved by Se application in sorghum under heat stress [77]. Se application has been associated with amelioration in photosynthesis and Chl protection and has been observed in sorrel (Rumex patientia × R. tianshanicus) [14] and cucumber [58] under saline conditions. Our study showed that salt stress severely affected the photosynthetic process by causing a significant reduction in Chl content and Fv/Fm in non-Se-treated black gram plants, whereas Se-treated (1.5 ppm) black gram plants exhibited higher Chl content and Fv/Fm under salt stress. Se application has been reported to protect the function of Chl and photosystem II from salt-induced oxidative damage through improvement in antioxidant enzyme activities in tomato seedlings [6], which supports our findings. Under salt stress, a disturbance in sucrose metabolism, as demonstrated by a decrease in sucrose and reducing sugar content in leaves of salt-exposed black gram plants, could inhibit photosynthesis in leaves. Salt stress has been described to have a negative impact on sucrose metabolism by influencing genes and enzymes associated with sucrose synthesis in tomato [78]. However, we observed that the Se application significantly restricted salt-induced decline in sucrose and reducing sugars in black gram leaves. An earlier study found that 0.75 ppm of Se improved sucrose and reducing sugar content, and thus provided more energy for mungbean (Phaseolus aureus.) growth under normal conditions [37]. There is a need to further explore the contribution of Se-regulated sugar metabolism to salt tolerance in black gram.
5. Conclusions
The current study exposed that the addition of Se at a low concentration (1.5 ppm) to black gram plants growing in saline soil hindered Na uptake, resulting in amelioration of physiological and biochemical responses such as antioxidant defense, Chl concentration, gs, photochemical efficiency, sucrose levels, and reducing sugars. Se improved antioxidant enzyme activities (SOD, CAT, APX, GPX, and GR), followed by a significant reduction in ROS accumulation and membrane lipid peroxidation in salt-stressed black gram plants, which could be beneficial to the maintenance of higher Fv/Fm and sugar content under salt stress. Se fertilization at a low concentration (1.5 ppm) could be one of the potential approaches to minimize the effects of salt toxicity in black gram plants growing under salt-affected soils.
Author Contributions
Conceptualization, M.A. (Mukhtar Ahmad), M.J.H., M.A. (Muhammad Ansar), and G.A.S.; methodology, M.A.R., I.K., T.A.M., and M.J.H.; formal analysis, N.K., I.K., and S.A.A.; investigation, Z.L., N.I., and Muhammad Ansar.; writing—original draft preparation, M.J.H.; writing—review and editing, Z.L., Y.P., and G.A.S.; funding acquisition, Z.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Chunhui Program of the Ministry of Education (Z2017095) and the National Natural Science Foundation of China (31702182).
Conflicts of Interest
All authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Zhu J.-K. Plant salt tolerance. Trends Plant Sci. 2001;6:66–71. doi: 10.1016/S1360-1385(00)01838-0. [DOI] [PubMed] [Google Scholar]
- 2.Luo Q., Yu B., Liu Y. Differential sensitivity to chloride and sodium ions in seedlings of Glycine max and G. soja under NaCl stress. J. Plant Physiol. 2005;162:1003–1012. doi: 10.1016/j.jplph.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 3.Hussain T.M., Hazara M., Sultan Z., Saleh B.K., Gopal G.R. Recent advances in salt stress biology a review. Biotechnol. Mol. Biol. Rev. 2008;3:8–13. [Google Scholar]
- 4.Mittler R., Vanderauwera S., Gollery M., Van Breusegem F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004;9:490–498. doi: 10.1016/j.tplants.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 5.Munns R., Tester M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- 6.Diao M., Ma L., Wang J., Cui J., Fu A., Liu H.-Y. Selenium promotes the growth and photosynthesis of tomato seedlings under salt stress by enhancing chloroplast antioxidant defense system. J. Plant Growth Regul. 2014;33:671–682. doi: 10.1007/s00344-014-9416-2. [DOI] [Google Scholar]
- 7.Chaves M.M., Flexas J., Pinheiro C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009;103:551–560. doi: 10.1093/aob/mcn125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang C., Zheng Q., Liu Z., Xu W., Liu L., Zhao G., Long X. Overexpression of Arabidopsis thaliana Na+/H+ antiporter gene enhanced salt resistance in transgenic poplar (Populus× euramericana ‘Neva’) Trees. 2012;26:685–694. doi: 10.1007/s00468-011-0635-x. [DOI] [Google Scholar]
- 9.Brugnoli E., Lauteri M. Effects of salinity on stomatal conductance, photosynthetic capacity, and carbon isotope discrimination of salt-tolerant (Gossypium hirsutum L.) and salt-sensitive (Phaseolus vulgaris L.) C3 non-halophytes. Plant Physiol. 1991;95:628–635. doi: 10.1104/pp.95.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mittal S., Kumari N., Sharma V. Differential response of salt stress on Brassica juncea: Photosynthetic performance, pigment, proline, D1 and antioxidant enzymes. Plant Physiol. Biochem. 2012;54:17–26. doi: 10.1016/j.plaphy.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Yang X., Lu C. Photosynthesis is improved by exogenous glycinebetaine in salt-stressed maize plants. Physiol. Plant. 2005;124:343–352. doi: 10.1111/j.1399-3054.2005.00518.x. [DOI] [Google Scholar]
- 12.Shu S., Yuan L.-Y., Guo S.-R., Sun J., Yuan Y.-H. Effects of exogenous spermine on chlorophyll fluorescence, antioxidant system and ultrastructure of chloroplasts in Cucumis sativus L. under salt stress. Plant Physiol. Biochem. 2013;63:209–216. doi: 10.1016/j.plaphy.2012.11.028. [DOI] [PubMed] [Google Scholar]
- 13.Sairam R., Tyagi A. Physiology and molecular biology of salinity stress tolerance in plants. Curr. Sci. 2004;86:407–421. [Google Scholar]
- 14.Kong L., Wang M., Bi D. Selenium modulates the activities of antioxidant enzymes, osmotic homeostasis and promotes the growth of sorrel seedlings under salt stress. Plant Growth Regul. 2005;45:155–163. doi: 10.1007/s10725-005-1893-7. [DOI] [Google Scholar]
- 15.Elkhatib H., Elkhatib E., Khalaf Allah A., El-Sharkawy A. Yield response of salt-stressed potato to potassium fertilization: A preliminary mathematical model. J. Plant Nutr. 2004;27:111–122. doi: 10.1081/PLN-120027550. [DOI] [Google Scholar]
- 16.Kaya C., Higgs D., Sakar E. Response of two leafy vegetables grown at high salinity to supplementary potassium and phosphorus during different growth stages. J. Plant Nutr. 2002;25:2663–2676. doi: 10.1081/PLN-120015530. [DOI] [Google Scholar]
- 17.Kaya C., Higgs D. Calcium nitrate as a remedy for salt-stressed cucumber plants. J. Plant Nutr. 2002;25:861–871. doi: 10.1081/PLN-120002965. [DOI] [Google Scholar]
- 18.Tuna A.L., Kaya C., Ashraf M., Altunlu H., Yokas I., Yagmur B. The effects of calcium sulphate on growth, membrane stability and nutrient uptake of tomato plants grown under salt stress. Environ. Exp. Bot. 2007;59:173–178. doi: 10.1016/j.envexpbot.2005.12.007. [DOI] [Google Scholar]
- 19.Yang G., Ge K., Chen J., Chen X. Selenium-related endemic diseases and the daily selenium requirement of humans. World Rev. Nutr. Diet. 1988;55:98–152. doi: 10.1159/000415560. [DOI] [PubMed] [Google Scholar]
- 20.Allan C.B., Lacourciere G.M., Stadtman T.C. Responsiveness of selenoproteins to dietary selenium. Annu. Rev. Nutr. 1999;19:1–16. doi: 10.1146/annurev.nutr.19.1.1. [DOI] [PubMed] [Google Scholar]
- 21.Combs G.F. Selenium in global food systems. Br. J. Nutr. 2001;85:517–547. doi: 10.1079/BJN2000280. [DOI] [PubMed] [Google Scholar]
- 22.Combs G., Jr. Status of selenium in prostate cancer prevention. Br. J. Cancer. 2004;91:195–199. doi: 10.1038/sj.bjc.6601974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turakainen M., Hartikainen H., Seppänen M.M. Effects of selenium treatments on potato (Solanum tuberosum L.) growth and concentrations of soluble sugars and starch. J. Agric. Food Chem. 2004;52:5378–5382. doi: 10.1021/jf040077x. [DOI] [PubMed] [Google Scholar]
- 24.Terry N., Zayed A., De Souza M., Tarun A. Selenium in higher plants. Annu. Rev. Plant Biol. 2000;51:401–432. doi: 10.1146/annurev.arplant.51.1.401. [DOI] [PubMed] [Google Scholar]
- 25.Sors T.G., Ellis D.R., Na G.N., Lahner B., Lee S., Leustek T., Pickering I.J., Salt D.E. Analysis of sulfur and selenium assimilation in Astragalus plants with varying capacities to accumulate selenium. Plant J. 2005;42:785–797. doi: 10.1111/j.1365-313X.2005.02413.x. [DOI] [PubMed] [Google Scholar]
- 26.Dumont E., Vanhaecke F., Cornelis R. Selenium speciation from food source to metabolites: A critical review. Anal. Bioanal. Chem. 2006;385:1304–1323. doi: 10.1007/s00216-006-0529-8. [DOI] [PubMed] [Google Scholar]
- 27.Pilon-Smits E.A., Quinn C.F., Tapken W., Malagoli M., Schiavon M. Physiological functions of beneficial elements. Curr. Opin. Plant Biol. 2009;12:267–274. doi: 10.1016/j.pbi.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Germ M., Kreft I., Osvald J. Influence of UV-B exclusion and selenium treatment on photochemical efficiency of photosystem II, yield and respiratory potential in pumpkins (Cucurbita pepo L.) Plant Physiol. Biochem. 2005;43:445–448. doi: 10.1016/j.plaphy.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 29.Xue T., Hartikainen H., Piironen V. Antioxidative and growth-promoting effect of selenium on senescing lettuce. Plant Soil. 2001;237:55–61. doi: 10.1023/A:1013369804867. [DOI] [Google Scholar]
- 30.Djanaguiraman M., Devi D.D., Shanker A.K., Sheeba J.A., Bangarusamy U. Selenium—An antioxidative protectant in soybean during senescence. Plant Soil. 2005;272:77–86. doi: 10.1007/s11104-004-4039-1. [DOI] [Google Scholar]
- 31.Kamran M., Parveen A., Ahmar S., Malik Z., Hussain S., Chattha M.S., Saleem M.H., Adil M., Heidari P., Chen J.-T. An Overview of Hazardous Impacts of Soil Salinity in Crops, Tolerance Mechanisms, and Amelioration through Selenium Supplementation. Int. J. Mol. Sci. 2020;21:148. doi: 10.3390/ijms21010148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arvy M. Some factors influencing the uptake and distribution of selenite in the bean plant (Phaseolus vulgaris) Plant Soil. 1989;117:129–133. doi: 10.1007/BF02206265. [DOI] [Google Scholar]
- 33.Mazzafera P. Growth and biochemical alterations in coffee due to selenite toxicity. Plant Soil. 1998;201:189–196. doi: 10.1023/A:1004328717851. [DOI] [Google Scholar]
- 34.Hu Q., Xu J., Pang G. Effect of selenium on the yield and quality of green tea leaves harvested in early spring. J. Agric. Food Chem. 2003;51:3379–3381. doi: 10.1021/jf0341417. [DOI] [PubMed] [Google Scholar]
- 35.Munshi C.B., Mondy N.I. Glycoalkaloid and nitrate content of potatoes as affected by method of selenium application. Biol. Trace Elem. Res. 1992;33:121–127. doi: 10.1007/BF02784000. [DOI] [PubMed] [Google Scholar]
- 36.Pennanen A., Tailin X., Hartikainen H. Protective role of selenium in plant subjected to severe UV irradiation stress. J. Appl. Bot. 2002;76:66–76. [Google Scholar]
- 37.Malik J.A., Kumar S., Thakur P., Sharma S., Kaur N., Kaur R., Pathania D., Bhandhari K., Kaushal N., Singh K. Promotion of growth in mungbean (Phaseolus aureus Roxb.) by selenium is associated with stimulation of carbohydrate metabolism. Biol. Trace Elem. Res. 2011;143:530–539. doi: 10.1007/s12011-010-8872-1. [DOI] [PubMed] [Google Scholar]
- 38.Hartikainen H., Xue T. The promotive effect of selenium on plant growth as triggered by ultraviolet irradiation. J. Environ. Qual. 1999;28:1372–1375. doi: 10.2134/jeq1999.00472425002800040043x. [DOI] [Google Scholar]
- 39.Hartikainen H., Xue T., Piironen V. Selenium as an anti-oxidant and pro-oxidant in ryegrass. Plant Soil. 2000;225:193–200. doi: 10.1023/A:1026512921026. [DOI] [Google Scholar]
- 40.Seppänen M., Turakainen M., Hartikainen H. Selenium effects on oxidative stress in potato. Plant Sci. 2003;165:311–319. doi: 10.1016/S0168-9452(03)00085-2. [DOI] [Google Scholar]
- 41.Duke J.A. Handbook of Legumes of World Economic Importance. Springer; Berlin/Heidelberg, Germany: 1981. Legume species; pp. 5–310. [Google Scholar]
- 42.Sharma P., Sekhon H., Bains T. Performance and growth analysis in mash bean genotypes. World J. Agril. Sci. 2012;8:303–308. [Google Scholar]
- 43.Djanaguiraman M., Prasad P.V., Seppanen M. Selenium protects sorghum leaves from oxidative damage under high temperature stress by enhancing antioxidant defense system. Plant Physiol. Biochem. 2010;48:999–1007. doi: 10.1016/j.plaphy.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 44.Allen S.E., Grimshaw H.M., Parkinson J.A., Quarmby C. Chemical Analysis of Ecological Materials. Blackwell Scientific Publications; Hoboken, NJ, USA: 1974. [Google Scholar]
- 45.Turner N.C. Techniques and experimental approaches for the measurement of plant water status. Plant Soil. 1981;58:339–366. doi: 10.1007/BF02180062. [DOI] [Google Scholar]
- 46.Arnon D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Vos C., Schat H., De Waal M., Vooijs R., Ernst W. Increased resistance to copper-induced damage of the root cell plasmalemma in copper tolerant Silene cucubalus. Physiol. Plant. 1991;82:523–528. doi: 10.1034/j.1399-3054.1991.820407.x. [DOI] [Google Scholar]
- 48.Velikova V., Yordanov I., Edreva A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000;151:59–66. doi: 10.1016/S0168-9452(99)00197-1. [DOI] [Google Scholar]
- 49.Nayyar H., Gupta D. Differential sensitivity of C3 and C4 plants to water deficit stress: Association with oxidative stress and antioxidants. Environ. Exp. Bot. 2006;58:106–113. doi: 10.1016/j.envexpbot.2005.06.021. [DOI] [Google Scholar]
- 50.Change B., Maehly A. Assay of catalases and peroxidase. Methods Enzym. 1955;2:764–775. [Google Scholar]
- 51.Nakano Y., Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. [Google Scholar]
- 52.Foyer C.H., Halliwell B. The presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic acid metabolism. Planta. 1976;133:21–25. doi: 10.1007/BF00386001. [DOI] [PubMed] [Google Scholar]
- 53.Elia A.C., Galarini R., Taticchi M.I., Dörr A.J.M., Mantilacci L. Antioxidant responses and bioaccumulation in Ictalurus melas under mercury exposure. Ecotoxicol. Environ. Saf. 2003;55:162–167. doi: 10.1016/S0147-6513(02)00123-9. [DOI] [PubMed] [Google Scholar]
- 54.Jones M.G., Outlaw W.H., Lowry O.H. Enzymic assay of 10−7 to 10−14 moles of sucrose in plant tissues. Plant Physiol. 1977;60:379–383. doi: 10.1104/pp.60.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Feng R., Wei C., Tu S. The roles of selenium in protecting plants against abiotic stresses. Environ. Exp. Bot. 2013;87:58–68. doi: 10.1016/j.envexpbot.2012.09.002. [DOI] [Google Scholar]
- 56.Wu L., Huang Z.-Z., Burau R.G. Selenium accumulation and selenium-salt cotolerance in five grass species. Crop. Sci. 1988;28:517–522. doi: 10.2135/cropsci1988.0011183X002800030019x. [DOI] [Google Scholar]
- 57.Suárez N., Medina E. Salinity effects on leaf ion composition and salt secretion rate in Avicennia germinans (L.) L. Braz. J. Plant Physiol. 2008;20:131–140. doi: 10.1590/S1677-04202008000200005. [DOI] [Google Scholar]
- 58.Hawrylak-Nowak B. Beneficial effects of exogenous selenium in cucumber seedlings subjected to salt stress. Biol. Trace Elem. Res. 2009;132:259–269. doi: 10.1007/s12011-009-8402-1. [DOI] [PubMed] [Google Scholar]
- 59.Jiang C., Zu C., Lu D., Zheng Q., Shen J., Wang H., Li D. Effect of exogenous selenium supply on photosynthesis, Na+ accumulation and antioxidative capacity of maize (Zea mays L.) under salinity stress. Sci. Rep. 2017;7:42039. doi: 10.1038/srep42039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Parida A.K., Das A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005;60:324–349. doi: 10.1016/j.ecoenv.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 61.Ashraf M. Biotechnological approach of improving plant salt tolerance using antioxidants as markers. Biotechnol. Adv. 2009;27:84–93. doi: 10.1016/j.biotechadv.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 62.Mansour M. Plasma membrane permeability as an indicator of salt tolerance in plants. Biol. Plant. 2013;57:1–10. doi: 10.1007/s10535-012-0144-9. [DOI] [Google Scholar]
- 63.Sairam R., Srivastava G. Changes in antioxidant activity in sub-cellular fractions of tolerant and susceptible wheat genotypes in response to long term salt stress. Plant Sci. 2002;162:897–904. doi: 10.1016/S0168-9452(02)00037-7. [DOI] [Google Scholar]
- 64.Dogan M., Tipirdamaz R., Demir Y. Salt resistance of tomato species grown in sand culture. Plant Soil Environ. 2010;56:499–507. doi: 10.17221/24/2010-PSE. [DOI] [Google Scholar]
- 65.Van Raamsdonk J.M., Hekimi S. Superoxide dismutase is dispensable for normal animal lifespan. Proc. Natl. Acad. Sci. USA. 2012;109:5785–5790. doi: 10.1073/pnas.1116158109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405–410. doi: 10.1016/S1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- 67.Pukacka S., Malec M., Ratajczak E. ROS production and antioxidative system activity in embryonic axes of Quercus robur seeds under different desiccation rate conditions. Acta Physiol. Plant. 2011;33:2219. doi: 10.1007/s11738-011-0761-5. [DOI] [Google Scholar]
- 68.Hasanuzzaman M., Hossain M.A., Fujita M. Nitric oxide modulates antioxidant defense and the methylglyoxal detoxification system and reduces salinity-induced damage of wheat seedlings. Plant Biotechnol. Rep. 2011;5:353–365. doi: 10.1007/s11816-011-0189-9. [DOI] [PubMed] [Google Scholar]
- 69.Malik J.A., Goel S., Kaur N., Sharma S., Singh I., Nayyar H. Selenium antagonises the toxic effects of arsenic on mungbean (Phaseolus aureus Roxb.) plants by restricting its uptake and enhancing the antioxidative and detoxification mechanisms. Environ. Exp. Bot. 2012;77:242–248. doi: 10.1016/j.envexpbot.2011.12.001. [DOI] [Google Scholar]
- 70.Kaydan D., Yagmur M. Germination, seedling growth and relative water content of shoot in different seed sizes of triticale under osmotic stress of water and NaCl. Afr. J. Biotechnol. 2008;7:2862–2868. [Google Scholar]
- 71.Siddiqi E.H., Ashraf M. Can leaf water relation parameters be used as selection criteria for salt tolerance in safflower (Carthamus tinctorius L.) Pak. J. Bot. 2008;40:221–228. [Google Scholar]
- 72.Asfaw K.G. Effects of salinity on seedling biomass production and relative water content of twenty sorghum (Sorghum biolor L. Moench) accessions. Asian J. Agric. Sci. 2011;3:242–249. [Google Scholar]
- 73.Jamil M., Rha E.S. NaCl stress-induced reduction in grwoth, photosynthesis and protein in mustard. J. Agric. Sci. 2013;5:114. doi: 10.5539/jas.v5n9p114. [DOI] [Google Scholar]
- 74.Zhu M., Zhou M., Shabala L., Shabala S. Linking osmotic adjustment and stomatal characteristics with salinity stress tolerance in contrasting barley accessions. Funct. Plant Biol. 2015;42:252–263. doi: 10.1071/FP14209. [DOI] [PubMed] [Google Scholar]
- 75.Germ M., Kreft I., Stibilj V., Urbanc-Berčič O. Combined effects of selenium and drought on photosynthesis and mitochondrial respiration in potato. Plant Physiol. Biochem. 2007;45:162–167. doi: 10.1016/j.plaphy.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 76.Proietti P., Nasini L., Del Buono D., D’Amato R., Tedeschini E., Businelli D. Selenium protects olive (Olea europaea L.) from drought stress. Sci. Hortic. 2013;164:165–171. doi: 10.1016/j.scienta.2013.09.034. [DOI] [Google Scholar]
- 77.Abbas S.M. Effects of low temperature and selenium application on growth and the physiological changes in sorghum seedlings. J. Stress Physiol. Biochem. 2012;8:268–286. [Google Scholar]
- 78.Lu S., Li T., Jiang J. Effects of salinity on sucrose metabolism during tomato fruit development. Afr. J. Biotechnol. 2010;9:842–849. [Google Scholar]