Summary
Alcoholic hepatitis is an acute, inflammatory liver disease associated with high morbidity and mortality, both short and long term. Alcoholic hepatitis often arises from a background of chronic liver disease, characterized by rapid onset of jaundice and development of a myriad of complications. Current medical therapy for severe alcoholic hepatitis relies on corticosteroids, which have modest efficacy. Alcohol abstinence is of critical importance in alcoholic hepatitis, but recidivism is high. Due to the lack of efficacious medical treatments for alcoholic hepatitis and alcohol chemical dependency, there is a pressing need to develop new therapeutics. Backed by promising preliminary and preclinical studies, many clinical trials are currently ongoing in alcoholic hepatitis and are discussed further in this review.
Keywords: Alcoholic liver disease, alcohol use disorder, microbiome, gut-liver axis, severe alcoholic hepatitis, corticosteroids, STOPAH, pentoxifylline, antioxidant, regeneration, inflammation
Introduction
Excessive alcohol consumption is one of the leading causes of liver diseases and the seventh leading cause of premature death around the world (1). Alcohol abuse causes a spectrum of liver injuries collectively termed alcohol-associated liver diseases (ALD). ALD encompasses a range of histological findings, including steatosis, steatohepatitis and fibrosis. Symptomatic steatohepatitis or cirrhosis may present acutely, as an inflammation-driven condition termed alcoholic hepatitis. Alcoholic hepatitis is associated with high morbidity and mortality, especially when severe. The average 30-day mortality for severe alcoholic hepatitis patients may be as high as 17–50% (2, 3). Despite decades of research, there continues to be a clinical void in highly efficacious treatment for alcoholic hepatitis. In recent years, many novel therapeutics targeting the pathogenesis of alcoholic hepatitis have emerged, and some have successfully entered into clinical trials. In this review, we will systematically highlight the most promising novel therapeutics on the horizon.
Pathophysiology
Intense research efforts focused on elucidating mechanisms of liver injury in alcoholic hepatitis have greatly advanced our understanding of the pathophysiology of the disease [Figure 1]. Ethanol-induced liver damage occurs via both direct hepatocyte injury and inflammation (4). Metabolism of ethanol also changes the redox state of hepatocytes, which interferes with carbohydrate and lipid metabolisms contributing to hepatic steatosis (5). Alcohol increases hepatocyte vulnerability to free-radicals as a result of enzyme CYP2E1 induction, mitochondrial dysfunction, depletion of anti-oxidants storage, and recruitment of inflammatory cells (4). Chronic alcohol abuse, particularly when combined with malnutrition, often compound the effect of oxidative injury by further lowering cellular resilience to oxidative stress and depleting anti-oxidant storage (4, 5). Proteasome dysfunction also plays a role in exacerbating oxidative stress and cellular injury (4).
Figure 1: Pathophysiology of Alcoholic Hepatitis and Mechanisms of Action of Novel Therapeutic Agents.
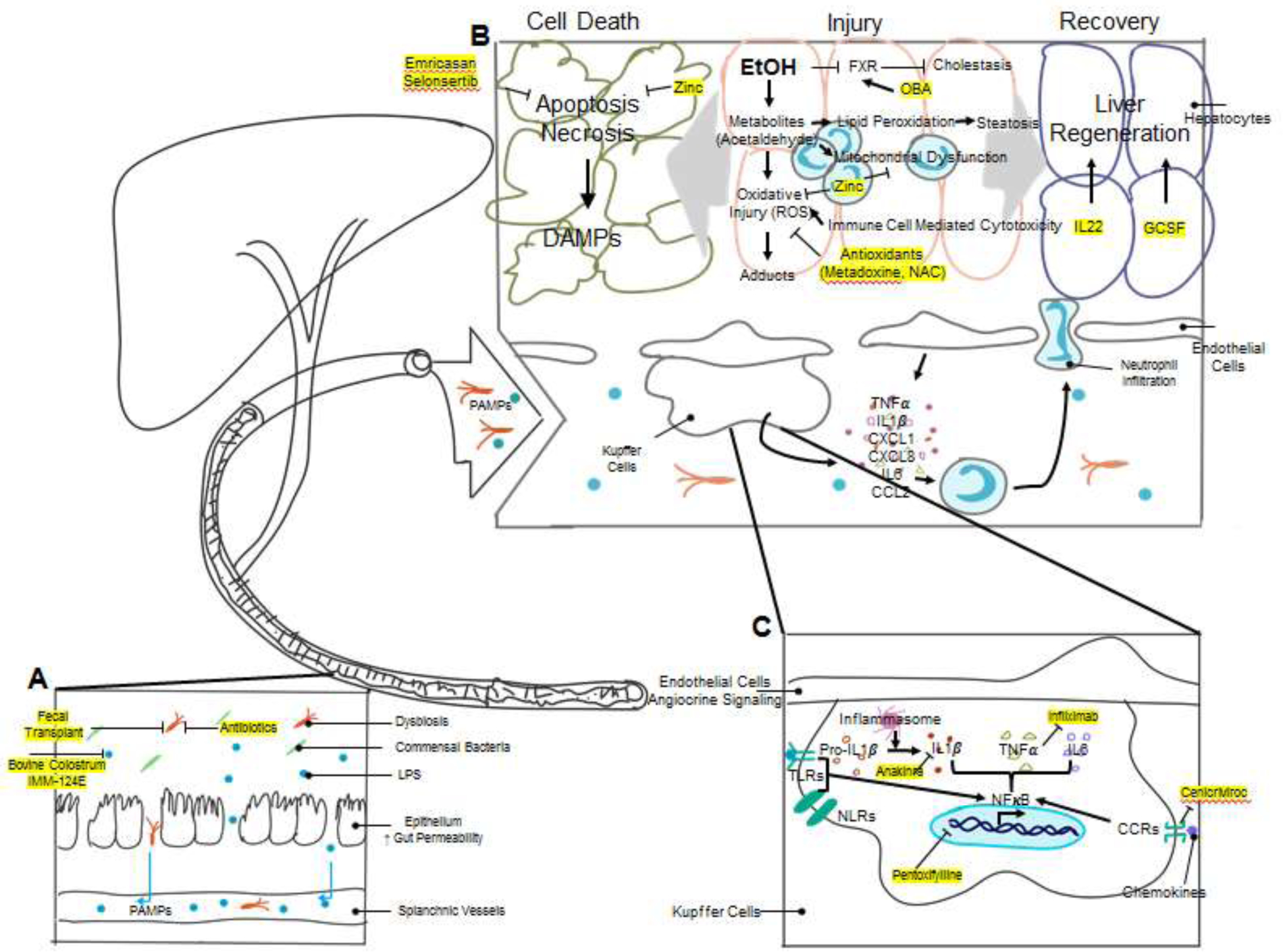
The pathogenesis of alcoholic hepatitis involves the interplay of multiple complex mechanisms. A. Chronic alcohol use causes changes in the gut microbiome composition (dysbiosis) and breakdown of gut barrier function. LPS and other bacterial products can potently activate the innate immune system and are collectively called Pathogen-Associated Molecular Patterns (PAMPs). Increased gut permeability allows for the translocation of bacteria and PAMPs to the liver via splanchnic vasculature. Multiple therapeutics, including antibiotics, fecal transplantation, bovine colostrum, and hyperimmunized bovine colostrum IMM-124E target dysbiosis and reduce endotoxemia. B. Excessive alcohol consumption leads to liver injury by multiple mechanisms. The toxic metabolites of ethanol, particularly acetaldehyde, cause direct hepatocyte oxidative injury as well as injury via formation of protein/DNA adducts. Ethanol metabolites also cause mitochondrial dysfunction and lipid peroxidation which leads to steatosis. Activated immune cells induce cell-mediated cytotoxicity by release of reactive oxygen species (ROS), further exacerbating oxidative injury. Antioxidants have been trialed in alcoholic hepatitis to attenuate oxidative stress. Zinc, in addition to being an antioxidant, is also protective against mitochondrial dysfunction and apoptosis. Cholestasis is another target for alcoholic hepatitis therapy. Obeticholic acid (OCA) is currently in trial as an agonist to the farnesoid X receptor (FXR), which has activity against bile synthesis. The injured liver has two different clinical outcomes, cell death and organ failure vs. liver regeneration and recovery. Hepatocyte injury activates apoptosis and necrosis pathways and releases Damage-Associated Molecular Patterns (DAMPs), which are cell derived molecules capable of activating the immune system. Emricasan and Selonsertib are two inhibitors to apoptosis signaling studied in alcoholic hepatitis. Liver injury also stimulates liver regeneration. Many cytokines, including TNFα and Interleukin-6 (IL-6), are potent activators of liver regeneration. Another cytokine, IL-22, has also been shown to stimulate liver regeneration. Growth colony stimulating factor (G-CSF) and its derivative pegylated G-CSF have shown promising results in multiple early clinical trials. C. The accumulation of DAMPs and PAMPs in the liver activates resident liver immune cells, particularly Kupffer cells, by activation of toll-like receptors (TLRs) and nucleotide-binding oligomerization domain-like receptors (NLRs). Receptor activation enhances NFκB signaling and results in expression of pro-inflammatory molecules, including IL-1β. Pro-IL-1β undergo cleavage by caspase-1, which is activated by the inflammasome complex, to become activated IL-1β. An inhibitor to IL-1β, Anakinra, is currently studied in clinical trials. TNFα inhibitors, including Infliximab, have been tested in clinical trials. Pentoxifylline is another extensively studied compound in the treatment of alcoholic hepatitis. It suppresses NFκB signaling and reduces the production of cytokines, including TNFα. Chemokine receptors, such as C-C chemokine receptors (CCRs), promote inflammatory signaling via stimulation of the NFκB pathway, and a CCR2/CCR5 inhibitor, Cenicriviroc, has been proposed as another target for alcoholic hepatitis therapy. Inflammatory cytokines and chemokines released by Kupffer cells and other liver cells recruit circulating immune cells such as neutrophils and monocytes and promote chemotaxis and infiltration into liver parenchyma. Immune cell infiltration causes secondary cell-mediated injury to the liver parenchymal cells by oxidative injury as described above.
Secondary damage on hepatocytes mediated by inflammatory cells also plays a central role in pathophysiology of alcoholic hepatitis. Chronic exposure can lead to increased gut permeability and elevated circulating pathogen products, such as LPS (lipopolysaccharide), which are also known as PAMPs (Pathogen Associated Molecular Patterns) (6). Ethanol injured hepatocytes release aseptic inflammatory mediators, or DAMPs (Damage Associated Molecular Patterns). DAMPs and PAMPs bind to pathogen-pattern receptors such as TLRs (Toll-like Receptors) and NLRs (Nucleotide-binding Oligomerization Domain-like Receptors) on immune cells and liver parenchymal cells and potently stimulate the innate immune response. This leads to dense neutrophil infiltrations which is a hallmark of alcoholic hepatitis (4, 6). Adaptive immune responses, mediated by B cells, T cells, and natural killer cells, also contribute to the storm of hepatic inflammation (7). Delineating the pathophysiological mechanisms of alcoholic hepatitis has provided many new targets for therapeutic targeting as discussed later in this review.
Current Diagnosis and Management of Alcoholic Hepatitis
While the gold-standard for diagnosing alcoholic hepatitis remains the liver biopsy, the diagnosis of alcoholic hepatitis is often made based solely on clinical and laboratory findings in the United States. This has led to significant heterogeneity among patients diagnosed with alcoholic hepatitis and added to the difficulty in clinical research of the disease. In an effort to standardize this diagnosis for clinical research, recent clinical trials on alcoholic hepatitis has mostly adopted the alcoholic hepatitis diagnostic criteria from National Institute of Alcoholism and Alcohol Abuse (NIAAA) consensus committee (8). According to this guideline, diagnosis of alcoholic hepatitis is made based on 1) alcohol use within 60 days of presentation; 2) presence of elevated liver enzymes with AST and ALT greater than 50 but less than 400 IU/L, with AST/ALT ratio of >1.5, 3) worsening jaundice, with bilirubin greater than 3, and 4) absence of any other causes of liver disease [Figure 2]. However, although clinical and laboratory information is usually sufficient to diagnose alcoholic hepatitis, liver biopsy is sometimes needed to discriminate from other causes of liver diseases as there can be other contributors to jaundice in alcoholic liver disease. Given presence of coagulopathy and ascites in many alcoholic hepatitis patients, transjugular approach to liver biopsy is sometimes preferred over percutaneous approach when liver biopsy is necessary. On histology, alcoholic hepatitis is characterized by neutrophil infiltration, steatosis, ballooning degeneration of hepatocyte, and Mallory-Denk bodies (9). Steatosis or Fibrosis may be present in patient with concomitant nonalcoholic steatohepatitis or cirrhosis. While alcoholic hepatitis can occur with or without cirrhosis, liver biopsies of alcoholic hepatitis patients demonstrated that a majority have clinically silent liver fibrosis or cirrhosis at the time of presentation with alcoholic hepatitis. Alcoholic hepatitis patients with underlying liver cirrhosis have worse prognosis compared to those without (10). Therefore, an evaluation for the presence of liver cirrhosis should be obtained at the time of alcoholic hepatitis diagnosis to aid prognostication and to direct subsequent follow-up. Other factors such as obesity, metabolic syndrome, hepatitis C infection, and genetic traits likely also contribute to individual susceptibility to alcoholic hepatitis (11). The presence of cofactors may lower the threshold level of alcohol. Expression of certain variants of genes such as PNPLA3, HSD17β13, TM6SF2, MBOAT7 etc. have been observed in patients with alcoholic hepatitis, and research in this area has the potential to uncover novel therapeutic targets (12).
Figure 2:
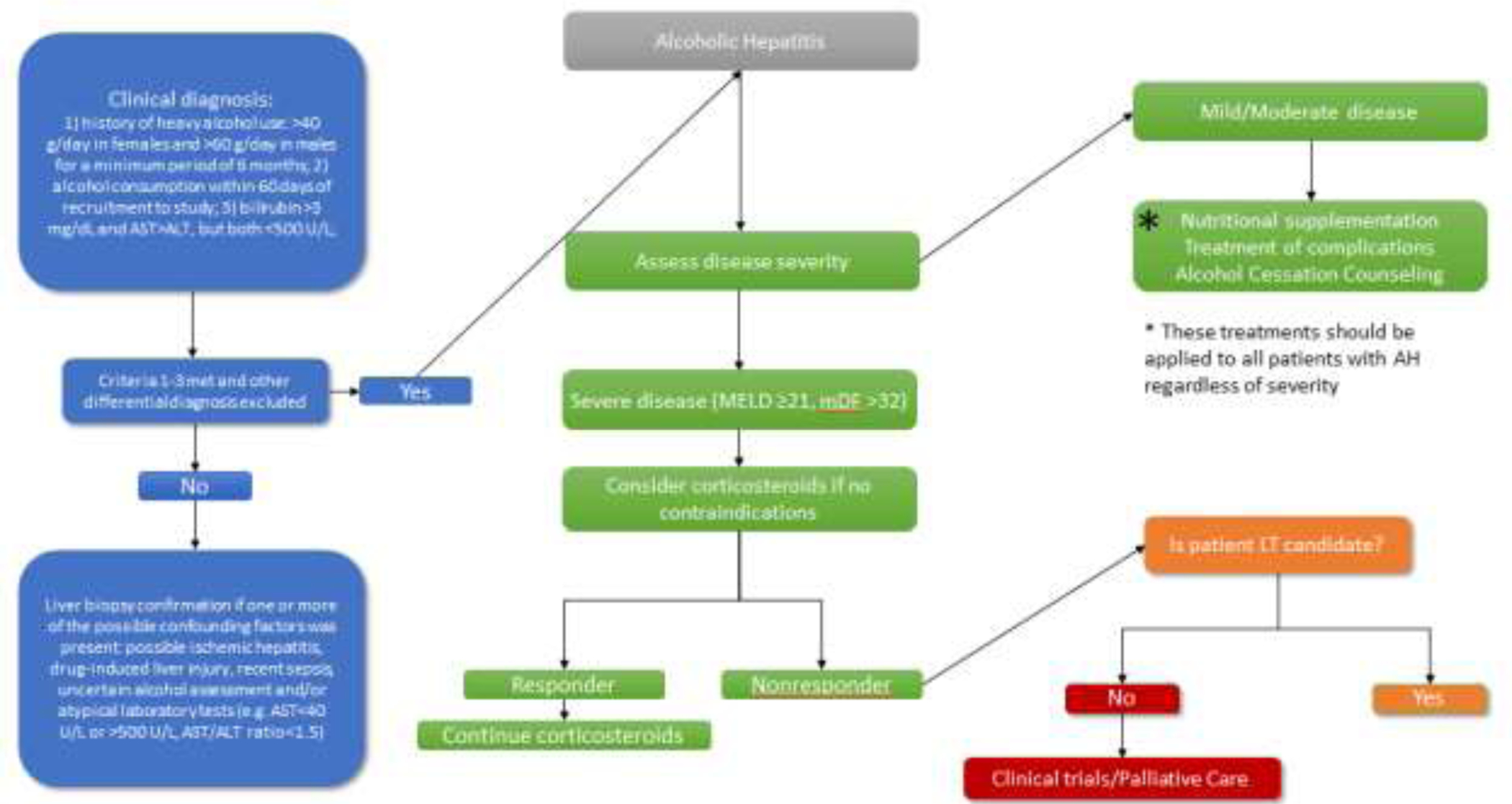
Current Clinical Treatment Algorithm for Alcoholic Hepatitis
Despite advantages of liver biopsy in diagnosing alcoholic hepatitis, its wide clinical use is limited by invasiveness, cost, and inaccuracies that arise from inter-observer and sampling errors. As systemic inflammatory symptoms in alcoholic hepatitis, including fever, leukocytosis, and abdominal pain, can be difficult to distinguish from signs of infection, another diagnostic challenge in alcoholic hepatitis patients is the detection of concomitant infections. To tackle issues associated with current diagnostic strategies there is a major drive in the field to discover novel and non-invasive biomarkers. Imaging and liquid biopsy based biomarkers, including but not limited to magnetic resonance elastography, FibroTest, extracellular vesicles, and peripheral leucocyte count have all shown promise as potential biomarkers that are currently being validated in multiple studies (13, 14). Some studies have utilized cytokine levels, cytokeratins and other serum metabolites for possible detection of infection but it still remains to be seen if they can provide disease specific information.
Disease stratification based on severity is another necessary step in the treatment of alcoholic hepatitis. Severity of alcoholic hepatitis greatly impacts prognosis and treatment options. A Maddrey’s Discriminant Function (mDF) score ≥32 and/or Model for End-Stage Liver Disease (MELD) score ≥21 have been most commonly used as cutoffs to identify those with severe disease.
Treatment algorithms differ based on disease severity stratification (15). Mild to moderate alcoholic hepatitis is generally managed conservatively with alcohol cessation and monitored for development of complications [Figure 2]. Mortality risk increases significantly in patients with severe alcoholic hepatitis, but few pharmacotherapies are available in addition to alcohol cessation. For decades, corticosteroids have been used for the treatment of severe alcoholic hepatitis, relying on their potent systemic anti-inflammatory effects. Recent meta-analysis of multiple clinical trials that included data from the landmark STOPAH (Steroids or Pentoxifylline for Alcoholic Hepatitis) trial has concluded that corticosteroids improve only short-term outcomes in severe alcoholic hepatitis and only modestly (16). Furthermore, the use of corticosteroids is restricted to patients in whom infection has been ruled out, which eliminates a significant proportion of alcoholic hepatitis patients. Finally, corticosteroids are only effective in a subset of patients and have little impact on long term survival. The Lille score is a commonly used clinical calculator to identify the patients most likely to respond to corticosteroid therapy after a 7-day trial. In patients whose Lille score is >0.45 after 7 days of corticosteroid therapy, further corticosteroids may lead to worse outcomes and thus is generally discontinued (17).
Liver transplantation is another effective treatment for alcoholic hepatitis. Clinical studies have shown improved outcomes with early liver transplantation in patients who do not respond to corticosteroids (18). However, liver transplantation for treatment of alcoholic hepatitis raises several concerns. Liver transplantation is a scarce resource and thus by default cannot be generalized to all patients with severe alcoholic hepatitis who may potentially benefit. In addition, recidivism after transplantation is a significant concern as alcoholic hepatitis patients often cannot complete alcohol rehabilitation and counseling programs generally required for others with alcoholic cirrhosis which generally requires an abstinent period of 6- months prior to liver transplant. Utilization of liver transplantation in the treatment of alcoholic hepatitis is an actively-evolving area as an increasing number of transplant centers are considering acute alcoholic hepatitis patients in their transplant programs. In patients who have undergone liver transplantation for alcoholic hepatitis, a multidisciplinary approach is needed for close monitoring. In addition to routine post-transplant care with surveillance and treatment of infections, renal injury, cardiovascular disease, osteoporosis, and de novo malignancies, patients undergoing liver transplantation for alcoholic hepatitis should receive alcohol-use disorder treatment to prevent post-transplant relapse (19, 20).
Regardless of severity, all patients with alcoholic hepatitis should be counseled to cease alcohol use completely. Inpatients and outpatient supervised counseling programs are available and widely used to assist with alcohol cessation. Pharmaceutical agents that treat alcohol dependency have also been explored. Agents such as acamprosate, naltrexone, disulfiram, topiramate, and baclofen have been shown in small trials to improve rates of cessation (21, 22). However, clinical utilization of these medications is limited and further research in the area of chemical dependency is greatly needed.
Novel therapeutics for treatment of severe alcoholic hepatitis
Many clinical trials are currently ongoing evaluating novel therapeutics in alcoholic hepatitis that are pathophysiologically relevant and have proven to be successful in pre-clinical studies. The major focus of this review is to highlight some of these emerging therapies and how these novel agents may possibly impact clinical care for treatment of alcoholic hepatitis [Figure 1] and [Table 1].
Table 1:
Highlighted Clinical Trials of Novel Therapeutic Agents for the Treatment of Alcoholic Hepatitis
| Therapy | Mechanism | Administration | Trial Design | Inclusion Criteria | Study Size | Primary Endpoint | Trial Status | Location | Clinical Trial ID# | Published Results | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Antibiotics | |||||||||||
| Rifaximin | Gutsterilzation | Combination with steroids | Open label, single arm | mDF ≥ 32 | 19 pts | Bacterial infections after 3-mo | Phase 2 Completed | Multicenter, Spain | NCT02116556 | No change in rate of infection | |
| Vancomycin, Gentamycin and Meropenem | Gutsterilzation | Intervention | Open label, single arm | AH | 15 pts | Macrophage activation | Completed | Single center, Denmark | NCT03157388 | Unknown | |
| Augmentin | Gut sterilzation | Combination with steroids; vs. Placebo | RCT, double blinded | mDF ≥ 32 or MELD ≥21 | 280 pts | 2-mo survival | Phase 3 Recruiting | Multicenter, France | NCT02281929 | ||
| Ciprofloxicin | Gutsterilzation | Combination with steroids; vs. Placebo | Open label, randomized | Severe AH | 22 pts | 1-, 3-, and 6-mo survival | Phase 1 Completed | Single center, Finland | NCT02326103 | Unknown | |
| Endotoxemia | |||||||||||
| Bovine Colostrum | Decrease LPS | 1. Combination with steroids | 2. Intervention; vs. Placebo | RCT, double blinded | 1.mDF > 54 | 2. DF>32 or MELD >=21 | 1. 10 | 2.111 pts | 1. Change in mDf after 8 weeks | 2. 3 mo survival | 1. Phase 2 Completed | 2. Phase 3. Recruiting | Single Center, India | 1.NCT02265328 | 2. NCT02473341 | 1. Improved liver function | |
| IMM 124-E | Decrease LPS | Intervention; vs. Placebo | RCT. double blinded | 20<= MELD <=28 | 56 pts | Circulating endotoxin level at 7-mo | Phase 2 Completed | Multicenter, US | NCT01968382 | Unknown | |
| Gut Microbiome | |||||||||||
| Lactobacillus subtilis/Streptoco ecus faecium | Modify gut microbiome | Intervention; vs. Placebo | RCT, double blinded | mDF ≥ 32 | 117 pts | LPS level | Phase 4 Completed | Single Center, Korea | NCT01501162 | LPS level decreased | |
| Lactobacillus Rhamnosus GG | Modify gut microbiome | Intervention; vs. Placebo | RCT, double blinded | AH, MELD <21 | 130 pts | Change in MELD at 1-mo | Recruiting | Multicenter, US | NCT01922895 | ||
| Fecal Microbiota Transplantation | Modify gut microbiome | Intervention; vs. Steroids | RCT | Severe AH | 1.8; 2. 130 pts | Survival at 3 months | NA | Single center, India | 1. NCT03091010; 2. NCT03091010 | 1. Improved survival at 1 year | |
| IL-1 Inhibitor | |||||||||||
| Canakinumab | IL-1 Inhibition | Intervention; vs. Placebo | RCT, double blinded | mDF ≥ 32 and MELD ≤25 | 52 pts | Histological improvement at 1-mo | Phase 2 Recruiting | Multiple Sites London | NCT03775109 | ||
| Anakinra, Pentoxifylline, and Zinc | IL-1 Inhibition (Anakinra) | Intervention; vs. Steroids | RCT, double blinded | mDF ≥ 32 and MELD ≥21 | 104 pts | 6-mo survival | Phase 2/3 Completed | Multicenter, US | NCT01809132 | No change in survival | |
| Liver Regeneration | |||||||||||
| IL-22 (F-652) | Liver regeneration | I ntervention | Open label, single arm | 11<= MELD <=28 | 24 pts | Safety and toleratability | Phase 2a Completed, Phase 2b pending | Single center, US (Phase 2b Multicenter US) | NCT02655510 | Well tolerated, decrease in MELD | |
| GCSF | Liver regeneration | 1–2. Combination with pentoxifylline | 3. Combination with steroids; vs. Placebo 4. Intervention; vs.Placebo | 5. Combination with either steroids or pentoxifylline | 1–3. Open label, randomized, placebo controlled | 4–5. RCT, double blinded | 1–2. Inpatient severe AH | 3. mDF≥32 and Lille >0.16 | 4. Steroid Nonresponder, Lille >0.45 | 5. mDF≥32 | 1.46 | 100 | 268 | 33 nonresponders | 78 pts | 1–2. 3-mo survival | 3. 2-mo survival | 4.1-mo survival | 5. 3-mo survival | 1. Phase 4 Completed | 2–3. Phase 4 Recruiting | 5. Phase 2 Recruiting | 1–2,4. Single Center, India | 3. Multicenter, Korea, 5. Multicenter, US | 1. NCT03703674 | 3. NCT02442180 | 4. NCT01820208 | 5. NCT02776059 | 1. Increased survival 4. No change in 30 day mortality, but increased survival at 90 days | |
| GCSF+NAC | Liver regeneration | Combination with pentoxifylline; vs. GCSF alone vs. placebo | Open label, randomized, placebo controlled | Inpatient severe AH | 57 pts | 3-mo survival | Phase 4 Complete | Single center, India | NCT02971306. | Increased survival in GCSF alone and GCSF+NAC | |
| Antioxidants | |||||||||||
| NAC | Antioxidant | Combination with steroids; vs. Steroid alone | RCT, double blinded | Biopsy proven AH and mDF≥32 | 180 pts | 6-mo survival | Phase 3 Completed | Multicenter, France | NCT00863785 | No change in survival at 6-mo, increased survival at 1-mo | |
| NAC + enteric nutrition | Antioxidant | Intervention, with enteral nutrition | Open label | Biopsy proven AH and mDF≥32 | 52 pts | 6-mo survival | Phase 3 Completed | Single center, Belgium | NCT00962442 | No change in survival | |
| Metacloxine | Antioxidant | 1. Combination with steroids; vs Steroids alone | 2. Combination with steroids/pentoxyfyll in | Open label, randomized | mDF≥32 | 1.70 | 2. 135 pts | 1.1- and 3-mo survival | 2. 3- and 6-mo survival | Phase 4 Completed | Single center, Mexico | 1. NCT02161653. | 2. NCT02161653. | Improved survival | |
| Omega 5 Fatty Acid | Antioxidant | Combination with steroids; vs. Steroids alone | RCT. double blinded | mDF≥32 | 40 pts | 30 Day Survival | Recruiting | Multiple Sites, Mexico | NCT03732586 | ||
| Supplemental Nutrition | |||||||||||
| Intensive Enteral Nutrition | Nutrition | Tube Feeding; vs. Usual meals | RCT | mDF≥32 | 136 pts | 6-mo survival | Completed | Multicenter, Belgium and France | NCT01801332 | No change in survival | |
| Apoptosis | |||||||||||
| Selonsertib (GS-4997) | ASK-1 inhibitor | Combination with steroids; vs. Steroids alone | RCT, double blinded | Biopsy proven severe AH | 104 pts | Safety and toleratability | Phase 2 Completed | Multicenter, Multinational | NCT02854631 | No difference in safety, mortality, Lille response, and changes in MELD | |
| Emricasan (IDN-6556) | Pan-caspase inhibitor | Intervention; vs. Placebo | RCT, double blinded | 20< MELD <35 | 5 pts | 1-mo survival | Phase 2 Terminated | Multicenter, US | NCT01937130 | Termination due to high systemic drug levels | |
| Bile Induced Injury | |||||||||||
| ObeticholicAcid | Synthetically modified bile acid | Intervention; vs. Placebo | RCT, double blinded | 11< MELD <20 | 19 pts | Safety and toleratability; Change in MELD at 6wk | Phase 2 Completed | Multicenter, US | NCT02039219 | Unknown | |
| Lipid Metabolism | |||||||||||
| DS102 | Bioactive lipid | Intervention; vs. Placebo | RCT, double blinded | Probable or definite AH | 120 pts | Change in MELD at 1-mo | Phase 2 Not yet recruiting | NCT03452540 | |||
| DUR-928 | Small molecule epigenetic regulator | Dose escalation | Open label | 11<= MELD <=30 | 36 pts | Safety and toleratability | Phase 2 Recruiting | Multicenter, US | NCT03432260 | ||
Anti-inflammatory Agents
Alcoholic hepatitis is a highly inflammatory condition involving complex crosstalks among various signaling pathways at the cellular level and multiple organs at the macro level. Liver Kupffer cells are believed to play an important role in instigating this inflammatory response. LPS-TLR4 binding on Kupffer cells leads to the activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) signaling and expression of a slew of NFκB regulated genes (23). Among them are pro-inflammatory cytokines and chemokines, including TNFα and Interleukin-6 (IL-6). Interleukin-1 (IL-1) is also produced in precursor form downstream of NFκB signaling. Pro-IL-1β is activated and secreted upon cleavage with caspase 1, which gains function via recruitment to a multi-protein complex termed the inflammasome (24). TNFα release can be further amplified by a self-reinforcing positive feed-back loop in Kupffer cells, and TNFα signaling results in upregulation of chemokines, including C-C chemokine ligand 2 (CCL2) and C-X-C motif ligand 1 (CXCL1), leading to the recruitment of macrophages and neutrophils in propagation of liver inflammatory responses (6, 25).
Given these observations, anti-inflammatory agents have been attractive therapeutic targets (26). Unfortunately, despite the clinical success of anti-TNFα agents in treatment of autoimmune diseases, TNFα inhibitors Infliximab and Etanercept failed in clinical trials for treatment of alcoholic hepatitis [Figure 1]. The use of TNFα inhibitors was associated with increased risk of infection and poor clinical outcomes (27, 28). TNFα plays an important role not only in inflammation but also in hepatic regeneration. It was hypothesized that the suppression of TNFα-mediated liver regeneration combined with profound immunosuppression may have led to poor outcomes. Pentoxifylline, a nonselective phosphodiesterase (PDE) inhibitor, is another agent that has been extensively studied in treatment of alcoholic hepatitis. Pentoxifylline increases cellular cAMP, which has been shown to decrease TNFα and other pro-inflammatory cytokine production in immune cells. A multitude of clinical trials have been carried out with pentoxifylline either alone or in combination with steroids in the treatment of alcoholic hepatitis, and results have been conflicting. Initial clinical trials on pentoxifylline suggested a mortality benefit in severe alcoholic hepatitis compared to placebo, and its benefit seemed to be related to prevention of hepatorenal syndrome (29). However, pentoxifylline was shown to be inferior to steroids in a head-to-head trial of severe alcoholic hepatitis patients (30), and pentoxifylline rescue was ineffective in steroid nonresponders (31). The effects of pentoxifylline alone and in combination with prednisolone were examined in the STOPAH trial, and despite lower incidence of hepatorenal syndrome, pentoxifylline failed to show mortality benefit (16). Given these results, clinical practice has moved away from use of pentoxifylline in severe alcoholic hepatitis.
Another cytokine, IL-1β, has also been studied as a therapeutic target for alcoholic hepatitis treatment. In preclinical models, IL-1 receptor antagonists were shown to attenuate liver injury caused by alcohol (32). Anakinra, an IL-1 receptor antagonist, has been studied in clinical trials in combination with zinc sulfate and pentoxifylline compared to corticosteroids in patients with severe alcoholic hepatitis. This trial did not meet statistical significance in mortality benefit but showed a treatment effect in favor of the combination group (33). Another NIAAA funded multicenter, randomized clinical trial examining the combination of Anakinra, zinc, and G-CSF is set to begin recruitment soon. More narrowly targeted and selective anti-inflammatory agents such as chemokine receptor antagonists have been used in preclinical setting with some success. Cenicriviroc, a CCR2/CCR5 receptor antagonist, was recently shown to reduce steatosis and fibrosis in a Phase II cohort with Nonalcoholic Steatohepatitis (NASH) (34). Clinical trial for treatment of alcoholic hepatitis with Cenicriviroc is needed to explore its applicability to alcoholic hepatitis.
Gut-Liver axis dysfunction and dysbiosis
Recent advances in our understanding of pathophysiology of alcoholic hepatitis have demonstrated that gut-liver axis dysfunction, particularly microbial dysbiosis and impaired gut barrier function, plays a significant role in development of liver diseases (35, 36). Gut permeability increases after alcohol binge or chronic consumption and grants intestinal lumen products access to the portal circulation, leading to the propagation of inflammation and cell injury (37, 38). Lipopolysaccharide (LPS), a component of gram-negative bacterial cell wall, and other PAMPs have been well documented as perpetuating agents in this process (39). PAMPs activate resident Kupffer cells in the liver through activation of TLR4 (40), and preclinical studies have shown attenuation of alcohol induced liver damage by inhibiting binding of endotoxin to these Kupffer cells (41). Thus, gut-liver axis derangement is being increasingly recognized as an important driver of alcohol induced liver damage.
Recognition of gut-liver axis dysfunction in alcoholic hepatitis has led to the development of many potential therapeutics over the last two decades. A good example of gut endotoxins targeted therapy is the use of bovine colostrum, which is rich in immunoglobulins and antimicrobials thought to decrease porto-systemic endotoxemia (42). A hyperimmune bovine colostrum, IMM 124-E is further enriched for anti-LPS IgG antibodies and shown to alleviate liver injury in a clinical trial in NASH patients, possibly through its modulation of natural killer and T cells function (43). There are multiple trials underway to study the benefit of bovine colostrum in alcoholic hepatitis, including a well- designed phase II placebo-controlled RCT (Randomized Controlled Trial) currently underway studying the use of IMM-124E in alcoholic hepatitis [Table 1].
Impaired gut barrier function and dysbiosis leads not only to endotoxin but also bacterial translocation [Figure 1]. Combined with immune dysfunction present in alcoholic hepatitis, severe alcoholic hepatitis patients are at significantly elevated risk of infections, as high as 42% incidence of infections in severe alcoholic hepatitis patients within 90 days of the diagnosis (44). This risk is further exacerbated by corticosteroids treatment (44). These observations prompted many clinical trials exploring the possibility of empiric antibiotics or probiotics as treatments for alcoholic hepatitis. A small pilot study with the prophylactic use of rifaximin showed a trend toward benefit compared to control (45). Currently, multiple large trials are underway studying the prophylactic use of a variety of antibiotics including Augmentin, Ciprofloxacin, Vancomycin, Gentamycin, and Meropenem. The use of probiotics has been evaluated as well with promising preliminary data. Murine studies showed benefit with VSL3 and lactobacillus rhamnosus GG (46). A RCT is now underway at NIAAA studying the use of Lactobacillus rhamnosus in alcoholic hepatitis. Fecal transplantation, which has shown great potential in treating gut dysbiosis, was also examined in open label trials with promising results, showing a mortality benefit despite very small sample size (36, 47). Given these early positive trends, further studies targeting gut microbiome in alcoholic hepatitis are hotly anticipated.
Anti-oxidants
Multiple modes of cellular injury in alcoholic hepatitis result in hepatocyte oxidative injury, including toxic ethanol metabolites, inflammatory cell-mediated cytotoxicity, as well as mitochondrial and proteasome dysfunctions. Given the central role of oxidative injury in the pathogenesis of alcoholic hepatitis, the use of anti-oxidants in alcoholic hepatitis has garnered much attention [Figure 1]. Unfortunately, as a group, anti-oxidants have largely failed to demonstrate a significant mortality benefit although a few treatments showed promise. N-acetylcysteine (NAC) is an anti-oxidant widely used in the treatment of acute liver injury. A randomized control trial of NAC infusion in combination with prednisolone showed mortality benefit at 30 days compared to prednisolone alone, but this effect was not seen at 3 months, which was the primary endpoint (48). Another study that combined the use of NAC and GCSF failed to show improvement of outcome beyond GCSF alone (49). Another example of antioxidant used to treat alcoholic hepatitis with promising trend is metadoxine, which is thought to increase hepatic glutathione concentrations. Metadoxine used in combination with steroid or pentoxifylline improved survival at 3 and 6 months in small open labeled trials (50). Interestingly, patients receiving metadoxine attained higher rate of sobriety in this trial, an effect that has also been observed in other retrospective analyses on alcohol dependency (51). If confirmed in larger studies, the dual antioxidative and abstinence-maintenance properties of metadoxine along with a very favorable side-effect profile, make it an attractive option for alcoholic hepatitis treatment.
Apoptosis and Liver Regeneration
The balance of signals promoting cell death and adaptive survival determines the presence of liver failure in alcoholic hepatitis. Hepatocyte death is induced by alcohol via apoptosis and necrosis processes, involving the mitochondrial pathway, caspase-dependent pathway, or ER stress (52). Caspases are a group of 11 intracellular cysteine proteases that are involved in apoptosis, profibrotic gene expression, and activation of IL-1β upon association with the inflammasome (24). Emricasan is an oral small-molecule caspase protease inhibitor currently in clinical trials. After promising preclinical studies, it was brought into trials for multiple liver diseases including alcoholic hepatitis (53). Emricasan was shown to improve liver function in patients liver fibrosis (54), but unfortunately, a recent phase II trial in severe alcoholic hepatitis patients was terminated due to concerns about liver toxicity. Another oral apoptosis inhibitor, apoptosis signal regulating kinase-1 (ASK-1) inhibitor, Selonsertib (GS-4997) was found to be safe but failed to improve mortality or liver function compared to steroids (55).
The counterbalance to cell death in alcoholic hepatitis is liver regeneration, which is the mechanism involved in recovery of liver function after toxic insults. Preclinical studies have implicated NF-kB, STAT3 and downstream pathways in the regeneration process (56, 57). TNFα is an important player to maintain an equilibrium between proliferation, survival and apoptosis as it can induce different responses depending on the cellular context (58). Interleukin-22 (IL-22) is a promising novel therapeutic agent from the IL-10 family that has been shown in preclinical studies as having anti-apoptosis, anti-inflammation, anti-steatosis and proliferative effects (59, 60). Preliminary data from an open-label, small cohort study with IL-22 showed efficacy signals and good safety profile in treatment of alcoholic hepatitis patients (61). A follow up phase IIb RCT on IL-22 is in preparation [Table 1]. Granulocyte-colony stimulating factor (G-CSF) is another promising therapeutic agent being evaluated in clinical trials [Table 1]. G-CSF was shown to mobilize hematopoietic stem cells and stimulate the proliferation of liver progenitor cells in animal models of acute liver failure (62). The activation of hematopoietic stem cells may assist with the recovery of immune dysfunction, and the proliferation of liver progenitor cells was thought to mediate liver regeneration. Several small clinical studies in India showed mortality benefit with G-CSF (63–65). Multiple clinical trials in Western populations are underway, and results are eagerly anticipated.
Bile metabolism has also been the target of research in alcoholic hepatitis treatment. Alcoholic hepatitis is characterized by cholestasis, and it has been hypothesized that Farnesoid X receptor (FXR) agonist obeticholic acid (OCA) protects hepatocytes against bile toxicity (62). OCA has shown promising results in treating chronic cholangiopathies (66, 67). Results from a recent phase II study looking at safety and efficacy is complete but unpublished as of now.
Supplemental Nutrition
Macro- and micronutrient malnutrition is a common feature in alcoholic hepatitis (68, 69). Decreased oral intake, decreased gut absorption and a hypermetabolic state perpetuate nutritional deficiencies in alcoholic hepatitis. Although the ideal nutrition intake goals for alcoholic hepatitis have not been studied in clinical trials, low nutritional intake, less than 21.5 kcal/kg/day, was associated with worse outcomes in severe alcoholic hepatitis (70). Current guidelines generally recommend daily protein intake of 1.2–1.5 g/kg/day and caloric intake of 30- 40 kcal/kg/day in alcoholic hepatitis patients (70, 71). Data on the role of intensive enteric feeding has been conflicting. An early Veteran’s Affair’s (VA) hospital prospective study showed improved liver function with supplemental enteric feeding via nasogastric tube (72). However, a recent European multicenter RCT failed to show mortality benefit with tube-feeding supplementation (73). This study suffered from a high rate of discontinuation of intervention in the nasogastric feeding arm, with 3 cases of aspiration pneumonia which led to 1 fatality. These results raised a valid concern of using nasogastric tube feeding in this patient population with a high incidence of encephalopathy. Total parenteral nutrition has also been proposed for the treatment of alcoholic hepatitis, but concerns regarding complications, particularly increased risk of infections and liver injury, limit its adaptation. Aside from protein-calorie malnutrition, deficiencies in many vitamins and minerals may impede recovery as well (70). Zinc is a trace element, and its deficiency is very common in patients with chronic alcohol use and has been shown to increase gut permeability and promote apoptosis (74). Zinc deficiency in endoplasmic reticulum and mitochondria has been shown to activate intrinsic cell death signaling in response to ethanol injury, which could not be effectively rescued by antioxidant treatment (74). A RCT studying the effects of combination therapy with zinc sulfate, pentoxifylline and Anakinra showed a trend toward decreased mortality as discussed above [Table 1]. It is of course difficult to draw conclusions about an individual intervention in a combination treatment group, but given the benign nature of the intervention, alcoholic hepatitis patients with macro and micronutrient deficiencies should undergo replacement. Empiric replacement of micronutrients may be appropriate as well depending on the cost of testing. Future trials addressing optimal strategies in treatment of macro- and micronutrient malnutrition in alcoholic hepatitis will help to direct clinical practice.
Alcohol use disorder (AUD)
Clinical guidelines from multiple international societies have recognized importance of treatment of AUD in alcoholic liver disease (71, 75). Alcoholic hepatitis diagnostic criteria include description of heavy alcohol use, which requires a background of more than 40–60 g of alcohol per day for a period of 5 years or more with heavy drinking for more than 6 months at least 2 months prior to diagnosis (8). Patients with alcoholic hepatitis by definition has AUD. Concern of recidivism has historically precluded these patients from being considered for liver transplantation, though recent data have had a positive trend on this practice (18).
Abstinence is a clear goal for the treatment of alcoholic hepatitis, and alcoholic hepatitis patients with relapsed alcohol use suffer from high mortality (76). Although there is no standard of care pharmaceuticals in maintenance of alcohol sobriety, research is ongoing in this area (76). Disulfiram, acamprosate, naloxone, naltrexone, baclofen, topiramate, gabapentin and sertraline have been associated with benefit in AUD (21); however, none have been definitively tested in ALD or alcoholic hepatitis patients. Indeed, many of these agents have a black box warning of associated hepatotoxicity. A RCT with alcoholic cirrhosis patients who were given baclofen showed 71% achievement and maintenance of abstinence in the treatment arm compared to 29% in the placebo group (77). However, the follow-up period was short and larger trials are needed to establish benefit in mortality. Another, single-center, open, retrospective study showed 97% maintenance of abstinence at 12 months in alcoholic hepatitis patients on Baclofen (78). Even though Baclofen has been approved in some countries and has received conditional recommendation in clinical guidelines, it is still not FDA approved for treatment of AUD due to low level of evidence. There is also some conflicting evidence regarding safety of baclofen (79). There is potential for treatment of AUD to positively impact not just outcomes of clinical episodes of alcoholic hepatitis but also outcomes post-transplantation. There is a notable lack of data regarding pharmacological therapy use in the post-liver transplantation population.
Alcohol rehabilitation is associated with decreased readmission, relapse and mortality in alcoholic hepatitis patients (80). Cognitive behavioural therapy, motivational interviewing, supportive therapy and psychoeducation are important treatment methodologies. Unfortunately, of date, no psychosocial intervention is able to consistently reduce relapse in alcoholic liver disease patients (81).
Conclusion
Conducting well designed and controlled clinical trials has traditionally proven to be difficult in alcoholic hepatitis due to numerous barriers. This situation has improved owing to relative standardization of study designs which has led to an exponential growth in ongoing registered trials. Currently novel therapeutics targeting oxidative damage, inflammation, apoptosis, reduced regenerative capacity, alcohol use disorder, dysbiosis and gut-liver axis dysfunction are being tested in alcoholic hepatitis. These therapeutics, whether standalone or in combination, hold tremendous potential to improve outcomes for these patients. Randomized clinical trials and further systematic analyses of results will dictate their acceptance and integration into routine clinical practice.
Search Strategy and Selection Criteria
References for this Review were identified through searches of PubMed with combinations of the search terms “alcoholic hepatitis”, “alcoholic liver disease”, “treatment” and “therapy” from inception until 14th June, 2019. Articles were also identified through searches of the authors’ own files as well as going through reference lists of reviews published in the past 10 years for identifying further relevant references. Only papers published in English were reviewed. The final reference list was generated on the basis of originality and relevance to the broad scope of this Review. We also searched abstracts from major international meetings in the field of gastroenterology and hepatology, such as The Liver Meeting (American Association of the Study of the Liver), Digestive Diseases Week, and the International Liver Congress (European Association for the Study of the Liver), held in the past 2 years. Clinicaltrials.gov was the source of information about relevant clinical trials.
Financial Support
This work was supported in part by National Institutes of Health (NIH) grants to Prof. V.H.S. including R37 AA021171 and R01 DK59615.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
All authors declared no conflict of interest for the work under consideration for publication. Outside submitted work, Prof. V.H.S. reported personal fees from Novartis Pharmaceuticals, Merck Research Laboratories, Afimmune, Ltd., Durect Corporation, Enterome SAB, and Vital Therapies.
References
- 1.Alcohol use and burden for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2018;392(10152):1015–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mathurin P, Duchatelle V, Ramond MJ, Degott C, Bedossa P, Erlinger S, et al. Survival and prognostic factors in patients with severe alcoholic hepatitis treated with prednisolone. Gastroenterology. 1996;110(6):1847–53. [DOI] [PubMed] [Google Scholar]
- 3.Thursz MR, Richardson P, Allison M, Austin A, Bowers M, Day CP, et al. Prednisolone or Pentoxifylline for Alcoholic Hepatitis. New England Journal of Medicine. 2015;372(17):1619–28. [DOI] [PubMed] [Google Scholar]
- 4.McClain RL CaCJ. Alcoholic Liver Disease In: Feldman M MD; Friedman Lawrence S. MD; Brandt Lawrence J. MD, editor. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease. e5 ed: Saunders; 2016. p. 1409–27. [Google Scholar]
- 5.Lieber CS. Alcoholic fatty liver: its pathogenesis and mechanism of progression to inflammation and fibrosis. Alcohol. 2004;34(1):9–19. [DOI] [PubMed] [Google Scholar]
- 6.Gao B, Ahmad MF, Nagy LE, Tsukamoto H. Inflammatory pathways in alcoholic steatohepatitis. J Hepatol. 2019;70(2):249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koskinas J, Kenna JG, Bird GL, Alexander GJ, Williams R. Immunoglobulin A antibody to a 200-kilodalton cytosolic acetaldehyde adduct in alcoholic hepatitis. Gastroenterology. 1992;103(6):1860–7. [DOI] [PubMed] [Google Scholar]
- 8.Crabb DW, Bataller R, Chalasani NP, Kamath PS, Lucey M, Mathurin P, et al. Standard Definitions and Common Data Elements for Clinical Trials in Patients With Alcoholic Hepatitis: Recommendation From the NIAAA Alcoholic Hepatitis Consortia. Gastroenterology. 2016;150(4):785–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Theise ND. Histopathology of alcoholic liver disease. Clinical Liver Disease. 2013;2(2):64–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morgan MY. The prognosis and outcome of alcoholic liver disease. Alcohol Alcohol Suppl. 1994;2:335–43. [PubMed] [Google Scholar]
- 11.Altamirano J, Michelena J. Alcohol consumption as a cofactor for other liver diseases. Clin Liver Dis (Hoboken). 2013;2(2):72–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atkinson SR, Way MJ, McQuillin A, Morgan MY, Thursz MR. Homozygosity for rs738409:G in PNPLA3 is associated with increased mortality following an episode of severe alcoholic hepatitis. J Hepatol. 2017;67(1):120–7. [DOI] [PubMed] [Google Scholar]
- 13.Moreno C, Mueller S, Szabo G. Non-invasive diagnosis and biomarkers in alcohol-related liver disease. J Hepatol. 2019;70(2):273–83. [DOI] [PubMed] [Google Scholar]
- 14.Roth NC, Saberi B, Macklin J, Kanel G, French SW, Govindarajan S, et al. Prediction of histologic alcoholic hepatitis based on clinical presentation limits the need for liver biopsy. Hepatol Commun. 2017;1(10):1070–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu M, Shah VH. New Prospects for Medical Management of Acute Alcoholic Hepatitis. Clinical Liver Disease. 2019;13(5):131–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Louvet A, Thursz MR, Kim DJ, Labreuche J, Atkinson SR, Sidhu SS, et al. Corticosteroids Reduce Risk of Death Within 28 Days for Patients With Severe Alcoholic Hepatitis, Compared With Pentoxifylline or Placebo—a Meta-analysis of Individual Data From Controlled Trials. Gastroenterology. 2018;155(2):458–68.e8. [DOI] [PubMed] [Google Scholar]
- 17.Louvet A, Naveau S, Abdelnour M, Ramond MJ, Diaz E, Fartoux L, et al. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology. 2007;45(6):1348–54. [DOI] [PubMed] [Google Scholar]
- 18.Mathurin P, Moreno C, Samuel D, Dumortier J, Salleron J, Durand F, et al. Early Liver Transplantation for Severe Alcoholic Hepatitis. New England Journal of Medicine. 2011;365(19):1790–800. [DOI] [PubMed] [Google Scholar]
- 19.Cuadrado A, Fabrega E, Casafont F, Pons-Romero F. Alcohol recidivism impairs long-term patient survival after orthotopic liver transplantation for alcoholic liver disease. Liver Transpl. 2005;11(4):420–6. [DOI] [PubMed] [Google Scholar]
- 20.Sheiner PA, Magliocca JF, Bodian CA, Kim-Schluger L, Altaca G, Guarrera JV, et al. Long-term medical complications in patients surviving > or = 5 years after liver transplant. Transplantation. 2000;69(5):781–9. [DOI] [PubMed] [Google Scholar]
- 21.Mellinger JL, Winder GS. Alcohol Use Disorders in Alcoholic Liver Disease. Clinics in Liver Disease. 2019;23(1):55–69. [DOI] [PubMed] [Google Scholar]
- 22.de Beaurepaire R, Sinclair JMA, Heydtmann M, Addolorato G, Aubin H-J, Beraha EM, et al. The Use of Baclofen as a Treatment for Alcohol Use Disorder: A Clinical Practice Perspective. Frontiers in psychiatry. 2019;9:708-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neuman MG, Maor Y, Nanau RM, Melzer E, Mell H, Opris M, et al. Alcoholic Liver Disease: Role of Cytokines. Biomolecules. 2015;5(3):2023–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szabo G, Csak T. Inflammasomes in liver diseases. J Hepatol. 2012;57(3):642–54. [DOI] [PubMed] [Google Scholar]
- 25.Hilscher MB, Sehrawat T, Arab Verdugo JP, Zeng Z, Gao J, Liu M, et al. Mechanical Stretch Increases Expression of CXCL1 in Liver Sinusoidal Endothelial Cells to Recruit Neutrophils, Generate Sinusoidal Microthombi, and Promote Portal Hypertension. Gastroenterology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greuter T, Malhi H, Gores GJ, Shah VH. Therapeutic opportunities for alcoholic steatohepatitis and nonalcoholic steatohepatitis: exploiting similarities and differences in pathogenesis. JCI Insight. 2017;2(17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boetticher NC, Peine CJ, Kwo P, Abrams GA, Patel T, Aqel B, et al. A Randomized, Double-Blinded, Placebo-Controlled Multicenter Trial of Etanercept in the Treatment of Alcoholic Hepatitis. Gastroenterology. 2008;135(6):1953–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naveau S, Chollet-Martin S, Dharancy S, Mathurin P, Jouet P, Piquet M-A, et al. A double-blind randomized controlled trial of infliximab associated with prednisolone in acute alcoholic hepatitis. Hepatology. 2004;39(5):1390–7. [DOI] [PubMed] [Google Scholar]
- 29.Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: A double-blind, placebo-controlled trial. Gastroenterology. 2000;119(6):1637–48. [DOI] [PubMed] [Google Scholar]
- 30.Park SH, Kim DJ, Kim YS, Yim HJ, Tak WY, Lee HJ, et al. Pentoxifylline vs. corticosteroid to treat severe alcoholic hepatitis: a randomised, non-inferiority, open trial. J Hepatol. 2014;61(4):792–8. [DOI] [PubMed] [Google Scholar]
- 31.Louvet A, Diaz E, Dharancy S, Coevoet H, Texier F, Thévenot T, et al. Early switch to pentoxifylline in patients with severe alcoholic hepatitis is inefficient in non-responders to corticosteroids. Journal of Hepatology. 2008;48(3):465–70. [DOI] [PubMed] [Google Scholar]
- 32.Petrasek J, Bala S, Csak T, Lippai D, Kodys K, Menashy V, et al. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. The Journal of Clinical Investigation. 2012;122(10):3476–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Szabo G, Mitchell MC, McClain CJ, Dasarathy S, McCullough AJ, Nagy L, et al. IL-1 Receptor Antagonist in Combination with Pentoxifylline and Zinc for Severe Alcoholic Hepatitis: A Multicenter Randomized Double-Bind Placebo-Controlled Clinical Trial. Hepatology. 2018;68(6):1444A–71A. [Google Scholar]
- 34.Lefebvre E, Ratziu V, Sanyal AJ, Francque SM, Wong V, Loomba R, et al. 357 - Cenicriviroc Treatment for Adults with Non-Alcoholic Steatohepatitis: Year 2 Analysis of the Phase 2B Centaur Study. Gastroenterology. 2018;154(6, Supplement 1):S–1085. [Google Scholar]
- 35.Wiest R, Albillos A, Trauner M, Bajaj JS, Jalan R. Targeting the gut-liver axis in liver disease. J Hepatol. 2017;67(5):1084–103. [DOI] [PubMed] [Google Scholar]
- 36.Bajaj JS. Alcohol, liver disease and the gut microbiota. Nature Reviews Gastroenterology & Hepatology. 2019;16(4):235–46. [DOI] [PubMed] [Google Scholar]
- 37.Basuroy S, Sheth P, Mansbach CM, Rao RK. Acetaldehyde disrupts tight junctions and adherens junctions in human colonic mucosa: protection by EGF and L-glutamine. Am J Physiol Gastrointest Liver Physiol. 2005;289(2):G367–75. [DOI] [PubMed] [Google Scholar]
- 38.Tamai H, Horie Y, Kato S, Yokoyama H, Ishii H. Long-term ethanol feeding enhances susceptibility of the liver to orally administered lipopolysaccharides in rats. Alcohol Clin Exp Res. 2002;26(8 Suppl):75s–80s. [DOI] [PubMed] [Google Scholar]
- 39.Bala S, Marcos M, Gattu A, Catalano D, Szabo G. Acute binge drinking increases serum endotoxin and bacterial DNA levels in healthy individuals. PLoS One. 2014;9(5):e96864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uesugi T, Froh M, Arteel GE, Bradford BU, Thurman RG. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology. 2001;34(1):101–8. [DOI] [PubMed] [Google Scholar]
- 41.Adachi Y, Bradford BU, Gao W, Bojes HK, Thurman RG. Inactivation of Kupffer cells prevents early alcohol-induced liver injury. Hepatology. 1994;20(2):453–60. [PubMed] [Google Scholar]
- 42.Döhler JR, Nebermann L. Bovine colostrum in oral treatment of enterogenic endotoxaemia in rats. Critical care (London, England). 2002;6(6):536–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mizrahi M, Shabat Y, Ben Ya’acov A, Lalazar G, Adar T, Wong V, et al. Alleviation of insulin resistance and liver damage by oral administration of Imm124-E is mediated by increased Tregs and associated with increased serum GLP-1 and adiponectin: results of a phase I/II clinical trial in NASH. Journal of inflammation research. 2012;5:141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vergis N, Atkinson SR, Knapp S, Maurice J, Allison M, Austin A, et al. In Patients With Severe Alcoholic Hepatitis, Prednisolone Increases Susceptibility to Infection and Infection-Related Mortality, and Is Associated With High Circulating Levels of Bacterial DNA. Gastroenterology. 2017;152(5):1068–77.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jimenez C, Ventura M, Sala M, Cañete N, Poca M, Simón-Talero M, et al. Use of rifaximin in alcoholic hepatitis: Pilot study. Journal of Hepatology. 2018;68:S816. [Google Scholar]
- 46.Ritze Y, Bárdos G, Claus A, Ehrmann V, Bergheim I, Schwiertz A, et al. Lactobacillus rhamnosus GG Protects against Non-Alcoholic Fatty Liver Disease in Mice. PLOS ONE. 2014;9(1):e80169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Philips CA, Pande A, Shasthry SM, Jamwal KD, Khillan V, Chandel SS, et al. Healthy Donor Fecal Microbiota Transplantation in Steroid-Ineligible Severe Alcoholic Hepatitis: A Pilot Study. Clin Gastroenterol Hepatol. 2017;15(4):600–2. [DOI] [PubMed] [Google Scholar]
- 48.Nguyen-Khac E, Thevenot T, Piquet M-A, Benferhat S, Goria O, Chatelain D, et al. Glucocorticoids plus N-Acetylcysteine in Severe Alcoholic Hepatitis. New England Journal of Medicine. 2011;365(19):1781–9. [DOI] [PubMed] [Google Scholar]
- 49.Singh V, Keisham A, Bhalla A, Sharma N, Agarwal R, Sharma R, et al. Efficacy of Granulocyte Colony-Stimulating Factor and N-Acetylcysteine Therapies in Patients With Severe Alcoholic Hepatitis. Clin Gastroenterol Hepatol. 2018;16(10):1650–6.e2. [DOI] [PubMed] [Google Scholar]
- 50.Higuera-de la Tijera F, Servin-Caamano AI, Serralde-Zuniga AE, Cruz-Herrera J, Perez-Torres E, Abdo-Francis JM, et al. Metadoxine improves the three- and six-month survival rates in patients with severe alcoholic hepatitis. World J Gastroenterol. 2015;21(16):4975–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leggio L, Kenna GA, Ferrulli A, Zywiak WH, Caputo F, Swift RM, et al. Preliminary findings on the use of metadoxine for the treatment of alcohol dependence and alcoholic liver disease. Hum Psychopharmacol. 2011;26(8):554–9. [DOI] [PubMed] [Google Scholar]
- 52.Nagy LE, Ding WX, Cresci G, Saikia P, Shah VH. Linking Pathogenic Mechanisms of Alcoholic Liver Disease With Clinical Phenotypes. Gastroenterology. 2016;150(8):1756–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mehta G, Rousell S, Burgess G, Morris M, Wright G, McPherson S, et al. A Placebo-Controlled, Multicenter, Double-Blind, Phase 2 Randomized Trial of the Pan-Caspase Inhibitor Emricasan in Patients with Acutely Decompensated Cirrhosis. J Clin Exp Hepatol. 2018;8(3):224–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Frenette CT, Morelli G, Shiffman ML, Frederick RT, Rubin RA, Fallon MB, et al. Emricasan Improves Liver Function in Patients With Cirrhosis and High Model for End-Stage Liver Disease Scores Compared With Placebo. Clin Gastroenterol Hepatol. 2019;17(4):774–83.e4. [DOI] [PubMed] [Google Scholar]
- 55.Mathurin P, Bzowej NH, Shiffman ML, Arterburn S, Nguyen T, Billin A, et al. Selonsertib in Combination with Prednisolone for the Treatment of Severe Alcoholic Hepatitis: A Phase 2 Randomized Controlled Trial. Hepatology. 2018;68(S1):1–183. [Google Scholar]
- 56.Freimuth J, Bangen J-M, Lambertz D, Hu W, Nevzorova YA, Sonntag R, et al. Loss of caspase-8 in hepatocytes accelerates the onset of liver regeneration in mice through premature nuclear factor kappa B activation. Hepatology. 2013;58(5):1779–89. [DOI] [PubMed] [Google Scholar]
- 57.Wang H, Park O, Lafdil F, Shen K, Horiguchi N, Yin S, et al. Interplay of hepatic and myeloid signal transducer and activator of transcription 3 in facilitating liver regeneration via tempering innate immunity. Hepatology. 2010;51(4):1354–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamada Y, Kirillova I, Peschon JJ, Fausto N. Initiation of liver growth by tumor necrosis factor: deficient liver regeneration in mice lacking type I tumor necrosis factor receptor. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(4):1441–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ki SH, Park O, Zheng M, Morales-Ibanez O, Kolls JK, Bataller R, et al. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology. 2010;52(4):1291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu Y, Verma VK, Malhi H, Gores GJ, Kamath PS, Sanyal A, et al. Lipopolysaccharide downregulates macrophage-derived IL-22 to modulate alcohol-induced hepatocyte cell death. Am J Physiol Cell Physiol. 2017;313(3):C305–c13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arab JP, Sehrawat T, Simonetto DA, Verma VK, Feng D, Tang T, et al. An Open Label, Cohort Dose Escalation Study to Assess the Safety and Efficacy of IL‐ 22 Agonist F‐ 652 in Patients with Alcoholic Hepatitis. Hepatology. 2018;68(S1):1–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yannaki E, Athanasiou E, Xagorari A, Constantinou V, Batsis I, Kaloyannidis P, et al. G-CSF-primed hematopoietic stem cells or G-CSF per se accelerate recovery and improve survival after liver injury, predominantly by promoting endogenous repair programs. Exp Hematol. 2005;33(1):108–19. [DOI] [PubMed] [Google Scholar]
- 63.Shasthry SM, Sharma MK, Shasthry V, Pande A, Sarin SK. Efficacy of Granulocyte Colony-stimulating Factor in the Management of Steroid-Nonresponsive Severe Alcoholic Hepatitis: A Double-Blind Randomized Controlled Trial. Hepatology. 2019. [DOI] [PubMed] [Google Scholar]
- 64.Singh V, Sharma AK, Narasimhan LR, Bhalla A, Sharma N, Sharma R. Granulocyte Colony-Stimulating Factor in Severe Alcoholic Hepatitis: A Randomized Pilot Study. American Journal of Gastroenterology. 2014;109(9):1417–23. [DOI] [PubMed] [Google Scholar]
- 65.Spahr L, Lambert JF, Rubbia-Brandt L, Chalandon Y, Frossard JL, Giostra E, et al. Granulocyte-colony stimulating factor induces proliferation of hepatic progenitors in alcoholic steatohepatitis: a randomized trial. Hepatology. 2008;48(1):221–9. [DOI] [PubMed] [Google Scholar]
- 66.Trauner M, Gulamhusein A, Hameed B, Caldwell S, Shiffman ML, Landis C, et al. The Nonsteroidal Farnesoid X Receptor Agonist Cilofexor (GS-9674) Improves Markers of Cholestasis and Liver Injury in Patients With Primary Sclerosing Cholangitis. Hepatology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Verbeke L, Farre R, Trebicka J, Komuta M, Roskams T, Klein S, et al. Obeticholic acid, a farnesoid X receptor agonist, improves portal hypertension by two distinct pathways in cirrhotic rats. Hepatology. 2014;59(6):2286–98. [DOI] [PubMed] [Google Scholar]
- 68.Cheung K, Lee SS, Raman M. Prevalence and Mechanisms of Malnutrition in Patients With Advanced Liver Disease, and Nutrition Management Strategies. Clinical Gastroenterology and Hepatology. 2012;10(2):117–25. [DOI] [PubMed] [Google Scholar]
- 69.Mendenhall CL, Anderson S, Weesner RE, Goldberg SJ, Crolic KA. Protein-calorie malnutrition associated with alcoholic hepatitis: Veterans administration cooperative study group on alcoholic hepatitis. The American Journal of Medicine. 1984;76(2):211–22. [DOI] [PubMed] [Google Scholar]
- 70.Plauth M, Bernal W, Dasarathy S, Merli M, Plank LD, Schutz T, et al. ESPEN guideline on clinical nutrition in liver disease. Clin Nutr. 2019;38(2):485–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Singal AK, Bataller R, Ahn J, Kamath PS, Shah VH. ACG Clinical Guideline: Alcoholic Liver Disease. Am J Gastroenterol. 2018;113(2):175–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kearns PJ, Young H, Garcia G, Blaschke T, O’Hanlon G, Rinki M, et al. Accelerated improvement of alcoholic liver disease with enteral nutrition. Gastroenterology. 1992;102(1):200–5. [DOI] [PubMed] [Google Scholar]
- 73.Moreno C, Deltenre P, Senterre C, Louvet A, Gustot T, Bastens B, et al. Intensive Enteral Nutrition Is Ineffective for Patients With Severe Alcoholic Hepatitis Treated With Corticosteroids. Gastroenterology. 2016;150(4):903–10.e8. [DOI] [PubMed] [Google Scholar]
- 74.Sun Q, Zhong W, Zhang W, Li Q, Sun X, Tan X, et al. Zinc deficiency mediates alcohol-induced apoptotic cell death in the liver of rats through activating ER and mitochondrial cell death pathways. Am J Physiol Gastrointest Liver Physiol. 2015;308(9):G757–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.EASL Clinical Practice Guidelines: Management of alcohol-related liver disease. Journal of Hepatology; 2018. 2018/07/01/. [DOI] [PubMed] [Google Scholar]
- 76.Szabo G, Kamath PS, Shah VH, Thursz M, Mathurin P. Alcohol-Related Liver Disease: Areas of Consensus, Unmet Needs and Opportunities for Further Study. Hepatology. 2019;69(5):2271–83. [DOI] [PubMed] [Google Scholar]
- 77.Addolorato G, Leggio L, Ferrulli A, Cardone S, Vonghia L, Mirijello A, et al. Effectiveness and safety of baclofen for maintenance of alcohol abstinence in alcohol-dependent patients with liver cirrhosis: randomised, double-blind controlled study. The Lancet. 2007;370(9603):1915–22. [DOI] [PubMed] [Google Scholar]
- 78.Yamini D, Lee SH, Avanesyan A, Walter M, Runyon B. Utilization of Baclofen in Maintenance of Alcohol Abstinence in Patients with Alcohol Dependence and Alcoholic Hepatitis with or without Cirrhosis. Alcohol and Alcoholism. 2014;49(4):453–6. [DOI] [PubMed] [Google Scholar]
- 79.Naudet F, Braillon A. Baclofen and alcohol in France. Lancet Psychiatry. 2018;5(12):961–2. [DOI] [PubMed] [Google Scholar]
- 80.Peeraphatdit TB, Kamath PS, Karpyak VM, Davis B, Desai V, Liangpunsakul S, et al. Alcohol Rehabilitation Within 30 Days of Hospital Discharge Is Associated With Reduced Readmission, Relapse, and Death in Patients With Alcoholic Hepatitis. Clin Gastroenterol Hepatol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Khan A, Tansel A, White DL, Kayani WT, Bano S, Lindsay J, et al. Efficacy of Psychosocial Interventions in Inducing and Maintaining Alcohol Abstinence in Patients With Chronic Liver Disease: A Systematic Review. Clin Gastroenterol Hepatol. 2016;14(2):191–202.e1–4; quiz e20. [DOI] [PMC free article] [PubMed] [Google Scholar]


