Abstract
To tackle the complexity of the global obesity epidemic, it is important to consider the many predisposing factors that underlie progressive and sustained weight gain. Some of the biological drivers for weight gain following initial weight loss include persistent changes in appetite hormones [including ghrelin and postprandial plasma peptide YY (PYY)], and ‘persistent metabolic adaptation’. However, many factors within our busy, stressful modern-day environment seem to conspire towards promotion of weight gain. These include the effects of sleep deprivation on appetite regulation, and the effects of modern-day technology on ‘attention competition’. These factors, combined with cultural and societal factors can result in a ‘mindless’ attitude regarding eating-related behaviour that is likely to predispose to weight gain. In addition to the external environment, our internal environment within the gut has also changed radically within the last few decades, resulting from changes in fibre intake, and increased ingestion of highly refined, sterilised and processed foods. Although contentious, these dietary changes have implications for our gut microbiota, and possible downstream effects on control of appetite and metabolism. In this brief review, we consider some of the novel predisposing factors for weight gain within our modern-day 21st century environments (both external and internal), and explore how legal terminology can help to conceptualise the numerous factors that contribute towards weight gain, and, ultimately the global obesity epidemic.
Keywords: actus reus, manner, mens rea, obesity
Introduction
Why is weight maintenance following weight loss so difficult to achieve?
The global obesity epidemic that has ensued over the last half century is likely to have complex and multi-factorial determinants.1 Obesity accounts for much chronic ill health globally, and associates with at least 50 obesity-related co-morbidities.2–5 There is also a substantial global socio-economic burden from obesity, with a substantial proportion of healthcare costs stemming from management strategies required for obesity-related conditions. Cardiometabolic dysfunction mediates most obesity-related diseases, including type 2 diabetes mellitus (T2D), hypertension and other features of the metabolic syndrome, and obesity-related malignancies such as endometrial carcinoma.6
For decades, attainment of a healthy weight has represented and continues to represent a key goal of health, and is thought to improve future development of chronic ill health. Evidence to support beneficial effects of weight loss in obesity, especially following bariatric surgery, is well established.7 However, lifestyle approaches to weight loss through change in diet and physical activity, appear to be unsuccessful when viewed from a perspective of weight maintenance following initial weight loss. Recent examples include the Diabetes Prevention Program, which demonstrated that, despite initial weight loss in the lifestyle group, gradual weight re-gain occurred over the 10 years following the initial lifestyle intervention.8 A further example is the Look AHEAD study, in which 44% of the intensive lifestyle intervention group had weight regain at 1-year follow up.9 Furthermore, an analysis of 14 long-term studies demonstrated that, following initial weight loss with follow up for up to 7 years, one-third to two-thirds of dieters actually regained more weight than they lost on their diets.10
It is pertinent to consider why weight maintenance appears to be so difficult to achieve in many of us. It is tempting to attribute this to lack of willpower, and other vices such as greed and sloth. However, this perspective is simplistic considering the strong biological drivers for weight regain that occur following weight loss. These biological drivers include ‘persistent metabolic adaptation’, defined as a persistent reduction in resting metabolic rate following adjustment for changes in body composition and age,11 and sustained enhancement of appetitive and hedonic effects of food. Possibly contributory to the latter is a sustained increase in ghrelin, and suppression of postprandial plasma peptide YY (PYY) and leptin within the serum following dietary-induced weight loss in individuals (compared with baseline levels prior to weight loss), that each persist for at least a year following weight loss. However, other factors are also likely to be involved.12,13 It is likely that changes in serum levels of PYY play an important role in this context, although this remains controversial.14 These changes in appetite hormones following weight loss drive enhanced appetite and a sustained increase in caloric intake.13 It is these potent and sustained changes in underlying biological drive in response to initial weight loss that underlie the challenge of long-term weight maintenance, and a good explanation for why lifestyle changes alone, at least in their current recommended form, appear ineffective for many who employ them.
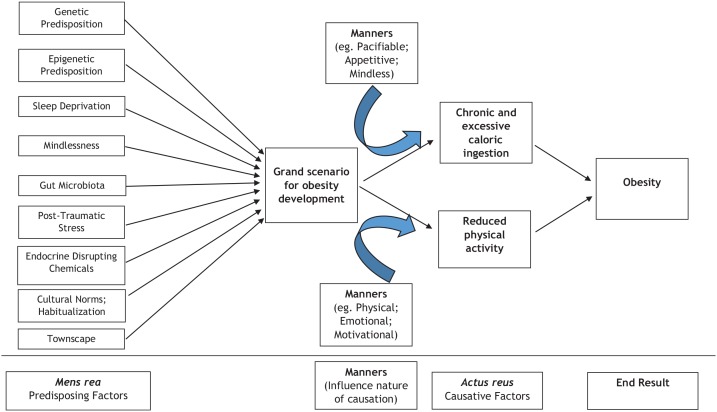
In this brief review, we discuss some unusual and novel factors that may contribute towards obesity development, and difficulty in achieving effective weight maintenance following initial weight loss. The novel factors discussed are summarized in Figure 1. It should be noted that the predisposing and causative factors discussed are not all inclusive. Some of these factors are contentious, with limited supporting evidence. Our aim is not to provide a comprehensive and exhaustive exposition of the entire obesity field regarding underlying pathogenesis, but rather to select some novel factors that may challenge prevailing dogmatic belief. Although not a focus in our review, we acknowledge that use of certain drug classes such as some anti-depressant and anti-psychotic therapies, are known to predispose to weight gain and obesity, with good evidence from the literature. Such data has been reviewed comprehensively elsewhere, including a systematic review reported by Leslie and colleagues.15 We hope our review will provide some future direction for ongoing research in this field, and ultimately benefit patient care.
Figure 1.
Overview of main factors implicated in the development of obesity.
Predisposing and causative factors implicated in the development of obesity are not all-inclusive.
External environment
Our modern day environment is noisy, stressful, light, busy, sleep-deprived, 24-h, ‘instant-access’ and technology-driven. The many distractions of modern daily living limit opportunities to engage with healthy lifestyle changes in a mindful way. Rather, our modern stressful environment with its many 24-h distractions fosters a mental landscape that is antithetic to mindfulness: that of ‘mindlessness’. We consider some novel aspects of our modern-day environment that may have contributed towards weight gain, and hamper attempts at weight maintenance following initial weight loss. We then consider implications for ‘mindlessness’.
Sleep deprivation
Sleep is important. Sleep influences virtually every aspect of physiology, including appetite control. Sleep deprivation, even for two nights, results in enhanced appetite, increased caloric ingestion, a preference for sweet and fatty foods, and changes in appetite hormones that include increased levels of serum ghrelin and reduced levels of serum leptin.16,17 Sleep deprivation is used here as an umbrella term, to include reduced duration of sleep, and impairment of sleep quality (including sleep disturbance and fragmentation). There is some evidence to link sleep disturbance with higher daytime salivary cortisol levels that may in turn contribute towards cardio-metabolic disease.18 Interestingly, there is some controversy in the current literature regarding association of sleep duration with body mass index (BMI), with some studies showing a direct inverse relationship between these two variables, others showing a ‘U-shaped’ relationship and yet others showing no relationship at all.19,20 Association between sleep duration and BMI appears stronger in younger adults.19 In children, one report showed a ‘U-shaped’ relationship between sleep duration and obesity risk amongst girls, with lowest risk for those who slept for 8 h.21 Other studies in children and young adults, have shown a direct linear association between short sleep duration and development of obesity.22 In a large systematic review on data from >690,000 children and adolescents from across 20 countries between 1905 and 2008, there has been a rapid and consistent decline in sleep duration, with an average decrease of >1 h per night for the duration of the study.23
In one study, it was shown that the success of lifestyle attempts at weight loss, including dietary modification, is very much dependent on sleep sufficiency as a pre-requisite.24 Whilst observational in nature, data from this study are consistent with sleep sufficiency as a determinant of appetite. In a further fascinating study on overweight individuals sleeping <6.5 h per night, individualised counselling on sleep hygiene was provided, resulting in sleep duration of 7–8 h per night.25 During this 2-week intervention, there was a 7% increase in daytime activity, improved sleepiness, and a 14% reduction in overall appetite ratings (including 62% reduction in appetite for sweet and salty foods).25 Therefore, based on current evidence from the literature, sleep deprivation appears to hinder attempts at weight loss,26 and improved sleep duration appears to have a beneficial effect on appetite and overall wellbeing. Effects of sleep deprivation on ability to maintain body weight are less clear, although it is a reasonable hypothesis that such a scenario pertains.
Our modern-day environment has become ‘24-h’ and ‘instant access’. There has been an encroachment on our time for sleep. Availability of provisions and entertainment around the clock and increased demands on our time have perhaps contributed towards sleep deprivation. Natural lighting, and especially blue light from screens, interferes with melatonin production thereby reducing sleepiness during late evening.27 To compound excessive light, noisiness of city life is relevant given that more of us now live in cities than at any other time in human history.28 Excessive light, noise pollution and the inherent stress of modern daily life has created a perfect storm in which sleep deprivation is a natural outcome. To compound environmental factors, we hypothesize that our modern culture seems to have lost an appreciation for, and understanding of the value of, adequate sleep as an important public health message, especially amongst our children. In the drive for capitalism, there is a danger that corporations may value ‘time-on-task’ more than ‘time-on-sleep’. Whilst many businesses seem to look after the wellbeing of their employees, encouragement of sleep sufficiency, and education regarding optimization of sleep hygiene, are perhaps less of a priority. Such de-valuation of sleep is short-sighted in the extreme, given the clear evidence in the literature to show that work productivity and effectiveness diminish with sleep deprivation.29
To optimize success of weight-loss strategies through changes in diet and physical activity, and success of maintenance of body weight following initial weight loss, it is imperative that we optimize sleep sufficiency within the populace. In our opinion, to achieve this will require multifactorial changes, including cultural beliefs regarding the importance of sleep, education of the populace regarding the importance of sleep and strategies to optimize sleep quality, particularly for our children. Our sleeping environments need optimization, with limitation of noise and light. Furthermore, it is known that the body needs to cool prior to sleep, therefore a cool bedroom temperature is important. (We discuss further the impact of ambient environmental temperature on metabolic rate below). Finally, our daily routines need modification to facilitate sleep. This includes avoidance of food, exercise, blue and bright light in the hour before sleep for example.30 Employers should adopt an appreciation for the importance of sleep, especially within profit-driven commercial corporations. Through avoidance of sleep deprivation, enhanced wellbeing of employees would likely translate into improved work ethic and efficiency.31 Finally, there is a need for more sleep-based research generally, including exploration of the importance of sleep sufficiency for maintenance of weight following weight loss.
Technology
When viewed from a broad perspective, it seems that the tsunami of technological advances that have swept over humanity over recent decades has ultimately improved our lives. Life has perhaps become more comfortable as a result and less effortful physically. Lack of physical activity and exercise are often blamed as predisposing factors for the obesity epidemic. Whilst many modern-day occupations are perhaps more sedentary than a century ago, resulting from the rise of technology and automation, it is important to counter such rhetoric with the increasing emphasis on physical activity in our leisure time, including gym culture in recent decades.
Furthermore, there is a common ‘linear misconception’ regarding the impact of physical activity on body weight: a belief that with increments in physical activity, total energy expenditure and overall metabolic rate also increases in a linear way that is commensurate with the increased amount of physical activity. In fact, recent data suggest that a ‘constrained’ model of metabolism regarding physical activity is more likely. A study that compared daily energy expenditure between African hunter-gatherer tribes and those with westernised sedentary lifestyles showed that daily energy expenditure in our species is much more constant, and less dependent upon total physical activity than previously believed.32,33 Furthermore, metabolic studies on those training for a marathon have also demonstrated that metabolic rate tends to reach a plateau with increasing levels of physical activity beyond a certain level.32,33 The determinants of this constrained model of human metabolism and implications of excessive physical activity on other components of metabolism remain important unanswered research questions.
The benefits of physical activity are legion,34 and go well beyond the scope of this review. Encouragement of physical activity and avoidance of physical inactivity should continue to form a pillar of healthy lifestyle advice. The data outlined above confirm that physical activity does indeed influence metabolic rate, but that this relationship does not appear to be linear.32,33 In fact, the incremental metabolic benefits of physical activity appear to diminish with increasing amounts of physical activity, akin to a law of diminishing returns. The threshold of daily physical activity beyond which metabolic rate plateaus may be different between individuals and may even vary in the same individual over time.
Environmental thermo-neutrality
Homo sapiens prefers to live in a ‘thermo-neutral environment’, defined by a temperature range such that metabolism is not increased either by cold or heat stress. For humans, this temperature range falls between around 22°C (71.6°F) and 32°C (89.6°F). In recent decades, there has been an increase in average indoor temperatures: between 1970 and 2000, average temperature inside UK-based homes has risen by 5°C from 13°C (55.4°F) to 18°C (64.4°F).35 This has mirrored a substantial increase in central heating within UK-based homes over a similar timescale. Ambient temperature influences metabolic rate. In one study on men, an increase of just 6°C ambient temperature [between 16°C (60.8°F) and 22°C (71.6°F)] for a 24-h period, resulted in a reduction in overall metabolic rate of 167 kcal for that 24-h period.35 Increased ambient indoor living temperature over recent decades may have predisposed to reduced overall energy expenditure that may manifest in gradual weight gain. It is important to emphasise, however, that this hypothesis is entirely speculative, and proof of a difference in overall energy expenditure according to ambient temperature is not the same as proof of predisposition to weight gain. Without such evidence, it is difficult to base any meaningful public health message regarding optimal environmental temperature, particularly in view of the risks of hypothermia amongst certain population groups, including the elderly population.
Endocrine disrupting chemicals
The Endocrine Society published a scientific statement on the effects of endocrine disrupting chemicals (EDCs; xenobiotic chemicals that can disrupt normal development and homeostatic control) on physiological processes, including association with obesity development.36 There are many thousands of EDCs that we are all exposed to in our environments every day. Accordingly, study of EDCs is inherently difficult, especially with regards to proving causality, with most studies only showing association-type data. Some epidemiological studies have shown association of bisphenol A (BPA) with human obesity. In a cross-sectional analysis of data from the US National Health and Nutrition Examination Survey, urinary BPA concentration seemed to correlate directly with BMI and waist circumference.37 A similar association was shown in a study from China.38 However, contrary to what would be expected, a prospective study showed that in utero exposure to BPA had an inverse association with BMI at the age of 9 years.39 Other EDCs shown to be associated with obesity include phthalates and persistent organic pollutants.36
Cellular models have shown that activation of peroxisome proliferator-activated receptor gamma (PPARγ) from numerous EDCs, including phthalates and BPA can increase adipogenesis in pre-adipocyte lines.36 This mechanism offers a plausible cellular process by which certain EDCs may contribute towards weight gain.36 Animal models of metabolic effects of EDCs provide some compelling evidence. Rodent models of EDC effects, including perinatal exposure to BPA reveal increased body weight and adiposity, and reduced glucose tolerance and insulin sensitivity.40,41 Such effects may depend on dosing and timing of exposure.36
It is premature to formulate firm conclusions regarding effects of EDC exposure on weight gain in humans, given a lack of prospective studies in this field, and contradictions that exist in the current literature. Although the rodent and cellular-based models provide compelling data and a plausible mechanism, it is important not to over-extrapolate data from rodent studies to humans. However, it is quite possible that exposure to certain EDCs, either in utero, during childhood or adulthood may contribute towards human obesity. It is important to explore this complex field further, with more prospectively designed studies. It should be acknowledged, however, that even if enough compelling evidence implicates a certain EDC as a contributor to human obesity, it would be difficult to envision an effective public health program that eliminates human exposure from such a chemical, given the sheer ubiquity of EDCs in our environment, including food and water supplies that we ingest, and the air that we breathe. Unless there is a radical change in global industry and manufacture, EDCs in all of their glorious complexity, are here to stay.
Post-traumatic stress
Our external environment manifests in many forms. We have already discussed the physical nature of this environment, with its noise and light pollution, ambient temperature, advanced technology and EDCs as products of our modern global industry and manufacturing processes. However, another aspect of our external environment is its capacity to inflict trauma on us. Such trauma includes both physical and emotional components, and can be inflicted deliberately by others, self-inflicted, or simply occur through witnessing events unfold in our external environment. In a study of >20,000 participants from a US adult population, association of post-traumatic stress disorder (PTSD) with obesity was shown [odds ratio (OR) 1.51]. Obesity affected 32.6% of those with PTSD in the last year, and 24.1% of those without a lifetime history of PTSD.42 The association of PTSD with obesity is likely mediated through complex pathways, and likely differs between individuals according to the nature of the trauma, personality type and psychosocial factors. Using food as a pacifier for mental distress resulting from prior trauma, is a common observation in clinical practice. Food may also be used as a distractor, or even as a replacement for love (resulting from childhood experiences of a neglectful parent for example). However, whatever the mechanism(s), it is important to be mindful of the potential for prior traumatic experiences to result in weight gain and obesity, and for this to be revealed through a careful history. Once traumatic experiences have been elicited, a compassionate approach to management is required, which goes well beyond simple dietary and lifestyle advice, but includes focused psychological support, and where possible identification and implementation of alternate healthy coping strategies.
Mindlessness
Mindfulness is a heightened awareness of one’s own internal emotions, psychology and immediate surroundings.43 Mindfulness is a type of reflection that occurs in the present. We use the term ‘mindlessness’ to describe an absence of mindfulness: for example, eating-related behaviour whilst not focusing and concentrating on the act of eating, but instead focusing on some other environmental or internal stimulus or nothing at all. An example could be watching TV whilst eating snacks. It is important to clarify that ‘mindlessness’ does not refer to underlying subconscious determinants of appetite and behaviour such as hypothalamic regulation, that have a firm basis in behavioural and neuroscientific research. Rather, mindlessness in the context that we use the term here, refers to reduced conscious awareness of one’s current emotions, drives and behaviour such as appetite and eating-related behaviours.
For many of us, modern-day busy and stressful environments are distracting. The modern-day environment we live in tends to bombard us with continuous information and updates, whether this be through social media, news updates, shopping opportunities or booking our next holiday. Execution of these activities can occur literally at any time of day. Our technology often persuades us to act now, and not to delay. It seems that our modern-day environment and culture leaves little time for quiet contemplative reflection, and engagement in mindful activities. This includes engagement with two pillars of healthy lifestyle: diet and physical exercise. Our environs have an abundance of food outlets, and food often forms an important component of any social interaction and event. For many of us, there is habitualisation of eating-related behaviour into a ‘three-meals-a-day’ endeavour. Often we eat simply because of social norms and expectations, or through habit rather than necessarily in response to appetitive cues.44,45 In a culture and society that relies so heavily on social cues and norms, it is easy to adopt a mindless approach to eating.
It is important to question the utility of mindfulness adoption as a useful strategy to facilitate engagement with lifestyle advice. Our own group demonstrated that adoption of mindfulness techniques to healthy eating applied in a tier 3 obesity setting, improved eating-related behaviours, and was effective in promoting weight loss.43 In a meta-analysis on adoption of mindfulness techniques in overweight and obese adults, there was a reduction of binge and impulsive eating-related behaviours, and increases in physical activity, although no effects on weight loss.46 The longer-term effects of mindfulness adoption following its implementation, on eating-related behaviours and body weight should be a focus for future research. Furthermore, it is important to acknowledge the limitations of mindfulness research reported to date, given that mindfulness is used as an umbrella term in the literature to cover a broad range of practices, and the difficulty in attributing outcomes to application of mindfulness, versus some other confounding effect of an applied intervention.47 Therefore, future research in this field should address this difficulty, with studies designed to explore effects of mindfulness interventions whilst eliminating other confounding factors.
Internal environment
Gut microbiota
In addition to factors in our external environment, it is also necessary to consider what potential factors in our internal environment, especially within our gut, may predispose to weight gain. Over recent years, data have emerged to support an important role for the gut faecal microbiota as a determinant of health, and as a contributor towards much 21st-century chronic disease, including obesity and metabolic dysfunction.48 Rodent-based studies have shown that microbial diversity within the gut is important for energy homeostasis.49 Human studies have highlighted the importance of gut faecal microbial diversity for general health, including metabolic health and fat deposition.49 Human-based studies have shown a typical signature for gut microbiota in obesity, particularly a reduced ratio of Bacteroides/Firmicutes species.49 However, such correlational data, typical of much of the faecal microbiota data published to date, preclude any firm assignment of causality regarding the effects of faecal microbiota signature and diversity on metabolic health and body weight.
There is evidence to suggest that changes in the gut faecal floral signature contribute towards a breach in the protective epithelial lining of the gut wall, and that development of intestinal inflammation following dysregulation of faecal microbiota associates with aberrant translocation of bacteria into the bowel wall.50 Such bacterial translocation may influence hypothalamic regulation of appetite and metabolic processes through a ‘microbiota-gut-brain’ axis, and thereby contribute towards body weight. Translocated bacteria from the gut may have direct hypothalamic effects, or act indirectly through mediation of changes in incretin release, inflammatory status, vagal afferent signals or some hitherto unknown mechanism(s). The effects of translocated bacteria on human physiology and hypothalamic regulation of appetite and metabolism remain speculative, and should form a focus for future research.
Data from rodent studies show effects of dietary fibre on metabolism. In one such study, fermentation of soluble fibre by gut microbiota produced short-chain fatty acids (SCFAs; propionate and butyrate) that in turn activated intestinal gluconeogenesis.51 The authors speculated that vagal afferents (stimulated by portal glucose sensors) mediate central control of metabolic changes.51 In a further study in mice, early exposure to high-fat diet repressed health-enhancing bacteria such as Bifidobacterium and Akkermansia, and promoted health-detracting bacteria such as Dorea.52 In a human-based study on normoglycaemic overweight and obese men, Canfora and colleagues reported on the metabolic effects of rectal administration of physiologically relevant SCFA mixtures, in a randomised double blind crossover design.53 The SCFA mixtures included acetate, propionate, butyrate or placebo. There was an increase in fasting fat oxidation and fasting and postprandial PYY, and attenuation of fasting free glycerol concentration in response to all three SCFA mixtures.53 Furthermore, resting energy expenditure increased after rectal infusion of acetate and propionate SCFA mixtures compared with placebo.53
Given the likely importance of gut microbiota for metabolic health, it is interesting to speculate on a potential role for oral antibiotic usage (since their widespread introduction in the 1940s), as a possible contributory factor for obesity development. In a recent study of >8000 children, it was shown that number of antibiotic courses in early life may increase risk of subsequent childhood obesity.54 In a further study on growth trajectory in the first year of life in term infants, although decreased growth was observed after antibiotics in the first week of life, increased growth occurred after later antibiotic courses.55 However, other data do not support a clinically meaningful effect of short-term oral antibiotic usage on metabolic health: Reijners and colleagues reported on the metabolic effects of a 7-day treatment course of oral antibiotics (randomised to amoxicillin, vancomycin or placebo).56 Although vancomycin reduced microbial diversity, there was no effect of oral antibiotics on tissue-specific insulin sensitivity, energy or substrate metabolism, postprandial hormones, systemic inflammation, gut permeability or adipocyte size.56 Further human-based studies are required to elucidate any potential for harmful metabolic effects of longer-term oral antibiotics, possibly mediated through changes in the gut microbiota.
Gut microbiota are remarkably sensitive to the food that we eat, and we can each therefore modify our own gut microbiota through our diet.57 Our understanding is that, with the exception of the neonatal period,58 microbial diversity generally promotes health and wellbeing, and certain microbial groups associate with health and disease.49 However, our understanding of the gut microbiota is very much in its infancy. Although mechanisms remain speculative, data outlined above including those from rodent-based and human-based studies suggest a link between gut microbiota and metabolic health, although results from previous studies have not been conclusive.59–62
The mechanisms implicated and the role of diet in mediating effects of gut microbiota on metabolic health (through for example changes in the levels of colonic SCFA) remain incompletely understood, and should be a focus for future research. What is incontrovertible is that there has been a radical change in our westernised diet over recent decades, including an impoverishment of dietary fibre intake and increased ingestion of sterilized and refined foods,63 coupled with the emergence of increased usage of oral antibiotics. It is reasonable to speculate and hypothesize that our gut microbiota profiles would have changed over this time. Whether these hypothesized changes in gut flora have also influenced our metabolic health, including propensity for weight gain remains open to question, and should form a focus for further research. Such research is likely to inform future guidance on healthy lifestyle, with dietary advice likely to be aligned to optimization of not just the functionality of our own cells, but also the >100 trillion microbes that reside in our gut. This research is also important to explore a possible future role for faecal transplantation to improve future risk for cardiometabolic disease. Based on current evidence however, the best advice we have is to increase intake of fibre and whole-food and reduce intake of fat, sugar and processed food, and to maintain a healthy and diverse diet including fermentable and non-sterilised foods.64
Obesity predisposition using legal terminology
Having outlined evidence for various novel and unusual predisposing factors for development of obesity, use of legal terminology as an analogy can help to build a mental scaffold to enable us to conceptualize this process. There are three separate and distinct legal terms in our analogy: actus reus, mens rea and manner. In legal terms, causation (actus reus or action) refers to the causal relationship between conduct and end-result. In addition to, and separate from, causation there is ‘mind-set’ (mens rea). Manner simply refers to the manner (or ‘influencer’) in which causation occurred. In the case of a death, causation is the specific injury or disease that leads to death; manner is how the injury or disease actually leads to death (natural, accidental, suicide, homicide and undetermined). In the case of homicide, mens rea would be the mind-set of the assailant, underlying their behaviour. The same cause of death can occur through multiple manners in different cases.
Using our legal analogy, the end-result is obesity. Obesity itself is, of course, a cause of multiple weight-related conditions,65 but we should ignore that for this analogy and focus only on obesity itself. It is widely accepted that an important actus reus (causative factor) in this context is chronic and excessive caloric ingestion (CECI).66 The manner in which CECI occurs includes use of food as a coping strategy and pacifier for some underlying emotional distress, often resulting from previous adverse life experiences (especially during childhood).67,68 An alternate manner would be through enhanced appetite following initial weight loss.13 Another example would be mindlessness, from simply being unaware of and/or unconcerned with the effects of CECI, or habitualised to social and cultural norms.69 In each of these scenarios, the cause of obesity (CECI) is the same, but the manner is very different. If we use sedentariness as another actus reus (causative factor) for obesity,66 there are also multiple manners (influencers) in which this may manifest that could include, for example, reduced physical ability to engage in physical activity, reduced motivation for physical activity or emotional resistance to physical activity.70,71
It is important to consider carefully and identify the manner in which obesity develops, as this is likely to inform alignment of management strategies (including for example psychological support or mindfulness techniques). There may be multiple manners (influencers) to consider. Our own group demonstrated that adoption of mindfulness approaches to eating behaviour, applied in a group-based tier 3 obesity management setting, resulted in significant and healthful changes in eating-related behaviour and improvements in body weight.43 In addition to application to eating-related behaviour, other aspects of lifestyle (including physical activity and sleep) could also benefit from mindfulness techniques.
In our analogy, mens rea (predisposing factors) refers to all factors that predispose to a scenario in which causation of obesity ensues, divided broadly into genetic and environmental factors. We already considered some of the novel obesogenic environmental factors earlier in this review. The former accounts for our biological predisposition towards weight gain and obesity. These multiple factors provide a perfect storm in which weight gain and obesity emerge from causal factors, enacted through multiple manners (influencers).
Genetic predisposing factors
We all share some genetic propensity for weight gain, although this varies across the population.72 Data from genome-wide association studies (GWAS) and genome-wide linkage studies (GWLS) on many thousands of participants from multiple continents have identified at least 127 sites in the human genome that associate with development of obesity.72 The first such genome sequence variant to associate with risk for common polygenic obesity was within FTO. In fact, FTO variants have the largest effect on obesity phenotype to date, each risk allele associating with 1–1.5 kg increase in body weight.72,73 Other genetic variants identified from GWAS to associate with weight gain and obesity include those implicated in the beta-adrenergic receptor family that regulates energy expenditure and lipolysis.72,74
Further obesity-susceptibility variants from GWAS include those implicated in regulation of uncoupling proteins, thereby providing a potential link between activity of brown adipose tissue (BAT) and energy expenditure with propensity for weight gain.72,75 Activation of BAT (shown by our own group for the first time in a living human to be discernible from white adipose tissue on magnetic resonance scanning) generates heat.76–78 Human studies demonstrate an inverse association between BAT volume with BMI and fat mass percentage.79,80 A cold environment activates BAT,77 and this could be one explanation for enhanced metabolic rate in response to drop in environmental temperature.35
GWAS data have also identified genetic variants that regulate tryptophan accessibility for serotonin synthesis, and thereby influence appetite control and energy balance.72,81 In addition to genetic variants, there is also evidence to support a role for epigenetic factors in predisposition to weight gain.72 Some genes, such as those encoding melanocortin 4 receptor (MC4R) and leptin, show altered methylation patterns in response to high-fat diet.72,82 Many other genes also appear to be epigenetic targets for obesity (epi-obesogenic genes).83
Data from GWAS have provided novel insight into the genetic predisposition for weight gain and obesity. There is strong support for an important role of the central nervous system in susceptibility for obesity, including pathways that implicate control of appetite, energy metabolism, adipogenesis and insulin secretion and action.84 However, despite all the recent progress in genetic research, the genetic variants identified from GWAS still only account for around 3% of the estimated 47–80% heritability of BMI.85 In a recently reported meta-analysis of GWAS for height and BMI in 700,000 individuals of European ancestry, 941 near-independent single nucleotide polymorphisms (SNPs) associated with BMI.86 However, even with this size of meta-analysis, these SNPs still only explained around 6% of the variance of BMI in an independent sample of individuals from the Health and Retirement Study.86 It is possible that epigenetic factors account for some of the missing link. The genetic determinants and heritability of obesity imply a spectrum of genetic predisposition for obesity within any population. However, even in those with the lowest genetic predisposition to weight gain, biology seems ‘hard-wired’ to mitigate against the harmful effects of starvation. From evolutionary first principles, we should not be surprised that the body responds to weight loss with enhanced appetite and persistent metabolic adaptation as a protective mechanism.11,13 It is only when viewed from the highly unusual modern-day obesogenic environment that such physiological responses appear harmful. The central control of appetite and metabolism appear important mediators of these genetic effects.
Conclusion
The pathogenesis of obesity is complex. In this brief review, we have used the analogy of legal terminology to dissect the different elements of obesity pathogenesis. Although direct causes of weight gain and obesity (including CECI and sedentariness) are fairly well established, consideration of these causal factors alone do not provide a deeper insight into the underlying mechanisms that result in obesity. The manners (influencers) in which CECI and sedentariness manifest are plethoric, and multiple manners can exist in each individual. Furthermore, the background ‘mind-set’ in which weight gain ensues, is complex and includes a genotype and epigenotype that promote weight gain especially following weight loss, and an obesogenic environment.
It is beyond the scope of this review to consider management strategies for obesity. However, it seems logical that management strategies should align to the complexity of each individual case. Given the complex manner in which CECI occurs, it is not appropriate to adopt a generalized approach in which everyone receives standard dietary advice for example. Contrarily, there is a need for an individualized approach to effective obesity management that aligns to manner of causation. This may require other approaches such as focused psychological support or mindfulness techniques in some cases. Furthermore, we need to be mindful of the many mens rea (predisposing) factors within our genetics and environment that seem to conspire towards weight gain, and hamper attempts at successful weight loss and weight maintenance. The common misconception that weight regain following initial weight loss is simply due to lack of willpower and motivation needs compassionate attention. Such misconceptions need to be re-framed in the context of understanding and insights that stem from application of a biopsychosocial model of obesity. Accordingly, the reality and challenge of weight maintenance following initial weight loss, and implications for behaviour change needs discussion with each patient undergoing lifestyle change for weight loss.
On a population level, a bigger challenge will be to address and modify environmental, cultural and social factors that underlie predisposition to obesity, and hamper successful weight loss. A good starting point would be to foster more compassion and understanding of obesity amongst society, starting in our schools. Through compassion, we can foster a shared understanding that no one is immune from the development of obesity, and hope for a future in which our shared collective insights and efforts instil the necessary changes that are so desperately required.
Footnotes
Author contributions: Petra Hanson: Data curation; Formal analysis; Methodology; Writing-original draft.
Martin O. Weickert: Formal analysis; Methodology; Writing-review and editing.
Thomas M. Barber: Data curation; Investigation; Methodology; Writing-original draft; Writing-review and editing.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Barber, TM  https://orcid.org/0000-0003-0689-9195
https://orcid.org/0000-0003-0689-9195
Contributor Information
Petra Hanson, Clinical Sciences Research Laboratories, Warwick Medical School, University Hospitals Coventry and Warwickshire, Clifford Bridge Road, Coventry, CV2 2DX; Warwickshire Institute for the Study of Diabetes, Endocrinology and Metabolism, University Hospitals Coventry and Warwickshire, Clifford Bridge Road, Coventry, CV2 2DX.
Martin O. Weickert, Clinical Sciences Research Laboratories, Warwick Medical School, University Hospitals Coventry and Warwickshire, Clifford Bridge Road, Coventry, CV2 2DX Warwickshire Institute for the Study of Diabetes, Endocrinology and Metabolism, University Hospitals Coventry and Warwickshire, Clifford Bridge Road, Coventry, CV2 2DX; Centre of Applied Biological & Exercise Sciences (ABES), Faculty of Health & Life Sciences, Coventry University, Coventry, UK.
Thomas M. Barber, Clinical Sciences Research Laboratories, Warwick Medical School, University Hospitals Coventry and Warwickshire, Clifford Bridge Road, Coventry, CV2 2DX; Warwickshire Institute for the Study of Diabetes, Endocrinology and Metabolism, University Hospitals Coventry and Warwickshire, Clifford Bridge Road, Coventry, CV2 2DX.
References
- 1. Barber TM, Hanson P, Weickert MO, et al. Obesity and polycystic ovary syndrome: implications for pathogenesis and novel management strategies. Clin Med Insights Reprod Health 2019; 13: 1179558119874042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guh DP, Zhang W, Bansback N, et al. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health 2009; 9: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barber TM, McCarthy MI, Wass JA, et al. Obesity and polycystic ovary syndrome. Clin Endocrinol (Oxf) 2006; 65: 137–145. [DOI] [PubMed] [Google Scholar]
- 4. Saboor Aftab SA, Kumar S, Barber TM. The role of obesity and type 2 diabetes mellitus in the development of male obesity-associated secondary hypogonadism. Clin Endocrinol (Oxf) 2013; 78: 330–337. [DOI] [PubMed] [Google Scholar]
- 5. Barber TM, McCarthy MI, Franks S, et al. Metabolic syndrome in polycystic ovary syndrome. Endokrynol Pol 2007; 58: 34–41. [PubMed] [Google Scholar]
- 6. Passarello K, Kurian S, Villanueva V. Endometrial cancer: an overview of pathophysiology, management, and care. Semin Oncol Nurs 2019; 35: 157–165. [DOI] [PubMed] [Google Scholar]
- 7. Wolfe BM, Kvach E, Eckel RH. Treatment of obesity: weight loss and bariatric surgery. Circ Res 2016; 118: 1844–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Diabetes Prevention Program Research Group, Knowler WC, Fowler SE, et al. 10-year follow-up of diabetes incidence and weight loss in the diabetes prevention program outcomes study. Lancet 2009; 374: 1677–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Beavers KM, Neiberg RH, Houston DK, et al. Body weight dynamics following intentional weight loss and physical performance: the look AHEAD movement and memory study. Obes Sci Pract 2015; 1: 12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mann T, Tomiyama AJ, Westling E, et al. Medicare’s search for effective obesity treatments: diets are not the answer. Am Psychol 2007; 62: 220–233. [DOI] [PubMed] [Google Scholar]
- 11. Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med 1995; 332: 621–628. [DOI] [PubMed] [Google Scholar]
- 12. Arafat AM, Weickert MO, Adamidou A, et al. The impact of insulin-independent, glucagon-induced suppression of total ghrelin on satiety in obesity and type 1 diabetes mellitus. J Clin Endocrinol Metab 2013; 98: 4133–4142. [DOI] [PubMed] [Google Scholar]
- 13. Sumithran P, Prendergast LA, Delbridge E, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med 2011; 365: 1597–1604. [DOI] [PubMed] [Google Scholar]
- 14. Pfluger PT, Kampe J, Castaneda TR, et al. Effect of human body weight changes on circulating levels of peptide YY and peptide YY3-36. J Clin Endocrinol Metab 2007; 92: 583–588. [DOI] [PubMed] [Google Scholar]
- 15. Leslie WS, Hankey CR, Lean ME. Weight gain as an adverse effect of some commonly prescribed drugs: a systematic review. QJM 2007; 100: 395–404. [DOI] [PubMed] [Google Scholar]
- 16. Spiegel K, Tasali E, Penev P, et al. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med 2004; 141: 846–850. [DOI] [PubMed] [Google Scholar]
- 17. Spiegel K, Leproult R, L’Hermite-Baleriaux M, et al. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab 2004; 89: 5762–5771. [DOI] [PubMed] [Google Scholar]
- 18. Morgan E, Schumm LP, McClintock M, et al. Sleep characteristics and daytime cortisol levels in older adults. Sleep 2017; 40: zsx043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Theorell-Haglow J, Lindberg E. Sleep duration and obesity in adults: what are the connections? Curr Obes Rep 2016; 5: 333–343. [DOI] [PubMed] [Google Scholar]
- 20. Taheri S, Lin L, Austin D, et al. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med 2004; 1: e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang H, Hu R, Du H, et al. The relationship between sleep duration and obesity risk among school students: a cross-sectional study in Zhejiang, China. Nutr Metab (Lond) 2018; 15: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nielsen LS, Danielsen KV, Sorensen TI. Short sleep duration as a possible cause of obesity: critical analysis of the epidemiological evidence. Obes Rev 2011; 12: 78–92. [DOI] [PubMed] [Google Scholar]
- 23. Matricciani L, Olds T, Petkov J. In search of lost sleep: secular trends in the sleep time of school-aged children and adolescents. Sleep Med Rev 2012; 16: 203–211. [DOI] [PubMed] [Google Scholar]
- 24. Pagliai G, Dinu M, Casini A, et al. Relationship between sleep pattern and efficacy of calorie-restricted Mediterranean diet in overweight/obese subjects. Int J Food Sci Nutr 2018; 69: 93–99. [DOI] [PubMed] [Google Scholar]
- 25. Tasali E, Chapotot F, Wroblewski K, et al. The effects of extended bedtimes on sleep duration and food desire in overweight young adults: a home-based intervention. Appetite 2014; 80: 220–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. St-Onge MP. Sleep-obesity relation: underlying mechanisms and consequences for treatment. Obes Rev 2017; 18(Suppl. 1): 34–39. [DOI] [PubMed] [Google Scholar]
- 27. Ostrin LA. Ocular and systemic melatonin and the influence of light exposure. Clin Exp Optom 2019; 102: 99–108. [DOI] [PubMed] [Google Scholar]
- 28. Hou B, Nazroo J, Banks J, et al. Are cities good for health? A study of the impacts of planned urbanization in China. Int J Epidemiol 2019; 48: 1083–1090. [DOI] [PubMed] [Google Scholar]
- 29. Webb WB, Levy CM. Effects of spaced and repeated total sleep deprivation. Ergonomics 1984; 27: 45–58. [DOI] [PubMed] [Google Scholar]
- 30. Agaronov A, Ash T, Sepulveda M, et al. Inclusion of sleep promotion in family-based interventions to prevent childhood obesity. Child Obes 2018; 14: 485–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Redeker NS, Caruso CC, Hashmi SD, et al. Workplace interventions to promote sleep health and an alert, healthy workforce. J Clin Sleep Med 2019; 15: 649–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Melanson EL. The effect of exercise on non-exercise physical activity and sedentary behavior in adults. Obes Rev 2017; 18(Suppl. 1): 40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pontzer H, Durazo-Arvizu R, Dugas LR, et al. Constrained total energy expenditure and metabolic adaptation to physical activity in adult humans. Curr Biol 2016; 26: 410–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Febbraio MA. Exercise metabolism in 2016: health benefits of exercise - more than meets the eye! Nat Rev Endocrinol 2017; 13: 72–74. [DOI] [PubMed] [Google Scholar]
- 35. McAllister EJ, Dhurandhar NV, Keith SW, et al. Ten putative contributors to the obesity epidemic. Crit Rev Food Sci Nutr 2009; 49: 868–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gore AC, Chappell VA, Fenton SE, et al. EDC-2: the endocrine society’s second scientific statement on endocrine-disrupting chemicals. Endocr Rev 2015; 36: E1–E150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Carwile JL, Michels KB. Urinary bisphenol A and obesity: NHANES 2003-2006. Environ Res 2011; 111: 825–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang T, Li M, Chen B, et al. Urinary bisphenol A (BPA) concentration associates with obesity and insulin resistance. J Clin Endocrinol Metab 2012; 97: E223–E227. [DOI] [PubMed] [Google Scholar]
- 39. Harley KG, Aguilar Schall R, Chevrier J, et al. Prenatal and postnatal bisphenol A exposure and body mass index in childhood in the CHAMACOS cohort. Environ Health Perspect 2013; 121: 514–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wei J, Lin Y, Li Y, et al. Perinatal exposure to bisphenol A at reference dose predisposes offspring to metabolic syndrome in adult rats on a high-fat diet. Endocrinology 2011; 152: 3049–3061. [DOI] [PubMed] [Google Scholar]
- 41. Somm E, Schwitzgebel VM, Toulotte A, et al. Perinatal exposure to bisphenol a alters early adipogenesis in the rat. Environ Health Perspect 2009; 117: 1549–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pagoto SL, Schneider KL, Bodenlos JS, et al. Association of post-traumatic stress disorder and obesity in a nationally representative sample. Obesity (Silver Spring) 2012; 20: 200–205. [DOI] [PubMed] [Google Scholar]
- 43. Hanson P, Shuttlewood E, Halder L, et al. Application of mindfulness in a tier 3 obesity service improves eating behavior and facilitates successful weight loss. J Clin Endocrinol Metab 2019; 104: 793–800. [DOI] [PubMed] [Google Scholar]
- 44. Martin RE, Villanueva Y, Stephano T, et al. Social influence shifts valuation of appetitive cues in early adolescence and adulthood. J Exp Psychol Gen 2018; 147: 1521–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schuz B, Papadakis T, Ferguson SG. Situation-specific social norms as mediators of social influence on snacking. Health Psychol 2018; 37: 153–159. [DOI] [PubMed] [Google Scholar]
- 46. Ruffault A, Czernichow S, Hagger MS, et al. The effects of mindfulness training on weight-loss and health-related behaviours in adults with overweight and obesity: a systematic review and meta-analysis. Obes Res Clin Pract 2017; 11(5 Suppl. 1): 90–111. [DOI] [PubMed] [Google Scholar]
- 47. Tapper K. Can mindfulness influence weight management related eating behaviors? If so, how? Clin Psychol Rev 2017; 53: 122–134. [DOI] [PubMed] [Google Scholar]
- 48. Gentile CL, Weir TL. The gut microbiota at the intersection of diet and human health. Science 2018; 362: 776–780. [DOI] [PubMed] [Google Scholar]
- 49. Compare D, Rocco A, Sanduzzi Zamparelli M, et al. The gut bacteria-driven obesity development. Dig Dis 2016; 34: 221–229. [DOI] [PubMed] [Google Scholar]
- 50. Chiriac MT, Mahapatro M, Neurath MF, et al. The microbiome in visceral medicine: inflammatory bowel disease, obesity and beyond. Visc Med 2017; 33: 153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. De Vadder F, Kovatcheva-Datchary P, Goncalves D, et al. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 2014; 156: 84–96. [DOI] [PubMed] [Google Scholar]
- 52. Villamil SI, Huerlimann R, Morianos C, et al. Adverse effect of early-life high-fat/high-carbohydrate (“Western”) diet on bacterial community in the distal bowel of mice. Nutr Res 2018; 50: 25–36. [DOI] [PubMed] [Google Scholar]
- 53. Canfora EE, van der Beek CM, Jocken JWE, et al. Colonic infusions of short-chain fatty acid mixtures promote energy metabolism in overweight/obese men: a randomized crossover trial. Sci Rep 2017; 7: 2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kelly D, Kelly A, O’Dowd T, et al. Antibiotic use in early childhood and risk of obesity: longitudinal analysis of a national cohort. World J Pediatr 2019; 15: 390–397. [DOI] [PubMed] [Google Scholar]
- 55. Kamphorst K, Oosterloo BC, Vlieger AM, et al. Antibiotic treatment in the first week of life impacts the growth trajectory in the first year of life in term infants. J Pediatr Gastroenterol Nutr 2019; 69: 131–136. [DOI] [PubMed] [Google Scholar]
- 56. Reijnders D, Goossens GH, Hermes GD, et al. Effects of gut microbiota manipulation by antibiotics on host metabolism in obese humans: a randomized double-blind placebo-controlled trial. Cell Metab 2016; 24: 63–74. [DOI] [PubMed] [Google Scholar]
- 57. Lyte JM. Eating for 3.8 × 1013: examining the impact of diet and nutrition on the microbiota-gut-brain axis through the lens of microbial endocrinology. Front Endocrinol (Lausanne) 2018; 9: 796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Singh A, Mittal M. Neonatal microbiome - a brief review. J Matern Fetal Neonatal Med 2019: 1–8. [DOI] [PubMed] [Google Scholar]
- 59. Russell WR, Baka A, Bjorck I, et al. Impact of diet composition on blood glucose regulation. Crit Rev Food Sci Nutr 2016; 56: 541–590. [DOI] [PubMed] [Google Scholar]
- 60. Isken F, Klaus S, Osterhoff M, et al. Effects of long-term soluble vs. insoluble dietary fiber intake on high-fat diet-induced obesity in C57BL/6J mice. J Nutr Biochem 2010; 21: 278–284. [DOI] [PubMed] [Google Scholar]
- 61. Weickert MO, Arafat AM, Blaut M, et al. Changes in dominant groups of the gut microbiota do not explain cereal-fiber induced improvement of whole-body insulin sensitivity. Nutr Metab (Lond) 2011; 8: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Weickert MO, Pfeiffer AFH. Impact of dietary fiber consumption on insulin resistance and the prevention of type 2 diabetes. J Nutr 2018; 148: 7–12. [DOI] [PubMed] [Google Scholar]
- 63. Kopp W. How western diet and lifestyle drive the pandemic of obesity and civilization diseases. Diabetes Metab Syndr Obes 2019; 12: 2221–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zinocker MK, Lindseth IA. The Western diet-microbiome-host interaction and its role in metabolic disease. Nutrients. Epub ahead of print 17 March 2018. DOI: 10.3390/nu10030365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kyrou I, Randeva HS, Tsigos C, et al. Clinical problems caused by obesity. In: Feingold KR, Anawalt B, Boyce A, et al. , (eds) Endotext. South Dartmouth, MA: MDText.com, Inc, 2000. [Google Scholar]
- 66. Engin A. Eat and death: chronic over-eating. Adv Exp Med Biol 2017; 960: 53–80. [DOI] [PubMed] [Google Scholar]
- 67. Hemmingsson E. A new model of the role of psychological and emotional distress in promoting obesity: conceptual review with implications for treatment and prevention. Obes Rev 2014; 15: 769–779. [DOI] [PubMed] [Google Scholar]
- 68. Hemmingsson E, Johansson K, Reynisdottir S. Effects of childhood abuse on adult obesity: a systematic review and meta-analysis. Obes Rev 2014; 15: 882–893. [DOI] [PubMed] [Google Scholar]
- 69. Ogden J, Coop N, Cousins C, et al. Distraction, the desire to eat and food intake. Towards an expanded model of mindless eating. Appetite 2013; 62: 119–126. [DOI] [PubMed] [Google Scholar]
- 70. Panahi S, Tremblay A. Sedentariness and health: is sedentary behavior more than just physical inactivity? Front Public Health 2018; 6: 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. O’Donoghue G, Perchoux C, Mensah K, et al. A systematic review of correlates of sedentary behaviour in adults aged 18–65 years: a socio-ecological approach. BMC Public Health 2016; 16: 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Singh RK, Kumar P, Mahalingam K. Molecular genetics of human obesity: a comprehensive review. C R Biol 2017; 340: 87–108. [DOI] [PubMed] [Google Scholar]
- 73. Frayling TM, Timpson NJ, Weedon MN, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 2007; 316: 889–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lowell BB, Bachman ES. Beta-Adrenergic receptors, diet-induced thermogenesis, and obesity. J Biol Chem 2003; 278: 29385–29388. [DOI] [PubMed] [Google Scholar]
- 75. Ochoa MC, Marti A, Azcona C, et al. Gene-gene interaction between PPAR gamma 2 and ADR beta 3 increases obesity risk in children and adolescents. Int J Obes Relat Metab Disord 2004; 28(Suppl. 3): S37–S41. [DOI] [PubMed] [Google Scholar]
- 76. Reddy NL, Jones TA, Wayte SC, et al. Identification of brown adipose tissue using MR imaging in a human adult with histological and immunohistochemical confirmation. J Clin Endocrinol Metab 2014; 99: E117–E121. [DOI] [PubMed] [Google Scholar]
- 77. Reddy NL, Tan BK, Barber TM, et al. Brown adipose tissue: endocrine determinants of function and therapeutic manipulation as a novel treatment strategy for obesity. BMC Obes 2014; 1: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lowell BB, Flier JS. Brown adipose tissue, beta 3-adrenergic receptors, and obesity. Annu Rev Med 1997; 48: 307–316. [DOI] [PubMed] [Google Scholar]
- 79. Wang Q, Zhang M, Xu M, et al. Brown adipose tissue activation is inversely related to central obesity and metabolic parameters in adult human. PLoS One 2015; 10: e0123795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Saito M, Okamatsu-Ogura Y, Matsushita M, et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 2009; 58: 1526–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Suviolahti E, Oksanen LJ, Ohman M, et al. The SLC6A14 gene shows evidence of association with obesity. J Clin Invest 2003; 112: 1762–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Widiker S, Karst S, Wagener A, et al. High-fat diet leads to a decreased methylation of the Mc4r gene in the obese BFMI and the lean B6 mouse lines. J Appl Genet 2010; 51: 193–197. [DOI] [PubMed] [Google Scholar]
- 83. Campion J, Milagro FI, Martinez JA. Individuality and epigenetics in obesity. Obes Rev 2009; 10: 383–392. [DOI] [PubMed] [Google Scholar]
- 84. Locke AE, Kahali B, Berndt SI, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 2015; 518: 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Albuquerque D, Nobrega C, Manco L, et al. The contribution of genetics and environment to obesity. Br Med Bull 2017; 123: 159–173. [DOI] [PubMed] [Google Scholar]
- 86. Yengo L, Sidorenko J, Kemper KE, et al. Meta-analysis of genome-wide association studies for height and body mass index in approximately 700000 individuals of European ancestry. Hum Mol Genet 2018; 27: 3641–3649. [DOI] [PMC free article] [PubMed] [Google Scholar]