Abstract
Hypoglycaemia remains an inevitable risk in insulin-treated type 1 diabetes and type 2 diabetes and has been associated with multiple adverse outcomes. Whether hypoglycaemia is a cause of fatal cardiac arrhythmias in diabetes, or merely a marker of vulnerability, is still unknown. Since a pivotal report in 1991, hypoglycaemia has been suspected to induce cardiac arrhythmias in patients with type 1 diabetes, the so-called ‘dead-in-bed syndrome’. This suspicion has subsequently been supported by the coexistence of an increased mortality and a three-fold increase in severe hypoglycaemia in patients with type 2 diabetes receiving intensive glucose-lowering treatment in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Studies have investigated the association between hypoglycaemia-induced cardiac arrhythmias. In a rat-model, severe hypoglycaemia resulted in a specific pattern of cardiac arrhythmias including QT-prolongation, ventricular tachycardia, second- and third-degree AV block and ultimately cardiorespiratory arrest. In clinical studies of experimentally induced hypoglycaemia, QTc-prolongation, a risk factor of ventricular arrhythmias, is an almost consistent finding. The extent of QT-prolongation seems to be modified by several factors, including antecedent hypoglycaemia, diabetes duration and cardiac autonomic neuropathy. Observational studies indicate diurnal differences in the pattern of electrocardiographic alterations during hypoglycaemia with larger QTc-prolongations during daytime, whereas the risk of bradyarrhythmias may be increased during sleep. Daytime periods of hypoglycaemia are characterized by shorter duration, increased awareness and a larger increase in catecholamines. The counterregulatory response is reduced during nightly episodes of hypoglycaemia, resulting in prolonged periods of hypoglycaemia with multiple nadirs. An initial sympathetic activity at plasma glucose nadir is replaced by increased vagal activity, which results in bradycardia. Here, we provide an overview of the existing literature exploring potential mechanisms for hypoglycaemia-induced cardiac arrhythmias and studies linking hypoglycaemia to cardiac arrhythmias in patients with diabetes.
Keywords: cardiac arrhythmias, diabetes complications, hypoglycaemia, type 1 diabetes, type 2 diabetes
Introduction
Type 1 and type 2 diabetes are characterized by elevated plasma glucose levels and increased cardiovascular mortality.1 Since the UK Prospective Diabetes Study (UKPDS) was published in 1998, it has been recognised that intensive glycaemic control reduces the risk of microvascular disease in patients with newly diagnosed type 2 diabetes.2 Moreover, the 10-year follow up demonstrated a significant reduction in myocardial infarction and cardiovascular mortality in patients receiving intensive therapy.3 Nevertheless, in several subsequent trials exploring the effect of intensive glycaemic control versus less intensive glycaemic control, intensive glucose-lowering therapy failed to reduce the risk of cardiovascular disease and cardiovascular mortality (Table 1). Notably, in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial, intensive glycaemic control resulted in a significant increase in all-cause mortality, driven mainly by a 35% increase in cardiovascular mortality compared with patients on standard therapy, which led to an early discontinuation of the trial.4 Both groups in the ACCORD trial were treated with insulin, sulphonylureas, metformin and thiazolidinediones. Since the ACCORD trial was discontinued, two new classes of glucose-lowering drugs have been shown to have beneficial effects on cardiovascular morbidity and mortality in patients with type 2 diabetes in large-scale cardiovascular outcome trials.5,6 Interestingly, the beneficial effects on cardiovascular disease seem to be independent of HbA1c reductions, and the cardiovascular effect of intensive glucose-lowering therapy is still a subject of some controversy. Importantly, patients randomized to intensive glycaemic control in the ACCORD trial had a threefold increase in incidence of severe hypoglycaemia, and it has been hypothesized that the marked increase in incidence of hypoglycaemia may account for some of the observed increase in cardiovascular mortality.4 However, it has also been argued that the association between hypoglycaemia and cardiovascular disease may be explained largely by an increased vulnerability in patients experiencing hypoglycaemia.7 Nevertheless, in a meta-analysis of retrospective and prospective cohort studies in patients with type 2 diabetes, severe hypoglycaemia was found to double the risk of cardiovascular disease, which could not be explained entirely by differences in comorbidities.8
Table 1.
Large-scale clinical trials comparing intensive glucose-lowering therapy with standard therapy.
| Study | Population | Design | Hypoglycaemia | CVD | All-cause mortality |
|---|---|---|---|---|---|
| DCCT/EDIC 9,10 | Patients with T1D aged 13–39 years (N = 1441) | Conventional treatment with 1–2 daily insulin injections
versus intensive treatment with ⩾3 daily
injections or insulin pump Mean follow up of 6.5 years |
Threefold increase in severe hypoglycaemia in the intensive treatment group (p < 0.001) | No difference after the initial 6.5 years of
follow-up. 57% reduction in in MACE (p = 0.02) after median follow up of 17 years |
No difference between groups |
| UKPDS3 11,12 | Patients with newly diagnosed T2D (N = 4209) | Intensive treatment (metformin and SU/insulin)
versus conventional therapy (primarily
diet) Median follow up of 10 years |
Two-fold increase in severe hypoglycaemia in the intensive treatment group (p < 0.0001) | No difference after the initial 10-years of follow
up Significant reductions in both SU/insulin and metformin group after 10 years of post-trial follow up MI SU/insulin: RR 0.85 (p = 0.01) Metformin: RR 0.67 (p = 0.005) Stroke SU/insulin: RR 0.91 (p = 0.39) Metformin: RR 0.80 (p = 0.35) |
No difference for Insulin/SU group 36% reduction in the obese metformin group (p = 0.01) 13% (p = 0.007) and 17% (p = 0.002) reduction after 10 years of post-trial follow up |
| ACCORD 4 | Patients with T2D and established CVD (35%) or high CV risk (N = 10,251) | Intensive glycaemic control (HbA1c<6.0%)
versus standard therapy (HbA1c
7.0–7.9%) All glucose-lowering therapies allowed. Discontinued after a mean follow up of 3.5 years |
Percentage of patients experiencing at least one episode of
severe hypoglycaemia 16.2 versus 5.1% (p < 0.001), respectively |
MACE HR 0.90 (p = 0.16) CV mortality HR 1.35 (p = 0.02) Nonfatal MI HR 0.76 (p = 0.004) Nonfatal stroke HR 1.06 (p = 0.74) CV mortality remained significantly increased after a mean 7.7 years of follow up HR 1.20 (p = 0.02) |
HR 1.22 (p = 0.04) All-cause mortality normalized after a mean of 7.7 years of follow up HR 1.01 (p = 0.91) |
| ADVANCE 13,14 | Patients with T2D and microvascular or macrovascular complication or ⩾ 1 risk factors (N = 11,140) | Intensive treatment (HbA1c<6.5%)
versus standard treatment. Median follow up of 5 years |
Significant increase in severe hypoglycaemia in the intensive
treatment group HR 1.86 (p < 0.001) |
MACE HR 0.94 (p = 0.37) CV mortality HR 0.88 (p = NS) Nonfatal MI HR 1.02 (p = NS) Nonfatal stroke HR 0.98 (p = NS) No effect observed after 5.4 years of post-trial follow up |
HR 0.93 (p = 0.28) No effect observed after 5.4 years of post-trial follow up |
| VADT 15,16 | Patients with T2D treated with insulin or maximal-dose oral agent (N = 1791) | Intensive treatment (HbA1c<6.0%)
versus standard therapy. Median follow up of 5.6 years |
Significant increase in hypoglycaemia in the intensive treatment group (p < 0.001) | No difference in the primary composite CV endpoint HR 0.88 (p = 0.14) Significant reduction in the primary composite CV endpoint after 9.8 years of follow up HR 0.83 (p = 0.04) |
HR 1.07 (p = 0.62) No reduction after 9.8 years of follow up HR 1.05 (p = 0.54) |
| ORIGIN 17 | Patients with IFG, IGT or T2D and CV risk factors (N = 12,537) | Early treatment with insulin glargine (target FPG 5.3 mmol/l)
versus standard care. Median follow up of 6.2 years |
Threefold increase in severe hypoglycaemia (p < 0.001) | MACE HR 1.03 (p = 0.63) CV death HR 1.00 (p = 0.98) |
HR 0.98 (p = 0.70) |
CV, cardiovascular; CVD, cardiovascular disease; FPG, fasting plasma glucose; HbA1c, glycated haemoglobin; HR, hazard ratio; IFG, impaired fasting glucose; IGT impaired glucose tolerance; MACE, major adverse cardiovascular event (composite endpoint of cardiovascular death, nonfatal myocardial infarction and nonfatal stroke); MI, myocardial infarction; NS, nonsignificant; RR, relative risk; SU, sulphonylurea; T1D, type 1 diabetes; T2D, type 2 diabetes.
A potential mechanism by which hypoglycaemia may increase cardiovascular mortality in patients with diabetes is through induction of fatal cardiac arrhythmias. Since a case series of unexplained deaths among patients with type 1 diabetes (most of whom had gone to bed in apparently good health and been found dead in the morning) was published 1991, insulin-induced hypoglycaemia has been associated with sudden overnight death in patients with type 1 diabetes, the so-called ‘dead-in-bed syndrome’.18 Epidemiological data have shown that sudden unexplained death may be increased 10-fold in type 1 diabetes compared with individuals without diabetes.19 However, since cardiac arrhythmias are relatively rare and may be clinically asymptomatic, it has proved difficult to demonstrate a direct causal relationship. The present review aims to describe potential mechanisms by which hypoglycaemia may induce cardiac arrhythmias and provide an overview of the current available data linking hypoglycaemia to cardiac arrhythmias in patients with diabetes.
Preclinical studies
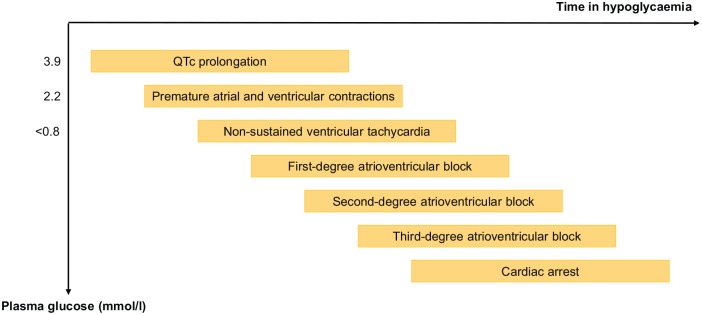
The full pathway from hypoglycaemia to fatal cardiac arrhythmia has been explored in preclinical studies in rats. During a hyperinsulinaemic hypoglycaemic clamp targeting a plasma glucose of 0.6–0.8 mmol/l, a typical pattern of electrocardiographic abnormalities was observed (Figure 1).20 Initial changes consisted of prolongation of corrected QT (QTc) interval, which was followed by an increasing number of premature atrial and ventricular contractions and first-degree atrioventricular (AV) block. This was followed occasionally by nonsustained ventricular tachycardia before evolving to high-grade second-degree AV block and third-degree AV block. Before going into cardiorespiratory arrest, a lower-voltage electrocardiogram (ECG) with a markedly reduced frequency was noted. Interestingly, when cerebral glucose levels were maintained at basal levels through an intracerebroventricular glucose infusion, the adrenergic response during hypoglycaemia was blunted markedly, and incidence of cardiac arrhythmias and mortality reduced significantly. Similarly, the intravenous infusion of either alpha/beta-blockers or beta-blockers completely prevented death during hypoglycaemia (p = 0.029) and intravenous supplementation of plasma potassium tended to reduce mortality (p < 0.08). These findings indicate that cardiac arrhythmias during insulin-induced hypoglycaemia are mediated through central activation of the sympathetic nervous system, which results in direct stimulation of the heart as well as an indirect effects through a marked increase in plasma catecholamines. The role of reductions in plasma potassium is less clear.20
Figure 1.
Pattern of electrocardiographic changes during insulin-induced severe hypoglycaemia in a rat model as described by Reno and colleagues.20
QTc, corrected QT interval.
In a diabetic rodent model with streptozotocin-treated diabetic rats, insulin-deficiency more than doubled the risk of third-degree AV block and death during severe hypoglycaemia compared with nondiabetic rats.21 However, exposure of streptozotocin-treated rats to antecedent moderate hypoglycaemia the days before being exposed to hypoglycaemia resulted in reduced sympatoadrenergic response during severe hypoglycaemia and a significant reduction in third-degree AV block and mortality compared with nondiabetic rats.21 Hence, while diabetes might increase the risk of fatal cardiac arrhythmias during severe hypoglycaemia, antecedent hypoglycaemia blunts the sympatoadrenergic response to hypoglycaemia, which might serve as a mediator of cardiac arrhythmias during severe hypoglycaemia.21
Although the abovementioned findings point towards a central role for catecholamines and the sympathetic nervous system in cardiac arrhythmias during severe hypoglycaemia, they have recently been contradicted by a study from the same group.22 When performing hyperinsulinaemic hypoglycaemic clamps in nondiabetic rats, neither adrenal demedullation nor chemical sympathectomy reduced the incidence of cardiac arrhythmias or death or prevented QTc prolongation. On the contrary, left-side vagotomy led to a seven-fold decrease in mortality, a four-fold reduction in first- and second-degree AV block and a complete absence of third-degree AV block. Likewise, pharmacological blockade of nicotinic receptors completely prevented death and nearly prevented any arrhythmia. These findings indicate a strong role for the parasympathetic nervous systems as a mediator of cardiac arrhythmias during hypoglycaemic conditions.22
Experimentally induced hypoglycaemia in clinical studies
The electrocardiographic changes in humans during hypoglycaemia have been explored in several studies employing experimental, insulin-induced hypoglycaemia.23–33 In studies applying the hyperinsulinaemic hypoglycaemic clamp, QTc interval prolongation is an almost consistent finding in patients with type 1 diabetes and type 2 diabetes, respectively, as well as healthy control subjects.23–31 QTc interval has previously been demonstrated to be a predictor of cardiovascular mortality and all-cause mortality in patients with diabetes34,35 and QTc interval prolongation increases the risk of developing ventricular arrhythmias.36 However, the magnitude of QTc interval prolongation during experimental hypoglycaemia varies with the applied method of correcting for heart rate. Multiple methods for heart rate correction have been developed, and, while Bazett’s correction is most widely used, it is generally recognized to overcorrect when the heart rate is in the high range, whereas Fridericia’s correction remains reliable in the high range.37 Indeed, in one study, a significant change in QTc interval during hypoglycaemia compared with baseline was found only when Bazett’s formula for correction of heart rate was applied, whereas Fridericia’s formula and the nomogram method both resulted in nonsignificant changes.32
As observed in some preclinical studies, QTc interval prolongation during hypoglycaemia appears to be mediated through sympathoadrenal stimulation.30 It has been demonstrated that 1 week of beta-adrenergic blockade with atenolol prevented QTc interval prolongation during hypoglycaemia in healthy individuals.30 This explains the large variation in QTc interval prolongation during hypoglycaemia, since the magnitude of the sympathoadrenal response during hypoglycaemia depends on several factors (Table 2). It is well-established that frequent or recent exposure to hypoglycaemia blunts the sympathoadrenal response during an episode of hypoglycaemia.38 Accordingly, patients with type 1 diabetes exhibit a reduced release of catecholamines and a reduced prolongation of QTc interval during hypoglycaemia compared with healthy subjects, and this trend is amplified by disease duration.25,29 Additionally, the development of cardiac autonomic neuropathy has been shown to reduce the QTc interval prolongation during hypoglycaemia.31 Taken together, these results indicate that the largest QTc interval prolongations during a spontaneous episode of insulin-induced hypoglycaemia might be found in relatively young patients with short diabetes duration.
Table 2.
Factors modifying QTc prolongation during hypoglycaemia in clinical studies.
| Factors decreasing QTc interval prolongation | Beta-blockade Antecedent hypoglycaemia Diabetes duration (T1D) Cardiac autonomic neuropathy |
| Factors increasing QTc interval prolongation | Time in hypoglycaemia Rate of plasma glucose decline (affects the onset of QTc interval prolongation) |
T1D, type 1 diabetes; QTc, corrected QT interval.
Time spent in hypoglycaemia may also play an important role in the extent of QTc interval prolongation. In one study, a progressive prolongation of QTc interval was observed, when plasma glucose was kept at a stable level of <3.0 mmol/l in 80 min, indicating an increased risk of cardiac arrhythmias during prolonged periods of hypoglycaemia.29 Furthermore, one study has suggested that the rate of decline in plasma glucose might affect the time of onset of QTc interval prolongation.26 In the latter study, an intravenous bolus of insulin resulted in a rapid decline in plasma glucose and a significant QTc prolongation at a plasma glucose level of 7.2 mmol/l. While these results have not yet been reproduced, it indicates a role for poor glycaemic control with large and rapid glycaemic fluctuations in QTc prolongations.
In addition to changes in QTc interval, several other electrocardiographic changes occur during hypoglycaemia, including increased QT dispersion and changes to T-wave morphology.24,28 Although these measures of cardiac repolarization have attracted less attention than the QTc interval, these findings add to the concept of an abnormal cardiac repolarization during hypoglycaemia. Another consistent finding during experimental hypoglycaemia is a significant reduction in plasma potassium, which is known to potentially induce QTc prolongations.23,24,28,30,31,39 However, in a study including patients with type 2 diabetes, changes in plasma potassium was found not to correlate with QTc prolongation, and, whereas increases in QTc dispersion were prevented by a potassium infusion in a study including healthy individuals, QTc interval remained unaffected.24,30
The studies described above all utilized intravenously administrated insulin. The findings have been reproduced in a study aiming to mimic a spontaneous episode of hypoglycaemia. An individualized subcutaneous bolus of insulin was administrated in 10 patients with type 1 diabetes, targeting a plasma glucose of 2.4 mmol/l, and significant QTc prolongations were found during hypoglycaemia compared with euglycaemia.33
Spontaneous hypoglycaemia in clinical studies
Several studies link spontaneous episodes of hypoglycaemia to cardiac arrhythmias.40–48 As in studies of experimentally induced hypoglycaemia, QTc prolongation is an almost consistent finding in both patients with either type 1 and type 2 diabetes.40–45 Nevertheless, the results are conflicting, and a study by Koivikko and colleagues including patients with type 1 diabetes (n = 11) for overnight continuous glucose monitoring (CGM) and ECG monitoring found a significant reduction in QTc interval during hypoglycaemia.42,46,47 These conflicting results may largely be explained by methodological problems. As alluded to above, several tools have been developed to correct QTc interval for differences in heart rate, and the use of Bazett’s correction, which is widely used, results in greater estimates of QTc interval than the remaining methods.37 Furthermore, T-wave morphology is changed markedly during hypoglycaemia, resulting in flattening of the T-wave, which complicates accurate measurement of the QTc interval. Nevertheless, Koivikko and colleagues still found significant changes in cardiac repolarisation consistent with increased risk of cardiac arrhythmias and cardiovascular mortality.47
In an attempt to further link spontaneous hypoglycaemia to clinical episodes of cardiac arrhythmias under real-life circumstances, several studies employing concomitant Holter and CGM monitoring in an ambulatory setting in patients with type 1 and type 2 diabetes, respectively, have been performed.43,44,48 In patients with insulin-treated type 2 diabetes (N = 25) undergoing 5 days of concomitant CGM and Holter monitoring, the incidence of bradycardia (defined as ⩾4 consecutive beats at <45 bpm) was eightfold higher during nocturnal hypoglycaemia compared with euglycaemic periods.43 Furthermore, a significant increase in atrial ectopic beats and ventricular premature beats were detected during nocturnal hypoglycaemia. During the day, a small, but significant, increase in ventricular premature beats was found, whereas no episodes of bradycardia were detected. The differences between daytime and nocturnal episodes have been explained by an insufficient counterregulatory response during sleep resulting in a prolonged duration of nocturnal hypoglycaemic episodes with multiple and lower nadirs.43 During the nocturnal episodes of hypoglycaemia, an initial pattern of transient cardiac acceleration at plasma glucose nadir followed by an increased vagal counteraction associated with bradycardia was found. Hence, bradycardia during nocturnal hypoglycaemia may be explained by sympathetic withdrawal followed by vagal overcompensation.43 A similar pattern of an initial increase in heart rate followed by vagal reactivation and a decline in heart rate was subsequently reproduced in patients with type 2 diabetes during a hypoglycaemic clamp.28 Interestingly, healthy control participants demonstrated continued vagal withdrawal throughout the hypoglycaemic period.
In a study involving 4 days of concomitant CGM and Holter monitoring in patients with type 1 diabetes (N = 37), a sixfold increase in risk of bradycardia during nocturnal hypoglycaemia was found when compared with nocturnal euglycaemia.44 Furthermore, atrial ectopics were more frequent during daytime hypoglycaemia when compared with daytime euglycemia. However, a subsequent analysis of data from this study has shown that the increase in bradycardia during nocturnal hypoglycaemia was driven primarily by a single participant with a markedly higher number of bradycardia beats per hour than the remaining study participants.44 Accordingly, no increased risk of bradycardia during hypoglycaemia was found when this specific participant was removed from the analysis.49 This led the authors to conclude that increased risk of bradycardia may apply only to certain individuals highly susceptible to bradycardia.
In another study, patients with type 2 diabetes (N = 94) and established cardiovascular disease underwent 5 days of concomitant CGM and Holter monitoring. It was found that patients experiencing episodes of hypoglycaemia had a significantly higher number of ventricular tachycardias.48 Whereas the study did not investigate the time relation between episodes of hypoglycaemia and initiation of cardiac arrhythmias, duration of hypoglycaemia was found to be an independent risk factor of ventricular tachycardia. Although the latter study has some obvious methodological limitations since it did not investigate the time-relation between hypoglycaemic episodes and arrhythmic events, the hypoglycaemia group and the no-hypoglycaemia group were similar in age, diabetes duration, diabetic complications, diabetes treatment and prevalence of cardiovascular disease. This indicates that hypoglycaemia is not only a marker of vulnerability to cardiac arrhythmias.
Discussion
Although considerable advances in the treatment of diabetes have been made during recent years including more stable insulins analogues, insulin pumps and CGM monitoring, hypoglycaemia remains an inevitable risk in patients with insulin-treated diabetes.50 Presumably, only a small proportion of patients with diabetes are likely to develop clinically significant, potentially fatal cardiac arrhythmias during hypoglycaemic conditions, and these patients are difficult to identify.49 Multiple factors may affect the individual risk of developing cardiac arrhythmias in relation to an episode of hypoglycaemia. Genetic factors affecting the function of cardiac ion channels and cardiac fibrosis have been suggested as an underlying predisposing factor.51 Furthermore, in patients with type 2 diabetes or longstanding type 1 diabetes and established cardiovascular disease, functional and structural remodelling resulting in an ischaemic substrate to ventricular tachycardias may also be an important factor.
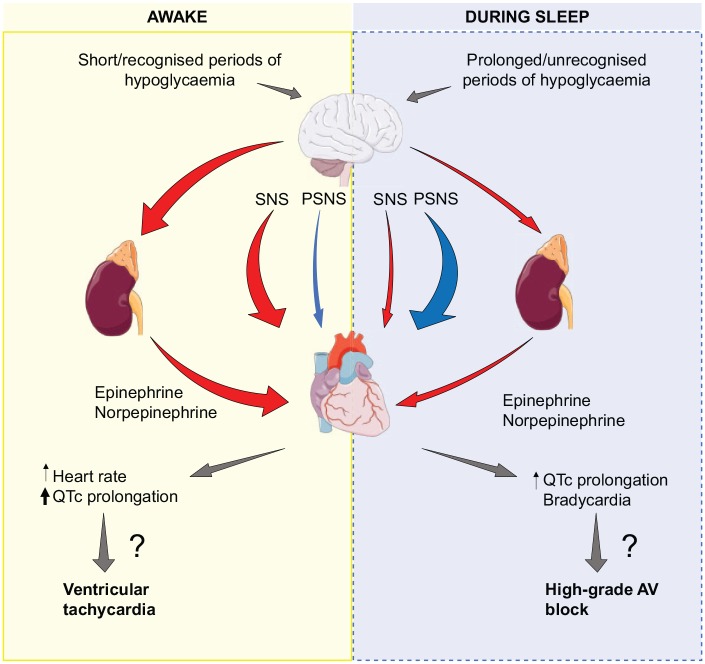
At least during daytime, a maintained counterregulatory response during hypoglycaemia with a large sympathoadrenal response appears to be an essential factor to the electrocardiographic changes found in clinical studies (Figure 2).25,29 Hence, theoretically, daytime cardiac arrhythmias are most likely to occur in patients not frequently exposed to hypoglycaemia who are experiencing an episode of severe hypoglycaemia, such as young, newly diagnosed patients with type 1 diabetes. Interestingly, in a post hoc analysis of data from the ACCORD study, the cardiovascular event rate was significantly lower in patients treated with beta-blockers in the intensive treatment arm compared with patients treated with beta-blockers and receiving standard therapy.52 Furthermore, in patients receiving beta-blockers, no difference in all-cause mortality was found between the intensively treated arm and the standard therapy arm. While these findings indicate that beta-blockers may counteract the deleterious effects of hypoglycaemia, the use of betablockers itself may dampen early symptoms of hypoglycaemia and thereby promote hypoglycaemia. In another post hoc analysis of the ACCORD study, the use of beta-blockers was found to increase the incidence of severe hypoglycaemia and increase the risk of cardiovascular events.53 Hence, the cardiovascular effect of beta-blockers in patients with diabetes is ambiguous, and randomized, controlled trials are needed.
Figure 2.
Proposed mechanism for hypoglycaemia-induced cardiac arrhythmias. Sympathoadrenal activity and parasympathetic activity is depicted with red arrows and blue arrows, respectively. When awake, a marked increase in catecholamines during episodes of hypoglycaemia results in an increase in heart rate and QTc, which is a well-stablished risk factor of ventricular arrhythmias. At night, the sympathoadrenal response is blunted and QTc prolongations less pronounced. The initial sympathoadrenal response is followed by increased vagal activity resulting in bradycardia. Preclinical studies have indicated that bradycardia may be the initial step leading to high-grade AV-block and cardiac arrest during severe hypoglycaemia.
AV block, atrioventricular block; PSNS, parasympathetic nervous system; QTc, corrected QT interval; SNS, sympathetic nervous system.
Whereas sympathoadrenal activation traditionally have been suspected to be the key mediator of hypoglycaemia-induced cardiac arrhythmias, several studies suggest that the parasympathetic nervous system may be responsible for nocturnal brady-arrhythmias.22,28,43,44 These studies indicate two completely different mechanisms for the development of cardiac arrhythmias during the day and at night. A relatively blunted sympathetic response during prolonged episodes of hypoglycaemia may lead to sympathoadrenal exhaustion and an over compensatory parasympathetic stimulation, and eventually a cascade of bradyarrhythmias and cardiac arrest as seen in preclinical studies. Nevertheless, it should be recognized that a large gap exists between the increased risk of bradycardia during nocturnal hypoglycaemia found in observational real-life studies, and the fatal bradyarrhythmias observed during very severe hypoglycaemia in a rodent model.
Although a considerable amount of evidence supports the view that hypoglycaemia may induce cardiac arrhythmias in patients with diabetes, a direct causal link between hypoglycaemia and clinically relevant cardiac arrhythmias requiring intervention has not yet been demonstrated. Since prospective randomized trials would be unethical, future research should focus on establishing whether such a link exists by employing an observational design and taking advantage of modern tools for long-term, real-life monitoring. Additionally, further research is needed to delineate the role of the autonomic nervous system and catecholamines as mediators of hypoglycaemia-induced cardiac arrhythmia.
Footnotes
Authors Contribution: AA researched data for the article and wrote the first draft. AA, PGJ, FKK and TV contributed substantially to the discussion of content and reviewed and/or edited the article before submission.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflicts of interest statement: A.A. has no conflicts of interest. P.G.J. has received lecture fee from Novo Nordisk. F.K.K. has served on scientific advisory panels and been part of speaker’s bureaus for, served as a consultant to or received research support from Amgen, AstraZeneca, Boehringer Ingelheim, Carmot Therapeutics, Eli Lilly, Gubra, Lupin, MedImmune, MSD/Merck, Mundipharma, Norgine, Novo Nordisk, Sanofi and Zealand Pharma. T.V. declares personal fees from Amgen, Boehringer Ingelheim, Eli Lilly, AstraZeneca, Merck Sharp and Dohme, Mundipharma, Sanofi, Sun Pharma, Novo Nordisk and Bristol-Myers Squibb, and grants (to her institution) from Eli Lilly, Boehringer Ingelheim, and Novo Nordisk.
ORCID iDs: Andreas Andersen  https://orcid.org/0000-0001-8190-5140
https://orcid.org/0000-0001-8190-5140
Peter G. Jørgensen  https://orcid.org/0000-0002-1217-8944
https://orcid.org/0000-0002-1217-8944
Filip K. Knop  https://orcid.org/0000-0002-2495-5034
https://orcid.org/0000-0002-2495-5034
Contributor Information
Andreas Andersen, Steno Diabetes Center Copenhagen, Gentofte Hospital, Hellerup, Denmark; Center for Clinical Metabolic Research, Herlev and Gentofte Hospital, University of Copenhagen, Hellerup, Denmark.
Peter G. Jørgensen, Department of Cardiology, Herlev and Gentofte Hospital, University of Copenhagen, Hellerup, Denmark
Filip K. Knop, Center for Clinical Metabolic Research, Herlev and Gentofte Hospital, University of Copenhagen, Hellerup, Denmark Steno Diabetes Center Copenhagen, Gentofte Hospital, Hellerup, Denmark; Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Novo Nordisk Foundation Center for Basic Metabolic Research, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark.
Tina Vilsbøll, Steno Diabetes Centre Copenhagen, Gentofte Hospital, Kildegårdsvej 28, Hellerup, 2900, Denmark; Center for Clinical Metabolic Research, Herlev and Gentofte Hospital, University of Copenhagen, Hellerup, Denmark; Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark.
References
- 1. Raghavan S, Vassy JL, Ho Y. et al. Diabetes mellitus–related all-cause and cardiovascular mortality in a national cohort of adults. J Am Heart Assoc; 8 Epub ahead of print 19 February 2019. DOI: 10.1161/JAHA.118.011295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998; 352: 854–865. [PubMed] [Google Scholar]
- 3. Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359: 1577–1589. [DOI] [PubMed] [Google Scholar]
- 4. Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358: 2545–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Andersen A, Lund A, Knop FK, et al. Glucagon-like peptide 1 in health and disease. Nat Rev Endocrinol 2018; 14: 390–403. [DOI] [PubMed] [Google Scholar]
- 6. Chilton RJ. Effects of sodium-glucose cotransporter-2 inhibitors on the cardiovascular and renal complications of type 2 diabetes. Diabetes Obes Metab. Epub ahead of print 13 August 2019. DOI: 10.1111/dom.13854. [DOI] [PubMed] [Google Scholar]
- 7. Zoungas S, Patel A, Chalmers J, et al. Severe hypoglycemia and risks of vascular events and death. N Engl J Med 2010; 363: 1410–1418. [DOI] [PubMed] [Google Scholar]
- 8. Goto A, Arah OA, Goto M, et al. Severe hypoglycaemia and cardiovascular disease: systematic review and meta-analysis with bias analysis. BMJ 2013; 347: f4533. [DOI] [PubMed] [Google Scholar]
- 9. Diabetes Control and Complications Trial Research Group, Nathan DM, Genuth S, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329: 977–986. [DOI] [PubMed] [Google Scholar]
- 10. Nathan DM, Cleary PA, Backlund J-YC, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353: 2643–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352: 837–853. [PubMed] [Google Scholar]
- 12. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998; 352: 854–865. [PubMed] [Google Scholar]
- 13. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358: 2560–2572. [DOI] [PubMed] [Google Scholar]
- 14. Zoungas S, Chalmers J, Neal B, et al. Follow-up of blood-pressure lowering and glucose control in type 2 diabetes. N Engl J Med 2014; 371: 1392–1406. [DOI] [PubMed] [Google Scholar]
- 15. Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360: 129–139. [DOI] [PubMed] [Google Scholar]
- 16. Hayward RA, Reaven PD, Wiitala WL, et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015; 372: 2197–2206. [DOI] [PubMed] [Google Scholar]
- 17. ORIGIN Trial Investigators, Gerstein HC, Bosch J, et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med 2012; 367: 319–328. [DOI] [PubMed] [Google Scholar]
- 18. Tattersall RB, Gill GV. Unexplained deaths of type 1 diabetic patients. Diabet Med J Br Diabet Assoc 1991; 8: 49–58. [DOI] [PubMed] [Google Scholar]
- 19. Secrest AM, Becker DJ, Kelsey SF, et al. Characterizing sudden death and dead-in-bed syndrome in Type 1 diabetes: analysis from two childhood-onset Type 1 diabetes registries. Diabet Med J Br Diabet Assoc 2011; 28: 293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Reno CM, Daphna-Iken D, Chen YS, et al. Severe hypoglycemia-induced lethal cardiac arrhythmias are mediated by sympathoadrenal activation. Diabetes 2013; 62: 3570–3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reno CM, VanderWeele J, Bayles J, et al. Severe hypoglycemia–induced fatal cardiac arrhythmias are augmented by diabetes and attenuated by recurrent hypoglycemia. Diabetes 2017; 66: 3091–3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Reno CM, Bayles J, Huang Y, et al. Severe hypoglycemia induced fatal cardiac arrhythmias are mediated by the parasympathetic nervous system in rats. Diabetes 2019; 68: 2107–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Marques JL, George E, Peacey SR, et al. Altered ventricular repolarization during hypoglycaemia in patients with diabetes. Diabet Med J Br Diabet Assoc 1997; 14: 648–654. [DOI] [PubMed] [Google Scholar]
- 24. Landstedt-Hallin L, Englund A, Adamson U, et al. Increased QT dispersion during hypoglycaemia in patients with type 2 diabetes mellitus. J Intern Med 1999; 246: 299–307. [DOI] [PubMed] [Google Scholar]
- 25. Limberg JK, Farni KE, Taylor JL, et al. Autonomic control during acute hypoglycemia in type 1 diabetes mellitus. Clin Auton Res 2014; 24: 275–283. [DOI] [PubMed] [Google Scholar]
- 26. Christensen TF, Lewinsky I, Kristensen LE, et al. QT interval prolongation during rapid fall in blood glucose in type I diabetes. In: 2007. Computers in Cardiology. Durham, NC, USA: IEEE, pp. 345–348. [Google Scholar]
- 27. Færch LH, Thorsteinsson B, Tarnow L, et al. Effects of angiotensin II receptor blockade on cerebral, cardiovascular, counter-regulatory, and symptomatic responses during hypoglycaemia in patients with type 1 diabetes. J Renin Angiotensin Aldosterone Syst 2015; 16: 1036–1045. [DOI] [PubMed] [Google Scholar]
- 28. Chow E, Bernjak A, Walkinshaw E, et al. Cardiac Autonomic regulation and repolarization during acute experimental hypoglycemia in type 2 diabetes. Diabetes 2017; 66: 1322–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lipponen JA, Kemppainen J, Karjalainen PA, et al. Dynamic estimation of cardiac repolarization characteristics during hypoglycemia in healthy and diabetic subjects. Physiol Meas 2011; 32: 649–660. [DOI] [PubMed] [Google Scholar]
- 30. Robinson RTCE, Harris ND, Ireland RH, et al. Mechanisms of abnormal cardiac repolarization during insulin-induced hypoglycemia. Diabetes 2003; 52: 1469–1474. [DOI] [PubMed] [Google Scholar]
- 31. Lee SP, Yeoh L, Harris ND, et al. Influence of autonomic neuropathy on QTc interval lengthening during hypoglycemia in type 1 diabetes. Diabetes 2004; 53: 1535–1542. [DOI] [PubMed] [Google Scholar]
- 32. Koivikko ML, Karsikas M, Salmela PI, et al. Effects of controlled hypoglycaemia on cardiac repolarisation in patients with type 1 diabetes. Diabetologia 2008; 51: 426–435. [DOI] [PubMed] [Google Scholar]
- 33. Christensen TF, Cichosz SL, Tarnow L, et al. Hypoglycaemia and QT interval prolongation in type 1 diabetes-bridging the gap between clamp studies and spontaneous episodes. J Diabetes Complications 2014; 28: 723–728. [DOI] [PubMed] [Google Scholar]
- 34. Cox AJ, Azeem A, Yeboah J, et al. Heart rate–corrected qt interval is an independent predictor of all-cause and cardiovascular mortality in individuals with type 2 diabetes: the diabetes heart study. Diabetes Care 2014; 37: 1454–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rossing P, Breum L, Major-Pedersen A, et al. Prolonged QTc interval predicts mortality in patients with Type 1 diabetes mellitus. Diabet Med 2001; 18: 199–205. [DOI] [PubMed] [Google Scholar]
- 36. Al-Khatib SM, LaPointe NMA, Kramer JM, et al. What clinicians should know about the QT interval. JAMA 2003; 289: 2120–2127. [DOI] [PubMed] [Google Scholar]
- 37. Vandenberk B, Vandael E, Robyns T, et al. Which QT correction formulae to use for QT monitoring? J Am Heart Assoc Cardiovasc Cerebrovasc Dis; 5 Epub ahead of print 17 June 2016. DOI: 10.1161/JAHA.116.003264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dagogo-Jack SE, Craft S, Cryer PE. Hypoglycemia-associated autonomic failure in insulin-dependent diabetes mellitus. Recent antecedent hypoglycemia reduces autonomic responses to, symptoms of, and defense against subsequent hypoglycemia. J Clin Invest 1993; 91: 819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lindström T, Jorfeldt L, Tegler L, et al. Hypoglycaemia and cardiac arrhythmias in patients with type 2 diabetes mellitus. Diabet Med J Br Diabet Assoc 1992; 9: 536–541. [DOI] [PubMed] [Google Scholar]
- 40. Robinson RTCE, Harris ND, Ireland RH, et al. Changes in cardiac repolarization during clinical episodes of nocturnal hypoglycaemia in adults with Type 1 diabetes. Diabetologia 2004; 47: 312–315. [DOI] [PubMed] [Google Scholar]
- 41. Gill GV, Woodward A, Casson IF, et al. Cardiac arrhythmia and nocturnal hypoglycaemia in type 1 diabetes—the ‘dead in bed’ syndrome revisited. Diabetologia 2008; 52: 42. [DOI] [PubMed] [Google Scholar]
- 42. Christensen TF, Tarnow L, Randløv J, et al. QT interval prolongation during spontaneous episodes of hypoglycaemia in type 1 diabetes: the impact of heart rate correction. Diabetologia 2010; 53: 2036–2041. [DOI] [PubMed] [Google Scholar]
- 43. Chow E, Bernjak A, Williams S, et al. Risk of cardiac arrhythmias during hypoglycemia in patients with type 2 diabetes and cardiovascular risk. Diabetes 2014; 63: 1738–1747. [DOI] [PubMed] [Google Scholar]
- 44. Novodvorsky P, Bernjak A, Chow E, et al. Diurnal differences in risk of cardiac arrhythmias during spontaneous hypoglycemia in young people with type 1 diabetes. Diabetes Care 2017; 40: 655–662. [DOI] [PubMed] [Google Scholar]
- 45. Lee AS, Brooks BA, Simmons L, et al. Hypoglycaemia and QT interval prolongation: detection by simultaneous Holter and continuous glucose monitoring. Diabetes Res Clin Pract 2016; 113: 211–214. [DOI] [PubMed] [Google Scholar]
- 46. Middleton TL, Wong J, Molyneaux L, et al. Cardiac effects of sulfonylurea-related hypoglycemia. Diabetes Care 2017; 40: 663–670. [DOI] [PubMed] [Google Scholar]
- 47. Koivikko ML, Kenttä T, Salmela PI, et al. Changes in cardiac repolarisation during spontaneous nocturnal hypoglycaemia in subjects with type 1 diabetes: a preliminary report. Acta Diabetol 2017; 54: 251–256. [DOI] [PubMed] [Google Scholar]
- 48. Pistrosch F, Ganz X, Bornstein SR, et al. Risk of and risk factors for hypoglycemia and associated arrhythmias in patients with type 2 diabetes and cardiovascular disease: a cohort study under real-world conditions. Acta Diabetol 2015; 52: 889–895. [DOI] [PubMed] [Google Scholar]
- 49. Campbell M, Heller SR, Jacques RM, et al. Response to comment on Novodvorsky et al. Diurnal differences in risk of cardiac arrhythmias during spontaneous hypoglycemia in young people with type 1 diabetes. Diabetes Care 2017; 40: 655–662. Diabetes Care 2018; 41: e65–e66. [DOI] [PubMed] [Google Scholar]
- 50. Frier BM, Jensen MM, Chubb BD. Hypoglycaemia in adults with insulin-treated diabetes in the UK: self-reported frequency and effects. Diabet Med 2016; 33: 1125–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tu E, Bagnall RD, Duflou J, et al. Post-mortem pathologic and genetic studies in “dead in bed syndrome” cases in type 1 diabetes mellitus. Hum Pathol 2010; 41: 392–400. [DOI] [PubMed] [Google Scholar]
- 52. Tsujimoto T, Sugiyama T, Noda M, et al. Intensive glycemic therapy in patients with type 2 diabetes on β-blockers. Diabetes Care 2016; 39: 1818–1826. [DOI] [PubMed] [Google Scholar]
- 53. Tsujimoto Tetsuro, Sugiyama Takehiro, Shapiro Martin F, et al. Risk of cardiovascular events in patients with diabetes mellitus on β-blockers. Hypertension 2017; 70: 103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]