Abstract
Background:
The 5 m gait speed (5MGS), a simple and reliable performance metric and surrogate indicator of frailty, consistently predicts adverse events in elders. Additionally, MELD-Na (model for end-stage liver disease-sodium) scores fail to capture nutritional and functional decline of cirrhotic patients that may confer excess mortality. We hypothesized that 5MGS might be associated with all-cause mortality, and that inclusion of frailty assessment within MELD-Na could improve the prediction of mortality in cirrhosis.
Methods:
5MGS was measured at baseline in 113 hospitalized cirrhotic patients. Survival status over 2 years and cirrhosis-related complications were recorded. We evaluated the prognostic value of 5MGS (as a continuous variable and as a dichotomous variable). The definition of slow versus preserved 5MGS was 0.8 ms−1 based on previous publication. Using Cox proportional hazards regression, a novel MELDNa-5MGS score was derived. Receiver operating characteristics (ROC) curves estimated discrimination between the new score model and established prognostic indices.
Results:
The continuous 5MGS and slow 5MGS were independent predictors of all-cause mortality [5MGS: hazard ratio (HR) 0.133 (0.047–0.347), p < 0.001; slow 5MGS: HR 4.805 (1.536–15.026), p < 0.007]. The equation derived from Cox regression analysis was as follows: MELDNa-5MGS: MELD-Na score + 11 × slow 5MGS. The 2-year mortality in patients with high MELDNa-5MGS score was significantly higher (p < 0.001). Discriminatory power was significantly better for MELDNa-5MGS than MELD-Na score (AUC: 0.802 versus 0.724, p = 0.014 for 1 year; 0.773 versus 0.709, p = 0.044 for 2 years).
Conclusion:
In cirrhotic patients, 5GMS is an independent risk factor of mortality. Modification of MELD-Na to include frailty estimated by low 5GMS is related to improved prognostication of mortality.
Keywords: gait speed, frailty, cirrhosis, model for end-stage liver disease
Introduction
Frailty, a biological syndrome of decreased reserve and resistance to stressors, has been well and widely investigated in the realm of geriatrics.1 It has also been recognized that physical frailty applies in chronic liver disease and contributes to prediction of mortality and morbidity.2–4 Conventional scores used to prognosticate mortality, such as model for end-stage liver disease (MELD) and Child-Turcotte-Pugh (CTP) classification, may fail to reflect global status of a cirrhotic patient due to their sole reliance on laboratory parameters, and the inability to quantify physical performance. As frailty serves as a modifiable risk factor to an extent, appropriate identification gives rise to a target for optimization and, in the context of cirrhosis, potentially allows more feasible follow-up trajectory and exercise intervention.5
Although frailty is gaining more attention with respect to its pivotal role in cirrhosis, we still lack a unanimous consensus on the most appropriate measurement. The 5-m gait speed (5MGS) test is a surrogate indicator of physical frailty.6,7 This metric is quick to determine and acceptable to inpatients. Gait speed has been proved to be a consistent risk factor of adverse events and poor outcomes including non-elective hospitalization, disability, and all-cause mortality.8,9 More recently, Dunn et al. found frailty as measured by 5MGS is an independent and potential modifiable predictor for complications requiring hospitalization, in contrast to hand grip.4 Frailty and sarcopenia, two interrelated concepts, share similarities in their etiological roots. Sarcopenia may be a contributor to the development of physical frailty. Additionally, the European Working Group on Sarcopenia in Older People (EWGSOP) group also advise measures of low physical performance to assess severe sarcopenia by applying gait speed.10 Notably, gait speed has also been recognized as the best validated functional performance estimation for pharmacological trials in frailty and sarcopenia.11 Taken together, we hypothesized that 5MGS could more effectively predict all-cause mortality in cirrhosis. We also explored whether the inclusion of frailty assessment within MELD-Na could improve the prediction of mortality in cirrhosis. Our preliminary results may facilitate further clinical trials by balancing cirrhotic individuals at high risk of adverse outcome or treatment arms at baseline.
Methods
Study population
Adult cirrhotic patients were enrolled prospectively at the Department of Gastroenterology and Hepatology, Tianjin Medical University General Hospital between 2016 and 2017. This study was conducted in accordance with the Declaration of Helsinki and was approved by Ethics Committee of Tianjin Medical University General Hospital (2015-024). Written informed consent was obtained from all participants. We identified subjects with cirrhosis by collecting medical history, laboratory examinations, imaging results, endoscopy data, and/or liver biopsy. Meanwhile, the presence of cirrhosis-associated complications, such as gastroesophageal varices, hepatic encephalopathy (HE), ascites, hypersplenism, and hypoalbuminemia, were reviewed. The exclusion criteria were as follows: (1) presence of acute-on-chronic liver failure (ACLF) on admission; (2) presence of severe HE (as recognized by the time to complete a numbers connection test of > 120 s)12; (3) primary liver cancer or extrahepatic malignancies; (4) liver transplantation; (5) refusal to follow-up trajectory. A total of 137 patients with cirrhosis were recruited prospectively at initial assessment, 4, 2, 9, 2, and 7 cases were excluded owing to admission ACLF, severe HE, malignancies, liver transplantation, and refusal to follow-up trajectory. Finally, a total of 113 participants were left for final analysis (Figure S1). Liver failure for definition of ACLF include jaundice (serum total bilirubin ⩾ 5 mg/dl) and coagulation dysfunction [international normalized ratio (INR) > 1.5] complicated within 4 weeks by clinical ascites and/or HE in a patient with previously diagnosed or undiagnosed chronic liver disease/cirrhosis [Asian Pacific Association for the Study of the Liver (APASL) definition].
Clinical and laboratory measurement
Demographic information, clinical characteristics, and laboratory parameters, including age, gender, etiology of cirrhosis, presence of complications, complete blood count, liver function tests, prothrombin-international normalized ratio (PT-INR), CTP classification and MELD-Na (MELD-sodium) score were retrieved within 24 h after hospitalization. The primary outcome of interest was patient death over 2 years. Patients who underwent liver transplantation or were diagnosed with malignancies during follow-up period were censored.
Physical frailty measurement
We detected self-selected (usual pace) gait speed (5MGS) over a 5-m marked distance from a fixed start in our wards for patients with cirrhosis who could ambulate (more information in Supplementary File). The average of three consecutive trials were calculated and illustrated in ms−1 for final analysis.4 We selected gait speed on account of its ease and validity across a variety of studies in multiple populations as being indicative of health situations and physical performance decline.9,13 This parameter was measured by well-trained nurses. Prior to study initiation, two nurses simultaneously tested gait speed in a sample of 10 subjects in order to identify intra-observer agreement for the metrics as described by Bland and Altman.14 Patients unable to walk at all were assigned a gait speed of 0.01 ms−1 according to previous publication.15
Statistical analysis
Data were presented as mean ± standard deviation (SD), median [interquartile range (IQR)], simple frequencies, or percentages (%) as appropriate. Continuous data were compared using an independent Student t test or the Mann–Whitney test for groups with non-normal distribution. Categorical variables were compared by chi-square test or Fisher’s exact test. Correlations between continuous variables were assessed using Pearson correlation coefficients. Multivariable analysis performed by Cox proportional hazard analysis was used to identify the independent predictors of 2-year mortality. Hazard ratio (HR) and 95% confidence interval (CI) were calculated. The survival rate was calculated using the Kaplan–Meier method and compared in order to detect statistically significant differences using the log-rank test. The receiver operating characteristics (ROC) curve was used to evaluate sensitivity and specificity of all predictive indices.
5MGS was evaluated as a continuous measure but also a binary variable [slow 5MGS (<0.8 ms−1) versus preserved 5MGS (⩾0.8 ms−1)]. We have selected this threshold since international consensus statements have implied that it is a consistent indicator of adverse outcomes,9,16 and is advised by EWGSOP2 on account of simplicity.10
Using Cox proportional hazard regression, we developed a novel MELDNa-5MGS score based on the prediction of all-cause mortality according to the formula: MELDNa-5MGS = MELD-Na score + [β(slow 5MGS)/β(MELD-Na score)] × slow 5MGS. Discriminative ability (referring to the potential of a model to correctly distinguish between distinct outcomes), was determined using the area under ROC curve (AUC). The p-value for comparison of AUCs was calculated using the DeLong method. Clinical usefulness and net benefit of MELDNa-5MGS score were estimated with decision-curve analysis.17 The trends of mortality with respect to 5MGS and MELDNa-5MGS score increment were determined using the Cochran-Armitage test as previously described.18 We considered p < 0.05 as statistically significant. All statistical analyses were carried out using Stata 14.0 (Stata Corporation, College Station, TX, USA), MedCalc 15.2.2 (MedCalc, Mariakerke, Belgium), or R 3.3.2 (http://www.r-project.org/).
Results
Characteristics of patients
The baseline characteristics and laboratory data are shown in Table 1. A total of 113 cirrhotic patients (males: n = 60, 53%) with an average age of 64 ± 11 years were recruited to the study. The etiology of liver cirrhosis (LC) was attributed to chronic viral hepatitis in 43 (38%), alcoholism/nonalcoholic steatohepatitis (NASH) in 14 (12%), autoimmune liver disease in 16 (14%), and cryptogenic in 40 participants (35%). The cirrhosis-associated complications consisted of gastroesophageal varices in 45, HE in 21, ascites in 36, hypersplenism in 35, and hypoalbuminemia in 48 patients, respectively. Of these patients, 42 were classified as CTP-A, 51 as CTP-B, and 20 as CTP-C. The mean MELD-Na at admission was 11.4 ± 4.7 points; 25% (n = 28) of the cohort died during the 2-year follow-up period. When stratified by 5MGS (slow: n = 80; preserved: n = 33), there was a trend toward worse survival in patients with slow 5MGS compared with patients with preserved 5MGS, but without statistical significance (slow: 30% versus preserved: 12%; p = 0.056).
Table 1.
Baseline characteristics of cirrhotic patients.
| Characteristic | Total (n = 113) |
Surviving (n = 85) | Deceased (n = 28) | p value |
|---|---|---|---|---|
| Age, years | 64 ± 11 | 63 ± 11 | 67 ± 11 | 0.65 |
| Sex, n (%) | 0.196 | |||
| Female | 53 (47) | 43 (51) | 10 (36) | |
| Male | 60 (53) | 42 (49) | 18 (64) | |
| Etiology, n (%) | 0.215 | |||
| Chronic viral hepatitis | 43 (38) | 31 (26) | 12 (21) | |
| Alcohol | 14 (12) | 8 (9) | 6 (21) | |
| Autoimmune | 16 (14) | 14 (16) | 2 (7) | |
| Cryptogenic | 40 (35) | 32 (48) | 8 (50) | |
| CTP, n (%) | 0.009 | |||
| CTP-A | 42 (37) | 36 (42) | 6 (21) | |
| CTP-B | 51 (45) | 39 (46) | 12 (43) | |
| CTP-C | 20 (18) | 10 (12) | 10 (36) | |
| MELD-Na | 11.4 ± 4.7 | 11.5 ± 4.3 | 13.9 ± 4.9 | 0.001 |
| Albumin, g/l | 30.6 ± 5.9 | 31.5 ± 5.5 | 27.5 ± 5.8 | 0.001 |
| PT-INR | 1.4 ± 0.6 | 1.4 ± 0.7 | 1.4 ± 0.4 | 0.674 |
| TBIL, mg/dl | 2.0 ± 2.4 | 1.8 ± 2.1 | 2.8 ± 3.1 | 0.111 |
| Creatinine, μmol/l | 70.2 ± 33.9 | 63.2 ± 26.7 | 91.4 ± 42.7 | 0.003 |
| Sodium, mmol/l | 139.7 ± 5.0 | 140.1 ± 4.7 | 138.4 ± 5.3 | 0.106 |
| 5MGS, ms−1 | 0.56 ± 0.35 | 0.64 ± 0.31 | 0.34 ± 0.36 | <0.0001 |
| Complications, n (%) | ||||
| Hepatic encephalopathy | 21 (19) | 9 (11) | 12 (43) | 0.0004 |
| Gastroesophageal varices | 45 (40) | 34 (40) | 11 (39) | 0.999 |
| Hypersplenism | 35 (31) | 27 (32) | 8 (29) | 0.818 |
| Hypoalbuminemia | 48 (42) | 29 (34) | 19 (68) | 0.002 |
| Ascites | 36 (32) | 26 (31) | 10 (36) | 0.645 |
| Hematological indices | ||||
| NLR | 5.3 ± 6.9 | 5.0 ± 7.3 | 6.0 ± 5.1 | 0.472 |
| PLR | 136.3 ± 124.9 | 138.4 ± 106.3 | 130.0 ± 167.5 | 0.808 |
| LMR | 2.3 ± 2.1 | 2.5 ± 2.3 | 1.7 ± 1.0 | 0.011 |
| ALT, U/l | 19 (14,32) | 19 (14,36) | 18 (14,28) | 0.485 |
| AST, U/l | 30 (22,44) | 30 (21,48) | 29 (25,40) | 0.869 |
| GGT, U/l | 39 (25,79) | 39 (24,81) | 40 (28,56) | 0.861 |
5MGS, 5-m gait speed; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CTP, Child-Turcotte-Pugh classification; GGT, gamma glutamyl transferase; IQR, interquartile range; LMR, lymphocyte-to-monocyte ratio; MELD-Na, model for end-stage liver disease-sodium; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; PT-INR, prothrombin-international normalized ratio; SD, standard deviation; TBIL, total bilirubin.
Percentages might not add up to 100% because of rounding.
Values are presented as the mean ± SD, median (IQR), or number of patients (%).
MELDNa-5MGS score
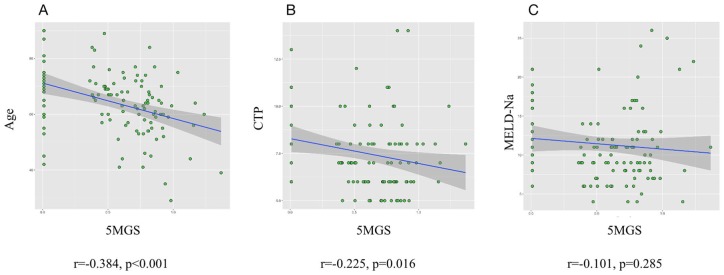
We first determined the correlation between 5MGS and conventional scoring system as well as age. Figure 1 shows that MELD-Na score and 5MGS was not significantly related. The univariable analysis for risk of all-cause mortality is shown in Table 2. The crude HR for mortality decreased for each 1 ms−1 increase in 5MGS (0.100, 95% CI: 0.034–0.299, p < 0.001). Similarly, when estimated as a binary measure, those with slow 5MGS had an increased crude HR compared with participants with preserved 5MGS (2.881, 95% CI: 0.999–8.309, p = 0.005). Further multivariable analysis confirmed that 5MGS (binary and continuous) remained an independent predictor for 2-year mortality (Table 2). Moreover, the Kaplan–Meier analysis demonstrated a shorter survival time in those with slow compared with preserved 5MGS (log-rank: p = 0.04, Figure S2). Cox regression analysis generated the following equation for MELDNa-5MGS:
Figure 1.
Correlations among 5MGS and age (A) as well as conventional scores (B, C). No significant correlation was identified between MELD-Na and 5MGS.
CTP, Child-Turcotte-Pugh classification; 5MGS, 5-m gait speed; MELD-Na, model for end-stage liver disease-sodium.
Table 2.
Univariable and multivariable analyses for 2-year all-cause mortality.
| Univariable analysis |
Multivariable Analysis-model 1* |
Multivariable Analysis-model 2* |
||||
|---|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | |
| 5MGS (ms−1) | 0.100 (0.034–0.299) |
<0.001 | 0.133(0.047–0.347) | <0.001 | ||
| Slow 5MGS (reference: preserved 5MGS) | 2.881 (0.999–8.309) |
0.005 | 4.805(1.536–15.026) | 0.007 | ||
| Age | 1.034 (0.999–1.071) |
0.056 | ||||
| Sex | 0.593 (0.274–1.284) |
0.185 | ||||
| CTP | 0.229 (0.083–0.631) |
0.012 | ||||
| MELD-Na | 1.103 (1.036–1.174) |
0.002 | 1.104(1.027–1.187) | 0.008 | 1.153(1.070–1.243) | 0.001 |
Multivariable model 1: 5MGS (continuous format); age; sex; CTP; MELD-Na. *Final model presented.
Multivariable model 2: Slow 5MGS (binary format); age; sex; CTP; MELD-Na. *Final model presented.
5MGS, 5-m gait speed; CI, confidence interval; CTP, Child-Turcotte-Pugh classification; HR, hazard ratio; MELD-Na, model for end-stage liver disease-sodium.
The median MELDNa-5MGS score was 20 points and the AUC revealed a cut-off MELDNa-5MGS score of 23 (AUC = 0.773, p < 0.001, sensitivity 0.64, specificity 0.84), resulting in 32 patients having high MELDNa-5MGS scores. Table 3 shows the parameters of patients stratified according to MELDNa-5MGS scores. Apart from the components of MELDNa-5MGS, CTP classification (p < 0.0001), HE (p < 0.0001), hypoalbuminemia (p < 0.0001), neutrophil-to-lymphocyte ratio (p = 0.032), and lymphocyte-to-monocyte ratio (p < 0.0001) differed significantly between patients with low and high MELDNa-5MGS scores.
Table 3.
Baseline characteristics of cirrhotic patients stratified according to MELDNa-5MGS score.
| Characteristics | High MELDNa-5MGS (n = 32) | Low
MELDNa-5MGS (n = 81) |
p value |
|---|---|---|---|
| Age, years | 65 ± 10 | 63 ± 12 | 0.404 |
| Sex, n (%) | 0.531 | ||
| Female | 13 (41) | 40 (49) | |
| Male | 19 (59) | 41 (51) | |
| Etiology, n (%) | 0.195 | ||
| Chronic viral hepatitis | 9 (28) | 34 (42) | |
| Alcohol | 6 (19) | 8 (10) | |
| Autoimmune | 7 (22) | 9 (11) | |
| Cryptogenic | 10 (31) | 30 (37) | |
| CTP, n (%) | <0.0001 | ||
| CTP-A | 1 (3) | 41 (51) | |
| CTP-B | 15 (47) | 36 (44) | |
| CTP-C | 16 (50) | 4 (5) | |
| MELD-Na | 16.3 ± 4.0 | 9.5 ± 3.3 | <0.0001 |
| Albumin, g/l | 26.1 ± 5.0 | 32.3 ± 5.2 | 0.005 |
| PT-INR | 1.7 ± 1.1 | 1.2 ± 0.2 | 0.012 |
| TBIL, mg/dl | 3.1 ± 3.0 | 1.6 ± 2.1 | 0.018 |
| Creatinine, μmol/l | 91.1 ± 50.0 | 61.9 ± 19.8 | 0.003 |
| Sodium, mmol/l | 138.1 ± 4.5 | 140.4 ± 5.0 | 0.026 |
| 5MGS, ms−1 | 0.35 ± 0.36 | 0.65 ± 0.31 | <0.0001 |
| Complications, n (%) | |||
| Hepatic encephalopathy | 15 (47) | 6 (7) | <0.0001 |
| Gastroesophageal varices | 13 (41) | 32 (40) | 0.999 |
| Hypersplenism | 7 (22) | 28 (35) | 0.259 |
| Hypoalbuminemia | 25 (78) | 23 (28) | <0.0001 |
| Ascites | 13 (41) | 23 (32) | 0.263 |
| Hematological indices | |||
| NLR | 8.6 ± 11.5 | 4.0 ± 3.0 | 0.032 |
| PLR | 175.1 ± 195.2 | 120.9 ± 79.0 | 0.137 |
| LMR | 1.5 ± 0.9 | 2.6 ± 2.4 | <0.0001 |
| ALT, U/l | 14 (23,36) | 18 (14,29) | 0.390 |
| AST, U/l | 36 (25,64) | 30 (21,41) | 0.118 |
| GGT, U/l | 35 (28,49) | 43 (24,83) | 0.610 |
5MGS, 5-m gait speed; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CTP, Child-Turcotte-Pugh classification; GGT, gamma glutamyl transferase; IQR, interquartile range; LMR, lymphocyte-to-monocyte ratio; MELD-Na, model for end-stage liver disease-sodium; MELDNa-5MGS, MELDNa-5 meter gait speed; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; PT-INR, prothrombin-international normalized ratio; SD, standard deviation; TBIL, total bilirubin.
Percentages might not add up to 100% because of rounding.
Values are presented as the mean ±SD, median (IQR), or number of patients (%).
Discriminatory power and clinical utility of MELDNa-5MGS score
Supplementary Table and Figure 2 depict the AUC of MELDNa-5MGS, the conventional MELD-Na, and CTP classification for predicting 3-momth, 6-month, 1-year, and 2-year all-cause mortality. MELDNa-5MGS scores predicted mortality more accurately than MELD-Na (p = 0.014 for 1-year, p = 0.044 for 2-years). In addition, our decision curve analysis implicated the net benefit achieved from the MELDNa-5MGS than MELD-Na were higher, with threshold probabilities of 0.16–0.6 (Figure 3).
Figure 2.
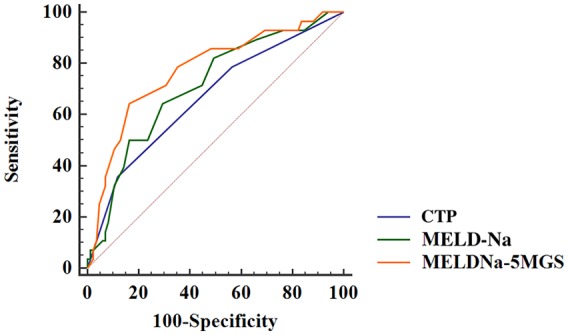
Cut-off values for MELDNa-5MGS determined by ROC analysis.
CTP, Child-Turcotte-Pugh classification; MELD-Na, model for end-stage liver disease-sodium; MELDNa-5MGS, MELDNa-5-m gait speed; ROC, receiver operating characteristics curve.
Figure 3.
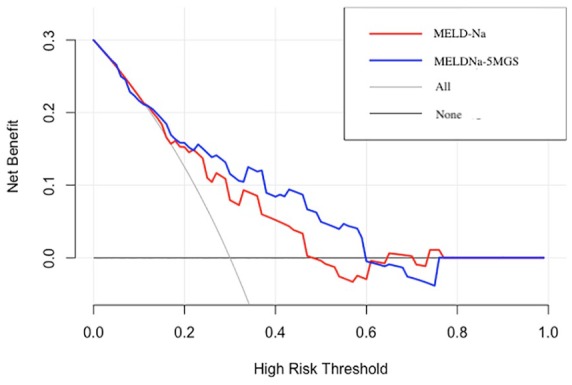
Decision-curve analysis of MELDNa-5MGS versus MELD-Na for the prediction of 2-year mortality in cirrhosis.
MELD-Na, model for end-stage liver disease-sodium; MELDNa-5MGS, MELDNa-5-m gait speed.
Prediction of mortality on account of MELDNa-5MGS score
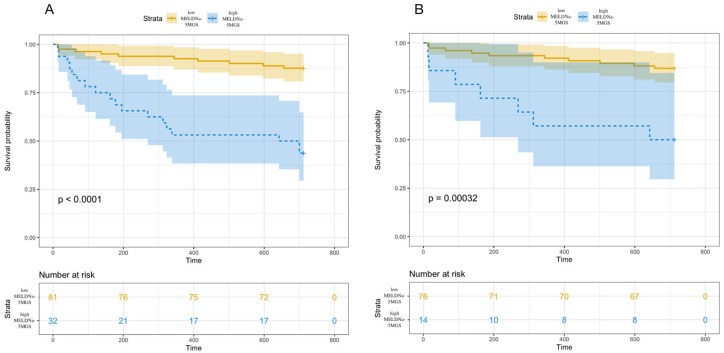
All-cause mortality was significantly higher in patients with high (⩾15) (n = 23) than low (<15) (n = 90) MELD-Na scores (p = 0.0028, Figure S3).19 Additionally, all-cause mortality was significantly higher among patients with high (n = 32) than low (n = 81) MELDNa-5MGS scores (p < 0.0001, Figure 4A). The 6-month, 1- and 2-year mortality rates with high and low MELDNa-5MGS scores were 31%, 47% and 56% versus 5%, 7% and 12%, respectively. Furthermore, even among patients with low MELD-Na scores (n = 90), mortality rates were significantly higher in 14 patients with high MELDNa-5MGS scores compared with 76 patients with low MELDNa-5MGS scores (p = 0.0003, Figure 4B).
Figure 4.
Survival rates among cirrhotic patients classified according to MELDNa-5MGS. (A) Survival rates were significantly higher in patients with low (n = 81), than high (n = 32) MELDNa-5MGS scores (p < 0.0001). (B) Among 90 patients with low MELD-Na scores, mortality rates were significantly higher among those who also had high MELDNa-5MGS scores, than among patients with low MELD-Na and low MELD-Na scores (p = 0.0003).
MELD-Na, model for end-stage liver disease-sodium; MELDNa-5MGS, MELDNa-5-m gait speed.
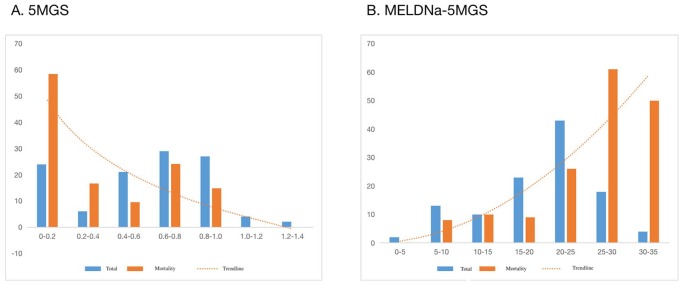
Distribution of 5MGS and MELDNa-5MGS scores
The distribution of 5MGS and MELDNa-5MGS in our patients is shown in Figure 5. The mortality rate decreased significantly (p = 0.003 for trend) from 58% (14/24) in patients in the 0–0.2 5MGS range to 0% (0/2) in the 1.2–1.4 5MGS range. Moreover, the mortality rate increased significantly (p < 0.001 for trend) from 0% (0/2) in patients in the 0–5 MELDNa-5MGS score range to 50% (2/4) in the 30–35 MELDNa-5MGS score range.
Figure 5.
Two-year all-cause mortality related to 5MGS and MELDNa-5MGS. The vertical bars show the percentages of patients (blue: all patients; orange: deceased patients), scaled on the y-axis, and corresponded to the indicated intervals of 5MGS (A) and MELDNa-5MGS (B) on the x-axis. The trend was statistically significant for 5MGS (p = 0.003) and MELDNa-5MGS (p < 0.001) using the Cochran-Armitage test.
MELD-Na, model for end-stage liver disease-sodium; MELDNa-5MGS, MELDNa-5-m gait speed.
Discussion
As far as we can determine, MELDNa-5MGS is the first scoring system to include frailty estimated by gait speed, and it was more useful in predicting all-cause mortality than the conventional MELD-Na score. The MELD is derived from three objective parameters, including serum bilirubin, creatinine, and PT-INR. It is reliable for predicting short- and mid-term mortality in cirrhosis,20,21 and modification of MELD to include sodium has been implemented in standard practice to increase the prognostic value.22 However, one flaw of scoring systems based on MELD is that they fail to completely capture elements contributing to functional status or physical reserve of patients with cirrhosis. Frailty has been increasingly recognized to apply in advanced liver disease, and is useful in heralding prognosis and predicting outcomes.2,4,15,23 Among various measurement tools, gait speed has been implemented individually or within an amalgamation of methods for grading frailty.5,24
Studies assessing the prognostic ability of gait speed in patients with cirrhosis is limited. Dunn and colleagues measures 5MGS in 373 cirrhotic patients evaluated for, or awaiting, liver transplantation, and uncovered that gait speed was a risk factor for complications requiring hospitalization.4 Moreover, each 0.1 ms−1 gait speed decrease was related to 22% greater hospital days.4 Ribeiro et al. determined gait speed based on a 6-min walk test in 73 participants.25 They found that slow gait speed was an independent predictor of death, and showed the highest predictive power of decease concomitantly linked to other malnourished indices. Lai et al. set out to develop a novel frailty index and implied that cirrhotics with poor frailty scores exhibited more impaired gait speed.15 Their final model embodies three performance-based tests (grip strength, chair stands, and balance) and enhances mortality prediction in combination with MELD-Na score over MELD-Na alone. Notwithstanding, substituting walk speed for grip strength would impair the balance between statistical accuracy and ease of use in clinical practice. Intriguingly, another study from Japan found grip strength might be a suitable proxy for gait speed in hospitalized patients with chronic liver disease for hepatocellular carcinoma treatment.26 Our results implicated that both continuous and slow 5MGS were independently associated with all-cause mortality after adjustment for potential confounders. Taken together, we hypothesized the above-mentioned difference is attributed to a study population with distinct clinical features (clinic versus hospitalization) and outcomes of interest (1-year waitlist mortality versus 2-year all-cause mortality). Notably, our scoring system might be more feasible among acutely ill inpatients with cirrhosis.
Our results indicated that 5MGS may represent a stratification tool of all-cause mortality in cirrhosis. According to purpose, 5MGS could have the potential to delete or enrich a clinical trial with those at high risk of adverse outcome, or to balance therapy arms at baseline. For instance, an outstanding study by Kitajima et al. implied that dietary supplementation with branched-chain amino acids (BCAA) was associated with amelioration of hypoalbuminemia, decreased fat deposits in skeletal muscle, maintenance of skeletal muscle mass, and improved glucose sensitivity.27 Thus BCAA supplementation may represent a less invasive and a more consistent treatment to inhibit or prevent the pathogenesis of cirrhosis and result in a favorable outcome.27 However, the authors addressed that the amount of physical activity was not investigated, where we propose that 5MGS may be a valid and feasible endpoint in their study. Indeed, it should be emphasized that gait speed has been implemented as a trial endpoint in other chronic entities and gave rise to the regulatory approval of medications for multiple sclerosis.28
Frailty and sarcopenia in cirrhosis are closely related, and sarcopenia likely serves a significant contributor. It is believed by some practitioners that frailty is the result of prolonged sarcopenia.29 Thus, measuring sarcopenia, by means of radiographic image analysis [computed tomography (CT) or magnetic resonance imaging (MRI)], may encapsulate physical frailty to some extent. However, there are potential limitations associated with using imaging-based results as surrogate parameter for assessment. Specifically, missing values are not avoided because a proportion of patients are unable to perform serial measurements due to expense or concerns about radiation. As frailty occurs on a continuum, gait speed has advantage of being simple, reliable, and reproducible in serial tests. Furthermore, frailty is not a single phenotype but rather varying clusters of multiple traits of vulnerabilities, weaknesses, instabilities, and limitations30; these manifestations are not captured by laboratory/radiographic data. Of note, although MELD-Sarcopenia score was shown to be an independent predictor of mortality by Montano-Loza et al., the inclusion of 5MGS in our novel scoring system dramatically improved predictive capacity.31
The other disadvantage is that patients with lower MELD-Na scores might not be at low risk for mortality. Our proposed MELDNa-5MGS score extracted patients with high all-cause mortality among those considered to be at low risk according to MELD-Na scores. These results suggested that the MELDNa-5MGS score could serve as a favorable prognostication system that could precisely predict mid-term mortality.
Our study has some limitations. First, we examined baseline 5MGS only at index hospitalization of cirrhotic inpatients, whereas change in gait speed may be more reflective of frailty circumstance, which warrants exploration in future study. Second, patients unable to finish 5MGS were assigned a gait speed of 0.01 ms−1, which may result in selection bias. Third, we did not externally validate our results. However, given the consistent demonstration of the predictive capacity of MELD-Na and gait speed in cirrhosis as well as other populations, the need for external validation is less pronounced.9,16 Finally, the vast majority of published articles have concentrated on frailty assessment in outpatients. Taking account of frailty as a universal condition among subjects with various chronic diseases, we aimed to explore the utility and effectiveness of gait speed for predicting long-term mortality in cirrhosis. It is inevitable to address the inherent limitation of this frailty metric itself, given the instability of testing gait speed in hospitalized critical patients. Moreover, our group has conducted a series of baseline and longitudinal studies to investigate the impact of frailty screening/assessment upon long-term mortality, such as self-reported frailty index. Further study is warranted to confirm our findings.
In conclusion, we refined the conventional MELD-Na score by incorporating assessment of frailty by gait speed. Moreover, the novel MELDNa-5MGS score is more accurately predictive of mid-term mortality than MELD-Na scores. It is thus plausible to include 5MGS as a surrogate indicator of frailty for cirrhosis in clinic settings.
Supplemental Material
Supplemental material, Supplementary_File_revised for Incorporation of frailty estimated by gait speed within MELD-Na and the predictive potential for mortality in cirrhosis by You Deng, Lin Lin, Xiaofei Fan, Binxin Cui, Lijun Hou, Tianming Zhao, Junjie Hou, Lihong Mao, Xiaoyu Wang, Wei Zhao, Bangmao Wang, Qingxiang Yu and Chao Sun in Therapeutic Advances in Chronic Disease
Acknowledgments
We thank all the nurses who took part in the current study.
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Chao Sun  https://orcid.org/0000-0002-0380-7999
https://orcid.org/0000-0002-0380-7999
Supplemental material: Supplemental material for this article is available online.
Contributor Information
You Deng, Department of Gastroenterology and Hepatology, Tianjin Medical University General Hospital, Heping District, Tianjin, China; Tianjin Institute of Digestive Disease, Tianjin Medical University General Hospital, Heping District, Tianjin, China.
Lin Lin, Department of Gastroenterology, Tianjin Medical University General Hospital Airport Hospital, Tianjin, China.
Xiaofei Fan, Department of Gastroenterology and Hepatology, Tianjin Medical University General Hospital, Heping District, Tianjin, China; Tianjin Institute of Digestive Disease, Tianjin Medical University General Hospital, Heping District, Tianjin, China.
Binxin Cui, Department of Gastroenterology, Tianjin Medical University General Hospital Airport Hospital, Tianjin, China.
Lijun Hou, Department of Gastroenterology and Hepatology, Tianjin Medical University General Hospital, Heping District, Tianjin, China; Tianjin Institute of Digestive Disease, Tianjin Medical University General Hospital, Heping District, Tianjin, China.
Tianming Zhao, Department of Gastroenterology and Hepatology, Tianjin Medical University General Hospital, Heping District, Tianjin, China; Tianjin Institute of Digestive Disease, Tianjin Medical University General Hospital, Heping District, Tianjin, China.
Junjie Hou, Department of Gastroenterology and Hepatology, Tianjin Medical University General Hospital, Heping District, Tianjin, China; Tianjin Institute of Digestive Disease, Tianjin Medical University General Hospital, Heping District, Tianjin, China.
Lihong Mao, Department of Gastroenterology and Hepatology, Tianjin Medical University General Hospital, Heping District, Tianjin, China; Tianjin Institute of Digestive Disease, Tianjin Medical University General Hospital, Heping District, Tianjin, China.
Xiaoyu Wang, Department of Gastroenterology and Hepatology, Tianjin Medical University General Hospital, Heping District, Tianjin, China; Tianjin Institute of Digestive Disease, Tianjin Medical University General Hospital, Heping District, Tianjin, China.
Wei Zhao, Department of Gastroenterology and Hepatology, Tianjin Medical University General Hospital, Heping District, Tianjin, China; Tianjin Institute of Digestive Disease, Tianjin Medical University General Hospital, Heping District, Tianjin, China.
Bangmao Wang, Department of Gastroenterology and Hepatology, Tianjin Medical University General Hospital, Heping District, Tianjin, China; Tianjin Institute of Digestive Disease, Tianjin Medical University General Hospital, Heping District, Tianjin, China.
Qingxiang Yu, Department of Gastroenterology and Hepatology, Tianjin Medical University General Hospital, Anshan Road 154, Heping District, Tianjin 300052, China; Tianjin Institute of Digestive Disease, Tianjin Medical University General Hospital, Heping District, Tianjin, China.
Chao Sun, Department of Gastroenterology and Hepatology, Tianjin Medical University General Hospital, Anshan Road 154, Heping District, Tianjin 300052, China; Tianjin Institute of Digestive Disease, Tianjin Medical University General Hospital, Heping District, Tianjin, China; Department of Gastroenterology, Tianjin Medical University General Hospital Airport Hospital, Tianjin, China.
References
- 1. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56: M146–M156. [DOI] [PubMed] [Google Scholar]
- 2. Tandon P, Tangri N, Thomas L, et al. A rapid bedside screen to predict unplanned hospitalization and death in outpatients with cirrhosis: a prospective evaluation of the clinical frailty scale. Am J Gastroenterol 2016; 111: 1759–1767. [DOI] [PubMed] [Google Scholar]
- 3. Sinclair M, Poltavskiy E, Dodge JL, et al. Frailty is independently associated with increased hospitalisation days in patients on the liver transplant waitlist. World J Gastroenterol 2017; 23: 899–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dunn MA, Josbeno DA, Tevar AD, et al. Frailty as tested by gait speed is an independent risk factor for cirrhosis complications that require hospitalization. Am J Gastroenterol 2016; 111: 1768–1775. [DOI] [PubMed] [Google Scholar]
- 5. Laube R, Wang H, Park L, et al. Frailty in advanced liver disease. Liver Int 2018; 38: 2117–2128. [DOI] [PubMed] [Google Scholar]
- 6. Bergquist CS, Jackson EA, Thompson MP, et al. Understanding the association between frailty and cardiac surgical outcomes. Ann Thorac Surg 2018; 106: 1326–1332. [DOI] [PubMed] [Google Scholar]
- 7. Afilalo J, Kim S, O’Brien S, et al. Gait speed and operative mortality in older adults following cardiac surgery. JAMA Cardiol 2016; 1: 314–321. [DOI] [PubMed] [Google Scholar]
- 8. Steffen TM, Hacker TA, Mollinger L. Age- and gender-related test performance in community-dwelling elderly people: Six-Minute Walk Test, Berg Balance Scale, Timed Up & Go Test, and gait speeds. Phys Ther 2002; 82: 128–137. [DOI] [PubMed] [Google Scholar]
- 9. Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA 2011; 305: 50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019; 48: 16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Working Group on Functional Outcome Measures for Clinical Trials. Functional outcomes for clinical trials in frail older persons: time to be moving. J Gerontol A Biol Sci Med Sci 2008; 63: 160–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weissenborn K, Ruckert N, Hecker H, et al. The number connection tests A and B: interindividual variability and use for the assessment of early hepatic encephalopathy. J Hepatol 1998; 28: 646–653. [DOI] [PubMed] [Google Scholar]
- 13. Pamoukdjian F, Paillaud E, Zelek L, et al. Measurement of gait speed in older adults to identify complications associated with frailty: a systematic review. J Geriatr Oncol 2015; 6: 484–496. [DOI] [PubMed] [Google Scholar]
- 14. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986; 1: 307–310. [PubMed] [Google Scholar]
- 15. Lai JC, Covinsky KE, Dodge JL, et al. Development of a novel frailty index to predict mortality in patients with end-stage liver disease. Hepatology 2017; 66: 564–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Abellan van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) task force. J Nutr Health Aging 2009; 13: 881–889. [DOI] [PubMed] [Google Scholar]
- 17. Deng Y, Fan X, Ran Y, et al. Prognostic impact of neutrophil-to-lymphocyte ratio in cirrhosis: a propensity score matching analysis with a prespecified cut-point. Liver Int 2019; 39: 2153–2163. [DOI] [PubMed] [Google Scholar]
- 18. Yang F, Lin L, Jiang X, et al. Increasing diverticulosis in an aging population: a colonoscopy-based study of 5-year trends in 26 463 patients in northern China. Med Sci Monit 2018; 24: 2825–2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mazumder NR, Atiemo K, Daud A, et al. Patients with persistently low MELD-Na scores continue to be at risk of liver related death. Transplantation. Epub ahead of print. 21 October 2019. DOI: 10.1097/TP.0000000000002997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kamath PS, Kim WR. and Advanced Liver Disease Study Group. The model for end-stage liver disease (MELD). Hepatology 2007; 45: 797–805. [DOI] [PubMed] [Google Scholar]
- 21. Biggins SW, Kim WR, Terrault NA, et al. Evidence-based incorporation of serum sodium concentration into MELD. Gastroenterology 2006; 130: 1652–1660. [DOI] [PubMed] [Google Scholar]
- 22. Huo TI, Wang YW, Yang YY, et al. Model for end-stage liver disease score to serum sodium ratio index as a prognostic predictor and its correlation with portal pressure in patients with liver cirrhosis. Liver Int 2007; 27: 498–506. [DOI] [PubMed] [Google Scholar]
- 23. Lai JC, Feng S, Terrault NA, et al. Frailty predicts waitlist mortality in liver transplant candidates. Am J Transplant 2014; 14: 1870–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kok B, Tandon P. Frailty in patients with cirrhosis. Curr Treat Options Gastroenterol. 2018; 16: 215–225. [DOI] [PubMed] [Google Scholar]
- 25. Ribeiro HS, Mauricio SF, Antonio da, Silva T, et al. Combined nutritional assessment methods to predict clinical outcomes in patients on the waiting list for liver transplantation. Nutrition 2018; 47: 21–26. [DOI] [PubMed] [Google Scholar]
- 26. Nagamatsu A, Kawaguchi T, Hirota K, et al. Slow walking speed overlapped with low handgrip strength in chronic liver disease patients with hepatocellular carcinoma. Hepatol Res 2019; 49: 1427–1440. [DOI] [PubMed] [Google Scholar]
- 27. Kitajima Y, Takahashi H, Akiyama T, et al. Supplementation with branched-chain amino acids ameliorates hypoalbuminemia, prevents sarcopenia, and reduces fat accumulation in the skeletal muscles of patients with liver cirrhosis. J Gastroenterol 2018; 53: 427–437. [DOI] [PubMed] [Google Scholar]
- 28. Goodman AD, Brown TR, Edwards KR, et al. A phase 3 trial of extended release oral dalfampridine in multiple sclerosis. Ann Neurol 2010; 68: 494–502. [DOI] [PubMed] [Google Scholar]
- 29. Landi F, Calvani R, Cesari M, et al. Sarcopenia as the biological substrate of physical frailty. Clin Geriatr Med. 2015; 31: 367–374. [DOI] [PubMed] [Google Scholar]
- 30. Fried LP, Hadley EC, Walston JD, et al. From bedside to bench: research agenda for frailty. Sci Aging Knowledge Environ 2005; 2005: pe24. [DOI] [PubMed] [Google Scholar]
- 31. Montano-Loza AJ, Duarte-Rojo A, Meza-Junco J, et al. Inclusion of sarcopenia within MELD (MELD-sarcopenia) and the prediction of mortality in patients with cirrhosis. Clin Transl Gastroenterol 2015; 6: e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_File_revised for Incorporation of frailty estimated by gait speed within MELD-Na and the predictive potential for mortality in cirrhosis by You Deng, Lin Lin, Xiaofei Fan, Binxin Cui, Lijun Hou, Tianming Zhao, Junjie Hou, Lihong Mao, Xiaoyu Wang, Wei Zhao, Bangmao Wang, Qingxiang Yu and Chao Sun in Therapeutic Advances in Chronic Disease