Abstract
Obstructive sleep apnea (OSA) predominantly during rapid eye movement (REM) sleep may have impacts on brain health, even in milder OSA cases. Here, we evaluated whether REM sleep OSA is associated with abnormal daytime cerebral functioning using high-resolution single-photon emission computed tomography (SPECT). We tested 96 subjects (25 F, age: 65.2 ± 6.4) with a wide range of OSA severity from no OSA to severe OSA (apnea–hypopnea index: 0–97 events/h). More respiratory events during REM sleep were associated with reduced daytime regional cerebral blood flow (rCBF) in the bilateral ventromedial prefrontal cortex and in the right insula extending to the frontal cortex. More respiratory events during non-REM (NREM) sleep were associated with reduced daytime rCBF in the left sensorimotor and temporal cortex. In subjects with a lower overall OSA severity (apnea–hypopnea index<15), more respiratory events during REM sleep were also associated with reduced daytime rCBF in the insula and extending to the frontal cortex. Respiratory events that characterized OSA during NREM versus REM sleep are associated with distinct patterns of daytime cerebral perfusion. REM sleep OSA could be more detrimental to brain health, as evidenced by reduced daytime rCBF in milder forms of OSA.
Keywords: Functional neuroimaging, perfusion, rapid eye movement-predominant obstructive sleep apnea, sleep-disordered breathing, single-photon emission computed tomography
Introduction
Previous studies using quantitative neuroimaging techniques have shown that obstructive sleep apnea (OSA) is associated with altered daytime cerebral functioning over many different cortical and subcortical regions.1–6 Impacts of OSA on brain function also include daytime sleepiness and altered mood.7 Recent studies showed that OSA increases the risk of accelerated cognitive decline and dementia.8 Improving our understanding of how OSA causes daytime cerebral dysfunctions, particularly in older individuals, is needed to identify patients at risk of adverse long-term neurocognitive consequences.
The sleep stage during which apneas and hypopneas occur might significantly affect how OSA will impact daytime cerebral functioning. About 14 to 37% of individuals with OSA have most of their apneas and hypopneas during rapid eye movement (REM) sleep, and this proportion is higher for milder OSA cases.9–13 Because REM sleep represents only a small proportion of total sleep duration, many respiratory events predominantly during REM sleep may still result in a low overall apnea–hypopnea index (AHI), leading to a milder OSA diagnosis. This poses a clinical dilemma as to whether we should treat milder OSA cases that features respiratory events predominantly during REM sleep.14 This dilemma can only be resolved if we understand the specific impact of OSA during REM sleep versus non-REM (NREM) sleep on general health. Aiming at answering this question, previous studies have shown that obstructive respiratory events during REM sleep are generally longer, more hypoxic and cause higher blood pressure elevation than those occurring in NREM sleep.15,16 OSA during REM sleep was also repeatedly associated with cardiovascular and metabolic consequences, including a higher incidence of hypertension,17,18 loss of nighttime blood pressure dipping,19 poor glycemic control in type 2 diabetes patients,20 and insulin resistance.21 Because all these conditions may affect brain functioning, these recent discoveries suggest that REM sleep OSA may be especially detrimental to brain health.
The present study aimed at determining whether respiratory events, i.e. apneas and hypopneas, during REM sleep are associated with abnormal daytime cerebral functioning. To achieve this goal, the association between OSA during REM versus NREM sleep was investigated with single photon emission computed tomography (SPECT) during wakefulness to measure regional cerebral blood flow (rCBF), as this technique was shown to be sensitive to cerebral changes in OSA.1 Resting-state cerebral perfusion is considered a marker of brain functioning because it is closely related to neuronal and astroglial activity via the neuro-vascular coupling.22 We hypothesized that (1) the severity of OSA during REM versus NREM sleep would be characterized by distinct daytime rCBF patterns, and that (2) OSA during REM sleep would be associated with daytime rCBF anomalies even in milder cases for whom there is a clinical dilemma. Additional daytime cerebral functioning measurements were used to assess sleepiness, mood, and cognition.
Material and methods
Recruitment
We recruited subjects aged between 55 and 85 years old with a wide range of OSA severities from ads in media, although some subjects (n = 21) were recruited from a waiting list for OSA screening in a pulmonary clinic. Recruitment continued until all OSA severity groups (from healthy controls to severe OSA individuals) were represented. Exclusion criteria were psychiatric (e.g. diagnosed depression and anxiety disorders), neurologic (e.g. dementia, stroke) and pulmonary diseases (e.g. chronic obstructive pulmonary disease); sleep disorders other than OSA (e.g. restless leg syndrome, insomnia, REM sleep behavior disorder, parasomnias); morbid obesity ( ≥ 40 kg/m2); drug and alcohol abuse; and medications known to influence sleep or cerebral functioning (e.g. antidepressants, benzodiazepines, opioids, antiepileptics, antipsychotics). Cardiovascular risk factors and diseases were assessed with the index of vascular burden.23 Participants with controlled diabetes or hypertension were not excluded, but their conditions were documented in that index. All OSA subjects included in our study were newly diagnosed and none of them were treated for OSA. The Hôpital du Sacré-Coeur de Montréal Ethics' Committee approved the research protocol (#2012-697). The present research and the Ethics' Committee follow the guidelines of the Canadian Tri-Council Policy Statement: Ethical Conduct for Research and Involving Humans, as well as the Declaration of Helsinki. Written informed consent was obtained from each participant.
Overview of the protocol
Participants were evaluated with a polysomnographic recording, questionnaires, and a SPECT imaging session. In order to characterize our sample, we used questionnaires, namely the Epworth Sleepiness Scale (ESS)24; the Beck Depression Inventory-II (BDI-II)25; the Beck Anxiety Inventory (BAI)26; and a cognitive screening test, the Montreal cognitive assessment (MoCA).27 Questionnaires were administered the evening before polysomnography, except for the MoCA that was performed the morning after by a trained neuropsychologist. The daytime SPECT imaging was performed a month on average after the polysomnographic recording (26.2 ± 30.2 days).
Polysomnographic recording
The full-night polysomnographic recording was performed in a sleep laboratory and included an 18-channel electroencephalogram, an electrooculogram, chin and anterior tibialis electromyograms, and an electrocardiogram. Apneas and hypopneas were recorded with thoracic-abdominal strain gauges, oronasal cannula and thermal sensors, and a transcutaneous finger pulse oximeter. Sleep stages as well as apneas and hypopneas were scored by a medical electrophysiology technologist with extensive training in sleep according to the 2007 American Academy of Sleep Medicine Manual for the scoring of sleep28 and their 2012 update for the scoring of respiratory events.29 Apneas and hypopneas were scored if they lasted >10 s. An airflow reduction of ≥90% and ≥30% defined an apnea and a hypopnea, respectively. A hypopnea had to be accompanied by either an oxygen desaturation of ≥3% or an arousal.
The REM-AH was computed by adding the total number of apneas and hypopneas during REM sleep (and vice versa for NREM-AH). The REM-AHI was calculated as follows: apneas+hypopneas during REM sleep/REM sleep duration in hours (and vice versa for NREM-AHI). Apneas and hypopneas that occurred either completely or partially during REM sleep were computed in the REM-AH and REM-AHI, while others were computed in the NREM-AH and NREM-AHI. Although these variables include central events, all OSA participants had mostly obstructive rather than central events (9.8 ± 15.0% of the AHI were central events).
SPECT image acquisition, processing, and data analysis
To assess rCBF during wakefulness, all subjects underwent technetium-99m-hexamethylpropyleneamine oxime (99mTc-HMPAO) SPECT scanning using a high-resolution brain-dedicated scanner (NeuroFOCUS, NeuroPhysics, Shirley, USA). This procedure was described in a previous study by our group.1 While subjects lay awake with their eyes closed, an average dose of 750 MBq of 99mTc-HMPAO was administered intravenously followed by a saline flush. 99mTc-HMPAO uptake in the brain takes about a minute. This fast uptake allows reducing to a maximum the potential effect of drowsiness on scanning. Twenty minutes later, projections were acquired statically for 30 min. Individual images were reconstructed in a 128 × 128 matrix to achieve 32 slices with a filtered back projection and an attenuation correction (Chang's absorption coefficient: 0.01 cm−1). Because this SPECT scanner does not record the whole cerebellum, this region was excluded from analyses. All acquired individual images were visually inspected for gross abnormalities. Individual SPECT images were registered and spatially normalized (co-registered and warped) to the standard SPECT template included in SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). Normalized images were smoothed using a 14-mm full-width half-maximum filter and proportionally scaled with a grand mean scaling of 50 ml/min/100g for individual global mean signals. Therefore, rCBF changes are relative to the mean of each subject rather than absolute rCBF changes. This grand mean scaling allows reducing inter-individual variations regarding the amount of radioactivity detectable. Final individual images were equally smoothed and normalized into the same common space in order to perform voxel-based statistical comparisons.
Statistical analyses
Statistics were performed with SPSS 19 (IBM SPSS Statistics, New York, USA), except for rCBF analyses that were performed within SPM. Comparisons between control, mild, moderate and severe OSA groups were performed with one-way ANOVA for continuous variables and Chi-square for categorical variables. These variables included clinical, demographic, polysomnographic and cognitive data (see Table 1).
Table 1.
Clinical, polysomnographic, and respiratory variables for healthy control subjects and OSA groups.
| Variables | Controls [A] | Mild OSA [B] | Moderate OSA [C] | Severe OSA [D] | p-value | Post-hoc tests |
|---|---|---|---|---|---|---|
| AHI criteria for groups | <5 | ≥5 to < 15 | ≥15 to < 30 | ≥30 | n/a | |
| Number of subjects | 16 | 37 | 20 | 23 | n/a | |
| Sex (#; %Male) | 10; 62.5% | 24; 64.9% | 17; 85.0% | 20; 87.0% | ns | |
| Age (years) | 64.4 (6.7) | 64.4 (5.9) | 64.6 (6.3) | 67.0 (7.0) | ns | |
| Body mass index (kg/m2) | 25.9 (3.4) | 26.7 (3.6) | 28.9 (3.6) | 28.6 (2.5) | <0.05 | A < C |
| Vascular burden index | 0.9 (1.1) | 1.3 (1.4) | 1.3 (1.0) | 1.4 (1.3) | ns | |
| Epworth sleepiness scale | 7.8 (5.7) | 7.5 (4.6) | 9.1 (5.2) | 8.4 (4.8) | ns | |
| Beck depression inventory II | 5.4 (5.4) | 6.4 (5.4) | 7.1 (5.4) | 7.0 (5.2) | ns | |
| Beck anxiety inventory | 4.7 (4.7) | 4.0 (4.0) | 5.2 (5.7) | 4.1 (4.1) | ns | |
| Montreal cognitive assessment | 27.4 (2.2) | 27.6 (2.0) | 27.2 (2.4) | 26.5 (3.0) | ns | |
| Polysomnographic variables | ||||||
| TST (min) | 367.0 (54.9) | 358.7 (74.0) | 347.1 (49.1) | 370.5 (62.6) | ns | |
| Sleep efficiency (%) | 78.4 (8.8) | 79.1 (12.5) | 78.7 (11.2) | 79.2 (11.0) | ns | |
| MAI (MA/h) | 11.1 (4.0) | 13.5 (5.4) | 17.0 (6.6) | 23.8 (10.8) | <0.001 | A, B, C < D |
| NREM 1 (min) | 58.5 (28.1) | 62.4 (23.3) | 78.3 (30.5) | 121.0 (51.7) | <0.001 | A, B, C < D |
| NREM 2 (min) | 209.7 (44.2) | 197.7 (61.6) | 196.7 (47.6) | 180.8 (57.5) | ns | |
| NREM 3 (min) | 40.3 (31.7) | 41.4 (39.0) | 21.1 (24.5) | 16.3 (18.7) | <0.01 | B > D |
| NREM (min) | 308.5 (52.9) | 301.4 (60.1) | 296.0 (42.7) | 318.1 (54.6) | ns | |
| NREM (%) | 83.9 (4.6) | 84.5 (5.5) | 85.5 (5.7) | 86.0 (5.7) | ns | |
| REM (min) | 58.5 (16.5) | 57.2 (24.0) | 51.0 (20.5) | 52.3 (24.3) | ns | |
| REM duration < 30 (%) | 0.0% | 13.5% | 20.0% | 13.0% | ns | |
| REM (%) | 16.1 (4.6) | 15.5 (5.5) | 14.5 (5.7) | 14.0 (5.7) | ns | |
| Respiratory variables | ||||||
| AHI (AH/hour) | 2.0 (1.3) | 9.3 (2.8) | 22.3 (4.6) | 46.6 (17.9) | <0.001 | A < B < C < D |
| AH duration (s) | 20.3 (5.2) | 22.3 (4.6) | 20.7 (3.6) | 25.9 (5.2) | <0.001 | A, B, C < D |
| NREM-AHI (AH/h) | 1.4 (1.2) | 7.5 (3.5) | 20.3 (5.9) | 46.5 (18.2) | <0.001 | A, B < C < D |
| #NREM-AH | 6.8 (5.5) | 37.0 (18.8) | 101.1 (37.1) | 246.0 (107.7) | <0.001 | A, B < C < D |
| REM-AHI (AH/h) | 5.4 (5.1) | 22.7 (22.2) | 40.1 (28.2) | 49.5 (30.2) | <0.001 | A < C, D; B < D |
| #REM-AH | 5.3 (5.4) | 18.0 (14.1) | 28.2 (17.0) | 38.0 (22.4) | <0.001 | A < B, C, D; B<D |
| Minimal SpO2 (%) | 90.4 (2.9) | 87.0 (4.5) | 82.9 (5.6) | 82.5 (5.5) | <0.001 | A, B > C, D |
| Minimal NREM SpO2 (%) | 91.3 (2.9) | 88.2 (4.6) | 84.1 (5.8) | 83.9 (4.8) | <0.001 | A, B > C, D |
| Minimal REM SpO2 (%) | 91.4 (2.3) | 89.6 (3.5) | 85.2 (6.3) | 84.7 (6.1) | <0.001 | A, B > C, D |
| TST SpO2 < 90% (min) | 0.1 (0.2) | 2.1 (5.1) | 6.0 (5.1) | 16.1 (18.9) | <0.001 | A, B, C < D |
Note: Results are presented as mean (SD).
OSA: obstructive sleep apnea; AHI: apnea–hypopnea index; N/A: not applicable; TST: Total sleep time; MAI: micro-arousal index; NREM: non-rapid eye movement sleep; REM: rapid eye movement sleep; AH: apneas and hypopneas; SpO2: oxygen desaturation.
Use of a regression approach in REM sleep OSA
We assessed the relationship between REM sleep OSA and daytime cerebral functioning mostly using regression models. Although groups are clinically important, there is many different criteria that exist in the current literature with arguably arbitrary cut-offs to define the presence of REM sleep OSA.10 Moreover, some studies showed a dose–response relationship between various physiological functions and respiratory events during REM sleep.18–21 Regression models are not limited by arbitrary cut-offs and may represent more closely the physiopathology compared to a group analysis. Regressions models used in the present study were based on those performed in large cohort studies that have investigated the effect of REM sleep OSA on hypertension, glucose metabolism as well as sleepiness and quality of life.18,19,21,30
Independent variables for statistical models
The relationship between REM sleep OSA and daytime cerebral functioning is evaluated with statistical models presented below. REM sleep OSA was assessed with the REM-AHI and the REM-AH. Because REM sleep duration is the denominator to compute the REM-AHI, a very short duration may lead to an overestimation of REM sleep OSA. Therefore, for analyses using the REM-AHI, subjects with very short REM sleep duration (<30 min) during their polysomnographic recording were excluded. This threshold was used in previous studies investigating the impacts of REM sleep OSA.17,18 Moreover, the REM-AHI is highly dependent of the REM sleep duration whether it is very short or very long. Because the REM-AHI is limited by the REM sleep duration to accurately assessed OSA during REM sleep, we also performed statistical models with the REM-AH instead of the REM-AHI. With models performed with the REM-AH, all subjects were included regardless of REM sleep duration since there is no denominator effect. Thus, subjects with less than 30 min of REM sleep were included in all analyses with the REM-AH. However, total sleep time was included as a covariate in regression models with the REM-AH to adjust for sleep duration. Indeed, because total sleep time and REM sleep duration are highly correlated, including total sleep time in models partially adjusts for REM sleep duration.
Respiratory variables representing OSA were negatively skewed, did not have a normal distribution, and contained at least one value equal to zero. Thus, the formula “log10(variable + 1)” was used. Similar data transformation for regression models was used in previous studies investigating the impacts of REM sleep OSA.18–21,30 Statistics that were performed between variables included in regression models suggested no multicollinearity (variance inflation factor < 1.1). Age was included as a covariate in all models. Because the prevalence of REM sleep OSA is reported to be closely associated with sex,12 we did not include it as a covariate upfront since it could have removed an REM sleep OSA effect. We, however, performed supplementary analyses that included both age and sex as covariates for each statistical model.
Model 1: Regression between REM sleep OSA and daytime rCBF in all subjects
To assess whether OSA during REM versus NREM sleep is associated with distinct patterns of daytime cerebral functioning, linear regressions were used across all subjects from controls to severe OSA individuals. Since both REM and NREM sleep OSA variables were included in the same model simultaneously (REM-AH and NREM-AH, or REM-AHI and NREM-AHI), significant regressions with REM sleep OSA were controlled for NREM sleep OSA, and vice versa. This allowed investigating OSA effect during each sleep type independently on daytime rCBF as a dependent variable. In a supplementary analysis, we also divided the sample into quartiles of REM sleep OSA severity and compared them regarding their daytime rCBF values (see Model S1 in supplementary material online).
Model 2: Regression between REM sleep OSA and daytime rCBF in subjects with an AHI<15
OSA that is predominantly during REM sleep can result in a low overall OSA severity. To assess whether REM sleep OSA affects daytime cerebral functioning in milder OSA severity, REM and NREM sleep OSA variables (REM-AH and NREM-AH, or REM-AHI and NREM-AHI) were included simultaneously in a regression model with daytime rCBF as a dependent variable in subjects with an AHI<15 only, thus excluding subjects with an overall moderate and severe OSA. We also investigated the association between daytime rCBF and REM sleep OSA in subjects with no or mild NREM sleep OSA in a supplementary analysis (NREM-AHI < 15, see Model S2 in supplementary material online).
Model 3: Regression between REM sleep OSA and symptomatology
To investigate whether REM sleep OSA (or NREM sleep OSA) is associated with global cognitive performance, depressive and anxious symptoms or with daytime sleepiness, we performed regression analyses across all subjects including both REM sleep and NREM sleep OSA variables simultaneously in regression models (REM-AH and NREM-AH, or REM-AHI and NREM-AHI) with MoCA, BDI-II, BAI and ESS as dependent variables.
Statistical thresholds
For daytime rCBF analyses performed within SPM, models were applied with the voxel-based technique. Final processed individual SPECT images have thousands of rCBF values: one single rCBF value for every voxel (2 mm3). This type of neuroimaging technique applies the statistical models on all voxels specified. We used a mask covering gray matter in the cerebrum to apply constructed models on these voxels only, and thus excluding the cerebellum, the white matter and cerebrospinal fluid. Significance was set at p < 0.001 for voxel peaks combined with an extent threshold of clusters containing ≥100 contiguous voxels. This specific combination of a liberal p-value uncorrected for multiple comparisons combined with a restrictive cluster size was shown to better find and localizes regions with changed metabolism, without increasing false positives.31 Significant regions were identified using PickAtlas with the automated anatomical labeling (AAL) atlas (http://fmri.wfubmc.edu/software/pickatlas; version 3.0). For analyses with sleepiness, mood, and cognition as dependent variables, a threshold of p < 0.05 was chosen.
Results
Sample characteristics
The sample included 96 subjects (25 females; 71 males; mean age: 65.2 ± 6.4 years; age range: 55–82 years). In order to describe our sample, we divided subjects into four groups according to their AHI: control group (17% of the sample) and mild (38%), moderate (21%), and severe (24%) OSA groups. Detailed participant characteristics are presented in Table 1. At study entry, 44% of the sample reported an OSA-related complaint, including snoring, daytime sleepiness or bed partner witnessing respiratory disturbances. The average apnea and hypopnea duration as well as mean and minimal oxygen saturation did not differ significantly between REM and NREM sleep in our sample.
No differences were found between controls and OSA groups for sleepiness, mood, or cognition. In our sample, around a third presented daytime sleepiness (33% with the ESS ≥ 10), whereas about a fifth presented cognitive deficits (21% with the MoCA < 26). Low levels of depression and anxiety (9% with the BDI-II ≥14 and 16% with the BAI ≥ 10) were observed. No difference between groups was found for the vascular burden as well.
Model 1: Regression between REM sleep OSA and daytime rCBF in all subjects
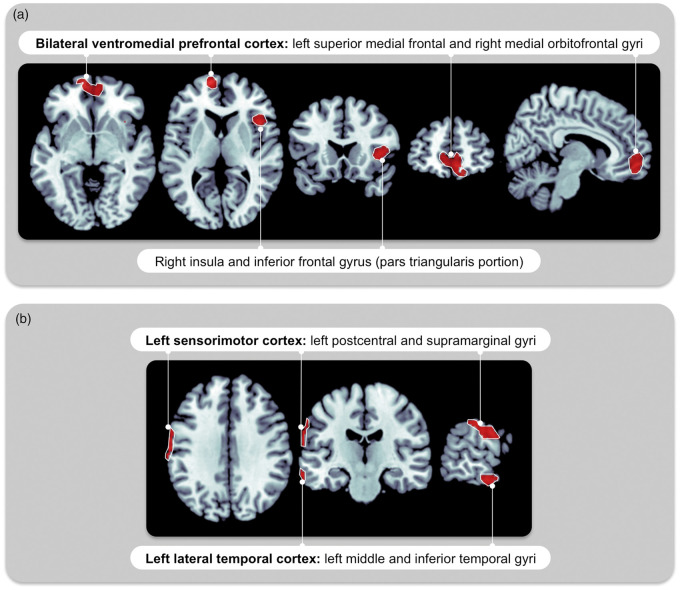
In all subjects (n = 96) including those with less than 30 min of REM sleep, the REM-AH and NREM-AH were associated independently with distinct patterns of reduced daytime rCBF (see Figure 1 and Table 2). Reduced daytime rCBF was observed in association with REM-AH in the bilateral ventromedial prefrontal cortex and the right insula extending to the inferior frontal gyrus. Reduced daytime rCBF with NREM-AH was present in the left sensorimotor cortex and left lateral temporal cortex. No region of increased daytime rCBF was observed. When sex was added as a covariate, a similar pattern of hypoperfusion was observed with REM-AH, whereas no daytime rCBF changes were observed with NREM-AH (see Table 2).
Figure 1.
Daytime brain perfusion patterns associated with OSA during REM versus NREM sleep. In the complete sample representing all OSA severities (n = 96, AHI from 0 to 97, model 1), reduced daytime rCBF is associated with (a) a higher REM-AH in the bilateral ventromedial prefrontal cortex and right insula extending to the frontal cortex; (b) a higher NREM-AH in the left sensorimotor and lateral temporal cortex. Regressions model were between REM-AH and daytime rCBF, adjusted for age, total sleep duration and NREM-AH (a); and between NREM-AH and daytime rCBF, adjusted for age, total sleep duration and REM-AH (b). Statistical analyses were performed on every voxel of gray matter. Significant regions of abnormal rCBF were identified when single voxels showed a significant regression with the OSA variable (statistical threshold at p < 0.001) and when these were surrounded by a cluster of ≥100 voxels with rCBF values that behave similarly. Similar regions of daytime hypoperfusion were observed when REM-AHI and NREM-AHI were used instead of REM-AH and NREM-AH. rCBF: regional cerebral blood flow; NREM: non-rapid eye movement sleep; REM: rapid eye movement sleep; AH: apneas + hypopneas; AHI: apnea–hypopnea index; OSA: obstructive sleep apnea.
Table 2.
Significant clusters of reduced daytime rCBF with REM-AH and NREM-AH in all subjects.
| Cluster size (k) | T | MNI coordinates |
Peak location with AAL atlas | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Model 1: Reduced daytime rCBF with REM-AH | |||||
| 647*† | 4.6 | −12 | 62 | 2 | L superior frontal medial |
| 4.0 | 6 | 54 | −8 | R medial orbitofrontal | |
| 221* | 4.1 | 42 | 20 | 4 | R inferior frontal pars triangularis (extending to the R insula) |
| Model 1: Reduced daytime rCBF with NREM-AH | |||||
| 140 | 4.1 | −68 | −16 | −20 | L middle temporal |
| 3.8 | −68 | −26 | −20 | L inferior temporal | |
| 225† | 3.9 | −66 | −14 | 22 | L postcentral (parietal) |
| 3.9 | −66 | −16 | 30 | L postcentral (parietal) | |
| 3.9 | −64 | −20 | 38 | L supramarginal (parietal) | |
| Model 2: Reduced daytime rCBF with REM-AH in subjects with AHI<15 | |||||
| 271*† | 4.4 | 40 | 18 | 6 | R inferior frontal pars opercularis (extending to the R insula) |
| 100 | 3.9 | −30 | 22 | 10 | L insula |
Note: Regression models included both REM-AH and NREM-AH as well as age and total sleep duration as covariates. Clusters marked with (*) were still significant when sex was added as a covariate (cluster size: 146, 133 and 200 voxels). Clusters marked with (†) were still significant with REM-AHI or NREM-AHI instead of REM-AH and NREM-AH in subjects with REM sleep duration ≥ 30 minutes (cluster size: 207, 193 and 185 voxels). Significant regions of daytime rCBF were obtained with the following threshold: p < 0.001 uncorrected for peaks voxels found within a cluster of ≥100 continuous voxels.
rCBF: regional cerebral blood flow; NREM: non-rapid eye movement sleep; REM: rapid eye movement sleep; AH: apneas + hypopneas; AHI; apnea–hypopnea index; MNI: Montreal Neurological Institute; AAL: automated anatomical labeling; OSA: obstructive sleep apnea; L: left; R: right.
We performed the same model with REM-AHI and NREM-AHI (instead of REM-AH and NREM-AH) in subjects with an REM sleep duration ≥30 min (n = 84). Of the 96 subjects recruited, 12 subjects had < 30 min of REM sleep during their in-laboratory polysomnographic recording. As stated earlier, a very short REM sleep duration led to a very high REM-AHI, and therefore an overestimation of OSA during REM. These subjects with short REM sleep duration had a higher AHI (p < 0.05) and shorter total sleep time (p < 0.001) than subjects with more than 30 min of REM sleep. Unsurprisingly, subjects with short REM sleep duration had a higher REM-AHI compared to other subjects (64.6 events/h versus 24.9 events/h respectively, p < 0.001).
Distinct patterns of daytime rCBF were also associated with REM-AHI and NREM-AHI. Reduced daytime rCBF in the right medial orbitofrontal cortex was associated with REM-AHI, and reduced daytime rCBF in the left postcentral gyrus was correlated with NREM-AHI. Therefore, the same regions of hypoperfusion were found with the absolute amount of AHs than with the AHIs. However, less and smaller clusters were found with REM-AHI and NREM-AHI compared to analyses performed in the complete sample with REM-AH and NREM-AH (see Table 2).
In our quartiles supplementary analysis, similar regions of daytime hypoperfusion (frontal cortex, insula and basal ganglia) were observed in the second and fourth quartiles of REM sleep OSA compared to the first quartile (see Model S1 in supplementary material online).
Model 2: Regression between REM sleep OSA and daytime rCBF in subjects with an AHI<15
In subjects with an overall OSA severity considered as no or mild OSA (n = 54), including those with REM sleep duration <30 min, the REM-AH was associated with reduced daytime rCBF in the bilateral insula extending to the inferior frontal gyrus on the right hemisphere, adjusted for NREM-AH, age and total sleep duration (see Table 2). Similar results on the right hemisphere were observed when sex was entered as a covariate or when the REM-AHI was used instead in subjects with a REM sleep duration ≥30 min (see Table 2). No region of increased daytime rCBF was found, and no association was observed between daytime rCBF and NREM-AH.
In our supplementary analysis, similar regions of daytime hypoperfusion (frontal cortex and insula) were observed with REM sleep OSA in subjects with either no or mild NREM sleep OSA (see Model S2 in supplementary material online).
Model 3: Regression between REM sleep OSA and symptomatology
In the complete sample (n = 96) including those with REM sleep duration <30 minutes, only a trend was observed: a higher REM-AH was associated with a worse MoCA (r = −0.197, p = 0.06) when adjusting for NREM-AH, age and total sleep time. When sex was entered as a covariate, the same trend remained (r = −0.206, p = 0.05). No association was found with the BDI-II, BAI and ESS scores with REM-AH, and no association was found between any questionnaire score and the NREM-AH.
In the sample corresponding to either no or mild OSA (AHI<15, n = 54), including those with REM sleep duration <30 min, a trend was found with a higher REM-AH associated with more depressive symptoms (r = 0.261, p = 0.07). However, that trend was not present when adjusted for sex. No association was found between NREM-AH and any questionnaire score. No association was found with the MoCA, BAI and ESS scores.
None of these trends were observed with the REM-AHI in subjects with a REM sleep duration ≥30 min instead of the REM-AH adjusted for total sleep duration.
Discussion
Summary of the cerebral perfusion findings
In this study, we aimed to clarify the impacts of OSA during REM sleep on daytime cerebral functioning. We found that more respiratory events during REM or NREM sleep are associated with different patterns of daytime brain perfusion. In fact, the presence of more apneas and hypopneas during REM sleep was associated with daytime hypoperfusion in the ventromedial prefrontal and fronto-insular regions, whereas more respiratory events during NREM sleep were associated with daytime hypoperfusion in the left sensorimotor and temporal cortex. Another significant finding is that reduced daytime brain perfusion was also observed in fronto-insular regions in association with REM sleep OSA among subjects without moderate to severe OSA. This result suggests that when the overall OSA severity is low, REM sleep OSA can still affect daytime cerebral functioning, a finding that was not observed for NREM sleep OSA. This also suggests that the presence of REM sleep OSA exclusively can affect daytime cerebral functioning. The novelty of our study lies in the use of neuroimaging to investigate whether REM sleep OSA affects daytime cerebral functioning in a large sample of participants, including subjects with milder forms of OSA.
SPECT as a sensitive tool to detect cerebral dysfunctions in OSA
Resting-state cerebral perfusion is considered as a marker of brain functioning because it is closely related to neuronal and astroglial activity,22 although other contributing factors to cerebral perfusion include vascular reactivity and cerebral autoregulation.32 Because neurovascular coupling was reported to be preserved in OSA individuals,33 we can hypothesize that altered daytime rCBF represents, in part at least, metabolic demands of neurons and astrocytes. Hypoperfusions in the ventral, medial and orbital prefrontal cortex, as well as in the insula were found in previous OSA studies.1,2,4–6 Similarly, reduced rCBF in temporal and sensorimotor regions was also reported in OSA subjects.1–3,5,6 Therefore, our study brings new insight into the discrepancies across brain regions that have been reported as hypoperfused in previous OSA studies: differences in the numbers of apneas and hypopneas during REM and NREM sleep could explain why different brain locations show abnormal daytime rCBF in different studies.
Characteristics of OSA during REM sleep and daytime cerebral functioning
During REM sleep, upper airway muscle activity is reduced and their collapsibility increases,10,18,34 which may lead to more apneas and hypopneas during REM sleep, even in milder OSA cases. The literature is scarce on the consequences of REM sleep OSA with a mild overall severity. In addition, it is unclear whether these patients should they be treated or not.14 Our results showed that REM sleep OSA could be more harmful to brain functioning than NREM sleep OSA. Previous authors have suggested that the harmful impact of REM sleep OSA could be due to the fact that respiratory events during REM sleep last longer and provoke more hypoxemia.15 In the present study, however, it was not the case. Apneas and hypopneas duration as well as mean and minimal saturation did not differ statistically between REM and NREM sleep in our sample. Instead, another hypothesis could be that the impact of REM sleep OSA could be related to the physiological response to apneas and hypopneas rather than their characteristics like duration and oxygen saturation. Compared to NREM sleep, REM sleep is characterized by a high level of brain activity, higher sympathetic activity, and irregular heart rate.34,35 These REM sleep particularities could put the brain in a vulnerable state to the effects of OSA. In fact, blood pressure fluctuations are higher for apneas and hypopneas during REM sleep than NREM sleep.16 Moreover, increased activity during REM sleep has been previously reported for the ventral and orbital prefrontal cortex as well as for the anterior portion of the insula.36 Areas with increased metabolic requirements during apneas and hypopneas could be more susceptible to injury provoked by oxygen shortage, arousals, and hemodynamic fluctuations. These areas of high activity during REM sleep could show neuronal dysfunction that can then be manifested during daytime as reduced activity. Thus, OSA during REM sleep could lead to vascular and metabolic consequences to which the brain is especially vulnerable. Consistently, OSA during REM sleep was recently associated with impaired cardiovascular and metabolic function.17–21
Because we observed larger and more regions of reduced rCBF, our analyses were stronger when using the absolute number of apneas and hypopneas during REM sleep rather than the REM-AHI. This could be due to the exclusion of subjects with very short REM sleep duration in order to accurately compute the REM-AHI: indeed, subjects with very short REM sleep duration may be the ones that are most affected by OSA during REM sleep, which could be characterized by a perturbed and short REM sleep. Moreover, stronger results may also suggest that the absolute amount (AH) rather than the density (AHI) of apneas and hypopneas during REM sleep is more representative of underlying processes that may be detrimental to brain health. Overall, although the AHI is the standard measurement of OSA severity, REM-AH may not be the best variable to assess REM sleep OSA giving the high influence of REM sleep duration on its value.
Clinical significance of REM sleep OSA
While some previous studies did not observe any association between OSA symptomatology and REM sleep OSA,9,37,38 others found that OSA during REM sleep was associated with depression.11,12 Moreover, a study used a unique design in OSA individuals by applying CPAP during NREM sleep and removing it during REM sleep. They found that spatial navigation memory was impaired after this specific REM sleep disruption by OSA.39 In the present study, we found associations at a trend level between REM sleep OSA and worse cognitive performance in the complete sample and more depressive symptoms in subjects without moderate to severe OSA. Worse cognitive performance with REM sleep OSA was observed only in analyses that included the complete sample with all levels of OSA severity, where reduced rCBF in the ventromedial prefrontal cortex was also observed. The ventromedial prefrontal cortex may be involved in cognitive deficits associated with REM sleep OSA, since it is involved in various cognitive functions, including executive functions and memory.40 On the other hand, associations between REM sleep OSA and depressive symptoms were observed only in the subsample including subjects with a milder form of OSA, in whom reduced rCBF with a fronto-insular distribution was also observed. Reduced insular perfusion may be involved in depressive symptoms because the insula is structurally included in the limbic system and plays a role in emotional behavior and feelings.41 These associations at a trend level between REM sleep OSA with a specific symptomatology may suggest that REM sleep OSA is more detrimental to brain health than NREM sleep OSA, for which no association was observed with symptomatology. Of note, only a fifth and a tenth of our sample showed cognitive deficits and mild depression levels, which may explain why only trends were observed. These trends obviously need to be replicated in a sample that is more symptomatic. Moreover, we did not observe any associations between OSA and daytime sleepiness. Our sample could be composed of OSA individuals with less clinical dysfunctions since a prevalence as high as 87% for daytime sleepiness and 59% for cognitive deficits were reported in other samples.42,43 However, clinical dysfunctions were measured differently than in ours in these studies: excessive daytime sleepiness was considered as present when a mean sleep latency <10 was observed on the daytime Multiple Sleep Latency Test, while cognitive deficits were identified with an impairment in one or more cognitive functions measured with a neuropsychological assessment.42,43
Although our sample was relatively asymptomatic, OSA is known to affect sleepiness, mood and cognition.7,8 Thus, in addition to worse global cognitive performance and depressive symptoms associated with REM sleep OSA, various symptoms may arise from prolonged preclinical periods of neuronal dysfunction evidenced by areas of decreased daytime rCBF associated with REM sleep OSA. The ventromedial prefrontal cortex plays a key role in emotional processing and its dysfunction has been observed with mood, moral cognition and personality changes, and various psychiatric disorders.44,45 The anterior insula is involved in a wide range of cognitive processes, including language, interoception and emotions.41 Although previous studies showed that OSA during NREM sleep was associated with sleepiness,13,30,46 we did not observe this relationship. However, prolonged preclinical periods of reduced perfusion in the parieto-temporal regions with NREM sleep OSA may also lead to various symptoms. In fact, sensorimotor and lateral temporal regions are involved in daytime sleepiness via their connection to the thalamus.47 The sensorimotor region and other parietal areas are constituents of the fronto–parietal attention network involved in working memory, episodic retrieval, and mental imagery,48 while the lateral temporal cortex plays an important role in memory.49 Consequently, further studies should investigate whether changes in rCBF in these particular regions predict the occurrence of OSA symptomatology and decline in these cognitive functions.
Therefore, abnormal rCBF in these specific cerebral areas may lead to symptoms following chronic REM or NREM sleep OSA over months or even years. However, the duration of the disease is difficult to assess in the OSA population. In fact, determining at which age the OSA condition started is very hard to know, since many people are not aware of their condition. OSA is often undiagnosed and asymptomatic, particularly in the elderly.50,51 Thus, although OSA was newly diagnosed in our sample, some individuals could have had OSA for years. Longitudinal studies are needed to understand the impacts of chronic and untreated OSA on the brain over time.
As other authors have suggested,10,18–20 we propose that individuals with several apneas and hypopneas during REM sleep, even with milder OSA forms, could benefit from treatment to reduce harmful effects to brain health. Consistently, continuous positive airway pressure (CPAP) was previously shown to be effective in reversing at least partially daytime rCBF changes in OSA individuals.4,6 Moreover, many OSA patients treated with CPAP remove their mask before the end of the night, which is where most REM sleep occurs. This could lead to up to 60% of REM sleep untreated with an average CPAP use.20 A poor CPAP adherence near the morning could make these treated patients vulnerable to the effects of OSA during REM sleep. This is problematic since subjects with OSA exclusively during REM sleep have a lower adherence to CPAP than other OSA patients.52
Strengths and limitations
To our knowledge, this is the first study to assess the specific effects of REM sleep OSA on brain health using neuroimaging. Our study adds to the current literature that underlines the detrimental effects of REM sleep OSA on physiological processes. The large sample investigated in this study, which included subjects with all levels of OSA severity, allowed us to investigate the specific effects of REM sleep OSA in milder cases.
REM sleep OSA was shown to be more prevalent in younger individuals and women,13 two populations that are either not or only poorly represented in the present study. Nevertheless, sex was entered as a covariate in our additional analyses, and we found less but similar results.
Conclusions
We found that apneas and hypopneas during REM versus NREM sleep were associated with distinct patterns of daytime regional cerebral perfusion, with a hypoperfused ventromedial prefrontal and fronto-insular distribution associated with REM sleep OSA as well as a hypoperfused left sensorimotor and temporal cortex associated with NREM sleep OSA. Only REM sleep OSA was independently associated with altered rCBF during wakefulness in subjects with a milder OSA forms. Our results suggest that OSA during REM sleep could be more detrimental to brain health. Therefore, subjects with OSA predominantly during REM sleep, but with low overall OSA severity could benefit from treatment. As suggested by others,10,18–20 prolonged use of CPAP therapy throughout the night may also be beneficial, as most REM sleep occurs near morning. However, before justifying treatment for all REM sleep predominant OSA patients, our results need to be replicated in longitudinal treatment studies to obtain a more complete understanding of the impacts of REM sleep OSA on the brain.
Supplemental Material
Supplementary Material for Obstructive sleep apnea during REM sleep and daytime cerebral functioning: A regional cerebral blood flow study using high-resolution SPECT by Andrée-Ann Baril, Katia Gagnon, Pauline Brayet, Jacques Montplaisir, Julie Carrier, Jean-Paul Soucy, Chantal Lafond, Hélène Blais, Caroline d'Aragon, Jean-François Gagnon and Nadia Gosselin in Journal of Cerebral Blood Flow & Metabolism
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by grants from government granting agencies: the Canadian Institutes of Health Research (CIHR) and the Fonds de la recherche en santé du Québec (FRQ-S).
Acknowledgments
The authors wish to thank Dominique Petit; Joëlle Robert; Sarah-Hélène Julien; Maxime Fortin; Maria Tuineag; Fatma Ben Aissa; Madiha Akesbi; Jessica Cole; Nazmiye Uzun; Marjolaine Lafortune; and Frédérique Escudier.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: P. Brayet and Dr. Gosselin received a scholarship/salary award from the FRQ-S. K. Gagnon, Dr. Soucy, H. Blais, and C. d'Aragon have no financial disclosures. A.A. Baril, Drs. Carrier and J.F. Gagnon received a scholarship or a salary award from the CIHR. Dr. Carrier is the principal investigator of the Canadian Sleep and Circadian Network, which received grants from RANA, Respironics and Meck. Dr. J.F. Gagnon holds a Canada Research Chair in Cognitive Decline in Pathological Aging. Dr. Montplaisir holds a Canada Research Chair in Sleep Medicine. Dr. Montplaisir serves as a consultant for Merck Pharmaceutical, Jazz Pharmaceutical, UBC Canada and Valeant Pharmaceutical. Dr. Lafond evaluated sleep tests for Biron. These associations with commercial entities from Drs. Montplaisir, Carrier, and Lafond did not support the present work.
Authors' contributions
A.A. Baril: study conception and design; data acquisition, analysis, and interpretation; drafting of the paper and revised it following co-authors' comments; K. Gagnon: study design; data acquisition and interpretation; critical revision of the manuscript; Ms. Brayet: study design; data acquisition; critical revision of the manuscript; Dr. Montplaisir: study conception; data interpretation; critical revision of the manuscript; Dr. Carrier: study conception and design; data interpretation; critical revision of the manuscript; Dr. Soucy: study conception; data interpretation; critical revision of the manuscript; Dr. Lafond: study conception; data interpretation; critical revision of the manuscript; H. Blais: study design; data acquisition and interpretation; critical revision of the manuscript; C. d'Aragon: data acquisition, analysis and interpretation; critical revision of the manuscript; Dr. J-F. Gagnon: study conception; critical revision of the manuscript; Dr. Gosselin: study conception and design; data interpretation; participating to draft the paper; revised it critically. All authors approved the final version of the manuscript, and take responsibility for the research.
Supplemental material
Supplemental material for this paper can be found at the journal website: http://journals.sagepub.com/home/jcb
References
- 1.Baril AA, Gagnon K, Arbour C, et al. Regional cerebral blood flow during wakeful rest in older subjects with mild to severe obstructive sleep apnea. Sleep 2015; 38: 1439–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Innes CR, Kelly PT, Hlavac M, et al. Decreased regional cerebral perfusion in moderate-severe obstructive sleep apnoea during wakefulness. Sleep 2015; 38: 699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joo EY, Tae WS, Han SJ, et al. Reduced cerebral blood flow during wakefulness in obstructive sleep apnea-hypopnea syndrome. Sleep 2007; 30: 1515–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shiota S, Inoue Y, Takekawa H, et al. Effect of continuous positive airway pressure on regional cerebral blood flow during wakefulness in obstructive sleep apnea. Sleep Breath 2014; 18: 289–295. [DOI] [PubMed] [Google Scholar]
- 5.Chen HL, Lin HC, Lu CH, et al. Systemic inflammation and alterations to cerebral blood flow in obstructive sleep apnea. J Sleep Res 2017; 26: 789–798. [DOI] [PubMed] [Google Scholar]
- 6.Kim JS, Seo JH, Kang MR, et al. Effect of continuous positive airway pressure on regional cerebral blood flow in patients with severe obstructive sleep apnea syndrome. Sleep Med 2017; 32: 122–128. [DOI] [PubMed] [Google Scholar]
- 7.Sanchez AI, Martinez P, Miro E, et al. CPAP and behavioral therapies in patients with obstructive sleep apnea: effects on daytime sleepiness, mood, and cognitive function. Sleep Med Rev 2009; 13: 223–233. [DOI] [PubMed] [Google Scholar]
- 8.Leng Y, McEvoy CT, Allen IE, et al. Association of sleep-disordered breathing with cognitive function and risk of cognitive impairment: a systematic review and meta-analysis. JAMA Neurol 2017; 74: 1237–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haba-Rubio J, Janssens JP, Rochat T, et al. Rapid eye movement-related disordered breathing: clinical and polysomnographic features. Chest 2005; 128: 3350–3357. [DOI] [PubMed] [Google Scholar]
- 10.Alzoubaidi M, Mokhlesi B. Obstructive sleep apnea during rapid eye movement sleep: clinical relevance and therapeutic implications. Curr Opin Pulm Med 2016; 22: 545–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conwell W, Patel B, Doeing D, et al. Prevalence, clinical features, and CPAP adherence in REM-related sleep-disordered breathing: a cross-sectional analysis of a large clinical population. Sleep Breath 2012; 16: 519–526. [DOI] [PubMed] [Google Scholar]
- 12.Lee SA, Paek JH, Han SH. REM-related sleep-disordered breathing is associated with depressive symptoms in men but not in women. Sleep Breath 2016; 20: 995–1002. [DOI] [PubMed] [Google Scholar]
- 13.Pamidi S, Knutson KL, Ghods F, et al. Depressive symptoms and obesity as predictors of sleepiness and quality of life in patients with REM-related obstructive sleep apnea: cross-sectional analysis of a large clinical population. Sleep Med 2011; 12: 827–831. [DOI] [PubMed] [Google Scholar]
- 14.Ganguly G. The clinical dilema: to treat or not to treat REM related obstructive sleep apnea?. Sleep 2012; 35: 755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muraki M, Kitaguchi S, Ichihashi H, et al. Apnoea-hypopnoea index during rapid eye movement and non-rapid eye movement sleep in obstructive sleep apnoea. J Int Med Res 2008; 36: 906–913. [DOI] [PubMed] [Google Scholar]
- 16.Garpestad E, Ringler J, Parker JA, et al. Sleep stage influences the hemodynamic response to obstructive apneas. Am J Respir Crit Care Med 1995; 152: 199–203. [DOI] [PubMed] [Google Scholar]
- 17.Appleton SL, Vakulin A, Martin SA, et al. Hypertension is associated with undiagnosed OSA during rapid eye movement sleep. Chest 2016; 150: 495–505. [DOI] [PubMed] [Google Scholar]
- 18.Mokhlesi B, Finn LA, Hagen EW, et al. Obstructive sleep apnea during REM sleep and hypertension. results of the Wisconsin Sleep Cohort. Am J Respir Crit Care Med 2014; 190: 1158–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mokhlesi B, Hagen EW, Finn LA, et al. Obstructive sleep apnoea during REM sleep and incident non-dipping of nocturnal blood pressure: a longitudinal analysis of the Wisconsin Sleep Cohort. Thorax 2015; 70: 1062–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grimaldi D, Beccuti G, Touma C, et al. Association of obstructive sleep apnea in rapid eye movement sleep with reduced glycemic control in type 2 diabetes: therapeutic implications. Diab Care 2014; 37: 355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chami HA, Gottlieb DJ, Redline S, et al. Association between glucose metabolism and sleep-disordered breathing during REM sleep. Am J Respir Crit Care Med 2015; 192: 1118–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sestini S. The neural basis of functional neuroimaging signal with positron and single-photon emission tomography. Cell Mol Life Sci 2007; 64: 1778–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Villeneuve S, Belleville S, Massoud F, et al. Impact of vascular risk factors and diseases on cognition in persons with mild cognitive impairment. Dement Geriatr Cogn Disord 2009; 27: 375–381. [DOI] [PubMed] [Google Scholar]
- 24.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991; 14: 540–545. [DOI] [PubMed] [Google Scholar]
- 25.Beck AT, Steer RA, Ball R, et al. Comparison of beck depression inventories -IA and -II in psychiatric outpatients. J Pers Assess 1996; 67: 588–597. [DOI] [PubMed] [Google Scholar]
- 26.Beck AT, Epstein N, Brown G, et al. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol 1988; 56: 893–897. [DOI] [PubMed] [Google Scholar]
- 27.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005; 53: 695–699. [DOI] [PubMed] [Google Scholar]
- 28.Iber C, Ancoli-Israel S, Chesson ALJ, et al. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications, 1st ed. Westchester: American Academy of Sleep Medicine, 2007. [Google Scholar]
- 29.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. deliberations of the sleep apnea definitions task force of the american academy of sleep medicine. J Clin Sleep Med 2012; 8: 597–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chami HA, Baldwin CM, Silverman A, et al. Sleepiness, quality of life, and sleep maintenance in REM versus non-REM sleep-disordered breathing. Am J Respir Crit Care Med 2010; 181: 997–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mayoral M, Marti-Fuster B, Carreno M, et al. Seizure-onset zone localization by statistical parametric mapping in visually normal (18) F-FDG PET studies. Epilepsia 2016; 57: 1236–1244. [DOI] [PubMed] [Google Scholar]
- 32.Macey PM. Altered resting cerebral blood flow in obstructive sleep apnea: a helpful change or not?. Sleep 2015; 38: 1345–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tekgol Uzuner G, Uzuner N. Cerebrovascular reactivity and neurovascular coupling in patients with obstructive sleep apnea. Int J Neurosci 2017; 127: 59–65. [DOI] [PubMed] [Google Scholar]
- 34.Moszczynski A, Murray BJ. Neurobiological aspects of sleep physiology. Neurol Clin 2012; 30: 963–985. [DOI] [PubMed] [Google Scholar]
- 35.Chouchou F, Desseilles M. Heart rate variability: a tool to explore the sleeping brain?. Front Neurosci 2014; 8: 402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hobson JA, Stickgold R, Pace-Schott EF. The neuropsychology of REM sleep dreaming. Neuroreport 1998; 9: R1–14. [DOI] [PubMed] [Google Scholar]
- 37.Khan A, Harrison SL, Kezirian EJ, et al. Obstructive sleep apnea during rapid eye movement sleep, daytime sleepiness, and quality of life in older men in osteoporotic fractures in men (MrOS) sleep study. J Clin Sleep Med 2013; 9: 191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Y, Su C, Liu R, et al. NREM-AHI greater than REM-AHI versus REM-AHI greater than NREM-AHI in patients with obstructive sleep apnea: clinical and polysomnographic features. Sleep Breath 2011; 15: 463–470. [DOI] [PubMed] [Google Scholar]
- 39.Varga AW, Kishi A, Mantua J, et al. Apnea-induced rapid eye movement sleep disruption impairs human spatial navigational memory. J Neurosci 2014; 34: 14571–14577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zald DH, Andreotti C. Neuropsychological assessment of the orbital and ventromedial prefrontal cortex. Neuropsychologia 2010; 48: 3377–3391. [DOI] [PubMed] [Google Scholar]
- 41.Gasquoine PG. Contributions of the insula to cognition and emotion. Neuropsychol Rev 2014; 24: 77–87. [DOI] [PubMed] [Google Scholar]
- 42.Pierobon A, Giardini A, Fanfulla F, et al. A multidimensional assessment of obese patients with obstructive sleep apnoea syndrome (OSAS): a study of psychological, neuropsychological and clinical relationships in a disabling multifaceted disease. Sleep Med 2008; 9: 882–889. [DOI] [PubMed] [Google Scholar]
- 43.Seneviratne U, Puvanendran K. Excessive daytime sleepiness in obstructive sleep apnea: prevalence, severity, and predictors. Sleep Med 2004; 5: 339–343. [DOI] [PubMed] [Google Scholar]
- 44.Barbas H. Connections underlying the synthesis of cognition, memory, and emotion in primate prefrontal cortices. Brain Res Bull 2000; 52: 319–330. [DOI] [PubMed] [Google Scholar]
- 45.Young L, Koenigs M. Investigating emotion in moral cognition: a review of evidence from functional neuroimaging and neuropsychology. Br Med Bull 2007; 84: 69–79. [DOI] [PubMed] [Google Scholar]
- 46.Punjabi NM, Bandeen-Roche K, Marx JJ, et al. The association between daytime sleepiness and sleep-disordered breathing in NREM and REM sleep. Sleep 2002; 25: 307–314. [PubMed] [Google Scholar]
- 47.Killgore WD, Vanuk JR, Knight SA, et al. Daytime sleepiness is associated with altered resting thalamocortical connectivity. Neuroreport 2015; 26: 779–784. [DOI] [PubMed] [Google Scholar]
- 48.Luckmann HC, Jacobs HI, Sack AT. The cross-functional role of frontoparietal regions in cognition: internal attention as the overarching mechanism. Prog Neurobiol 2014; 116: 66–86. [DOI] [PubMed] [Google Scholar]
- 49.Cheung MC, Chan AS. Memory impairment in humans after bilateral damage to lateral temporal neocortex. Neuroreport 2003; 14: 371–374. [DOI] [PubMed] [Google Scholar]
- 50.Morrell MJ, Finn L, McMillan A, et al. The impact of ageing and sex on the association between sleepiness and sleep disordered breathing. Eur Respir J 2012; 40: 386–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simpson L, Hillman DR, Cooper MN, et al. High prevalence of undiagnosed obstructive sleep apnoea in the general population and methods for screening for representative controls. Sleep Breath 2013; 17: 967–973. [DOI] [PubMed] [Google Scholar]
- 52.Almeneessier AS, Almousa Y, Hammad O, et al. Long-term adherence to continuous positive airway pressure in patients with rapid eye movement-only obstructive sleep apnea: a prospective cohort study. J Thorac Dis 2017; 9: 3755–3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material for Obstructive sleep apnea during REM sleep and daytime cerebral functioning: A regional cerebral blood flow study using high-resolution SPECT by Andrée-Ann Baril, Katia Gagnon, Pauline Brayet, Jacques Montplaisir, Julie Carrier, Jean-Paul Soucy, Chantal Lafond, Hélène Blais, Caroline d'Aragon, Jean-François Gagnon and Nadia Gosselin in Journal of Cerebral Blood Flow & Metabolism