Abstract
Hybrid imaging using PET/MRI has emerged as a platform for elucidating novel neurobiology, molecular and functional changes in disease, and responses to physiological or pharmacological interventions. For the central nervous system, PET/MRI has provided insights into biochemical processes, linking selective molecular targets and distributed brain function. This review highlights several examples that leverage the strengths of simultaneous PET/MRI, which includes measuring the perturbation of multi-modal imaging signals on dynamic timescales during pharmacological challenges, physiological interventions or behavioral tasks. We discuss important considerations for the experimental design of dynamic PET/MRI studies and data analysis approaches for comparing and quantifying simultaneous PET/MRI data. The primary focus of this review is on functional PET/MRI studies of neurotransmitter and receptor systems, with an emphasis on the dopamine, opioid, serotonin and glutamate systems as molecular neuromodulators. In this context, we provide an overview of studies that employ interventions to alter the activity of neuroreceptors or the release of neurotransmitters. Overall, we emphasize how the synergistic use of simultaneous PET/MRI with appropriate study design and interventions has the potential to expand our knowledge about the molecular and functional dynamics of the living human brain. Finally, we give an outlook on the future opportunities for simultaneous PET/MRI.
Keywords: Dopamine, pharmacology, opioid, serotonin, simultaneous PET/MRI
Introduction
Positron emission tomography (PET) is a non-invasive imaging technology with high sensitivity for tracing very low concentrations of radiolabeled molecules in the human body. This allows us to selectively image different targets, such as the distribution of receptors in the brain, and related biological processes in vivo. Magnetic resonance imaging (MRI) provides exquisite spatial and temporal resolution, in comparison to PET, with versatile imaging contrasts. Despite not having comparable molecular specificity to PET, functional MRI (fMRI) offers the sensitivity to detect small signal fluctuations based on hemodynamic responses in order to infer neuronal activity. With the introduction of PET/MR scanners (simultaneous or sequential), it has become possible to combine molecular target specificity with local brain function in vivo across not only spatial but also temporal scales.
Since its introduction almost a decade ago, approximately 70 simultaneous PET/MRI scanners1 exist across the globe and now serve as a valuable research tool. While PET/computed tomography (CT) is likely to remain a workhorse for diagnostic PET, PET/MRI has the potential to add clinical value in niche applications.2–4 In its infancy, the integration of PET and MRI required development of new hardware and software solutions: To date, the combined effort of research groups around the world has resolved many of the technical challenges, resulting in improved image quality of simultaneous PET/MRI of the brain.5–7 For example, in the absence of CT, new MR-based methods had to be developed for attenuation correction.8 Ladefoged et al.9 compared 11 different MR-based methods for attenuation correction of the brain and found that several of these methods performed comparable to the gold-standard CT-based attenuation correction.9 Furthermore, because head motion is a confounder in many imaging studies, MR-based motion correction of PET data has been developed.10 Continuous acquisition of MR sequences allows for tracking of motion, which can be applied during PET reconstruction.11
As the exclusively available non-invasive tool to image neuroreceptors in the living brain, PET combined with fMRI has tremendous value in investigating neurotransmitter systems. The power of multi-modal imaging with PET/fMRI has the potential to inform about molecular and neural mechanisms underlying sensory stimuli, behavior, and cognition. Simultaneous rather than sequential PET/MRI increases reproducibility by decreasing potential confounds introduced by physiology, adaptation or biases. Our current limited knowledge about neurotransmission imbalance and how it can lead to neurologic or psychiatric disorders emphasizes the importance to improve and utilize in vivo imaging methods and models to elucidate this enigma.
While pharmacological PET is commonly used, especially to evaluate target engagement or dose-occupancy of new drugs, the field of pharmacological MRI (phMRI) is less established even though it can contribute important biological information about drug–receptor interactions.12 The need for novel drugs to treat neurological and neuropsychiatric disorders is high but is challenged by being complex, costly and time-consuming.13 Drug-induced functional signaling measures together with receptor occupancy is how PET/MRI can help bridge the knowledge from in vitro to in vivo (pre-)clinical studies, thus being a valuable add-on in phase 0 drug studies. Overall, pharmacological PET/MRI provides a platform for characterizing both existing and novel drugs.14,15
In this review, we first discuss considerations that should be made when designing dynamic, functional simultaneous PET/MRI studies. We then provide an overview that focuses on combined PET/MRI studies investigating neurotransmission, with emphasis on the dopamine, opioid, serotonin and glutamate neurotransmitter systems. Lastly, we highlight exciting advances and opportunities for further development of receptor-specific simultaneous PET/MRI.
Practical aspects of dynamic PET/MRI experiments
Thoughtful experimental design is critical before conducting any studies. In the following, we will touch upon questions and considerations that are important for dynamic and functional PET/MRI studies for neuroreceptor imaging (Table 1). Note that the following section is not a comprehensive review and thus references represent examples of the described method. Example study designs are also illustrated in Figure 1.
Table 1.
Important considerations for dynamic functional PET/MRI experimental design.
| 1 | What opportunities exist for PET/MR to give new insights into receptor biology? |
| 2 | Choice of (a) PET radiotracer based on target and (b) functional MRI techniques. |
| 3 | Which pharmacological, physiological, or behavioral interventions are appropriate for the scientific question? |
| 4 | (a) What is the optimal timing to introduce an intervention during the PET scan?(b) What is the optimal start and duration of the corresponding fMRI acquisition? |
| 5 | PET and MRI data analysis and quantification: Methods for integration of multi-modal data analysis. |
| 6 | Practical consequences for patients and personnel taking part in PET/MRI studies. |
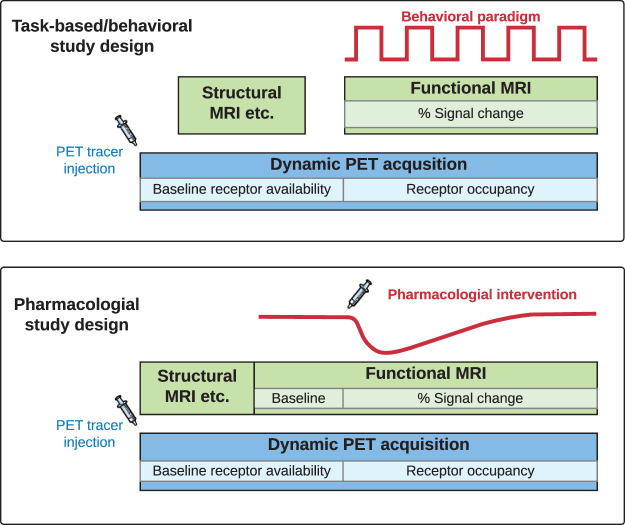
Figure 1.
Schematic representation of example task-based/behavioral or pharmacological study designs. Lighter colors are examples of outcome measures from each imaging modality for receptor-binding radiotracers. PET: positron emission tomography; MRI: magnetic resonance imaging.
1. PET/MRI is a non-invasive imaging tool with strong translational value. PET imaging serves as a bridge from in vitro pharmacology to in vivo molecular readouts, whereas fMRI is an indicator of dynamic brain function with a close link to behavior. There are a wide range of potential opportunities for PET/MRI, such as investigating the coupling between drug binding and efficacy, relating neurotransmitter release to cognition and disease states, and linking receptor densities to behavioral phenotypes. Examples of interventions suictable for PET/MRI are amphetamine for studying neurotransmitter release,16–18 nicotine or marihuana smoking before or during a PET/MRI scan,19 and working memory tasks.20
2a. We typically begin with choosing a PET radiotracer that is appropriate for a study since there are limited number of PET radiotracers that fulfill the criteria for successful radiotracers.21 Radiotracers exist for wide variety of targets in the brain, e.g. receptor, enzymes and misfolded proteins22 and development of new radiotracers is an active field of research. It is not within the scope of this review to give an overview of all available radioligands for neuroimaging targets and so we refer to several other excellent reviews on this topic.23–25 For receptor imaging, both agonist and antagonist radiotracers can exist for the same target. Agonist radiotracers potentially offer the advantage of binding to activated (high-affinity state) receptors, whereas antagonist radiotracers bind to the total pool of receptors.26–28 If the study only aims at quantifying the availability of receptors, an antagonist radiotracer would be a good choice. However, if the study aims at quantifying the amount of endogenous transmitter release, agonist radiotracers have been shown to be more sensitive.29,30
2b. A variety of fMRI techniques exist for addressing neuroscience questions. In particular, fMRI methods are commonly used to map the effects of explicit tasks and behaviors to corresponding cortical or subcortical areas. FMRI usually detects changes in hemodynamic responses based on blood oxygenated level dependent (BOLD) signals,31–33 cerebral blood flow (CBF),34–36 or cerebral blood volume (CBV)37–39 contrasts. While BOLD-fMRI offers the advantages of high sensitivity and ease of implementation over CBF- and CBV-fMRI, the BOLD signal reflects a composite change in CBF, CBV, and oxygen metabolism. CBF- and CBV-fMRI provide functional readouts based on single quantitative physiological parameter. However, CBF- and CBV-fMRI techniques usually require specialized pulse sequences, and careful optimization of sequence parameters is needed to achieve sufficient imaging contrast. MR contrast agents have been used for CBV-fMRI to improve detection power,37,40 but the utilization of contrast agents may limit its application in human subjects.
Besides fMRI techniques for addressing the scientific questions, a high-resolution T1-weighted anatomical scan is usually required, for the purpose of performing an MR-based attenuation correction of the PET data or for delineating anatomical regions of interests (ROI).9 Alternatively, an ultrashort (or zero) echo time image can be used to derive an attenuation correction map.41–43 Both structural and functional MR images can also be used to perform MR-based motion correction of PET.10
3. An intervention can be pharmacological (e.g. drug injection),44 physiological (e.g. hypo-/hypercapnic gas challenges)45 or task-based/behavioral (e.g. sensory-motor stimuli or cognitive tasks).46 The purpose of an intervention is usually to compare changes in imaging signals that change in time, i.e. before vs. after the intervention. Functional PET/MRI studies can also be carried out without any intervention (e.g. resting-state connectivity or baseline receptor availability), and serve to compare varying mental states, their connectivity patterns and underlying receptor distributions.47–49
4a. The timing of any intervention needs careful consideration. For pharmacological challenges, the time and drug administration route are tightly interlinked. The drug is most often administered orally or intravenously, although other options such as intranasal or intramuscular administration are possible.50 The intervention can be given as a pretreatment before the start of the PET/MRI to allow for the plasma concentration to, e.g., reach maximal or stable levels. If the drug is administrated during the scan as a within-scan challenge (preferably intravenously), it is possible to dynamically measure drug occupancy and time-dependent changes in drug-induced functional brain responses44,51 (Figure 1). In this scenario, it is important to first establish a stable baseline outcome measure prior to the time of challenge; methods for determining the exact timing of the introduction include realistic forward-model stimulations or analysis of real data in the absence of a challenge.52 A dynamic within-scan challenge offers the advantage of obtaining a baseline and dynamic changes due to an intervention in a single imaging session. However, this needs to be weighed against increased scan time, which may not be well tolerated by patients. In addition, the combination of long scan times and short-lived radiotracers will introduce noise to the PET images at late time points.
The type of radiotracer administration (either as a bolus injection or as a bolus followed by a constant infusion) is an important decision that goes hand in hand with the timing of the intervention. Conventionally, PET scans are performed with a single bolus injection. A different approach is to deliver the radioactive tracer as an infusion to achieve constant radioactivity levels in the ROI and in the plasma. A bolus-infusion approach is thought to provide better sensitivity for tracking dynamic changes in occupancy due to an intervention by providing continuous delivery of radioligand.53 However, to date, no systematic study has directly compared a dynamic challenge with a bolus versus a bolus-infusion. The standard approach is to calculate the theoretical bolus-infusion scheme (Kbol) based on an initial bolus baseline study,53 and the Kbol can then be further adjusted in repeated scans. It is important to note that the Kbol is region-specific, and therefore a uniform Kbol may not exist.
4b. Depending on the PET radiotracer and intervention chosen, the timing for introducing the fMRI sequence will vary. Unlike PET (as discussed in 4a), where a baseline outcome measure is obtained before interventions, the typical outcome measure for fMRI is percent signal changes (or activation maps based on statistical parametric mapping). A stable baseline signal is needed in order to calculate percent signal change or derive activation map based on general linear modeling (GLM). Pharmacological studies require a longer baseline (at least 10–15 min) to be collected before a drug challenge,12 whereas task-based fMRI has interleaved (e.g., an ON-OFF paradigm) baseline measurements. The total acquisition time for dynamic PET is on the corder of 30–120 min, depending on the radionuclide and pharmacokinetics of the tracer. For simplified semi-quantification without the use of kinetic models, 20–40 min acquisition time is often sufficient once validated. For fMRI, the acquisition time can be short (5 min) or long (90 min) depending on the MR sequence and the chosen intervention. For task-based stimuli, the acquisition time can be on the shorter end (5-10 min), with repeated fMRI runs performed to increase statistical power (Figure 1). For pharmacological challenges (phMRI), the acquisition time is often longer (30-120 min), depending on pharmacokinetics and experimental design.
5. For PET data quantification, kinetic modeling should be applied, with the model choice depending on the radiotracer and possible intervention. The most common fMRI outcome measure is a relative change in hemodynamic parameters, although quantitative CBF and CBV can also be calculated. Both modalities need to use robust statistics for group-level analyses. Combining parameters across PET and fMRI can aid the modeling and the interpretation of imaging data.14,54 In the next section below, a brief overview of existing dynamic models for PET and fMRI analysis will be provided.
6. The magnetic field associated with PET/MR imaging may exclude certain patients if they have magnetic implants, pacemakers, etc. The magnetic field also requires that any equipment in the scanner room, such as an emergency bed, anesthesia or blood sampling machines must be non-magnetic. These changes in daily procedures and extra safety issues require additional training of personnel. For pediatric and geriatric patients, obtaining PET and MRI data in a single imaging session increases patient comfort and reduces the amount of ionizing radiation exposure (compared to PET/CT), as well as sedation in these fragile patient populations. Sequential same-day imaging on separate systems can also constitute a reliable and cost-effective alternative for non-fragile patient populations.55,56
Dynamic models for PET receptor quantification and fMRI analysis
In simultaneous PET/MRI studies that explore temporal dynamics of signal changes and aim to draw comparisons between PET and fMRI signals, appropriate analysis techniques and models need to be considered. Absolute quantification of PET imaging data requires kinetic modeling, and changes in radiotracer signals are dependent on the pharmacokinetics of radiotracers, which tends to be on a slower timescale than typical fMRI experiments. FMRI experiments typically employ block or event-related design techniques relying on repeated and short stimuli. Conversely, conventional competition or displacement studies in PET are geared at detecting changes over several tens of minutes or hours and are thus not optimized for fMRI experimental design. In this section, we summarize existing dynamic PET models and MR techniques that have been or can be used to evaluate simultaneous signal changes in time (Table 2 and 3).
Table 2.
Comparison of key models for PET quantification and fMRI analysis that capture time-dependent changes during a scan.
| Name of PET model | Description | Key assumptions | Features | Limitations |
|---|---|---|---|---|
| SRTM-CR61 | A tissue concentration ratio method to compute changes in BPND, based on an initial estimate from SRTM. | Steady state of tracer kinetics is attained for a duration before and after an intervention. | Simple to compute. Close relation to the raw data. | Bias is unknown since the baseline parameter is derived from kinetic modeling and the post intervention parameter is based on raw data. Steady state needs to be achieved experimentally after the intervention. |
| ESRTM61 | A composite of two SRTM models with two BPND values as outcomes: One for the baseline, the other for the post-intervention. | Instantaneous change in BPND due to the intervention. | Simple to compute with two implementations of SRTM. | Not all interventions may cause an instantaneous change in binding potential, and last for the duration of the scan. |
| LSRRM57 | Linear extension of SRTM that accounts for within-scan challenge by cognitive task or drug. | Instantaneous maximal change in BPND at onset of the intervention, which decays exponentially. | Only one additional model parameter. | The shape/timecourse of the intervention is assumed to follow an exponential function. |
| lp-ntPET59,60 | Extension of LSRRM using gamma-variate basis functions, representing the timecourse of neurotransmitter release. | Basis functions are computed based on information content in time-activity curves and changes due to assumed endogenous dopamine release. | Data-driven approach. No assumptions about the shape/timecourse of the intervention. | Fitting of many parameters. Limited robustness of outcome measures in the context of noisy data. Model is tailored specifically for dopamine release. |
| SRTM2 with dynamic binding term to compute DBPND44,51 | Linearized SRTM2 implemented with a time-dependent k2a binding term. Combines elements from both LSRRM and lp-ntPET. | A priori knowledge or assumption about the shape/timecourse of the intervention. | Choice of analytical function for modeling the shape/timecourse of the intervention. Timecourse of occupancy can be computed from DBPND. Peak or average occupancy can be computed. | A priori information about the shape/timecourse of the intervention is needed. |
| rFRTM262 | Regularized full reference tissue model. | Same as SRTM2, except for the instantaneous exchange between the tissue compartments: k4 is not infinite. | Reduces some bias compared to the dynamic SRTM2, specifically in regions of small BPND values. | More complex computation with additional iterations to estimate k4 required. |
Table 3.
Comparison of key models for PET quantification and fMRI analysis that capture time-dependent changes during a scan.
| Functional MRI method | Description | Key assumptions | Features | Limitations |
|---|---|---|---|---|
| Functional MRI: block or event-related31,33 | Task-based or repeated stimulus intervention assessed by a general linear model (GLM). | Shape of the hemodynamic response function due to neuronal activation. | Robust outcome measures together with appropriate statistics and with sufficient repetitions within an experiment. | Interventions that are long-lived may not be distinguishable from baseline. Physiological noise can be a barrier in certain cases. |
| Pharmacological MRI12 | Functional MRI with a pharmaceutical as the intervention. | Timecourse of the pharmacological intervention may be data-driven or based on knowledge from PK/PD. | Evaluation of hemodynamic changes due to a pharmaceutical. | Effects of drugs that have many targets may be more complex to interpret with phMRI alone. |
| Functional connectivity139 | Resting-state or task-activated functional connectivity between brain regions with synchronous temporal activity or fluctuations. | Brain regions that display synchronous activity are functionally connected. | Mainly a data-driven approach with several analysis technique choices. | No directionality. |
PET dynamic kinetic models
The simplest way to analyze dynamic PET data is to calculate concentration ratios from raw time-activity curves. This has the advantage of staying as close as possible to the data, without introducing model biases. Yet, sensitivity to smaller changes and dynamic timecourses may be limited with this approach.
Several models have been proposed for estimating a temporal change in specific binding using kinetic modeling. To date, these types of modeling approaches have solely been developed for reference tissue models. Table 2 summarizes PET kinetic models from the literature that incorporate a time-dependent parameter in the context of a within-scan challenge. A time-dependent specific binding term was first proposed for the purpose of estimating displacement induced by a cognitive paradigm using [11C]raclopride.57 This kinetic modeling method is based on the simplified reference tissue model (SRTM58) and fits the specific binding term (k2a) with an exponential at the start of the challenge. The ntPET model59 focuses on modeling endogenous dopamine release and the lp-ntPET model60 enables linearization and a set of basis functions that model an assumed endogenous dopamine timecourse. Given the number of parameters to be fitted, noise levels and potential biases are an important consideration for these types of models. The extended SRTM (ESRTM)61 assumes a constant baseline binding rate which at intervention instantaneously changes to a new constant binding rate. To reduce bias introduced by assumptions on the specific binding dissociation rate constant (k4) when converting the full reference tissue model (FRTM) to SRTM, the regularized FRTM (rFRTM62) was introduced using an estimate for a realistic k4 value. As a descriptive outcome parameter that captures the dynamic nature of these models, the “dynamic binding potential” or DBP44 was proposed as a time-varying description of binding potential, which can be compared to BPND. This description delineates that a time-dependent specific binding term is employed during the analysis. Finally, DBP(t) enables the computation of changes in receptor occupancy over time that can be compared to fMRI timecourses. We refer to Table 2 for features and limitations of each model.
fMRI and phMRI methods and analysis
Traditional fMRI studies use a task (e.g. finger tapping) or a cognitive challenge (e.g. working memory) to modulate neuronal activity and map the corresponding hemodynamic changes. This type of repeated stimulus that elicits repeated brain activation patterns has statistical advantages: A GLM is most often used to look for activation patterns at a voxelwise level and more repetitions of the same activation event lead to higher statistical power. In addition, these types of repeated challenges usually occur on the order to several seconds33 (fast stimuli compared to PET) and thus are easily distinguishable from baseline drift in hemodynamic signals (such as BOLD, CBF or CBV). Table 3 provides an overview of commonly used fMRI methods and analysis approaches.
A slightly distinct form of fMRI that is of particular interest in the context of PET/fMRI neuroreceptor studies is the use of pharmacological challenges rather than a behavioral task – this type of fMRI is also referred to as phMRI12. The analysis of phMRI uses similar tools as task-based fMRI but has important distinctions: GLM is also used as the basis for analysis but instead of fitting fast repeated patterns that are convolved with a hemodynamic response function, an analytical function such as a gamma-variate or sigmoidal curve is fitted to the signal.12,14 The choice of the best mathematical function is informed by knowledge about the pharmacokinetics of the drug and the fit to the data. Hemodynamic changes elicited through pharmacological challenges are often larger in magnitude compared to task-based fMRI, although they certainly depend on the type of drug and respective dose.40
With slow-onset drugs, or non-intravenous administrations, a real difficulty in phMRI can be the differentiation of signal changes of interest from the baseline drift. Unlike in PET, fMRI analysis is usually not tailored to detect slower temporal changes, such as those caused by a pharmacological challenge or spontaneous fluctuation. While correction methods exist for correcting baseline drift,63,64 slow signal changes in fMRI can sometimes be difficult to be distinguished from signal drift. For this reason, arterial spin labeling (ASL, a technique for measuring changes in CBF) can be a good alternative for some studies: The upside is that baseline drift is taken out during subtraction of control and labelled images, yet this need to be weighed versus the downside of less signal to noise in ASL compared to BOLD. It is important to keep these analysis tradeoffs in mind when designing new experiments.
Effects of physiology on PET/MR signals after an intervention
An intervention designed to change receptor occupancy usually also induces changes in physiology. By nature of its measurement, fMRI signals either directly measure changes in physiology (CBF, CBV) or are influenced by a combination of physiological changes (BOLD). While a large body of research has shown the link between fMRI and neuronal activity,65 combined PET has the potential to further aid in disentangling pure physiology from molecular and neural components.
Measurements by PET itself, however, ideally should not depend on physiology. One of the main confounds that has been debated in the literature is the potential effects of blood flow changes on the delivery rate constant of radiotracers and thus PET outcome measures. This question has been investigated through simulations57,60 but the latest evidence has come from a simultaneous PET/MR study: Increases in blood flow were induced using hypercapnia while observing dopamine receptor availability using PET radiotracers. No changes in PET signals were found despite very large changes in blood flow.45
Truly integrated data analysis has the potential to explore multi-modal variables from simultaneous PET/MR studies. For example, measurements of blood flow can provide insights into delivery and washout of PET radiotracers. From a technical and practical perspective, data quality can be improved by integrating motion estimates from both modalities in the data reconstruction. Additional ideas are provided in the advances and opportunities section, highlighting the importance of methodological advances.
Pharmacological PET/MRI
In the following sections, we present simultaneous PET/MRI studies of neuroreceptor systems. The emphasis will be on the dopamine, opioid and serotonin systems because these neurotransmitter systems are common targets for neurological and psychiatric therapeutics. For this reason, abundant literature describing pharmacology and in vivo imaging exist. Examples of simultaneous PET/MRI studies with acute exogenous drug challenges that induced changes in radiotracer binding and caused time-varying pharmacological MRI responses are shown in Figures 2 and 3.
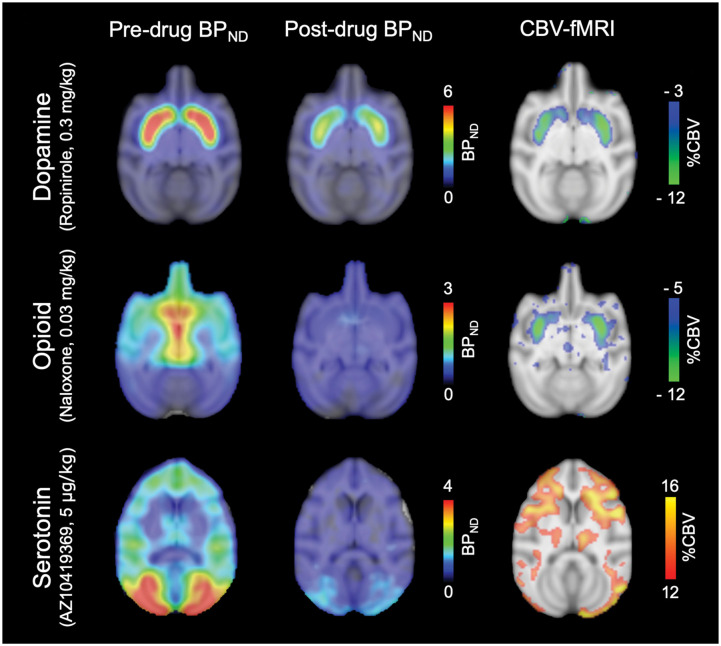
Figure 2.
Pharmacological PET/fMRI maps from three different neurotransmitter systems in response to pharmacological challenges in non-human primates. For imaging the dopamine, opioid and serotonin systems, the radiotracers [11C]raclopride, [11C]carfentanil and [11C]AZ10419369 were used, respectively. Left and middle column: Non-displaceable binding potential (BPND) maps are shown at baseline (pre-drug) and after administration of ropinirole, naloxone and AZ10419369, respectfully. Right column: Voxelwise maps show simultaneously acquired peak percent cerebral blood volume (%CBV) changes due to the respective pharmacological challenges. Corresponding timecourses from the same experiments are shown in Figure 3.
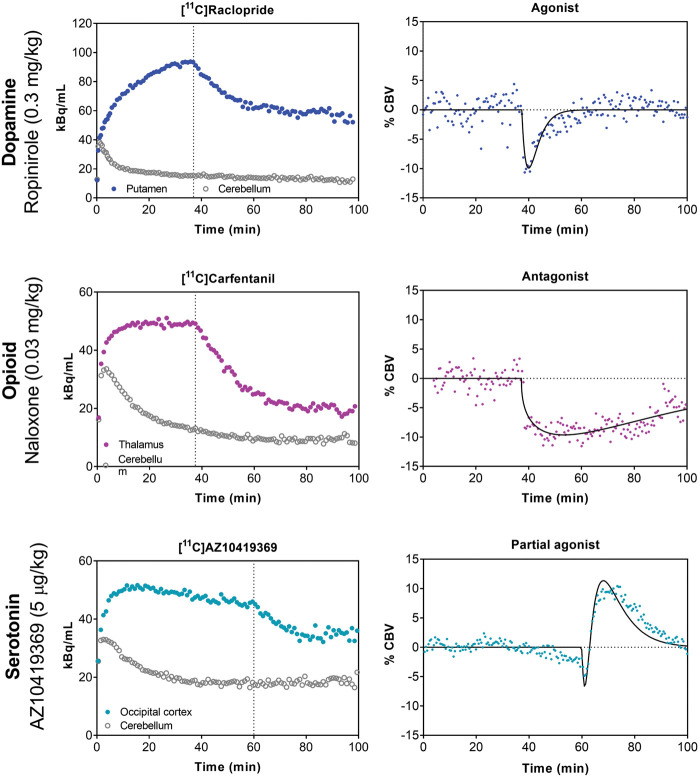
Figure 3.
Examples of dynamic timecourses of simultaneous PET and fMRI experiments with pharmacological challenges. Left column: In all three cases, the radiotracer was administered as a bolus-infusion and a within-scan pharmacological challenge was injected at the dotted vertical line. Right column: Simultaneously acquired fMRI signals are shown as percent cerebral blood volume (%CBV). For the dopamine system, the D2/D3 receptor agonist ropinirole was used to displace [11C]raclopride binding and elicited a short-lived negative %CBV response. For the opioid system, the µ-opioid receptor antagonist naloxone was used to displace [11C]carfentanil binding and elicited a longer-lasting negative %CBV response. For the serotonin system, the 5-HT1B receptor partial agonist AZ10419369 was used to displace [11C]AZ10419369 binding and elicited a biphasic %CBV response. Corresponding voxelwise maps to these timecourses are shown in Figure 2.
The dopamine system
The neurotransmitter dopamine plays a key role in a number of neuromodulatory functions, including reward and attention. The vast majority of clinically approved therapeutic drugs in psychiatric and movement disorders target dopamine receptors. Radiotracers for the excitatory D1-like receptors (D1/D5), the inhibitory D2-like receptors (D2/D3/D4) and the dopamine transporter exist and have been used to study receptor-specific function.66 PhMRI studies together with microdialysis studies have shown that functional signals tightly correlate with dopamine release.12,67 Using simultaneous PET/MRI to study the dopamine system, advances have been made to understand the molecular component of fMRI and to characterize the downstream signaling of dopamine receptor binding, which is summarized below.
The most frequently employed radiotracer in the context of dopamine receptors is [11C]raclopride,68 an antagonist that targets D2/D3 receptors. This radiotracer is favored due to its ability to reliably quantify receptor availability in the striatum and its relatively fast kinetics. The first study that integrated dynamic PET and phMRI for mapping neuroreceptor function used a D2/D3 receptor antagonist drug challenge44: [11C]Raclopride was used both as a radiotracer and pharmacological challenge while acquiring PET and fMRI signals simultaneously, and demonstrated neurovascular coupling to any receptor occupancy for the first time. Coupling between D2/D3 receptor occupancy and fMRI signal due to pharmacological injection of unlabeled raclopride was shown in anatomical space, in time and with dose.44
As a counterpart to the antagonist effects, the functional effects of D2/D3-specific receptor agonists have been also been examined using simultaneous PET/fMRI. A study of the D2/D3 receptor agonist quinpirole demonstrated neurovascular coupling to receptor occupancy in anatomical space and with dose,14 similar to the antagonist challenge. However, the direction of the fMRI signal was shown to be the opposite to an antagonist challenge, i.e. receptor occupancy correlated with negative cerebral blood volume changes in striatal regions, consistent with the inhibitory nature of D2/D3 receptors (Figure 3). Discrepancies in dynamic timecourses between PET and fMRI due to the agonist drug challenge provided measures of receptor desensitization and internalization14 (Figure 3), a quantity not otherwise accessible in vivo.
The link between D2 neuroreceptor distribution, drug action and brain function, evaluated as changes in CBF, was also investigated in the context of antipsychotics: Given a single effective dose of an antipsychotic, an increase in striatal CBF was found that could also be correlated with receptor distribution and DRD2 mRNA expression profiles.69 Consistent with other pharmacological studies, all four investigated antipsychotics showed a spatial neurovascular coupling to baseline receptor density profiles. A similar study that investigated seven clinical drugs relevant in psychiatry showed that coupling between receptors and neurovascular responses is applicable to several receptor targets. Distinct relationships were found between drug-induced CBF changes and a range of associated underlying receptor densities.70
Simultaneous PET/fMRI in these pharmacological studies was critical in demonstrating neurovascular coupling to dopamine receptor occupancies, and to decipher differential effects of dopaminergic drug function and receptor trafficking. Combined multi-modal imaging is likely going to deliver further insights into detailed dopamine receptor dynamics, adaptations and function in the living brain.
The opioid system
The opioid receptor (OR) system comprises mu- (MOR), delta- (DOR), kappa- (KOR), and orphan receptor subtypes with corresponding endogenous peptides endorphins, enkephalins and dynorphins. Because of the significant role of ORs in mediating pain, addiction, placebo effects, as well as affective and reward processes, several OR-selective radiotracers have been developed. [11C]carfentanil, a MOR agonist, has been widely used in human research because of its favorable kinetic properties. KOR-selective agonist and antagonist radiotracers were developed in the past few years resulting in ongoing preclinical and clinical studies investigating the KOR system.
Opioid antagonists, such as naltrexone, have been shown to attenuate reward properties possibly through opioid-mediated effects on the dopamine system. In a sequential PET/MRI study, a pharmacological challenge experiment with the opioid inverse agonist GSK1521498 was performed in healthy subjects to evaluate whether naltrexone attenuates behavior-reinforcing effects.71 Both naltrexone and GSK1521498 showed a dose-dependent reduction in [11C]carfentanil binding, confirming target engagement. However, only GSK1521498, but not naltrexone, showed significant attenuation of fMRI responses to palatable food stimuli in the amygdala. The lack of naltrexone-related modulation on fMRI signals was attributed to the generation of metabolites that complicate the pharmacodynamic profile of naltrexone. Although done separately, this study is an example for the power of combining PET and fMRI data to quantify and characterize drug properties. Future experiments examining the temporal signal changes of both PET and fMRI may offer new insights into pharmacokinetics and pharmacodynamics (Figures 2 and 3).
Given the effectiveness of MOR agonists for pain management, it is surprising that only a few pharmacological PET (using [11C]diprenorphine) and fMRI studies investigated the effects of MOR agonist/partial agonists on brain function in animal models or humans.72–74 Preliminary studies by our group have identified paradoxical PET signals change induced by MOR agonists. We attribute the results to agonist-induced receptor trafficking such as receptor desensitization and internalization. Studies are currently ongoing to validate this hypothesis.75 Concurrent PET and fMRI measurements may offer a new way to help differentiate drug-radiotracer competition vs. receptor trafficking.
The serotonin system
Serotonin (5-HT) is a neurotransmitter that signals through a total of 14 different receptors to modulate brain function. Here, we will focus on the 5-HT1A and 5-HT1B receptors, both of which have been tied to a variety of physiological and pathological processes, notably in anxiety, mood and cognition.76–80
Two studies used simultaneous PET/MRI to characterize the functionality of 5-HT1A and 5-HT1B receptor drugs in vivo. Vidal et al.81 studied two selective and structurally related 5-HT1A agonists (NLX-101 and NLX-112), which were shown to preferentially stimulate different signaling pathways in vitro.82,83 Significant positive and negative BOLD signal changes were found at low 5-HT1A receptor occupancies (<20%) in 5-HT1A receptor-rich areas of the cat brain. The spatial pattern of the drug-induced fMRI responses was compared between the two drugs to infer differential signaling patterns. The comparison of drug-induced BOLD signal changes was based on the administered dose (in mg/kg). However, given that the two agonists have different affinities for their respective targets, equal drug doses could result in different receptor occupancies. Hence, it would have been advantageous to compare the BOLD signal changes based on the measured 5-HT1A receptor occupancy. This study highlights the fact that structurally very similar drugs can have differential effects in vivo, and it presents one example of how PET/MRI can be used to investigate functional selectivity of drugs quantitatively and in vivo. Hansen et al.15 used a partial agonist AZ-10419369 to investigate the functional consequences of activating the 5-HT1B receptors. A dose-dependent effect of AZ10419369 on receptor occupancy (Figure 2) and a linear correlation between the drug dose and the peak changes in CBV was found. Taking advantage of the high temporal resolution and the long dynamic phMRI scans, it was possible to follow the functional effect of the drug: a bi-phasic response was observed with a short-lasting decrease in CBV and a longer lasting increase in CBV (Figure 3). The biphasic response was thought to be a combination of postsynaptic 5-HT1B receptor activation and a 5-HT1B receptor-mediated decrease in glutamate release, respectively. These two studies show how simultaneous PET/MRI can inform us about different aspects of pharmacology – not just the functionality of the drugs but also the consequences of activation of different downstream mechanisms and how the temporal information of the phMRI response plays an important role in the interpretation of the drug responses.
Simultaneous PET/MRI can also be used to investigate the mechanism of action of existing drugs beyond target engagement. Citalopram, a serotonin transporter reuptake inhibitor, is a well-known treatment option for patients with major depressive disorder. In a simultaneous PET/MRI study, the effects of citalopram on functional connectivity84 and serotonin transporter occupancy85 were studied in healthy volunteers. The strength of this study is that the authors employ three different models to resting state fMRI analysis: (1) Modeling using individual plasma concentrations of citalopram, (2) a linear ramp function representing the infusion of citalopram or (3) modeling using individual changes in [11C]DASB binding (i.e. transporter occupancy of citalopram). By employing these models, the authors are not only taking advantage of the spatial co-registration but more excitingly also the temporal information by which subjects may react differently in magnitude and timing to the drug intervention. Other examples of incorporating PET into fMRI analysis, are the studies of changes in functional connectivity following administration of MDMA (3,4-methylendioxy-N-methylamfetamin, also known as ecstasy)86 and lysergic acid diethylamide (LSD),87 respectively. They used an in vivo serotonergic atlas88 to inform the fMRI analysis about the distribution of various serotonergic receptors and transporter. Both studies find that the change in functional connectivity induced by the drugs can be explained through the binding to their respective targets. Although these two examples are not simultaneous PET/MRI studies, they combine information about neuronal activity, anatomy, specific serotonin receptor density, and advanced computational models to define new fingerprints of the drugs and potentially result in a better understanding of response to treatment.
PET/MRI of endogenous neurotransmitter release by pharmacological challenge or behavioral modulation
Endogenous neurotransmitter release can be induced not only by pharmacological challenges, but also through sensory-motor stimuli or behavioral tasks. While fMRI studies are widely used to localize areas of brain activity, PET studies, albeit smaller in numbers, have been used to pinpoint and quantify neurotransmitter release. When used in isolation, each technique mainly provides correlational measurements to the task. The power of multi-modal imaging with PET/fMRI, however, is the potential to inform about molecular and neural mechanisms associated with a cognitive task. While such studies could theoretically be carried out sequentially, the uncertainty about robust reproducibility of an effect and potential changes in physiology are a major limitation, especially in complex cognitive tasks or when signal changes are small. The use of a hybrid PET/MR system decreases intrasubject variability by avoiding repeated measurements on separate imaging systems, as well as the accompanied attentional bias and adaptation, which would in turn directly affect task performance and hence brain activation.
The dopamine system
Since the neurotransmitter dopamine is involved in a vast range of behavioral or cognitive functions and is disturbed in brain dysfunction,89,90 it has been a target of interest in a range of functional imaging studies. Combined PET/fMRI studies that employ radiotracers targeting dopamine receptors can shed a light on the underlying molecular nature of fMRI signatures and provide mechanistic interpretation. In this section, we focus on studies that used combined PET/MRI in order to learn more about endogenous dopamine function and integrated information from both modalities.
Endogenous dopamine levels can be increased through stimulants, which stimulate the release of dopamine and/or block the reuptake of dopamine in the synapse. The effects of oral methylphenidate, which blocks the dopamine transporter, were investigated with the high affinity D2/D3 receptor radiotracer [18F]fallypride and functional connectivity in non-human primates.91 Methylphenidate decreased [18F]fallypride binding potential in the head of caudate, which was then shown to negatively correlate with dorsolateral prefrontal cortex, precuneus and hippocampus using functional connectivity. A similar negative relationship between dopamine release and functional connectivity was also demonstrated in healthy human volunteers after oral methylphenidate administration.92 As more studies investigate the consequences of dopamine release on functional networks, we may be able to determine whether dopamine is a major modulator in this context.
Dopamine levels have also been shown to be altered in several psychiatric disease states,93,94 although the exact mechanisms and functional consequences are still not understood. Specifically, amphetamine-induced dopamine release has been used as a model to ask the question whether the capacity to release dopamine is altered.18,95,96 In PET/MRI studies that used sequential acquisitions in schizophrenia patients, the dopamine response was blunted and showed a significant association to working-memory BOLD activation in the prefrontal cortex.18 In a study with similar methodological approaches but in the context of major depressive disorder that used the D3-preferring agonist radiotracer [11C]PHNO, neither receptor availability nor dopamine release capacity showed correlations to a ventrostriatal BOLD response to reward prediction error.96 Using a dual-tracer approach to measure both dopamine synthesis using [18F]DOPA and release capacity with [11C]PHNO in healthy subjects, the former was associated with greater salience network connectivity, whereas dopamine release capacity was associated with weaker connectivity.47 Overall, these findings demonstrate that altered dopamine release affects cortical function. Certainly, further investigations are needed to understand the intricacies of dopamine release and function in these complex disorders.
There are several studies that have investigated the involvement of endogenous dopamine release on cognitive functions.97 In a multi-modal study using [11C]raclopride, striatal dopamine release was detected during normal speaking and correlated to fMRI activity in the anterior putamen.98 Since dopamine function is altered in aging, dopamine may also play a role in tuning striatal circuits to adjust cognitive flexibility.99 Paired with behavioral interventions, PET/fMRI has the potential to elucidate the involvement of dopamine in cognition and its role in modulating distributed brain function and networks.
PET/MRI has also been used to study the relationship between baseline or altered dopamine receptor availability and modulation of functional networks. Using the D1 radiotracer [11C]NNC112 and a working memory task to modulate functional networks, correlations between striatal and cortical D1 receptor availability and prefrontal connectivity were found to be positive during a task and negative at rest only for striatal D1 receptor availability.20 Using the D2 radiotracer [11C]raclopride, however, an increased striatal D2 receptor availability correlated with decreased functional connectivity.100 In a separate study, increased connectivity between striatal and extrastriatal D2 receptor availability was determined in patients with schizophrenia with a high-affinity radiotracer.101 Whether the positive vs. negative correlations found in these studies are driven by excitatory vs. inhibitory dopamine projections, and correlate with behavioral measures,102 is an intriguing area of research. Due to whole-brain coverage that both PET and fMRI can provide, comparing striatal vs. extrastriatal or excitatory vs. inhibitory dopaminergic and functional network modulations is an exciting research area that PET/MR can be used to expand to.
The opioid system
A few studies have investigated the effects of behavioral stimuli (such as pain, placebo, and reward) on modulating endogenous MOR release.46,103–106 Both simultaneous and sequential PET/fMRI have been used with a common goal of exploring the correlation of task-evoked fMRI response and the decrease in PET binding potential (as an indicator of endogenous opioid release). It was found that experimental pain and vicarious pain activate similar brain regions responsible for pain processing46,104 and positive correlation between colocalized fMRI and PET signal changes was found in the thalamus suggesting that pressure pain causes changes in opioid neurotransmission that contribute a significant component of the fMRI signal change in this region.46 On the other hand, a negative correlation between cerebral MOR availability and BOLD responses was observed in sensorimotor regions, insula, and prefrontal cortex when seeing others in pain, suggesting that the endogenous opioid system also plays a role in vicarious pain.104 In addition to pain, MOR is also believed to be involved in placebo analgesia and analgesic acupuncture.103,107 However, no correlation between fMRI and PET signal changes was reported in two studies investigating placebo analgesia in healthy volunteers and patients with migraine.103,107 A small cortical region in the orbital frontal cortex showed colocalized fMRI and PET signal changes (fMRI activation and reduction in PET binding potential as measured with [11C]diprenorphine) to verum acupuncture but not placebo acupuncture.103
Opioid receptors and peptides are abundantly expressed in the human reward and reinforcement circuit. Therefore, a few studies investigating the role of MOR system to reward stimuli have also been conducted.105,106 It has been shown that endogenous MOR availability is associated with anticipatory reward responses to palatable (versus non-palatable) foods. Furthermore, changes in MOR binding after aerobic exercise are associated with changes in brain responses to foods versus nonfoods, and to palatable versus nonpalatable foods as measured using fMRI.105,106
These PET and fMRI studies using physiological challenges or behavioral tasks to induce endogenous opioid release have enhanced our understanding of the opioid systems in pain and reward processes. Future studies taking advantage of simultaneous PET/MRI capability to investigate the temporal evolution of PET and fMRI signal changes to challenges/behavioral tasks would be of great interest.
The serotonin system
While measurements of changes in endogenous neurotransmitter with PET have been successfully performed in the dopamine and opioid system, so far only two serotonergic radiotracers have shown the necessary sensitivity: [11C]AZ10419369, a 5-HT1B receptor partial agonist and [11C]Cimbi-36, a 5-HT2A receptor agonist.30 To the best of our knowledge, only one simultaneous PET/MR study has been conducted including a task-based/behavioral intervention. In this study, subjects were presented with autobiographical images during the scan, and a subsequent increase in CBF and 5-HT release (as measured with [11C]AZ10409369) was found. Changes in BPND and CBF were strongly correlated in the occipital cortex indicating that 5-HT signaling may be involved in the processing of visual stimuli and/or visual attention.108 Simultaneous PET/MRI studies leveraging radiotracers that are sensitive to changes in endogenous 5-HT and appropriate interventions could give insight into the 5-HT’s role in sensory and behavioral processing.
A few sequential PET/MRI studies are also worth mentioning because the experimental design would be interesting to translate into a simultaneous PET/MR imaging setting. For example, several studies have investigated the relationship between the 5-HT1A receptor, in particular the autoreceptors located in the raphe nuclei, and emotional processing.109–112 A behavioral paradigm well-suited for simultaneous PET/MRI is the point-subtraction aggression paradigm (PSAP). The PSAP was used in a sequential PET/MR study to explore the link between amygdala reactivity to provocations and 5-HT1B receptor binding in a cohort of men displaying a wide range of aggressive behavior.113 Investigating the 5-HT1A and 5-HT1B autoreceptors with behavioral paradigms in different patient populations or with pharmacological interventions could pinpoint 5-HT’s role in emotional processing. Furthermore, this could inform about receptor subtype involvements and enable investigating timescales of emotional processing and its regulation.
The glutamate system
As the main excitatory neurotransmitter in the human brain, glutamate is intimately involved in a multitude of homeostatic and cognitive functions. Given glutamate’s vital role, observed correlations between decreased functional connectivity and reduced glutamate receptor availability are plausible,114 although other studies cross-validating this with PET/MRI are still in its infancy. From a technical perspective, magnetic resonance spectroscopy (MRS) is an established technique to image glutamate, glutamine and other metabolites.115 Conversely, PET radiotracers that target the glutamate system are still relatively novel. Notwithstanding this, the potential to cross-validate and measure glutamate using PET/MRI is obviously an exciting arena for an integrated scanner. This has been exploited by evaluating metabotropic glutamate receptor subtype 5 (mGluR5) availability while measuring glutamate-to-glutamine ratio during recovery of sleep. Interestingly, prolonged wakefulness increased striatal glutamate while also elevating mGluR5 availability,116 thereby posing a question mark on a common interpretation of linking glutamate release with a reduction in metabotropic receptor availability. Another simultaneous PET/MRI study by Tuura et al.117 demonstrates an acute decrease in striatal glutamate after stimulation with N-acetyl-cysteine was observed with MRS, while no changes in mGluR5 availability were measured by PET. Certainly, the relationship between changes in glutamate as measured by MRS and mGluR5 availability is intriguing and likely to be the subject of future PET/MRS studies.
Advances and opportunities for simultaneous PET/MRI
The reviewed literature in this article is meant to provide an overview of combined PET/MRI studies that investigate the dopamine, opioid, serotonin and glutamate systems. Continuing this line of work has tremendous potential, but it is equally important to enhance our in vivo understanding of other neurotransmitter systems, such as the acetylcholine or the GABA systems. We eagerly await studies on all neurotransmitter systems, and we encourage the continued discussion on optimized experimental designs, analysis methods and interpretation of data.
Novel MRI and PET techniques translatable for human use
To further advance the field of PET/MRI, it is important to continue developing new tools and methods that enable neuroscientific discovery and address clinical needs. Improved spatial and temporal resolution is of broad interest and can be achieved by, e.g., novel MR pulse sequences or novel PET radiotracer administration methods. This may enable detection of small signal changes due to small dose or efficacy of drugs, or possibly from small anatomical areas, such as small subcortical structures or laminar organization of the cortex.
Mapping neuronal activity based on glucose utilization with [18F]FDG is an established method from the 1980s for human brain mapping.118 Novel methods, based on continuous infusion of [18F]FDG, have been developed that allow PET/fMRI acquisitions with repeated stimuli during one imaging session to infer dynamic changes in brain metabolism.119–124 To date, this has been mainly shown for selective sensory stimuli but this type of method may bring together cognitive neuroscience approaches from fMRI and PET measures of metabolism.
New MRI methods providing novel contrasts could offer additional neurochemical measurements that complement PET. Using PET and MRS, Brownell et al.125 showed that the neurochemical changes following pharmacological degeneration of dopaminergic neurons are dynamic and complex. As another example, glutamate chemical exchange saturation transfer (CEST) MRI has demonstrated feasibility to map glutamate distribution in human subjects.126,127 However, how the total glutamate concentration (as measured by MRI/MRS) compares to the availability of glutamate receptor subtypes (as measured by PET) remains to be explored. Manganese-enhanced MRI has been translated to a PET imaging tool, which now allows for clinical studies exploring anatomy, functionality and connectivity.128 Finally, combining receptor-specific PET/MRI with physiology or other (non)-invasive neuronal measurements in a trimodal approach, e.g. by adding electroencephalography (EEG),129 may further expand our understanding of receptor-driven brain function.
Despite extensive efforts, there are still many receptors for which molecular imaging probes do not exist. Radiotracer development is, much like central nervous system drug development, a complex and costly process, but fortunately new radiotracers for existing and new targets continue to emerge.22 It will be exciting to see on how simultaneous PET/MRI can be used in a synergistic way to investigate other protein targets.
Interventions with novel pharmacological challenges
Pharmacological imaging enables us to translate knowledge about drug–receptor interactions from in vitro studies to in vivo systems. To date, the majority of drugs can be classified as antagonists or agonists, with a further subclassification into partial and inverse agonists. In recent years, we have learned that agonists can possess functional selectivity, i.e. agonists can exhibit a preference or bias for specific downstream mechanisms.130 The above-mentioned drugs bind to orthosteric sites, yet, allosteric binding sites also exist for some targets. Studies investigating the in vivo behaviors of biased agonists and allosteric modulators are still awaiting.
Receptor conformation can be modulated by specific drugs and affect functional responses. Our understanding of how drugs affect receptor states and subsequent signaling pathways is still limited. Simultaneous PET/MRI would be a powerful technique for investigating this plethora of receptor pharmacology.
Genetic manipulations of the central nervous system
The advancement of genetic and chemogenetic technologies, such as gene knock-in/knock-out animal models, gene-editing by CRISPR-Cas9, or pharmacogenetic approaches (Designer Receptors Exclusively Activated by Designer Drugs, DREADDs) has revolutionized how we can selectively silence or activate specific cell-types in the central nervous system.131 Combining these novel genetic tools with PET/MRI provides new opportunities to investigate neuronal circuits during selective activation or inhibition of specific cells. For example, a study has demonstrated that activating the serotonin-generating neurons induces different fMRI signal patterns compared to systematic administration of serotonin reuptake inhibitor.132 Chemogenetically silencing neurons that express endogenous KOR peptide precursors resulted in altered metabolic activity, as measured with [18F]FDG PET.133 Using the CRISPR-Cas9 technique, modified antibodies equipped with a tag for site-specific bioconjugation of radioisotopes have been shown to reduce non-specific binding in excretion organs in preclinical PET imaging.134 The CRISPR-Cas9 technique has also been applied to create a non-human primate model of autism spectrum disorder by disrupting the SHANK3 gene. The genetically modified non-human primate had no obvious difference in brain morphology; however, decreased metabolic activity was measured with [18F]FDG PET.135 These early animal studies are excellent examples demonstrating the feasibility of integrating novel biological tools to precisely manipulate neuronal systems while monitoring the functional consequences in vivo using imaging methods.
Novel models for integrated PET and MRI data quantification
Quantification of dynamic PET and MRI data requires advanced models. However, to date, there are only a few examples that combine PET and MRI data within a given model. Scott et al. investigated the relationship between PET radiotracer delivery and quantitative CBF with ASL MRI. A modified SRTM was developed to reduce the acquisition time of [18F]florbetapir by exploiting the simultaneously acquired CBF data.54 In this model, the CBF information is used to derive a delivery-related kinetic parameter by assuming linearity. By incorporating CBF information obtained by MRI, the authors demonstrated comparable PET outcome measures with 50% reduced PET scan time.
Novel models can also be created to infer biological phenomena. Sander et al.14 demonstrated that dynamic PET together with time-varying phMRI data can estimate receptor desensitization and internalization rates, a quantity not previously accessible in vivo. This and other types of adaptation mechanisms may also be influenced by acute versus chronic dosing schemes or routes of administration. New models that integrate information from PET can help differentiate how, e.g., D1 versus D2 receptor subtypes contribute to fMRI signals.51 Additional development of models incorporating temporal changes that exploit the simultaneity of PET and MRI signals is encouraged. These may inform us about more detailed pharmacokinetic profiles of administered drugs or represent the timescales of downstream signaling pathways. Finally, machine learning tools may provide a powerful way of extracting maximal information from PET/MRI.136
Creative experimental design to probe receptor systems interactions
Receptor systems do not work in isolation. When a pharmacological or behavioral intervention perturbs one neurotransmitter system, interconnected systems can be modulated at the same time. One example is amphetamine, a dopamine releaser and reuptake inhibitor, which also releases norepinephrine, serotonin and endogenous opioid peptides.17,137,138 Thus, caution should be exercised when interpreting the functional response to complex pharmacological challenges or behavioral tasks. While PET could quantify receptor occupancy when a selective radiotracer is used, fMRI responses may capture the combined downstream effects of multiple receptor systems. Despite this potentially painting a more complicated assessment, experiments can be designed in such a way to build a neurochemical connectome by using specifically targeted drugs,14,44 genetic animal models or stimulation of specific neuronal population.
Conclusion
Simultaneous PET/MRI is a powerful technique with a unique capacity to improve our understanding of the in vivo functionality of different pharmacological tools. Receptor-specific measures from multi-modal imaging studies have already advanced our understanding of networks and brain function. Taking advantage of temporal domains from simultaneous acquisitions has provided a window into molecular and brain functional dynamics, and future studies would benefit from further exploration of temporal dynamics in experimental design and analysis. Appropriate tools and powerful interventions can take us a step forward in understanding endogenous neurotransmitter (dys)function in health and disease. Altogether, PET/MRI imaging is likely to play a key role in deciphering in vivo neurobiology through novel insights into receptor pharmacology, signaling pathways and brain function modulated by the plethora of neurotransmitters.
Acknowledgements
We would like to thank Drs. Jacob M. Hooker, Gitte M. Knudsen, Julie C. Price, and Joseph B. Mandeville for their constructive feedbacks and suggestions.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: CYS received support from the NIH grants K99DA043629, R00DA043629 and R01NS112295. HDH is supported by the Lundbeck Foundation (R-293-2018-738) and Innovation Fund Denmark (4108-00004B). HYW is supported by the NIH grants R00DA037928, R21DA047133, R61DA048485, and UH2AR076741. The following NIH grants further supported this work: P41EB015896, S10RR026666, S10RR022976, S10RR019933 and S10RR017208.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iDs
Hanne D Hansen https://orcid.org/0000-0001-5564-7627
Hsiao-Ying Wey https://orcid.org/0000-0002-1425-8489
References
- 1.Fendler WP, Czernin J, Herrmann K, et al. Variations in PET/MRI operations: results from an international survey among 39 active sites. J Nucl Med 2016; 57: 2016–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nensa F, Bamberg F, Rischpler C, et al. Hybrid cardiac imaging using PET/MRI: a joint position statement by the European Society of Cardiovascular Radiology (ESCR) and the European Association of Nuclear Medicine (EANM). Eur Radiol 2018; 28: 4086–4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guberina N, Hetkamp P, Ruebben H, et al. Whole-body integrated [68 Ga]PSMA-11-PET/MR imaging in patients with recurrent prostate cancer: comparison with whole-body PET/CT as the standard of reference. Mol Imaging Biol. Epub ahead of print 3 September 2019. DOI: 10.1007/s11307-019-01424-4 [DOI] [PubMed] [Google Scholar]
- 4.Catalano OA, Daye D, Signore A, et al. Staging performance of whole-body DWI, PET/CT and PET/MRI in invasive ductal carcinoma of the breast. Int J Oncol 2017; 51: 281–288. [DOI] [PubMed] [Google Scholar]
- 5.Aiello M, Cavaliere C, Marchitelli R, et al. Hybrid PET/MRI Methodology. Int Rev Neurobiol 2018; 141: 97–128. [DOI] [PubMed]
- 6.Bailey DL, Pichler BJ, Gückel B, et al. Combined PET/MRI: global warming-summary report of the 6th international workshop on PET/MRI, March 27–29, 2017, Tübingen, Germany. Mol Imaging Biol 2018; 20: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hope TA, Fayad ZA, Fowler KJ, et al. Summary of the first ISMRM–SNMMI workshop on PET/MRI: applications and Limitations. J Nucl Med 2019; 60: 1340–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Izquierdo-Garcia D, Catana C.MR imaging–guided attenuation correction of PET data in PET/MR imaging. PET Clin 2016; 11: 129–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ladefoged CN, Law I, Anazodo U, et al. A multi-centre evaluation of eleven clinically feasible brain PET/MRI attenuation correction techniques using a large cohort of patients. Neuroimage 2017; 147: 346–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Catana C, Benner T, Van Der Kouwe A, et al. MRI-assisted PET motion correction for neurologic studies in an integrated MR-PET scanner. J Nucl Med 2011; 52: 154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen KT, Salcedo S, Chonde DB, et al. MR-assisted PET motion correction in simultaneous PET/MRI studies of dementia subjects. J Magn Reson Imaging 2018; 48: 1288–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jenkins BG.Pharmacologic magnetic resonance imaging (phMRI): imaging drug action in the brain. Neuroimage 2012; 62: 1072–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindsley CW.New statistics on the cost of new drug development and the trouble with CNS drugs. ACS Chem Neurosci 2014; 5: 1142–1142. [DOI] [PubMed] [Google Scholar]
- 14.Sander CY, Hooker JM, Catana C, et al. Imaging agonist-induced D2/D3 receptor desensitization and internalization in vivo with PET/fMRI. Neuropsychopharmacology 2016; 41: 1427–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hansen HD, Mandeville JB, Sander CY, et al. Functional characterization of 5-HT 1B receptor drugs in non-human primates using simultaneous PET-MR. J Neurosci 2017; 37: 1971–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erritzoe D, Ashok AH, Searle GE, et al. Serotonin release measured in the human brain: a PET study with [11C]CIMBI-36 and d-amphetamine challenge. Neuropsychopharmacology. Epub ahead of print 12 November 2019. DOI: 10.1038/s41386-019-0567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mick I, Myers J, Ramos AC, et al. Blunted endogenous opioid release following an oral amphetamine challenge in pathological gamblers. Neuropsychopharmacology 2016; 41: 1742–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slifstein M, van de Giessen E, Van Snellenberg J, et al. Deficits in prefrontal cortical and extrastriatal dopamine release in schizophrenia. JAMA Psychiatry 2015; 72: 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim SJ, Sullivan JM, Wang S, et al. Voxelwise lp-ntPET for detecting localized, transient dopamine release of unknown timing: sensitivity analysis and application to cigarette smoking in the PET scanner. Hum Brain Mapp 2014; 35: 4876–4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roffman JL, Tanner AS, Eryilmaz H, et al. Dopamine D 1 signaling organizes network dynamics underlying working memory. Sci Adv 2016; 2: e1501672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pike VW.Considerations in the development of reversibly binding PET radioligands for brain imaging. Curr Med Chem 2016; 23: 1818–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCluskey SP, Plisson C, Rabiner EA, et al. Advances in CNS PET: the state-of-the-art for new imaging targets for pathophysiology and drug development. Eur J Nucl Med Mol Imaging 2020; 47: 451–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paterson LM, Kornum BR, Nutt DJ, et al. 5-HT radioligands for human brain imaging with PET and SPECT. Med Res Rev 2013; 33: 54–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prante O, Maschauer S, Banerjee A.Radioligands for the dopamine receptor subtypes. J Label Compd Radiopharm 2013; 56: 130–148. [DOI] [PubMed] [Google Scholar]
- 25.Mach RH.Small molecule receptor ligands for PET studies of the central nervous system-focus on g protein coupled receptors. Semin Nucl Med 2017; 47: 524–535. [DOI] [PubMed] [Google Scholar]
- 26.Fitzgerald LW, Conklin DS, Krause CM, et al. High-affinity agonist binding correlates with efficacy (intrinsic activity) at the human serotonin 5-HT2A and 5-HT2C receptors: evidence favoring the ternary complex and two-state models of agonist action. J Neurochem 1999; 72: 2127–2134. [DOI] [PubMed] [Google Scholar]
- 27.Park PS-H, Lodowski DT, Palczewski K.Activation of G protein-coupled receptors: beyond two-state models and tertiary conformational changes. Annu Rev Pharmacol Toxicol 2008; 48: 107–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skinbjerg M, Sibley DR, Javitch JA, et al. Imaging the high-affinity state of the dopamine D2 receptor in vivo: fact or fiction? Biochem Pharmacol 2012; 83: 193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Narendran R, Hwang D-R, Slifstein M, et al. in vivo vulnerability to competition by endogenous dopamine: comparison of the D2 receptor agonist radiotracer (-)-N-[11C]propyl-norapomorphine ([11C]NPA) with the D2 receptor antagonist radiotracer [11C]-raclopride. Synapse 2004; 52: 188–208. [DOI] [PubMed] [Google Scholar]
- 30.Yang K-C, Stepanov V, Martinsson S, et al. Fenfluramine reduces [11C]cimbi-36 binding to the 5-HT2A receptor in the nonhuman primate brain. Int J Neuropsychopharmacol 2017; 20: 683–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ogawa S, Tank DW, Menon R, et al. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci USA 1992; 89: 5951–5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ogawa S.Finding the BOLD effect in brain images. Neuroimage 2012; 62: 608–609. [DOI] [PubMed] [Google Scholar]
- 33.Kwong KK, Belliveau JW, Chesler DA, et al. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci USA 1992; 89: 5675–5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aguirre GK, Detre JA.The development and future of perfusion fMRI for dynamic imaging of human brain activity. Neuroimage 2012; 62: 1279–1285. [DOI] [PubMed] [Google Scholar]
- 35.Williams DS, Detre JA, Leigh JS, et al. Magnetic resonance imaging of perfusion using spin inversion of arterial water. Proc Natl Acad Sci USA 1992; 89: 212–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koretsky AP.Early development of arterial spin labeling to measure regional brain blood flow by MRI. Neuroimage 2012; 62: 602–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Belliveau J, Kennedy D, McKinstry R, et al. Functional mapping of the human visual cortex by magnetic resonance imaging. Science 1991; 254: 716–719. [DOI] [PubMed] [Google Scholar]
- 38.Lu H, van Zijl PCM.A review of the development of vascular-space-occupancy (VASO) fMRI. Neuroimage 2012; 62: 736–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu H, Golay X, Pekar JJ, et al. Functional magnetic resonance imaging based on changes in vascular space occupancy. Magn Reson Med 2003; 50: 263–274. [DOI] [PubMed] [Google Scholar]
- 40.Mandeville JB.IRON fMRI measurements of CBV and implications for BOLD signal. Neuroimage 2012; 62: 1000–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mehranian A, Arabi H, Zaidi H.Vision 20/20: magnetic resonance imaging-guided attenuation correction in PET/MRI: challenges, solutions, and opportunities. Med Phys 2016; 43: 1130–1155. [DOI] [PubMed] [Google Scholar]
- 42.Wiesinger F, Bylund M, Yang J, et al. Zero TE-based pseudo-CT image conversion in the head and its application in PET/MR attenuation correction and MR-guided radiation therapy planning. Magn Reson Med 2018; 80: 1440–1451. [DOI] [PubMed] [Google Scholar]
- 43.Catana C, van der Kouwe A, Benner T, et al. Toward implementing an MRI-based PET attenuation-correction method for neurologic studies on the MR-PET brain prototype. J Nucl Med 2010; 51: 1431–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sander CY, Hooker JM, Catana C, et al. Neurovascular coupling to D2/D3 dopamine receptor occupancy using simultaneous PET/functional MRI. Proc Natl Acad Sci USA 2013; 110: 11169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sander CY, Mandeville JB, Wey H-Y, et al. Effects of flow changes on radiotracer binding: simultaneous measurement of neuroreceptor binding and cerebral blood flow modulation. J Cereb Blood Flow Metab 2019; 39: 131–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wey HY, Catana C, Hooker JM, et al. Simultaneous fMRI-PET of the opioidergic pain system in human brain. Neuroimage 2014; 102: 275–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McCutcheon RA, Nour MM, Dahoun T, et al. Mesolimbic dopamine function is related to salience network connectivity: an integrative positron emission tomography and magnetic resonance study. Biol Psychiatry 2019; 85: 368–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tomasi D, Wang G-J, Volkow ND.Energetic cost of brain functional connectivity. Proc Natl Acad Sci USA 2013; 110: 13642–13647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Drzezga A, Becker JA, Van Dijk KRA, et al. Neuronal dysfunction and disconnection of cortical hubs in non-demented subjects with elevated amyloid burden. Brain 2011; 134: 1635–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bardal SK, Waechter JE and Martin DS. Chapter 2 - Pharmacokinetics. In: Applied Pharmacology. Elsevier Health Sciences, 2011, pp.17–34.
- 51.Mandeville JB, Sander CYM, Jenkins BG, et al. A receptor-based model for dopamine-induced fMRI signal. Neuroimage 2013; 75: 46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ikoma Y, Ito H, Arakawa R, et al. Error analysis for PET measurement of dopamine D2 receptor occupancy by antipsychotics with [11C]raclopride and [11C]FLB 457. Neuroimage 2008; 42: 1285–1294. [DOI] [PubMed] [Google Scholar]
- 53.Carson RE.PET physiological measurements using constant infusion. Nucl Med Biol 2000; 27: 657–660. [DOI] [PubMed] [Google Scholar]
- 54.Scott CJ, Jiao J, Melbourne A, et al. Reduced acquisition time PET pharmacokinetic modelling using simultaneous ASL–MRI: proof of concept. J Cereb Blood Flow Metab 2019; 39: 2419–2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marner L, Henriksen OM, Lundemann M, et al. Clinical PET/MRI in neurooncology: opportunities and challenges from a single-institution perspective. Clin Transl Imaging 2017; 5: 135–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mayerhoefer ME, Prosch H, Beer L, et al. PET/MRI versus PET/CT in oncology: a prospective single-center study of 330 examinations focusing on implications for patient management and cost considerations. Eur J Nucl Med Mol Imaging 2020; 47: 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alpert NM, Badgaiyan RD, Livni E, et al. A novel method for noninvasive detection of neuromodulatory changes in specific neurotransmitter systems. Neuroimage 2003; 19: 1049–1060. [DOI] [PubMed] [Google Scholar]
- 58.Lammertsma AA, Hume SP.Simplified reference tissue model for PET receptor studies. Neuroimage 1996; 4: 153–158. [DOI] [PubMed] [Google Scholar]
- 59.Morris ED, Yoder KK, Wang C, et al. ntPET: a new application of PET imaging for characterizing the kinetics of endogenous neurotransmitter release. Mol Imaging 2005; 4: 473–489. [DOI] [PubMed] [Google Scholar]
- 60.Normandin MD, Schiffer WK, Morris ED.A linear model for estimation of neurotransmitter response profiles from dynamic PET data. Neuroimage 2012; 59: 2689–2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou Y, Chen MK, Endres CJ, et al. An extended simplified reference tissue model for the quantification of dynamic PET with amphetamine challenge. Neuroimage 2006; 33: 550–563. [DOI] [PubMed] [Google Scholar]
- 62.Mandeville JB, Sander CYM, Wey HY, et al. A regularized full reference tissue model for PET neuroreceptor mapping. Neuroimage 2016; 139: 405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Evans JW, Kundu P, Horovitz SG, et al. Separating slow BOLD from non-BOLD baseline drifts using multi-echo fMRI. Neuroimage 2015; 105: 189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Caballero-Gaudes C, Reynolds RC.Methods for cleaning the BOLD fMRI signal. Neuroimage 2017; 154: 128–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Logothetis NK, Pauls J, Augath M, et al. Neurophysiological investigation of the basis of the fMRI signal. Nature 2001; 412: 150–157. [DOI] [PubMed] [Google Scholar]
- 66.Ginovart N.Imaging the dopamine system with in vivo [11C]raclopride displacement studies: understanding the true mechanism. Mol Imaging Biol 2005; 7: 45–52. [DOI] [PubMed] [Google Scholar]
- 67.Chen YCI, Galpern WR, Brownell A-L, et al. Detection of dopaminergic neurotransmitter activity using pharmacologic MRI: correlation with PET, microdialysis, and behavioral data. Magn Reson Med 1997; 38: 389–398. [DOI] [PubMed] [Google Scholar]
- 68.Farde L, Hall H, Ehrin E, et al. Quantitative analysis of D2 dopamine receptor binding in the living human brain by PET. Science 1986; 231: 258–261. [DOI] [PubMed] [Google Scholar]
- 69.Selvaggi P, Hawkins PCT, Dipasquale O, et al. Increased cerebral blood flow after single dose of antipsychotics in healthy volunteers depends on dopamine D2 receptor density profiles. Neuroimage 2019; 188: 774–784. [DOI] [PubMed] [Google Scholar]
- 70.Dukart J, Holiga Š, Chatham C, et al. Cerebral blood flow predicts differential neurotransmitter activity. Sci Rep 2018; 8: 4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rabiner EA, Beaver J, Makwana A, et al. Pharmacological differentiation of opioid receptor antagonists by molecular and functional imaging of target occupancy and food reward-related brain activation in humans. Mol Psychiatry 2011; 16: 826–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Melichar JK, Hume SP, Williams TM, et al. Using [11C]diprenorphine to image opioid receptor occupancy by methadone in opioid addiction: clinical and preclinical studies. J Pharmacol Exp Ther 2005; 312: 309–315. [DOI] [PubMed] [Google Scholar]
- 73.Liu CH, Greve DN, Dai G, et al. Remifentanil administration reveals biphasic phMRI temporal responses in rat consistent with dynamic receptor regulation. Neuroimage 2007; 34: 1042–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Becerra L, Upadhyay J, Chang P-C, et al. Parallel buprenorphine phMRI responses in conscious rodents and healthy human subjects. J Pharmacol Exp Ther 2013; 345: 41–51. [DOI] [PubMed] [Google Scholar]
- 75.Wey H-Y, Placzek MS, Hooker JM, et al. Measure and validate agonist-induced mu-opioid receptor desensitization using simultaneous PET/MRI. Neuropsychopharmacology 2017; 42: S476–S652.
- 76.Kaufman J, DeLorenzo C, Choudhury S, et al. The 5-HT1A receptor in major depressive disorder. Eur Neuropsychopharmacol 2016; 26: 397–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Muller CP, Jacobs BL. Handbook of the behavioral neurobiology of serotonin. Cambridge, MA: Academic Press, 2010. [Google Scholar]
- 78.Tiger M, Varnäs K, Okubo Y, et al. The 5-HT1B receptor – a potential target for antidepressant treatment. Psychopharmacology 2018; 235: 1317–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Albert PR, Vahid-Ansari F.The 5-HT1A receptor: signaling to behavior. Biochimie 2019; 161: 34–45. [DOI] [PubMed] [Google Scholar]
- 80.Bouhelal R, Smounya L, Bockaert J.5-HT1B receptors are negatively coupled with adenylate cyclase in rat substantia nigra. Eur J Pharmacol 1988; 151: 189–196. [DOI] [PubMed] [Google Scholar]
- 81.Vidal B, Fieux S, Redouté J, et al. in vivo biased agonism at 5-HT 1A receptors: characterisation by simultaneous PET/MR imaging. Neuropsychopharmacology 2018; 43: 2310–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Newman-Tancredi A, Martel J-C, Cosi C, et al. Distinctive in vitro signal transduction profile of NLX-112, a potent and efficacious serotonin 5-HT 1A receptor agonist. J Pharm Pharmacol 2017; 69: 1178–1190. [DOI] [PubMed] [Google Scholar]
- 83.Newman-Tancredi A, Martel J-C, Assié M-B, et al. Signal transduction and functional selectivity of F15599, a preferential post-synaptic 5-HT 1A receptor agonist. Br J Pharmacol 2009; 156: 338–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gryglewski G, Klöbl M, Berroterán-Infante N, et al. Modeling the acute pharmacological response to selective serotonin reuptake inhibitors in human brain using simultaneous PET/MR imaging. Eur Neuropsychopharmacol 2019; 29: 711–719. [DOI] [PubMed] [Google Scholar]
- 85.Silberbauer LR, Gryglewski G, Berroterán-Infante N, et al. Serotonin transporter binding in the human brain after pharmacological challenge measured using PET and PET/MR. Front Mol Neurosci 2019; 12: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dipasquale O, Selvaggi P, Veronese M, et al. Receptor-enriched analysis of functional connectivity by targets (REACT): a novel, multimodal analytical approach informed by PET to study the pharmacodynamic response of the brain under MDMA. Neuroimage 2019; 195: 252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Deco G, Cruzat J, Cabral J, et al. Whole-brain multimodal neuroimaging model using serotonin receptor maps explains non-linear functional effects of LSD. Curr Biol 2018; 28: 3065–3074.e6. [DOI] [PubMed] [Google Scholar]
- 88.Beliveau V, Ganz M, Feng XL, et al. A high-resolution in vivo atlas of the human brain’s serotonin system. J Neurosci 2017; 37: 120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bromberg-Martin ES, Matsumoto M, Hikosaka O.Dopamine in motivational control: rewarding, aversive, and alerting. Neuron 2010; 68: 815–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tritsch NX, Sabatini BL.Dopaminergic modulation of synaptic transmission in cortex and striatum. Neuron 2012; 76: 33–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Birn RM, Converse AK, Rajala AZ, et al. Changes in endogenous dopamine induced by methylphenidate predict functional connectivity in nonhuman primates. J Neurosci 2019; 39: 1436–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Berry AS, White RL, Furman DJ, et al. Dopaminergic mechanisms underlying normal variation in trait anxiety. J Neurosci 2019; 39: 2735–2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McCutcheon RA, Abi-Dargham A, Howes OD.Schizophrenia, dopamine and the striatum: from biology to symptoms. Trends Neurosci 2019; 42: 205–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Volkow ND, Morales M.The brain on drugs: from reward to addiction. Cell 2015; 162: 712–725. [DOI] [PubMed] [Google Scholar]
- 95.Laruelle M, Iyer RN, Al-Tikriti MS, et al. Microdialysis and SPECT measurements of amphetamine-induced dopamine release in nonhuman primates. Synapse 1997; 25: 1–14. [DOI] [PubMed] [Google Scholar]
- 96.Schneier FR, Slifstein M, Whitton AE, et al. Dopamine release in antidepressant-naive major depressive disorder: a multimodal [11C]-(+)-PHNO positron emission tomography and functional magnetic resonance imaging study. Biol Psychiatry 2018; 84: 563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Badgaiyan RD.Detection of dopamine neurotransmission in “real time”. Front Neurosci 2013; 7: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Simonyan K, Herscovitch P, Horwitz B.Speech-induced striatal dopamine release is left lateralized and coupled to functional striatal circuits in healthy humans: a combined PET, fMRI and DTI study. Neuroimage 2013; 70: 21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Berry AS, Shah VD, Jagust WJ.The influence of dopamine on cognitive flexibility is mediated by functional connectivity in young but not older adults. J Cogn Neurosci 2018; 30: 1330–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hamilton JP, Sacchet MD, Hjørnevik T, et al. Striatal dopamine deficits predict reductions in striatal functional connectivity in major depression: a concurrent 11 C-raclopride positron emission tomography and functional magnetic resonance imaging investigation. Transl Psychiatry 2018; 8: 264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim JH, Cumming P, Son YD, et al. Altered connectivity between striatal and extrastriatal regions in patients with schizophrenia on maintenance antipsychotics: an [18F]fallypride PET and functional MRI study. Synapse 2018; 72: 1–15. [DOI] [PubMed] [Google Scholar]
- 102.Kim JH, Choe YS, Cumming P, et al. Relationship of self-transcendence traits with in vivo dopamine D2/3 receptor availability and functional connectivity: an [18F]fallypride PET and fMRI study. Synapse 2019; 73: 1–14. [DOI] [PubMed] [Google Scholar]
- 103.Dougherty DD, Kong J, Webb M, et al. A combined [11C]diprenorphine PET study and fMRI study of acupuncture analgesia. Behav Brain Res 2008; 193: 63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Karjalainen T, Karlsson HK, Lahnakoski JM, et al. Dissociable roles of cerebral µ-opioid and type 2 dopamine receptors in vicarious pain: a combined PET-fMRI study. Cereb Cortex 2017; 27: 4257–4266. [DOI] [PubMed] [Google Scholar]
- 105.Nummenmaa L, Saanijoki T, Tuominen L, et al. Μ-opioid receptor system mediates reward processing in humans. Nat Commun 2018; 9: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Saanijoki T, Nummenmaa L, Tuulari JJ, et al. Aerobic exercise modulates anticipatory reward processing via the μ-opioid receptor system. Hum Brain Mapp 2018; 39: 3972–3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Linnman C, Catana C, Petkov MP, et al. Molecular and functional PET-fMRI measures of placebo analgesia in episodic migraine: preliminary findings. NeuroImage Clin 2018; 17: 680–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hansen HD, Lindberg U, Fisher PM, et al. Simultaneous measurement of cerebral blood flow and 5-HT1B receptor binding changes during a visual stimulus. In: The 12th international symposium of functional neuroreceptor mapping of the living brain (NRM18), London, 9–12 July 2018, pp.21–22.
- 109.Selvaraj S, Mouchlianitis E, Faulkner P, et al. Presynaptic serotoninergic regulation of emotional processing: a multimodal brain imaging study. Biol Psychiatry 2015; 78: 563–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Selvaraj S, Walker C, Arnone D, et al. Effect of citalopram on emotion processing in humans: a combined 5-HT1A [11C]CUMI-101 PET and functional MRI study. Neuropsychopharmacology 2017; 43: 655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kranz GS, Hahn A, Kraus C, et al. Probing the association between serotonin-1A autoreceptor binding and amygdala reactivity in healthy volunteers. Neuroimage 2018; 171: 1–5. [DOI] [PubMed] [Google Scholar]
- 112.Fisher PM, Price JC, Meltzer CC, et al. Medial prefrontal cortex serotonin 1A and 2A receptor binding interacts to predict threat-related amygdala reactivity. Biol Mood Anxiety Disord 2011; 1: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.da Cunha-Bang S, Fisher PM, Hjordt LV, et al. Men with high serotonin 1B receptor binding respond to provocations with heightened amygdala reactivity. Neuroimage 2018; 166: 79–85. [DOI] [PubMed] [Google Scholar]
- 114.Kim JH, Joo YH, Son YD, et al. in vivo metabotropic glutamate receptor 5 availability-associated functional connectivity alterations in drug-naïve young adults with major depression. Eur Neuropsychopharmacol 2019; 29: 278–290. [DOI] [PubMed] [Google Scholar]
- 115.Ramadan S, Lin A, Stanwell P.Glutamate and glutamine: a review of in vivo MRS in the human brain. NMR Biomed 2013; 26: 1630–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Weigend S, Holst SC, Treyer V, et al. Dynamic changes in cerebral and peripheral markers of glutamatergic signaling across the human sleep–wake cycle. Sleep 2019; 42: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.O’Gorman Tuura R, Warnock G, Ametamey S, et al. Imaging glutamate redistribution after acute N-acetylcysteine administration: a simultaneous PET/MR study. Neuroimage 2019; 184: 826–833. [DOI] [PubMed] [Google Scholar]
- 118.Fox PT, Raichle ME, Mintun MA, et al. Nonoxidative glucose consumption during focal physiologic neural activity. Science 1988; 241: 462–464. [DOI] [PubMed] [Google Scholar]
- 119.Villien M, Wey H-Y, Mandeville JB, et al. Dynamic functional imaging of brain glucose utilization using fPET-FDG. Neuroimage 2014; 100: 192–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Riedl V, Utz L, Castrillón G, et al. Metabolic connectivity mapping reveals effective connectivity in the resting human brain. Proc Natl Acad Sci USA 2016; 113: 428–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hahn A, Gryglewski G, Nics L, et al. Task-relevant brain networks identified with simultaneous PET/MR imaging of metabolism and connectivity. Brain Struct Funct 2018; 223: 1369–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wehrl HF, Hossain M, Lankes K, et al. Simultaneous PET-MRI reveals brain function in activated and resting state on metabolic, hemodynamic and multiple temporal scales. Nat Med 2013; 19: 1184–1189. [DOI] [PubMed] [Google Scholar]
- 123.Marchitelli R, Aiello M, Cachia A, et al. Simultaneous resting-state FDG-PET/fMRI in Alzheimer disease: relationship between glucose metabolism and intrinsic activity. Neuroimage 2018; 176: 246–258. [DOI] [PubMed] [Google Scholar]
- 124.Jamadar SD, Ward PG, Li S, et al. Simultaneous task-based BOLD-fMRI and [18-F] FDG functional PET for measurement of neuronal metabolism in the human visual cortex. Neuroimage 2019; 189: 258–266. [DOI] [PubMed] [Google Scholar]
- 125.Brownell A-L, Jenkins BG, Elmaleh DR, et al. Combined PET/MRS brain studies show dynamic and long-term physiological changes in a primate model of Parkinson disease. Nat Med 1998; 4: 1308–1312. [DOI] [PubMed] [Google Scholar]
- 126.van Zijl PCM, Lam WW, Xu J, et al. Magnetization transfer contrast and chemical exchange saturation transfer MRI. Features and analysis of the field-dependent saturation spectrum. Neuroimage 2018; 168: 222–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cai K, Singh A, Roalf DR, et al. Mapping glutamate in subcortical brain structures using high-resolution GluCEST MRI. NMR Biomed 2013; 26: 1278–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Saar G, Millo CM, Szajek LP, et al. Anatomy, functionality, and neuronal connectivity with manganese radiotracers for positron emission tomography. Mol Imaging Biol 2018; 20: 562–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Del Guerra A, Ahmad S, Avram M, et al. TRIMAGE: a dedicated trimodality (PET/MR/EEG) imaging tool for schizophrenia. Eur Psychiatry 2018; 50: 7–20. [DOI] [PubMed] [Google Scholar]
- 130.Berg KA, Clarke WP.Making sense of pharmacology: inverse agonism and functional selectivity. Int J Neuropsychopharmacol 2018; 21: 962–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Roth BL.DREADDs for neuroscientists. Neuron 2016; 89: 683–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Giorgi A, Migliarini S, Galbusera A, et al. Brain-wide mapping of endogenous serotonergic transmission via chemogenetic fMRI. Cell Rep 2017; 21: 910–918. [DOI] [PubMed] [Google Scholar]
- 133.Michaelides M, Anderson SAR, Ananth M, et al. Whole-brain circuit dissection in free-moving animals reveals cell-specific mesocorticolimbic networks. J Clin Invest 2013; 123: 5342–5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Khoshnejad M, Brenner JS, Motley W, et al. Molecular engineering of antibodies for site-specific covalent conjugation using CRISPR/Cas9. Sci Rep 2018; 8: 1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tu Z, Zhao H, Li B, et al. CRISPR/Cas9-mediated disruption of SHANK3 in monkey leads to drug-treatable autism-like symptoms. Hum Mol Genet 2019; 28: 561–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zaharchuk G.Next generation research applications for hybrid PET/MR and PET/CT imaging using deep learning. Eur J Nucl Med Mol Imaging 2019; 46: 2700–2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mick I, Myers J, Stokes PRA, et al. Amphetamine induced endogenous opioid release in the human brain detected with [11C]carfentanil PET: replication in an independent cohort. Int J Neuropsychopharmacol 2014; 17: 2069–2074. [DOI] [PubMed] [Google Scholar]
- 138.Ferrucci M, Limanaqi F, Ryskalin L, et al. The effects of amphetamine and methamphetamine on the release of norepinephrine, dopamine and acetylcholine from the brainstem reticular formation. Front Neuroanat 2019; 13: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Biswal B, Zerrin Yetkin F, et al. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 1995; 34: 537–541. [DOI] [PubMed] [Google Scholar]