Abstract
The association between impaired brain perfusion, cerebrovascular reactivity status and the risk of ictal events in patients with subarachnoid hemorrhage is unknown. We identified 13 subarachnoid hemorrhage (SAH) patients with seizures and 22 with ictal-interictal continuum (IIC), and compared multimodality physiological recordings to 38 similarly poor-grade SAH patients without ictal activity. We analyzed 10,179 cumulative minutes of seizure and 12,762 cumulative minutes of IIC. Cerebrovascular reactivity (PRx) was not different between subjects with seizures, IIC, or controls. Cerebral perfusion pressure (CPP) was higher in patients with seizures [99 ± 6.5, p = .005] and IIC [97 ± 8.5, p = .007] when compared to controls [89 ± 12.3]. DeltaCPP, defined as actual CPP minus optimal CPP (CPPopt), was also higher in the seizure group [8.3 ± 7.9, p = .0003] and IIC [8.1 ± 10.3, p = .0006] when compared to controls [−0.1 ± 5]. Time spent with supra-optimal CPP was higher in the seizure group [342 ± 213 min/day, p = .002] when compared to controls [154 ± 120 min/day]. In a temporal examination, a supra-optimal CPP preceded increased seizures and IIC in SAH patients, an hour before and continued to increase during the events [p < .0001].
Keywords: Seizures, ictal-interictal continuum, electroencephalogram, cerebral perfusion, subarachnoid hemorrhage
Introduction
Cerebral perfusion pressure (CPP) is an important parameter in the critical care management of the acutely brain injured patient, as an essential guide to minimize secondary brain injury. High CPP is associated with increased morbidity in patients with traumatic brain injury (TBI) and subarachnoid hemorrhage (SAH). On the other hand, low CPP predisposes to cerebral ischemia.1,2 Cerebral blood flow (CBF) is maintained constant with fluctuation of systemic blood pressure (SBP) in normal individuals. This is regulated via a metabolic effect on the arterioles (dependent on arterial CO2 PaCO2 and oxygen PaO2 levels)3 and a myogenic response of the carotids in response to blood pressure fluctuations. When the lower limit of autoregulation is breached, CBF drops resulting in ischemia. A higher CBF (hyperemia) occurs when the upper limit of autoregulation is surpassed.4,5
Seizures have demonstrable effects on the brain and systemic physiology of animals6,7 and humans.8–11 These include metabolic coupling11–13 with increased blood flow to the seizure focus,13 spreading depolarization, spreading ischemia,14,15 tachycardia, tachypnea, and increased blood pressure.8 Seizures and ictal-interictal continuum (IIC) are common in SAH patients, and they are more prominent in high-grade patients.16–19 These are associated with secondary brain injury and poor outcome.9,10,20,21 It is unknown how brain perfusion affects the risk of seizures and IIC in acute brain injury patients. Posterior reversible leukoencephalopathy syndrome (PRES),22 hypertensive encephalopathy,23 hypertension in pregnancy,24 and reperfusion syndrome after carotid surgery25 are some examples of syndromes with coexistence of increased CPP, impaired autoregulation, and seizures.
We hypothesized that impaired autoregulation, and non-optimal cerebral perfusion may be associated with increased risk of seizures and IIC in patients with SAH. We investigated this hypothesis by evaluating the relationship between CPP, optimal CPP (CPPopt), deltaCPP (CPP – CPPopt) and cerebrovascular reactivity (autoregulation) during the hospitalization of SAH patients with seizures and IIC.
Materials and methods
Study population
We studied all poor-grade SAH patients admitted to the Neurological Intensive Care Unit between June 2006 and June 2013 who underwent invasive brain multimodality monitoring following institutional protocol.26,27 Patients were assessed for multimodality monitoring when the Glasgow Coma Scale (GCS) was 8 or lower at ICU admission and without expected improvement of consciousness, clinical worsening, and or death for at least 48 h. This decision was made jointly by the neurointensivist and the neurosurgeon. We included all patients with seizures (13) and/or IIC (22) on intracortical EEG during the period of multimodality monitoring. We compared these patients to 38 controls with poor-grade SAH, multimodality monitoring and no seizure or IIC events during their hospitalization. Age less than 18 years old and pregnant state were exclusion criteria. The study was approved by the institutional review board of Columbia University Medical Center. Informed consent was obtained from patient representatives. The study is in accordance with the ethical standards of the Helsinki Declaration of 1975 (and as revised in 1983).
Management plan
Our institution follows the guidelines from the American Heart Association for medical and surgical management for patients with SAH.18,28 Patients were given anticonvulsant medication for a week as prophylaxis regardless of their EEG, which was discontinued if there was no further indication. Periodic epileptiform discharges (PEDs) were not considered seizures, and there was no attempt to eliminate them with medications; however, patients with PEDs were maintained on anticonvulsants to prevent seizures. Anticonvulsants were not altered for isolated minidepth electrode findings.26 In case of vasospasm concern (clinical symptoms, elevated transcranial Doppler blood flow velocity, or vessel narrowing on vascular imaging), blood pressure was optimized to enhance CPP. Nimodipine was given for 21 days total unless significant blood pressure drops were experienced after dosing (e.g. requiring escalating vasopressors).
Intracranial monitoring
Intracranial pressure (ICP) was monitored using Integra Neuroscience catheters (Plainsborough, NJ). Electroencephalogram (EEG) was recorded using minidepth electrodes (8-contact Spencer depth electrode with 2.2 mm center-to-center intercontact spacing, contact width 5 1.32 mm, 0.9 mm spacing between electrodes; ADTech Racine, WI). The EEG minidepth electrode, designed for clinical intracranial EEG recording, is placed at the bedside; details of the placement have been described.26,27 Minidepth electrodes were placed to span the cortical ribbon with the goal of having one electrode in the skull, two to three in the cortical gray matter, and the remaining four to five electrodes in the white matter. Partial brain tissue oxygenation (pbtO2) was recorded using a flexible polarographic LICOX Clark-type electrode probe. Monitoring probes were placed ipsilateral to the aneurysm in patients who underwent coiling. In patients who had clipping, probes were placed contralateral to the bone flap. The location of the probe was confirmed with a computed tomography scan immediately after the procedure.
Data collection
Data collection methods for the prospective outcomes database and digital physiological data have been reported previously.8 All digital physiologic data were from General Electric Solar 8000i monitors (Milwaukee, WI) and acquired at a sampling frequency of 0.2 Hz using BedmasterEX (Excel Medical Electronics, Jupiter, FL). End-tidal carbon dioxide (ETCO2) data were collected through continuous capnography monitoring as an indirect monitor of the CO2 partial pressure in the arterial blood. For EEG recordings, we used a digital bedside video monitoring system (XLTEK, Excel-Tech Corp., Natus Medical Incorporated, Oakville, Ontario, Canada).
EEG definitions
Each minute of EEG was classified as ictal, IIC, or non-ictal by a board-certified neurophysiologist with critical care competencies (AA). Ictal was defined as any spikes, sharp waves, or sharp and slow wave complexes lasting for 10 s or more at either a frequency of at least 3 per second or a frequency of at least 1 per second with clear evolution in frequency, morphology, or location. IIC was defined as repetitive generalized or focal spikes, sharp-waves, spike-and wave or sharp-and-slow wave complexes that lasted for 10 s or more with a frequency between 1 and 3 per second without clear evolution in frequency, morphology, or location. Non-ictal was defined as conditions not having been met for either ictal or IIC.8 We included events (seizure and IIC) that are longer than 10 min. We considered 5 min or less between seizure or IIC events as a single event. We analyzed the hemodynamic data before the first ever event of seizure or IIC (baseline), before the event during 1 h free of ictal events, during the event (seizure or IIC), and 1 h after the event.
Cerebrovascular monitoring-computing PRx, CPP, and CPPopt
Cerebrovascular reactivity can be continuously evaluated in the intensive care clinical setting using the pressure reactivity index (PRx).29,30 The PRx is the correlation between the slow-wave components of arterial blood pressure and intracranial pressure. It measures a component of autoregulation and has shown good correlation with more direct measures of autoregulation.31 Higher values have been associated with worse outcome and mortality.32 As a non-dynamic measure that does not require a potentially harmful stimulus, it has shown value and gained favor as a continuously calculable physiologic feature in severe acute brain injury.31,33
The CPP value at which the lowest value of PRx is experienced in a period of time is considered the “optimal CPP;34” the “delta CPP” (actual CPP – optimal CPP) is associated with outcome after acute brain injury.2,35 The greater the absolute “delta CPP,” the worse the outcome, while negative “delta CPP” is associated with higher mortality.2,35
Arterial blood pressure (ABP) was monitored through an arterial line if clinically indicated (Transpac IV Monitoring Kit, ICU Medical, San Clemente, CA USA). The arterial line was zeroed at the level of the phlebostatic axis. ABP and ICP data were processed using ICM Plus software (University of Cambridge, Cambridge Enterprise, Cambridge, UK, http://www.neurosurg.cam.ac.uk/icmplus). PRx was calculated as the Pearson correlation coefficient using 10-s averages of ICP and ABP over a 5-min moving window with 80% overlap.29,30 A 5-min median CPP trend was calculated (ABP-ICP) and PRx values were divided and averaged into CPP bins spanning 5 mm Hg. A parabolic curve was applied and the CPP bin with the lowest PRx value was recorded as the CPPopt.35,36 We also calculated the time spent with PRx more than 0.2, as it correlates with increased mortality in ABI patients.37 DeltaCPP was calculated as actual CPP – CPPopt at baseline (prior to seizure or IIC). In the control group, the parameters were averaged over their multimodality recordings.
Statistical analysis
Frequency comparisons for categorical variables were performed by Fisher exact test. Two-group comparisons of continuous variables were performed with the Mann–Whitney U test. All statistical tests were two-tailed and a p-value < 0.05 was considered statistically significant.
Multilevel linear regression method
The study employs a repeated-measures design due to the repeated outcomes over time.38 Since the physiological data were a time series, we used a longitudinal mixed effects model to assess the relationship between seizures and physiological data over time. With longitudinal mixed-effects models, it is possible to study whether expected values of the intercepts and slopes (i.e. the level and shape of recovery trajectories) are affected by other variables. The mixed effects linear model enables us to model the intra-patient and inter-patient variability. This allows for the assessment of differences within a patient over the time series, and differences between patients over the time series. In doing so, the mixed effects model assumes the intercept is random for every person instead of assuming a single intercept for everyone. The model for the linear mixed effects model is
where is one of the physiological data points (CPP, CPPopt, deltaCPP, MAP, SBP, DBP, and PRx) for the i-th individual at time point j. is the exposure metric of seizure for the i-th individual at time j, is the person-specific slope, represents the measurement of the exposure time, and is a subject-specific random effect that accounts for correlation among the repeated measurements in the same subject. is the error term for i-th individual at time point j. We used a mixed effects model for each outcome of interest over time, and assessed how the groups differed overall as well as before, during, and after seizure onset.
Data availability statement
Data that support the results of this study are available from the corresponding author on request from any qualified investigator.
Results
Between June 2006 and June 2013, 13 seizure patients, 22 IIC patients, and 38 control patients with SAH met the inclusion criteria. We analyzed 10,179 cumulative minutes of seizure and 12,762 cumulative minutes of IIC. Baseline characteristics for all patients included in the study are demonstrated in Table 1. One patient had seizures without IIC, and 10 patients had IIC without seizure.
Table 1.
Patients' characteristics.
| Characteristica | Seizure (N = 13) | IIC (N = 22) | Control (N = 38) | p-value (seizure/IIC) |
|---|---|---|---|---|
| Age, mean (SD), y | 58 (11) | 62 (15) | 49 (13) | 0.029/0.004** |
| Female | 8 (61.5%) | 14 (63.6%) | 27 (71.1%) | 0.730/0.577 |
| HH on admission | 0.701/0.744 | |||
| 1–3 | 3 (23.1%) | 5 (22.7%) | 7 (18.4%) | |
| 4–5 | 10 (76.9%) | 17 (77.3%) | 31 (81.6%) | |
| MFS | 0.311/0.148 | |||
| 0–2 | 0 (0%) | 0 (0%) | 5 (13.2%) | |
| 3–4 | 13 (100%) | 22 (100%) | 33 (86.8%) | |
| WFNS | 0.352/0.449 | |||
| 1–3 | 3 (23.1%) | 4 (18.2%) | 4 (10.5%) | |
| 4–5 | 10 (76.9%) | 18 (81.8%) | 34 (86.8%) | |
| GCS on presentation | 0.145/0.191 | |||
| 3–8 | 10 (76.9%) | 17 (77.3%) | 26 (68.4%) | |
| 9–12 | 0 (0%) | 1 (4.6%) | 8 (21.1%) | |
| 13–15 | 3 (23.1%) | 4 (18.2%) | 4 (10.5%) | |
| History of smoking | 7 (53.8%) | 9 (40.9%) | 20 (52.6%) | 1/0.431 |
| History of anticonvulsants use | 0 (0%) | 1 (4.5%) | 0 (0%) | 0.156/0.452 |
| History of seizure | 0 (0%) | 0 (0%) | 0 (0%) | 0.255/0.367 |
| Pressor use | 8/10 (80%) | 17/20 (85%) | 27/33 (82%) | 0.897/0.765 |
| History of HTN | 7 (53.8%) | 13 (59.1%) | 10 (26.3%) | 0.023/0.008 |
| History of DM | 4 (30.8%) | 4 (18.2%) | 3 (7.9%) | 0.019/0.143 |
| History of liver disease | 1 (7.7%) | 1 (4.5%) | 0 (0%) | 0.061/0.131 |
| History of kidney disease | 0 (0%) | 0 (0%) | 0 (0%) | 0.255/0.367 |
| IVH | 13 (100%) | 19 (86.4%) | 31 (81.6%) | 0.169/0.732 |
| Cerebral edema | 11 (84.6%) | 20 (90.9%) | 36 (94.7%) | 0.266/0.619 |
| Hemi-craniectomy | 1 (7.7%) | 1 (4.5%) | 6 (15.8%) | 0.662/0.246 |
| Hydrocephalus | 11 (84.6%) | 19 (86.4%) | 33 (86.8%) | 1/1 |
| Pulmonary edema | 4 (30.8%) | 9 (40.9%) | 17 (44.7%) | 0.518/0.794 |
| DCI | 5 (38.5%) | 8 (36.4%) | 16 (42.1%) | 1/0.787 |
| Disposition at ICU discharge | 0.010/0.143 | |||
| Home/acute rehab facility | 5 (38.5%) | 6 (27.3%) | 14 (36.8%) | |
| SNF | 8 (61.5%) | 10 (45.5%) | 9 (23.7%) | |
| Hospital | 0 (0.0%) | 22 (9.1%) | 1 (2.6%) | |
| Expired | 0 (0.0%) | 4 (18.2%) | 14 (36.8%) | |
| MRS at 3 months | 0.712/0.859 | |||
| 0–2 | 1 (7.7%) | 2 (9.1%) | 6 (15.8%) | |
| 3–6 | 10 (76.9%) | 15 (68.2%) | 23 (60.5%) | |
| Follow-up unavailable | 2 (15.4%) | 5 (22.7%) | 9 (23.7%) | |
| MRS at 12 months | 0.691 /0.587 | |||
| 0–2 | 2 (15.4%) | 4 (18.2%) | 6 (15.8%) | |
| 3–6 | 8 (61.5%) | 13 (59.1%) | 18 (47.4%) | |
| Follow-up unavailable | 3 (23.1%) | 5 (22.7%) | 14 (36.8%) | |
| LOS, mean (SD), d | 36.2 (15.5) | 28.8 (16.0) | 22.5 (12.7) | 0.006/0.144 |
IIC; ictal-interictal continuum; SD; standard deviation; HH; Hunt and Hess scale; MFS; Modified Fisher Scale; WFNS; World Federation of Neurosurgical Societies score; GCS; Glasgow Coma Scale; HTN; hypertension; DM; diabetes mellitus; IVH; intraventricular hemorrhage; DCI; delayed cerebral ischemia; SNF; skilled nursing facility; MRS; Modified Rankin Scale; LOS; length of hospital stay.
Data are presented as number (%) unless otherwise specified.
First p value is for seizure vs. control comparison, second p value is for IIC vs. control comparison. Significant differences (p > 0.05) are in bold.
Cerebral perfusion and cerebrovascular reactivity in patient with seizures
All results are summarized in Table 2 and Figures 1, 2(a), and 3. Please see supplementary appendix for detailed statistical analysis.
Table 2.
Physiologic data results in patient with seizures.
| Dataa | Baseline | An hour before the seizure | During the seizure | An hour after the seizure | Control group |
|---|---|---|---|---|---|
| CPP | 94 (11) | 100 (13) | 102 (13) | 102 (12) | 89 (14) |
| CPPopt | 93 (15) | 98 (11) | 99 (12) | 97 (11) | 92 (12) |
| DeltaCPP | 3 (10) | 6 (14) | 8 (15) | 8 (14) | 0.1 (5) |
| MAP | 104 (13) | 108 (15) | 111 (15) | 108 (14) | 100 (13) |
| SBP | 153 (24) | 166 (29) | 169 (30) | 166 (29) | 151 (22) |
| DBP | 76 (15) | 74 (17) | 73 (14) | 73 (15) | 73 (12) |
| ICP | 8 (4) | 4 (6) | 5 (6) | 4 (6) | 11 (4) |
| PRx | 0.1 (0.19) | 0.13 (0.21) | 0.16 (0.22) | 0.14 (0.20) | 0.12 (0.14) |
| ETCO2 | 33 (5) | 33 (5) | 33 (6) | 34 (5) | 31 (5) |
| PbtO2 | 23 (9) | 23 (11) | 22(10) | 22 (9) | 25 (20) |
CPP; cerebral perfusion pressure; CPPopt; optimal cerebral perfusion pressure; DeltaCPP: CPP – CPPopt; MAP; mean arterial pressure; SBP; systolic blood pressure; DBP; diastolic blood pressure; ICP; intracranial pressure; PRx; cerebrovascular pressure reactivity index; ETCO2; end-tidal carbon dioxide; PbtO2; partial brain tissue oxygenation.
Data are presented as numbers-median (standard deviation). All units are in mmHg except PRx which is in A.U.
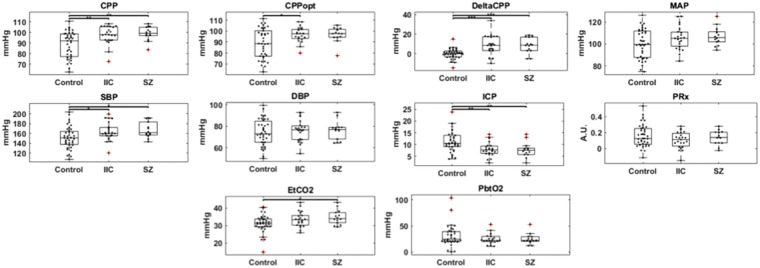
Figure 1.
Physiologic data figures in all groups during the entire recording. Boxplot of the median values of physiologic changes in SAH patients with seizures and IIC compared to control group without seizures/IIC.
CPP; cerebral perfusion pressure; CPPopt; optimal cerebral perfusion pressure; DeltaCPP: CPP – CPPopt; MAP; mean arterial pressure; SBP; systolic blood pressure; DBP; diastolic blood pressure; ICP; intracranial pressure; PRx; cerebrovascular pressure reactivity index; ETCO2; end-tidal carbon dioxide; PbtO2; partial brain tissue oxygenation; Sz; seizure; IIC; ictal-interictal continuum. *p ≤ 0.05, **p ≤ .01, ***p ≤ .001
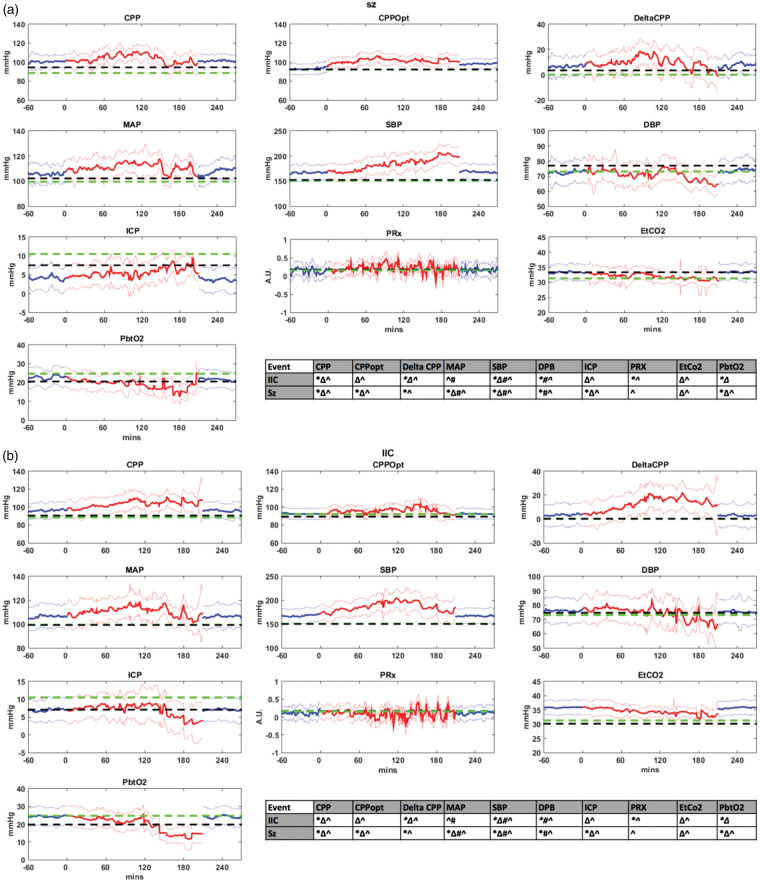
Figure 2.
(a) Physiologic data figures in patients with seizures. (b) Physiologic data figures in patients with IIC. (a, b) Physiologic changes in SAH patients with seizures and IIC. X axis represents the timeline, and Y axis represents the values. Black dashed line represents all values at baseline (prior to seizure or IIC). Blue line represents an hour prior to the event (seizure/IIC) and an hour after the event, respectively. Red line represents the values during the event. The green dotted line represents the median of all patients in the control group. Physiologic graphs are displayed as medians with 1 standard error of the median (shaded areas). Statistical significance for all values is represented in the tables for both seizure and IIC figures.
All statistically significant values have p < 0.05. *During event compared to an hour prior to seizure (red with blue). Δ an hour before seizure when compared to the control subset (blue with green). #Baseline in patients with events compared to the control subset (black with green). ^Baseline compared to an hour before the event (black with blue).
CPP; cerebral perfusion pressure; CPPopt; optimal cerebral perfusion pressure; DeltaCPP: CPP – CPPopt; MAP; mean arterial pressure; SBP; systolic blood pressure; DBP; diastolic blood pressure; ICP; intracranial pressure; PRx; cerebrovascular pressure reactivity index; ETCO2; end-tidal carbon dioxide; PbtO2; partial brain tissue oxygenation; Sz; seizure; IIC; ictal-interictal continuum.
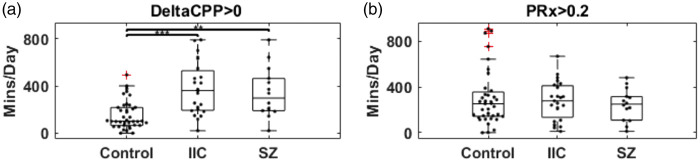
Figure 3.
(a) Boxplot for time burden (min/day) for DeltaCPP > 0 (CPP > CPPopt) for the control group, patients with IIC and patients with seizures. (b) Boxplot for time burden (min/day) for impaired autoregulation (PRx > 0.2 A.U.) for the control group, patients with IIC and patients with seizures. *p ≤ 0.05, ** p ≤ .01, *** p ≤ .001.
Throughout the entire recording, CPP was higher in the seizure group [99 ± 6.5, p = .005] when compared to controls [89 ± 12.3]. DeltaCPP was also higher [8.3 ± 7.9, p = .0003] when compared to controls [−0.1 ± 5]. PRx values were not statistically significant between the two groups [seizure 0.13 ± 0.09, controls 0.16 ± 0.14, p = .8]. Time spent with supra-optimal CPP was higher in the seizure group [342 ± 213 min/day, p = .002] when compared to controls [154 ± 120 min/day]. Time spent with PRx > 0.2 (impaired autoregulation) was not statistically significant between the seizure group [244 ± 140 min/day, p = .8] and the control group [298 ± 237 min/day].
CPP increased an hour before seizure [p = 0.001], and continued to increase during seizure [p < 0.0001]. DeltaCPP increased an hour before seizure [p < 0.0001] and continued to increase during seizure [p < 0.0001]. PRx values decreased an hour before the seizure compared to baseline [p < 0.0001]. There was a trend toward increased PRx values during seizure without statistical significance. MAP and SBP were higher at baseline compared to the control group [MAP p = 0.001, SBP p < 0.0001]. Both values continued to increase an hour prior to seizure and during the seizure, then decreased after seizure but were still higher when compared to the control group and the seizure group at baseline. ICP values were lower than the control group an hour before, during and after the seizure. During seizure, there was a trend toward higher ICP with more seizure time (>100 min). ETCO2 increased an hour before seizure [p < 0.0001] with a trend toward decreased value with more seizure time. The values were increased after the cessation of the seizure event back to pre-seizure CO2 levels. PbtO2 showed a trend toward lower values with more seizure time (>120 min).
Cerebral perfusion and cerebrovascular reactivity in patient with IIC
All results are summarized in Table 3 and Figures 1, 2(b) and 3. Please see supplementary appendix for detailed statistical analysis.
Table 3.
Physiologic data results in patient with IIC.
| Dataa | Baseline | An hour before the IIC | During the IIC | An hour after the IIC | Control group |
|---|---|---|---|---|---|
| CPP | 93 (13) | 97 (16) | 98 (16) | 97 (16) | 89 (14) |
| CPPopt | 93 (11) | 92 (12) | 92 (13) | 92 (12) | 92 (12) |
| DeltaCPP | 1 (14) | 4 (17) | 6 (18) | 3 (17) | 0.1 (5) |
| MAP | 101 (15) | 107 (17) | 109 (19) | 105 (17) | 100 (13) |
| SBP | 153 (24) | 167 (28) | 172 (30) | 168 (30) | 151 (22) |
| DBP | 76 (15) | 77 (16) | 77 (17) | 76 (16) | 73 (12) |
| ICP | 8 (4) | 7 (6) | 7 (7) | 7 (6) | 11 (4) |
| PRx | 0.1 (0.2) | 0.1 (0.19) | 0.11 (0.23) | 0.1 (0.21) | 0.12 (0.14) |
| ETCO2 | 30 (5) | 36 (6) | 36 (5) | 36 (5) | 31 (5) |
| PbtO2 | 22 (8) | 24 (10) | 25 (11) | 24 (10) | 25 (20) |
CPP; cerebral perfusion pressure; CPPopt; optimal cerebral perfusion pressure; DeltaCPP: CPP – CPPopt; MAP; mean arterial pressure; SBP; systolic blood pressure; DBP; diastolic blood pressure; ICP; intracranial pressure; PRx; cerebrovascular pressure reactivity index; ETCO2; end-tidal carbon dioxide; PbtO2; partial brain tissue oxygenation.
Data are presented as numbers-median (standard deviation). All units are in mmHg except PRx which is in A.U.
Throughout the entire recording, CPP was higher in the IIC group [97 ± 8.5, p = .007] when compared to controls [89 ± 12.3]. DeltaCPP was also higher [8.1 ± 10.3, p = .0006] when compared to controls [−0.1 ± 5]. PRx values were not statistically significant between the two groups [IIC 0.11 ± 0.1, controls 0.16 ± 0.14, p = .2]. Time spent with supra-optimal CPP was higher in the IIC group [377 ± 222 min/day, p < .0001] when compared to controls [154 ± 120 min/day]. Time spent with PRx > 0.2 (impaired autoregulation) was not statistically significant between the IIC group [280 ± 170 min/day, p = .7] and the control group [298 ± 237 min/day].
CPP increased an hour before IIC [p = 0.001], and continued to increase during IIC [p < 0.0001]. DeltaCPP increased an hour before IIC [p < 0.0001] and continued to increase during the event [p < 0.0001]. PRx values decreased an hour before the IIC compared to baseline [p < 0.0001] and during IIC compared to an hour before the event (p = 0.04). SBP values were higher at baseline compared to the control group [p < 0.0001]. The values continued to increase an hour prior to IIC, during the IIC, then decreased after IIC but were still higher when compared to the control group and the IIC group at baseline. MAP values were higher at baseline when compared to the control subset [p = 0.001], and continued to increase an hour before the IIC [p < 0.0001]. ICP values were lower than the control group at baseline and an hour before the IIC [p < 0.0001]. During IIC, there was a trend toward lower ICP with more IIC time (>120 min). ETCO2 increased an hour before the IIC [p < 0.0001] with higher CO2 levels during and after IIC compared to the control subset. PbtO2 showed a trend toward lower values with more IIC time (>120 min).
Outcome data
All outcome data are demonstrated in Table 1. Overall, DCI was the same among all groups. In terms of discharge disposition, patients with seizures were discharged more often to home or an acute rehabilitation facility versus skilled nursing facility [p = 0.01]. Modified Rankin scale at 3 months and 12 months was the same among all groups. Length of stay was higher in seizure patients [p = 0.006].
Discussion
To our knowledge, this study is the first in humans to address the relationship of optimal values of cerebral perfusion with seizures and IIC in patients with acute brain injury. We analyzed physiologic and correlated EEG data, and showed that higher than optimal CPP is associated with increased risk of seizures and IIC in patients with SAH.
The exact pathophysiology of seizures in acute brain injury, while unknown, is likely multifactorial. Its resulting effects on the brain and body are complex, including increased metabolic demand, increased sympathetic drive leading to tachycardia and increased blood pressure, spreading depolarization and ischemia, and increased blood supply in the seizure focus.8,12–14,39 The current study demonstrates that for at least an hour prior to the onset of seizure or IIC, there is an increase in SBP, MAP, and CPP, when compared to the control group. PbtO2 decreased with more seizure time, possibly as a result of increased metabolic demand.9
Accordingly, deltaCPP was higher at least an hour before the event, meaning there was relative hyperemia prior to seizure onset. Relative hyperemia preceding seizure events was appropriately associated with increased ETCO2 values. Increased ETCO2 may happen as a result of increased metabolism or decreased respiratory rate leading to CO2 retention. Throughout the recording, CPP and deltaCPP were higher in patients with ictal events compared to the control group. In addition, they spent more time in hyperemic state (CPP > CPPopt). This is consistent with our hypothesis and with the reported literature in PRES,22 hypertensive encephalopathy,23 hypertension in pregnancy,24 and reperfusion syndrome after carotid surgery25. All of these conditions are examples of the association between increased CPP and resulting ictal events.
Impaired vasoreactivity has been reported in patients with acute brain injury, and is a predictor of worse functional outcome.32 In our cohort, cerebrovascular reactivity overall was similar between patients with seizure/IIC and those without. It is not clear what the significance of these results is, future studies are needed on this topic. In addition, we demonstrated a trend of rising ICP with cumulative seizures corroborating prior studies.8,10,40 This may be related to increased cerebral perfusion during seizures.
Our study has limitations. First, identification of seizures based on minidepth EEG has limitations as some seizures and IIC events might start remotely and later spread to the depth electrode. However, we noticed the hemodynamic changes prior to the events onset by at least an hour. This supports our hypothesis that non-optimal cerebral perfusion may be associated with increased risk of seizures and IIC. Second, our results are not necessarily generalizable to all acute brain injury, as our cohort represents poor grade SAH patients. Third, the optimal CPP in SAH patients is complicated by the presence of various states of proximal vessel narrowing (vasospasm) and the changes in cerebrovascular reserve. CPPopt opt may not be the CPP with the lowest PRx, as has been defined in TBI.34
We provide evidence that cerebral hyperemia (supra-optimal CPP) is associated with an increased risk of seizures and IIC in SAH patients. This is important in the context of induced hypertensive therapy which is oft used for the prevention and treatment of DCI. The study provides rationale to target an optimal CPP in guided therapy and is a starting point for future studies. A future intention to treat study could be done to determine if targeting optimal cerebral perfusion would result in less seizures/IIC in patients with acute brain injury.
Supplemental Material
Supplemental Material for Hyperemia in subarachnoid hemorrhage patients is associated with an increased risk of seizures by Ayham Alkhachroum, Murad Megjhani, Kalijah Terilli, Clio Rubinos, Jenna Ford, Brendan K Wallace, David J Roh, Sachin Agarwal, E Sander Connolly, Amelia K Boehme, Jan Claassen and Soojin Park in Journal of Cerebral Blood Flow & Metabolism
Acknowledgments
We thank the attending physicians, fellows, and residents of the neurology and neurosurgery departments for their support of this project.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Dr. Soojin Park NIH K01 ES026833.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors' contributions
AA, SP and MM have full access to all the data in the study and take responsibility for the integrity of the study. Study concept and design: AA and SP Acquisition of data was performed by AA, MM and KT. Drafting the manuscript: AA Critical revision of the manuscript: MM, KT, CR, JF, BW, DR, SA, SC, AB, JC, SP. Statistical analysis was conducted by MM and AB Study supervision: SP
Supplemental material
Supplemental material for this paper can be found at the journal website: http://journals.sagepub.com/home/jcb
References
- 1.Kinoshita K. Traumatic brain injury: pathophysiology for neurocritical care. J Intensive Care 2016; 4: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rasulo FA, Girardini A, Lavinio A, et al. Are optimal cerebral perfusion pressure and cerebrovascular autoregulation related to long-term outcome in patients with aneurysmal subarachnoid hemorrhage?. J Neurosurg Anesthesiol 2012; 24: 3–8. [DOI] [PubMed] [Google Scholar]
- 3.Willie CK, Tzeng YC, Fisher JA, et al. Integrative regulation of human brain blood flow. J Physiol 2014; 592: 841–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Budohoski KP, Czosnyka M, Smielewski P, et al. Impairment of cerebral autoregulation predicts delayed cerebral ischemia after subarachnoid hemorrhage: a prospective observational study. Stroke 2012; 43: 3230–3237. [DOI] [PubMed] [Google Scholar]
- 5.Budohoski KP, Czosnyka M, Kirkpatrick PJ, et al. Clinical relevance of cerebral autoregulation following subarachnoid haemorrhage. Nat Rev Neurol 2013; 9: 152–163. [DOI] [PubMed] [Google Scholar]
- 6.Meldrum BS, Nilsson B. Cerebral blood flow and metabolic rate early and late in prolonged epileptic seizures induced in rats by bicuculline. Brain 1976; 99: 523–542. [DOI] [PubMed] [Google Scholar]
- 7.Ingvar M, Siesjö BK. Local blood flow and glucose consumption in the rat brain during sustained bicuculline-induced seizures. Acta Neurol Scand 1983; 68: 129–144. [DOI] [PubMed] [Google Scholar]
- 8.Claassen J, Perotte A, Albers D, et al. Nonconvulsive seizures after subarachnoid hemorrhage: multimodal detection and outcomes. Ann Neurol 2013; 74: 53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Witsch J, Frey HP, Schmidt JM, et al. Electroencephalographic periodic discharges and frequency-dependent brain tissue hypoxia in acute brain injury. JAMA Neurol 2017; 74: 301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vespa PM, Miller C, McArthur D, et al. Nonconvulsive electrographic seizures after traumatic brain injury result in a delayed, prolonged increase in intracranial pressure and metabolic crisis. Crit Care Med 2007; 35: 2830–2836. [PMC free article] [PubMed] [Google Scholar]
- 11.Vespa P, Tubi M, Claassen J, et al. Metabolic crisis occurs with seizures and periodic discharges after brain trauma. Ann Neurol 2016; 79: 579–590. [DOI] [PubMed] [Google Scholar]
- 12.Geneslaw AS, Zhao M, Ma H, et al. Tissue hypoxia correlates with intensity of interictal spikes. J Cereb Blood Flow Metab 2011; 31: 1394–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao M, Nguyen J, Ma H, et al. Preictal and ictal neurovascular and metabolic coupling surrounding a seizure focus. J Neurosci 2011; 31: 13292–13300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dreier JP, Major S, Pannek HW, et al. Spreading convulsions, spreading depolarization and epileptogenesis in human cerebral cortex. Brain 2012; 135: 259–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Czosnyka M, Smielewski P, Czosnyka Z, et al. Continuous assessment of cerebral autoregulation: clinical and laboratory experience. Acta Neurochir Suppl 2003; 86: 581–585. [DOI] [PubMed] [Google Scholar]
- 16.Claassen J, Peery S, Kreiter KT, et al. Predictors and clinical impact of epilepsy after subarachnoid hemorrhage. Neurology 2003; 60: 208–214. [DOI] [PubMed] [Google Scholar]
- 17.Kim JA, Rosenthal ES, Biswal S, et al. Epileptiform abnormalities predict delayed cerebral ischemia in subarachnoid hemorrhage. Clin Neurophysiol 2017; 128: 1091–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Connolly ES, Rabinstein AA, Carhuapoma JR, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke 2012; 43: 1711–1737. [DOI] [PubMed] [Google Scholar]
- 19.Diringer MN, Bleck TP, Claude Hemphill J, et al. Critical care management of patients following aneurysmal subarachnoid hemorrhage: recommendations from the Neurocritical Care Society's Multidisciplinary Consensus Conference. Neurocrit Care 2011; 15: 211–240. [DOI] [PubMed] [Google Scholar]
- 20.Vespa P, Prins M, Ronne-Engstrom E, et al. Increase in extracellular glutamate caused by reduced cerebral perfusion pressure and seizures after human traumatic brain injury: a microdialysis study. J Neurosurg 1998; 89: 971–982. [DOI] [PubMed] [Google Scholar]
- 21.Claassen J, Hirsch LJ, Frontera JA, et al. Prognostic significance of continuous EEG monitoring in patients with poor-grade subarachnoid hemorrhage. Neurocrit Care 2006; 4: 103–112. [DOI] [PubMed] [Google Scholar]
- 22.Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med 1996; 334: 494–500. [DOI] [PubMed] [Google Scholar]
- 23.Vaughan CJ, Delanty N. Hypertensive emergencies. Lancet 2000; 356: 411–417. [DOI] [PubMed] [Google Scholar]
- 24.Cunningham FG, Lindheimer MD. Hypertension in pregnancy. N Engl J Med 1992; 326: 927–932. [DOI] [PubMed] [Google Scholar]
- 25.Karapanayiotides T, Meuli R, Devuyst G, et al. Postcarotid endarterectomy hyperperfusion or reperfusion syndrome. Stroke 2005; 36: 21–26. [DOI] [PubMed] [Google Scholar]
- 26.Waziri A, Claassen J, Stuart RM, et al. Intracortical electroencephalography in acute brain injury. Ann Neurol 2009; 66: 366–377. [DOI] [PubMed] [Google Scholar]
- 27.Stuart RM, Schmidt M, Kurtz P, et al. Intracranial multimodal monitoring for acute brain injury: a single institution review of current practices. Neurocrit Care 2010; 12: 188–198. [DOI] [PubMed] [Google Scholar]
- 28.Bederson JB, Connolly ES, Batjer HH, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke 2009; 40: 994–1025. [DOI] [PubMed] [Google Scholar]
- 29.Zweifel C, Lavinio A, Steiner LA, et al. Continuous monitoring of cerebrovascular pressure reactivity in patients with head injury. Neurosurg Focus 2008; 25: E2. [DOI] [PubMed] [Google Scholar]
- 30.Megjhani M, Terilli K, Martin A, et al. Deriving the PRx and CPPopt from 0.2-Hz data: establishing generalizability to bedmaster users. Acta Neurochir Suppl 2018; 126: 179–182. [DOI] [PubMed] [Google Scholar]
- 31.Czosnyka M, Smielewski P, Kirkpatrick P, et al. Continuous assessment of the cerebral vasomotor reactivity in head injury. Neurosurgery 1997; 41: 11–17. discussion 17–19. [DOI] [PubMed] [Google Scholar]
- 32.Rivera-Lara L, Zorrilla-Vaca A, Geocadin R, et al. Predictors of outcome with cerebral autoregulation monitoring: a systematic review and meta-analysis. Crit Care Med 2017; 45: 695–704. [DOI] [PubMed] [Google Scholar]
- 33.Donnelly J, Budohoski KP, Smielewski P, et al. Regulation of the cerebral circulation: bedside assessment and clinical implications. Crit Care 2016; 20: 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeiler FA, Ercole A, Cabeleira M, et al. Comparison of performance of different optimal cerebral perfusion pressure parameters for outcome prediction in adult TBI: a center-TBI study. J Neurotrauma 2019; 36: 1505–1517. [DOI] [PubMed] [Google Scholar]
- 35.Aries MJ, Czosnyka M, Budohoski KP, et al. Continuous determination of optimal cerebral perfusion pressure in traumatic brain injury. Crit Care Med 2012; 40: 2456–2463. [DOI] [PubMed] [Google Scholar]
- 36.Steiner LA, Czosnyka M, Piechnik SK, et al. Continuous monitoring of cerebrovascular pressure reactivity allows determination of optimal cerebral perfusion pressure in patients with traumatic brain injury. Crit Care Med 2002; 30: 733–738. [DOI] [PubMed] [Google Scholar]
- 37.Sorrentino E, Diedler J, Kasprowicz M, et al. Critical thresholds for cerebrovascular reactivity after traumatic brain injury. Neurocrit Care 2012; 16: 258–266. [DOI] [PubMed] [Google Scholar]
- 38.Gelman A, Jennifer Hill. Data analysis using regression and multilevel/hierarchical models, Cambridge: Cambridge University Press, 2006. [Google Scholar]
- 39.Dreier JP. The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat Med 2011; 17: 439–447. [DOI] [PubMed] [Google Scholar]
- 40.Ko SB, Ortega-Gutierrez S, Choi HA, et al. Status epilepticus-induced hyperemia and brain tissue hypoxia after cardiac arrest. Arch Neurol 2011; 68: 1323–1326. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Hyperemia in subarachnoid hemorrhage patients is associated with an increased risk of seizures by Ayham Alkhachroum, Murad Megjhani, Kalijah Terilli, Clio Rubinos, Jenna Ford, Brendan K Wallace, David J Roh, Sachin Agarwal, E Sander Connolly, Amelia K Boehme, Jan Claassen and Soojin Park in Journal of Cerebral Blood Flow & Metabolism
Data Availability Statement
Data that support the results of this study are available from the corresponding author on request from any qualified investigator.