Abstract
Collateral circulation plays a pivotal role in acute ischemic stroke due to large vessel occlusion (LVO) and may be affected by multiple variables during sedation for endovascular therapy (EVT). We conducted detailed analyses of the GOLIATH trial to identify predictors of collateral circulation grade and infarct growth. We also modified the ASITN collateral grading scale and sought to determine its impact on clinical outcome and infarct growth. Multivariable analysis was used to identify predictors of collaterals and infarct growth. Ordinal analysis demonstrated nominal, but non-significant association between modified ASITN scale and infarct growth. Among all analyzed baseline clinical and procedural variables, the most significant predictors of infarct growth at 24 h were phenylephrine dose (estimate 6.78; p = 0.014) and baseline infarct volume (estimate 0.93; p = 0.03). The most significant predictors of worse collateral grade were mean arterial pressure (MAP) <70 mmHg (OR 0.35; p = 0.048) and baseline infarct volume (OR 0.96; p = 0.003). Hypotension during sedation for EVT for LVO negatively impacts collateral circulation, while higher pressor dose is a strong predictor of infarct growth. Avoidance of anesthesia-induced hypotension and consequent need for pressor therapy may prevent collateral failure and minimize infarct growth.
Keywords: Anesthesia, collaterals, hypotension, stroke, thrombectomy
Introduction
Collateral circulation is a key determinant of acute ischemic stroke outcome: better collaterals are associated with smaller infarct size, higher revascularization rates, lower hemorrhagic transformation, and improved long-term clinical outcome after EVT for AIS.1–5 As such, any therapeutic intervention aimed to improve collaterals or delay/prevent collateral failure in the setting of EVT, has the potential advantage to improve clinical outcome by prevention of ischemic core expansion, lowering hemorrhage risk, and improving recanalization success. Despite the exhaustive literature and advanced understanding of the pathophysiologic factors influencing collateral flow, there is limited translational application of collateral therapeutic strategies in the current era of AIS treatment.6,7 A distinct advantage of monitored anesthesia is that it offers an ideal environment for investigating the effect of different physiologic parameters upon the collateral circulation during acute cerebral ischemia with the potential for therapeutic translation. Furthermore, prior studies have suggested that anesthesia-induced intra-procedural hypotension and low end-tidal carbon dioxide (ETCO2) prior to reperfusion are associated with worse clinical outcome after EVT, presumably due to collateral compromise.8–10 However, the association between collateral circulation and these important physiologic parameters remains theoretical. To this date, no studies have provided solid evidence of the direct impact of intraprocedural ETCO2 and blood pressure variations on collaterals. In addition, while the primary aim of all interventions for treatment of acute ischemic stroke (AIS) due to large vessel occlusion (LVO) is fast and efficient recanalization, little attention has been given to other potentially modifiable variables affecting the pathophysiology of cerebral ischemia. Factors, including blood pressure and ventilatory status, may be of particular relevance in establishing the best possible methods of sedation in the setting of EVT, especially in patients with tenuous collaterals who might be particularly vulnerable to accelerated infarct growth. Given rapid expansion of EVT for AIS and increasing interest in revascularizing patients with large baseline ischemic core, periprocedural management of these physiologic factors may be of particular importance for optimal clinical outcome.11–13
In this study, we aimed to identify specific intraprocedural physiologic predictors of collateral circulation strength and infarct growth immediately prior to thrombectomy attempts for AIS due to LVO. We hypothesized that intra-procedural hypotension and low pCO2 levels are associated with worse collaterals. In addition, we sought to further analyze the currently accepted reference standard ASITN collateral grading scale by introducing a novel subcategory based on the extend of the filling defect: we divided grade 2 into two separate categories: 2−(collateral filling in <50% of the ischemic territory) and 2+ (collateral filling in >50% of the territory) (Table 1). We hypothesized that ASITN grade 2+ collaterals will have more favorable impact on lesion growth and clinical outcome as compared to grade 2−.
Table 1.
Modified ASITN collateral grading scale.
| Grade | Angiographic assessment |
|---|---|
| 0 | No visible collaterals to the ischemic site |
| 1 | Slow collaterals to the periphery of the ischemic site with persistence of some defect |
| 2− | Rapid collaterals to the periphery of the ischemic site with collateral filling in <50% of the territory |
| 2+ | Rapid collaterals to the periphery of the ischemic site with collateral filling >50% of the territory |
| 3 | Collaterals with slow but complete angiographic blood flow of the ischemic bed by the venous phase |
| 4 | Complete and rapid collateral blood flow to the vascular bed in the entire ischemic territory by retrograde perfusion |
Materials and methods
We conducted a retrospective analysis of the complete GOLIATH trial database. The GOLIATH trial was a single-center, prospective parallel group, open-label randomized controlled trial with blinded end point evaluation (PROBE design). Details regarding trial design and outcome have been previously published.14 The study was approved by The Ethical Committee in Central Denmark Region in accordance with ethical standards of the Helsinki Declaration of 1975 (and as revised in 1983). The committee accepted a waiver of consent before randomization because eligible patients typically were not able to give informed consent and treatment was time critical. Patients or their next of kin were later required to give written informed consent to remain in the trial, and only one patient refused to give post-randomization consent because he/she did not want to undergo repeated magnetic resonance imaging (MRI) scans. No data monitoring board was involved. Briefly, patients were included from March 2015 to February 2017. Eligible patients with acute ischemic stroke presenting with anterior circulation large vessel occlusion were randomized for EVT under either general anesthesia (GA) or conscious sedation (CS). Total of 128 patients were included in the trial, of which 65 patients were randomized to receive GA and 63 patients were allocated to CS. Four patients (6%) crossed over from CS to GA. All patients were included in this analysis.
GA included rapid sequence induction with suxamethonium, alfentanil, and propofol followed by infusion of propofol and remifentanil.15 Following endotracheal intubation, controlled ventilation was applied to achieve normoventilation. CS included a fentanyl bolus dose, which was repeated if necessary and a low-dose propofol infusion with the infusion rate adjusted at the discretion of the anesthesiologist. Periprocedural monitoring consisted of continuous electrocardiogram, pulse oximetry, and ETCO2 monitoring. An arterial catheter was inserted before induction for continuous invasive arterial blood pressure measurements. Blood pressure variables including SBP, diastolic blood pressure, and mean arterial pressure (MAP) were measured every minute throughout the procedure. Immediately after termination of the procedure, the attending anesthesiologist calculated the total time the patient was below the prespecified blood pressure thresholds (SBP < 140 mm Hg, MAP < 70 mm Hg) and manually recorded blood pressure measurements for every minute during the first 5 min followed by recording of measurements for every 5 min. The data were stored in the GOLIATH database and used for calculation of blood pressure variability and analyses of the continuous hemodynamic variables. Detailed analysis of this data and the relationship of blood pressure variability with clinical outcome has been previously published.16
Per recommendations from the Society for Neuroscience in Anesthesiology and Critical Care,17 and the study by Whalin et al.18 who reported unfavorable outcomes with MABP < 70 mm Hg,18 the pre-specified trial protocol blood pressure goals were SBP > 140 mm Hg and MABP > 70 mm Hg. A reduction in SBP and MAP below these targets was treated with ephedrine, phenylephrine, or both, with choice of vasopressor per anesthesiologist’s discretion. Baseline blood pressure was defined as the blood pressure measured in the neurointerventional suite immediately before induction of GA or CS. MAP at the end of procedure was measured at the time of removal of the femoral artery sheath.
The pre-specified primary outcome of the trial was infarct growth at 48–72 h after EVT, measured in milliliters (mL). Infarct size before and after the procedure, modified TICI score, and procedural safety measures (dissection, perforation, and clot migration) were evaluated by a single independent core imaging laboratory investigator to ensure unbiased assessment of the study endpoints. Baseline infarct size was determined on diffusion-weighted imaging (DWI) or apparent diffusion coefficient (ADC) imaging. Final infarct size measurement was performed, using a T2 fluid-attenuated inversion recovery sequence with additional reference to the DWI or ADC imaging, and included regions of hemorrhagic conversion.
Secondary outcome measures were modified Rankin scale (mRS) scores at 90 days, process time intervals, blood pressure levels, and safety end points. The mRS score was evaluated at 90 days (80–100 days) after the stroke over the telephone by a certified study nurse who was blinded to randomization.
Angiographic collateral assessment was performed by two independent readers who were blinded to clinical and imaging outcome, followed by consensus adjudication. Collateral grade was scored only when assessable, prior to any thrombectomy attempt.
In this study, we introduced a novel sub classification of the grade 2 category of the originally described ASITN angiographic collateral flow grading scale.19 According to this originally proposed scale, grade 2 is described as “rapid collaterals to the periphery of the ischemic site with persistence of some of the defect and to only a portion of the ischemic territory.” Given the potential for high variability in the size of the ischemic territory, we further divided grade 2 into two separate categories (Table 1): 2− (collateral filling of <50% of the ischemic territory), and 2+ (collateral filling of >50% of the territory).
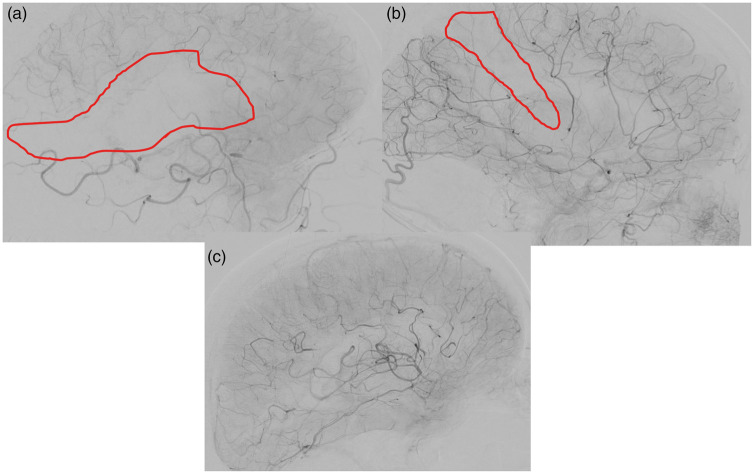
Examples of these two separate grades are depicted in Figure 1.
Figure 1.
Lateral projection angiographic images obtained in the venous phase in setting of M1 occlusions in three different patients. The images demonstrate examples of different collateral grades according to the modified ASITN grading scale: (a) Rapid collaterals to the periphery of the ischemic site with filling in <50% of the MCA territory, corresponding to ASITN grade 2−. (b) Rapid collaterals to the periphery of the ischemic site with filling in ≧50% of the MCA territory, corresponding to ASITN grade 2+ (c). Collaterals with slow but complete angiographic flow of the ischemic bed, corresponding to ASITN grade 3.
Statistical analysis
All analyzed clinical, imaging, and procedural anesthetic variables, including collaterals by modified ASITN grading scale, and infarct growth are listed in Table 2.
Table 2.
Variables analyzed.
| Variables included in the analysis | % (N); Mean ± SD (Median) |
|---|---|
| Age | 71.4 ± 11.4 (72.5) |
| Baseline NIHSS | 17.6 ± 4.3 (17.5) |
| History of HTN | 56% (71/127) |
| A-fib | 40% (51/127) |
| Smoker | 32% (40/126) |
| DM | 14% (128/127) |
| Baseline ASPECTS | 7.0 ± 1.8 (7.0) |
| Infarct growth (ml) | 45.6 ± 85.3 (14.7) |
| Time to groin (min) | 193.9 ± 71.7 (180.0) |
| Propofol dose (mg) | 249.3 ± 214.9 (213.0) |
| Phenylephrine % used; dose | 77% (99/128) |
| ETCO2 (kPa) | 4.5 ± 0.4 (4.5) |
| MAP < 70 mmHg | 26% (33/128) |
| Ephedrine % used; dose | 38% (48/128) |
| Evaluable collaterals | 62% (80/128) |
| M1 | 83% (44/53) |
| Tandem (ICA/M1) | 31% (9/29) |
| ICA T | 32% (6/19) |
| ICA neck | 50% (4/8) |
| M2 | 47% (9/19) |
| Modified ASITN grade | |
| 1 | 11% (9/80) |
| 2− | 24% (19/80) |
| 2+ | 39% (31/80) |
| 3 | 26% (21/80) |
| 4 | 0 |
Baseline characteristics are reported using standard summary statistics, including mean ± standard deviation and median for continuous variables, and frequency tabulations for discrete and ordinal variables.
Spearman’s rho for correlation was used to assess the relationship between the modified 4-point ASITN collateral score (no grade 4 was detected in this dataset) and: (a) mRS at 90 days (b) ETCO2, and (c) infarct growth.
Univariate analyses were run for each baseline variable to associate candidate clinical and procedural variables with two separate outcomes: (1) modified ASITN scale and (2) infarct growth. The multivariate models were built by first including typical covariates (e.g. age) and predictors of specific interest (e.g. MAP) to the research hypothesis, prior to the construction of the models. Any additional predictors with univariate p values < 0.20 were also included in the multivariable analyses. For the modified ASITN scale, ordinal logistic regression was employed, while the continuous outcome of infarct growth was analyzed with linear regression. Predictors in each model are presented with their estimated effects (odds ratios for ASITN and regression betas for infarct growth) along with their associated 95% confidence intervals and p-values.
Results
Among the 80 (63%) patients in whom collaterals were evaluable on pre-intervention angiography, the most common grade was 2 + (n = 31), followed by 3 (n = 21), and 2 − (n = 19). Only nine patients had grade 1 collaterals. Grade 4 was not observed in this cohort. Collaterals were most frequently evaluable in patients with M1 (83%), followed by ICA neck (50%), M2 (47%), ICA T (32%), and tandem occlusions (31%).
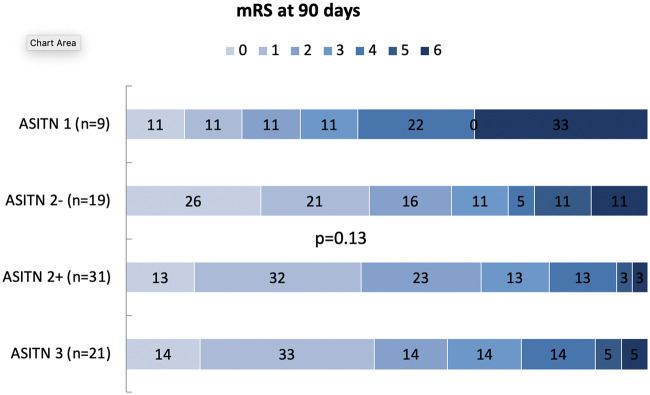
In the univariate ordinal analyses, we found no significant relationship between collaterals grading by modified ASITN and clinical outcome (Figure 2).
Figure 2.
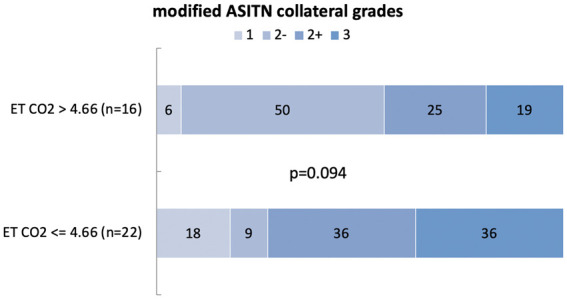
Modified ASITN grades distribution according to ETCO2 values. Numbers within each colored region represent the percentage of patients with the corresponding modified ASITN grades.
Collaterals and ETCO2
The median ETCO2 was 4.4 kPa (33 mmHG) (inter-quartile range, 4.2–4.8 kPa). Among all subjects with evaluable collaterals (n = 80), ETCO2 values were available only in 37 patients. To explore the potential association between collateral circulation and ETCO2 levels, we performed both continuous and categorical analyses. Spearman rho ordinal correlation coefficient test was used to assess the relationship between collateral grading and mean ETCO2 levels, revealing no significant association between the two variables (r = 0.19, p = 0.250) Furthermore, we dichotomized subjects into two separate categories, using the mean ETCO2 value of 4.66 kPa (35 mmhg) as a cutoff for definition of hypocapnia as follows (Figure 3)
Normocapnia > 4.7 kPa (n = 22).
Hypocapnia: ≤ 4.6 kPa (n = 16).
Figure 3.
Ninety-day mRS outcomes according to modified ASITN grading scale. Stacked bar graphs demonstrate full mRS outcome distributions for the examined cohort divided into four groups based on modified ASITN grading scale. Numbers within each colored region represent the percentage of patients with the corresponding mRS outcome grade for that group.
We found no relationship between hypocapnia and collaterals (p = 0.094).
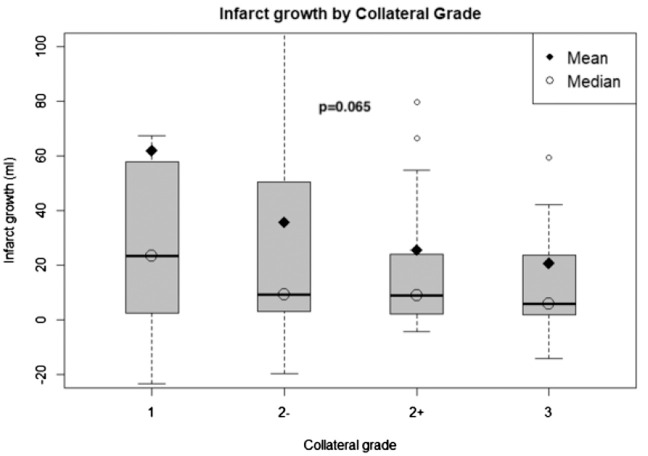
There was a distinct nominal, but non-statistically significant association between different collateral grades and infarct growth with apparent greater potential for infarct growth between grades 2− and 2+ (Figure 4).
Figure 4.
Infarct growth according to modified ASITN collateral grade. Bar plots represent infarct growth volume in each collateral grade category.
Results of the univariate analyses are included in Tables A and B in the Supplemental Material. Among all analyzed variables, the most significant predictors of infarct growth at 24 h were phenylephrine dose (estimate 6.78; p = 0.014) and baseline infarct volume (estimate 0.93; p = 0.03) (Table 3a). The most significant predictors of worse collateral grade were MAP < 70 mmHg (OR 0.35; p = 0.048) and baseline infarct volume (OR 0.96; p = 0.003) (Table 3b). Smaller baseline core volume was associated with better collaterals (OR 1.04; p = 0.003). MAP <70 mmHg occurred much more frequently in the GA arm (35.4%) as compared to CS arm (15.9%). IV pressors were required in almost all (98%) patients in the GA arm versus approximately half (57%) in the CS arm. Phenylephrine was used much more frequently than ephedrine (77% vs. 38%).
Table 3.
Results from multivariable analysis.
| Predictor | Estimate (beta) | LCL | UCL | p-value |
|---|---|---|---|---|
| A. Predictors of infarct growth | ||||
| Age (per year) | 0.80 | −0.54 | 2.13 | 0.245 |
| NIHSS at baseline (per point) | 3.23 | −0.26 | 6.72 | 0.072 |
| Baseline infarct volume (per ml) | 0.93 | 0.10 | 1.75 | 0.030 |
| Time to groin puncture (per min) | 0.12 | −0.08 | 0.33 | 0.233 |
| Propofol dose (per mg) | 0.02 | −0.05 | 0.10 | 0.509 |
| Phenylephrine dose (per mg) | 6.78 | 1.46 | 12.11 | 0.014 |
| MAP < 70 (yes vs. no) | −21.38 | −55.84 | 13.08 | 0.226 |
| Odds ratio |
LCL |
UCL |
p-value |
|
| B. Predictors of collateral grade. | ||||
| Age (per year) | 0.99 | 0.96 | 1.03 | 0.627 |
| NIHSS at baseline (per point) | 0.96 | 0.94 | 1.06 | 0.410 |
| Baseline infarct volume (per ml) | 0.96 | 1.00 | 0.99 | 0.003 |
| Time to groin puncture (per min) | 1.00 | 1.00 | 1.01 | 0.224 |
| Propofol dose (per mg) | 1.00 | 1.00 | 1.00 | 0.580 |
| Phenylephrine dose (per mg) | 0.98 | 0.84 | 1.14 | 0.778 |
| MAP < 70 (yes vs. no) | 0.35 | 0.12 | 0.99 | 0.048 |
Discussion
To our knowledge, this is the first study to demonstrate the detrimental impact of intra-procedural BP reduction upon collateral circulation. Data from physiologic and observational studies and more recently from single-center randomized trials provide insights into potential biologic advantages and disadvantages of both GA and CS during EVT for AIS. One of the main advantages of GA over CS is less patient motion facilitating catheterization and potentially leading to greater revascularization success. This notion is supported by the higher rate of successful reperfusion in the GA arm of the GOLIATH trial.14 Conversely, a potentially detrimental effect of GA is blood pressure reduction, as demonstrated in multiple retrospective studies.9,20–22 The presumed mechanism underlying the deleterious effect of GA-induced hypotension during EVT is collateral compromise leading to accelerated infarct growth. However, no prior studies have established a direct relationship between BP and collateral circulation. Of note, neither of these two factors (collaterals and hypotension) ultimately translated into worse clinical outcome in this and prior analyses of the GOLIATH data.16 A potential explanation is that the negative impact of hypotension on collaterals and clinical outcome was mitigated by the higher rate of successful reperfusion achieved in the GA arm. It is also important to emphasize that despite the pre-specified target BP parameters, hypotension occurred more frequently in the GA arm with a significantly higher rate of IV pressor administration as compared to CS. Moreover, MAP < 70 mmHg occurred mostly at the beginning of the procedure.16 The association between higher pressor dose and infarct growth indicates that the deleterious effect of BP drop during induction upon collateral circulation may be irreversible and may not be compensated by IV pressor therapy. Another theoretical mechanism by which vasopressors may directly influence infarct growth is impaired microvascular perfusion and capillary flow heterogeneity, leading to limited oxygen exchange and penumbral tissue reduction.23,24 Animal data support that leptomeningeal arterioles respond paradoxically to increased pressure with direct vasoconstriction, particularly in setting of common comorbid conditions like hypertension and aging.25 These findings are of paramount importance for future choice of induction strategies during GA. For example, an alternative medication to propofol for induction is Etomidate, which has been proven to preserve hemodynamic stability during GA in volume-depleted patients.26,47 The drug has become a logical choice for many anesthesiologists, intensivists, and ED providers in this patient population due to its unique ability to spare vascular tone, myocardial contractility, and heart rate, while rapidly achieving a hypnotic state.27,28 One notable side effect is the temporary suppression of cortisol synthesis, although no study has shown a definitive link between Etomidate-induced adrenal suppression and worse clinical outcome in non-septic patients. Another alternative medication for induction is ketamine, which is also has very stable cardiovascular properties and potential neuroprotective effects.29 However, this medication has been less popular among most practicing anesthesiologists due to its well-established side effects of dissociative state and hallucinations, which could be prevented with benzodiazepines administration.30
An important factor potentially affecting cerebral collateral circulation during GA is ventilation and ETCO2. Although systemic arterial blood pressure appears to play an important role in preservation and augmentation of downstream collateralization in setting of AIS,31,32 the hallmarks of sufficient collateral flow preserving penumbral tissue viability are adequate cerebral blood flow (CBF) and cerebral blood volume (CBV) at the level of the cerebral tissue microcirculation.33–35 Regional CBV and CBF are proportionally dependent on partial pressure of CO2 due to its vasodilatory properties.36,37 In addition, increased blood flow and vascular reactivity within collaterally perfused brain tissue after AIS have been shown to be more reliably evoked by CO2 than induced hypertension in patients with subacute strokes.38 In a retrospective study by Takahashi et al.,10 lower BP in patients with AIS undergoing EVT under GA had no clinical impact, whereas decreased ETCO2 was the only factor associated with poor outcome.10 Moreover, a recent retrospective single-center study demonstrated that higher end-tidal CO2 and early extubation were the most significant predictors of good neurological outcome after endovascular treatment of AIS under GA.39 Of note, the GA protocol in SIESTA included mild hypercapnia (ETCO2 target 40–45).40 The potential benefical effect of GA over CS was magnified in patients with worse collaterals on pre-treatment CT angiography, which the authors attributed to the mild hypercapnia and cerebral vasodilation.41 Despite these multiple mechanisms by which paCO2-induced vasodilation during AIS may be beneficial, there are additional mechanisms by which paCO2 might exert a detrimental effect. Greater responsiveness of normal cerebral vasculature than ischemic cerebral vasculature to vasodilatory signals could lead to “steal” phenomena, with paradoxical “shunting” of flow away from the ischemic tissue.42 However, we did not find a significant correlation between ETCO2 and collaterals in our analysis. There are several potential explanations. First, the number of patients with evaulable collaterals and available ETCO2 values in our study is very low (N = 37). Second, the median ETCO2 was 4.4 kPa (33 mmHG), i.e. consistent with mild hypocapnia, with very few patients in the more extreme ranges: only three patients had mean ETCO2 values < 4 kPa (30 mmhg) and only one patient had ETCO2 > 5.3 (40 mmhg). Third, we used mean ETCO2 values and we did not analyze detailed minute-by-minute ETCO2 data throughout the procedure. As such, no meaningful conclusion can be drawn from our data regarding the previously established relationship between CO2 and cerebral circulation, particularly with respect to the detrimental vasoconstrictive effect of hypocarbia. Further studies are needed to explore the therapeutic potential of hypercapnia on collaterals in setting of GA.
Our findings also highlight the importance of scrutinizing the angiographic assessment of cerebral collateral circulation. Among all diagnostic methods, catheter angiography provides the best spatial and dynamic visualization of the anatomic and functional capacity of collaterals.43 Several angiographic collateral grading scales have been proposed.19,44–46 In our opinion, the ASITN grading scale provides the most accurate description, incorporating both the dynamic and anatomic aspects of collaterals, focusing on the temporal relationship with the venous phase and the presence and extent of the collateral defect within the ischemic territory. In addition, the ASITN scale has been recommended by multi-society consensus as the most accurate method for collateral flow assessment.47 Furthermore, this scale has been used in most of the published studies describing various associations of collaterals with infarct size, successful reperfusion, hemorrhage rates, glucose levels, microcatheter flow patterns, and clinical outcome after EVT.3,48,49 However, the commonly used dichotomization between “poor” (grades 0–2) and “good” (grades 3–4) collaterals in these studies may not be optimal due to inclusion of the highly variable grade 2 in the “poor” category. Similar to the well-established angiographic and functional distinction between TICI grade 2a and 2b based on the size of the reperfused territory, the ischemic defect in ASITN grade 2 can also vary in size. Examples of two different patients with ASITN grade 2 are included in Figure 1, illustrating the difference in the size of the ischemic defect. This potential of high variability within a single collateral grade may hamper the prognostic accuracy of angiographic collateral assessment in clinical practice and research. As such, we pursued more detailed investigation of this category with respect to clinical (mRS) and biomarker (infarct growth) outcomes. Using the TICI scoring paradigm as a reference, we subdivided grade 2 based on the size of the ischemic defect into 2− (<50% collateral filling of the ischemic territory) and 2+ (>50%), and we explored the relationship of this modified ASITN grading with 90 days mRS and infarct growth. We did not find correlation between clinical outcome and collaterals, but we observed a potential relationship with infarct growth with more frequent instances of large infarct growth in grade 2− versus 2+ collaterals (Figure 4). Although this difference did not reach statistical significance, our findings demonstrate the potential utility of this modified ASITN grading for more precise collateral assessment findings and warrant further validation in larger cohorts.
Limitations
The main limitation of our study is the relatively small sample size. In addition, our analyses and findings are focused primarily on physiologic parameters without significant correlation with clinical outcome. Another important limitation is that the GOLIATH trial was a single center study and thus the findings reported here may not be generalizable to other centers with different anesthesia regimens. Finally, the ETCO2 analyses presented here may not accurately reflect its physiologic correlation with collaterals. We used mean ETCO2 values from the entire period of anesthesia, and thus we cannot detect potential variability which likely occurred at the beginning of the procedure. Similar to BP, profound temporary drops in the ETCO2 may hamper collaterals. Moreover, we did not measure the exact PaCO2 to ETCO2 gradient, which may influence the number of patients dichotomized to either normo- or hypocapnia in our association between ETCO2 and collateral flow.
Conclusions
Sedation-induced intraprocedural blood pressure drop has a deleterious effect on cerebral collateral circulation in setting of EVT, which may not be reversed by vasopressor administration. Avoidance of hypotension (particularly during induction) appears critical for preserving collaterals and minimizing infarct growth. These findings illustrate the significance of tight periprocedural hemodynamic control, especially in patients with larger.
Supplemental Material
Supplemental Material for Physiologic predictors of collateral circulation and infarct growth during anesthesia – Detailed analyses of the GOLIATH trial by Radoslav Raychev, David S Liebeskind, Albert J Yoo, Mads Rasmussen, Dimiter Arnaudov, Scott Brown, Jeffrey Saver and Claus Z Simonsen in Journal of Cerebral Blood Flow & Metabolism
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: Radoslav Raychev: Research grant, SVIN. Albert Yoo: Research grant, Medtronic, Cerenovus, Penumbra, Stryker, Genentech. Mads Rasmussen: Health Research Fund of Central Denmark Region. Dimiter Arnaudov: none. Claus Z Simonsen: Research Grant, Novo Nordisk Foundation.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: David Liebeskind: Consultant to Cerenovus, Genentech, Medtronic, Stryker and Vesalio as imaging core lab. Albert Yoo: Consultant to Cerenovus and Genentech; Equity interest: Insera Therapeutics. Jeffrey Saver: Consultant to Cerenovus, Genentech, Medtronic, Stryker. Scott Brown: Consultant for Medtronic, Stryker, Cerenovus.
Authors’ contributions
The main author (RR) interpreted the data, drafted the original manuscript version, reviewed all suggestions provided by all co-authors, approved the final version, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. SB conducted all statistical analyses. DS, AY, MR, DA, JS, CS provided substantial contribution to the interpretation of the provided data. DS, AY, MR, DA, JS, CS also contributed with revisions to the original draft, approved the final version of the manuscript, and agreed to be accountable for all aspects of the work.
Supplemental material
Supplemental material for this paper can be found at the journal website: http://journals.sagepub.com/home/jcb
References
- 1.Liebeskind DS. Collateral circulation. Stroke 2003; 34: 2279–2284. [DOI] [PubMed] [Google Scholar]
- 2.Campbell BC, Christensen S, Tress BM, et al. Failure of collateral blood flow is associated with infarct growth in ischemic stroke. J Cereb Blood Flow Metab 2013; 33: 1168–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bang OY, Saver JL, Kim SJ, et al. Collateral flow predicts response to endovascular therapy for acute ischemic stroke. Stroke 2011; 42: 693–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bang OY, Saver JL, Kim SJ, et al. Collateral flow averts hemorrhagic transformation after endovascular therapy for acute ischemic stroke. Stroke 2011; 42: 2235–2239. [DOI] [PubMed] [Google Scholar]
- 5.Leng X, Fang H, Leung TW, et al. Impact of collateral status on successful revascularization in endovascular treatment: a systematic review and meta-analysis. Cerebrovasc Dis 2016; 41: 27–34. [DOI] [PubMed] [Google Scholar]
- 6.Liebeskind DS. Collateral perfusion: time for novel paradigms in cerebral ischemia. Int J Stroke 2012; 7: 309–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheth SA, Liebeskind DS. Collaterals in endovascular therapy for stroke. Curr Opin Neurol 2015; 28: 10–15. [DOI] [PubMed] [Google Scholar]
- 8.Davis MJ, Menon BK, Baghirzada LB, et al. Anesthetic management and outcome in patients during endovascular therapy for acute stroke. Anesthesiology 2012; 116: 396–405. [DOI] [PubMed] [Google Scholar]
- 9.Jagani M, Brinjikji W, Rabinstein AA, et al. Hemodynamics during anesthesia for intra-arterial therapy of acute ischemic stroke. J Neurointerv Surg 2015; 8: 883–888. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi CE, Brambrink AM, Aziz MF, et al. Association of intraprocedural blood pressure and end tidal carbon dioxide with outcome after acute stroke intervention. Neurocrit Care 2014; 20: 202–208. [DOI] [PubMed] [Google Scholar]
- 11.Desilles JP, Consoli A, Redjem H, et al. Successful reperfusion with mechanical thrombectomy is associated with reduced disability and mortality in patients with pretreatment diffusion-weighted imaging-alberta stroke program early computed tomography score </=6. Stroke 2017; 48: 963–969. [DOI] [PubMed] [Google Scholar]
- 12.Leslie-Mazwi TM, Hamilton S, Mlynash M, et al. Defuse 3 non-dawn patients. Stroke 2019; 50: 618–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chalumeau V, Blanc R, Redjem H, et al. Anterior cerebral artery embolism during thrombectomy increases disability and mortality. J Neurointerv Surg 2018; 10: 1057–1062. [DOI] [PubMed] [Google Scholar]
- 14.Simonsen CZ, Yoo AJ, Sorensen LH, et al. Effect of general anesthesia and conscious sedation during endovascular therapy on infarct growth and clinical outcomes in acute ischemic stroke: a randomized clinical trial. JAMA Neurol 2018; 75: 470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simonsen CZ, Sorensen LH, Juul N, et al. Anesthetic strategy during endovascular therapy: general anesthesia or conscious sedation? (goliath – general or local anesthesia in intra arterial therapy) a single-center randomized trial. Int J Stroke 2016; 11: 1045–1052. [DOI] [PubMed] [Google Scholar]
- 16.Rasmussen M, Espelund US, Juul N, et al. The influence of blood pressure management on neurological outcome in endovascular therapy for acute ischaemic stroke. Br J Anaesth 2018; 120: 1287–1294. [DOI] [PubMed] [Google Scholar]
- 17.Talke PO, Sharma D, Heyer EJ, et al. Republished: society for neuroscience in anesthesiology and critical care expert consensus statement: anesthetic management of endovascular treatment for acute ischemic stroke. Stroke 2014; 45: e138–150. [DOI] [PubMed] [Google Scholar]
- 18.Whalin MK, Lopian S, Wyatt K, et al. Dexmedetomidine: a safe alternative to general anesthesia for endovascular stroke treatment. J Neurointerv Surg 2014; 6: 270–275. [DOI] [PubMed] [Google Scholar]
- 19.Higashida RT, Furlan AJ, Roberts H, et al. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke 2003; 34: e109–137. [DOI] [PubMed] [Google Scholar]
- 20.Lowhagen Henden P, Rentzos A, Karlsson JE, et al. Hypotension during endovascular treatment of ischemic stroke is a risk factor for poor neurological outcome. Stroke 2015; 46: 2678–2680. [DOI] [PubMed] [Google Scholar]
- 21.Whalin MK, Halenda KM, Haussen DC, et al. Even small decreases in blood pressure during conscious sedation affect clinical outcome after stroke thrombectomy: an analysis of hemodynamic thresholds. AJNR Am J Neuroradiol 2017; 38: 294–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Treurniet KM, Berkhemer OA, Immink RV, et al. A decrease in blood pressure is associated with unfavorable outcome in patients undergoing thrombectomy under general anesthesia. J Neurointerv Surg 2017; 10: 107–111. [DOI] [PubMed] [Google Scholar]
- 23.Ostergaard L, Jespersen SN, Mouridsen K, et al. The role of the cerebral capillaries in acute ischemic stroke: the extended penumbra model. J Cereb Blood Flow Metab 2013; 33: 635–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dubin A, Pozo MO, Casabella CA, et al. Increasing arterial blood pressure with norepinephrine does not improve microcirculatory blood flow: a prospective study. Crit Care 2009; 13: R92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan SL, Sweet JG, Bishop N, et al. Pial collateral reactivity during hypertension and aging: understanding the function of collaterals for stroke therapy. Stroke 2016; 47: 1618–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haessler R, Madler C, Klasing S, et al. Propofol/fentanyl versus etomidate/fentanyl for the induction of anesthesia in patients with aortic insufficiency and coronary artery disease. J Cardiothorac Vasc Anesth 1992; 6: 173–180. [DOI] [PubMed] [Google Scholar]
- 27.Oglesby AJ. Should etomidate be the induction agent of choice for rapid sequence intubation in the emergency department? Emerg Med J 2004; 21: 655–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benson M, Junger A, Fuchs C, et al. Use of an anesthesia information management system (aims) to evaluate the physiologic effects of hypnotic agents used to induce anesthesia. J Clin Monit Comput 2000; 16: 183–190. [DOI] [PubMed] [Google Scholar]
- 29.Bell JD. In vogue: ketamine for neuroprotection in acute neurologic injury. Anesth Analg 2017; 124: 1237–1243. [DOI] [PubMed] [Google Scholar]
- 30.Perumal DK, Adhimoolam M, Selvaraj N, et al. Midazolam premedication for ketamine-induced emergence phenomenon: a prospective observational study. J Res Pharm Pract 2015; 4: 89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Astrup J, Symon L, Branston NM, et al. Cortical evoked potential and extracellular k+ and h+ at critical levels of brain ischemia. Stroke 1977; 8: 51–57. [DOI] [PubMed] [Google Scholar]
- 32.Powers WJ. Acute hypertension after stroke: the scientific basis for treatment decisions. Neurology 1993; 43: 461–467. [DOI] [PubMed] [Google Scholar]
- 33.Wintermark M, Flanders AE, Velthuis B, et al. Perfusion-ct assessment of infarct core and penumbra: receiver operating characteristic curve analysis in 130 patients suspected of acute hemispheric stroke. Stroke 2006; 37: 979–985. [DOI] [PubMed] [Google Scholar]
- 34.Cortijo E, Calleja AI, Garcia-Bermejo P, et al. Relative cerebral blood volume as a marker of durable tissue-at-risk viability in hyperacute ischemic stroke. Stroke 2014; 45: 113–118. [DOI] [PubMed] [Google Scholar]
- 35.Consoli A, Andersson T, Holmberg A, et al. Ct perfusion and angiographic assessment of pial collateral reperfusion in acute ischemic stroke: the capri study. J Neurointerv Surg 2016; 8: 1211–1216. [DOI] [PubMed] [Google Scholar]
- 36.Grubb RL, Jr, Raichle ME, Eichling JO, et al. The effects of changes in paco2 on cerebral blood volume, blood flow, and vascular mean transit time. Stroke 1974; 5: 630–639. [DOI] [PubMed] [Google Scholar]
- 37.Cohen ER, Ugurbil K, Kim SG. Effect of basal conditions on the magnitude and dynamics of the blood oxygenation level-dependent fmri response. J Cereb Blood Flow Metab 2002; 22: 1042–1053. [DOI] [PubMed] [Google Scholar]
- 38.Olsen TS, Larsen B, Herning M, et al. Blood flow and vascular reactivity in collaterally perfused brain tissue. Evidence of an ischemic penumbra in patients with acute stroke. Stroke 1983; 14: 332–341. [DOI] [PubMed] [Google Scholar]
- 39.Athiraman U, Sultan-Qurraie A, Nair B, et al. Endovascular treatment of acute ischemic stroke under general anesthesia: predictors of good outcome. J Neurosurg Anesthesiol 2017; 21: 655–659. [DOI] [PubMed] [Google Scholar]
- 40.Schonenberger S, Uhlmann L, Hacke W, et al. Effect of conscious sedation vs general anesthesia on early neurological improvement among patients with ischemic stroke undergoing endovascular thrombectomy: a randomized clinical trial. JAMA 2016; 316: 1986–1996. [DOI] [PubMed] [Google Scholar]
- 41.Schonenberger S, Pfaff J, Uhlmann L, et al. The impact of conscious sedation versus general anesthesia for stroke thrombectomy on the predictive value of collateral status: a post hoc analysis of the siesta trial. AJNR Am J Neuroradiol 2017; 38: 1580–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alexandrov AV, Sharma VK, Lao AY, et al. Reversed robin hood syndrome in acute ischemic stroke patients. Stroke 2007; 38: 3045–3048. [DOI] [PubMed] [Google Scholar]
- 43.McVerry F, Liebeskind DS, Muir KW. Systematic review of methods for assessing leptomeningeal collateral flow. AJNR Am J Neuroradiol 2012; 33: 576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Christoforidis GA, Mohammad Y, Kehagias D, et al. Angiographic assessment of pial collaterals as a prognostic indicator following intra-arterial thrombolysis for acute ischemic stroke. AJNR Am J Neuroradiol 2005; 26: 1789–1797. [PMC free article] [PubMed] [Google Scholar]
- 45.Al-Ali F, Jefferson A, Barrow T, et al. The capillary index score: rethinking the acute ischemic stroke treatment algorithm. Results from the borgess medical center acute ischemic stroke registry. J Neurointerv Surg 2013; 5: 139–143. [DOI] [PubMed] [Google Scholar]
- 46.Qureshi AI. New grading system for angiographic evaluation of arterial occlusions and recanalization response to intra-arterial thrombolysis in acute ischemic stroke. Neurosurgery 2002; 50: 1405–1414. discussion 1414–1405. [DOI] [PubMed] [Google Scholar]
- 47.Zaidat OO, Yoo AJ, Khatri P, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke 2013; 44: 2650–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raychev R, Jahan R, Saver JL, et al. Microcatheter contrast injection in stent retriever neurothrombectomy is safe and useful: insights from swift prime. J Neurointerv Surg 2018; 10: 615–619. [DOI] [PubMed] [Google Scholar]
- 49.Kim JT, Liebeskind DS, Jahan R, et al. Impact of hyperglycemia according to the collateral status on outcomes in mechanical thrombectomy. Stroke 2018; 49: 2706–2714. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Physiologic predictors of collateral circulation and infarct growth during anesthesia – Detailed analyses of the GOLIATH trial by Radoslav Raychev, David S Liebeskind, Albert J Yoo, Mads Rasmussen, Dimiter Arnaudov, Scott Brown, Jeffrey Saver and Claus Z Simonsen in Journal of Cerebral Blood Flow & Metabolism