Abstract
Stroke induces cardiac dysfunction which increases post stroke mortality and morbidity particularly in aging population. Here, we investigated the effects of inflammatory responses as underlying mediators of cardiac dysfunction after stroke in adult mice. Adult (eight-to-nine months) male C57BL/6 mice were subjected to photothrombotic stroke. To test whether immunoresponse to stroke leads to cardiac dysfunction, splenectomy was performed with stroke. Immunohistochemistry, flow cytometry, PCR, ELISA and echocardiography were performed. We found marginal cardiac dysfunction at acute phase and significant cardiac dysfunction at chronic phase of stroke as indicated by significant decrease of left ventricular ejection fraction (LVEF) and shortening fraction (LVSF). Stroke significantly increases macrophage infiltration into the heart and increases IL-1β, IL-6, MCP-1, TGF-β and macrophage-associated inflammatory cytokine levels in the heart as well as induces cardiac-fibrosis and hypertrophy. Splenectomy with stroke significantly reduces macrophage infiltration into heart, decreases inflammatory factor expression in the heart, decreases cardiac hypertrophy and fibrosis, as well as significantly improves cardiac function compared to non-splenectomized adult stroke mice. Therefore, cerebral ischemic stroke in adult mice induces chronic cardiac dysfunction and secondary immune response may contribute to post stroke cardiac dysfunction.
Keywords: Stroke, immune system, macrophages, cardiac dysfunction, splenectomy
Introduction
Stroke is a prominent cause of mortality and long-term disability worldwide, and is accompanied by high lifetime medical costs. Accumulating clinical and experimental evidence indicates that stroke may induce myocardial damage and arrhythmias.1–5 Stroke-induced cardiac damage may lead to fatality, lifelong cardiac disease, or lead to mild and recoverable damage such as neurogenic stress cardiomyopathy and Takotsubo cardiomyopathy.5 We have previously demonstrated that experimental stroke in young adult mice with no primary cardiac disease or underlying vascular pathologies induces cardiac abnormalities including functional changes such as decreased cardiac ejection fraction and morphological changes such as cardiomyocyte hypertrophy and interstitial fibrosis.6 Ischemia-like electrocardiography (ECG) changes and arrhythmias are frequently seen in stroke patients, even in those without symptoms or history of primary heart disease, which support a central nervous system origin of these cardiac abnormalities.7 Therefore, it is likely that there exists a causal relationship between brain damage and heart dysfunction after stroke.
Aging increases the risk of cardiac dysfunction after stroke.8 There have been reports that stroke-induced chronic cardiac dysfunction is related to sympathetic nerve activity.9 However, age-related brain–heart interaction is not simply confined to progressive sympathetic system impairment, and inflammation may play an important role in mediating cardiac dysfunction post stroke.10–12 Stroke induces cardiac damage, and a magnified cardiac response is characterized by excessive inflammatory cell infiltration and release of inflammatory mediators.10 Moreover, inflammation of the heart causes trans-differentiation of fibroblasts to myofibroblasts, thereby increasing collagen accumulation in the heart, a key part of adverse cardiac remodeling.13 Aging increases the number of macrophages in heart.14 Macrophages are the primary immune cells that reside in the heart under normal physiological conditions, are locally replenished via proliferation, and perform immunosurveillance of cardiac tissue.15 However, under pathological conditions such as myocardial ischemia, the majority of cardiac macrophages are derived from blood monocytes.15 Increased macrophage activation may promote cardiac dysfunction. Monocyte-chemoattractant protein-1 (MCP-1), is a potent chemo-attractant for monocytes, natural killer cells and T cells, as well as directly promotes cardiac fibrosis by increasing collagen expression.16 During the acute phase of cardiac infarction, monocytes infiltrate into the myocardium in response to upregulation of MCP-1.17 In addition, aging-associated stress also stimulates proinflammatory responses within the heart and large arteries.10,18 Myocardial MCP-1 is increased with advancing age and promotes hypertrophy and senescence/apoptosis.11 Thus, increased age drives chronic low-grade systemic and cardiac inflammation.19
In this study, using adult (eight-to-nine months) mice, we tested the effects of brain–heart interaction after stroke and investigated inflammation and immune responses mediated by the spleen as potential mechanisms of stroke-induced cardiac dysfunction.
Materials and methods
All experiments were conducted in accordance with, and approval of the National Institutes of Health guidelines for the Animal Care and Use Committee of Tianjin Medical University General Hospital. The research adheres to the Transparency and Openness Promotion Guidelines. Appropriate measures were taken to ensure minimal pain or discomfort in animals. The manuscript was prepared in accordance with the ARRIVE guidelines.
Animals and experimental groups
Adult (eight-to-nine months) male C57/BL6 mice were purchased from HFK Bioscience Corporation (Beijing, China). Mice were randomized and divided into different groups: (1) sham-control (no stroke, no splenectomy); (2) stroke alone; (3) stroke with splenectomy and (4) only splenectomy. Four sets of mice (n = 4–6/group) were prepared: one set was sacrificed at three days after stroke for flow cytometry; one set sacrificed at three days after stroke to harvest heart tissue for real-time PCR, ELISA and immunostaining, third set sacrificed at one month after stroke for immunostaining evaluation of brain and cardiac tissues and fourth set for blood pressure and body temperature measurements.
Photothrombotic stroke
A model of photothrombotic stroke was employed to induce cortical infarcts in mice, as previously described.20 The photothrombotic stroke model was chosen over the intraluminal middle cerebral artery occlusion (MCAo) stroke model in this study because: (1) The MCAo model may directly affect the insular cortex which then affects cardiac function.21 In our pilot studies, we have found that the MCAo model in 8–10-month-old mice induces significant cardiac deficits, but also induces a high mortality rate which hinders long term cardiac function measurement. (2) The photothrombotic stroke model produces infarction of small size with well-delimited boundaries in the cortex and is minimally invasive without any mechanical manipulations with blood vessel and does not affect the insular cortex.20,22 (3) Mice subjected to the photothrombotic stroke model survive longer than mice subjected to the MCAo model and this permits us to investigate the chronic cardiac function after stroke. Briefly, mice were anesthetized with chloral hydrate (0.3 mg/kg, intraperitoneal injection). A concentration of 10 mg/mL solution of Rose Bengal dye (Sigma Aldrich, St Louis, MO) in saline was prepared and 150 µL administered via intraperitoneal injection 5 min prior to cold light exposure. The skull was exposed by a midline incision to the right side of the scalp. A roundabout rubber was placed on the skull surface to expose 0.7–2.7 mm right to the midline, −2.5 to 1 mm rostral to the bregma and illuminated for 15 min with a fiber-optic bundle of a cold light source (KL 1600 LED; Schott, Mainz, Germany). The incision was sutured and mouse moved to its home cage to recover. Sham-control mice underwent the same procedure without Rose Bengal dye injection or splenectomy.
Splenectomy
Splenectomy was performed prior to stroke induction. The splenic artery, veins, and nerves were ligated, and the spleen was removed through a small lateral peritoneal incision, as previously described.23 Photothrombotic stroke surgery was performed immediately after splenectomy in the stroke with splenectomy group.
Echocardiographic measurements
Animals were anesthetized with 1.5%–2.0% isoflurane administered via a face mask, and the chest was shaved and placed in the supine position on a 37 ℃ heating pad. Left ventricular cardiac function was measured by echocardiography at three days and one month after stroke using a Vevo 2100 ultra-high resolution small animal ultrasound imaging system in real time (VisualSonicsVevo 2100, Canada) with an MS-250 ultrasound scanning transducer (model C5) following previously described methods.24 The left ventricles, long axis Doppler mode images and M mode were recorded. The following parameters of left ventricular function and structure were recorded: left ventricular ejection fraction (LVEF), left ventricular shortening fraction (LVSF), interventricular septum thickness diastolic (IVS;d), interventricular septum thickness diastolic (IVS;s), left ventricular internal diameter diastolic (LVID;d), left ventricular internal diameter systolic (LVID;s), left ventricular posterior wall thickness diastolic (LVPW;d), left ventricular posterior wall thickness systolic (LVPW;s), left ventricular volume diastolic (LV VOL;d), left ventricular volume systolic (LV VOL;s). LVEF was measured using the formula: LVEF=[(LV VOL;d- LV VOL;s/ LV VOL;d × 100], LVSF=[(LVID;d-LVID;s)/LVID;d×100]. Echocardiography was performed by an investigator blinded to the experimental groups. All primary measurements were digitized by goal-directed, diagnostically driven software and three beats were averaged for each measurement.
Neurological function tests
An investigator who was blinded to the experimental groups performed a battery of functional tests, including modified neurological severity score25 and foot-fault test,26 prior to stroke and on days 1, 3, 7, 14, 21, 28 after stroke.
Quantitative real-time PCR
At three days after stroke, total RNA was isolated from heart with TRIzol reagent (Invitrogen) and quantified by ultraviolet spectrophotometry at 260/280 nm. Trans-Script First-Strand cDNA Synthesis SuperMix Kit (Transgen) was used to transcribe complementary DNA (cDNA). PCR was performed on an Opticon 2 Real-Time PCR Detection System (BioRad, Hercules, CA, USA) with the primers and SYBR green PCR Master Mix (Roche Diagnostics, Basel, Switzerland).Samples were conducted in duplicate and normalized to GAPDH using the 2–ΔΔCt method. The expression levels of mRNAs were calculated as fold changes vs. control. The following primer sequences were used:
ED-1: FWD:GAAGGAAAGAGCTGAAGAGCAG;
REV:AGGTTTAGGAGAGGGTTTCCAC;
MCP-1: FWD: CTGCTACTCATTCACCAGCAAG;
REV:CTCTCTCTTGAGCTTGGTGACA;
IL-1β: FWD:TCCAGGATGAGGACATGAGCAC;
REV:GAACGTCACACACCAGCAGGTTA;
IL-6: FWD:TGATGCACTTGCAGAAAACA;
REV:ACCAGAGGAAATTTTCAATAGGC;
IL-10: FWD: GTACAGCCGGGAAGACAATAAC
REV: GCATTAAGGAGTCGGTTAGCAG
GAPDH: FWD: GCCAAGGCTGTGGGCAAGGT;
REV: TCTCCAGGCGGCACGTCAGA.
Flow cytometry
Mice were sacrificed at three days after stroke and peripheral blood and heart tissue harvested and single cell suspensions prepared. Briefly, for each animal, the heart was excised and minced with a fine scissor prior to digestion in 125 U/ml collagenase II, 80 U/ml DNase I and 60 U/ml hyaluronidase (Sigma-Aldrich) for 1 h at 37 ℃. Tissues were triturated and cells filtered through 40 µm nylon mesh (BD Falcon), washed and centrifuged (10 min, 350 g, 4 ℃). The pellet was resuspended in 5 ml of 30% Percoll (GE Healthcare Bio Science AB, Uppsala, Sweden) and centrifuged at 700 × g for 10min. Cells were collected on the bottom and resuspended with 1% BSA. For blood samples, the mononuclear cells were isolated from the whole blood of angular vein. The cell suspensions were stained with fluorochrome and biotin-conjugated antibodies. Fluorochrome- and biotin-conjugated antibodies specific to mouse CD45 (30-F11), CD11b (M1/70) and F4/80 (BM8) were used. CD45 is a marker of leukocyte. Activated macrophages/monocytes were identified as CD45+CD11b+F4/80 + cells. Fluorescence minus one (FMO) controls were stained, respectively. Flow cytometry data were obtained on a FACSAria™ flow cytometer (BD Biosciences, San Jose, CA, USA) and analyzed with FlowJo software version 7.6.1.
Immunohistochemistry
Mice were anesthetized and transcardially perfused with cold PBS. Brain and heart were isolated and fixed in 4% paraformaldehyde before being embedded in paraffin. Heart coronal sections (6 µm thick) were cut and PicroSirius Red (PSR) staining was employed to assess myocyte cross-sectional area (MCSA)27 and interstitial collagen fraction measurement.28 Antibodies against monocyte chemotactic protein-1 (MCP-1, Abcam, 1:150), transforming growth factor (TGF-β; 1:250, Santa Cruz), CD45 (1:500, Abcam), Natural Killer Cell (NK cells, 1:1000, Abcam), CD3 (T cell marker, 1:500, Abcam) and IBA1 (1:1000, Wako) were employed. DAPI counter stain was used to stain nuclei in immunofluorescent staining. Three slides from each heart, with each slide containing 5 fields of view were digitized under 20 × magnification using a light microscope (Olympus, Tokyo, Japan) or fluorescence microscope. Positive areas of PSR, MCSA, and the number of positive cells of MCP-1, TGF-β, IBA1, NK and CD3 were calculated by Image Pro Plus 6.0. Immunohistochemical analysis was performed by an investigator who was blinded to the experimental groups.
Lesion volume measurement
Brain coronal sections (6 µm thick) were cut from a standard block obtained from the center of the lesion (bregma −2.5mm∼+1 mm). Seven coronal sections of tissue were stained with hematoxylin and eosin (H&E) for lesion volume calculation and presented as a percentage of lesion compared with the contralateral hemisphere. Measurements were performed by an investigator who was blinded to the experimental groups.
Blood pressure and body temperature measurements
To test whether stroke and/or splenectomy regulate blood pressure, diastolic arterial pressure (DAP), mean arterial pressure (MAP) and systolic arterial pressure (SAP) were measured in all four groups (sham-control, Spx-alone, stroke alone and stroke+Spx) by tail-cuff method (CODA 8-Channel High Throughput Non-Invasive Blood Pressure system, KENT scientific) on day 28 after stroke. The mice were habituated for 2–3 min in plastic restrainers for seven consecutive days before experiments were performed. Body temperature was maintained at 37 ℃ using a warming pad. Blood pressure was recorded and averaged over 15 consecutive readings. Body core temperature was monitored before stroke or splenectomy and on days 1, 3, 14, 21, and 28 days after stroke or/and splenectomy using a rectal temperature probe coupled to a data-acquisition system (ADInstruments) in awake mice. In order to rule out biorhythm, the core temperature was recorded at 2–4 pm.
ELISA
Protein from mouse hearts was isolated using TriZol (Thermo FisherSci) following standard protocol. Once the protein pellets from the TriZol isolation were resuspended in 1% SDS, 150 ug of protein/well was used in duplicate wells to run an MCP-1 ELISA (Thermo Fisher Sci, BMS6005) following standard protocol.
Statistical analysis
To test the main effect of group difference (1m-stroke vs. 1m-spx-stroke) in functional test, the repeated measure analysis of variance (ANCOVA) was used to study the difference in mNSS functional test and foot-fault functional test over time (time points: 1, 3, 7, 14, 21, and 28 days).
The one-way analysis of variance (ANOVA) was considered and analysis started testing the overall group effect using F-test, followed by Tukey's honest significance test for pairwise group comparisons on each outcome of interest including LVEF, LVFS, LV Vol;d, LV Vol;s, IVS;d, IVS;s, LVID;d, LVID;s, LVPW;d, LVPW;s, LV mass, fibrosis, heart weight, MCSA, MCP-1, IL-1β, IL-6, IL-10, ED-1, IL-10, lesion volume, DAP, SAP, MAP, TGF-β, flow cytometry, CD45, IBA-1, CD3 and NK cells at three days and/or one month mice, respectively. A significant pairwise group difference was detected if both the overall group and pairwise group comparison were significant with p value < 0.05, respectively. All the analyses were conducted without adjusting for multiple endpoints given this was a proof-of-concept study. All data are expressed as mean±SD.
Results
Cerebral ischemic stroke induces progressive cardiac dysfunction in adult mice, while splenectomy prior to stroke reduces cardiac damage without affecting blood pressure and body temperature
To test the effects of stroke on heart function, echocardiography was performed on days 3 and 1 month after stroke. Figure 1(a) and (b) and Table 1 show that stroke induces marginal cardiac dysfunction at three days after stroke, and significant cardiac dysfunction and LV enlargement at one month after stroke identified by decreased LVEF as well as increased LVID;s and LVVOL;s compared to sham-control mice. Splenectomy alone does not induce any significant changes in heart size or cardiac function.
Figure 1.
Splenectomy with stroke attenuates cerebral ischemic stroke-induced cardiac dysfunction in adult mice. (a) Representative images of echocardiography from sham control, splenectomy alone, stroke alone and stroke with splenectomy groups at one month after stroke in adult mice. (b) Echocardiography data at three days and one month after stroke shows that stroke induces marginal cardiac dysfunction at acute stage and significant cardiac dysfunction at one month after stroke Splenectomy with stroke in adult mice significantly improves cardiac function as indicated by increased LVEF and LVSF. The respective F values are 2.4, 2.92, 9.2, 7.96, sample size of n = 9/group was employed for all groups except 1m-spx n=6, 1m-spx-stroke n = 8, *p < 0.05, One-way ANOVA (Tukey). (c) Stroke or splenectomy with stroke does not alter blood pressure or body temperature compared to sham control mice. The respective F values were: 0.6, 0.85, 0.69. Sample size: control n = 5, 1m-spx n = 5, 1m-stroke n = 5, 1m-spx-stroke n = 4, *p < 0.05, One-way ANOVA (Tukey). (d) Splenectomy prior to stroke significantly improves neurological function (n = 9/group, ANCOVA, *p < 0.05) and decreases lesion volume (n = 6/group, *p < 0.05, One-way ANOVA (Tukey)) at one month after stroke. The respective F values are 27.59, 37.27, 0.6, 0.85, 0.64.
Table 1.
Echocardiography measurements at three days and one month after stroke in adult mice.
| Group | IVS;d (mm) | IVS;s (mm) | LVID;d (mm) | LVID;s (mm) | LVPW;d (mm) | LVPW;s (mm) | LV Vol;d (ul) | LV Vol;s (ul) |
|---|---|---|---|---|---|---|---|---|
| 3d-Control | 0.52 ± 0.03 | 0.87 ± 0.03 | 3.91 ± 0.12 | 2.629 ± 0.12 | 0.76 ± 0.03 | 1.06 ± 0.04 | 67.16 ± 4.76 | 26.05 ± 2.71 |
| 3d-Spx | 0.65 ± 0.02 | 1.02 ± 0.05 | 3.91 ± 0.11 | 2.63 ± 0.16 | 0.86 ± 0.03 | 1.21 ± 0.05 | 67.14 ± 4.23 | 26.64 ± 3.75 |
| 3d-Stroke | 0.581 ± 0.03 | 0.88 ± 0.04 | 3.89 ± 0.13 | 2.80 ± 0.13 | 0.79 ± 0.05 | 1.07 ± 0.05 | 66.63 ± 5.17& | 30.32 ± 3.28& |
| 3d-Spx-Stroke | 0.69 ± 0.04 | 0.93 ± 0.06 | 3.51 ± 0.10 | 2.27 ± 0.14 | 0.82 ± 0.05 | 1.22 ± 0.06 | 61.52 ± 1.59 | 22.59 ± 1.47 |
| 1m-Control | 0.52 ± 0.03 | 0.87 ± 0.03 | 3.91 ± 0.12 | 2.629 ± 0.12 | 0.76 ± 0.03 | 1.06 ± 0.04 | 67.16 ± 4.76 | 26.05 ± 2.71 |
| 1m-Spx | 0.55 ± 0.04$ | 0.98 ± 0.06 | 3.87 ± 0.12 | 2.57 ± 0.21$ | 0.79 ± 0.05 | 1.07 ± 0.05 | 66.24 ± 4.96 | 25.87 ± 4.67$ |
| 1m-Stroke | 0.55 ± 0.02 | 0.79 ± 0.02 | 4.26 ± 0.09 | 3.27 ± 0.09* | 0.71 ± 0.02 | 0.92 ± 0.05 | 81.60 ± 4.11 | 43.66 ± 2.93* |
| 1m-Spx-Stroke | 0.55 ± 0.02 | 0.86 ± 0.04 | 3.78 ± 0.12 | 2.72 ± 0.12# | 0.72 ± 0.01 | 1.03 ± 0.04 | 62.40 ± 4.91 | 28.55 ± 3.2# |
Note: Compared to sham control mice, stroke mice exhibit significant cardiac dysfunction at three days and one month after stroke. LV enlargement is significantly higher at one month compared to three days after stroke. Splenectomy with stroke significantly attenuates stroke-induced cardiac dysfunction at one month after stroke in adult mice. Splenectomy in sham control does not induce any significant changes in cardiac function. Spx: splenectomy; IVS;d: interventricular septum thickness diastolic, IVS;s: interventricular septum thickness systolic, LVID;d: left ventricular internal diameter diastolic, LVID;s: left ventricular internal diameter systolic, LVPW;d: left ventricular posterior wall thickness diastolic, LVPW;s: left ventricular posterior wall thickness systolic, LV Vol;d: left ventricular volume diastolic, LV Vol;s: left ventricular volume systolic. *p < 0.05 vs. Control; $p < 0.05 vs. 1m-stroke; #p < 0.05 vs. 1m-Stroke; &p < 0.05 vs. 1m-Stroke; One-way ANOVA (Tukey). The respective F values are 10.31, 5.17, 3.62, 7.94, 4.84, 5.41, 2.97, 4.64.
To suppress immune response, splenectomy was performed prior to stroke induction. Compared to stroke alone mice, splenectomy with stroke significantly attenuates cardiac dysfunction indicated by increased LVSF at acute stage of stroke, and significantly increases LVEF at chronic stage of stroke and decreases LVID and LVVOL at both acute and chronic stage of stroke. The data indicate that cerebral ischemic stroke induces significant and progressive cardiac dysfunction, while splenectomy reduces stroke-induced cardiac dysfunction in adult mice.
To test if stroke with or without splenectomy induces any hemodynamic changes and thereby affects cardiac function, we measured blood pressure and body core temperature at one month after stroke. Figure 1(c) shows that there were no significant changes among the four groups (sham control, stroke alone, splenectomy alone and stroke with splenectomy) in mean arterial BP, diastolic BP or systolic BP and body temperature. There were no mortalities in any of the four groups.
Splenectomy prior to stroke significantly decreases lesion volume and improves neurological function at one month after stroke
To test the effect of splenectomy on stroke outcome, we performed a battery of neurological function tests and measure lesion volume at one month after stroke. Figure 1(d) shows that splenectomy with stroke significantly improves neurological function indicated by lower mNSS and foot-fault score as well as decreases cerebral ischemic lesion volume compared to stroke mice without splenectomy.
Splenectomy reduces stroke-induced chronic heart damage
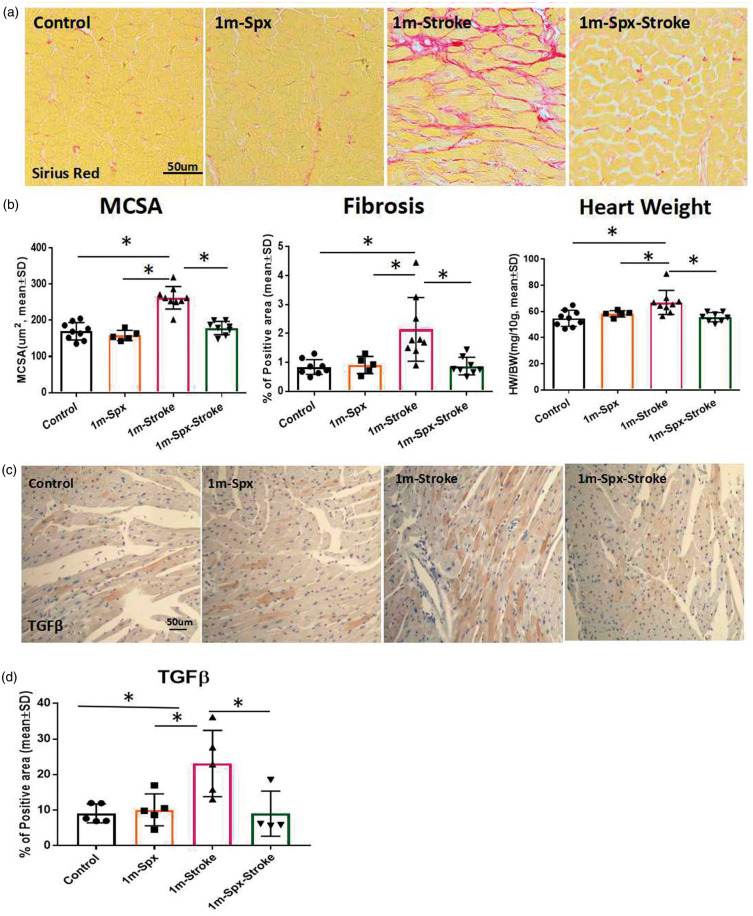
To test whether stroke affects cardiac hypertrophy, we measured heart weight, cardiac fibrosis and cardiomyocyte size at one month after stroke. Cardiac fibrosis is evident in the myocardial interstitium in the stroke group. Figure 2(a) and (b) shows that stroke significantly increases cardiac interstitial fibrosis and cardiomyocyte size compared to sham-control mice. We found a significant increase in heart weight after stroke compared with non-stroke control mice. These data indicate that stroke induces myocyte hypertrophy and interstitial fibrosis. However, stroke mice with splenectomy exhibited significantly decreases heart weight, hypertrophy and fibrosis compared to adult stroke mice. Splenectomy alone group does not increase heart weight, hypertrophy or fibrosis.
Figure 2.
Splenectomy with stroke decreases chronic cardiac fibrosis and hypertrophy in adult mice. (a) Representative images for PicroSirius Red immunostaining in heart tissue at one month after stroke. (b) Splenectomy with stroke in adult mice significantly attenuates stroke induced cardiomyocyte hypertrophy (MCSA), interstitial fibrosis (Sirius red), and increased heart weight at one month after stroke in adult mice. The respective F values are 31.87, 8.35, 6.65. Sample size: Control n = 9, 1m-Spx n = 5, 1m-Stroke n = 9, 1m-spx-stroke n = 8, *p < 0.05, One-way ANOVA (Tukey). (c) TGF-β immunostaining and (d) quantification data indicate that splenectomy with stroke in adult mice significantly decreases stroke-induced TGF-β expression in heart. F value = 6.023. Sample size: Control n = 5, 1m-Spx n = 5, 1m-Stroke n = 5, 1m-spx-stroke n = 4, *p < 0.05, One-way ANOVA (Tukey).
TGF-β plays an important role in mediating cardiac fibrosis.29 TGF-β immunostaining and quantification data obtained at one month after stroke indicate that stroke significantly increases TGF-β expression in heart compared to sham-control mice, while splenectomy with stroke significantly decreases TGF-β expression compared to non-splenectomy stroke mice (Figure 2(c) and (d)).
Splenectomy with stroke decreases stroke-induced cardiac inflammatory responses during acute phase of stroke
Stroke is known to cause systemic inflammation, therefore, to test whether stroke induces cardiac inflammation at an acute time point after stroke, inflammatory factor (IL-1β, IL-6, MCP-1 and ED1) and anti-inflammatory factor IL-10 gene expression were measured by PCR in heart tissue harvested at three days after stroke. Figure 3(a) to (c) shows that the gene expression of inflammatory cytokines IL-1β, IL-6 and MCP-1 was significantly increased in stroke mice compared to sham-control mice. Meanwhile, splenectomy with stroke significantly reduces IL-6, IL-1β and MCP-1 gene expression compared to stroke alone mice. However, stroke with or without splenectomy does not significantly affect IL-10 gene expression at three days after stroke compared to sham control mice (Figure 3(e)). Figure 3(d) shows that macrophage marker ED1 expression is significantly increased in the heart after stroke and decreased significantly in stroke mice with splenectomy. Therefore, to test whether stroke induces changes in macrophages, flow cytometry was employed.
Figure 3.
Splenectomy with stroke decreases stroke-induced acute cardiac inflammatory factor expression. PCR results indicate that splenectomy with stroke significantly decreases stroke-induced inflammatory factor gene expression such as (a) IL-1β, (b) IL-6, (c) MCP-1 and (d) ED1 compared to sham-control mice. However, stroke with or without splenectomy does not significantly alter (e) IL-10 gene expression at three days after stroke compared to sham control mice. F values are 7.66, 9.07, 22.96, and 17.09, respectively. Sample size: Control n = 6, 3d-Spx n = 4, 3d-stroke n = 6, 3d-Spx stroke n = 6; *p < 0.05, One-way ANOVA (Tukey).
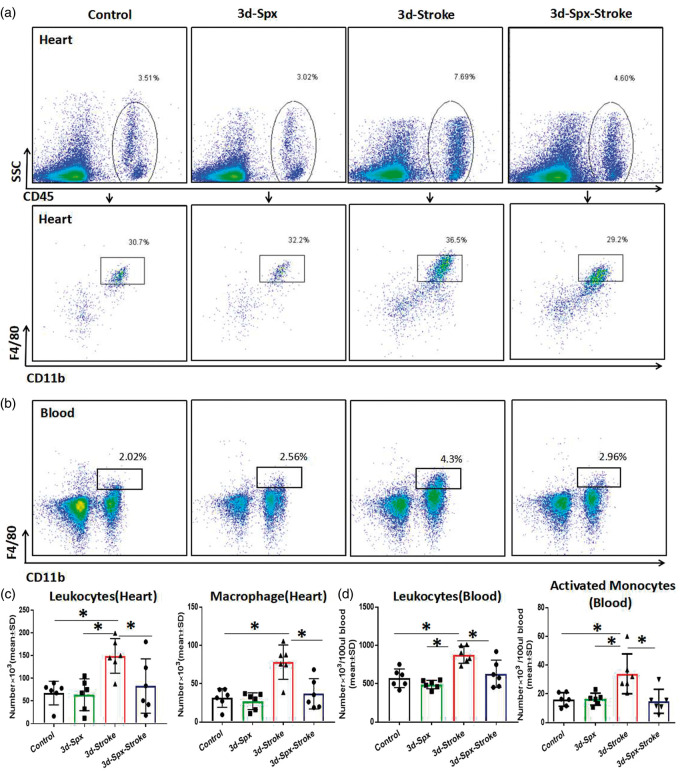
Splenectomy with stroke decreases stroke-induced inflammatory cell infiltration into heart during acute phase of stroke
CD45 is a leukocyte marker and macrophages are identified as cells with dual expression of CD11b and F4/80 surface markers.30 When monocytes migrate into heart, they become macrophages. To determine whether splenectomy regulates macrophages (CD45+CD11+bF4/80+) in heart, macrophages count were measured by flow cytometry. As shown in Figure 4(a) to (c), heart tissue harvested at three days after stroke exhibit a significantly increase in macrophages expression compared to sham control and splenectomy only mice. Splenectomy with stroke significantly reduced macrophages expression in the heart compared to stroke control mice. Stroke mice also exhibit a significant increase in circulating leukocytes and activated monocytes (CD11b+F4/80+) compared with sham-control and splenectomy only group (Figure 4(b) and (d)). Figure 4 also shows that stroke mice with splenectomy exhibit significantly reduced macrophage (CD11b+F4/80+) infiltration into heart as well as decreased circulating activated monocytes number compared to stroke mice. We found that splenectomy alone does not induce any significant differences in leukocytes and macrophage infiltrating into heart and circulation leukocytes and activated monocytes (Figure 4).
Figure 4.
Splenectomy with stroke decreases invasion of immune cells into heart. (a–b) Representative flow cytometry plots show the gating strategy of CD45 + leukocytes population and CD45+CD11b+F4/80 + macrophage population in heart, and activated monocytes (CD11b+F4/80+) in blood. (c) Splenectomy with stroke significantly decreases infiltration of leukocytes and macrophages into heart, compared to stroke alone mice. F values are 5.49, 11.42, respectively. (d) Splenectomy with stroke significantly decreases the leukocytes and activated monocytes in blood, compared to stroke alone mice. F values are 10.84 and 5.64, respectively. N=6/group, *p < 0.05, One-way ANOVA (Tukey).
To determine whether splenectomy regulates peripheral monocyte number (gated by their characteristic size and granularity in whole blood), blood monocyte count before and after splenectomy was measured by flow cytometry. We found that splenectomy alone did not induce any significant differences in peripheral monocyte number prior to and three days after splenectomy (Supplementary Figure 1).
To test whether stroke regulates other inflammatory cell infiltration into the heart tissue, NK cell and T cell immunostaining was employed. Stroke does not significantly increase NK and T cell number in the heart compared to sham control mice (Supplementary Figure 2). These data suggest that inflammation, especially macrophages, may play an important role in brain–heart interaction after stroke in adult mice.
Stroke induced increase of macrophage infiltration persist from acute to chronic phase of stroke
At the acute phase of stroke, augmented cardiac infiltration of leukocytes and macrophages was further confirmed by CD45 and IBA-1 immunostaining (Figure 5(a) and (b)), which revealed a significant increase in leukocytes and macrophages in heart tissue compared with sham control mice. Splenectomy with stroke significantly decreases leukocyte and macrophage number in heart after stroke. In the chronic phase of stroke, infiltrating macrophages in heart are still significantly increased compared to sham group, but less than three days after stroke (p < 0.05). Although stroke with splenectomy mice have reduced inflammatory cell infiltration into heart than stroke alone mice, there were no significant differences between chronic stroke and chronic stroke with splenectomy mice.
Figure 5.
Stroke induces macrophage infiltration into heart which persists from acute to chronic phase of stroke. (a) CD45 immunostaining and quantification data indicate that stroke significantly increases CD45 + cells in heart at three days and one month after stroke compared to sham control and splenectomy only group, respectively. F value=8.61. (b) IBA-1 immunostaining and quantification data indicate that stroke significantly increases IBA-1 positive macrophages in heart at three days as well as one month after stroke compared to sham control mice. Splenectomy with stroke significantly decreases IBA-1 positive macrophages at three days after stroke. F value=6.25. Sample size: Control n = 5, 3d-spx=4, 3d-stroke n = 5, 3d-spx-stroke n = 5, 1m-spx n = 5, 1m-stroke n = 5, 1m-spx-stroke=4. *p < 0.05, One-way ANOVA (Tukey).
MCP-1 immunofluorescence staining and quantification data obtained at three days and one month after stroke indicate that stroke increases MCP-1 expression in heart compared to sham-control mice, while splenectomy with stroke significantly decreases MCP-1 expression compared to stroke only mice (Figure 6(a) to (c)). It has been previously reported that MCP-1 is not only expressed in inflammatory cell, but also co-localizes with cardiomyocytes.31–33 We found that MCP1 is primarily expressed in cardiomyocyte in the heart tissue at one month after stroke as shown in Supplementary Figure 3. To confirm the immunostaining data, MCP-1 ELISA was performed. We found that stroke with splenectomy significantly decreased MCP-1 protein level compared to stroke alone mice at three days and one month after stroke (Figure 6(d)). These data indicate that immunoresponse may regulate cardiac pathological remodeling after stroke.
Figure 6.
Splenectomy with stroke decreases stroke-induced cardiac MCP-1 expression. Representative images for MCP-1 immunostaining in heart tissue at (a) three days and (b) one month after stroke and (c) quantification data. Stroke significantly increases cardiac MCP-1 expression compared to sham control mice as well as splenectomy alone mice. MCP-1 expression is significantly greater at one month after stroke compared to three days after stroke. Splenectomy with stroke significantly decreases MCP-1 expression compared to stroke alone group at three days as well as one month after stroke. F value = 28.54. Sample size: Control n = 6, 3d-spx=5, 3d-stroke n = 6, 3d-spx-stroke n = 6, 1m-spx n = 5, 1m-stroke n = 6, 1m-spx-stroke=6. *p < 0.05, One-way ANOVA (Tukey). (d) ELISA data indicate that splenectomy with stroke significantly decreases MCP-1 expression compared to stroke alone group at three days as well as one month after stroke in adult mice.
Discussion
In this study, we found that ischemic stroke in adult mice induces chronic heart dysfunction which may be attributed to macrophage infiltration and increased inflammatory factor expression in the heart. Splenectomy prior to stroke significantly decreases stroke-induced inflammatory cell infiltration and inflammatory factor expression in the heart and improves cardiac function as well as neurological functional outcome in adult mice. These data suggest that inflammation may contribute to the progression of cardiac dysfunction after stroke in adult mice.
Stroke induces progressive cardiac dysfunction in adult mice
Stroke can cause cardiac dysfunction even in the absence of risk factors and pre-existing heart disease, especially in stroke patients of advanced age.1–7,34 Therefore, investigating cardiac dysfunction after stroke in the older population is important and clinically relevant. Our previous study found that stroke induces mild chronic heart damage in healthy, young mice subject to stroke.6 However, this is not a clinically relevant population. Therefore, in our present study, we have employed adult (eight-to-nine months old) mice and investigated acute and chronic effects of stroke on cardiac function. Our results indicate significant cardiac dysfunction at three days after stroke without evident cardiac pathological remodeling. At one month after stroke, we found that stroke induces significant cardiac dysfunction which was worse compared to three days after stroke, as well as myocardial pathological remodeling. Myocardial pathological remodeling is characterized by rearrangement of the normal heart chamber wall, which includes cardiac hypertrophy, myocardial interstitial fibrosis, and apoptosis and necrosis.35 Myocardial interstitial fibrosis is an important and common pathological process that is closely related to rheumatic heart disease, hypertension, cardiac dysfunction, arrhythmia and sudden cardiac death, as well as reduces the ventricular systolic and diastolic compliance, damages the electrical coupling between myocardial cells, reduces capillary density, increases the oxygen diffusion distance of myocardial cells, and ultimately affects ventricle function.36 Myocardial interstitial fibrosis plays a crucial role in the change of cardiac function from compensatory to decompensatory, and in myocardial remodeling from reversible to irreversible. Our data show that stroke in adult mice induces chronic heart pathological remodeling, demonstrated by increased heart weight, LVVOL, cardiac interstitial fibrosis and hypertrophy without affecting blood pressure compared to control mice.
In the heart, TGF-β plays an important role in mediating cardiac fibrosis by regulating differentiation, migration, and collagen secretion of cardiac myofibroblasts.29 TGF-β is an important mediator of the hypertrophic growth response of the heart to Ang-II and decrease in TGF-β attenuates cardiac hypertrophy.37 Myocardial TGF-β expression is significantly increased under pathological conditions such as myocardial infarction, cardiac hypertrophy, and hypertrophic cardiomyopathy. Increased cardiac expression of TGF-β is associated with increased interstitial cardiac fibrosis and cardiac hypertrophy in mice.38,39 In addition, TGF-β overexpression can also increase myocardial stiffness and induce diastolic dysfunction.38 Our data indicate that stroke increases TGF-β expression in heart which may be mediated by splenic immune response and contribute to observed cardiac remodeling and functional deficits.
Stroke induces inflammatory responses in heart tissue
Many factors including post-stroke inflammation and immune response may mediate brain–heart interaction after stroke.5 Macrophages are the most prominent population among cardiac leukocytes.15 Macrophages help maintain cardiovascular health; however, activation of their inflammatory actions can promote heart disease.40,41 Macrophages expand in mice and humans with diastolic dysfunction, particularly in aged mice.42 Macrophages can stimulate myocardial fibroblasts and induce cardiac fibrosis.43 Cardiac fibrosis may cause systolic dysfunction through several distinct mechanisms, including impaired force generation by myocytes,44,45 disrupted normal coordination of myocardial excitation-contraction coupling,46 and an asynchronous contraction of the myocardium.47 Our data show that macrophages were significantly increased at an early stage after stroke compared with the non-stroke sham control. Cardiac macrophage expansion was associated with MCP-1, which promotes the migration of monocytes into the heart. Myocardial macrophage, the number of circulating activated monocytes and MCP-1 expression were significantly increased after stroke in adult mice compared to sham control. These results indicate that macrophages may play an important role in mediating cardiac dysfunction after stroke.
Immune response mediated by the spleen contributes to cardiac dysfunction after stroke in adult mice
In our present study, we found that splenectomy alone did not induce cardiac dysfunction at one month after surgery as measured by LVEF and LVSF, and did not increase cardiac fibrosis compare to sham control mice. However, splenectomy with stroke significantly attenuates stroke-induced cardiac dysfunction and inflammation in the heart, as well as reduced heart weight, remission of myocardial hypertrophy, and reduction of myocardial fibrosis compared with non-splenectomized stroke animals at one month after stroke. Our data suggest that chronic cardiac damage is inhibited by reduction of macrophage infiltration and anti-inflammatory effects of splenectomy.
Sager et al.48 found that myocardial infarction increased macrophage through both increased resident macrophage proliferation (about 9%) and monocyte/macrophage recruitment (1/3).48 Macrophages participate in the immunosurveillance of myocardial tissue.49 Except the resident macrophages (about 6–8% of the non-cardiomyocyte population in the healthy mouse myocardium),49 the spleen is the largest secondary lymphoid organ in the body, and serves as the major and immediate source of monocyte/macrophage deployment following injury.50,51 Increased macrophage density in heart is caused by monocyte recruitment and hematopoiesis activation in spleen and bone marrow.42 Activated splenic immune cells can traffic into the damaged heart and exhibit immune memory and are primed to induce tissue injury that promotes pathological cardiac remodeling.52 MCP-1 signaling pathway mediates cardiac macrophage expansion through recruitment of monocyte/macrophage.48 Our data are consistent with other publications that splenectomy alone does not significantly decrease peripheral monocytes.53 Compared to sham control but splenectomy with stroke significantly attenuates stroke-induced monocytes/macrophage infiltration into heart, and decreases MCP-1 expression in the heart tissue as well as improves cardiac function after stroke in adult mice. Therefore, the improved cardiac function after splenectomy is most likely not simply related to changes in circulating inflammatory cell number, but rather related to the loss of splenic mobilization and subsequent diminished tissue infiltration of inflammatory cells.54
MCP-1 plays an important role in a variety of pathophysiological processes in cardiovascular biology.31 However, why splenectomy causes a significant decrease in MCP-1 expression is not clear. Previous studies have found that inflammatory cytokines (IL-1 and TNF-α) stimulate cultured human cardiac cells expression of MCP-1.31 Oxidative stress is known to increase MCP-1 secretion from cardiomyocytes as well as monocytes.31–33 We found that stroke not only increases MCP-1 expression in monocytes/macrophages, but also in cardiomyocytes, which is consistent with previous studies showing that myocarditis increases cardiomyocyte MCP-1 expression.31 Damaged cardiomyocytes can express MCP-1 which promotes recruitment of monocytes into the heart.55 The recruited inflammatory cells can also secrete MCP1 in the heart and exacerbate myocardial damage, resulting in a cascade of inflammatory reactions. Brain-injury can induce cardiac oxidative stress which can injure cardiomyocytes.5,56,57 Splenectomy not only reduces monocyte infiltration into heart, but also reduces heart tissue oxidative stress and cardiomyocyte damage.56 Thus, reduced monocyte infiltration, oxidative stress and myocardial damage may play a role in MCP-1 reduction after splenectomy.
In this study, we also found that stroke significantly increased pro-inflammatory factors IL-1β and IL-6 expression, but did not regulate anti-inflammatory factor IL-10 expression in the heart. Splenectomy significantly attenuated stroke-induced pro-inflammatory factor IL-1β and IL-6 expression in the heart compared to stroke alone mice. Our data suggest that inflammation may contribute to stroke-induced cardiac deficits. In addition, we also found that splenectomy prior to stroke significantly decreases cerebral lesion volume compared to stroke control in adult mice. Therefore, we cannot exclude the possibility that decrease of lesion volume may also contribute to splenectomy induced improved cardiac function.
We found that splenectomy alone did not induce cardiac dysfunction at one month after surgery, measured by LVEF and LVSF and did not increase cardiac fibrosis measured by Sirius red staining. Kondo et al.58 found that splenectomy increases interstitial fibrosis in the left atrium in mice subjected to abdominal aortic constriction (AAC). However, splenectomy did not regulate heart fibrosis and macrophage infiltration at any time point in the sham-AAC control group.58 Ismahil et al.54 also found that splenectomy did not induce any changes in cardiac function in sham-operated mice. Splenectomy reduced the recruitment of macrophages into the heart and decreased interstitial/perivascular fibrosis as well as MCP-1 expression in four week Ang II infusion-induced cardiac fibrosis and hypertensive rats,59 and splenectomy reversed pathological cardiac remodeling as indicated by increased LVEF and decreased monocyte/DC infiltration in mice with chronic heart failure.54 Therefore, our results are consistent with others,54,58 and splenectomy may play different roles in cardiac function under different pathological conditions.
Splenectomy is associated with several risks such as immunosuppression and infection, thrombocytosis and venous thromboembolism with multiple pathophysiological mechanisms.60,61 We would like to highlight that this is a proof-of-concept study demonstrating the role of the spleen and immune response in mediating cardiac deficits after stroke. We do not suggest splenectomy as a therapeutic intervention, as the spleen exerts crucial hematological and immunological functions and splenectomy can induce immunosuppression and increase the risk of infection as well as induce thrombocytosis and venous thromboembolism. Rather, this study draws attention to cardiac deficits after stroke even in the absence of primary cardiac disease and suggests that it may be important to improve cardiac function (such as using immunomodulatory therapeutics) in addition to treating the injured brain.
In this study, we found that splenectomy significantly reduced lesion volume and improve neurological functional outcome after stroke compared to stroke alone mice. Previous studies have reported a lack of beneficial effect of splenectomy on either early or late outcomes after stroke in Lewis rats.62 In another study, although splenectomy decreased the number of infiltrating monocytes/macrophages in the ischemic brain, but did not alter infarct volume at seven days after MCAo in 10–11-week-old mice.63 In our study, we have employed a less severe stroke model (Photothrombotic stroke) in eight-to-nine-months-old mice and observe that splenectomy performed just before stroke decreases lesion volume and improves neurological recovery in addition to attenuating stroke-induced cardiac deficits. Thus, the apparent divergent results possibly may be attributed to the differences in strain, age, and stroke model, and further studies using multiple models of stroke including severe stroke are warranted.
Limitations
Investigation of how stroke in aged population mediates cardiac function is clinically relevant. Our pilot studies have found that aged mice alone have significant cardiac functional deficit compared to young adult or adult mice (data not shown). In the current study, we focus on testing whether stroke can cause cardiac dysfunction and whether immunoresponse contribute to stroke-induced cardiac deficit in the absence of pre-existing heart disease. Therefore, aged mice were not employed in this study. Further studies are warranted on direct comparisons on the progression of stroke-induced cardiac dysfunction as a function of age in male and female animals.
MCP-1 not only recruits macrophages, but also regulates natural killer cells and T cells recruit.64 IL-6 and IL-1β can be produced and secreted by a variety of cell types such as monocytes/macrophage, natural killer cells and T cells. Although the spleen serves as the major and immediate source of monocyte/macrophage deployment following injury,50,51 we cannot exclude the possibility that other inflammatory cells such as natural killer cells and T cells also play a role in splenectomy-induced improvement cardiac function and reduced inflammatory factor MCP1, IL-1β and IL-6 expression. Our supplementary data show that there are few CD3 and NK cells that infiltrate into the heart after stroke. Stroke did not significantly increase T cell and NK cell infiltration into heart, but significantly increased macrophage expression in the heart tissue when compared to sham control. Therefore, we focus our investigation on whether splenectomy regulates macrophage infiltration into heart after stroke. The potential effects of other inflammatory cells on cardiac damage post stroke warrant future study.
Using an in vivo rat model of Angiotensin (Ang) II infusion, others have demonstrated that the spleen is the source of macrophage accumulation in the heart, and following the release of monocytes by the spleen, activation of MCP-1 and the AT1 receptor in the heart promotes recruitment of macrophages into the heart and aorta and contributes to Ang II-induced cardiac fibrosis and hypertension.59,65 Ang II–AT-1 receptor signaling also induces splenic monocyte motility and tissue infiltration.51 Splenectomy or inhibition of the AT1 receptor pathway significantly reduced the number of macrophages in heart, decreased myofibroblast proliferation, inhibited collagen production and decreased cardiac tissue fibrosis through activating the AT2 receptor in an Ang II-induced myocardial fibrosis and hypertension model.59,66 The inflammatory responses mediated by the spleen-Ang II pathway may also play an important role in cardiovascular remodeling after vascular injury, which warrant future study.
Conclusions
In this study, we found that cerebral ischemic stroke induces chronic cardiac dysfunction in adult mice. Immune and inflammatory responses may be involved in stroke-induced cardiac dysfunction. Suppression of macrophage infiltration may be a therapeutic target to prevent or reduce heart dysfunction post stroke.
Supplemental Material
Supplemental Material for Inflammatory responses mediate brain–heart interaction after ischemic stroke in adult mice by Tao Yan, Zhili Chen, Michael Chopp, Poornima Venkat, Alex Zacharek, Wei Li, Yi Shen, Ruixia Wu, Linlin Li, Julie Landschoot-Ward, Mei Lu, Kuan-Han Hank, Jianning Zhang and Jieli Chen in Journal of Cerebral Blood Flow & Metabolism
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China grant 81671144 and 81720108015; Tianjin Municipal Science and Technology Commission (15ZXLCSY00060, 15ZXJSY00040 and 15ZXLCSY00060).
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors' contributions
TY: experimental design, wrote the manuscript, analyzed data and gave final approval of manuscript; ZC: performed experiments, analyzed data, prepared figures, and wrote the manuscript; MC: experimental design and gave final approval of manuscript; PV: analyzed data, prepared figures, and wrote the manuscript; AZ: performed experiments; WL: performed experiments, analyzed data; YS: performed experiments; RW: performed experiments; LL: performed experiments; JLW: performed immunostaining; ML: performed statistical analysis; KHW: performed statistical analysis; JZ: experimental design, wrote the manuscript, analyzed data and gave final approval of manuscript; JC was involved in experimental design, wrote the manuscript, analyzed data and gave final approval of manuscript.
Supplemental material
Supplemental material for this paper can be found at the journal website: http://journals.sagepub.com/home/jcb
References
- 1.Burch GE, Meyers R, Abildskov JA. A new electrocardiographic pattern observed in cerebrovascular accidents. Circulation 1954; 9: 719–723. [DOI] [PubMed] [Google Scholar]
- 2.Ay H, Koroshetz WJ, Benner T, et al. Neuroanatomic correlates of stroke-related myocardial injury. Neurology 2006; 66: 1325–1329. [DOI] [PubMed] [Google Scholar]
- 3.Oppenheimer SM. Neurogenic cardiac effects of cerebrovascular disease. Curr Opin Neurol 1994; 7: 20–24. [DOI] [PubMed] [Google Scholar]
- 4.Tokgozoglu SL, Batur MK, Topcuoglu MA, et al. Effects of stroke localization on cardiac autonomic balance and sudden death. Stroke 1999; 30: 1307–1311. [DOI] [PubMed] [Google Scholar]
- 5.Chen Z, Venkat P, Seyfried D, et al. Brain-heart interaction: cardiac complications after stroke. Circ Res 2017; 121: 451–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J, Cui C, Yang X, et al. MiR-126 affects brain-heart interaction after cerebral ischemic stroke. Transl Stroke Res 2017; 8: 374–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Togha M, Sharifpour A, Ashraf H, et al. Electrocardiographic abnormalities in acute cerebrovascular events in patients with/without cardiovascular disease. Ann Ind Acad Neurol 2013; 16: 66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prosser J, MacGregor L, Lees KR, et al. Predictors of early cardiac morbidity and mortality after ischemic stroke. Stroke 2007; 38: 2295–2302. [DOI] [PubMed] [Google Scholar]
- 9.Bieber M, Werner RA, Tanai E, et al. Stroke-induced chronic systolic dysfunction driven by sympathetic overactivity. Ann Neurol 2017; 82: 729–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toba H, Cannon PL, Yabluchanskiy A, et al. Transgenic overexpression of macrophage matrix metalloproteinase-9 exacerbates age-related cardiac hypertrophy, vessel rarefaction, inflammation, and fibrosis. Am J Physiol Heart Circ Physiol 2017; 312: H375–h383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiao YA, Dai Q, Zhang J, et al. Multi-analyte profiling reveals matrix metalloproteinase-9 and monocyte chemotactic protein-1 as plasma biomarkers of cardiac aging. Circ Cardiovasc Genet 2011; 4: 455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiao YA, Ramirez TA, Zamilpa R, et al. Matrix metalloproteinase-9 deletion attenuates myocardial fibrosis and diastolic dysfunction in ageing mice. Cardiovasc Res 2012; 96: 444–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindner D, Zietsch C, Tank J, et al. Cardiac fibroblasts support cardiac inflammation in heart failure. Basic Res Cardiol 2014; 109: 428. [DOI] [PubMed] [Google Scholar]
- 14.Toba H, de Castro Bras LE, Baicu CF, et al. Secreted protein acidic and rich in cysteine facilitates age-related cardiac inflammation and macrophage M1 polarization. Am J Physiol Cell Physiol 2015; 308: C972–C982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heidt T, Courties G, Dutta P, et al. Differential contribution of monocytes to heart macrophages in steady-state and after myocardial infarction. Circ Res 2014; 115: 284–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gharaee-Kermani M, Denholm EM, Phan SH. Costimulation of fibroblast collagen and transforming growth factor beta1 gene expression by monocyte chemoattractant protein-1 via specific receptors. J Biol Chem 1996; 271: 17779–17784. [DOI] [PubMed] [Google Scholar]
- 17.Dewald O, Zymek P, Winkelmann K, et al. CCL2/monocyte chemoattractant protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ Res 2005; 96: 881–889. [DOI] [PubMed] [Google Scholar]
- 18.Ungvari Z, Kaley G, de Cabo R, et al. Mechanisms of vascular aging: new perspectives. J Gerontol A Biol Sci Med Sci 2010; 65: 1028–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruunsgaard H, Pedersen BK. Age-related inflammatory cytokines and disease. Immunol Allergy Clin North Am 2003; 23: 15–39. [DOI] [PubMed] [Google Scholar]
- 20.Labat-gest V, Tomasi S. Photothrombotic ischemia: a minimally invasive and reproducible photochemical cortical lesion model for mouse stroke studies. J Vis Exp 2013; 76: 50370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Min J, Farooq MU, Greenberg E, et al. Cardiac dysfunction after left permanent cerebral focal ischemia: the brain and heart connection. Stroke 2009; 40: 2560–2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uzdensky AB. Photothrombotic stroke as a model of ischemic stroke. Transl Stroke Res 2018; 9(5): 437–451. [DOI] [PubMed] [Google Scholar]
- 23.Dotson AL, Wang J, Saugstad J, et al. Splenectomy reduces infarct volume and neuroinflammation in male but not female mice in experimental stroke. J Neuroimmunol 2015; 278: 289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ai D, Pang W, Li N, et al. Soluble epoxide hydrolase plays an essential role in angiotensin II-induced cardiac hypertrophy. Proc Natl Acad Sci U S A 2009; 106: 564–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen J, Sanberg PR, Li Y, et al. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke 2001; 32: 2682–2688. [DOI] [PubMed] [Google Scholar]
- 26.Yan T, Venkat P, Chopp M, et al. Neurorestorative therapy of stroke in type 2 diabetes mellitus rats treated with human umbilical cord blood cells. Stroke 2015; 46: 2599–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu J, Carretero OA, Liao TD, et al. Local angiotensin II aggravates cardiac remodeling in hypertension. Am J Physiol Heart Circ Physiol 2010; 299: H1328–H1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mito S, Ozono R, Oshima T, et al. Myocardial protection against pressure overload in mice lacking Bach1, a transcriptional repressor of heme oxygenase-1. Hypertension 2008; 51: 1570–1577. [DOI] [PubMed] [Google Scholar]
- 29.Salvarani N, Maguy A, De Simone SA, et al. TGF-beta1 (Transforming growth factor-beta1) plays a pivotal role in cardiac myofibroblast arrhythmogenicity. Circ Arrhythm Electrophysiol 2017; 10: e004567. [DOI] [PubMed] [Google Scholar]
- 30.Ismahil MA, Hamid T, Bansal SS, et al. Remodeling of the mononuclear phagocyte network underlies chronic inflammation and disease progression in heart failure: critical importance of the cardiosplenic axis. Circ Res 2014; 114: 266–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hohensinner PJ, Kaun C, Rychli K, et al. Monocyte chemoattractant protein (MCP-1) is expressed in human cardiac cells and is differentially regulated by inflammatory mediators and hypoxia. FEBS Lett 2006; 580: 3532–3538. [DOI] [PubMed] [Google Scholar]
- 32.Matsuda S, Umemoto S, Yoshimura K, et al. Angiotensin activates MCP-1 and induces cardiac hypertrophy and dysfunction via toll-like receptor 4. J Atheroscleros Thromb 2015; 22: 833–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han YL, Li YL, Jia LX, et al. Reciprocal interaction between macrophages and T cells stimulates IFN-gamma and MCP-1 production in Ang II-induced cardiac inflammation and fibrosis. PLoS One 2012; 7: e35506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Christensen H, Fogh Christensen A, Boysen G. Abnormalities on ECG and telemetry predict stroke outcome at 3 months. J Neurol Sci 2005; 234: 99–103. [DOI] [PubMed] [Google Scholar]
- 35.Distefano G, Sciacca P. Molecular pathogenesis of myocardial remodeling and new potential therapeutic targets in chronic heart failure. Ital J Pediatr 2012; 38: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Donekal S, Venkatesh BA, Liu YC, et al. Interstitial fibrosis, left ventricular remodeling, and myocardial mechanical behavior in a population-based multiethnic cohort: the Multi-Ethnic Study of Atherosclerosis (MESA) study. Circul Cardiovasc Imag 2014; 7: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schultz JEJ, Witt SA, Glascock BJ, et al. TGF-β1 mediates the hypertrophic cardiomyocyte growth induced by angiotensin II. J Clin Invest 2002; 109: 787–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dobaczewski M, Chen W, Frangogiannis NG. Transforming growth factor (TGF)-beta signaling in cardiac remodeling. J Mol Cell Cardiol 2011; 51: 600–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosenkranz S, Flesch M, Amann K, et al. Alterations of beta-adrenergic signaling and cardiac hypertrophy in transgenic mice overexpressing TGF-beta(1). Am J Physiol Heart Circ Physiol 2002; 283: H1253–H1262. [DOI] [PubMed] [Google Scholar]
- 40.Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science 2013; 339: 161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Epelman S, Liu PP, Mann DL. Role of innate and adaptive immune mechanisms in cardiac injury and repair. Nat Rev Immunol 2015; 15: 117–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hulsmans M, Sager HB, Roh JD, et al. Cardiac macrophages promote diastolic dysfunction. J Exp Med 2018; 215: 423–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma F, Li Y, Jia L, et al. Macrophage-stimulated cardiac fibroblast production of IL-6 is essential for TGF beta/Smad activation and cardiac fibrosis induced by angiotensin II. PLoS one 2012; 7: e35144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bartosova D, Chvapil M, Korecky B, et al. The growth of the muscular and collagenous parts of the rat heart in various forms of cardiomegaly. J Physiology 1969; 200: 285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reed AL, Tanaka A, Sorescu D, et al. Diastolic dysfunction is associated with cardiac fibrosis in the senescence-accelerated mouse. Am J Physiol Heart Circ Physiol 2011; 301: H824–H831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brown RD, Ambler SK, Mitchell MD, et al. The cardiac fibroblast: therapeutic target in myocardial remodeling and failure. Ann Rev Pharmacol Toxicol 2005; 45: 657–687. [DOI] [PubMed] [Google Scholar]
- 47.Winegrad S, Robinson TF. Force generation among cells in the relaxing heart. Eur J Cardiol 1978(7 Suppl,63–70. [PubMed] [Google Scholar]
- 48.Sager HB, Hulsmans M, Lavine KJ, et al. Proliferation and recruitment contribute to myocardial macrophage expansion in chronic heart failure. Circ Res 2016; 119: 853–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heidt T, Courties G, Dutta P, et al. Differential contribution of monocytes to heart macrophages in steady-state and after myocardial infarction. Circ Res 2014; 115: 284–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim E, Yang J, D Beltran C, et al. Role of spleen-derived monocytes/macrophages in acute ischemic brain injury. J Cereb Blood Flow Metab 2014; 34: 1411–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Swirski FK, Nahrendorf M, Etzrodt M, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 2009; 325: 612–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prabhu SD. The cardiosplenic axis is essential for the pathogenesis of ischemic heart failure. Transac Am Clin ClimatolAssoc 2018; 129: 202–214. [PMC free article] [PubMed] [Google Scholar]
- 53.Wohleb ES, McKim DB, Shea DT, et al. Re-establishment of anxiety in stress-sensitized mice is caused by monocyte trafficking from the spleen to the brain. Biol Psychiatr 2014; 75: 970–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ismahil MA, Hamid T, Bansal SS, et al. Remodeling of the mononuclear phagocyte network underlies chronic inflammation and disease progression in heart failure: critical importance of the cardiosplenic axis. Circ Res 2014; 114: 266–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deshmane SL, Kremlev S, Amini S, et al. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res 2009; 29: 313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qiang Z, Tao Y, Linlin L, et al. Immune response mediates cardiac dysfunction after traumatic brain injury. J Neurotrauma 2018; 35: 1–11. [DOI] [PubMed] [Google Scholar]
- 57.Larson BE, Stockwell DW, Boas S, et al. Cardiac reactive oxygen species after traumatic brain injury. J Surg Res 2012; 173: e73–e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kondo H, Takahashi N, Gotoh K, et al. Splenectomy exacerbates atrial inflammatory fibrosis and vulnerability to atrial fibrillation induced by pressure overload in rats: possible role of spleen-derived interleukin-10. Heart Rhythm 2016; 13: 241–250. [DOI] [PubMed] [Google Scholar]
- 59.Wang NP, Erskine J, Zhang WW, et al. Recruitment of macrophages from the spleen contributes to myocardial fibrosis and hypertension induced by angiotensin II. J Renin Angiotensin Aldosterone Syst 2017; 18: 1470320317706653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee DH, Barmparas G, Fierro N, et al. Splenectomy is associated with a higher risk for venous thromboembolism: a prospective cohort study. Int J Surg 2015; 24: 27–32. [DOI] [PubMed] [Google Scholar]
- 61.Crary SE, Buchanan GR. Vascular complications after splenectomy for hematologic disorders. Blood 2009; 114: 2861–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zierath D, Shen A, Stults A, et al. Splenectomy does not improve long-term outcome after stroke. Stroke 2017; 48: 497–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim E, Yang J, Beltran CD, et al. Role of spleen-derived monocytes/macrophages in acute ischemic brain injury. J Cereb Blood Flow Metab 2014; 34: 1411–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lehmann MH, Torres-Dominguez LE, Price PJ, et al. CCL2 expression is mediated by type I IFN receptor and recruits NK and T cells to the lung during MVA infection. J Leukoc Biol 2016; 99: 1057–1064. [DOI] [PubMed] [Google Scholar]
- 65.Dai Z, Aoki T, Fukumoto Y, et al. Coronary perivascular fibrosis is associated with impairment of coronary blood flow in patients with non-ischemic heart failure. J Cardiol 2012; 60: 416–421. [DOI] [PubMed] [Google Scholar]
- 66.Bai F, Pang XF, Zhang LH, et al. Angiotensin II AT1 receptor alters ACE2 activity, eNOS expression and CD44-hyaluronan interaction in rats with hypertension and myocardial fibrosis. Life Sci 2016; 153: 141–152. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Inflammatory responses mediate brain–heart interaction after ischemic stroke in adult mice by Tao Yan, Zhili Chen, Michael Chopp, Poornima Venkat, Alex Zacharek, Wei Li, Yi Shen, Ruixia Wu, Linlin Li, Julie Landschoot-Ward, Mei Lu, Kuan-Han Hank, Jianning Zhang and Jieli Chen in Journal of Cerebral Blood Flow & Metabolism