Abstract
Cells actively interact with their microenvironment, constantly sensing and modulating biochemical and biophysical signals. Blood comprises a variety of non-adherent cells that interact with each other and with endothelial and vascular smooth muscle cells of the blood vessel walls. Blood cells are further experiencing a range of external forces by the hemodynamic environment and they also exert forces to remodel their local environment. Therefore, the biophysics and material properties of blood cells and blood play an important role in determining blood behaviour in health and disease. In this Review, we discuss blood cells and tissues from a materials perspective, considering the mechanical properties and biophysics of individual blood cells and endothelial cells as well as blood cell collectives. We highlight how blood vessels provide a mechanosensitive barrier between blood and tissues and how changes in vessel stiffness and flow shear stress can be correlated to plaque formation and exploited for the design of vascular grafts. We discuss the effect of the properties of fibrin on blood clotting, and investigate how forces exerted by platelets are correlated to disease. Finally, we hypothesize that blood and vascular cells are constantly establishing a mechanical homeostasis, which, when imbalanced, can lead to hematologic and vascular diseases.
Introduction
Blood comprises trillions of cells that are pumped by the heart to circulate in the blood vessels throughout the body. Therefore, blood and vascular cells are constantly exposed to a hemodynamic microenvironment involving a range of external forces distinct from other tissue types. From a macroscale perspective, the mechanical properties of many blood-related components, such as blood pressure, fluid shear stress, blood viscosity, the stiffness of blood vessels as well as blood cells and clots, remain relatively stable in healthy tissue, suggesting that the key components of the circulatory system – vessels, blood and blood clots – are maintained in a state of mechanical equilibrium. Like many adherent cells, blood and vascular cells contain a pre-stressed cytoskeletal structure and a mechanotransductive machinery to sense, respond and modify the microenvironment1. Coordinated mechanosensitive and mechanoresponsive behaviour enables cells to provide regulatory feedback to the blood system. Therefore, we hypothesize that in a healthy circulatory system, a mechanical homeostasis is maintained at the cellular and tissue level.
Here, mechanical homeostasis is defined as the process that maintains the mechanical equilibrium of a biological system using negative feedback mechanisms. This concept of mechanical homeostasis has been demonstrated in other types of adherent cells, such as fibroblasts and mammary epithelial cells2, 3, and their associated tissues, such as skin and mammary gland3, 4, which can maintain normal physiological conditions against intra- and extracellular forces and deformation. Alterations of the mechanical properties of blood cells and vascular tissues can be linked to the pathogenesis of numerous cardiovascular and hematologic diseases, including pro-inflammatory vascular conditions, such as sickle cell disease and bleeding disorders, and uncontrolled blood clotting in atherosclerosis and/or stroke 4–9. Therefore, the specific mechanical properties of blood cells and tissues, such as blood vessel stiffness, blood cell contraction forces and vascular and blood cell stiffnesses, can be potentially used as biomarkers for diagnosing cardiovascular and hematologic diseases. Importantly, the mechanical disequilibrium associated with many of these diseases can be targeted as a treatment strategy 10, 11.
However, a comprehensive understanding of the mechanical homeostasis of blood and vascular tissues remains elusive thus far. Particularly, a quantitative characterization of the mechanical dynamics in the hemodynamic microenvironment at the cellular and molecular level is difficult. Thus, how alterations of cell–cell and cell–extracellular matrix (ECM) interactions can result in pathophysiology at the tissue and organ level is not yet understood. Materials-based techniques offer the possibility to characterize the mechanical dynamics at micro- and nanoscales, allowing the identification of mechanical biomarkers and therapeutics for haematological and vascular diseases. Moreover, sophisticated and smart materials could be used as tools to engineer in vitro models that better recapitulate the in vivo mechanical microenvironment for studying mechanical equilibrium states.
In this Review, we discuss techniques for measuring the mechanical properties of blood tissues and materials that can be used to recreate their mechanical microenvironment. We then examine the mechanical homeostasis hypothesis in three distinct anatomical regions: blood vessels, blood and blood clots, highlighting key findings and important knowledge gaps that need to be filled to validate the hypothesis. Finally, we investigate exciting future areas of research at the crossroads between materials science and the study of blood tissues and disease.
Tools to measure mechanical properties
To study the mechanics of blood tissues across different length scales (FIG. 1a), tools and techniques are required that operate with measurement resolutions at broad length scales, ranging from nm to cm, and at broad force scales, ranging from pN to N8, 9, 12, 13.
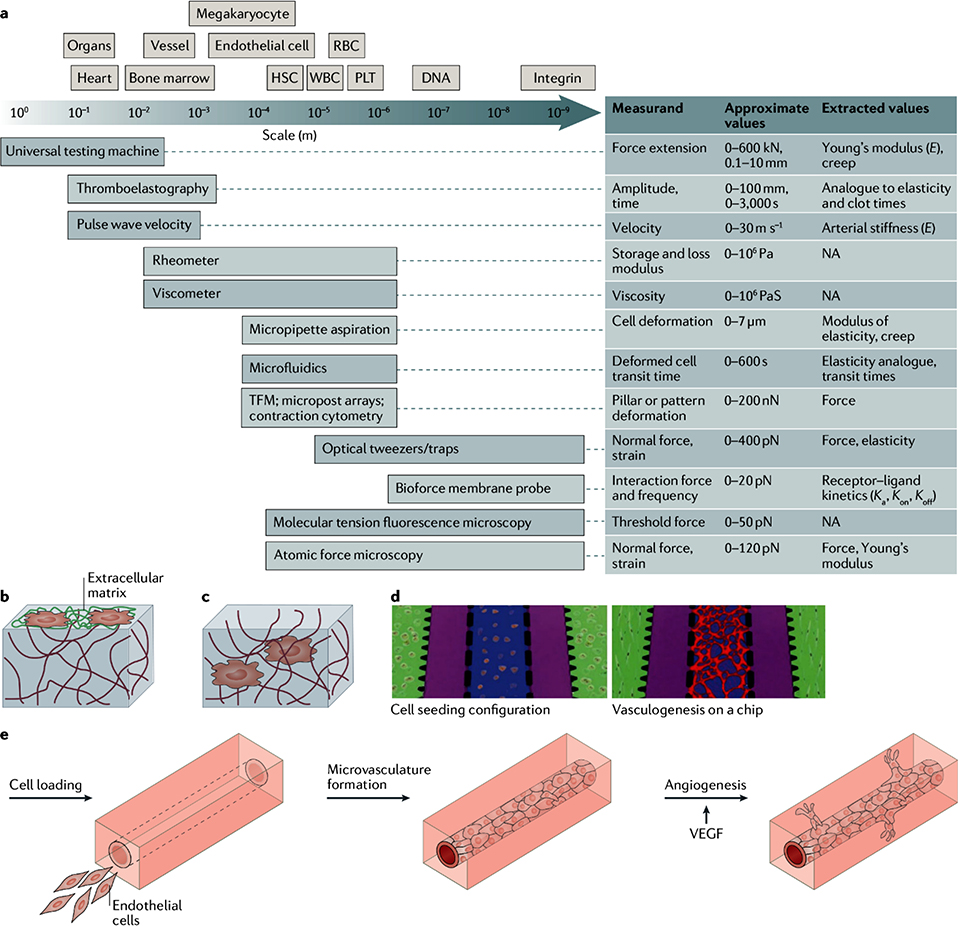
Figure 1: Tools to measure and recreate the mechanical properties of blood tissues.
a) The schematics compares the length scale of blood cells and tissues with the resolution of techniques for the investigation of the mechanical properties of blood cells and tissues. Typical measurements, values, and extracted values are also shown. b) Schematic of a 2D cell culture on a protein-conjugated hydrogel surface. c) Schematic of a 3D cell culture. Cells are encapsulated inside a hydrogel and can interact with the surrounding environment. d) Hydrogels can be used for the engineering of microvasculature by vasculogenesis (green: fibrin gels embedded with perivascular cells; purple: fluidic channels; blue: fibrin gels embedded with endothelial cells; red: microvascular network formed via vasculogenesis208. e) Hydrogels can also be applied for the engineering of microvasculature by angiogenesis, using vascular endothelial growth factor (VEGF)209.
Bulk mechanical properties
The characterization of bulk mechanical properties of tissues provides information on the correlation of tissue mechanics and the pathogenesis of diseases and aging processes. For example, stiffening of blood vessels has been identified as a mechanical biomarker of aging6 and diseases such as atherosclerosis7, 14. The bulk mechanical properties of blood vessels can be measured using tensile testing, in which mN to N forces are directly applied to the vessel wall, generating a strain–stress curve to characterize the elastic modulus of the vessels12, 15. Arterial stiffness is also clinically used by physicians and can be indirectly characterized by measuring the pulse wave velocity using a non-invasive approach. Thereby, the speed at which the elastic distortion of the vessel wall propagates is measured in response to the blood pressure pulse in the systole–diastole cycle16, 17. In addition, the compliance of blood vessels can be measured by analyzing the percentage of vessel radius increase using ultrasound in the clinic, which corresponds to the increase in intramural pressure18. The alteration of other bulk mechanical properties, such as blood viscosity and clot viscoelasticity, could also be associated with disease states. These properties can be measured by viscometry19 and rheometry20.
Some specialized tools have been developed specifically for measuring the bulk mechanical properties of blood. These tools have generally been optimized for use in a clinical setting. Thromboelastography bears some similarity to rheometry and measures clot mechanical properties over time, from initiation to lysis. In this tool, activated blood fills the space between two moveable surfaces. Initially, only one surface is moved back and forth in a steady function. As blood coagulates and solidifies, the motion of the moving surface is transferred to the other surface, which is free to move. By measuring the movement of the second plate and the timing of this movement, measurements about the clot initiation time (in s), clot maximum firmness (in mm), and clot lysis time can extracted 9, 21, 22. However, note that the clot firmness is given in terms of mm of movement of the second plate, not Pa as seen in rheometres. Another system, created by Hemodyne, provided information about clot stiffness (Pa) and bulk platelet contraction forces (0 – 0.2N) 23, although this tool is no longer available.
Mechanical properties at the micro- and nanoscale
The bulk mechanical properties of tissues can provide useful information for disease diagnosis and therapies; however, mechanical homeostasis is achieved through the interaction between cells and the extracellular environment at the micro- and nanoscale5, 24, 25. Therefore, these scales need to be probed to understand how the mechanical properties at the cellular and molecular levels cause the alteration of bulk mechanics in disease. For example, the stiffness of cells and ECMs can be tested at the microscale using atomic force microscopy (AFM). An atomic force microscope can be used to measure the length of cells at the nm to μm scale and force at the pN to nN scale, and to characterize the mechanics of compliant objectives, including the stiffness of ECMs and cells12, 26.
Cells generate forces at the single cell and the receptor level. The forces exerted by adherent cells can be assessed by traction force microscopy (TFM)27 and micropost array detectors2, 28 by measuring the displacement of beads embedded in hydrogels with a known elastic modulus or of microposts with a pre-determined stiffness. We recently developed a technique called contraction cytometry, in which adhesive protein microdots are conjugated to a hydrogel of known stiffness. The contractile forces generated by single platelets can then be measured by characterizing the change of distance between a microdot pair29. However, these techniques only provide nN sensitivity, which is not sufficient to determine the forces transmitted by individual receptors at the pN scale. AFM, magnetic or optical tweezers30, and biomembrane force probes31 can be used to measure forces with pN sensitivity, but these techniques are limited by low-throughput. Molecular tension fluorescence microscopy (MTFM) has recently been developed to map the spatiotemporal forces of single receptors across the cell surface at the pN scale and with high throughput, by measuring the fluorescence emission of immobilized MTFM probes32. Using these techniques, cell and tissue mechanics can be quantified across a broad length scale to investigate the multiscale nature of mechanical homeostasis and the disturbance of mechanical equilibrium in hematologic and vascular disease.
Mimicking the microenvironment
To investigate cell behaviour in vitro, it is important to recreate a physiologically-relevant microenvironment that contains the biochemical and biomechanical cues which modulate cell behaviour in vivo33. The incorporation of appropriate ligands into microenvironments is paramount, because by binding to specific receptors at distinct kinetics, ligands trigger specific downstream signalling pathways. For example, immobilized von Willebrand factor (vWF) and fibrinogen control platelet adhesion at high and low shear stress through binding to distinct platelet receptors34, 35.
Biomechanical cues, such as stiffness, regulate cellular tensegrity, affect cell behaviours and can be recapitulated using materials (TABLE 1). For example, polyacrylamide (PA) gels can be used to study the effect of ECM stiffness on cell behaviour36,37. The stiffness of this synthetic gel can be fine-tuned by controlling the crosslinking density to represent the stiffnesses of different tissues. PA hydrogels have been used for studying various types of cells, including endothelial cells38 and platelets39, 40, which play major roles in hematologic processes and disease. However, PA gels can only serve as 2D models owing to the incapability of biodegradation and cell encapsulation (FIG. 1b). By contrast, naturally-derived collagen and fibrin gels can be applied to investigate 3D cell behaviours (FIG. 1c); for example, white blood cell migration41, platelet contraction39 and angiogenesis42–44. However, biophysical properties, such as ligand density, ECM elasticity and porosity, are difficult to decouple in naturally-derived gels. Moreover, natural ECM-derived gels are too soft to be tuned to a broad stiffness range. Alternatively, alginate gels can be used to address these limitations. In alginate gels, ligand density can be easily controlled by titrating adhesive domains. In addition, the rigidity of the gel can be tuned by modulating the physical and chemical crosslinking density45, 46. Fluid shear stress is also an important mechanical cue, which can be recapitulated using macro- and microfluidics (FIG. 1d-e). Traditional fluidics are made of polydimethylsiloxanes (PDMS)47–50; however, fluidic systems can also be made of soft materials, such as collagen42–44, 51, fibrin52–54, alginate55 and agarose56, which allow the tuning of stiffness and permeability, making these systems more physiologically relevant.
TABLE 1.
Hydrogels for the investigation of blood tissues
| Materials | Pros | Cons | Applications | Refs |
|---|---|---|---|---|
| Polyacrylamide | • Well characterized for a wide physiologically-relevant range of stiffness (hundreds of Pa – hundreds of kPa); • Conjugation of adhesive proteins on the surface |
• Only suitable as 2D model • Not suitable for cell embedding • not degradable • Swelling ratio is not controllable |
• Cell adhesion, spreading and differentiation • Traction force microscopy • Contraction cytometry |
36–40,100 |
| Collagen and fibrin | • Natural proteins that contain adhesive domains for cells • Suitable for cell encapsulation and 3D study • Degradable • Cell migration and angiogenesis studies • Easy to incorporate into microfluidic systems |
• Soft and restricted stiffness range (< a few kPa) • Difficult to decouple stiffness and ligand density • Fibrin gel formation depends on thrombin and is thus difficult to control |
• Microvasculature-on-a-chip by top-down and bottom-up approaches • Cell migration • Platelet contraction in 3D (fibrin gel) |
39,41–44,51–54 |
| Gelatin-methacrylate (MA) | • Denatured collagen contains adhesive domains for cells • Wide range of compressive modulus (hundreds of Pa to ~ 35 kPa) • Suitable for cell encapsulation and 3D study • Degradable • Easy to incorporate into microfluidic systems |
• Difficult to decouple stiffness, ligand density and porosity • Degradability is compromised with increasing MA ratio • Swelling ratio is coupled to crosslinking and ligand density |
• Microvasculature-on-a-chip by top-down and bottom up approaches | 99–101 |
| Agarose-gelatine interpenetrating network | • Wide stiffness range Gelatine contains adhesive domains for cells • Easy to incorporate into microfluidic systems• Swelling is negligible |
• Not degradable • Not suitable for cell migration and angiogenesis studies • Requires post-crosslinking for the fabrication of microfluidics |
• Microvasculature-on-a-chip by top-down approach with well-controlled microvascular size scale and flow dynamics • Long-term culture with physiologically-relevant endothelial permeability |
106 |
| Chemically modified alginate | • Covalent and/or physical crosslinking • Ligand density can be decoupled from stiffness, crosslinking density and porosity • Suitable for cell encapsulation and 3D studies • Can be incorporated into microfluidic systems |
• Only degradable when modified with oxidization • Crosslinking may be affected by calcium in the medium |
• Microfluidic channels • Cell encapsulation in 3D |
45,46,55 |
| Chemically modified polyethylene glycol | • Ligand density can be decoupled from stiffness, crosslinking density and porosity • Can be used for cell encapsulationin 3D and angiogenesis • Can be incorporated into microfluidic systems |
• Depends on incorporation of degradable segments for degradability • Uncontrolled swelling |
• Microvasculature-on-a-chip by top-down and bottom-up approaches | 102–104 |
| Desired hydrogel materials | • Contains appropriate ligands or ligands are easy to incorporate • Easy to adjust stiffness to a wide range • Decoupled stiffness, ligand density and porosity • Negligible swelling • Degradable • easy cell encapsulation • Easy to apply to microfluidic systems and to connect with tubing and pumps |
• Microvasculature-on-a-chip by top-down and bottom-up approaches with well-controlled size and flow dynamics • Long-term culture with physiologically-relevant endothelial permeability • Cell encapsulation in 3D • Cell migration |
Blood vessels
Mechanosensitive barriers between blood and tissues
Blood vessels form a tree-like, pressurized circuit that circulates blood between the heart and other organs. The blood vessel wall contains mechanically responsive cells, including endothelial cells and vascular smooth muscle cells that actively respond to the hemodynamic environment, making the vessel wall a mechanosensitive barrier. Exertion of mechanical forces between these cells and between cells and the ECM as well as feedback mechanisms provide mechanical homeostasis in blood vessels. In healthy tissue, vascular cells maintain a specific mechanical microenvironment and dysregulation of this mechanical homeostasis leads to cardiovascular pathology, highlighting the importance of mechanical homeostasis in vascular physiology 4, 57. Therefore, understanding mechanical homeostasis and the key mechanical forces in blood vessels will greatly improve the engineering of blood vessels and vascular grafts.
The size, structure, cellular components and mechanical properties of blood vessels vary in the different tissues of the body depending on their function. For example, arteries carry pulsatile blood away from the heart as it contracts and generates pressure. As blood moves distally from the heart, the pressure drops along the vessel and the blood flow becomes less pulsatile. Eventually, the vessels branch into smaller arterioles and capillaries, which converge into larger venules and veins that return blood back into the atria (FIG. 2a)58. With the exception of capillaries, all blood vessels possess an intima, which is the innermost layer comprising of a sheet of flattened endothelial cells that rest on a thin layer of connective tissue, a layer called the media, which consists of spindle-shaped vascular smooth muscle cells that are embedded in a matrix of elastin and collagen fibres, and an adventitia, which is the outermost layer composed mainly of fibroblasts and collagen matrix59 (Fig. 2b). By contrast, capillaries only contain an intima that is covered with pericytes.
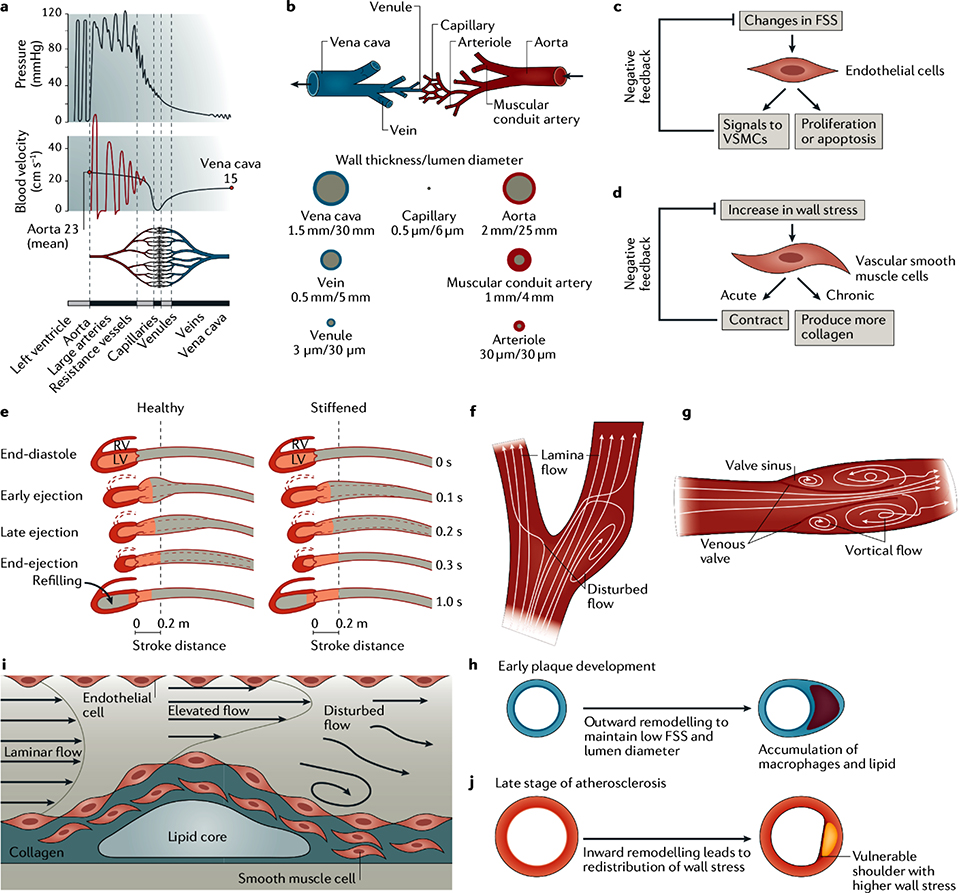
Figure 2: Mechanical forces and mechanical homeostasis in blood vessels.
a) The graphs show the distribution of blood pressure and blood velocity across a blood vessel. b) The tree-like vasculature has a heterogenic structure in arteries, microvasculature and veins210. c) A negative feedback mechanism regulates mechanical homeostasis in blood vessels. Flow shear stress triggers signalling pathways in endothelial cells, which leads to cell proliferation or apoptosis and has an effect on vascular smooth muscle cells. d) Similarly, vascular smooth muscle cells respond to an increase in vessel wall stress by contracting or by producing collagen. e) Pulse wave velocity is slower in healthy arteries compared to stiff arteries (modified based on Ref. 210), which is related to the probability of cardiovascular diseases, including atherosclerosis and stroke. f) Disturbed blood flow occurs in the bifurcation region of arteries, making them atherogenic. g) Two vortical flows form in the venous valve sinus owing to its geometry, which increases the risk of thrombosis. h) In the early stage of plaque development, outward remodelling occurs to maintain a constant low flow shear stress and lumen size. i) Inward growth of a vessel wall at a late stage of plaque development results in a local change of the flow shear stress. Elevated shear stress appears upstream of the bulge, while disturbed flow with low oscillatory shear stress occurs downstream of the plaque. j) Computational modelling demonstrates that inward growth of the vessel wall can lead to higher wall stress on the shoulder of plaque, which is more vulnerable to rupture61.
Each blood vessel type is structurally and mechanically different, but they all are exposed to various hemodynamic forces, including flow shear stress, frictional forces parallel to the vessel wall caused by flowing blood, and circumferential stress perpendicular to the vessel wall, generated by transmural pressure60–62. Vascular smooth muscle cells 63 and endothelial cells64 are sensitive to these physical forces; changes in force can trigger intracellular signalling pathways, which regulate cellular function and the structure of blood vessels.
Flow shear stress
The ability of endothelial cells to respond to physical perturbations and maintain the flow shear stress in a narrow physiological range by modulating the vascular lumen size was first reported decades ago57, 65, leading to the hypothesis of mechanical homeostasis in blood vessels3, 57, which can be regulated by vascular cells and endothelial cells through a negative feedback loop. For example, endothelial cells can sense the acute elevation of flow shear stress. They respond by producing nitric oxide (NO) and prostacyclin to relax the vascular smooth muscle cells66, which causes an increase in vessel diameter and an decrease in flow shear stress. Thereby, endothelial cells can regulate the flow shear stress67 (Fig. 2c). If the flow shear stress remains too high, endothelial cells induce the remodelling of the vessel wall, which can last for weeks or months, to widen the vessel lumen. Such changes in vessel wall and lumen diameter have been observed in ischemic injuries, to bypass the internal blockage of large arteries by arterialization of the collateral capillary and to carry more blood into the tissue68. Conversely, as the flow shear stress decreases, endothelial cell signalling causes a decrease in blood vessel diameter and thus, an increase in flow shear stress65.
Endothelial mechanotransduction and adaptive feedback signalling mechanisms5, 58, 69 are controlled by mechanosensitive protein machineries, including ion channels, G proteins, the apical glycocalyx, primary cilia, the platelet endothelial cell adhesion molecule (PECAM)-1/vascular endothelial (VE)-cadherin/vascular endothelial growth factor receptor (VEGFR) complex, integrins and NOTCH1, which are activated by apical flow shear stress. Downstream signalling pathways then lead to the production of NO and prostacyclin as well as endothelial cell proliferation and barrier function5, 70–72. However, the physiological range of flow shear stress differs in arteries, the microvasculature and veins. Therefore, the questions remain how the mechanosensors in the different vessel types respond to and regulate different levels of shear stress and whether distinct blood vessel types contain distinct mechanosensors or whether they express different levels of mechanosensitive proteins72, 73.
Vessel stiffness
Vascular smooth muscle cells also play a crucial role in maintaining a constant wall stress through a negative feedback mechanism. According to LaPlace’s law (S = P x r / w), the blood vessel wall stress (S) is proportional to the transmural pressure (P) and the radius of the blood vessel (r), and inversely proportional to the wall thickness (w)5. To keep the wall stress constant with increasing transmural pressure, vascular smooth muscle cells contract, which causes a decrease in vessel radius. By contrast, a chronic increase in transmural pressure, for example in patients with hypertension (high blood pressure), leads to the secretion of more collagen fibres by vascular smooth muscle cells, which causes thickening of the vessel wall and consequently, a decrease in wall stress6, 74 (FIG. 2d). However, the compliance of blood vessels is primarily established by elastin, which is a highly elastic protein of the connective tissue that is only minimally expressed after birth59. Therefore, the elastin content does not change much throughout life, and thus, the deposition of collagen fibres by vascular smooth muscle cells is sufficient to cause a decline in vessel compliance and an increase in vessel stiffness.
Blood vessel stiffening is also observed during aging. Aging leads to the breaking of elastin fibrils, which become disordered and weak owing to repetitive strain injury75. To compensate for the weaker vessel wall, vascular smooth muscle cells deposit more collagen fibres, which stiffens the vessel wall6. The stiffness of blood vessels can be clinically analyzed by measuring the pulse wave velocity, which is proportional to stiffness. For example, an inverse relationship between blood vessel stiffness and age has been demonstrated by reproducible pulse wave velocity analysis in humans12. Children at the age of 10 have a pulse wave velocity of ~6.5 m/s, whereas the pulse wave velocity is ~11 m/s in adults at the age of 50. Moreover, the pulse wave velocity continuously increases with age. A threshold of carotid-femoral pulse wave velocity of >12 m/s has been suggested as an indicator of significant alterations of aortic functions and cardiovascular diseases12 (FIG. 2e). In addition, the irreversible age- and hypertension-associated vessel stiffening leads to an increase in the resistance of the systemic circulation and thus, in the required cardiac work, which is associated with many cardiovascular diseases, for example, heart failure6.
Flow patterns and plaque formation
In addition to the magnitude of shear stress, endothelial cells are also sensitive to flow patterns, which play a crucial role in initiating dysregulation of mechanical homeostasis and in the development of atherosclerosis. Endothelial mechanosensors can respond to physiological laminar flow, which triggers the expression of transcription factor Kruppel-like factor (KLF2) and nuclear factor (erythroid-derived 2)-like (NRF2), as well as the downstream expression of anti-inflammatory, antithrombotic and antioxidative mediators76. By contrast, disturbed flow patterns, including slow, oscillatory and turbulent flow around bifurcations, bends (FIG. 2f) and valves 77, 78(FIG. 2g), inhibit the activation of KLF2 and NRF2 and continuously activate inflammatory pathways involving nuclear factor-κB (NF-κB)62, 79. Of note, in such atheroprone regions, antioxidant, protective pathways are also upregulated, which has been shown by gene expression analysis of endothelial cells 80, 81.
However, the upregulation of protective pathways is insufficient to balance the activated inflammatory pathways80, 81 resulting in a net chronic inflammatory phenotype in atheroprone regions. Although the chronic inflammation is mild, it increases vessel permeability and predisposes arteries to the risk of atherosclerosis at bifurcations and bends (BOX 1). Additional risk factors, such as high low-density lipoprotein (LDL) cholesterol and hyperglycemia5, promote the accumulation of apolipoprotein-B (apoB) containing lipoproteins (LPs) in these inflamed regions with higher permeability and slow shear stress. The retention of lipoproteins aggravates the inflammation and increases the expression of vascular cell adhesion protein (VCAM)-1 and intercellular adhesion molecule (ICAM)-1, which leads to the recruitment of monocytes that differentiate into macrophages. Lipid and macrophage accumulation eventually cause the formation of a plaque.
BOX 1. Mechanical imbalance and disease.
Atherosclerosis is a chronic disease that occurs in the focal regions of medium-sized arteries. The focal regions are either bends or bifurcation branches that are exposed to disturbed flow. This flow pattern can cause chronic inflammation of endothelial cells and thus, recruitment of monocytes and subendothelial retention of apolipoprotein B (apoB)-containing lipoproteins (LPs), resulting in plaque formation. Some atherosclerotic lesions undergo partial resolution by forming a fibrous cap, which prevents direct contact of the plaque with prothrombotic factors. Some atherosclerotic lesions develop a vulnerable plaque with a necrotic core and a thin fibrous cap, which results in the remodelling of the inner arterial wall, leading to a local change in flow shear stress. At regions with high flow shear stress, the size of the fibrous cap decreases owing to an increase in matrix metalloproteinase production, matrix degradation, intraplaque haemorrhage, accumulation of macrophages and microcalcification. The plaque ruptures once the wall stress exceeds the strength of the weak fibrous cap, causing life-threatening heart attacks and stroke.
Sickle cell disease stems from a single nucleotide polymorphism leading to the expression of haemoglobin that assembles and polymerizes in low oxygen conditions, resulting in a banana-like or sickle-like shape and the stiffening of red blood cells. A small fraction of red blood cells also become irreversibly sickled owing to repeated cycles of haemoglobin polymerization and depolymerization. Red blood cell membranes become fragile, which leads to cell lysis and the release of free haemoglobin, causing further inflammation. Red blood cells are also more adhesive than in healthy tissues, and thus, can adhere to endothelial and other cells, causing small occlusions. The increase in the number of platelets, increased cell adhesiveness and a proinflammatory environment can lead to stroke and undetectable microstrokes. However, defining the progression and frequency of these microstrokes remains elusive thus far.
Bleeding and thrombosis can be linked to the mechanical properties of blood clots. Plasma clots that are 2350% stiffer (3.1 vs 2.1 kPa) are associated with thrombotic disorders150. Whole blood clots measured using rotational thromboelastography, which provides measurements analogous to clot elasticity, are much softer in patients with bleeding disorders (10 mm vs 60 mm). Fibrin formation and clot contraction contribute to the stiffness of blot clots. For example, bleeding is associated with low fibrin formation, which is a symptom of patients with haemophilia. In thrombosis, blood clots have highly branched thin fibrin fibres that are resistant to lysis152. Changes or impairment of clot contraction, volume or forces are also related to various diseases, including acute ischemic stroke 189, acute uremia207, diabetes mellitus 207 and coronary artery disease 23. Moreover, patients missing highly contractile platelets and with average contractile forces lower than 25nN correlate with symptomatic bleeding29.
In most cases, early progression and remodelling of a plaque follows a negative feedback mechanism: outward remodelling of vessel matrix ensures maintenance of the lumen size and low shear stress82, 83. However, persistent low shear stress accelerates plaque development through promoting inflammation and matrix degradation, eventually augmenting endothelial dysfunction84, 85 and leading to the formation of a large necrotic core (FIG. 2h). Once vessel matrix outward remodelling can no longer compensate plaque growth, the plaque begins to grow into the lumen60. This inward lumen bulge substantially alters local shear stress and wall stress. Computational models have shown that high shear stress appears upstream of the bulge, low oscillatory shear stress occurs downstream of the plaque, and wall stress is higher at the shoulder regions of the plaque than the rest areas of the plaque60, 86 (FIG. 2i-j). In general, plaques are more vulnerable at the shoulder exhibiting high wall stress87 and at the upstream region showing high shear stress88 than at regions with low shear and wall stress. High shear stress increases matrix metalloproteinase production and thus, matrix degradation, initiates vascular smooth muscle cells apoptosis, induces intraplaque haemorrhage and promotes macrophage accumulation and microcalcification, causing the formation of thin and weak fibrous caps60, 61. Ultimately, once the wall stress exceeds the strength of the fibrous cap, the plaque ruptures and can cause life-threatening heart attacks and stroke.
Vascular grafts
Mechanical homeostasis are not only important in vascular physiology, but also for determining the long-term success of engineered vascular grafts. Vascular grafts are vascular substitutes that are widely used in patients with peripheral arterial disease, coronary artery disease and end stage renal disease89. Autografts are currently the gold standard in the clinic; however, they are often not available. Alternatively, synthetic grafts, such as expanded poly(ethylene terephthalate) (Dacron) or poly(tetrafluoethylene) (ePTFE), have been used since the mid-1970s90. However, these synthetic grafts are difficult to re-endothelialize and therefore, have been limited to applications in arteries with diameters >6 mm and to high flow and low resistance conditions. Although these synthetic materials have a suitable strength, their lack of appropriate compliance can lead to their failure91, because incompatible compliance can result in extra stress and disturbed flow patterns at the suture lines, which subsequently causes endothelial dysfunction, thrombosis, neo-intimal hyperplasia and anastomotic aneurysms90, 91. Moreover, synthetic grafts cannot undergo adaptive remodelling, and thus, cannot promote a state of mechanical homeostasis. Therefore, their stiff mechanical properties and low compliance lead to high resistance in the circulation, which can eventually cause various cardiovascular complications 90.
Tissue-engineered vascular grafts can overcome these issues. These vascular grafts can be engineered by assembling sheets of autologous cells cultured in vitro 92 or by fabricating a tubular ECM mesh from allogeneic smooth vascular muscle cells which are first grown on a rapidly degrading tubular polyglycolic acid scaffold in a bioreactor89 and then removed to render the grafts nonimmunogenic. Before implantation, these tissue-engineered vascular grafts have comparable strength and compliance (~3.5% per 100 mm Hg) to native vessels (human saphenous vein: 0.7–1.5 % per 100 mm Hg; human internal mammary artery: 11.5 ± 3.9 % per 100 mm Hg 89). They further allow fast endothelialization, infiltration and migration of native vascular smooth muscle cells and endothelial cells, and adaptive ECM remodelling after implantation 89, 92. Therefore, tissue-engineered vessels provide excellent patency and resist fatigue-induced aneurysms, dilatation and calcification. Tissue-engineered vascular grafts have been successfully applied in patients with end-stage renal disease93, 94 and they show great potential for coronary bypass procedures89, highlighting the importance of matching the mechanical properties of grafts with those of native vessels and of re-endothelialization and remodelling by the host cells.
Microvasculature
Mechanical homeostasis has mainly been studied in arteries; however, the concept of mechanical homeostasis can also be considered for the microvasculature. The microvasculature is composed of a network of small vessels, including arterioles, capillaries and venules, and forms a barrier responsible for the exchange of fluid and plasma metabolites in organs. Increased shear stress can induce capillary growth in skeletal muscle, whereas reduced flow leads to capillary retraction 95, 96. Capillary density is regulated by angiogenic factors, such as VEGF, which are induced by parenchymal hypoxia97. Whether a mechanical negative feedback process cooperates with angiogenic factors to modulate capillary density is difficult to study in vivo owing to the challenges associated with decoupling flow forces from angiogenic and other factors, such as changes in nutrient exchange.
Microfabrication techniques can be used to create microvasculature models in vitro that recapitulate microvascular geometry and that can be endothelialized. These models can be applied to address questions related to negative feedback mechanisms, because flow and nutrient exchange can be independently controlled43, 49, 98. Microfluidic devices with microvascular geometry can be fabricated using solid polymers, such as PDMS49 and polyester98, and more physiologically relevant hydrogels, such as collagen42–44, fibrin52, 53, alginate55, gelatin99–101 and polyethylene glycol (PEG)102–104 (TABLE 1). Top-down fabrication approaches, including 3D printing105, spatial laser degradation102 and soft-lithography-based layer-by-layer assembly106, can be used to create a variety of microfluidic geometries, on which endothelial cells can easily grow and form a monolayer. Microvasculature models can further be engineered in hydrogel-based materials by bottom-up approaches, for example, in situ vasculogenesis52, 53 (FIG. 1d) and angiogenesis42–44 (FIG. 1e). In these hydrogel-based microfluidic vascular models, physiological flow forces, such as interstitial shear stress and intraluminal flow shear stress, can be recreated.
Using microvasculature in vitro models, it has been demonstrated that interstitial flow directs endothelial morphogenesis and sprout formation97. Moreover, a flow shear stress threshold for angiogenesis44 has been proposed: a shear stress of > 10 dyn/cm2 triggers matrix metallopeptidase 1-mediated endothelial sprouting towards the direction of draining interstitial fluid flow, and the subsequent higher microvessel density reduces flow shear stress, suggesting a mechanical negative feedback mechanisms governing angiogenesis. Conversely, halted flow causes microvessels to retract. In vitro microvasculature models also enabled the identification of a NOTCH1-mediated mechanosensitive pathway in endothelial cells 107. Activation of NOTCH1 signalling by laminar shear stress leads to the formation of a multicomplex unit that regulates cytoskeletal remodelling and barrier functions.
In vitro models further allow the incorporation of substrates with physiological or pathological stiffnesses which can be accurately measured by performing AFM measurements6, 108. Using such models, it has been shown that integrins mediate the response of endothelial cells to the stiffness of the abluminal substrate, and that the endothelial permeability increases as substrates stiffen via a Rho-associated signalling pathway108. Stiff substrates induce excess activation of Rho, causing an increase in intracellular contractile forces. These forces pull cell–cell junctions apart, resulting in a leaky endothelium108. Since leaky endothelium is a hallmark of atherogenesis, it suggests that age-associated blood vessel stiffening is a direct cause of atherosclerosis development. Using an agarose–gelatin interpenetrating network, tuned to a physiological stiffness of ~ 20 kPa, we showed that an engineered microvasculature can exhibit a physiological endothelial barrier function for months106. However, most in vitro models only contain endothelial cells. Incorporating other cell types, such as pericytes and vascular smooth muscle cells, would enable the investigation of feedback loops and of crosstalk between the different cell types; for example, by using hydrogel materials, such as collagen, fibrin, and methacrylated gelatin that allow encapsulation of vascular smooth muscle cells and pericytes and that support long-term culture of endothelial monolayers. Importantly, the hydrogels need to be compatible with microfabrication techniques, provide appropriate mechanical properties and allow remodelling by cells to recreate mechanical homeostasis.
Blood
A flowing biomaterial
The concept of mechanical homeostasis can also be applied to blood, although the feedback mechanisms may be less complex and easier to perturb. The material properties and the mechanical equilibrium of single blood cells as well as of a collective of blood cells, contribute to the material properties and behaviour of blood, and can be related to blood pathologies.
Blood is a dynamic shear-thinning biofluid composed of a variety of proteins, small molecules, gases and mechanosensitive cells, including red blood cells, white blood cells and platelets, which are suspended in plasma (FIG. 3a). The size, shape and stiffness of blood cells remain uniform and are regulated by cytoskeletal proteins, ion pumps and the nucleus. Micropipette aspiration109, microfluidic deformation assays110, optical tweezes111 and atomic force microscopy112 have been used to measure the stiffness of blood cells, which range from 100’s of Pa112 to several kPa110. The cytoskeleton of blood cells influences the stiffness of the cells, which has been demonstrated by pharmacologically impairing or genetically modifying cytoskeleton proteins, such as spectrin, which is unique to red blood cells, actin113, microtubules and intermediate filaments114. Remarkably, each blood cell type has its own unique composition of cytoskeletal proteins influencing shape and stiffness; for example, discoid red blood cells uniquely rely on spectrin (FIG. 3a), spheroid white blood cells rely mainly on actin and intermediate filaments(FIG. 3b), and resting platelets contain an actin cytoskeleton with a prominent microtubule ring (FIG. 3c).
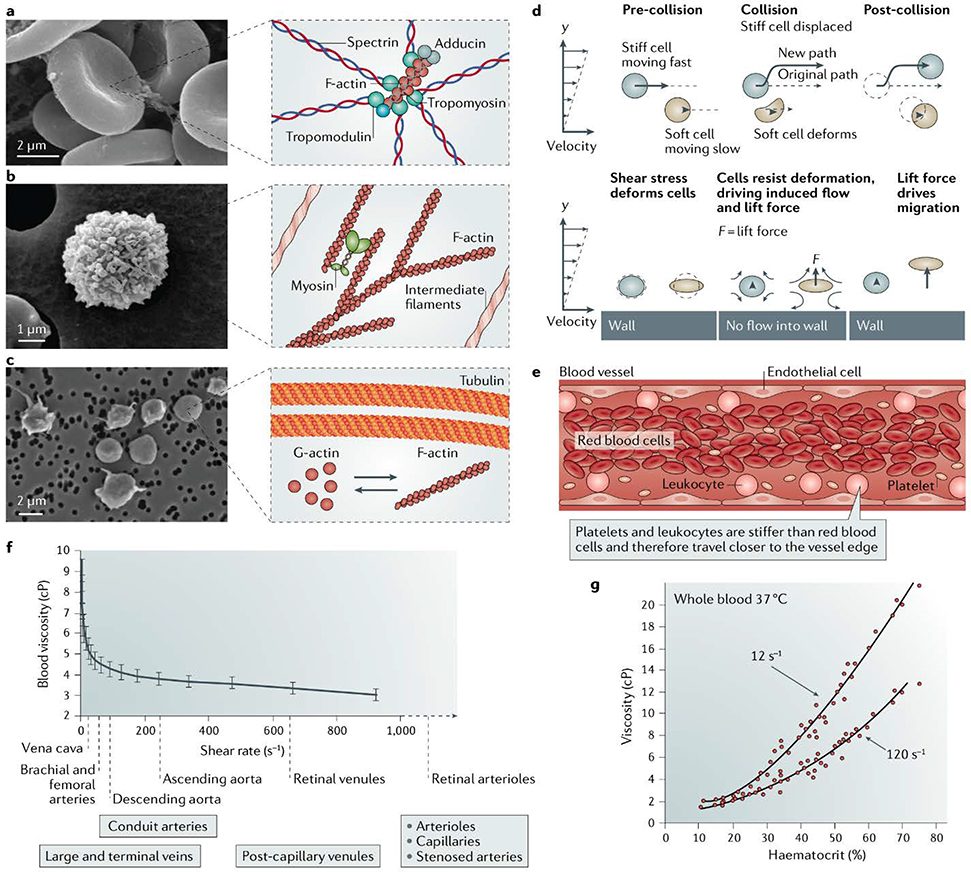
Figure 3: Material properties of individual blood cells.
Blood is a shear thinning fluid composed of cells suspended in a plasma. Each blood cell type is supported by specific cytoskeletal structures resulting in different shapes and material stiffness.211–213 a) The electron micrograph shows a red blood cell with a cytoskeleton mainly composed of a spectrin mesh and actin, which significantly contributes to this cell’s shape and exceptional deformability. b) The electron micrograph shows a white blood cell with a cytoskeleton mainly composed of actin and intermediate filaments. c) The electron micrograph shows platelets, which possess microtubules, and F-actin that rapidly disassembles and reassembles upon platelet activation. d) Margination is triggered by physical interactions between cells of different stiffness and the vessel wall, and is a purely passive phenomenon112. e) Margination leads to an increase in the concentration of white blood cells and platelets travelling along the periphery of the vessel. f) Blood viscosity as a function of shear stress is shown. The bulk material properties of blood, such as viscosity (shown), change in response to local shear stress rates214. g) Haematocrit and applied shear stress change the viscosity as measured with a viscometer. Many parameters, such as the number of cells 215, plasma volume and cell stiffness affect the bulk properties of blood. Changes to these parameters are often associated with pathological conditions.
Defining the mechanisms that regulate single cell stiffness is challenging owing to the complexity of cells in terms of protein composition, regulation and dynamics as well as cellular interactions. However, there is evidence that the dynamic rearrangement of structural components contributes to the regulation of the overall stiffness 115. For example, actin116 and spectrin117 are able to change behaviour in response to force116, 118. Unfortunately, deciphering the mechanical feedback of cytoskeletal networks remains difficult, owing to a lack of tools capable of examining nanoscale network formation during mechanical perturbation, and a lack of studies on other cytoskeletal and nuclear proteins, especially intermediate filaments. The nucleus also plays a dominant role in defining the mechanical properties of single cells. For example, red blood cells, which do not possess a nucleus, have an average stiffness of 150 Pa112, whereas neutrophils, which have lobed nuclei, have an average stiffness of 400 Pa112. Correspondingly, lymphocytes, which have a large central nucleus, have a stiffness on the order of 2900 Pa110. The nuclear proteins lamin A and C are important regulators of nuclear mechanics119, and lamin A is involved in mechanosensitive differentiation as shown in hematopoietic stem cells 120 and mesenchymal stem cells101. However, the underlying feedback mechanisms associated with nuclear stiffness121, especially in blood cells, remain elusive.
Mechanical properties of red blood cells
Healthy red blood cells have tightly controlled biophysical properties, and perturbations to the stiffness and shape of red blood cells are associated with impaired function, suggesting the existence of a state of mechanical equilibrium that is maintained in healthy tissue 122. Red blood cells have a discoid shape, maximizing their surface area to volume ratio, and a deformable soft cytoskeleton that is resistant to lysis and fracture, enabling them to pass through constricted spaces within the vasculature. Red blood cells do not possess a nucleus and have spectrin-based cytoskeletal architecture that provides less rigidity and is more deformable than other blood cells. Therefore, red cell mechanics influenced by the haemoglobin content membrane rigidity, hydration levels and ion pump function also impact cell stiffness and viscosity – parameters that are tightly regulated.
Spectrin is the major structural protein of red blood cells (FIG. 3a)122. Moreover, a variety of other cytoskeletal proteins, such as myosin IIA123, are incorporated into the spectrin network, affecting cellular structure. Owing to their specific cytoskeletal architecture, red blood cells are substantially softer than other blood constituents and able to undergo elastic deformations. The ability to maintain membrane integrity while undergoing deformation is crucial for the transport of oxygen through reactive haemoglobin, which is encapsulated in red blood cells. Free haemoglobin cannot be transported in blood, because it would cause acute and chronic vascular disease, inflammation, thrombosis and renal impairment 124. Therefore, pathological changes to the cytoskeletal structure that result in impaired membrane integrity are associated with multiple diseases that arise from different origins. For example, hereditary spherocytosis involves mutations to cytoskeletal proteins122 and sickle cell disease indirectly leads to changes in the cytoskeletal structure, yet both diseases disrupt the red cell membrane and are associated with haemolysis. The cargo encapsulated within red blood cells has an impact on the mechanical properties of red blood cells. Cargo type, concentration and structure affect the mechanical properties of the cell, which, if dysregulated, can cause pathological effects. For example, in patients with sickle cell disease125 (BOX 1), mutated haemoglobin polymerizes in low oxygen conditions leading to an increase in cell stiffness and reduced deformability. In addition, as the red cell continuously travels between low and high oxygen concentrations, the fluctuating mechanical loads to the membrane applied by polymerizing haemoglobin contribute to the aforementioned haemolysis126. Similarly, in the early stages of malaria infection, red blood cells that contain the parasite become stiffer 127, 128 and more adhesive, allowing them to stick to the wall of small blood vessels and evade detection by the immune system129.
Hydration levels also substantially impact the mechanical properties of red blood cells. For example, red blood cells contain Piezo1, a mechanically sensitive ion channel, which connects mechanical force to red blood cell volume 130. Piezo1 enables prolonged calcium entry in response to mechanical stretch and its dysfunction has been correlated to hereditary xerocytosis131–133. In murine red blood cells, this gain-of-function leads to excess calcium import, which activates the KCa3.1 Gardos channel, leading to potassium export, and thus, dehydration of the cells 130. Severe dehydration in adults and especially in neonates is associated with an increased risk of thrombosis134, 135, which may be related to the corresponding increase in red blood cell stiffness and viscosity; however, the exact underlying mechanisms remain unclear thus far. Even small changes in the microenvironment can affect red blood cell transport in the microvasculature; for example, the tonicity of a clinical intravenous fluid affects sickle red blood cell adhesion to endothelial cells and transport through capillary size microchannels11. Therefore, consistent material properties and thus, mechanical homeostasis, seem to be an important factor for healthy red blood cell function.
Emergent properties of the blood cell collective
Margination
Blood can also be considered as a collective of cells with emergent behaviours that arise from the mechanics of single cells and their interactions. For example, when traveling through a vessel, stiff leukocytes and small platelets marginate and predominantly travel near the periphery of the blood vessel. This effect is mediated by the differences in cell size, shape and stiffness of the different blood cell types136. Indeed, margination is facilitated by an asymmetry in cellular collisions and by lift forces generated on deformable cells 112. When a soft red blood cell and a stiff white blood cell collide, the soft cell primarily deforms and the stiff cell primarily displaces (FIG. 3d). Importantly, this displacement can push stiff cells across streamlines and towards the vessel wall (FIG. 3e). After many collisions, lift forces generated at the vessel wall have a smaller effect on stiffer white blood cells than on softer red blood cells112, which helps to trap the cells at the periphery (FIG. 3f). Owing to their high stiffness and small size, platelets also travel near the vessel periphery similar to white blood cells 137.
Therefore, the process of margination depends on the mechanical stiffness and size differences between single blood cells, which thus contribute to an emergent collective cell behaviour. There is further evidence that the mechanical properties of the blood cell collective are also regulated on a macroscopic level by the kidneys and spleen. The kidneys regulate plasma volume, red blood cell number138 and electrolyte levels, which influence single cell stiffness and contribute to blood viscosity (FIG. 3g)139. As the primary regulators of sodium and water excretion, the kidneys receive feedback on plasma volume by the hormone-based renin-angiotensin-aldosterone system and by the nervous system through baroreceptors, which can sense vessel pressures that are too high or too low138. In addition, peritubular fibroblasts in the kidneys are transcriptionally regulated by oxygen levels, and respond to low oxygen by producing erythropoietin, which stimulates red blood cell production in the bone marrow140, and therefore, changes the overall blood viscosity. Thus, the kidneys are involved in the regulation of the number of red blood cells and the plasma volume. The spleen further helps to control cell stiffness by mechanically filtering red blood cells that show reduced deformability141, 142.
Effect on haemostasis and the immune system
A change in the collective cell number may also impair haemostatic function126, 132. For example, abnormally high numbers of white blood cells can lead to leukostasis, a medical condition in which cells block blood flow, which can cause a transient ischemic attack and a stroke 143. Similarly, high numbers of red blood cells can promote blood clotting by increasing platelet margination, which increases platelet accumulation at injury sites137, 144. Although the mechanisms are unclear, thrombotic complications are a known concern for patients with polycythemia vera, which is a type of blood cancer, in which the bone marrow produces too many red blood cells145. A low number of red blood cells can also be expected to influence the margination process and possibly lead to more bleeding; indeed, anemia is a predictor for bleeding in patients with atrial fibrillation and in patients taking anticoagulants 146. However, studying the correlation between anemia and bleeding in a clinical setting is difficult, because excessive bleeding can also lead to low red blood cell counts, and therefore, confound measurements.
In addition to cell number, the material properties of cell collectives can impact haemostatic and/or immune function. For example, it has been demonstrated that an increase in red blood cell stiffness interferes with leukocyte adhesion147. We further showed that endothelial cell permeability106 increases with increasing red blood cell stiffness. Similarly, a systemic increase in white blood cell stiffness is associated with many pathological conditions, such as acute lung injury, sepsis, posttraumatic shock, diabetes and stroke148. We demonstrated that a decrease in leukocyte stiffness can be correlated with a decrease in margination, a decrease in the time leukocytes reside in capillary beds, which increases the number of circulating leukocytes112 and may have important implications for immune cell trafficking and susceptibility to infection.
Various blood pathologies can be associated with altered collective cell behaviour, which is modulated by the stiffness, deformability, number and interactions of the different blood cell types as well as by the plasma volume. Therefore, we hypothesize that an optimal mechanical equilibrium of the blood cell collective exists to ensure hemostasis and immune function, which is regulated by feedback mechanisms on the single cell level and macroscopically by the spleen and liver. However, more research is required to validate the concept of mechanical homeostasis and to decipher regulatory mechanisms in blood.
Blood clots
Blood clots are naturally occurring, self-assembled, active materials composed of contracting platelets, which pull on a nascent polymeric fibrin mesh that may contain entrapped red and white blood cells. Blood clots maintain vascular integrity by creating a plug capable of withstanding the forces applied by flowing blood. Soft clots are associated with bleeding149 and stiff clots are associated with thrombotic disorders150, suggesting that an optimal mechanical stiffness is required to facilitate healthy haemostasis (BOX 1). Although the mechanistic link between clot function and stiffness is still being elucidated, these findings allow for the hypothesis that blood clots must be stiff enough to prevent bleeding yet soft enough to not cause unhealthy blood clotting. In both conditions – pathological clotting and excessive bleeding – the mechanical equilibrium of platelets 23, 29 and/or of fibrin is perturbed 151, 152. Moreover, the material properties of blood clots change in disease.
Fibrin structure and formation
Blood clots contain a network of fibrin (FIG. 4a), which is a fibrous biopolymer that reinforces the clot. Fibrin is enzymatically formed from the soluble plasma protein fibrinogen in response to injuries in the vasculature. The concentration of fibrinogen in blood (1.5 – 4 g L−1) is an order of magnitude higher than that of other plasma proteins. Fibrin has interesting mechanical properties153–160; single fibrin fibres are elastic154, possess extraordinary extensibility161 and a stiffness that changes with diameter162, from ~1.5 MPa for a 1.4 nm fiber to ~250 kPa for a 2.2 nm fiber. Fibrin mechanics have been measured using numerous techniques including atomic force microscopy163, optical tweezers154, rheometers 164 and universal testing machines153. However, fibrin typically forms viscoelastic164 networks that stiffen when stretched153, 165 and align in response to applied loads153 or fluidic forces158. In addition, fibrin can self-assemble into a sheet at the air–blood interface in vitro and in vivo, which can encapsulate blood clots and protect against microbial invasion166. Fibrin formation and destruction are primarily controlled by thrombin and plasmin; however, biophysical cues also play a role in the regulation of fibrin network formation. For example, platelet contraction 167, fibre diameter and fibre strain impact fibrin lysis by plasmin168 and thus, blood clot lysis. Single molecule experiments have further demonstrated that the lifetime of fibrin–fibrin bonds increase, peak and decrease with increasingly high forces, suggesting that mechanical homeostasis may be established by a force-based regulatory mechanism169, 170.
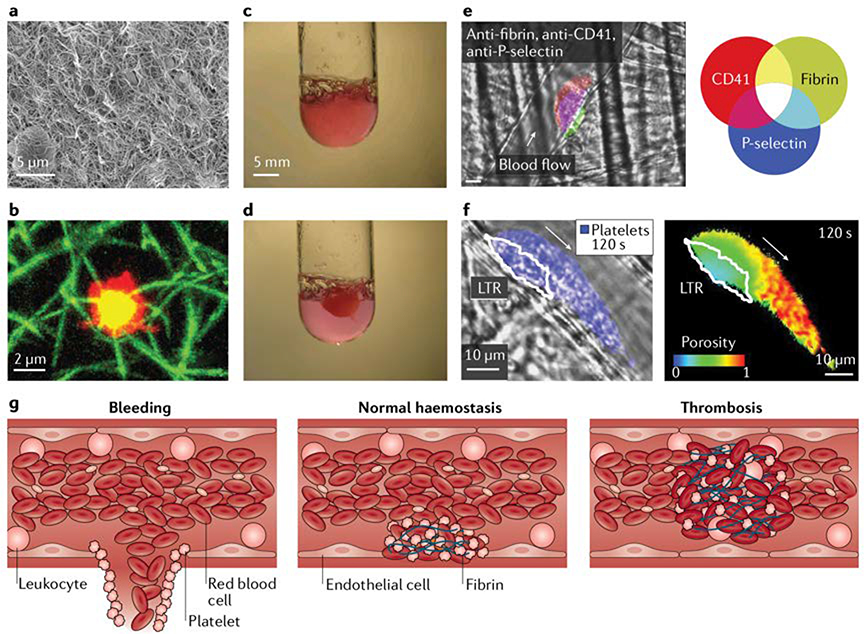
Figure 4: Blood clots.
Blood clots may contain red blood cells and white blood cells in addition to platelets and a fibrin mesh, which polymerizes from the plasma protein fibrinogen after tissue damage. a) The electron micrograph shows the fibrin matrix on the exterior of a blood clot formed in vitro. b) Platelets (red) can be thought of as actuators of clotting, contracting fibrin (green) triggered by local biochemical and biomechanical stimuli. Yellow indicates platelets and fibrin colocalization. c) Upon activation, platelets and nascent fibrin form into a gel structure. d) Contractile platelets can dramatically reduce the size of a clot. e) Blood clots can self-assemble in vivo into platelet-rich (CD41, red) structures with a layer of fibrin (green) anchoring the clot to the vessel183. In clots, platelets have different activation levels, a core of platelets is defined by a marker of platelet activation, P-selectin (blue), and is surrounded by a shell of p-selectin negative platelets. Overlay of platelets and P-selectin is purple and overlay of all 3 fluorophores is white. f) Platelet activation levels affect the porosity of a clot, which affects the transport of solutes in the clot. An in vivo image of a platelet (blue) overlaid with an outline of a low transport region (LTR), which coincides with the activated platelet core. To define the LTR, fluorescent albumin was incorporated into the initial clot and diffused out over time. A pseudo colored image of the porosity was created by monitoring the loss of fluorescent signal over time, which correlates with loss of albumin and is dictated by the local porosity.197. g) We hypothesize that mechanical homeostasis ensuring optimal forces, structure, porosity and stiffness enable correct clotting and that perturbations to this process can lead to either bleeding or excessive clotting.
The mechanisms of the regulation of fibrin structures are not yet fully understood; however, perturbations to fibrin formation can be associated with pathophysiological conditions and altered blood clot properties. For example, patients suffering from haemophilia, dysfibrinogenemia or afibrinogenemia, have impaired or no fibrin formation leading to the generation of unstable clots that are prone to rupture and that can break away. Structural changes to fibrin can further be related to multiple myeloma171, diabetes23, nephrotic syndrome172, deep vein thrombosis173, atrial fibrillation and stroke174. In all these conditions, the fibrin meshes are stiffer, more densely packed with fibres and/or less prone to fibrinolysis than in healthy controls. Although the underlying mechanisms for this altered structure remain unclear, these diseases can be associated with increased thrombin generation175, increased fibrinogen levels176 and/or biochemical changes of fibrin itself23, which all affect fibrin structure. Decoupling these different factors and identifying the dominant cause of altered fibrin structure remains challenging and requires the development of new tools and assays. Currently, fibrin formation and structure are mainly investigated by clotting platelet-poor plasma of patients, which contains a myriad of factors that can affect fibrin structure and which varies in fibrinogen, thrombin and coagulation factor concentrations. Therefore, using this approach, it is difficult if not impossible to isolate and test the effect of each condition on the final blood clot structure.
Platelets
Actuators of blood clotting
Nascent fibrin provides the structure of blood clots and reinforces the developing clot, whereas platelets can be thought of as the actuators of blood clotting, which can sense and apply force to equilibrate the local microenvironment (FIG. 4b). Sensing occurs biochemically on the platelet surface through a range of receptors, such as integrins, that can sense thrombin, ADP, epinephrine, collagen, vWF and fibrinogen. In addition, changing the biophysical arrangement of biochemical cues can affect the platelet response. For example, the extent of platelet spreading on glass surfaces depends on the concentration of fibrinogen on the surface177. Paradoxically, platelets spread more on surfaces with low fibrinogen concentrations than on surfaces with high fibrinogen concentrations, with differences in the activation of signalling pathways.
Mechanical and biophysical stimuli can trigger a response in platelets. We have shown that platelets adhere, progressively spread less and have lower levels of activation as the stiffness fibrinogen or collagen coated polyacrylamide surfaces decreases from 100 kPa to 0.25 kPa 39, 40. Interestingly, platelet mechanosensing is mediated by different pathways, depending on whether the platelet is undergoing initial adhesion or if it is actively spreading39. Platelets attached to a surface are further capable of applying substantial forces (1.5 to 79nN), comparable to muscle cells if cell size is considered178. Application of force by platelets is a mechanosensitive process, and the force increases with increasing substrate stiffness29, 178; however, biochemical and mechanical conditions synergize to maximize platelet forces29. In addition, there is an upper limit to the forces that a platelet can apply, which is defined by the total amount and the organization of actin and myosin. These studies suggest that platelets are actuators of blood clotting, which control clot stiffness and are regulated by signalling networks that respond to the biochemical and mechanical microenvironment.
Biophysical properties
We are only in the early stages of understanding platelet biophysics; however, it is known that perturbations to platelet forces are associated with pathophysiological conditions. In a blood clot, platelets facilitate a volumetric reduction 20 and concomitant stiffening 179, that can both be on the order of one magnitude. This process is referred to as the second phase of clot formation or clot contraction (FIG. 4c-d). Clot contraction which includes contributions from platelet biophysics, fibrin structure, and incorporated cells, are altered in many pathophysiological conditions, including severe coronary artery disease 180 and systemic lupus erythematosus 181. Microenvironmental cues, including the mechanical properties of the underlying matrix substrate39, 178, matrix geometry182, biochemical conditions183 and shear stress184, 185, mediate how platelets exert force on and contract other platelets and fibrin polymers.
Tools such as a rheometre179, hemostasis analysis system186, automated optical contraction analyzer20 and platelet contraction cytometer29 can be used to assess the biophysical properties of platelets. For example, platelets of patients with Glanzmann’s thrombasthenia23, cannot aggregate and apply force owing to defective and low levels of fibrinogen-binding integrin αIIbβ3. However, a thorough understanding of single platelet biophysics in diseases related to bleeding or clotting remains elusive.
Bulk platelet forces can be measured using a customized whole-blood assay of platelet function, which can also measure the elastic modulus of blood clots23. Using this assay, an increase in platelet force could be correlated to disorders with clotting complications, such as chest pain187, severe coronary artery disease180 and thromboangiitis obliterans188. Whole-blood clotting assays were also used to demonstrate that impaired clot contraction occurs in diseases with clotting complications, such as acute ischemic stroke 189, sickle cell disease 20, systemic lupus erythematosus 181 and polycythemia vera 190, 191. However, it is difficult to directly compare force and contraction assays. Moreover, numerous processes need to be coordinated to control blood clotting, which all affect the biophysical properties of platelets. Nevertheless, these results suggest that platelet function may differ in diseases related to blood clotting as compared to controls.
Microtechnology-based approaches can be applied to study platelet forces at the single cell level29, 192, 193. For example, we developed a hydrogel-based platelet contraction cytometer to measure contractile forces of individual platelets. As expected platelets have lost their ability to exert forces in patients symptomatic bleeding and an actin or myosin disorder. Interestingly, platelets also lost their ability to exert forces in patients that had unexplained bleeding29. Despite having normal values for all standard clinical tests of haemostasis, these patients still had symptomatic bleeding. More research is still required to understand mechanical homeostasis of whole blood clots; however, dysregulation of platelet force is certainly linked to bleeding and clotting.
Assembly and structure of clots
The initiation and assembly of blood clots impact clot properties. Blood clots have the remarkable ability to self-assemble and organize into a variety of architectures. Once coagulation begins, homogeneous, static blood forms a blood clot that contains a core of compressed red blood cells surrounded by a fibrin mesh194. In flowing conditions, in vivo experiments have demonstrated that blood clots composed predominantly of platelets adopt a core–shell architecture, established by the different activation levels of the platelets within the clots183. In this structure, a core of highly activated platelets that support fibrin formation is surrounded by a shell of less activated platelets195–197 (FIG. 4e). The level of platelet activation is controlled by biochemical signalling events; however, biophysical cues, such as platelet-mediated porosity of the blood clot, also play a key role in controlling gradients of various platelet activators, such as ADP and thrombin198 (FIG. 4f). Moreover, as coagulation is controlled by a cascade of chemical reactions that occur in the complex hemodynamic environment, conditions affecting mass transfer and chemical kinetics affect blood clot formation199. In addition, the presence of valves78 or certain geometries, such as aneurysms, stenoses and bifurcations 47, can influence the adhesion of blood cells and the initiation of clotting. As the clot matures, platelet contraction may redistribute the less adhesive “procoagulant” platelets to the clot periphery, which expose high amounts of phosphatidylserine and enhance fibrin formation200 The different clot architectures can be expected to affect the mechanical equilibrium of the clot, whereas the details of the structure-mechanics relationship remain to be elucidated.
Correlating the mechanical properties of blood clots and its various components with disease requires an understanding of the mechanical properties of the individual components, their interactions and assembly, and the contraction process. The feedback and regulatory mechanisms that maintain optimal stiffness values also need to be investigated; in particular, the individual contribution of each clot component to the overall clot stiffness throughout the clotting process, the interactions that drive clot contraction, the assembled architectures, the translation of microscale changes in cells, platelets and fibrin into macroscale behaviours, and the interactions between platelets, fibrin and other cells. For example, studies of clot component interactions have shown that platelets can fold fibrin into a structure featuring ‘kinks’201. Finally, more research is needed to understand whether pathophysiological changes to the mechanical properties of platelets, fibrin and blood clots are associative or causative.
Mechanical homeostasis in blood clots?
Defining mechanical homeostasis as the process by which a biological system maintains a mechanical equilibrium using feedback mechanisms, this concept may also be applied to blood clots (FIG. 4g). Blood clots have an optimal and regulated mechanical ‘set point’ or equilibrium, at which haemostasis is supported instead of thrombosis. Mechanosensitive platelets can decrease the size of clots and stabilize them by exerting forces to regulate their mechanical equilibrium. Therefore, we hypothesize that the blood clot maintains mechanical homeostasis, through mechanical feedback loops. For example, the mechanosensitive behaviour of platelets contributes to a uniform clot contraction process178. During clot development, activated platelets in the heterogeneous fibrin gel can pull more strongly on denser and stiffer fibrin regions than on softer fibrin, with both regions experiencing the same net changes in strain. Similarly, the mechanosensitivity of platelets39 and the fact that they stiffen with contraction178 could provide a positive feedback loop mechanism of platelet activation within the growing thrombus, to further activate platelets and trigger the release of granules. Biomechanical and biochemical pathways might then coordinate to maximize platelet forces29. As forces increase, the shorter bond lifetimes of fibrin-fibrin interactions may help to limit the maximum clot stiffness by altering the fibrin architecture. Towards the end of the life of a platelet, when phosphatidylserine is exposed, platelets may even stop applying forces32, suggesting that platelets innately have the ability to tune the mechanical stiffness of the system. Forces applied by platelets may even regulate the growth of a clot since contraction moves less adhesive, soft, procoagulant platelets to the clot periphery, which could play a role in halting the uncontrolled growth of a clot. Blood clots are transient structures that block the flow of blood at the early stages of wound healing. Given the importance of clotting and the involvement of mechanosensitive cells and proteins, mechanical feedback mechanisms, similar to the ones observed in blood vessels, might be involved in clot regulation.
Conclusions and perspective
Blood and blood tissues have mainly been investigated from a biological and biochemical perspective. However, a materials-based framework can offer important insight into such a dynamic system, which is also greatly regulated by physical interactions. In this Review, we discuss the concept of mechanical homeostasis for blood and blood tissues and the idea that competing mechanical interactions are balanced with associated feedback mechanisms. Similar to the concept of cellular tensegrity, which proposes a system in which cells are viewed as prestressed structures capable of sensing and responding to mechanical forces, the concept of mechanical homeostasis in blood suggests dynamic mechanically-driven sensing and response mechanisms of the various blood components. Acto-myosin forces prestress and regulate a structure that is composed of tensile cytoskeletal microfilaments and intermediate filaments that are balanced by elements resisting compression such as microtubule struts and ECM adhesions.. Similarly, blood cells and tissues can maintain a mechanical equilibrium with tensile and compressive elements, which is regulated by feedback mechanisms. Since mechanics is disrupted in disease, this suggests that a mechanical equilibrium may be required to ensure tissue function.
Investigating the concept of mechanical homeostasis in blood might provide new insights not only in basic haematology, but also for diagnostics and therapeutics. Blood vessels, blood and blood clots sense and respond to numerous biochemical and biomechanical stimuli, which are often altered in disease. Technologies based on better recapitulating the in vivo mechanics of blood and blood tissues have enabled new functional studies, such better understanding platelet adhesion, spreading 39, 40, and force 29. Soft materials, such as hydrogels, support the 3D culture of endothelial cells106 and thus, can be used to investigate how changes in red blood cell properties or haemolytic by-products influence endothelial cell permeability. Furthermore, blood microenvironments often feature multi-cellular structures with collective behaviours that cannot necessarily be extrapolated from single cell studies. For example, platelets and fibrin are relatively well studied at the single cell level, but much less is known about the interaction of these components and how they collectively lead to clot contraction.
Insights into the mechanical and material properties of blood components have led to exciting innovative approaches in diagnostics and therapeutics. For example, creating soft hydrogel microparticles with a similar size and shape than those of red blood cells and with low stiffness (7.8 kPa) allow the optimization of the biodistribution of microparticles in blood by mimicking the behaviour of red blood cells. Interestingly, the low elastic modulus of these hydrogel microparticles increased their circulation time in vivo, from a half-life of <10hrs for stiff particles (>17kPa) to a half-life of 93 hrs for low stiffness (7.8 kPa) particles. This enabled the particles to avoid sequestration in many organs including the lungs, which can lead to pulmonary embolism202. Similarly, by using red blood cells as carriers, clearance and non-optimal delivery of nanoparticles and viral vectors can be avoided203. Injecting red blood cells loaded with nanoparticles upstream of the target organ, enables delivery of the particles to this organ with substantially increased transfer efficiency. Combining knowledge about margination and fluid mechanics allows the design of a drug delivery system to transport drugs directly to areas of high shear stress. For example, aggregates of nanoparticles coated with therapeutic compounds can be engineered to break apart in high shear regions, allowing targeted drug delivery to the vessel wall204.
The mechanical forces exerted by platelets may provide a new type of biophysical biomarker. Collectives of platelets exerting low forces, typically with an average force below 25nN, are linked to symptomatic bleeding in patients, who show normal haemostasis using existing clinical tests29. Remarkably, this correlation is independent of platelet biomarkers of activation, showing that mechanical forces offer an independent measure of health. Platelet contraction could further be exploited for the design of a drug delivery platform capable of locally modifying the biochemical microenvironment of a clot205. Strategies using soft materials can further be applied to limit biomechanics-induced pathological clotting on implanted materials since platelet adhesion is reduced on soft surfaces39, 40. Similarly, ultrasoft microgels augment blood clotting under physiological flow conditions206. These spherical ultra-low cross-linked microgels with a diameter of 1 μm were capable of spreading into discs on glass that had a diameter of 2 μm and a height of 4 nm. By adding molecular-recognition motifs, these platelet-like particles collapse fibrin networks in vitro and reduced bleeding times in vivo. Therefore, the mechanics and materials properties of blood and blood tissues offer a new tool for diagnosis and treatment of blood-related disorders, showcasing the important contribution of materials science to medicine.
Acknowledgements
We’d like to acknowledge the Georgia Tech Institute for Electronics and Nanotechnology (a member of the National Nanotechnology Coordinated Infrastructure, which is supported by the National Science Foundation (grant ECCS-1542174), and the financial supports provided by National Science Foundation grants CAREER CBET-1150235 (to W.A.L.) and DMR-1809566 (to W.A.L and D.R.M..), National Institutes of Health grants R01HL140589 (to W.A.L.), R21MD011590 (to W.A.L.), R01HL130918 (to W.A.L.), U54HL141981 (to W.A.L.), R01HL121264 (to W.A.L.), K25HL141636 (to D.R.M.) and R21EB026591 (to D.R.M.) D.R.M. would like to thank C. Rebecca Dillon for advice and useful discussions.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Ingber DE, Wang N & Stamenovic D Tensegrity, cellular biophysics, and the mechanics of living systems. Reports on Progress in Physics 77 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weng SN, Shao Y, Chen WQ & Fu JP Mechanosensitive subcellular rheostasis drives emergent single-cell mechanical homeostasis. Nature Materials 15, 961–967 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paszek MJ et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 8, 241–254 (2005). [DOI] [PubMed] [Google Scholar]
- 4.Humphrey JD, Dufresne ER & Schwartz MA Mechanotransduction and extracellular matrix homeostasis. Nature Reviews Molecular Cell Biology 15, 802–812 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hahn C & Schwartz MA Mechanotransduction in vascular physiology and atherogenesis. Nature Reviews Molecular Cell Biology 10, 53–62 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kohn JC, Lampi MC & Reinhart-King CA Age-related vascular stiffening: causes and consequences. Frontiers in Genetics 6 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Llaurado G et al. Arterial Stiffness Is Increased in Patients With Type 1 Diabetes Without Cardiovascular Disease A potential role of low-grade inflammation. Diabetes Care 35, 1083–1089 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tomaiuolo G Biomechanical properties of red blood cells in health and disease towards microfluidics. Biomicrofluidics 8 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tran R et al. Biomechanics of haemostasis and thrombosis in health and disease: from the macro- to molecular scale. Journal of Cellular and Molecular Medicine 17, 579–596 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lampi MC & Reinhart-King CA Targeting extracellular matrix stiffness to attenuate disease: From molecular mechanisms to clinical trials. Science Translational Medicine 10 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Carden MA et al. Extracellular fluid tonicity impacts sickle red blood cell deformability and adhesion. Blood 130, 2654–2663 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akhtar R, Sherratt MJ, Cruickshank JK & Derby B Characterizing the elastic properties of tissues. Materials Today 14, 96–105 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu PH et al. A comparison of methods to assess cell mechanical properties. Nature Methods 15, 491-+ (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shirwany NA & Zou MH Arterial stiffness: a brief review. Acta Pharmacologica Sinica 31, 1267–1276 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Andel CJ, Pistecky PV & Borst C Mechanical properties of porcine and human arteries: Implications for coronary anastomotic connectors. Annals of Thoracic Surgery 76, 58–64 (2003). [DOI] [PubMed] [Google Scholar]
- 16.Hirata K, Kawakami M & O’Rourke MF Pulse wave analysis and pulse wave velocity - A review of blood pressure interpretation 100 years after Korotkov. Circulation Journal 70, 1231–1239 (2006). [DOI] [PubMed] [Google Scholar]
- 17.Wentland AL, Grist TM & Wieben O Review of MRI-based measurements of pulse wave velocity: a biomarker of arterial stiffness. Cardiovasc Diagn Ther 4, 193–206 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tai NR, Salacinski HJ, Edwards A, Hamilton G & Seifalian AM Compliance properties of conduits used in vascular reconstruction. British Journal of Surgery 87, 1516–1524 (2000). [DOI] [PubMed] [Google Scholar]
- 19.Stone MJ & Bogen SA Evidence-based focused review of management of hyperviscosity syndrome. Blood 119, 2205–2208 (2012). [DOI] [PubMed] [Google Scholar]
- 20.Tutwiler V et al. Kinetics and mechanics of clot contraction are governed by the molecular and cellular composition of the blood. Blood 127, 149–159 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartert H Blutgerinnungsstudien Mit Der Thrombelastographie, Einem Neuen Untersuchungsverfahren. Klinische Wochenschrift 26, 577–583 (1948). [DOI] [PubMed] [Google Scholar]
- 22.Whitten CW & Greilich PE Thromboelastography (R) - Past, present, and future. Anesthesiology 92, 1223–1225 (2000). [PubMed] [Google Scholar]
- 23.Carr ME Development of platelet contractile force as a research and clinical measure of platelet function. Cell biochemistry and biophysics 38, 55–78 (2003).This Review comprehensively describes the measurement of bulk platelet forces in a clinical setting, suggesting that forces exerted by platelet may be altered in pathophysiological conditions.
- 24.Qiu YZ, Ciciliano J, Myers DR, Tran R & Lam WA Platelets and physics: How platelets “feel” and respond to their mechanical microenvironment. Blood Reviews 29, 377–386 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Davies PF Flow-Mediated Endothelial Mechanotransduction. Physiological Reviews 75, 519–560 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muller DJ & Dufrene YF Atomic force microscopy: a nanoscopic window on the cell surface. Trends in Cell Biology 21, 461–469 (2011). [DOI] [PubMed] [Google Scholar]
- 27.Reinhart-King CA, Dembo M & Hammer DA The dynamics and mechanics of endothelial cell spreading. Biophysical Journal 89, 676–689 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu ZJ, Sniadecki NJ & Chen CS Mechanical Forces in Endothelial Cells during Firm Adhesion and Early Transmigration of Human Monocytes. Cellular and Molecular Bioengineering 3, 50–59 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Myers DR et al. Single-platelet nanomechanics measured by high-throughput cytometry. Nature materials 16, 230–235 (2017).In this paper, a novel cytometer is reported for characterizing platelet contraction forces at a single platelet level, demonstrating how low platelet contraction forces correlate with bleeding disorders and therefore showing the first demonstration of a biophysical biomarker.
- 30.Kim J, Zhang CZ, Zhang XH & Springer TA A mechanically stabilized receptor-ligand flex-bond important in the vasculature. Nature 466, 992–U123 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yago T et al. Platelet glycoprotein Ibα forms catch bonds with human WT vWF but not with type 2B von Willebrand disease vWF. The Journal of Clinical Investigation 118, 3195–3207 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y et al. Platelet integrins exhibit anisotropic mechanosensing and harness piconewton forces to mediate platelet aggregation. Proceedings of the National Academy of Sciences 115, 325 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun YB, Chen CS & Fu JP Forcing Stem Cells to Behave: A Biophysical Perspective of the Cellular Microenvironment. Annual Review of Biophysics, Vol 41 41, 519–542 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Savage B, Saldivar E & Ruggeri ZM Initiation of platelet adhesion by arrest onto fibrinogen or translocation on von Willebrand factor. Cell 84, 289–297 (1996). [DOI] [PubMed] [Google Scholar]
- 35.Ruggeri ZM Platelet Adhesion under Flow. Microcirculation 16, 58–83 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pelham RJ Jr. & Wang Y Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci U S A 94, 13661–5 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Engler AJ, Sen S, Sweeney HL & Discher DE Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 (2006). [DOI] [PubMed] [Google Scholar]
- 38.Yeh YT et al. Matrix Stiffness Regulates Endothelial Cell Proliferation through Septin 9. Plos One 7 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qiu YZ et al. Platelet mechanosensing of substrate stiffness during clot formation mediates adhesion, spreading, and activation. Proceedings of the National Academy of Sciences of the United States of America 111, 14430–14435 (2014).This paper is the first to describe how the stiffness of substrates affect platelet physiology, highlighting the role of the physical microenvironment in clot formation and the importance of adjusting mechanical properties for biomaterials in blood.
- 40.Kee MF, Myers DR, Sakurai Y, Lam WA & Qiu Y Platelet Mechanosensing of Collagen Matrices. PLoS ONE 10, e0126624 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lammermann T et al. Cdc42-dependent leading edge coordination is essential for interstitial dendritic cell migration. Blood 113, 5703–5710 (2009). [DOI] [PubMed] [Google Scholar]
- 42.Nguyen DHT et al. Biomimetic model to reconstitute angiogenic sprouting morphogenesis in vitro. Proceedings of the National Academy of Sciences of the United States of America 110, 6712–6717 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zheng Y et al. In vitro microvessels for the study of angiogenesis and thrombosis. Proceedings of the National Academy of Sciences of the United States of America 109, 9342–9347 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Galie PA et al. Fluid shear stress threshold regulates angiogenic sprouting. Proceedings of the National Academy of Sciences of the United States of America 111, 7968–7973 (2014).Using a hydrogel-based microvasculature model, the existence of a shear stress threshold in regulating angiogenic sprouting is demonstrated.
- 45.Chaudhuri O et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nature Materials 15, 326-+ (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huebsch N et al. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nature Materials 9, 518–526 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mannino RG et al. “Do-it-yourself in vitro vasculature that recapitulates in vivo geometries for investigating endothelial-blood cell interactions”. Scientific Reports 5, 12401 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qiu YZ et al. Magnetic forces enable controlled drug delivery by disrupting endothelial cell-cell junctions. Nature Communications 8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsai M et al. In vitro modeling of the microvascular occlusion and thrombosis that occur in hematologic diseases using microfluidic technology. Journal of Clinical Investigation 122, 408–418 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huh D et al. A Human Disease Model of Drug Toxicity-Induced Pulmonary Edema in a Lung-on-a-Chip Microdevice. Science Translational Medicine 4 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chrobak KM, Potter DR & Tien J Formation of perfused, functional microvascular tubes in vitro. Microvascular Research 71, 185–196 (2006). [DOI] [PubMed] [Google Scholar]
- 52.Chen MB et al. On-chip human microvasculature assay for visualization and quantification of tumor cell extravasation dynamics. Nat Protoc 12, 865–880 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jeon JS et al. Human 3D vascularized organotypic microfluidic assays to study breast cancer cell extravasation. Proceedings of the National Academy of Sciences of the United States of America 112, 214–219 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang XL et al. Engineering anastomosis between living capillary networks and endothelial cell-lined microfluidic channels. Lab on a Chip 16, 282–290 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Choi NW et al. Microfluidic scaffolds for tissue engineering. Nature Materials 6, 908–915 (2007). [DOI] [PubMed] [Google Scholar]
- 56.Ling Y et al. A cell-laden microfluidic hydrogel. Lab on a Chip 7, 756–762 (2007). [DOI] [PubMed] [Google Scholar]
- 57.Humphrey JD Vascular adaptation and mechanical homeostasis at tissue, cellular, and sub-cellular levels. Cell Biochemistry and Biophysics 50, 53–78 (2008). [DOI] [PubMed] [Google Scholar]
- 58.Baeyens N, Bandyopadhyay C, Coon BG, Yun S & Schwartz MA Endothelial fluid shear stress sensing in vascular health and disease. J Clin Invest 126, 821–8 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wagenseil JE & Mecham RP Vascular Extracellular Matrix and Arterial Mechanics. Physiological Reviews 89, 957–989 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kwak BR et al. Biomechanical factors in atherosclerosis: mechanisms and clinical implications. Eur Heart J 35, 3013–20, 3020a-3020d (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thondapu V et al. Biomechanical stress in coronary atherosclerosis: emerging insights from computational modelling. European Heart Journal 38, 81–92C (2017). [DOI] [PubMed] [Google Scholar]
- 62.Chiu JJ & Chien S Effects of Disturbed Flow on Vascular Endothelium: Pathophysiological Basis and Clinical Perspectives. Physiological Reviews 91, 327–387 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lacolley P, Regnault V, Nicoletti A, Li ZL & Michel JB The vascular smooth muscle cell in arterial pathology: a cell that can take on multiple roles. Cardiovascular Research 95, 194–204 (2012). [DOI] [PubMed] [Google Scholar]
- 64.Lu DS & Kassab GS Role of shear stress and stretch in vascular mechanobiology. Journal of the Royal Society Interface 8, 1379–1385 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Langille BL & Odonnell F Reductions in Arterial Diameter Produced by Chronic Decreases in Blood-Flow Are Endothelium-Dependent. Science 231, 405–407 (1986). [DOI] [PubMed] [Google Scholar]
- 66.Frangos JA, Eskin SG, Mcintire LV & Ives CL Flow Effects on Prostacyclin Production by Cultured Human-Endothelial Cells. Science 227, 1477–1479 (1985).This seminal contribution demonstrates how the application of forces influence the biochemical response of a cell, by showing that pulsatile shear stress applied to cultured endothelial cells increases production of PGI2, a vasodilator and potent endogenous inhibitor of platelet aggregation.
- 67.Sandoo A, van Zanten JJ, Metsios GS, Carroll D & Kitas GD The endothelium and its role in regulating vascular tone. Open Cardiovasc Med J 4, 302–12 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mac Gabhann F & Peirce SM Collateral Capillary Arterialization following Arteriolar Ligation in Murine Skeletal Muscle. Microcirculation 17, 333–347 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chien S Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. American Journal of Physiology-Heart and Circulatory Physiology 292, H1209–H1224 (2007). [DOI] [PubMed] [Google Scholar]
- 70.Kutys ML & Chen CS Forces and mechanotransduction in 3D vascular biology. Current Opinion in Cell Biology 42, 73–79 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu J et al. GPR68 Senses Flow and Is Essential for Vascular Physiology. Cell 173, 762-+ (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mack JJ et al. NOTCH1 is a mechanosensor in adult arteries. Nature Communications 8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Coon BG et al. Intramembrane binding of VE-cadherin to VEGFR2 and VEGFR3 assembles the endothelial mechanosensory complex. Journal of Cell Biology 208, 975–986 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schiffrin EL Vascular stiffening and arterial compliance - Implications for systolic blood pressure. American Journal of Hypertension 17, 39s–48s (2004). [DOI] [PubMed] [Google Scholar]
- 75.Duca L et al. Matrix ageing and vascular impacts: focus on elastin fragmentation. Cardiovascular Research 110, 298–308 (2016). [DOI] [PubMed] [Google Scholar]
- 76.SenBanerjee S et al. KLF2 is a novel transcriptional regulator of endothelial proinflammatory activation. Journal of Experimental Medicine 199, 1305–1315 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mackman N New insights into the mechanisms of venous thrombosis. Journal of Clinical Investigation 122, 2331–2336 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lehmann M et al. Platelets Drive Thrombus Propagation in a Hematocrit and Glycoprotein VI-Dependent Manner in an In Vitro Venous Thrombosis Model. Arteriosclerosis Thrombosis and Vascular Biology 38, 1052–1062 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Conway DE, Williams MR, Eskin SG & McIntire LV Endothelial cell responses to atheroprone flow are driven by two separate flow components: low time-average shear stress and fluid flow reversal. American Journal of Physiology-Heart and Circulatory Physiology 298, H367–H374 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Davies PF Endothelial transcriptome profiles in vivo in complex arterial flow fields. Ann Biomed Eng 36, 563–70 (2008). [DOI] [PubMed] [Google Scholar]
- 81.Davies PF, Civelek M, Fang Y, Guerraty MA & Passerini AG Endothelial heterogeneity associated with regional athero-susceptibility and adaptation to disturbed blood flow in vivo. Semin Thromb Hemost 36, 265–75 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Glagov S, Weisenberg E, Zarins CK, Stankunavicius R & Kolettis GJ Compensatory Enlargement of Human Atherosclerotic Coronary-Arteries. New England Journal of Medicine 316, 1371–1375 (1987). [DOI] [PubMed] [Google Scholar]
- 83.Inaba S et al. Compensatory enlargement of the left main coronary artery: insights from the PROSPECT study. Coronary Artery Disease 25, 98–103 (2014). [DOI] [PubMed] [Google Scholar]
- 84.Varnava AM, Mills PG & Davies MJ Relationship between coronary artery remodeling and plaque vulnerability. Circulation 105, 939–943 (2002). [DOI] [PubMed] [Google Scholar]
- 85.Samady H et al. Coronary Artery Wall Shear Stress Is Associated With Progression and Transformation of Atherosclerotic Plaque and Arterial Remodeling in Patients With Coronary Artery Disease. Circulation 124, 779–788 (2011). [DOI] [PubMed] [Google Scholar]
- 86.Stone PH et al. Prediction of Progression of Coronary Artery Disease and Clinical Outcomes Using Vascular Profiling of Endothelial Shear Stress and Arterial Plaque Characteristics The PREDICTION Study. Circulation 126, 172-+ (2012). [DOI] [PubMed] [Google Scholar]
- 87.Li ZY et al. Structural analysis and magnetic resonance imaging predict plaque vulnerability: A study comparing symptomatic and asymptomatic individuals. Journal of Vascular Surgery 45, 768–775 (2007). [DOI] [PubMed] [Google Scholar]
- 88.Gijsen FJH et al. Strain distribution over plaques in human coronary arteries relates to shear stress. American Journal of Physiology-Heart and Circulatory Physiology 295, H1608–H1614 (2008). [DOI] [PubMed] [Google Scholar]
- 89.Dahl SLM et al. Readily Available Tissue-Engineered Vascular Grafts. Science Translational Medicine 3 (2011). [DOI] [PubMed] [Google Scholar]
- 90.Spadaccio C et al. Old Myths, New Concerns: the Long-Term Effects of Ascending Aorta Replacement with Dacron Grafts. Not All That Glitters Is Gold. Journal of Cardiovascular Translational Research 9, 334–342 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hoenig MR, Campbell GR, Rolfe BE & Campbell JH Tissue-engineered blood vessels - Alternative to autologous grafts? Arteriosclerosis Thrombosis and Vascular Biology 25, 1128–1134 (2005). [DOI] [PubMed] [Google Scholar]
- 92.L’Heureux N et al. Human tissue-engineered blood vessels for adult arterial revascularization. Nat Med 12, 361–5 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McAllister TN et al. Effectiveness of haemodialysis access with an autologous tissue-engineered vascular graft: a multicentre cohort study. Lancet 373, 1440–1446 (2009). [DOI] [PubMed] [Google Scholar]
- 94.Lawson JH et al. Bioengineered human acellular vessels for dialysis access in patients with end-stage renal disease: two phase 2 single-arm trials. Lancet 387, 2026–2034 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Egginton S, Zhou AL, Brown MD & Hudlicka O Unorthodox angiogenesis in skeletal muscle. Cardiovascular Research 49, 634–646 (2001). [DOI] [PubMed] [Google Scholar]
- 96.Hudlicka O & Brown MD Adaptation of Skeletal Muscle Microvasculature to Increased or Decreased Blood Flow: Role of Shear Stress, Nitric Oxide and Vascular Endothelial Growth Factor. Journal of Vascular Research 46, 504–512 (2009). [DOI] [PubMed] [Google Scholar]
- 97.Song JW & Munn LL Fluid forces control endothelial sprouting. Proceedings of the National Academy of Sciences of the United States of America 108, 15342–15347 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang BY et al. Biodegradable scaffold with built-in vasculature for organ-on-a-chip engineering and direct surgical anastomosis. Nature Materials 15, 669-+ (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bertassoni LE et al. Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs. Lab on a Chip 14, 2202–2211 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.DiVito KA, Daniele MA, Roberts SA, Ligler FS & Adams AA Microfabricated blood vessels undergo neoangiogenesis. Biomaterials 138, 142–152 (2017). [DOI] [PubMed] [Google Scholar]
- 101.Nichol JW et al. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 31, 5536–5544 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Heintz KA et al. Fabrication of 3D Biomimetic Microfluidic Networks in Hydrogels. Advanced Healthcare Materials 5, 2153–2160 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cuchiara MP, Gould DJ, McHale MK, Dickinson ME & West JL Integration of Self-Assembled Microvascular Networks with Microfabricated PEG-Based Hydrogels. Advanced Functional Materials 22, 4511–4518 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brandenberg N & Lutolf MP In Situ Patterning of Microfluidic Networks in 3D Cell-Laden Hydrogels. Advanced Materials 28, 7450–7456 (2016). [DOI] [PubMed] [Google Scholar]
- 105.Miller JS et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat Mater 11, 768–74 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Qiu YZ et al. Microvasculature-on-a-chip for the long-term study of endothelial barrier dysfunction and microvascular obstruction in disease. Nature Biomedical Engineering 2, 453–463 (2018).A hydrogel-based microvasculature-on-a-chip device is reported showing for the first time long-term physiologically relevant endothelial barrier function in vitro and the potential to elucidate the role of microvascular dysfunction in the pathogenesis of hematological disorders.
- 107.Polacheck WJ et al. A non-canonical Notch complex regulates adherens junctions and vascular barrier function. Nature 552, 258-+ (2017).Using a hydrogel-based microvasculature model, NOTCH1 could be identified as a mechanosensor that is responsible for regulating endothelial barrier function by a novel transcription-independent signalling mechanism.
- 108.Huynh J et al. Age-Related Intimal Stiffening Enhances Endothelial Permeability and Leukocyte Transmigration. Science Translational Medicine 3 (2011).In this paper, the age-related stiffening of the arterial intima is characterized and increased endothelial permeability is associated with its stiffening, which is a hallmark of atherosclerosis.
- 109.Swift J et al. Nuclear Lamin-A Scales with Tissue Stiffness and Enhances Matrix-Directed Differentiation. Science 341 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zheng Y et al. Decreased deformability of lymphocytes in chronic lymphocytic leukemia. Scientific Reports 5, 7613 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dao M, Lim CT & Suresh S Mechanics of the human red blood cell deformed by optical tweezers. Journal of the Mechanics and Physics of Solids 51, 2259–2280 (2003). [Google Scholar]
- 112.Fay ME et al. Cellular softening mediates leukocyte demargination and trafficking, thereby increasing clinical blood counts. Proceedings of the National Academy of Sciences of the United States of America 113, 1987–1992 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tsai MA, Waugh RE & Keng PC Passive Mechanical Behavior of Human Neutrophils: Effects of Colchicine and Paclitaxel. Biophysical Journal 74, 3282–3291 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Brown MJ, Hallam JA, Colucci-Guyon E & Shaw S Rigidity of Circulating Lymphocytes Is Primarily Conferred by Vimentin Intermediate Filaments. The Journal of Immunology 166, 6640 (2001). [DOI] [PubMed] [Google Scholar]
- 115.Fletcher DA & Mullins D Cell mechanics and the cytoskeleton. Nature 463, 485–492 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Harris AR, Jreij P & Fletcher DA Mechanotransduction by the Actin Cytoskeleton: Converting Mechanical Stimuli into Biochemical Signals. Annual Review of Biophysics 47, 617–631 (2016). [Google Scholar]
- 117.Discher DE & Carl P New insights into red cell network structure, elasticity, and spectrin unfolding--a current review. Cell Mol Biol Lett 6, 593–606 (2001). [PubMed] [Google Scholar]
- 118.Johnson CP, Tang H-Y, Carag C, Speicher DW & Discher DE Forced Unfolding of Proteins Within Cells. Science 317, 663–666 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lammerding J et al. Lamins A and C but Not Lamin B1 Regulate Nuclear Mechanics. Journal of Biological Chemistry 281, 25768–25780 (2006). [DOI] [PubMed] [Google Scholar]
- 120.Shin JW et al. Lamins regulate cell trafficking and lineage maturation of adult human hematopoietic cells. Proceedings of the National Academy of Sciences of the United States of America 110, 18892–18897 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Feng Q & Kornmann B Mechanical forces on cellular organelles. Journal of Cell Science 131 (2018). [DOI] [PubMed] [Google Scholar]
- 122.Mohandas N & Gallagher PG Red cell membrane: past, present, and future. Blood 112, 3939–3948 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Smith AS et al. Myosin IIA interacts with the spectrin-actin membrane skeleton to control red blood cell membrane curvature and deformability. Proceedings of the National Academy of Sciences of the United States of America 115, E4377–E4385 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schaer DJ, Buehler PW, Alayash AI, Belcher JD & Vercellotti GM Hemolysis and free hemoglobin revisited: exploring hemoglobin and hemin scavengers as a novel class of therapeutic proteins. Blood 121, 1276–1284 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Presley TD et al. Effects of a Single Sickling Event on the Mechanical Fragility of Sickle Cell Trait Erythrocytes. Hemoglobin 34, 24–36 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Messmann R, Gannon S, Sarnaik S & Johnson R Mechanical properties of sickle cell membranes. Blood 75, 1711–1717 (1990). [PubMed] [Google Scholar]
- 127.Cranston H et al. Plasmodium falciparum maturation abolishes physiologic red cell deformability. Science 223, 400–403 (1984). [DOI] [PubMed] [Google Scholar]
- 128.Paulitschke M & Nash GB Membrane rigidity of red blood cells parasitized by different strains of Plasmodium falciparum. J Lab Clin Med 122, 581–9 (1993). [PubMed] [Google Scholar]
- 129.Raventos-Suarez C, Kaul DK, Macaluso F & Nagel RL Membrane knobs are required for the microcirculatory obstruction induced by Plasmodium falciparum-infected erythrocytes. Proceedings of the National Academy of Sciences 82, 3829–3833 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cahalan SM et al. Piezo1 links mechanical forces to red blood cell volume. eLife 4, e07370 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Glogowska E et al. Novel mechanisms of PIEZO1 dysfunction in hereditary xerocytosis. Blood (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Albuisson J et al. Dehydrated hereditary stomatocytosis linked to gain-of-function mutations in mechanically activated PIEZO1 ion channels (vol 4, pg 1884, 2013). Nature Communications 4 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bae C, Gnanasambandam R, Nicolai C, Sachs F & Gottlieb PA Xerocytosis is caused by mutations that alter the kinetics of the mechanosensitive channel PIEZO1. Proceedings of the National Academy of Sciences of the United States of America 110, E1162–E1168 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Locke C, Depani S & Gray M Extensive subclinical venous sinus thrombosis in the dehydrated infant. The Journal of Maternal-Fetal & Neonatal Medicine 23, 463–464 (2010). [DOI] [PubMed] [Google Scholar]
- 135.Hbibi M et al. Severe hypernatremic dehydration associated with cerebral venous and aortic thrombosis in the neonatal period. BMJ Case Reports 2012 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Schmid-Schönbein GW, Usami S, Skalak R & Chien S The interaction of leukocytes and erythrocytes in capillary and postcapillary vessels. Microvascular Research 19, 45–70 (1980).This is one of the earliest papers suggesting that mechanical differences in size and shape of red and white blood cells contribute to the margination phenomenon.
- 137.Walton BL et al. Elevated hematocrit enhances platelet accumulation following vascular injury. Blood 129, 2537–2546 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dunn A & Donnelly S The Role of the Kidney in Blood Volume Regulation: The Kidney as a Regulator of the Hematocrit. The American Journal of the Medical Sciences 334, 65–71 (2007). [DOI] [PubMed] [Google Scholar]
- 139.Connes P et al. The role of blood rheology in sickle cell disease. Blood Reviews 30, 111–118 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jelkmann W Regulation of erythropoietin production. The Journal of Physiology 589, 1251–1258 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pivkin IV et al. Biomechanics of red blood cells in human spleen and consequences for physiology and disease. Proceedings of the National Academy of Sciences 113, 7804–7809 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Deplaine G et al. The sensing of poorly deformable red blood cells by the human spleen can be mimicked in vitro. Blood 117, e88–e95 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rollig C & Ehninger G How I treat hyperleukocytosis in acute myeloid leukemia. Blood 125, 3246–3252 (2015). [DOI] [PubMed] [Google Scholar]
- 144.Byrnes JR & Wolberg AS Red blood cells in thrombosis. Blood 130, 1795–1799 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Vannucchi AM How I treat polycythemia vera. Blood 124, 3212–3220 (2014). [DOI] [PubMed] [Google Scholar]
- 146.Westenbrink BD et al. Anemia is associated with bleeding and mortality, but not stroke, in patients with atrial fibrillation: Insights from the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial. American Heart Journal 185, 140–149 (2017). [DOI] [PubMed] [Google Scholar]
- 147.Gutierrez M, Fish MB, Golinski AW & Eniola-Adefeso O Presence of Rigid Red Blood Cells in Blood Flow Interferes with the Vascular Wall Adhesion of Leukocytes. Langmuir 34, 2363–2372 (2018). [DOI] [PubMed] [Google Scholar]
- 148.Khismatullin DB in Current Topics in Membranes 47–111 (Academic Press, 2009). [Google Scholar]
- 149.Hvas AM & Sørensen HT Tranexamic acid combined with recombinant factor VIII increases clot resistance to accelerated fibrinolysis in severe hemophilia A. Tranexamic acid combined with recombinant factor VIII increases clot resistance to accelerated fibrinolysis in severe hemophilia A (2007). [DOI] [PubMed] [Google Scholar]
- 150.Collet JP et al. Altered fibrin architecture is associated with hypofibrinolysis and premature coronary atherothrombosis. Arteriosclerosis, thrombosis, and vascular biology 26, 2567–2573 (2006). [DOI] [PubMed] [Google Scholar]
- 151.Brummel-Ziedins KE, Branda RF, Butenas S & Mann KG Discordant fibrin formation in hemophilia. Journal of Thrombosis and Haemostasis 7, 825–832 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Undas A Fibrin clot properties and their modulation in thrombotic disorders. Thrombosis and haemostasis 112, 32–42 (2014). [DOI] [PubMed] [Google Scholar]
- 153.Brown AE, Litvinov RI, Discher DE, Purohit PK & Weisel JW Multiscale mechanics of fibrin polymer: gel stretching with protein unfolding and loss of water. Science (New York, N.Y.) 325, 741–744 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Collet J-P, Shuman H, Ledger RE, Lee S & Weisel JW The elasticity of an individual fibrin fiber in a clot. Proceedings of the National Academy of Sciences of the United States of America 102, 9133 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gersh KC, Nagaswami C & Weisel JW Fibrin network structure and clot mechanical properties are altered by incorporation of erythrocytes. Thrombosis and haemostasis 102, 1169–1175 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hategan A, Gersh KC, Safer D & Weisel JW Visualization of the dynamics of fibrin clot growth 1 molecule at a time by total internal reflection fluorescence microscopy. Blood 121, 1455–1458 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ryan EA, Mockros LF, Weisel JW & Lorand L Structural origins of fibrin clot rheology. Biophysical journal 77, 2813–2826 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wolberg AS Thrombin generation and fibrin clot structure. Blood reviews 21, 131–142 (2007). [DOI] [PubMed] [Google Scholar]
- 159.Weisel JW The mechanical properties of fibrin for basic scientists and clinicians. Biophysical chemistry 112, 267–276 (2004). [DOI] [PubMed] [Google Scholar]
- 160.Weisel JW Enigmas of Blood Clot Elasticity. Science 320, 456–457 (2008). [DOI] [PubMed] [Google Scholar]
- 161.Liu W et al. Fibrin Fibers Have Extraordinary Extensibility and Elasticity. Science (New York, N.Y.) 313, 634-634 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Li W et al. Fibrin Fiber Stiffness Is Strongly Affected by Fiber Diameter, but Not by Fibrinogen Glycation. Biophysical journal 110, 1400–1410 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hudson NE et al. Stiffening of Individual Fibrin Fibers Equitably Distributes Strain and Strengthens Networks. Biophysical Journal 98, 1632–1640 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Shah JV & Janmey PA Strain hardening of fibrin gels and plasma clots. Rheologica Acta 36, 262–268 (1997). [Google Scholar]
- 165.Piechocka IK, Bacabac RG, Potters M, MacKintosh FC & Koenderink GH Structural Hierarchy Governs Fibrin Gel Mechanics. Biophysical Journal 98, 2281–2289 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Macrae FL et al. A fibrin biofilm covers blood clots and protects from microbial invasion. The Journal of Clinical Investigation 128 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Carroll RC, Gerrard JM & Gilliam JM Clot Retraction Facilitates Clot Lysis. Blood 57, 44–48 (1981). [PubMed] [Google Scholar]
- 168.Bucay I et al. Physical Determinants of Fibrinolysis in Single Fibrin Fibers. PLOS ONE 10, e0116350 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Litvinov RI & Weisel JW Shear strengthens fibrin: the knob-hole interactions display ‘catch-slip’ kinetics. Journal of thrombosis and haemostasis : JTH 11, 1933–1935 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Litvinov RI et al. Regulatory element in fibrin triggers tension-activated transition from catch to slip bonds. Proceedings of the National Academy of Sciences 115, 8575 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Carr ME & Zekert SL Abnormal clot retraction, altered fibrin structure, and normal platelet function in multiple myeloma. American Journal of Physiology-Heart and Circulatory Physiology 266, H1195–H1201 (1994). [DOI] [PubMed] [Google Scholar]
- 172.Collet J-P et al. Abnormal Fibrin Clot Architecture in Nephrotic Patients Is Related to Hypofibrinolysis: Influence of Plasma Biochemical Modifications. Thromb Haemost 82, 1482–1489 (1999). [PubMed] [Google Scholar]
- 173.Cieslik J, Mrozinska S, Broniatowska E & Undas A Altered plasma clot properties increase the risk of recurrent deep vein thrombosis: a cohort study. Blood 131, 797–807 (2018). [DOI] [PubMed] [Google Scholar]
- 174.Drabik L, Wołkow P & Undas A Fibrin Clot Permeability as a Predictor of Stroke and Bleeding in Anticoagulated Patients With Atrial Fibrillation. Stroke 48, 2716–2722 (2017). [DOI] [PubMed] [Google Scholar]
- 175.Undas A, Wiek I, Stêpien E, Zmudka K & Tracz W Hyperglycemia is associated with enhanced thrombin formation, platelet activation, and fibrin clot resistance to lysis in patients with acute coronary syndrome. Diabetes care 31, 1590–1595 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Carmassi F et al. Coagulation and fibrinolytic system impairment in insulin dependent diabetes mellitus. Thrombosis Research 67, 643–654 (1992). [DOI] [PubMed] [Google Scholar]
- 177.Jirousková M, Jaiswal JK & Coller BS Ligand density dramatically affects integrin alpha IIb beta 3-mediated platelet signaling and spreading. Blood 109, 5260–5269 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lam WA et al. Mechanics and contraction dynamics of single platelets and implications for clot stiffening. Nature materials 10, 61–66 (2011).This is the first paper to report the measurement of the mechanics of platelet contraction at the single platelet level, providing insight into how the mechanosensitivity and mechanics of single platelets affect clot mechanics.
- 179.Jen Chauying J & McIntire Larry V The structural properties and contractile force of a clot. Cell Motility 2, 445–455 (1982). [DOI] [PubMed] [Google Scholar]
- 180.Greilich PE, Carr ME, Zekert SL & Dent RM Quantitative assessment of platelet function and clot structure in patients with severe coronary artery disease. Am J Med Sci 307, 15–20 (1994). [DOI] [PubMed] [Google Scholar]
- 181.Minh GL et al. Impaired contraction of blood clots as a novel prothrombotic mechanism in systemic lupus erythematosus. Clinical Science 132, 243–254 (2018). [DOI] [PubMed] [Google Scholar]
- 182.Kita A et al. Microenvironmental geometry guides platelet adhesion and spreading: a quantitative analysis at the single cell level. PloS one 6 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Stalker TJ et al. Hierarchical organization in the hemostatic response and its relationship to the platelet-signaling network. Blood 121, 1875–1885 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kroll MH, Hellums JD, McIntire LV, Schafer AI & Moake JL Platelets and shear stress. Blood 88, 1525–1541 (1996). [PubMed] [Google Scholar]
- 185.Nesbitt WS et al. A shear gradient-dependent platelet aggregation mechanism drives thrombus formation. Nature medicine 15, 665–673 (2009). [DOI] [PubMed] [Google Scholar]
- 186.Carr ME Measurement of platelet force: the Hemodyne hemostasis analyzer. Clin Lab Manage Rev 9, 9 (1995). [PubMed] [Google Scholar]
- 187.Krishnaswami A et al. Patients with coronary artery disease who present with chest pain have significantly elevated platelet contractile force and clot elastic modulus. Thromb Haemost 88, 739–44 (2002). [PubMed] [Google Scholar]
- 188.Carr ME et al. Enhanced platelet force development despite drug-induced inhibition of platelet aggregation in patients with thromboangiitis obliterans--two case reports. Vascular and endovascular surgery 36, 473–480 (2002). [DOI] [PubMed] [Google Scholar]
- 189.Tutwiler V et al. Contraction of Blood Clots Is Impaired in Acute Ischemic Stroke. Arteriosclerosis, thrombosis, and vascular biology 37, 271–279 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Rusak T et al. Evaluation of hemostatic balance in blood from patients with polycythemia vera by means of thromboelastography: The effect of isovolemic erythrocytapheresis. Platelets 23, 455–462 (2012). [DOI] [PubMed] [Google Scholar]
- 191.Rusak T, Piszcz J, Misztal T, Branska-Januszewska J & Tomasiak M Platelet-related fibrinolysis resistance in patients suffering from PV. Impact of clot retraction and isovolemic erythrocytapheresis. Thromb Res 134, 192–8 (2014). [DOI] [PubMed] [Google Scholar]
- 192.Feghhi S et al. Glycoprotein Ib-IX-V Complex Transmits Cytoskeletal Forces That Enhance Platelet Adhesion. Biophysical Journal 111, 601–608 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Feghhi S, Tooley WW & Sniadecki NJ Nonmuscle Myosin IIA Regulates Platelet Contractile Forces Through Rho Kinase and Myosin Light-Chain Kinase. Journal of Biomechanical Engineering 138, 104506–104506–4 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Cines DB et al. Clot contraction: compression of erythrocytes into tightly packed polyhedra and redistribution of platelets and fibrin. Blood 123, 1596–603 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Stalker TJ et al. A systems approach to hemostasis: 3. Thrombus consolidation regulates intrathrombus solute transport and local thrombin activity. Blood 124, 1824–1831 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Tomaiuolo M et al. A systems approach to hemostasis: 2. Computational analysis of molecular transport in the thrombus microenvironment. Blood 124, 1816–1823 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Welsh JD et al. A systems approach to hemostasis: 1. The interdependence of thrombus architecture and agonist movements in the gaps between platelets. Blood 124, 1808–1815 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.M. M et al. Platelet packing density is an independent regulator of the hemostatic response to injury. Journal of Thrombosis and Haemostasis 16, 973–983 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Rana K & Neeves KB Blood flow and mass transfer regulation of coagulation. Blood reviews 30, 357–368 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Nechipurenko DY et al. Clot Contraction Drives the Translocation of Procoagulant Platelets to Thrombus Surface. Arteriosclerosis Thrombosis and Vascular Biology 39, 37–47 (2019). [DOI] [PubMed] [Google Scholar]
- 201.Kim OV, Litvinov RI, Alber MS & Weisel JW Quantitative structural mechanobiology of platelet-driven blood clot contraction. Nature Communications 8, 1274 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Merkel TJ et al. Using mechanobiological mimicry of red blood cells to extend circulation times of hydrogel microparticles. Proceedings of the National Academy of Sciences 108, 586–591 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Brenner JS et al. Red blood cell-hitchhiking boosts delivery of nanocarriers to chosen organs by orders of magnitude. Nature Communications 9, 2684 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Korin N et al. Shear-activated nanotherapeutics for drug targeting to obstructed blood vessels. Science (New York, N.Y.) 337, 738–742 (2012). [DOI] [PubMed] [Google Scholar]
- 205.Hansen CE et al. Platelet–Microcapsule Hybrids Leverage Contractile Force for Targeted Delivery of Hemostatic Agents. ACS Nano 11, 5579–5589 (2017). [DOI] [PubMed] [Google Scholar]
- 206.Brown AC et al. Ultrasoft microgels displaying emergent platelet-like behaviours. Nat Mater 13, 1108–1114 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Brophy DF, Martin RJ, Gehr TW & Carr ME A hypothesis-generating study to evaluate platelet activity in diabetics with chronic kidney disease. Thrombosis journal 3, 3 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kim S, Lee H, Chung M & Jeon NL Engineering of functional, perfusable 3D microvascular networks on a chip. Lab on a Chip 13, 1489–1500 (2013). [DOI] [PubMed] [Google Scholar]
- 209.Takahashi H et al. Visualizing dynamics of angiogenic sprouting from a three-dimensional microvasculature model using stage-top optical coherence tomography. Scientific Reports 7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Herring N & Paterson DJ Levick’s Introduction to Cardiovascular Physiology 6th edition (CRC Press, 2018). [Google Scholar]
- 211.Carr J (2017) Human Red Blood Cells [image] Available at http://www.nisenet.org/catalog/scientific-image-human-red-blood-cells-sem [Accessed 2/9/2019]
- 212.Digital Microscopy Facility at Mount Allison University (2000) Human White Blood Cell [image] Available at https://www.mta.ca/dmf/white_blood_cell.html [Accessed 2/9/2019]
- 213.Peshkova AD et al. Reduced Contraction of Blood Clots in Venous Thromboembolism Is a Potential Thrombogenic and Embologenic Mechanism. TH Open 02, e104–e115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Connes P et al. The role of blood rheology in sickle cell disease. Blood Reviews 30, 111–118 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wells RE & Merrill EW Influence of Flow Properties of Blood Upon Viscosity-Hematocrit Relationships. Journal of Clinical Investigation 41, 1591-& (1962). [DOI] [PMC free article] [PubMed] [Google Scholar]