Abstract
Skin wounds have been extensively studied as their healing represents a critical step towards achieving homeostasis following a traumatic event. Dependent on the severity of the damage, wounds are categorized as either acute or chronic. To date, chronic wounds have the highest economic impact as long term increases wound care costs. Chronic wounds affect 6.5 million patients in the United States with an annual estimated expense of $25 billion for the health care system. Among wound treatment categories, active wound care represents the fastest-growing category due to its specific actions and lower costs. Within this category, proteases from various sources have been used as successful agents in debridement wound care. The wound healing process is predominantly mediated by matrix metalloproteinases (MMPs) that, when dysregulated, result in defective wound healing. Therapeutic activity has been described for animal secretions including fish epithelial mucus, maggot secretory products and snake venom, which contain secreted proteases (SPs). No further alternatives for use, sources or types of proteases used for wound healing have been found in the literature to date. Through the present review, the context of enzymatic wound care alternatives will be discussed. In addition, substrate homology of SPs and human MMPs will be compared and contrasted. The purpose of these discussions is to identify and propose the stages of wound healing in which SPs may be used as therapeutic agents to improve the wound healing process.
Keywords: enzymatic wound treatment, fish epithelial mucus, maggot secretory products, matrix metalloproteases, snake venom proteases
1. Introduction
A wound of the skin is generally described as the interruption of the epithelial surface caused by a physical or thermal challenge (1). Skin wounds have been extensively studied as their healing represents a critical step in achieving homeostasis following a traumatic event. Dependent on the severity of the damage, wounds are categorized into either acute or chronic (2). To date, chronic wounds have the highest economic impact as long term treatment increases wound care costs (3). It is estimated that 1-2% of the population of the developing world will experience a chronic wound in their lifetime (4). According to Brem et al (5), in 2007 chronic wounds had affected 6.5 million patients in the United States, with an annual estimated health care expense of $25 billion (6). However, to date, the actual cost of chronic wound care in the United States is unknown (7). There has been a relatively high increase in the incidence of chronic wounds, and this may be closely associated with the increase in factors which impair wound healing, such as diabetes, obesity, or therapeutics such as chemotherapy, steroids and non-steroidal anti-inflammatory drugs (6).
The cost of chronic wound care represents a complicated scenario for patients and health care systems, leading to a necessity for the development of healing solutions which are both quicker and more cost-effective. To date, the available wound treatment therapeutics are: dressings, such as antimicrobial, films and alginate; hydrocolloids, collagen products, gauze composites and hydrogels; and active wound care (8). Active wound care represents the fastest growth category (20.6% compound annual growth rate between 2016-2022) as it is an alternative that has a more specific action and is more cost-effective (9). Within the active wound care category, proteases from a range of sources have been employed as successful agents in debridement (10), enhancing wound healing (11), coagulation (12) and keloid scar treatments (13). Of these, debridement comprises the principal dermatological application in enzymatic wound care, a proven and well-established principle (14).
The wound healing process is predominantly mediated by matrix metalloproteinases (MMPs) (15-17). Dysregulation of MMPs results in defective wound healing, which has made them targets of study in cases of chronic wounds, diabetic foot injury, keloid healing and burned skin (10). The topical application of non-human proteases has demonstrated beneficial therapeutic effects in events where MMPs fail due to dysregulation, for example in hemostasis (18), wound closure (19) and debridement (20).
Debridement is the most widely explored enzymatic wound care application, in which the most frequently used proteases are collagenases, serine proteases and cysteine proteases. The therapeutic activity of animal secretions from fish epithelial mucus (21), maggot (Lucilia sericata) secretory products (22) and snake venom (23) have also been demonstrated. These secretions contain different types of proteases capable of degrading the same substrates as MMPs. Besides these, no further use cases, sources or types of proteases for wound healing were found based on the currently available literature.
Through the present review, the context of enzymatic wound care alternatives will be discussed, along with a comparison of substrate homology of secreted proteases (SPs) and human MMPs. This review will aid in the identification of which stages of the wound healing process SPs may be used as therapeutic agents.
2. Chronic wound healing management: Practical context of traditional and enzymatically based debridement approaches
Debridement is the first step to enhance repair of chronic wounds. According to the European Wound Management Association, this procedure is considered a basic necessity to induce the physiological process of tissue repair (24). Through debridement, necrotic tissue is removed by external means to create a stable and healthy scaffold for re-epithelialization (25). In healthy individuals under normal circumstances, debridement is performed naturally following clot formation by neutrophil-derived MMPs and other components (26). However, when the MMP machinery fails, there is an accumulation of devitalized tissue. As a consequence, the steadiness of prolonged catabolism diminishes re-epithelialization and results in chronic wounding (27).
This failure represents an important baseline to treat chronic wounds, as devitalized epithelium builds up a physical barrier that precludes the healing process by interfering with the repair machinery, mimicking signs of infection, providing nutrients to anaerobic pathogenic agents, such as Clostridium perfringens or Bacteroides sp., and promoting cytokine production that in severe cases generates a septic response (28).
Debridement can be performed through autolytic, surgical, biological or enzymatic means (28). Of these, autolytic debridement is the most conservative treatment strategy. It enhances the action of endogenous phagocytic cells and proteases such as MMPs through dressings that provide the ideal catalytic conditions for removal of necrotic tissue (29). Among the dressings available for autolysis, films (polydimethylsiloxane), gauzes, hydrocolloids, hydrogels, alginates, hydrofibers and foams have been proposed (25,30). This strategy is selective, painless, inexpensive and suitable for most types of wounds (31). However, this process is slow, dependent on suitable reaction conditions and on the physiological response of the patient, and carries the risk of skin degradation due to prolonged exposure to moisture (maceration) (32) within the surrounding skin (28).
Surgical debridement strategies are performed by excising necrotic tissue until only healthy skin regions are exposed (33). Available variants of surgical debridement include ultrasound debridement, plasma-mediated bipolar radio-frequency ablation, versa-jet (fluid jet technology) and hydrosurgery (34,35). Surgical debridement is the fastest and most effective route of treatment, but is an expensive method that requires a sterile surgical environment, trained practitioners, and specific instruments, and is contraindicated for patients with clotting disorders (28,36).
By contrast, biological debridement promotes the removal of devitalized epithelium through the digestive action of Lucilia sericata sterile maggots (31). Maggots are caged in wound-sized hydrocolloid dressings that are placed in the affected area (37). The secretion of several components including proteolytic enzymes, such as trypsin and chymotrypsin serine proteases, then catalyze non-viable skin into a liquid feedstock that facilitates maggot feed (38). This alternative has proved to be efficient in several types of chronic wounds (39) and ulcers (40,41) by providing quick wound debridement, reduction in the use of biofilms, disinfection from bacteria (40,42-45) and improved pain control (46). However, due to the negative image several societies impose on maggots, this alternative has not been well accepted by patients and practitioners (47). Furthermore, it is contraindicated for the treatment of fistulae, exposed vessels and wounds in proximity to vital organs (42).
A potential compromise is enzymatic debridement, in which proteases from different sources (bacterial, vegetal or animal) is applied to the wounded area to remove necrotic tissue (48,49). Enzymatic debridement is selective and suitable for infected wounds (36), without the need for complex equipment or application procedures. This alternative also takes less time and requires fewer applications to accomplish debridement compared with dressings used for autolytic treatments (50). Other reported enzymatic wound healing approaches are anti- or pro-coagulation through venom toxins from Bothrops sp. (51,52). These enzymes may frequently be inhibited by salts, temperature and hydrogen peroxide, which are common elements of aseptic solutions. A stinging sensation and exudate may also be observed as an after-effect of enzymatic treatment (36).
From these four mentioned alternatives, three are directly dependent on proteases to perform the debriding activity. The direct or indirect use of proteases is therefore the second most commonly used tool after surgical debridement. In the current literature, the most commonly used proteases in direct enzymatic debridement are bromelain, papain and bacterial collagenases (53). Other enzymes have been demonstrated to intervene as anti- or pro-coagulation agents and in non-specific wound healing from animal secretions. The most common commercially and non-commercially available proteases associated with wound healing are listed in Table I.
Table I.
Applications of proteases in wound healing treatments classified by their reported therapeutic effect.
| A, Debridement and skin burns | |||
|---|---|---|---|
| Author, year | Enzyme | Source | (Refs.) |
| Ford et al, 2006 | Papain + urea (Accuzyme SE) | Carica papaya | (152) |
| Ford et al, 2006 | Papain, Urea, Chlorophyllin Copper Complex Sodium (Panafil SE) | (152) | |
| Muhammad et al, 2014; Yaakobi et al, 2007 | Papain/Chymopapain | (20,153) | |
| Klasen, 2000 | Collagenase | Clostridium sp. | (14) |
| Smith & Nephew, Inc., 2014 | Collagenase (Santyl®) | C. histolyticum | (154) |
| Giudice et al, 2017 | Bromelain (NexoBrid) | Ananas comosus | (155) |
| Gorecki and Toren, 2005 | Bromelain cysteine protease | (156) | |
| Klein and Houck, 1980 | Bromelain cysteine protease | (157) | |
| Niehaus et al, 2012 | Debrilase | Lucilia sericata | (158) |
| Niehaus et al, 2012 | Serine protease | (159) | |
| Rosenberg, 2012 | Bromelain, trypsin enzyme H-4, collagenase, papain/papain-urea | Several | (160) |
| Freeman et al, 2012 | Collagenase, elastase, papain, bromelain, hydrolase, streptokinase | (161) | |
| B, Anticoagulation and procoagulation | |||
| Author, year | Enzyme | Source | (Refs.) |
| Waheed et al, 2017 | Moojenin (Defibrase®) | Bothrops moojeni | (51) |
| Waheed et al, 2017 | Batroxobin (Reptilase) | B. atrox | (51) |
| Chan et al, 2016 | Thromboplastin-like and thrombin-like (Hemocoagulase) | (52) | |
| De Marco Almeida et al, 2015 | Venom | B. alternatus | (18) |
| Yaakobi et al, 2004 | Collagenase | Non specified | (162) |
| Rodeheaver et al, 1974 | Trypsin/ADAMS SVMP | Bovine | (163) |
| Glyantsev et al, 1996 | Collagenase | Crab (specie non specified) | (27) |
| Ferreira et al, 2017 | Buffalo cryoprecipitate and Serine protease | Crotalus durissus terrificus | (59) |
| C, Enhancing wound healing | |||
| Author, year | Enzyme | Source | (Refs.) |
| Fierro-Arias et al, 2017 | Collagenase | C. histolyticum | (13) |
| Gao et al, 2015 | rMMP8 and MMP9 inhibitor | Non specified | (164) |
| Pasha et al, 2015 | Cream/composite | Channa striatus | (143) |
| Rilley and Herman, 2005 | Collagenase | Clostridium sp. | (19) |
| Ferreira et al, 2018 | Jararhagin | B. jararaca | (58) |
| Mukherjee et al, 2017 | Mucus | Echinoida sp. | (83) |
| Costa-Neto, 2004 | Globe eye | Netuma barba | (54) |
| Manan Mat Jais, 2007 | Mucus | C. striatus | (55) |
MMP, matrix metalloproteinase; SVMP snake venom metalloprotease; rMMP, recombinant MMP.
Animal secretions with high quantities of protease content, including fish epithelial mucus and snake venom, have been reported to enhance wound healing. Wound healing properties were reported for the secreted mucus of the fish species Netuma barba (54), Channa striatus (55) and Clarias gariepinus (56). A reduction in healing time of almost 60% was achieved following the topical application of mucus preparations in the wounds of mice, rats, guinea pigs and humans (57). For snake venom, anti- or pro-coagulation and epithelial cell migration properties were observed with the toxins from the venom of Bothrops moojeni, B. atrox (51), B. alternatus (18) and B. jararaca (58).
Thus far, the primary application of proteases in wound treatment has been debridement. Information regarding the use of proteases being used for other wound healing treatments is scarce, suggesting that relatively little attempt has been made to propose the use of proteases in different stages of the wound healing process (57,59). Several therapeutic benefits have been described from animal secretions, but studies on their possible use in wound healing stages are limited. It may be beneficial to determine whether the existing types of SPs present in animal secretions with reported therapeutic effects (maggots, fish and snakes), can mimic human MMPs.
3. MMPs in skin wound healing: Comparison and substrate homology with proteases secreted from other animals
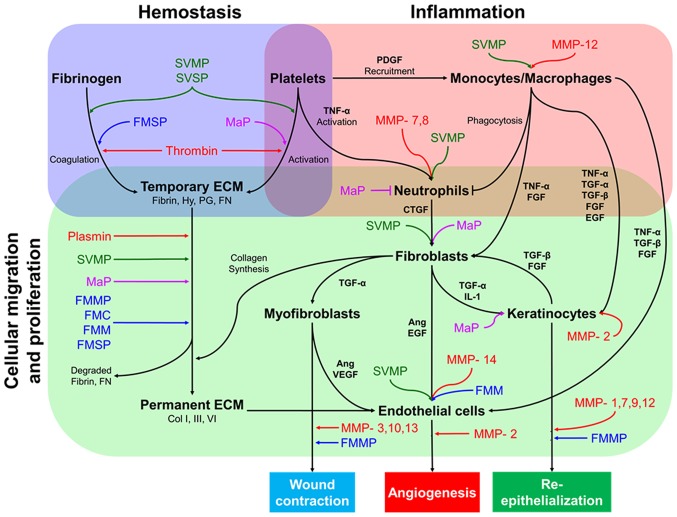
Wound healing is the process by which an epithelial discontinuity is closed and is divided into four major steps: Hemostasis, inflammation, cell migration-proliferation and skin remodeling (60,61). The interaction and co-ordination of several elements such as cytokines, growth factors, coagulation elements, extracellular matrix (ECM) components, parenchymal cells and MMPs (62,63) enable the correct progression of these major steps (Fig. 1).
Figure 1.
Simplified diagram of the interactions between different cell types during wound healing, the contribution of MMPs and proposed wound healing mechanisms of SPs. Skin injury repair begins with hemostasis, a process which stops blood loss and provides a temporary matrix facilitating further steps in wound healing. Fibrin-rich ECM formation stimulates neutrophil-activated monocyte recruitment through TNF-α and PDGF. Both neutrophils and monocytes produce several growth factors, such as TNF-α, TGF-α, TGF-β, EGF and FGF, to enhance migration and proliferation of fibroblasts, endothelial cells, and keratinocytes to the site of injury. Fibroblasts stimulate other cells to produce collagen deposits in the ECM, wound contraction, angiogenesis and re-epithelization. Studies suggest that SPs, such as FMC, FMMP, FMM, FMSP, MaP, SVMP and SVSP, may behave similarly to endogenous MMPs during these stages. Ang, angiopoietin; CTGF, connective tissue growth factor; Col, collagen; ECM, extracellular matrix; EGF, epidermal growth factor; FGF, fibroblast growth factor; FMC, fish mucus cathepsin; FMMP, fish mucus matrix metalloprotease; FMM, fish mucus meprin; FMSP, fish mucus serine protease; FN, fibronectin; Hy, hyaluronan; IL-1, interleukin-1; MaP, maggot protease; MMP, matrix metalloproteinase; PDGF, platelet-derived growth factor; PG, proteoglycan; SVMP, snake venom metalloprotease; SVSP, snake proteinase; TGF, transforming growth factor; TNF-α, tumor necrosis factor-α; VEGF, vascular endothelial growth factor.
It has been reported that MMPs predominantly mediate the wound healing process and are involved in several events in each stage, including ECM degradation (64), cell proliferation/migration, mesenchymal cell differentiation (65), wound contraction, angiogenesis and re-epithelialization (66-68). At present, 25 different MMP variants have been identified in the human genome (64,69). Of these, 11 are responsible for skin remodeling and wound healing (Table II).
Table II.
Classification and function of human MMPs involved in skin remodeling and wound healing.
| Family | Type | Function | Source | Substrates | Dysregulation effects | (Refs.) |
|---|---|---|---|---|---|---|
| Collagenases | 1 | Promotes re-epithelialization when cleaving native col 1 | Interstitial fibroblasts | Collagen I, II and III | In high levels generates chronic wounds | (66,165-171) |
| 8 | Regulation of neutrophil chemotaxis and effectors of inflammatory process | Neutrophils | Increased levels fibroblast lack apoptosis | |||
| 13 | Maturation of granular tissue and wound closure | Stromal fibroblasts Human chondrocytes | Collagen I, II, III, V and XI | Leads to arthritis, fibrosis, atherosclerosis and cancer | ||
| Gelatinases | 2 | Cleaves γ2 of laminin 332 promoting keratinocyte migration, angiogenesis regulation by p10 angiogenic cytokines | Fibroblasts, endo thelial cells alveolar epithelial cells | Collagen (IV, I), γ2 laminin 332, gelatin | Chronic wounds when MMP-2 is in high levels | (66-68,102,170,172-175) |
| 9 | Keratinocytes | Elastin, aggrecan, fibronectin and vitronectin | Wound closure impaired in MMP9 -/- | |||
| Stromelysins | 3 | Regulates wound healing (wound contraction), activate pro-MMPs and releases bioactive cytokines (HB-EGF, FGF) | Dermal fibroblasts and basal | Collagen (II, III, IV, IX, X) proteoglycans, laminin and fibronectin | Increased expression has been reported in dystrophic epidermolysis bullosa | (66-68,102,170,172-175) |
| 10 | Enhance migrating cell front in keratinocytes | Colocalized with MMP1 in leading edge of the wound keratinocytes | collagen III, IV and V | Disorganized cell migration, degradation of new matrix, aberrant cell to cell contact and increase in cell death of wound edge | ||
| 11 | Activation of pro-MMPs antiapoptotic | Peritumoral fibroblasts | α-I-antiprotease collagen VI | In increase expression promotes tumor development | ||
| Matrylisins | 7 | Wound re-epithelialization and neutrophil migration enhancing through chemokine processing. | Stromal fibroblasts in mucosal epithelia | Pro-MMP-1, gelatin, collagens | Innate immunity defects decreased re-epithelialization in lung injury | (19,176-178) |
| Membrane bound | 14 | Regulates epithelial cell prolif eration by altering KFG receptor and activates pro MMP2 | Cell membrane of keratinocytes of the migrating front | Collagen (I, II, III), gelatin, fibronectin, laminin | Defective collagen I production, loss of MMP2 and impaired wound healing | (66,102) |
| Other MMPs | 12 | Elastin degradation and microphage migration | Macrophages | Collagen (1, IV), elastin, fibronectin, laminin, vitronectin, proteoglycan | Increased angiogenesis because of decreased angiotensin | (173,177,179-182) |
MMP, metalloproteinase; HB-EGF, Heparin-binding EGF-like growth factor; FGF, fibroblast growth factor; KFG, KGF, Keratinocyte growth factor.
The presence of SPs has been reported in the secretions of fish (70), maggots (71,72) and snake venom (73). As MMPs are one of the primary participants of the wound healing process, a similarity may exist in the catalytic mechanisms of SPs and MMPs. This similarity may explain the therapeutic effect provided by these secretions.
Maggot therapy efficiency in the treatment of necrotic, infected chronic wounds is due to the activity of several SPs. This secretion consists of serine proteases (trypsin-like and chymotrypsin-like) and metalloproteases (71,72). As a secretion, maggot proteases (MaPs) contribute to the wound healing process, primarily in fibroblast stimulation and bacterial disinfection. MaPs degrade fibrin clots and fibronectin (74), enhancing fibroblast metabolism and migration (22,75). In addition, MaPs increase TGF-β (transforming growth factor-β) signaling in wounds treated with maggots (76), which enhances endothelial cell and keratinocyte migration, thus promoting wound closure. Furthermore, MaPs inhibit neutrophil migration and decrease the production of pro-inflammatory mediators in neutrophils and monocytes (44,77), leading to recruitment of pro-angiogenic growth factors (78) and healthy granulation tissue (79). MaPs are also considered antimicrobial enzymes (80), capable of eliminating Staphylococcus aureus and Pseudomonas aeruginosa (44,81) as well as degradation of biofilms produced by S. epidermidis and S. aureus (41).
From MaPs, only a chymotrypsin-like protease has been isolated from maggot secretions, which exhibited clotting and proteolytic activity in fibronectin, suggesting its use in hemostasis and for temporary collagen-rich replacement of ECM (74,82). These proteases also reduce biofilms in patients with leg ulcers (40,41).
Similar to maggot secretions, fish mucus and snake venom have been hypothesized as wound healing treatment agents. In traditional medicine, they have been used as a therapy for skin burns and hemostasis (51,55,56,83). Fish epithelial mucus consists primarily of glycoproteins and immune biomolecules (84). Immune components, metalloproteases, serine proteases, and cathepsins B, D and L, have been identified in fish epithelial mucus (85,86). Enzymatic components from crude secretions contribute to accelerated clot formation and agglutination of red cells (87).
In the case of metalloproteases, fish matrix metalloproteinases (FMMPs) 9 and 13 and fish mucus meprins (FMM) have been described as components of fish mucosal secretions (88,89). FMMPs 9 and 13 have analogous variants in human tissue, which participate in wound contraction and re-epithelialization (66,90). FMMs can degrade collagen IV, fibrillar procollagen and fibronectin (91-93), which are also degraded by MMPs 3, 10, 11 and 12 (Table II). These proteases are involved in wound contraction, monocyte/macrophage metabolism and re-epithelialization (66).
Cathepsins are a family of proteases that have been identified in fish epithelial mucus, and these cathepsins in fish mucus have not been characterized. It is hypothesized that the cathepsins in fish mucus may exhibit a therapeutic effect on wound healing based on the available data regarding their properties on human skin. These proteases are normally present in lysosomal vesicles, but their presence has also been demonstrated extracellularly (94). In human physiology, they participate in wound healing during hemostasis (95), ECM remodeling (96) and keratinocyte migration (97). Cathepsin-L substrate affinity has been described for laminins, fibronectin, elastin and collagen (98,99). Cathepsin-D has affinity for fibronectin, proteoglycans, and collagens I and II (100), while substrate affinity of Cathepsin-B has been described primarily for collagen II, IX and XI (101). These substrates are also target proteins for MMPs 1, 8, 13 and 14 (66,102), which supports the reported role of cathepsins in wound contraction and hemostasis.
Additionally, fish mucus serine proteases (FMSPs) are present in mucosal secretions (103), albeit with only poor substrate characterization thus far. Nevertheless, this family of proteases has reported activity on collagen, elastin, fibrin and fibrinogen (104,105). Thus, this protease may be useful during hemostasis, generating platelet aggregation and fibrin clot formation (106). Additionally, FMSPs degrade fibrin, which may assist in the change of ECM from temporary to collagen-rich, resulting in cellular proliferation and migration (107). This family of enzymes also interferes with the maturation of MMPs (66) and the desquamation processes (108).
Snake venoms, particularly from the Viperidae family, are rich in proteases. There secretion is comprised of two types of proteases: Snake venom metalloproteases (SVMPs) and snake venom serine proteinases (SVSPs) (73). These enzymes catalyze a broad range of ECM components, coagulation factors and proteins involved in platelet aggregation (109,110).
SVMPs can intervene in hemostasis, as these hydrolyze glycoprotein Ib and factor X, which promote coagulation (110-112) and platelet aggregation (113,114), respectively. During inflammation, SVMPs enhance the infiltration of inflammatory cells (115,116) as well as increasing neutrophil and macrophage numbers (117-119), which increases soluble collagen levels and enhances angiogenesis through increasing vascular endothelial growth factor (VEGF) and TGF-β1 release (58). During cell migration and proliferation, it has been demonstrated that SVMPs degrade fibrin and fibronectin (112,120), resulting in the change from temporary to collagen-rich ECM. SVMPs also activate migration of skin fibroblasts (121) and endothelial cells (111,122-124). In addition, SVSPs exhibit proteolytic activity on Factor V and fibrinogen, promoting fibrin clot formation (125-127). SVSPs also promote aggregation of platelets (128).
Following analysis of reported interventions of SPs in wound healing, it could be presumed that they can intervene as helpers in several intermediate steps of the wound healing processes including coagulation, ECM degradation for re-epithelialization, or wound contraction, among other steps. The hypothesized mechanisms of SPs during the process of wound healing are presented in Fig. 1. Study of these variants may assist in the development of novel specific alternatives for active chronic wound healing care.
4. Potential of SPs as novel alternatives for wound healing care
Substrate homology analysis among MMPs and SPs suggest that animal enzymes may act similarly to the ones physiologically present in human skin. As presented in Fig. 1, previously compared SPs may be used to facilitate several steps involved in the process of wound healing, or to compensate for the physiological variants when they do not function properly. To understand this from a clearer perspective, it is important to comprehend in which of the most common chronic wounds types SPs may serve as suitable co-adjuvants.
In the current literature, chronic wounds have been classified into pressure ulcers, venous ulcers or diabetic ulcers (129,130). Pressure ulcers are caused by pressure, shear force, friction or a combination of these (131). The prevention and cure of pressure ulcers is associated with daily movement of extremities and frequent body positioning during hospitalization (132). In this case, the use of proteases may serve as palliative care in bed preparation for wounded patients as opposed to assisting the metabolic processes of wound healing.
Chronic venous ulcers are associated with inflammation, mechanical damage and erratic structural remodeling of the vein. Pathological hemodynamics results in changes to microcirculation; this produces thrombosis, proinflammatory activity and impaired MMP-3 activity (133), leading to cell dysfunction and finally to ulceration (134). For ulceration and potential necrosis, maggot therapy has shown efficacy (40,41) by decreasing inflammation and neutrophil migration (77,135). It also degrades eschar, debrides the wound and serves as a bacterial disinfectant (40,42-45). Furthermore, fish mucus proteases have been shown to exhibit antibacterial activity (55,136), which may be useful for bacterial disinfection of ulcers.
Diabetic foot ulcers are wounds that manifest after a cascade of metabolic dysregulations initiated by long-term hyperglycemia (137). As a result of prolonged exposure to high blood sugar levels, there is a decrease in fibrinolytic activity, thus increasing blood viscosity and coagulation in this type of wound (138). In addition, hyperglycemia results in a reduction of growth factors and receptor levels (such as TGF-β1), accompanied by a prolonged inflammatory phase due to upregulation of MMP-9 (139,140), which interrupts the inflammatory and proliferative phases of wound healing (141).
As an alternative therapy for diabetic foot ulcers, maggot treatment has demonstrated improved efficacy and efficiency compared with conventional methods (142). Furthermore, MaPs (74), FMMPs (91) and a certain type of SVMP (112,120) have been reported to exhibit fibrinolytic activity which may ameliorate the characteristic viscosity of diabetic ulcers. Additionally, it has been reported that TGF-β signaling is increased in the presence of MaPs (76) and SVMPs (58), and this may also assist wound healing in this type of ulcer. However, certain SVMPs can promote coagulation (110-112,120); thus, meticulous care must be taken to separate and study each component embedded within the secretion instead of applying it as a whole.
In another report, fish mucus application enhanced the healing of laparotomy wounds (143). Therefore, SPs may be used to reduce the time taken for wound healing or for the removal of necrotic tissue, depending on the wound pathophysiology.
Despite the positive effects of SPs in wound healing, further research must be performed to determine the specific mechanisms of action, regulation, site delivery and bioavailability of proposed proteases before they may be recommended as feasible pharmacological candidates for treatment of chronic wounds. The application of SPs may be limited however, as its use for treatment of burn wounds exhibits highly variable results in patients (14).
It is also important to determine how SPs may affect other wound healing mechanisms when used as an adjuvant with other healing methods such as skin transplants. In this procedure, lost skin is covered with healthy tissue or artificial composites (144,145) that provide the necessary elements (cells, growth factors, MMPs and scaffolds) for the healing process (146). The success of a skin transplant is primarily dependent on angiogenesis between the skin graft and the injury, which is predominantly mediated by MMP-2, 9 and 14(147). Thus, SPs have been proposed as potential adjuvants to increase tissue compatibility during skin transplants.
Nevertheless, studies on SP-aided transplants is still ambiguous. For example, the use of botulinum toxin A during skin transplantation in murine models enhances the expression of VEGF and prolonged the survival of skin grafts (148). By contrast, Kucukkaya et al (149) demonstrated that the same toxin reduces wound-graft contraction. Thus, the effects of SPs on skin transplants requires additional studies to determine its benefits during skin transplantation.
5. Future perspectives
Studies and development of less expensive wound healing treatment alternatives must be encouraged. Treatment of all types of even the most common chronic wounds still incur a high cost, and the reported care expenses are $50,000 for a diabetic ulcer (25), $500-$70,000 dollars for a pressure ulcer (150) and $390-$50,967 dollars per venous ulcer (151). The proposal of proteases obtained from animal secretions is a promising area to explore, as these act on specific substrates involved in the wound healing process. Furthermore, it is important to determine the molecular events specific to each chronic wound case, as these may represent key tags on how the proposed SPs may intervene. Under these conditions, active wound care represents a viable solution if its use is based on specific requirements. Importantly, SP characterization is crucial to dispense with the use of secretions in wound repair, and instead use only the SPs. This may also allow heterologous production, immobilization or improvement of the therapeutic properties of the characterized SPs through mutagenesis. In addition, time-efficient diagnostic tests on for detection of molecular targets in skin wound healing may be developed to guide practitioners on which tool to use for chronic wound care, resulting in improved wound healing and thus restoration of homeostasis.
Acknowledgements
Not applicable.
Funding
The present study was funded by CONACYT (grant nos. 886264 and 548216).
Availability of data and materials
Not applicable.
Authors' contributions
All authors (MIAR, DMM, CLC, JMAY, JB and MLS) contributed to writing, editing and revising the manuscript. All authors approved the final version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Dhivya S, Padma VV, Santhini E. Wound dressings-a review. Biomedicine (Taipei) 2015;5(22) doi: 10.7603/s40681-015-0022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nicoli Aldini N, Fini M, Giardino R. From Hippocrates to tissue engineering: Surgical strategies in wound treatment. World J Surg. 2008;32:2114–2121. doi: 10.1007/s00268-008-9662-1. [DOI] [PubMed] [Google Scholar]
- 3.Sen CK. Human wounds and its burden: An updated compendium of estimates. Adv Wound Care (New Rochelle) 2019;8:39–48. doi: 10.1089/wound.2019.0946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Järbrink K, Ni G, Sönnergren H, Schmidtchen A, Pang C, Bajpai R, Car J. Prevalence and incidence of chronic wounds and related complications: A protocol for a systematic review. Syst Rev. 2016;5(152) doi: 10.1186/s13643-016-0329-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brem H, Stojadinovic O, Diegelmann RF, Entero H, Lee B, Pastar I, Golinko M, Rosenberg H, Tomic-Canic M. Molecular markers in patients with chronic wounds to guide surgical debridement. Mol Med. 2007;13:30–39. doi: 10.2119/2006-00054.Brem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson K, Hamm RL. Factors that impair wound healing. J Am Coll Clin Wound Spec. 2014;4:84–91. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nussbaum SR, Carter MJ, Fife CE, DaVanzo J, Haught R, Nusgart M, Cartwright D. An economic evaluation of the impact, cost, and medicare policy implications of chronic nonhealing wounds. Value Health. 2018;21:27–32. doi: 10.1016/j.jval.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Avila Rodríguez MI, Rodríguez Barroso LG, Sánchez ML. Collagen: A review on its sources and potential cosmetic applications. J Cosmet Dermatol. 2018;17:20–26. doi: 10.1111/jocd.12450. [DOI] [PubMed] [Google Scholar]
- 9.Malik M. Advanced wound care market by product type (Infection Management, Exudate Management, Active Wound Care, Therapy Devices), application (Chronic Wounds and Acute Wounds), end user (Hospitals and Community Centers)-global opportunity analysis and industry forecast, 2014-2022. 2016 [Google Scholar]
- 10.Khan W, Morgan-Jones R. Debridement: Defining something we all do. J Trauma Orthop. 2016;4(48) [Google Scholar]
- 11.Kwan SH, Ismail MN. Identification of the potential bio-active proteins associated with wound healing properties in snakehead fish (Channa striata) mucus. Curr Proteomics. 2018;15:299–312. doi: 10.2174/1570164615666180717143418. [DOI] [Google Scholar]
- 12.Fatima L, Fatah C. Pathophysiological and pharmacological effects of snake venom components: Molecular targets. J Clin Toxicol. 2014;4(190) [Google Scholar]
- 13.Fierro-Arias L, Campos-Cornejo NG, Contreras-Ruiz J, Espinosa-Maceda S, López-Gehrke I, Márquez-Cárdenas R, Ramírez-Padilla M, Veras-Castillo E, Rodríguez-Alcocer AN. Productos enzimáticos (hialuronidasa, colagenasa y lipasa) y su uso en dermatología. Dermatol Rev Mex. 2017;61:206–219. [Google Scholar]
- 14.Klasen HJ. A review on the nonoperative removal of necrotic tissue from burn wounds. Burns. 2000;26:207–222. doi: 10.1016/s0305-4179(99)00117-5. [DOI] [PubMed] [Google Scholar]
- 15.Gill SE, Parks WC. Metalloproteinases and their inhibitors: Regulators of wound healing. Int J Biochem Cell Biol. 2008;40:1334–1347. doi: 10.1016/j.biocel.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ayuk SM, Abrahamse H, Houreld NN. The role of matrix metalloproteinases in diabetic wound healing in relation to photobiomodulation. J Diabetes Res. 2016;2016(2897656) doi: 10.1155/2016/2897656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mclennan SV, Min D, Yue DK. Matrix metalloproteinases and their roles in poor wound healing in diabetes. Wound Pract Res. 2008;16:116–120. [Google Scholar]
- 18.De Marco Almeida F, de Castro Pimenta AM, Oliveira MC, De Lima ME. Venoms, toxins and derivatives from the Brazilian fauna: Valuable sources for drug discovery. Sheng Li Xue Bao. 2015;67:261–270. [PubMed] [Google Scholar]
- 19.Riley KN, Herman IM. Collagenase promotes the cellular responses to injury and wound healing in vivo. J Burns Wounds. 2005;4(e8) [PMC free article] [PubMed] [Google Scholar]
- 20.Muhammad I, Shaikh SA, Rashid HU. Role of papaya dressings in the management of diabetic foot ulcers. J Rawalpindi Med College. 2014;18:87–89. [Google Scholar]
- 21.Esteban MÁ. An overview of the immunological defenses in fish skin. ISRN Immunol. 2012;2012(853470) doi: 10.5402/2012/853470. [DOI] [Google Scholar]
- 22.Horobin AJ, Shakesheff KM, Pritchard DI. Maggots and wound healing: an investigation of the effects of secretions from Lucilia sericata larvae upon the migration of human dermal fibroblasts over a fibronectin-coated surface. Wound Repair Regen. 2005;13:422–433. doi: 10.1046/j.1365-2133.2003.05314.x. [DOI] [PubMed] [Google Scholar]
- 23.Rajesh R, Raghavendra Gowda CD, Nataraju A, Dhananjaya BL, Kemparaju K, Vishwanath BS. Procoagulant activity of Calotropis gigantea latex associated with fibrin(ogen)olytic activity. Toxicon. 2005;46:84–92. doi: 10.1016/j.toxicon.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 24.White R. The costs of wound debridement and exudate management. Br J Health Care Manag. 2015;21:172–175. doi: 10.12968/bjhc.2015.21.4.172. [DOI] [Google Scholar]
- 25.Han G, Ceilley R. Chronic wound healing: A review of current management and treatments. Adv Ther. 2017;34:599–610. doi: 10.1007/s12325-017-0478-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sinclair RD, Ryan TJ. Proteolytic enzymes in wound healing: The role of enzymatic debridement. Australas J Dermatol. 1994;35:35–41. doi: 10.1111/j.1440-0960.1994.tb01799.x. [DOI] [PubMed] [Google Scholar]
- 27.Glyantsev SP, Savvina TV, Zayets TL. Comparative study of proteolytic enzymes used for debridement of purulent wounds. Bull Exp Biol Med. 1996;121:646–650. doi: 10.1007/BF02447142. [DOI] [Google Scholar]
- 28.Gray D, Acton C, Chadwick P, Fumarola S, Leaper D, Morris C, Stang D, Vowden K, Vowden P, Young T. Consensus guidance for the use of debridement techniques in the UK. Wounds UK. 2011;7:77–84. [Google Scholar]
- 29.Atkin L. Understanding methods of wound debridement. Br J Nurs. 2014;S10-S12, S14-S15(23) doi: 10.12968/bjon.2014.23.sup12.S10. [DOI] [PubMed] [Google Scholar]
- 30.Dabiri G, Damstetter E, Phillips T. Choosing a wound dressing based on common wound characteristics. Adv Wound Care (New Rochelle) 2016;5:32–41. doi: 10.1089/wound.2014.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manna B, Morrison CA. Wond debridement. StatPearls. 2019 [PubMed] [Google Scholar]
- 32.Cutting K, White R. Maceration of the skin and wound bed. 1: Its nature and causes. J Wound Care. 2002;11:275–278. doi: 10.12968/jowc.2002.11.7.26414. [DOI] [PubMed] [Google Scholar]
- 33.Mahoney J, Ward J. Surgical debridement. In: Surgery in wounds. Téot L, Banwell PE and Ziegler UE (eds.) Springer Berlin Heidelberg, Berlin, Heidelberg. 2004:67–71. [Google Scholar]
- 34.Bekara F, Vitse J, Fluieraru S, Masson R, Runz A, Georgescu V, Bressy G, Labbé JL, Chaput B, Herlin C. New techniques for wound management: A systematic review of their role in the management of chronic wounds. Arch Plast Surg. 2018;45:102–110. doi: 10.5999/aps.2016.02019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu W, Ma K, Kwon SH, Garg R, Patta YR, Fujiwara T, Gurtner GC. The abnormal architecture of healed diabetic ulcers is the result of FAK degradation by calpain 1. J Invest Dermatol. 2017;137:1155–1165. doi: 10.1016/j.jid.2016.11.039. [DOI] [PubMed] [Google Scholar]
- 36.Ayello EA, Cuddigan JE. Debridement: Controlling the necrotic/cellular burden. Adv Skin Wound Care. 2004;17quiz:66–75. 76–78. doi: 10.1097/00129334-200403000-00012. [DOI] [PubMed] [Google Scholar]
- 37.Whitaker IS, Twine C, Whitaker MJ, Welck M, Brown CS, Shandall A. Larval therapy from antiquity to the present day: Mechanisms of action, clinical applications and future potential. Postgrad Med J. 2007;83:409–413. doi: 10.1136/pgmj.2006.055905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gray M. Is larval (maggot) debridement effective for removal of necrotic tissue from chronic wounds? J Wound Ostomy Continence Nurs. 2008;35:378–384. doi: 10.1097/01.WON.0000326655.50316.0e. [DOI] [PubMed] [Google Scholar]
- 39.Jordan A, Khiyani N, Bowers SR, Lukaszczyk JJ, Stawicki SP. Maggot debridement therapy: A practical review. Int J Acad Med. 2018;4:21–34. doi: 10.4103/IJAM.IJAM_6_18. [DOI] [Google Scholar]
- 40.Brown A, Horobin A, Blount DG, Hill PJ, English J, Rich A, Williams PM, Pritchard DI. Blow fly Lucilia sericata nuclease digests DNA associated with wound slough/eschar and with Pseudomonas aeruginosa biofilm. Med Vet Entomol. 2012;26:432–439. doi: 10.1111/j.1365-2915.2012.01029.x. [DOI] [PubMed] [Google Scholar]
- 41.Harris LG, Nigam Y, Sawyer J, Mack D, Pritchard DI. Lucilia sericata chymotrypsin disrupts protein adhesin-mediated staphylococcal biofilm formation. Appl Environ Microbiol. 2013;79:1393–1395. doi: 10.1128/AEM.03689-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parnés A, Lagan KM. Larval therapy in wound management: A review. Int J Clin Pract. 2007;61:488–493. doi: 10.1111/j.1742-1241.2006.01238.x. [DOI] [PubMed] [Google Scholar]
- 43.Cazander G, Pritchard DI, Nigam Y, Jung W, Nibbering PH. Multiple actions of Lucilia sericata larvae in hard-to-heal wounds: Larval secretions contain molecules that accelerate wound healing, reduce chronic inflammation and inhibit bacterial infection. Bioessays. 2013;35:1083–1092. doi: 10.1002/bies.201300071. [DOI] [PubMed] [Google Scholar]
- 44.van der Plas MJ, Jukema GN, Wai SW, Dogterom-Ballering HC, Lagendijk EL, van Gulpen C, van Dissel JT, Bloemberg GV, Nibbering PH. Maggot excretions/secretions are differentially effective against biofilms of Staphylococcus aureus and Pseudomonas aeruginosa. J Antimicrob Chemother. 2008;61:117–122. doi: 10.1093/jac/dkm407. [DOI] [PubMed] [Google Scholar]
- 45.Pritchard DI, Brown AP. Degradation of MSCRAMM target macromolecules in VLU slough by Lucilia sericata chymotrypsin 1 (ISP) persists in the presence of tissue gelatinase activity. Int Wound J. 2015;12:414–421. doi: 10.1111/iwj.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arabloo J, Grey S, Mobinizadeh M, Olyaeemanesh A, Hamouzadeh P, Khamisabadi K. Safety, effectiveness and economic aspects of maggot debridement therapy for wound healing. Med J Islam Repub Iran. 2016;30(319) [PMC free article] [PubMed] [Google Scholar]
- 47.Evans H. A treatment of last resort. Nurs Times. 1997;93:62–65. [PubMed] [Google Scholar]
- 48.Ramundo J, Gray M. Enzymatic wound debridement. J Wound Ostomy Continence Nurs. 2008;35:273–280. doi: 10.1097/01.WON.0000319125.21854.78. [DOI] [PubMed] [Google Scholar]
- 49.Madhok BM, Vowden K, Vowden P. New techniques for wound debridement. Int Wound J. 2013;10:247–251. doi: 10.1111/iwj.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ziegler B, Hundeshagen G, Cordts T, Kneser U, Hirche C. State of the art in enzymatic debridement. Plast Aesthet Res. 2018;5(33) doi: 10.20517/2347-9264.2018.46. [DOI] [Google Scholar]
- 51.Waheed H, Moin SF, Choudhary MI. Snake venom: From deadly toxins to life-saving therapeutics. Curr Med Chem. 2017;24:1874–1891. doi: 10.2174/0929867324666170605091546. [DOI] [PubMed] [Google Scholar]
- 52.Chan YS, Cheung RCF, Xia L, Wong JH, Ng TB, Chan WY. Snake venom toxins: Toxicity and medicinal applications. Appl Microbiol Biotechnol. 2016;100:6165–6181. doi: 10.1007/s00253-016-7610-9. [DOI] [PubMed] [Google Scholar]
- 53.Smith RG. Enzymatic debriding agents: An evaluation of the medical literature. Ostomy Wound Manage. 2008;54:16–34. [PubMed] [Google Scholar]
- 54.Costa-Neto EM. Implications and applications of folk zootherapy in the state of Bahia, Northeastern Brazil. Sust Dev. 2004;12:161–174. doi: 10.1002/sd.234. [DOI] [Google Scholar]
- 55.Manan Mat Jais A. Pharmacognosy and pharmacology of Haruan (Channa striatus), a medicinal fish with wound healing properties. Bol Latinoam Caribe Plant Med Aromaticas. 2007;6:52–60. [Google Scholar]
- 56.Akunne TC, Okafor SN, Okechukwu DC, Nwankwor SS, Emene JO, Okoro BN. Catfish (Clarias gariepinus) slime coat possesses antimicrobial and wound healing activities. UK J Pharm Biosci. 2016;4:81–87. doi: 10.20510/ukjpb/4/i3/108393. [DOI] [Google Scholar]
- 57.Al-Hassan J, Thomson M, Griddle RS. Accelerated wound healing by a preparation from skin of the Arabian gulf catfish. Lancet. 1983;321:1043–1044. doi: 10.1016/s0140-6736(83)92665-x. [DOI] [PubMed] [Google Scholar]
- 58.Ferreira BA, Deconte SR, de Moura FBR, Tomiosso TC, Clissa PB, Andrade SP, Araújo FA. Inflammation, angiogenesis and fibrogenesis are differentially modulated by distinct domains of the snake venom metalloproteinase jararhagin. Int J Biol Macromol. 2018;119:1179–1187. doi: 10.1016/j.ijbiomac.2018.08.051. [DOI] [PubMed] [Google Scholar]
- 59.Ferreira RS Jr, de Barros LC, Abbade LPF, Barraviera SRCS, Silvares MRC, de Pontes LG, Dos Santos LD, Barraviera B. Heterologous fibrin sealant derived from snake venom: From bench to bedside-an overview. J Venom Anim Toxins Incl Trop Dis. 2017;23(21) doi: 10.1186/s40409-017-0109-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang PH, Huang BS, Horng HC, Yeh CC, Chen YJ. Wound healing. J Chin Med Assoc. 2018;81:94–101. doi: 10.1590/abd1806-4841.20164741. [DOI] [PubMed] [Google Scholar]
- 61.Sorg H, Tilkorn DJ, Hager S, Hauser J, Mirastschijski U. Skin wound healing: An update on the current knowledge and concepts. Eur Surg Res. 2017;58:81–94. doi: 10.1159/000454919. [DOI] [PubMed] [Google Scholar]
- 62.Clark RAF. Wound repair: Overview and general considerations. In: Clark RAF (ed): The molecular, cellular biology of wound repair, Plenum Press, New York. 1996:3–55. [Google Scholar]
- 63.Martin P, Nunan R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br J Dermatol. 2015;173:370–378. doi: 10.1111/bjd.13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cui N, Hu M, Khalil RA. Biochemical and biological attributes of matrix metalloproteinases. Prog Mol Biol Transl Sci. 2017;147:1–73. doi: 10.1016/bs.pmbts.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Greaves NS, Ashcroft KJ, Baguneid M, Bayat A. Current understanding of molecular and cellular mechanisms in fibroplasia and angiogenesis during acute wound healing. J Dermatol Sci. 2013;72:206–217. doi: 10.1016/j.jdermsci.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 66.Caley MP, Martins VL, O'Toole EA. Metalloproteinases and wound healing. Adv Wound Care (New Rochelle) 2015;4:225–234. doi: 10.1089/wound.2014.0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Krampert M, Bloch W, Sasaki T, Bugnon P, Rülicke T, Wolf E, Aumailley M, Parks WC, Werner S. Activities of the matrix metalloproteinase stromelysin-2 (MMP-10) in matrix degradation and keratinocyte organization in wounded skin. Mol Biol Cell. 2004;15:5242–5254. doi: 10.1091/mbc.e04-02-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Matziari M, Dive V, Yiotakis A. Matrix metalloproteinase 11 (MMP-11; stromelysin-3) and synthetic inhibitors. Med Res Rev. 2007;27:528–552. doi: 10.1002/med.20066. [DOI] [PubMed] [Google Scholar]
- 69.Gomis-Rüth FX. Structural aspects of the metzincin clan of metalloendopeptidases. Mol Biotechnol. 2003;24:157–202. doi: 10.1385/MB:24:2:157. [DOI] [PubMed] [Google Scholar]
- 70.Subramanian S, MacKinnon SL, Ross NW. A comparative study on innate immune parameters in the epidermal mucus of various fish species. Comp Biochem Physiol B Biochem Mol Biol. 2007;148:256–263. doi: 10.1016/j.cbpb.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 71.Franta Z, Vogel H, Lehmann R, Rupp O, Goesmann A, Vilcinskas A. Next generation sequencing identifies five major classes of potentially therapeutic enzymes secreted by Lucilia sericata medical maggots. Biomed Res Int. 2016;2016(8285428) doi: 10.1155/2016/8285428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Valachova I, Majtan T, Takac P, Majtan J. Identification and characterisation of different proteases in Lucilia sericata medicinal maggots involved in maggot debridement therapy. J Appl Biomed. 2014;12:171–177. doi: 10.1016/j.jab.2014.01.001. [DOI] [Google Scholar]
- 73.Tasoulis T, Isbister GK. A review and database of snake venom proteomes. Toxins (Basel) 2017;9(pii: E290) doi: 10.3390/toxins9090290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chambers L, Woodrow S, Brown AP, Harris PD, Phillips D, Hall M, Church JC, Pritchard DI. Degradation of extracellular matrix components by defined proteinases from the greenbottle larva Lucilia sericata used for the clinical debridement of non-healing wounds. Br J Dermatol. 2003;148:14–23. doi: 10.1046/j.1365-2133.2003.04935.x. [DOI] [PubMed] [Google Scholar]
- 75.Polakovicova S, Polák Š, Kuniaková M, Čambal M, Čaplovičová M, Kozánek M, Danišovič L, Kopáni M. The effect of salivary gland extract of Lucilia sericata maggots on human dermal fibroblast proliferation within collagen/hyaluronan membrane in vitro: Transmission electron microscopy study. Adv Skin Wound Care. 2015;28:221–226. doi: 10.1097/01.ASW.0000461260.03630.a0. [DOI] [PubMed] [Google Scholar]
- 76.Li PN, Li H, Zhong LX, Sun Y, Yu LJ, Wu ML, Zhang LL, Kong QY, Wang SY, Lv DC. Molecular events underlying maggot extract promoted rat in vivo and human in vitro skin wound healing. Wound Repair Regen. 2015;23:65–73. doi: 10.1111/wrr.12243. [DOI] [PubMed] [Google Scholar]
- 77.van der Plas MJA, van der Does AM, Baldry M, Dogterom-Ballering HC, van Gulpen C, van Dissel JT, Nibbering PH, Jukema GN. Maggot excretions/secretions inhibit multiple neutrophil pro-inflammatory responses. Microbes Infect. 2007;9:507–514. doi: 10.1016/j.micinf.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 78.van der Plas MJ, van Dissel JT, Nibbering PH. Maggot secretions skew monocyte-macrophage differentiation away from a pro-inflammatory to a pro-angiogenic type. PLoS One. 2009;4(e8071) doi: 10.1371/journal.pone.0008071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Honda K, Okamoto K, Mochida Y, Ishioka K, Oka M, Maesato K, Ikee R, Moriya H, Hidaka S, Ohtake T, et al. A novel mechanism in maggot debridement therapy: Protease in excretion/secretion promotes hepatocyte growth factor production. Am J Physiol Cell Physiol. 2011;301(C1423-C1430) doi: 10.1152/ajpcell.00065.2011. [DOI] [PubMed] [Google Scholar]
- 80.Andersen AS, Sandvang D, Schnorr KM, Kruse T, Neve S, Joergensen B, Karlsmark T, Krogfelt KA. A novel approach to the antimicrobial activity of maggot debridement therapy. J Antimicrob Chemother. 2010;65:1646–1654. doi: 10.1093/jac/dkq165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Margolin L, Gialanella P. Assessment of the antimicrobial properties of maggots. Int Wound J. 2010;7:202–204. doi: 10.1111/j.1742-481X.2010.00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pöppel AK, Kahl M, Baumann A, Wiesner J, Gökçen A, Beckert A, Preissner KT, Vilcinskas A, Franta Z. A Jonah-like chymotrypsin from the therapeutic maggot Lucilia sericata plays a role in wound debridement and coagulation. Insect Biochem Mol Biol. 2016;70:138–147. doi: 10.1016/j.ibmb.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 83.Mukherjee S, Gomes A, Dasgupta S. Zoo therapeutic uses of snake body parts in folk & traditional medicine. J Zool Res. 2017;1:1–9. [Google Scholar]
- 84.Shephard KL. Functions for fish mucus. Rev Fish Biol Fisheries. 1994;4:401–429. doi: 10.1007/BF00042888. [DOI] [Google Scholar]
- 85.Dash S, Das SK, Samal J, Thatoi HN. Epidermal mucus, a major determinant in fish health: A review. Iran J Vet Res. 2018;19:72–81. [PMC free article] [PubMed] [Google Scholar]
- 86.Sveen L, Timmerhaus GF, Torgersen J, Ytteborg E, Jørgensen SM, Handeland SO, Stefansson SO, Nilsen TO, Calabrese S, Ebbesson LOE, et al. Impact of fish density and specific water flow on skin properties in Atlantic salmon (Salmo salar L.) post-smolts. Aquaculture. 2016;464:629–637. doi: 10.1016/j.aquaculture.2016.08.012. [DOI] [Google Scholar]
- 87.Al-Hassan JM, Thomson M, Criddle KR, Summers B, Criddle RS. Catfish epidermal secretions in response to threat or injury. Marine Biol. 1985;88:117–123. doi: 10.1007/BF00397158. [DOI] [Google Scholar]
- 88.Krasnov A, Skugor S, Todorcevic M, Glover KA, Nilsen F. Gene expression in Atlantic salmon skin in response to infection with the parasitic copepod Lepeophtheirus salmonis, cortisol implant, and their combination. BMC Genomics. 2012;13(130) doi: 10.1186/1471-2164-13-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schütte A, Lottaz D, Sterchi EE, Stöcker W, Becker-Pauly C. Two alpha subunits and one beta subunit of meprin zinc-endopeptidases are differentially expressed in the zebrafish Danio rerio. Biol Chem. 2007;388:523–531. doi: 10.1515/BC.2007.060. [DOI] [PubMed] [Google Scholar]
- 90.Nguyen TT, Mobashery S, Chang M. Roles of Matrix Metalloproteinases in Cutaneous Wound Healing. Wound Healing-New insights into Ancient Challenges. 2016 doi: 10.1089/wound.2014.0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sterchi EE, Stöcker W, Bond JS. Meprins, membrane-bound and secreted astacin metalloproteinases. Mol Aspects Med. 2008;29:309–328. doi: 10.1016/j.mam.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bertenshaw GP, Turk BE, Hubbard SJ, Matters GL, Bylander JE, Crisman JM, Cantley LC, Bond JS. Marked differences between metalloproteases meprin A and B in substrate and peptide bond specificity. J Biol Chem. 2001;276:13248–13255. doi: 10.1074/jbc.M011414200. [DOI] [PubMed] [Google Scholar]
- 93.Kruse MN, Becker C, Lottaz D, Köhler D, Yiallouros I, Krell HW, Sterchi EE, Stöcker W. Human meprin alpha and beta homo-oligomers: Cleavage of basement membrane proteins and sensitivity to metalloprotease inhibitors. Biochem J. 2004;378:383–389. doi: 10.1042/BJ20031163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sun H, Lou X, Shan Q, Zhang J, Zhu X, Zhang J, Wang Y, Xie Y, Xu N, Liu S. Proteolytic characteristics of cathepsin D related to the recognition and cleavage of its target proteins. PLoS One. 2013;8(e65733) doi: 10.1371/journal.pone.0065733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wolters BK. Cathepsin L and V in human keratinocytes. J Univ. 2006 doi: 10.1078/0171-9335-00404. [DOI] [PubMed] [Google Scholar]
- 96.Vidak E, Javoršek U, Vizovišek M, Turk B. Cysteine cathepsins and their extracellular roles: Shaping the microenvironment. Cells. 2019;8(pii: E264) doi: 10.3390/cells8030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Reinheckel T, Hagemann S, Dollwet-Mack S, Martinez E, Lohmüller T, Zlatkovic G, Tobin DJ, Maas-Szabowski N, Peters C. The lysosomal cysteine protease cathepsin L regulates keratinocyte proliferation by control of growth factor recycling. J Cell Sci. 2005;118:3387–3395. doi: 10.1242/jcs.02469. [DOI] [PubMed] [Google Scholar]
- 98.Mason RW. Interaction of lysosomal cysteine proteinases with α2-macroglobulin: Conclusive evidence for the endopeptidase activities of cathepsins B and H. Arch Biochem Bioph. 1989;273:367–374. doi: 10.1016/0003-9861(89)90495-5. [DOI] [PubMed] [Google Scholar]
- 99.Maciewicz RA, Etherington DJ, Kos J, Turk V. Collagenolytic cathepsins of rabbit spleen: A kinetic analysis of collagen degradation and inhibition by chicken cystatin. Coll Relat Res. 1987;7:295–304. doi: 10.1016/S0174-173X(87)80035-3. [DOI] [PubMed] [Google Scholar]
- 100.Benes P, Vetvicka V, Fusek M. Cathepsin D-many functions of one aspartic protease. Crit Rev Oncol Hematol. 2008;68:12–28. doi: 10.1016/j.critrevonc.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cavallo-Medved D, Moin K, Sloane B. Cathepsin B: Basis sequence: Mouse. AFCS Nat Mol Pages. 2011;2011(pii: A000508) [PMC free article] [PubMed] [Google Scholar]
- 102.Krejner A, Litwiniuk M, Grzela T. Matrix metalloproteinases in the wound microenvironment: Therapeutic perspectives. Chronic Wound Care Manag Res. 2016;3:29–39. doi: 10.2147/CWCMR.S73819. [DOI] [Google Scholar]
- 103.Kim GY, Kim HY, Kim HT, Moon JM, Kim CH, Kang S, Rhim H. HtrA1 is a novel antagonist controlling fibroblast growth factor (FGF) signaling via cleavage of FGF8. Mol Cell Biol. 2012;32:4482–4492. doi: 10.1128/MCB.00872-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Meyer-Hoffert U, Schröder JM. Epidermal proteases in the pathogenesis of rosacea. J Investig Dermatol Symp Proc. 2011;15:16–23. doi: 10.1038/jidsymp.2011.2. [DOI] [PubMed] [Google Scholar]
- 105.Kim SK, Park PJ, Kim JB, Shahidi F. Purification and characterization of a collagenolytic protease from the filefish, Novoden modestrus. J Biochem Mol Biol. 2002;35:165–171. doi: 10.5483/bmbrep.2002.35.2.165. [DOI] [PubMed] [Google Scholar]
- 106.Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258–264. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- 107.Perona JJ, Craik CS. Structural basis of substrate specificity in the serine proteases. Protein Sci. 1995;4:337–360. doi: 10.1002/pro.5560040301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rawlings AV, Voegeli R. Stratum corneum proteases and dry skin conditions. Cell Tissue Res. 2013;351:217–235. doi: 10.1007/s00441-012-1501-x. [DOI] [PubMed] [Google Scholar]
- 109.Gutiérrez JM, Escalante T, Rucavado A, Herrera C, Fox JW. A Comprehensive view of the structural and functional alterations of extracellular matrix by snake venom metalloproteinases (SVMPs): Novel perspectives on the pathophysiology of envenoming. Toxins (Basel) 2016;8(pii: E304) doi: 10.3390/toxins8100304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kini RM, Koh CY. Metalloproteases affecting blood coagulation, fibrinolysis and platelet aggregation from snake venoms: Definition and nomenclature of interaction sites. Toxins (Basel) 2016;8(pii: E284) doi: 10.3390/toxins8100284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Silva MB, Schattner M, Ramos CR, Junqueira-de-Azevedo IL, Guarnieri MC, Lazzari MA, Sampaio CA, Pozner RG, Ventura JS, Ho PL, Chudzinski-Tavassi AM. A prothrombin activator from Bothrops erythromelas (jararaca-da-seca) snake venom: Characterization and molecular cloning. Biochem J. 2003;369:129–139. doi: 10.1042/BJ20020449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sanchez EF, Richardson M, Gremski LH, Veiga SS, Yarleque A, Niland S, Lima AM, Estevao-Costa MI, Eble JA. Data for a direct fibrinolytic metalloproteinase, barnettlysin-I from Bothrops barnetti (barnett(,)s pitviper) snake venom with anti-thrombotic effect. Data Brief. 2016;7:1609–1613. doi: 10.1016/j.dib.2016.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kamiguti AS. Platelets as targets of snake venom metalloproteinases. Toxicon. 2005;45:1041–1049. doi: 10.1016/j.toxicon.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 114.Howes JM, Kamiguti AS, Theakston RD, Wilkinson MC, Laing GD. Effects of three novel metalloproteinases from the venom of the West African saw-scaled viper, Echis ocellatus on blood coagulation and platelets. Biochim Biophys Acta. 2005;1724:194–202. doi: 10.1016/j.bbagen.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 115.Fernandes CM, Zamuner SR, Zuliani JP, Rucavado A, Gutiérrez JM, Teixeira Cde F. Inflammatory effects of BaP1 a metalloproteinase isolated from Bothrops asper snake venom: Leukocyte recruitment and release of cytokines. Toxicon. 2006;47:549–559. doi: 10.1016/j.toxicon.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 116.Silva A, Gunawardena P, Weilgama D, Maduwage K, Gawarammana I. Comparative in-vivo toxicity of venoms from South Asian hump-nosed pit vipers (Viperidae: Crotalinae: Hypnale) BMC Res Notes. 2012;5(471) doi: 10.1186/1756-0500-5-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mariano-Oliveira A, Coelho ALJ, Terruggi CH, Selistre-de-Araújo HS, Barja-Fidalgo C, De Freitas MS. Alternagin-C, a nonRGD-disintegrin, induces neutrophil migration via integrin signaling. Eur J Biochem. 2003;270:4799–4808. doi: 10.1046/j.1432-1033.2003.03867.x. [DOI] [PubMed] [Google Scholar]
- 118.Silva CA, Zuliani JP, Assakura MT, Mentele R, Camargo ACM, Teixeira CFP, Serrano SMT. Activation of αMβ2-mediated phagocytosis by HF3, a P-III class metalloproteinase isolated from the venom of Bothrops jararaca. Biochem Biophys Res Commun. 2004;322:950–956. doi: 10.1016/j.toxicon.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 119.Tseng YL, Lee CJ, Huang TF. Effects of a snake venom metalloproteinase, triflamp, on platelet aggregation, platelet-neutrophil and neutrophil-neutrophil interactions: Involvement of platelet GPIbalpha and neutrophil PSGL-1. Thromb Haemost. 2004;91:315–324. doi: 10.1160/TH03-07-0426. [DOI] [PubMed] [Google Scholar]
- 120.Bernardes CP, Menaldo DL, Camacho E, Rosa JC, Escalante T, Rucavado A, Lomonte B, Gutiérrez JM, Sampaio SV. Proteomic analysis of Bothrops pirajai snake venom and characterization of BpirMP, a new P-I metalloproteinase. J Proteomics. 2013;80:250–267. doi: 10.1016/j.jprot.2013.01.021. [DOI] [PubMed] [Google Scholar]
- 121.Zigrino P, Kamiguti AS, Eble J, Drescher C, Nischt R, Fox JW, Mauch C. The reprolysin jararhagin, a snake venom metalloproteinase, functions as a fibrillar collagen agonist involved in fibroblast cell adhesion and signaling. J Biol Chem. 2002;277:40528–40535. doi: 10.1074/jbc.M202049200. [DOI] [PubMed] [Google Scholar]
- 122.Costa ÉP, Santos MF. Jararhagin, a snake venom metalloproteinase-disintegrin, stimulates epithelial cell migration in an in vitro restitution model. Toxicon. 2004;44:861–870. doi: 10.1016/j.toxicon.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 123.Cominetti MR, Terruggi CH, Ramos OH, Fox JW, Mariano-Oliveira A, De Freitas MS, Figueiredo CC, Morandi V, Selistre-de-Araujo HS. Alternagin-C, a disintegrin-like protein, induces vascular endothelial cell growth factor (VEGF) expression and endothelial cell proliferation in vitro. J Biol Chem. 2004;279:18247–18255. doi: 10.1074/jbc.M311771200. [DOI] [PubMed] [Google Scholar]
- 124.Schattner M, Fritzen M, Ventura Jde S, de Albuquerque Modesto JC, Pozner RG, Moura-da-Silva AM, Chudzinski-Tavassi AM. The snake venom metalloproteases berythractivase and jararhagin activate endothelial cells. Biol Chem. 2005;386:369–374. doi: 10.1515/BC.2005.044. [DOI] [PubMed] [Google Scholar]
- 125.Siigur E, Tõnismägi K, Trummal K, Samel M, Vija H, Subbi J, Siigur J. Factor X activator from Vipera lebetina snake venom, molecular characterization and substrate specificity. Biochim Biophys Acta. 2001;1568:90–98. doi: 10.1016/s0304-4165(01)00206-9. [DOI] [PubMed] [Google Scholar]
- 126.Markland FS, Kettner C, Schiffman S, Shaw E, Bajwa SS, Reddy KN, Kirakossian H, Patkos GB, Theodor I, Pirkle H. Kallikrein-like activity of crotalase, a snake venom enzyme that clots fibrinogen. Proc Natl Acad Sci USA. 1982;79:1688–1692. doi: 10.1073/pnas.79.6.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang Y, Wisner A, Xiong Y, Bon C. A novel plasminogen activator from snake venom. Purification, characterization, and molecular cloning. J Biol Chem. 1995;270:10246–10255. doi: 10.1074/jbc.270.17.10246. [DOI] [PubMed] [Google Scholar]
- 128.Serrano SM, Matos MF, Mandelbaum FR, Sampaio CA. Basic proteinases from Bothrops moojeni (caissaca) venom-I. Isolation and activity of two serine proteinases, MSP 1 and MSP 2, on synthetic substrates and on platelet aggregation. Toxicon. 1993;31:471–481. doi: 10.1016/0041-0101(93)90182-i. [DOI] [PubMed] [Google Scholar]
- 129.Frykberg RG, Banks J. Challenges in the treatment of chronic wounds. Adv Wound Care (New Rochelle) 2015;4:560–582. doi: 10.1089/wound.2015.0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Telgenhoff D, Shroot B. Cellular senescence mechanisms in chronic wound healing. Cell Death Differ. 2005;12:695–698. doi: 10.1038/sj.cdd.4401632. [DOI] [PubMed] [Google Scholar]
- 131.Lumbers M. Pressure ulcers: An overview of risk. Br J Nurs. 2017;26(S49-S50) doi: 10.12968/bjon.2017.26.15.S49. [DOI] [PubMed] [Google Scholar]
- 132.Secretariat MA. Management of chronic pressure ulcers: An evidence-based analysis. Ont Health Technol Assess Ser. 2009;9:1–203. [PMC free article] [PubMed] [Google Scholar]
- 133.Comerota A, Lurie F. Pathogenesis of venous ulcer. Semin Vasc Surg. 2015;28:6–14. doi: 10.4103/2229-5178.137819. [DOI] [PubMed] [Google Scholar]
- 134.Mannello F, Raffetto JD. Matrix metalloproteinase activity and glycosaminoglycans in chronic venous disease: The linkage among cell biology, pathology and translational research. Am J Transl Res. 2011;3:149–158. [PMC free article] [PubMed] [Google Scholar]
- 135.van der Plas MJ, Baldry M, van Dissel JT, Jukema GN, Nibbering PH. Maggot secretions suppress pro-inflammatory responses of human monocytes through elevation of cyclic AMP. Diabetologia. 2009;52:1962–1970. doi: 10.1007/s00125-009-1432-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wei OY, Xavier R, Marimuthu K. Screening of antibacterial activity of mucus extract of snakehead fish, Channa striatus (Bloch) Eur Rev Med Pharmacol Sci. 2010;14:675–681. [PubMed] [Google Scholar]
- 137.Jhamb S, Vangaveti VN, Malabu UH. Genetic and molecular basis of diabetic foot ulcers: Clinical review. J Tissue Viability. 2016;25:229–236. doi: 10.1016/j.jtv.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 138.Su N, Tong N, Du L, Wu B, Xu T. Heparin and related substances for treating diabetic foot ulcers. Cochrane Database Syst Rev. 2017;2017(CD011087) doi: 10.1002/14651858.CD011087.pub2. [DOI] [Google Scholar]
- 139.Bruhn-Olszewska B, Korzon-Burakowska A, Gabig-Ciminska M, Olszewski P, Wegrzyn A, Jakóbkiewicz-Banecka J. Molecular factors involved in the development of diabetic foot syndrome. Acta Biochim Pol. 2012;59:507–513. [PubMed] [Google Scholar]
- 140.Blakytny R, Jude EB. Altered molecular mechanisms of diabetic foot ulcers. Int J Low Extrem Wounds. 2009;8:95–104. doi: 10.1177/1534734609337151. [DOI] [PubMed] [Google Scholar]
- 141.Patel S, Srivastava S, Singh MR, Singh D. Mechanistic insight into diabetic wounds: Pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed Pharmacother. 2019;112(108615) doi: 10.1016/j.biopha.2019.108615. [DOI] [PubMed] [Google Scholar]
- 142.Sherman RA. Maggot therapy for treating diabetic foot ulcers unresponsive to conventional therapy. Diabetes Care. 2003;26:446–451. doi: 10.2337/diacare.26.2.446. [DOI] [PubMed] [Google Scholar]
- 143.Pasha M, Husin RA, Hassan S. The influence of oral and topical Channa striatus on laparotomy wound healing in malnourished wistar rats. Int J Pharm Pharm Sci Invent. 2015;4:37–41. doi: 10.6084/m9.figshare.1473070.v1. [DOI] [Google Scholar]
- 144.Anish S. Skin substitutes in dermatology. Indian J Dermatol Venereol Leprol. 2015;81:175–178. doi: 10.4103/0378-6323.152288. [DOI] [PubMed] [Google Scholar]
- 145.Kordestani SS. Chapter 5-wound care management. In: Atlas of wound healing. Kordestani SS (ed). Elsevier. 2019:31–47. [Google Scholar]
- 146.Sun BK, Siprashvili Z, Khavari PA. Advances in skin grafting and treatment of cutaneous wounds. Science. 2014;346:941–945. doi: 10.1126/science.1253836. [DOI] [PubMed] [Google Scholar]
- 147.Knapik A, Hegland N, Calcagni M, Althaus M, Vollmar B, Giovanoli P, Lindenblatt N. Metalloproteinases facilitate connection of wound bed vessels to pre-existing skin graft vasculature. Microvasc Res. 2012;84:16–23. doi: 10.1016/j.mvr.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 148.Park YJ, Lee JW, Chong Y, Park TH. Botulinum toxin A increases allograft tolerance in an experimental transplantation model: A preliminary study. Biosci Rep. 2018;38(pii: BSR20171721) doi: 10.1042/BSR20171721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kucukkaya D, Irkoren S, Ozkan S, Sivrioglu N. The effects of botulinum toxin A on the wound and skin graft contraction. J Craniofac Surg. 2014;25:1908–1911. doi: 10.1097/SCS.0000000000000941. [DOI] [PubMed] [Google Scholar]
- 150.Boyko TV, Longaker MT, Yang GP. Review of the current management of pressure ulcers. Adv Wound Care (New Rochelle) 2018;7:57–67. doi: 10.1089/wound.2016.0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ma H, O'Donnell TF Jr, Rosen NA, Iafrati MD. The real cost of treating venous ulcers in a contemporary vascular practice. J Vasc Surg Venous Lymphat Disord. 2014;2:355–361. doi: 10.1016/j.jvsv.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 152.Ford CN, Reinhard ER, Yeh D, Syrek D, De Las Morenas A, Bergman SB, Williams S, Hamori CA. Interim Analysis of a Prospective, Randomized Trial of Vacuum-Assisted Closure Versus the Healthpoint System in the Management of Pressure Ulcers. Ann Plast Surg. 2002;49(1):55–61. doi: 10.1097/00000637-200207000-00009. [DOI] [PubMed] [Google Scholar]
- 153.Yaakobi T, Cohen-Hadar N, Yaron H, Hirszowicz E, Simantov Y, Bass A, Freeman A. Wound debridement by continuous streaming of proteolytic enzyme solutions: Effects on experimental chronic wound model in porcin. Wounds. 2007;19:192–200. [PubMed] [Google Scholar]
- 154.2014 Smith & Nephew, Inc.: Enzymatic debridement with collagenase SANTYL® Ointment, [Google Scholar]
- 155.Giudice G, Filoni A, Maggio G, Bonamonte D, Vestita M. Cost analysis of a novel enzymatic debriding agent for management of burn wounds. Biomed Res Int. 2017;2017(9567498) doi: 10.1155/2017/9567498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gorecki M, Toren A. 2005 Debriding composition from bromelain and methods of production thereof, Patent Appl Publ. [Google Scholar]
- 157.Klein GKV, Houck JC. 1980 Hydrolytic enzyme material. [Google Scholar]
- 158.Niehaus F, Eck J, Schulze R, Krohn M. Proteasa para el acondicionamiento de heridas y el cuidado de la piel. Brain Biotechnol Res Inf Netw. 2012 [Google Scholar]
- 159.Niehaus F, Eck J, Schulze R, Krohn M. Protease for wound conditioning and skin care. Brain Biotechnol Res Inf Netw. 2012 [Google Scholar]
- 160.Rosenberg L. Aparato y procedimientos para su uso en escarotomía enzimática en síndrome de compartimento inducido por quemaduras. MediWound. 2012 [Google Scholar]
- 161.Freeman A, Hirszowicz E, Be'eri-lipperman M. Apparatus and method for the enzymatic debridement of skin lesions, Ramot At Tel-Aviv Univ. 2012 [Google Scholar]
- 162.Yaakobi T, Roth D, Chen Y, Freeman A. Streaming of proteolytic enzyme solutions for wound debridement: A feasibility study. Wounds. 2004;16:201–205. [Google Scholar]
- 163.Rodeheaver G, Edgerton MT, Elliott MB, Kurtz LD, Edlich RF. Proteolytic enzymes as adjuncts to antibiotic prophylaxis of surgical wounds. Am J Surg. 1974;127:564–572. doi: 10.1016/0002-9610(74)90318-3. [DOI] [PubMed] [Google Scholar]
- 164.Gao M, Nguyen TT, Suckow MA, Wolter WR, Gooyit M, Mobashery S, Chang M. Acceleration of diabetic wound healing using a novel protease-anti-protease combination therapy. Proc Natl Acad Sci USA. 2015;112:15226–15231. doi: 10.1073/pnas.1517847112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gutiérrez-Fernández A, Fueyo A, Folgueras AR, Garabaya C, Pennington CJ, Pilgrim S, Edwards DR, Holliday DL, Jones JL, Span PN, et al. Matrix metalloproteinase-8 functions as a metastasis suppressor through modulation of tumor cell adhesion and invasion. Cancer Res. 2008;68:2755–2763. doi: 10.1158/0008-5472.CAN-07-5154. [DOI] [PubMed] [Google Scholar]
- 166.Hartenstein B, Dittrich BT, Stickens D, Heyer B, Vu TH, Teurich S, Schorpp-Kistner M, Werb Z, Angel P. Epidermal development and wound healing in matrix metalloproteinase 13-deficient mice. J Invest Dermatol. 2006;126:486–496. doi: 10.1038/sj.jid.5700084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kudo Y, Iizuka S, Yoshida M, Tsunematsu T, Kondo T, Subarnbhesaj A, Deraz EM, Siriwardena SB, Tahara H, Ishimaru N, et al. Matrix metalloproteinase-13 (MMP-13) directly and indirectly promotes tumor angiogenesis. J Biol Chem. 2012;287:38716–38728. doi: 10.1074/jbc.M112.373159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rohani MG, Parks WC. Matrix remodeling by MMPs during wound repair. Matrix Biol. 2015:44–46. 113–121. doi: 10.1016/j.matbio.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 169.Thirkettle S, Decock J, Arnold H, Pennington CJ, Jaworski DM, Edwards DR. Matrix Matrix metalloproteinase 8 (collagenase 2) induces the expression of interleukins 6 and 8 in breast cancer cells. J Biol Chem. 2013;288:16282–16294. doi: 10.1074/jbc.M113.464230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Utz ER, Elster EA, Tadaki DK, Gage F, Perdue PW, Forsberg JA, Stojadinovic A, Hawksworth JS, Brown TS. Metalloproteinase expression is associated with traumatic wound failure. J Surg Res. 2010;159:633–639. doi: 10.1016/j.jss.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 171.Yamamoto K, Okano H, Miyagawa W, Visse R, Shitomi Y, Santamaria S, Dudhia J, Troeberg L, Strickland DK, Hirohata S, Nagase H. MMP-13 is constitutively produced in human chondrocytes and co-endocytosed with ADAMTS-5 and TIMP-3 by the endocytic receptor LRP1. Matrix Biol. 2016;56:57–73. doi: 10.1016/j.matbio.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Motrescu ER, Blaise S, Etique N, Messaddeq N, Chenard MP, Stoll I, Tomasetto C, Rio MC. Matrix metalloproteinase-11/stromelysin-3 exhibits collagenolytic function against collagen VI under normal and malignant conditions. Oncogene. 2008;27:6347–6355. doi: 10.1038/onc.2008.218. [DOI] [PubMed] [Google Scholar]
- 173.Pittayapruek P, Meephansan J, Prapapan O, Komine M, Ohtsuki M. Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int J Mol Sci. 2016;17(pii: e868) doi: 10.3390/ijms17060868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Saarialho-Kere UK, Pentland AP, Birkedal-Hansen H, Parks WC, Welgus HG. Distinct populations of basal keratinocytes express stromelysin-1 and stromelysin-2 in chronic wounds. J Clin Invest. 1994;94:79–88. doi: 10.1172/JCI117351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sato T, Nomura K, Hashimoto I. Expression of collagenase and stromelysin in skin fibroblasts from recessive dystrophic epidermolysis bullosa. Arch Dermatol Res. 1995;287:428–433. doi: 10.1007/bf00373423. [DOI] [PubMed] [Google Scholar]
- 176.Kren L, Goncharuk V, Krenová Z, Stratil D, Hermanová M, Skricková J, Sheehan CE, Ross JS. Expression of matrix metalloproteinases 3, 10 and 11 (stromelysins 1, 2 and 3) and matrix metalloproteinase 7 (matrilysin) by cancer cells in non-small cell lung neoplasms. Clinicopathologic studies. Cesk Patol. 2006;42:16–19. [PubMed] [Google Scholar]
- 177.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Purcell WT, Hidalgo M. Matrix metalloproteinase inhibitors in cancer therapy. In: Proteases in tissue remodelling of lung and heart. Lendeckel U and Hooper NM (eds). Springer US, Boston, MA. 2003:pp75–118. [Google Scholar]
- 179.Herouy Y. The role of matrix metalloproteinases (MMPs) and their inhibitors in venous leg ulcer healing. Phlebolymphology. 2004;44:231–243. [Google Scholar]
- 180.Lagente V, Manoury B, Nenan S, Le Quement C, Martin-Chouly C, Boichot E. Role of matrix metalloproteinases in the development of airway inflammation and remodeling. Braz J Med Biol Res. 2005;38:1521–1530. doi: 10.1590/s0100-879x2005001000009. [DOI] [PubMed] [Google Scholar]
- 181.van Marion MMH. Matrix metalloproteinases and collagen remodeling. A Literature Review. 2006 [Google Scholar]
- 182.Tewari A, Grys K, Kollet J, Sarkany R, Young AR. Upregulation of MMP12 and its activity by UVA1 in human skin: potential implications for photoaging. J Invest Dermatol. 2014;134:2598–2609. doi: 10.1038/jid.2014.173. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.