Abstract
Mosquitoes are important vectors of several diseases, and control of these insects is imperative for human health. Insecticides have proven useful in controlling mosquito populations, but insecticide resistance and environmental concerns are increasing. Additionally, emerging and re-emerging microbial infections are problematic. Essential oils have been shown to be promising mosquito larvicidal agents as well as antimicrobial agents. In this work, the essential oils from four species of Myrtaceae (Baeckea frutescens, Callistemon citrinus, Melaleuca leucadendra, and Syzygium nervosum) growing wild in central Vietnam have been obtained by hydrodistillation and analyzed by gas chromatographic techniques. The essential oils have been screened for mosquito larvicidal activity against Aedes aegypti, Aedes albopictus, and Culex quinquefasciatus, and for antimicrobial activity against Enterococcus faecalis, Staphylococcus aureus, and Candida albicans. Callistemon citrinus fruit essential oil, rich in α-pinene (35.1%), 1,8-cineole (32.4%), limonene (8.2%), and α-terpineol (5.8%) showed good larvicidal activity with 24-h LC50 = 17.3 μg/mL against both Ae. aegypti and Cx. quinquefasciatus, and good antibacterial activity against E. faecalis (minimum inhibitory concentration (MIC) = 16 μg/mL) The 48-h larvicidal activities of M. leucadendra leaf essential oil, rich in α-eudesmol (17.6%), guaiol (10.9%), linalool (5.1%), (E)-caryophyllene (7.0%), and bulnesol (3.6%) were particularly notable, with LC50 of 1.4 and 1.8 μg/mL on Ae. aegypti and Cx. quinquefasciatus. Similarly, M. leucadendra bark essential oil, with α-eudesmol (24.1%) and guaiol (11.3%), showed good antibacterial activity against. E. faecalis. Both B. frutescens and C. citrinus leaf essential oils demonstrated anti-Candida activities with MIC values of 16 μg/mL. The results of this investigation suggest that essential oils derived from the Myrtaceae may serve as “green” alternatives for the control of mosquitoes and/or complementary antimicrobial agents.
Keywords: Baeckea frutescens, Callistemon citrinus, Melaleuca leucadendra, Syzygium nervosum
1. Introduction
Mosquitoes are important vectors of diseases and kill more humans than any other animal. Aedes aegypti (L.) and Ae. albopictus (Skuse) (Diptera: Culicidae) are vectors of the yellow fever, dengue, Zika, and chikungunya viruses [1,2,3]; Culex quinquefasciatus (Say) is the primary vector of the Saint Louis encephalitis and West Nile viruses, as well as the filarial nematode Wuchereria bancrofti, and may also be a vector of the Zika virus [4].
Microbial infections continue to be a problem, for humans [5], as well as for livestock and other agriculture settings [6,7,8]. Compounding this problem are newly emerging pathogenic microorganisms, in addition to re-emerging multidrug-resistant pathogens [9,10].
The Myrtaceae is comprised of 131 genera and around 5500 species, all of which are woody trees or shrubs and contain essential oils [11]. Several members of the family are commercially important for their medicinal essential oils, such as clove (Syzygium aromaticum (L.) Merr. & L.M. Perry), tea tree (Melaleuca alternifolia Cheel), allspice (Pimenta dioica (L.) Merr.), and Eucalyptus. In this work, we present the essential oil compositions of four species of Myrtaceae growing wild in central Vietnam, their larvicidal activities against Ae. aegypti, Ae. albopictus, and Cx. quinquefasciatus, and their antimicrobial activities against Enterococcus faecalis, Staphylococcus aureus, and Candida albicans.
Baeckea frutescens L. (syn. Baeckea chinensis Gaertn., Baeckea cochinchinensis Blume, Baeckea sumatrana Blume) is a shrub or small tree that ranges throughout southeastern China (including the provinces of Fujian, Guangdong, Guangxi, Hainan, Jaingxi, and Zhejiang), Burma, Cambodia, India, the Philippines, Thailand, and Vietnam [12].
Callistemon citrinus (Curtis) Skeels (syn. Melaleuca citrina (Curtis) Dum. Cours., Callistemon lanceolatus DC., Callistemon lanceolatus Sweet, Metrosideros citrinus Curtis, Metrosideros lanceolata Sm.) is a shrub or small tree, native to Australia, but has been introduced to tropical and subtropical regions worldwide [13].
Melaleuca leucadendra (L.) L. (syn. Melaleuca viridiflora C.F. Gaertn., Myrtus leucadendra L.) is a tree growing as large as 40 m in height, native to tropical Australia (Queensland, Northern Territory, and Western Australia, New Guinea, and islands of eastern Indonesia [14]. The tree has been introduced to other tropical areas [12], including Vietnam, where it is grown for use as poles and construction materials [14].
Syzygium nervosum DC. (syn. Cleistocalyx operculatus (Roxb.) Merr. & L.M.Perry, Eugenia operculata Roxb.) is a medium-sized tree native to the Asian tropics, from southern China (Guangdong, Guangxi, Hainan, Xizang Zizhiqu, and Yunnan provinces), India, Burma, Sri Lanka, Thailand, and Vietnam [12], and south into eastern Australia [15].
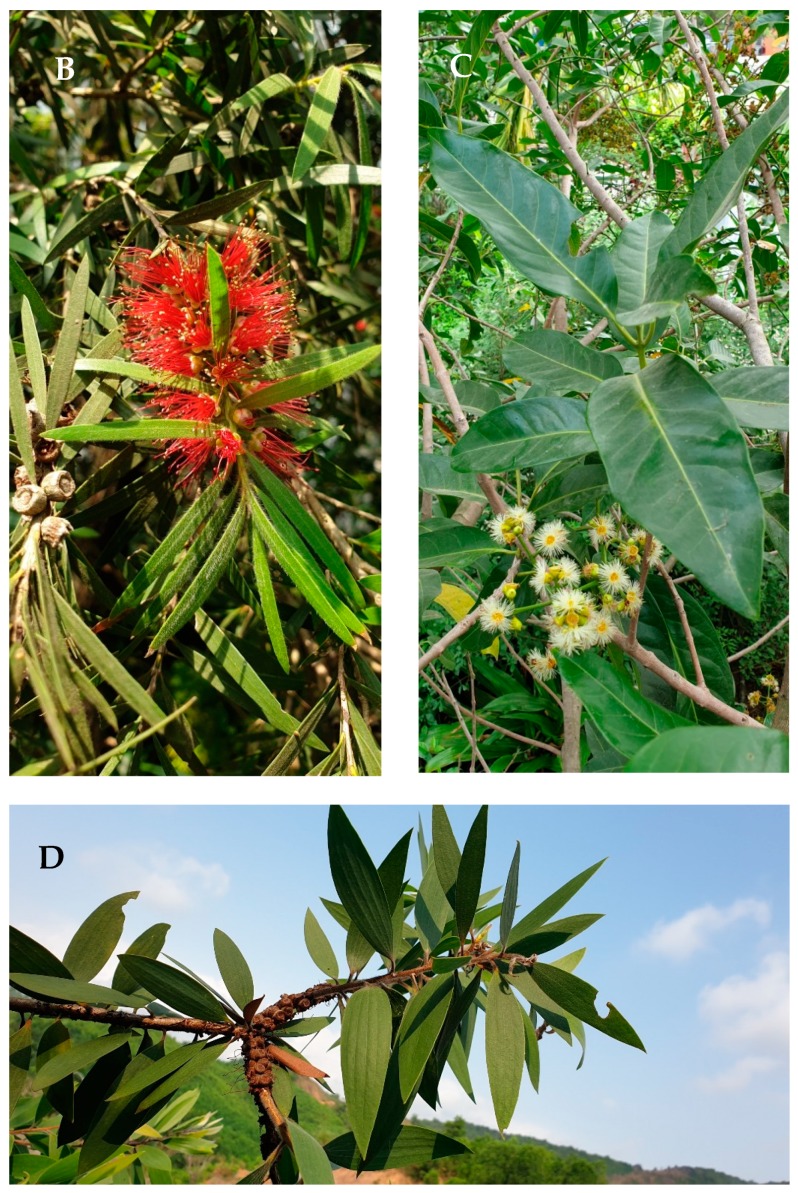
Photographs of the plants presented in this work are shown in Figure 1.
Figure 1.
Photographs of the plants examined in this work. A: Baeckea frutescens, B: Callistemon citrinus, C: Syzygium nervosum, D: Melaleuca leucadendra.
2. Results and Discussion
2.1. Chemical Compositions
The essential oil from the fresh leaves of Baeckea frutescens was obtained in a yield of 2.23%. The leaf essential oil composition of B. frutescens is presented in Table 1. A total of 88 compounds were identified accounting for 100% of the essential oil composition, with monoterpene hydrocarbons (55.6%) predominating. The major components were α-pinene (11.1%), β-pinene (19.0%), p-cymene (8.9%), 1,8-cineole (10.1%), γ-terpinene (11.7%), (E)-caryophyllene (7.1%), and α-humulene (9.9%). Leaf essential oil compositions have previously been reported from Vietnam [16,17,18], China [19], and from Malaysia [20]. The compositions of these essential oils have shown remarkable chemical variation. Nevertheless, the composition of B. frutescens in this present study is very similar to that found in a sample collected from Đồng Hới, Quảng Bình Province [16], and sample 2 (from Sóc Sơn District, Hanoi) reported by Tam and co-workers [17].
Table 1.
Chemical composition of Baeckea frutescens leaf essential oil from central Vietnam.
| RI(calc) | RI(db) | Compounds | % | RI(calc) | RI(db) | Compounds | % |
|---|---|---|---|---|---|---|---|
| 922 | 927 | α-Thujene | 1.8 | 1370 | 1375 | α-Copaene | 0.2 |
| 930 | 933 | α-Pinene | 11.1 | 1398 | 1405 | (Z)-Caryophyllene | tr |
| 943 | 948 | α-Fenchene | tr | 1401 | 1406 | α-Gurjunene | tr |
| 945 | 953 | Camphene | 0.1 | 1415 | 1417 | (E)-Caryophyllene | 7.1 |
| 968 | 972 | Sabinene | tr | 1433 | 1438 | Aromadendrene | 0.1 |
| 975 | 978 | β-Pinene | 19.0 | 1452 | 1454 | α-Humulene | 9.9 |
| 984 | 991 | Myrcene | 0.3 | 1455 | 1457 | allo-Aromadendrene | 0.1 |
| 1000 | 1004 | p-Mentha-1(7),8-diene | tr | 1466 | 1472 | trans-Cadina-1(6),4-diene | 0.1 |
| 1003 | 1007 | α-Phellandrene | 0.1 | 1469 | 1478 | γ-Muurolene | tr |
| 1005 | 1009 | δ-3-Carene | tr | 1483 | 1487 | β-Selinene | 0.1 |
| 1013 | 1018 | α-Terpinene | 0.3 | 1485 | 1490 | γ-Amorphene | tr |
| 1021 | 1025 | p-Cymene | 8.9 | 1490 | 1501 | α-Selinene | 0.1 |
| 1025 | 1030 | Limonene | 1.7 | 1492 | 1497 | α-Muurolene | 0.1 |
| 1029 | 1030 | 1,8-Cineole | 10.1 | 1500 | 1507 | Geranyl isobutyrate | 0.1 |
| 1030 | 1034 | (Z)-β-Ocimene | tr | 1506 | 1512 | γ-Cadinene | 0.2 |
| 1041 | 1045 | (E)-β-Ocimene | tr | 1509 | 1519 | Cubebol | tr |
| 1055 | 1057 | γ-Terpinene | 11.7 | 1512 | 1518 | δ-Cadinene | 0.9 |
| 1065 | 1069 | cis-Linalool oxide (furanoid) | tr | 1515 | 1519 | trans-Calamenene | 0.1 |
| 1081 | 1086 | Terpinolene | 0.7 | 1516 | 1521 | Zonarene | 0.1 |
| 1085 | 1093 | p-Cymenene | tr | 1526 | 1536 | trans-Cadine-1,4-diene | 0.1 |
| 1096 | 1101 | Linalool | 4.4 | 1530 | 1538 | α-Cadinene | tr |
| 1098 | 1104 | Hotrienol | tr | 1534 | 1544 | α-Calacorene | tr |
| 1114 | 1119 | endo-Fenchol | 0.1 | 1541 | 1549 | α-Elemol | tr |
| 1133 | 1139 | Nopinone | tr | 1545 | 1551 | (Z)-Caryphyllene oxide | 0.1 |
| 1136 | 1141 | trans-Pinocarveol | tr | 1554 | 1562 | (E)-Nerolidol | 0.5 |
| 1150 | 1156 | Camphene hydrate | tr | 1570 | 1576 | Spathulenol | tr |
| 1165 | 1170 | δ-Terpineol | 0.1 | 1576 | 1587 | Caryophyllene oxide | 2.0 |
| 1167 | 1170 | Borneol | 0.1 | 1579 | 1590 | Globulol | 0.1 |
| 1169 | 1171 | cis-Linalool oxide (pyranoid) | tr | 1592 | 1592 | Humulene epoxide I | 0.3 |
| 1173 | 1179 | 2-Isopropenyl-5-methyl-4-hexenal | 0.1 | 1598 | 1605 | Ledol | 0.1 |
| 1176 | 1180 | Terpinen-4-ol | 0.7 | 1604 | 1613 | Humulene epoxide II | 2.4 |
| 1178 | 1188 | Naphthalene | tr | 1619 | 1624 | Muurola-4,10(14)-dien-1β-ol | tr |
| 1181 | 1186 | p-Cymen-8-ol | tr | 1621 | 1628 | 1-epi-Cubenol | 0.3 |
| 1190 | 1195 | α-Terpineol | 1.7 | 1625 | 1611 | Germacra-1(10),5-dien-4α-ol | 0.3 |
| 1198 | 1203 | p-Cumenol | tr | 1626 | 1632 | Humulenol II | 0.3 |
| 1219 | 1229 | Nerol | tr | 1630 | 1636 | Caryophylla-4(12),8(13)-dien-5β-ol | 0.1 |
| 1234 | 1240 | Ascaridole | tr | 1634 | 1643 | τ-Cadinol | 0.2 |
| 1244 | 1244 | Geraniol | 0.1 | 1636 | 1645 | τ-Muurolol | 0.1 |
| 1261 | 1268 | Geranial | tr | 1639 | 1651 | α-Muurolol (= δ-Cadinol) | 0.1 |
| 1268 | 1275 | trans-Ascaridol glycol | tr | 1648 | 1652 | α-Eudesmol | 0.5 |
| 1274 | 1284 | p-Cymen-7-ol | tr | 1841 | 1837 | Homoisobaeckeol | 0.5 |
| 1284 | 1289 | Thymol | tr | Monoterpene hydrocarbons | 55.6 | ||
| 1291 | 1399 | Carvacrol | tr | Oxygenated monoterpenoids | 17.5 | ||
| 1298 | 1306 | Isoascaridole | tr | Sesquiterpene hydrocarbons | 19.1 | ||
| 1314 | 1320 | Methyl geranate | 0.1 | Oxygenated sesquiterpenoids | 7.3 | ||
| 1341 | 1349 | α-Cubebene | tr | Benzenoid aromatics | 0.5 | ||
| 1344 | 1357 | Eugenol | tr | Others | tr | ||
| Total identified | 100.0 |
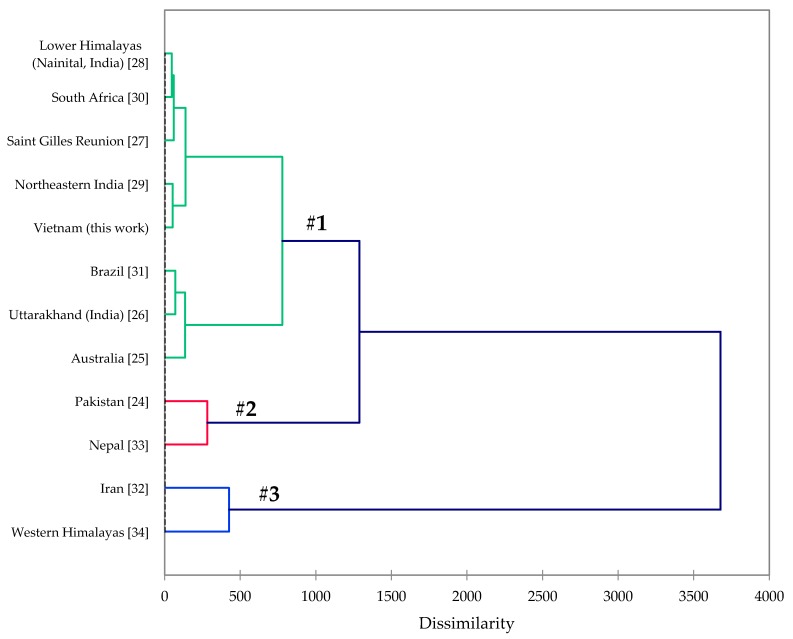
The leaf and fruit essential oils of Callistemon citrinus were obtained in yields of 0.62% and 0.34%, respectively. A total of 53 compounds were identified in the leaf essential oil of C. citrinus, and 63 compounds were identified in the fruit essential oil, accounting for 99.6% and 99.4% of the compositions, respectively. Monoterpene hydrocarbons (27.6% and 53.8%) and oxygenated monoterpenoids (69.9% and 41.3%) dominated the leaf and fruit oils, respectively. The major components in C. citrinus leaf and fruit essential oils were α-pinene (18.1% and 35.1%, respectively), limonene (5.4% and 8.2%), 1,8-cineole (56.3% and 32.4%), and α-terpineol (11.2% and 5.8%) (Table 2). There have been several previous examinations of the composition of C. citrinus leaf essential oil from various geographical locations [24,25,26,27,28,29,30,31,32,33,34]. An agglomerative hierarchical cluster analysis based on the compositions of the leaf essential oils (Figure 2) reveals three well-defined clusters: (#1) 1,8-cineole >> α-pinene > α-terpineol, (#2) 1,8-cineole > α-terpineol >> eugenol, and (#3) α-pinene > 1,8-cineole > α-terpineol. The C. citrinus leaf essential oil from Vietnam (this study) falls into cluster #1.
Table 2.
Chemical compositions of the leaf and fruit essential oils of Callistemon citrinus from central Vietnam.
| RI(calc) | RI(db) | Compound | % Composition | |
|---|---|---|---|---|
| Leaf | Fruit | |||
| 793 | 791 | 2,4-Dimethyl-3-pentanone | 0.3 | tr |
| 912 | 913 | Isobutyl isobutyrate | 0.2 | 0.3 |
| 924 | 927 | α-Thujene | 0.3 | 0.8 |
| 932 | 933 | α-Pinene | 18.1 | 35.1 |
| 946 | 948 | α-Fenchene | tr | tr |
| 948 | 953 | Camphene | 0.1 | 0.1 |
| 971 | 972 | Sabinene | tr | tr |
| 976 | 978 | β-Pinene | 0.6 | 0.7 |
| 987 | 989 | Myrcene | 0.1 | 0.5 |
| 999 | 1000 | δ-2-Carene | tr | 0.1 |
| 1004 | 1004 | p-Mentha-1(7),8-diene | 0.1 | 0.1 |
| 1006 | 1007 | α-Phellandrene | 0.4 | 1.6 |
| 1008 | 1009 | δ-3-Carene | 0.1 | 0.1 |
| 1011 | 1014 | Isoamyl isobutyrate | 0.2 | 0.3 |
| 1014 | 1018 | α-Terpinene | --- | 0.2 |
| 1014 | 1015 | 2-Methylbutyl isobutyrate | tr | 0.1 |
| 1021 | 1022 | Ethyl 3-methylbut-3-enyl carbonate | 0.1 | 0.1 |
| 1024 | 1025 | p-Cymene | 2.2 | 4.6 |
| 1029 | 1030 | Limonene | 5.4 | 8.2 |
| 1030 | 1032 | 1,8-cineole | 56.3 | 32.4 |
| 1032 | 1034 | (Z)-β-Ocimene | --- | 0.1 |
| 1044 | 1046 | (E)-β-Ocimene | tr | 0.2 |
| 1051 | 1050 | Prenyl isobutyrate | tr | 0.1 |
| 1057 | 1057 | γ-Terpinene | 0.3 | 1.0 |
| 1084 | 1087 | Terpinolene | 0.1 | 0.6 |
| 1088 | 1093 | p-Cymenene | --- | 0.1 |
| 1099 | 1101 | Linalool | 0.5 | 1.4 |
| 1119 | 1119 | endo-Fenchol | 0.1 | 0.1 |
| 1140 | 1141 | trans-Pinocarveol | 0.3 | tr |
| 1155 | 1156 | Camphene hydrate | tr | tr |
| 1163 | 1164 | Pinocarvone | tr | --- |
| 1170 | 1170 | δ-Terpineol | 0.2 | 0.1 |
| 1170 | 1165 | iso-Borneol | --- | 0.1 |
| 1173 | 1173 | Borneol | 0.1 | 0.1 |
| 1179 | 1179 | 2-Isopropenyl-5-methyl-4-hexenal | 0.1 | tr |
| 1180 | 1180 | Terpinen-4-ol | 0.5 | 0.6 |
| 1185 | 1188 | Naphthalene | 0.1 | --- |
| 1186 | 1189 | p-Cymen-8-ol | --- | tr |
| 1188 | 1187 | trans-p-Mentha-1(7),8-dien-2-ol | 0.1 | --- |
| 1194 | 1195 | α-Terpineol | 11.2 | 5.8 |
| 1202 | 1202 | cis-Sabinol | --- | 0.1 |
| 1219 | 1223 | trans-Carveol | 0.1 | tr |
| 1230 | 1230 | cis-p-Mentha-1(7),8-dien-2-ol | tr | --- |
| 1249 | 1249 | Geraniol | 0.5 | 0.6 |
| 1298 | 1300 | Carvacrol | tr | 0.1 |
| 1351 | 1356 | Eugenol | 0.1 | 0.1 |
| 1385 | 1390 | β-Elemene | --- | 0.1 |
| 1392 | 1395 | Phenylethyl isobutyrate | tr | tr |
| 1417 | 1417 | (E)-Caryophyllene | 0.1 | 0.2 |
| 1436 | 1438 | Aromadendrene | 0.1 | 0.2 |
| 1452 | 1454 | α-Humulene | --- | 0.1 |
| 1458 | 1458 | allo-Aromadendrene | 0.1 | 0.1 |
| 1477 | 1480 | Germacrene D | --- | tr |
| 1487 | 1491 | Viridiflorene | --- | 0.1 |
| 1500 | 1503 | (E,E)-α-Farnesene | --- | 0.1 |
| 1505 | 1507 | Geranyl isobutyrate | 0.1 | --- |
| 1505 | 1508 | β-Bisabolene | --- | 0.1 |
| 1514 | 1518 | δ-Cadinene | --- | tr |
| 1535 | 1539 | Flavesone | 0.3 | 0.3 |
| 1557 | 1561 | (E)-Nerolidol | --- | 0.1 |
| 1575 | 1578 | Spathulenol | 0.4 | 1.3 |
| 1580 | 1577 | Caryophyllene oxide | tr | 0.1 |
| 1584 | 1590 | Globulol | 0.1 | 0.2 |
| 1593 | 1594 | Viridiflorol | 0.1 | 0.1 |
| 1595 | 1599 | Cubeban-11-ol | tr | 0.1 |
| 1609 | 1614 | iso-Leptospermone | tr | 0.1 |
| 1619 | 1626 | Leptospermone | tr | 0.2 |
| 1629 | 1629 | iso-Spathulenol | --- | 0.2 |
| Monoterpene hydrocarbons | 27.6 | 53.8 | ||
| Oxygenated monoterpenoids | 69.9 | 41.3 | ||
| Sesquiterpene hydrocarbons | 0.2 | 0.8 | ||
| Oxygenated sesquiterpenoids | 0.5 | 2.0 | ||
| Others | 1.4 | 1.4 | ||
| Total identified | 99.6 | 99.4 | ||
Figure 2.
Dendrogram obtained from the agglomerative hierarchical cluster analysis of Callistemon citrinus leaf essential oil compositions.
Essential oils were obtained from six different tissues of Melaleuca leucadendra, young leaves, old leaves, stem bark, fruits, and branch tips, in yields of around 1%. A total of 104 compounds were identified in the M. leucadendra essential oils. Sesquiterpene hydrocarbons (18.8%–31.0%) and oxygenated sesquiterpenoids (35.6%–69.5%) were the dominant chemical classes. The essential oil compositions are compiled in Table 3.
Table 3.
Chemical compositions of essential oils from Melaleuca leucadendra from central Vietnam.
| RI(calc) | RI(db) | Compound | % Composition | ||||
|---|---|---|---|---|---|---|---|
| Young Leaf | Old Leaf | Stem Bark | Fruit | Branch Tips | |||
| 923 | 927 | α-Thujene | 0.8 | 0.4 | 0.1 | tr | 1.2 |
| 931 | 933 | α-Pinene | 0.7 | 0.6 | 0.8 | 0.2 | 1.4 |
| 947 | 953 | Camphene | --- | tr | tr | tr | --- |
| 960 | 960 | Benzaldehyde | 0.1 | 0.1 | --- | --- | tr |
| 975 | 978 | β-Pinene | 0.1 | 0.2 | 0.3 | 0.1 | 0.1 |
| 987 | 991 | Myrcene | 0.2 | 0.3 | 0.2 | 0.1 | 0.2 |
| 1003 | 1004 | p-Mentha-1(7),8-diene | --- | --- | tr | --- | --- |
| 1005 | 1007 | α-Phellandrene | 0.3 | 0.2 | --- | --- | 0.3 |
| 1007 | 1009 | δ-3-carene | 0.1 | tr | tr | --- | 0.1 |
| 1015 | 1018 | α-Terpinene | 0.4 | 0.3 | --- | --- | 0.4 |
| 1023 | 1025 | p-Cymene | 3.9 | 1.7 | 1.3 | 0.5 | 8.7 |
| 1027 | 1030 | Limonene | 0.3 | 0.8 | 1.4 | 0.4 | 0.7 |
| 1029 | 1031 | β-Phellandrene | tr | 0.1 | tr | --- | 0.1 |
| 1030 | 1030 | 1,8-cineole | --- | 5.2 | 1.8 | 0.2 | tr |
| 1033 | 1034 | (Z)-β-Ocimene | --- | tr | --- | --- | tr |
| 1043 | 1045 | (E)-β-Ocimene | --- | tr | --- | --- | tr |
| 1056 | 1057 | γ-Terpinene | 2.2 | 1.3 | tr | --- | 3.3 |
| 1068 | 1069 | cis-Linalool oxide (furanoid) | --- | --- | --- | --- | tr |
| 1084 | 1086 | Terpinolene | 3.0 | 1.6 | 0.1 | tr | 4.4 |
| 1089 | 1093 | p-Cymenene | 0.1 | tr | tr | --- | 0.2 |
| 1099 | 1101 | Linalool | 4.9 | 5.1 | 1.4 | 0.4 | 4.2 |
| 1103 | 1107 | Nonanal | --- | --- | 0.1 | --- | --- |
| 1110 | 1110 | 1,3,8-p-Menthatriene | tr | tr | --- | --- | --- |
| 1122 | 1124 | cis-p-Menth-2-en-1-ol | tr | tr | --- | --- | --- |
| 1141 | 1142 | Epoxyterpinolene | 0.3 | tr | --- | --- | 0.6 |
| 1147 | 1149 | iso-Pulegol | --- | tr | --- | --- | tr |
| 1168 | 1170 | δ-Terpineol | --- | tr | --- | --- | --- |
| 1170 | 1170 | Borneol | --- | tr | --- | --- | --- |
| 1177 | 1179 | 2-Isopropenyl-5-methyl-4-hexenal | 0.2 | 0.1 | --- | --- | 0.3 |
| 1179 | 1180 | Terpinen-4-ol | 0.9 | 0.4 | tr | tr | 1.1 |
| 1183 | 1188 | Naphthalene | --- | --- | 0.1 | 0.1 | 0.2 |
| 1184 | 1188 | 4’-Methylacetophenone | 0.1 | tr | --- | --- | 0.1 |
| 1186 | 1188 | p-Cymen-8-ol | 1.0 | 0.2 | 0.1 | 0.1 | 1.2 |
| 1194 | 1195 | α-Terpineol | 0.7 | 1.8 | 0.5 | 0.1 | 0.6 |
| 1198 | 1195 | p-Menth-3-en-7-al | --- | --- | --- | --- | 0.1 |
| 1202 | 1203 | p-Cumenol | 0.1 | 0.1 | --- | --- | 0.1 |
| 1222 | 1222 | iso-Ascaridol | --- | tr | --- | --- | 0.1 |
| 1223 | 1226 | Nerol | --- | tr | tr | --- | --- |
| 1225 | 1227 | Citronellol | --- | tr | tr | tr | 0.1 |
| 1248 | 1249 | Geraniol | 0.2 | 0.6 | 0.4 | 0.1 | 0.2 |
| 1266 | 1266 | Geranial | --- | tr | tr | --- | --- |
| 1273 | 1275 | trans-Ascaridol glycol | 0.2 | tr | --- | --- | 0.1 |
| 1290 | 1291 | cis-Ascaridol glycol | 0.1 | --- | --- | --- | 0.1 |
| 1293 | 1305 | Benzophenone | ---- | tr | --- | --- | --- |
| 1318 | 1318 | 3-Hydroxycineole | 0.2 | --- | --- | --- | 0.1 |
| 1348 | 1356 | Eugenol | --- | 0.1 | --- | --- | --- |
| 1367 | 1371 | α-Ylangene | 0.4 | 0.6 | 0.9 | 0.6 | 0.7 |
| 1373 | 1375 | α-Copaene | 0.2 | 0.3 | 0.8 | 0.3 | 0.3 |
| 1375 | 1380 | Geranyl acetate | --- | 0.1 | 0.2 | tr | 0.1 |
| 1381 | 1382 | β-Bourbonene | --- | --- | tr | --- | --- |
| 1387 | 1390 | β-Elemene | 0.1 | 0.1 | 0.1 | tr | 0.1 |
| 1389 | 1394 | Sativene | 0.1 | 0.1 | 0.1 | tr | 0.1 |
| 1401 | 1405 | (Z)-Caryophyllene | --- | --- | tr | tr | --- |
| 1417 | 1417 | (E)-Caryophyllene | 3.8 | 7.0 | 5.5 | 4.3 | 5.7 |
| 1421 | 1428 | 8-Hydroxycarvotanacetone | 0.1 | --- | --- | --- | 0.1 |
| 1426 | 1427 | γ-Elemene | 0.2 | 0.3 | 0.1 | 0.1 | 0.1 |
| 1432 | 1436 | α-Guaiene | 0.1 | 0.2 | 0.2 | 0.2 | 0.2 |
| 1438 | 1444 | Guaia-6,9-diene | 0.2 | 0.2 | 0.1 | 0.1 | 0.2 |
| 1444 | 1448 | cis-Muurola-3,5-diene | 0.2 | 0.2 | 0.1 | 0.1 | 0.2 |
| 1446 | 1447 | iso-Germacrene D | 0.1 | 0.2 | 0.1 | 0.1 | 0.2 |
| 1453 | 1454 | α-Humulene | 2.8 | 4.4 | 3.5 | 2.8 | 3.7 |
| 1467 | 1473 | Drima-7,9(11)-diene | 0.1 | 0.2 | 0.2 | 0.2 | 0.2 |
| 1470 | 1476 | Selina-4,11-diene | 0.2 | 0.5 | 0.5 | 0.4 | 0.6 |
| 1474 | 1476 | γ-Gurjunene | 0.6 | 1.1 | 1.1 | 0.9 | 1.4 |
| 1476 | 1479 | α-Amorphene | 0.7 | 1.2 | 1.5 | 0.9 | 1.2 |
| 1484 | 1488 | δ-Selinene | 1.0 | 1.6 | 0.7 | 0.6 | 1.3 |
| 1487 | 1492 | β-Selinene | 2.4 | 3.7 | 4.8 | 3.1 | 4.2 |
| 1490 | 1490 | γ-Amorphene | 0.2 | 0.3 | 0.4 | 0.3 | 0.5 |
| 1494 | 1501 | α-Selinene | 2.1 | 3.7 | 3.6 | 2.5 | 4.1 |
| 1495 | 1496 | trans-Muurola-4(14),5-diene | --- | 0.2 | --- | --- | 0.1 |
| 1496 | 1497 | α-Muurolene | --- | --- | 0.2 | 0.1 | --- |
| 1499 | 1505 | α-Bulnesene | --- | 0.1 | 0.1 | 0.2 | 0.1 |
| 1499 | 1506 | δ-Amorphene | --- | 0.2 | --- | --- | --- |
| 1500 | 1502 | trans-β-Guaiene | --- | 0.3 | --- | --- | --- |
| 1501 | 1501 | β-Dihydroagarofuran | --- | --- | 0.2 | 0.2 | --- |
| 1515 | 1518 | δ-Cadinene | --- | --- | 0.2 | 0.1 | --- |
| 1516 | 1520 | 7-epi-α-Selinene | --- | --- | --- | 0.2 | --- |
| 1517 | 1519 | trans-Calamenene | --- | --- | 0.7 | 0.4 | --- |
| 1534 | 1540 | Selina-4(15),7(11)-diene | 0.5 | 0.6 | 0.6 | 0.6 | 0.7 |
| 1539 | 1541 | α-Calacorene | 0.3 | 0.6 | 0.8 | 0.5 | 0.6 |
| 1539 | 1546 | Selina-3,7(11)-diene | 0.3 | 0.2 | --- | 0.3 | 0.3 |
| 1545 | 1546 | α-Elemol | 0.3 | 0.1 | 0.3 | 0.4 | --- |
| 1556 | 1557 | Germacrene B | 0.4 | 0.4 | 0.1 | --- | 0.1 |
| 1580 | 1587 | Caryophyllene oxide | 1.8 | 2.3 | 3.3 | 3.2 | 4.0 |
| 1590 | 1600 | Khusimone | 0.2 | 0.3 | 0.4 | 0.3 | 0.3 |
| 1595 | 1603 | Guaiol | 12.5 | 10.9 | 11.3 | 10.4 | 7.3 |
| 1607 | 1613 | Humulene epoxide II | 0.8 | 0.9 | 1.5 | 1.3 | 1.6 |
| 1610 | 1609 | Rosifoliol | 0.5 | 0.4 | 0.5 | 0.5 | 0.2 |
| 1620 | 1611 | Germacra-1(10),5-dien-4α-ol | 0.2 | 0.2 | 0.2 | --- | 0.2 |
| 1623 | 1624 | Selina-6-en-4β-ol | 2.0 | 1.6 | 1.7 | 2.2 | 1.2 |
| 1624 | 1629 | iso-Spathulenol | 0.2 | --- | --- | --- | --- |
| 1628 | 1631 | Eremoligenol | 3.4 | 3.4 | 4.9 | 6.5 | 2.7 |
| 1630 | 1633 | γ-Eudesmol | 3.9 | 2.8 | 3.5 | 5.3 | 1.9 |
| 1632 | 1634 | cis-Cadin-4-en-7-ol | 3.5 | 3.0 | 3.3 | 3.5 | 2.2 |
| 1635 | 1636 | Caryophylla-4(12),8(13)-dien-5β-ol | --- | 0.2 | 0.2 | 0.1 | 0.2 |
| 1638 | 1645 | Hinesol | 1.0 | 0.9 | 1.2 | 1.6 | 0.7 |
| 1645 | 1644 | Selina-3,11-dien-6α-ol | --- | 0.2 | 0.3 | --- | 0.2 |
| 1653 | 1652 | α-Eudesmol | 21.2 | 17.6 | 24.1 | 30.7 | 13.7 |
| 1657 | 1660 | Selin-11-en-4α-ol | 1.9 | 1.5 | 1.3 | 1.6 | 1.0 |
| 1663 | 1673 | Bulnesol | 5.3 | 3.6 | 3.3 | 4.4 | 2.2 |
| 1668 | 1671 | 14-Hydroxy-9-epi-(E)-caryophyllene | --- | --- | 0.5 | --- | --- |
| 1670 | 1677 | Cadalene | --- | --- | 0.3 | 0.2 | --- |
| 1695 | 1696 | Juniper camphor | --- | 0.2 | 0.1 | 0.2 | 0.1 |
| 1918 | 1929 | Carissone | --- | --- | 0.1 | 0.4 | --- |
| Monoterpene hydrocarbons | 11.9 | 7.2 | 4.2 | 1.3 | 21.2 | ||
| Oxygenated monoterpenoids | 8.8 | 13.5 | 4.4 | 0.8 | 9.0 | ||
| Sesquiterpene hydrocarbons | 18.8 | 30.8 | 30.5 | 23.4 | 31.0 | ||
| Oxygenated sesquiterpenoids | 56.9 | 47.6 | 59.1 | 69.5 | 35.6 | ||
| Benzenoid aromatics | 0.2 | 0.1 | 0.0 | 0.0 | 0.1 | ||
| Others | 0.0 | 0.0 | 0.2 | 0.1 | 0.2 | ||
| Total identified | 96.6 | 99.3 | 98.4 | 95.2 | 97.1 | ||
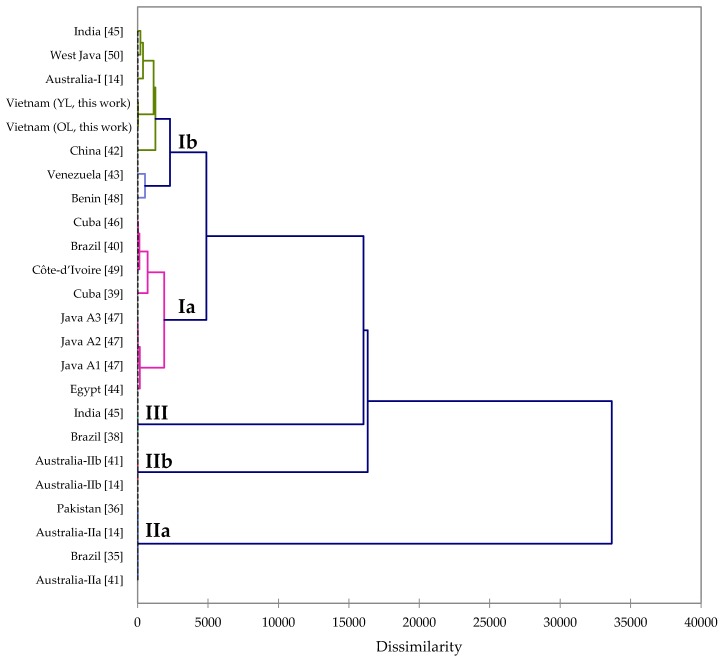
Brophy has described two different chemotypes of M. leucadendra from Australia, based on leaf essential oil composition [14]. Chemotype I, from Western Australia, is rich in monoterpenoids, e.g., 1,8-cineole (10–45%), p-cymene (5–22%), α-pinene (4–19%), limonene (3–6%), and α-terpineol (6–9%). Chemotype II, from eastern Australia, is dominated by phenylpropanoids, which was divided into two subtypes: IIa, eugenol methyl ether (95%–97%), and IIb, (E)-iso-eugenol methyl ether (74%–88%) subtype). Chemotype IIa has also been represented by samples from Minas Gerais, Brazil [35], and from Lahore, Pakistan [36]. There is a third chemotype, dominated by (E)-nerolidol (>90%), which has been described from Uttarakhand, India [37] and from Pernambuco, Brazil [38]. Chemotype I has also been found in Cuba [39] and Rio de Janeiro, Brazil [40]. They were both dominated by 1,8-cineole (43.0% and 48.7%, respectively), but these two samples were also rich in viridiflorol (24.2% and 27.8%, respectively), and therefore, may represent a subtype of chemotype I.
An agglomerative hierarchical cluster analysis was carried out using the M. leucadendra leaf essential oil compositions reported in the literature [14,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50] (Figure 3). The cluster analysis reveals two sub-types of chemotype I, the two sub-types of chemotype II, as described by Brophy [14], and chemotype III, the nerolidol chemotype. The leaf essential oils of M. leucadendra from Vietnam, fall into sub-type Ib; the leaf oils were rich in α-eudesmol (17.6%–21.2%), guaiol (10.9%–12.5%), with lesser concentrations of linalool (4.9%–5.1%), (E)-caryophyllene (3.8%–7.0%), and bulnesol (3.6%–5.3%). Concentrations of 1,8-cineole were low (0.0%–5.2%), and (E)-nerolidol and viridiflorol were not observed at all.
Figure 3.
Dendrogram obtained from the agglomerative hierarchical cluster analysis of Melaleuca leucadendra leaf essential oil compositions.
The leaf essential oil of Syzygium nervosum was obtained in 0.2% yield. A total of 61 compounds were identified in the leaf oil of S. nervosum, accounting for 90.9% of the composition, with 31.7% monoterpene hydrocarbons, 24.3% sesquiterpene hydrocarbons, and 27.9% oxygenated sesquiterpenoids predominating. The leaf essential oil of S. nervosum was rich in (Z)-β-ocimene (20.3%), caryophyllene oxide (13.2%), (E)-caryophyllene (12.1%), and α-pinene (5.2%) (Table 4). The leaf essential oil composition is qualitatively similar, but quantitatively different, to a previous report on the leaf essential oil from Lê Mao District, Vinh City, Vietnam [51]. Both samples had relatively high concentrations of α-pinene, (Z)-β-ocimene, (E)-β-ocimene, and (E)-caryophyllene (3.7%, 32.1%, 9.4%, and 14.5%, respectively, in the Vinh City sample), but the concentration of myrcene was much higher (24.6%) in the sample from Vinh City. The leaf essential oil S. nervosum from Nepal showed a very different composition with myrcene (69.7%), (E)-β-ocimene (12.2%), (Z)-β-ocimene (4.8%), and linalool (4.1%) [52].
Table 4.
Chemical compositions of essential oils from Syzygium nervosum from central Vietnam.
| RI(calc) | RI(db) | Compound | % | RI(calc) | RI(db) | Compound | % |
|---|---|---|---|---|---|---|---|
| 930 | 933 | α-Pinene | 5.2 | 1486 | 1492 | β-Selinene | 0.9 |
| 968 | 971 | Tetrahydrofurfuryl acetate | 0.2 | 1492 | 1501 | α-Selinene | 0.9 |
| 975 | 978 | β-Pinene | 1.0 | 1494 | 1500 | α-Muurolene | 0.4 |
| 986 | 991 | Myrcene | 0.4 | 1509 | 1512 | γ-Cadinene | 0.9 |
| 1022 | 1025 | p-Cymene | 0.1 | 1514 | 1518 | δ-Cadinene | 1.0 |
| 1027 | 1030 | Limonene | 0.2 | 1533 | 1538 | α-Cadinene | 0.4 |
| 1033 | 1034 | (Z)-β-Ocimene | 20.3 | 1538 | 1541 | α-Calcorene | 0.4 |
| 1043 | 1045 | (E)-β-Ocimene | 3.5 | 1557 | 1560 | (E)-Nerolidol | 0.1 |
| 1089 | 1091 | Rosefuran | 0.7 | 1559 | 1560 | β-Calacorene | 0.5 |
| 1092 | 1101 | α-Pinene oxide | 1.3 | 1573 | 1576 | Spathulenol | 0.6 |
| 1097 | 1101 | Linalool | 0.3 | 1579 | 1587 | Caryophyllene oxide | 13.2 |
| 1101 | 1102 | 6-Methyl-3,5-heptadien-2-one | 0.5 | 1582 | 1590 | Globulol | 1.2 |
| 1125 | 1127 | allo-Ocimene | 0.8 | 1591 | 1592 | Viridiflorol | 0.4 |
| 1127 | 1128 | (Z)-Epoxy ocimene (= (Z)-Myroxide) | 0.5 | 1593 | 1593 | Guaiol | 0.5 |
| 1137 | 1137 | (E)-Epoxy ocimene (= (E)-Myroxide) | 0.4 | 1595 | 1592 | Humulene epoxide I | 0.2 |
| 1167 | 1169 | Rosefuran epoxide | 0.3 | 1603 | 1607 | β-Oplopenone | 0.8 |
| 1170 | 1171 | p-Mentha-1,5-dien-8-ol | 0.2 | 1606 | 1613 | Humulene epoxide II | 1.8 |
| 1182 | 1188 | Naphthalene | 0.4 | 1623 | 1624 | Selina-6-en-4β-ol | 3.4 |
| 1193 | 1195 | α-Terpineol | 0.1 | 1624 | 1628 | 1-epi-Cubenol | 0.6 |
| 1199 | --- | (3Z)-Octenyl acetate | 0.4 | 1631 | 1634 | cis-Cadin-4-en-7-ol | 0.4 |
| 1199 | 1205 | cis-4-Caranone | 0.1 | 1634 | 1636 | Caryophylla-4(12),8(13)-dien-5β-ol | 0.5 |
| 1206 | 1207 | (3E)-Octenyl acetate | 0.7 | 1638 | 1643 | τ-Cadinol | 0.7 |
| 1353 | 1349 | α-Terpinyl acetate | 0.7 | 1640 | 1644 | τ-Muurolol | 0.2 |
| 1366 | 1367 | Cyclosativene | 0.2 | 1643 | 1651 | α-Muurolol (= δ-Cadinol) | 0.2 |
| 1372 | 1375 | α-Copaene | 0.4 | 1645 | 1645 | Selina-3,11-dien-6α-ol | 0.4 |
| 1374 | 1380 | Geranyl acetate | 0.4 | 1652 | 1655 | α-Cadinol | 1.7 |
| 1417 | 1417 | (E)-Caryophyllene | 12.1 | 1655 | 1660 | Selin-11-en-4α-ol | 0.6 |
| 1426 | 1433 | β-Copaene | 0.3 | 1698 | 1697 | (E)-trans-α-Bergamota-2,10-dien-12-ol | 0.4 |
| 1435 | 1438 | Aromadendrene | 0.6 | Monoterpene hydrocarbons | 31.7 | ||
| 1452 | 1454 | α-Humulene | 2.7 | Oxygenated monoterpenoids | 4.9 | ||
| 1471 | 1478 | γ-Muurolene | 0.9 | Sesquiterpene hydrocarbons | 24.3 | ||
| 1473 | 1476 | γ-Gurjunene | 1.4 | Oxygenated sesquiterpenoids | 27.9 | ||
| 1475 | 1482 | α-Amorphene | 0.3 | Others | 2.1 | ||
| Total identified | 90.9 |
2.2. Mosquito Larvicidal Activity
The 24-h and 48-h larvicidal activities are presented in Table 5 and Table 6, respectively. The Myrtaceae essential oils presenting the best 24-h larvicidal activities were C. citrinus fruit essential oil (LC50 = 17.3 μg/mL against both Ae. aegypti and Cx. quinquefasciatus), M. leucadendra stem bark essential oil (LC50 = 17.1, 19.3, and 21.4 μg/mL against Ae. aegypti, Ae. albopictus, and Cx. quinquefasciatus, respectively), M. leucadendra fruit essential oil (LC50 = 13.9, 19.2, and 26.2 μg/mL against Ae. aegypti, Ae. albopictus, and Cx. quinquefasciatus, respectively), and, especially, M. leucadendra old leaf essential oil (LC50 = 7.4 and 6.6 μg/mL against Ae. aegypti and Cx. quinquefasciatus, respectively). The 48-h larvicidal activities of M. leucadendra old leaf essential oil are particularly notable with LC50 of 1.4 and 1.8 μg/mL on Ae. aegypti and Cx. quinquefasciatus.
Table 5.
Twenty-four-hour mosquito larvicidal activities of Myrtaceae essential oils.
| Essential Oil | LC50 (95% Fiducial Limits) | LC90 (95% Fiducial Limits) | χ2 | p |
|---|---|---|---|---|
| Aedes aegypti | ||||
| Baeckea frutescens leaf EO | 23.00 (20.38–25.75) | 40.05 (35.75–46.71) | 6.512 | 0.039 |
| Callistemon citrinus leaf EO | 22.37 (18.62–25.88) | 57.34 (50.00–69.06) | 0.6655 | 0.717 |
| Callistemon citrinus fruit EO | 17.27 (15.30–19.03) | 33.02 (29.82–38.04) | 0.4348 | 0.805 |
| Melaleuca leucadendra young leaf EO | nt | nt | --- | --- |
| Melaleuca leucadendra old leaf EO | 7.400 (6.308–8.612) | 18.29 (16.05–21.47) | 30.77 | 0.000 |
| Melaleuca leucadendra stem bark EO | 17.14 (14.73–19.21) | 36.25 (32.42–42.31) | 2.244 | 0.326 |
| Melaleuca leucadendra fruit EO | 13.90 (11.03–16.02) | 31.76 (28.40–37.25) | 0.5750 | 0.750 |
| Melaleuca leucadendra branch tip EO | 21.99 (19.80–24.57) | 37.63 (33.67–43.39) | 2.277 | 0.517 |
| Syzygium nervosum leaf EO | 28.63 (24.83–32.87) | 61.41 (53.99–72.38) | 3.792 | 0.285 |
| Aedes albopictus | ||||
| Baeckea frutescens leaf EO | 25.73 (23.68–28.39) | 37.01 (33.33–43.13) | 0.4209 | 0.810 |
| Callistemon citrinus leaf EO | nt | nt | --- | --- |
| Callistemon citrinus fruit EO | nt | nt | --- | --- |
| Melaleuca leucadendra young leaf EO | nt | nt | --- | --- |
| Melaleuca leucadendra old leaf EO | nt | nt | --- | --- |
| Melaleuca leucadendra stem bark EO | 19.31 (16.83–21.60) | 40.91 (36.56–47.59) | 0.5986 | 0.741 |
| Melaleuca leucadendra fruit EO | 19.17 (16.89–21.32) | 39.08 (34.96–45.47) | 4.7420 | 0.093 |
| Melaleuca leucadendra branch tip EO | nt | nt | --- | --- |
| Syzygium nervosum leaf EO | nt | nt | --- | --- |
| Culex quinquefasciatus | ||||
| Baeckea frutescens leaf EO | 81.72 (76.16–87.75 | 112.7 (104.7–123.6) | 3.097 | 0.078 |
| Callistemon citrinus leaf EO | 73.60 (64.87–85.83) | 172.2 (135.9–249.1) | 57.10 | 0.000 |
| Callistemon citrinus fruit EO | 17.30 (11.04–22.56) | 77.42 (66.07–95.50) | 63.93 | 0.000 |
| Melaleuca leucadendra young leaf EO | 46.62 (42.65–51.45) | 70.10 (62.93–82.10) | 0.2083 | 0.648 |
| Melaleuca leucadendra old leaf EO | 6.618 (3.635–9.183) | 32.80 (27.99–40.13) | 5.474 | 0.361 |
| Melaleuca leucadendra stem bark EO | 21.35 (13.62–28.02) | 100.2 (84.4–126.2) | 86.78 | 0.000 |
| Melaleuca leucadendra fruit EO | 26.20 (19.47–32.30) | 91.81 (78.04–114.46) | 46.32 | 0.000 |
| Melaleuca leucadendra branch tip EO | 43.69 (40.13–47.81) | 64.43 (58.27–74.71) | 0.02181 | 0.883 |
| Syzygium nervosum leaf EO | 46.09 (40.59–52.38) | 95.07 (84.44–109.96) | 1.061 | 0.786 |
LC50 and LC90 in μg/mL. nt = not tested.
Table 6.
Forty-eight-hour mosquito larvicidal activities of Myrtaceae essential oils.
| Essential Oil | LC50 (95% Confidence Limits) | LC90 (95% Confidence Limits) | χ2 | p |
|---|---|---|---|---|
| Aedes aegypti | ||||
| Baeckea frutescens leaf EO | 15.31 (11.25–18.31) | 34.69 (30.31–42.30) | 2.418 | 0.298 |
| Callistemon citrinus leaf EO | 21.60 (17.74–25.13) | 56.87 (49.55–68.64) | 1.104 | 0.576 |
| Callistemon citrinus fruit EO | 16.80 (14.85–18.50) | 31.91 (28.87–36.66) | 0.2493 | 0.883 |
| Melaleuca leucadendra young leaf EO | nt | nt | --- | --- |
| Melaleuca leucadendra old leaf EO | 1.379 (1.127–1.626) | 5.066 (4.173–6.551) | 119.9 | 0.000 |
| Melaleuca leucadendra stem bark EO | 13.96 (10.91–16.21) | 33.15 (29.54–39.08) | 1.115 | 0.573 |
| Melaleuca leucadendra fruit EO | 9.071 (3.729–12.276) | 30.90 (27.21–37.34) | 1.180 | 0.554 |
| Melaleuca leucadendra branch tip EO | 15.79 (14.01–17.73) | 28.64 (25.53–33.35) | 2.103 | 0.551 |
| Syzygium nervosum leaf EO | 11.97 (5.54–16.89) | 53.97 (45.87–67.18) | 5.746 | 0.125 |
| Aedes albopictus | ||||
| Baeckea frutescens leaf EO | 23.98 (21.76–26.57) | 37.63 (33.75–43.80) | 1.375 | 0.503 |
| Callistemon citrinus leaf EO | nt | nt | --- | --- |
| Callistemon citrinus fruit EO | nt | nt | --- | --- |
| Melaleuca leucadendra young leaf EO | nt | nt | --- | --- |
| Melaleuca leucadendra old leaf EO | nt | nt | --- | --- |
| Melaleuca leucadendra stem bark EO | 17.09 (14.89–19.01) | 34.53 (31.02–40.08) | 1.050 | 0.592 |
| Melaleuca leucadendra fruit EO | 17.34 (14.79–19.55) | 37.85 (33.75–44.37) | 3.9440 | 0.139 |
| Melaleuca leucadendra branch tip EO | nt | nt | --- | --- |
| Syzygium nervosum leaf EO | nt | nt | --- | --- |
| Culex quinquefasciatus | ||||
| Baeckea frutescens leaf EO | 64.06 (56.83–72.12) | 116.6 (103.4–137.2) | 4.937 | 0.026 |
| Callistemon citrinus leaf EO | 49.18 (39.75–60.67) | 227.8 (147.4–549.1) | 16.79 | 0.000 |
| Callistemon citrinus fruit EO | 16.02 (12.54–19.77) | 72.19 (60.64–91.68) | 61.56 | 0.000 |
| Melaleuca leucadendra young leaf EO | 30.37 (21.56–36.81) | 72.32 (63.07–88.25) | 4.561 | 0.033 |
| Melaleuca leucadendra old leaf EO | 1.819 (1.262–2.394) | 14.40 (11.04–20.43) | 30.79 | 0.000 |
| Melaleuca leucadendra stem bark EO | 12.02 (5.71–16.91) | 64.16 (55.04–78.56) | 55.71 | 0.000 |
| Melaleuca leucadendra fruit EO | 17.38 (12.96–21.46) | 88.42 (65.61–143.30) | 17.23 | 0.000 |
| Melaleuca leucadendra branch tip EO | 23.78 (12.17–31.00) | 66.12 (57.18–82.37) | 2.383 | 0.123 |
| Syzygium nervosum leaf EO | 22.74 (16.64–28.33) | 75.02 (64.50–91.30) | 11.25 | 0.010 |
LC50 and LC90 in μg/mL. nt = not tested.
The larvicidal activities of M. leucadendra essential oils are likely due to the high concentrations of α-eudesmol and guaiol, or possibly synergistic effects involving these compounds. Unfortunately, there appear to be no reports on the larvicidal activities of these compounds.
It is tempting to suggest that the sensitivity of mosquito larvae to C. citrinus fruit essential oil is due to the combination of α-pinene and 1,8-cineole. 1,8-Cineole, (+)-α-pinene, and (–)-α-pinene have been screened against Ae. aegypti larvae, and showed modest larvicidal activities (LC50) of 74.9, 50.9, and 64.8 μg/mL, respectively [53]. Furthermore, Hedychium bousigonianum cv. “Tai Emperor” rhizome essential oil, with 16.7% α-pinene and 25.5% 1,8-cineole, showed only marginal larvicidal activity against Ae. aegypti (80% lethality at 125 μg/mL) [54]. In addition, Pavela has shown that α-pinene has marginal larvicidal activity against Cx. quinquefasciatus (LC50 = 95 μg/mL), 1,8-cineole is inactive (LC50 > 250 μg/mL), and a binary mixture of the two compounds does not demonstrate synergistic activity [55]. The observed larvicidal activities of C. citrinus fruit essential oil is apparently due to synergistic activities involving minor components. It has been shown that Musca domestica preferentially metabolizes the major components in an essential oil, which leaves the components of lower concentrations to act as the toxic agents [56].
Baeckea frutescens and Callistemon citrinus leaf essential oils were relatively inactive against Cx. quinquefasciatus, with 24-h LC50 values of 81.7 μg/mL and 73.6 μg/mL, respectively. However, both of those essential oils showed high concentrations of α-pinene (11.1% and 18.1%, respectively) and 1,8-cineole (10.1% and 56.3%, respectively). The leaf oil of B. frutescens also had high concentrations of β-pinene (19.0%), γ-terpinene (11.7%), α-humulene (9.9%), and (E)-caryophyllene (7.1%). The relative inactivity of B. frutescens against Cx. quinquefasciatus is difficult to explain. Both β-pinene and γ-terpinene have shown good larvicidal activity against Cx. pipiens pallens with 24-h LC50 of 21.1, 12.9, and 12.6 μg/mL for (+)-β-pinene, (–)-β-pinene, and γ-terpinene, respectively [53]. (E)-Caryophyllene showed only weak larvicidal activity (LC50 = 93.7 μg/mL), however [53], and α-humulene was found to be inactive against this mosquito [57]. The major components of C. citrinus leaf essential oil and C. citrinus fruit essential oil are qualitatively similar. It is not obvious why the larvicidal activities of these two oils against Cx. quinquefasciatus are so different, but it may be due to synergistic effects of minor components present in the fruit essential oil but absent in the leaf essential oil. Apparently, there is more involved in the larvicidal activities of these essential oils than the major components.
Syzygium nervosum essential oil larvicidal activity is also difficult to explain. There were high concentrations of (Z)-β-ocimene (20.3%), (E)-caryophyllene (12.1%), and caryophyllene oxide (13.2%). Unfortunately, we have found no larvicidal screening of (Z)-β-ocimene in the literature. Note, however, that Syzygium jambolana essential oil, rich in (Z)-β-ocimene (27.2%), was inactive against Ae. aegypti larvae (LC50 = 433 μg/mL) [58]. Furthermore, (E)-caryophyllene and caryophyllene oxide have shown only marginal larvicidal activities against Ae. aegypti or Cx. pipiens pallens [53,57].
2.3. Antimicrobial Activity
The Myrtaceae essential oils were screened for antibacterial activity against Enterococcus faecalis (ATCC 29912) and Staphylococcus aureus (ATCC 25923), and for antifungal activity against Candida albicans (ATCC 10231). The antimicrobial activities are summarized in Table 7.
Table 7.
Antimicrobial activities of Myrtaceae essential oils.
| Sample | Enterococcus faecalis | Staphylococcus aureus | Candida albicans |
|---|---|---|---|
| MIC (μg/mL) | |||
| Baeckea frutescens leaf EO | 64 | nt | 16 |
| Callistemon citrinus leaf EO | 32 | 256 | 16 |
| Callistemon citrinus fruit EO | 16 | nt | 128 |
| Melaleuca leucadendra old leaf EO | 32 | 64 | 128 |
| Melaleuca leucadendra stem bark EO | 16 | 64 | 64 |
| Melaleuca leucadendra fruit EO | 32 | 64 | 256 |
| Syzygium nervosum leaf EO | 32 | nt | 128 |
| Streptomycin | 256 | 256 | nt |
| Nistatin | nt | nt | 8 |
| IC50 (μg/mL) | |||
| Baeckea frutescens leaf EO | 33.56 | nt | 8.67 |
| Callistemon citrinus leaf EO | 16.67 | 128.00 | 8.67 |
| Callistemon citrinus fruit EO | 8.89 | nt | 32.67 |
| Melaleuca leucadendra old leaf EO | 16.72 | 33.23 | 65.56 |
| Melaleuca leucadendra stem bark EO | 8.32 | 32.23 | 34.22 |
| Melaleuca leucadendra fruit EO | 15.98 | 32.89 | 128.35 |
| Syzygium nervosum leaf EO | 17.00 | nt | 65.33 |
MIC = minimum inhibitory concentration, EO = essential oil, nt = not tested, IC50 = median inhibitory concentration.
The leaf essential oils of B. frutescens and C. citrinus both showed excellent anti-Candida activity, with minimum inhibitory concentration (MIC) values of 16 μg/mL. van Zyl and co-workers have screened several monoterpenoids against C. albicans, and many of the major components that were found in B. frutescens and C. citrinus leaf essential oils did show notable activities, including α-pinene (MIC 12.0 μg/mL), β-pinene (MIC 1.0 μg/mL), limonene (MIC 10.0 μg/mL), and γ-terpinene (MIC 6.0 μg/mL) [59]. 1,8-Cineole and α-terpineol are relatively inactive against C. albicans, however [60,61]. A perusal of the literature reveals a broad spectrum of reported antimicrobial activities for terpenoid constituents against E. faecalis, S. aureus, and C. albicans (Table 8). There are several potential reasons for the apparent discrepancies, including variation in antimicrobial assay protocols, different susceptibilities of different strains of a particular microorganism, mathematical errors in calculating dilutions and MIC values.
Table 8.
Antimicrobial activities (MIC, μg/mL) of essential oil components from the literature.
| Compound | Enterococcus faecalis [Ref] | Staphylococcus aureus [Ref] | Candida albicans [Ref] |
|---|---|---|---|
| α-pinene | 8000 [62] >4000 [63] inactive [64] |
13.6 [65] 45.7 [66] 312 [60] 800 [62] 1600 [67] 1300–2500 [68] >32 [59] |
12 [59] 156 [60] 800 [67] >1000 [69] |
| β-pinene | 60 [70] 2500 [71] >4000 [63] |
3.0 [59] 41.3 [66] 600 [70] 1600 [67] >20 [65] |
1.0 [59] 60 [70] 100 [69] 1600 [67] |
| p-cymene | 600 [72] inactive [73] |
2000 [67] >32 [59] >10,000 [68] >80,000 [74] |
100 [69] 1600 [67] >32 [59] >80,000 [61] |
| limonene | 27,000 [75] | 24 [59] 32.1 [66] 312 [60] >20 [65] >10,000 [68] |
10 [59] 1000 [69] 1250 [60] |
| 1,8-cineole | 7500 [64] 23,000 [75] >8000 [76] inactive [62] |
32 [59] 625 [60] 5000 [74] >10,000 [68] |
312 [60] 10,000 [74] 40,000 [61] >32 [59] >1000 [69] |
| γ-terpinene | no data | >32 [59] >80,000 [74] |
6.0 [59] 100 [69] >80,000 [61] |
| α-terpineol | >1000 [77] | 1250 [60] 2500 [74] >20 [65] |
1200 [61] 1250 [60] 2500 [74] |
| (E)-caryophyllene | 6 [78] 60 [70] 2500 [71] >4000 [63] inactive [79] |
5.1 [65] 30.3 [66] 60 [78] 312 [60] 9100 [79] >10,000 [68] |
1250 [60] >1000 [69] inactive [78] inactive [79] |
| α-humulene | 6 [70] >400 [80] |
2.6 [65] 312 [60] >10,000 [68] inactive [70] |
625 [60] inactive [70] |
Callistemon citrinus fruit essential oil, dominated by α-pinene (35.1%) and 1,8-cineole (32.4%), was particularly active against E. faecalis. Neither of these compounds have shown notable activity against E. faecalis, however (Table 8); the activity observed for C. citrinus fruit essential oil must be attributed to synergistic activity of less abundant components. Melaleuca leucadendra bark essential oil, which was rich in α-eudesmol (24.1%) and guaiol (11.3%), also exhibited notable activity against E. faecalis, possibly due to the high concentrations of sesquiterpene alcohols present.
3. Materials and Methods
3.1. Plant Collection
Plant materials were collected from wild-growing plants in the Hoa Vang and Hoa Khanh districts of Da Nang city. The plants were identified by Do Ngoc Dai. In each case, the fresh plant material was chopped, and 2.0 kg was subjected to hydrodistillation using a Clevenger-type apparatus (Table 9).
Table 9.
Collection details and essential oil yields of four species of Myrtaceae from central Vietnam.
| Species | Vietnamese Name | Collection Site | Voucher Number | Part | % Yield |
|---|---|---|---|---|---|
| Baeckea frutescens L. | Chổi xể, Chổi trện, Chóp máu, Thanh hao, Thanh liễu | Hoa Vang district, Da Nang city (16°1′10.1″ N, 108°06′01.3″ E, elev. 27 m), in January 2019. | NHH7 | Leaf | 2.23 |
| Melaleuca leucadendra (L.) L. | Tràm lá dài, tràm lá hẹp | Hoa Vang district, Da Nang city (16°1′10.1″ N, 108°06′01.3″ E, elev. 27 m), in February 2019. | NHH4 | Young leaf | 1.22 |
| Old leaf | 1.43 | ||||
| Stem bark | 0.91 | ||||
| Fruit | 1.12 | ||||
| Branch tip | 1.10 | ||||
| Callistemon citrinus (Curtis) Skeels | Tràm bông đỏ, Tràm liễu, Kiều nhụy, Kiều hùng | Garden for Medicinal Plant Conservation, Duy Tan University, Hoa Khanh district, Da Nang city (16°02′57.6″ N, 108°09′34.5″ E, elev 8 m), in November 2018. | NHH6 | Leaf | 0.62 |
| Fruit | 0.34 | ||||
| Syzygium nervosum DC. | Vối, Trâm vối, Trâm nắp | Garden for Medicinal Plant Conservation, Duy Tan University, Hoa Khanh district, Da Nang city (16°02′57.6″ N, 108°09′34.5″ E, elev. 8 m), in January 2019. | NHH10 | Leaf | 0.20 |
3.2. Gas Chromatographic – Mass Spectral Analysis
Each of the essential oils was analyzed by gas chromatography-mass spectrometry (GC-MS), as previously reported [81], using a Shimadzu GCMS-QP2010 Ultra, fitted with a ZB-5 column. Identification of the oil components was based on their retention indices determined by reference to a homologous series of n-alkanes, and by comparison of their mass spectral fragmentation patterns with those in the NIST [21] and FFSNC [22] databases and our own Sat-Set library [23].
3.3. Mosquito Larvicidal Assays
Mosquito colonies of Aedes aegypti, Aedes albopictus, and Culex quinquefasciatus were obtained and maintained as previously described [82].
Larvicidal activities of the essential oils were evaluated according to the protocol of Liu and co-workers [83] with slight modifications. For each assay, 150 mL of water containing 20 fourth-instar mosquito larvae was placed into 250-mL beakers and aliquots of the essential oils dissolved in EtOH (1% stock solution) were then added. A set of controls using EtOH only (negative control) and permethrin (positive control) were included for comparison. Mortality was recorded after 24 h and after 48 h of exposure, during which no nutritional supplement was added. The experiments were carried out at 25 ± 2 °C. Each test was conducted in quadruplicate with five concentrations (100, 50, 25, 12.5 and 6 μg/mL). The data obtained were subjected to log-probit analysis [84] to obtain LC50 values, LC90 values and 95% confidence limits using Minitab® 19 (Minitab, LLC, State College, PA, USA).
3.4. Antimicrobial Screening
The antimicrobial activity of the essential oils was evaluated using two bacteria (Enterococcus faecalis, ATCC 299212, and Staphylococcus aureus, ATCC 25923) and one yeast (Candida albicans, ATCC 10231) using the microdilution broth susceptibility assay, as previously reported [82]. Stock solutions of the each of the essential oils were prepared in dimethylsulfoxide. Dilution series were prepared from 16,384 to 2 μg/mL (214, 213, 212, 211, 210, 29, 27, 25, 23 and 21 µg/mL) in sterile distilled water in micro-test tubes from where they were transferred to the 96-well microtiter plates for the assays.
3.5. Agglomerative Hierarchical Cluster Analysis
The essential oil compositions from this work and from the published literature were treated as operational taxonomic units (OTUs). The percentage composition of the major components of the essential oils was used to determine the chemical relationship between the various essential oil samples by agglomerative hierarchical cluster (AHC) analysis, using the XLSTAT software, version 2018.1.1.6097 (Addinsoft™, Paris, France). Euclidean distance was used to measure dissimilarity, and Ward’s method was used for cluster definition.
4. Conclusions
Essential oils derived from Baeckea frutescens, Callistemon citrinus, Melaleuca leucadendra, and Syzygium nervosum have shown larvicidal activities against the mosquito species tested. In most cases, the larvicidal activities cannot be attributed to the major components, and synergistic interactions with minor components are likely responsible. Likewise, all of the Myrtaceae essential oils examined for antimicrobial activity showed promise. Thus, these essential oils may serve as “green” vector control agents and/or complementary antimicrobial agents, as well as providing value-added commodities for harvested timbers (e.g., Melaleuca leucadendra).
Acknowledgments
P.S. and W.N.S. participated in this work as part of the activities of the Aromatic Plant Research Center (APRC, https://aromaticplant.org/).
Author Contributions
Conceptualization, N.H.H.; methodology, N.H.H., P.S., W.N.S., N.T.G.A., D.N.D; software, P.S.; validation, N.H.H., P.S., and W.N.S.; formal analysis, W.N.S.; investigation, N.T.G.A., L.T.H., T.A.T., N.H.H., D.N.D., N.T.B.N.; resources, N.H.H. and P.S.; data curation, W.N.S.; writing—original draft preparation, W.N.S.; writing—review & editing, N.H.H., P.S., and W.N.S.; visualization, W.N.S.; supervision, N.H.H.; project administration, N.H.H.; funding acquisition, N.H.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Duy Tan University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Tilak R., Ray S., Tilak V.W., Mukherji S. Dengue, chikungunya … and the missing entity—Zika fever: A new emerging threat. Med. J. Armed Forces India. 2016;72:157–163. doi: 10.1016/j.mjafi.2016.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mayer S.V., Tesh R.B., Vasilakis N. The emergence of arthropod-borne viral diseases: A global prospective on dengue, chikungunya and zika fevers. Acta Trop. 2017;166:155–163. doi: 10.1016/j.actatropica.2016.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilder-Smith A., Gubler D.J., Weaver S.C., Monath T.P., Heymann D.L., Scott T.W. Epidemic arboviral diseases: Priorities for research and public health. Lancet Infect. Dis. 2017;17:e101–e106. doi: 10.1016/S1473-3099(16)30518-7. [DOI] [PubMed] [Google Scholar]
- 4.Samy A.M., Elaagip A.H., Kenawy M.A., Ayres C.F.J., Peterson A.T., Soliman D.E. Climate change influences on the global potential distribution of the mosquito Culex quinquefasciatus, vector of West Nile virus and lymphatic filariasis. PLoS ONE. 2016;11:e0163863. doi: 10.1371/journal.pone.0163863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miró-Canturri A., Ayerbe-Algaba R., Smani Y. Drug repurposing for the treatment of bacterial and fungal infections. Front. Microbiol. 2019;10:41. doi: 10.3389/fmicb.2019.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang Y., Ashworth A.J., Willett C., Cook K., Upadhyay A., Owens P.R., Ricke S.C., DeBruyn J.M., Moore P.A. Review of antibiotic resistance, ecology, dissemination, and mitigation in U.S. broiler poultry systems. Front. Microbiol. 2019;10:2639. doi: 10.3389/fmicb.2019.02639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bennani H., Mateus A., Mays N., Eastmure E., Stärk K.D.C., Häsler B. Overview of evidence of antimicrobial use and antimicrobial resistance in the food chain. Antibiotics. 2020;9:49. doi: 10.3390/antibiotics9020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vidovic N., Vidovic S. Antimicrobial resistance and food animals: Influence of livestock environment on the emergence and dissemination of antimicrobial resistance. Antibiotics. 2020;9:52. doi: 10.3390/antibiotics9020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geddes-McAlister J., Shapiro R.S. New pathogens, new tricks: Emerging, drug-resistant fungal pathogens and future prospects for antifungal therapeutics. Ann. N. Y. Acad. Sci. 2019;1435:57–78. doi: 10.1111/nyas.13739. [DOI] [PubMed] [Google Scholar]
- 10.Mulani M.S., Kamble E.E., Kumkar S.N., Tawre M.S., Pardesi K.R. Emerging strategies to combat ESKAPE pathogens in the era of antimicrobial resistance: A review. Front. Microbiol. 2019;10:539. doi: 10.3389/fmicb.2019.00539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mabberley D.J. Mabberley’s Plant-Book. 3rd ed. Cambridge University Press; Cambridge, UK: 2008. [Google Scholar]
- 12.Missouri Botanical Garden Tropicos.org. [(accessed on 11 February 2020)]; Available online: www.tropicos.org.
- 13.Brophy J.J., Craven L.A., Doran J.C. Melaleucas: Their Botany, Essential Oils and Uses. Volume 156. Australian Centre for International Agricultural Research; Canberra, Australia: 2013. [Google Scholar]
- 14.Brophy J.J. Potentially commercial Melaleucas. In: Southwell I., Lowe R., editors. The Genus Melaleuca. Harwood; Amsterdam, The Netherlands: 1999. pp. 247–274. [Google Scholar]
- 15.Hyland B.P.M., Whiffen T. Australian Tropical Rainforest Trees, Volume 2. CSIRO Publications; East Melbourne, Australia: 1993. [Google Scholar]
- 16.Son P.T., Giang P.M., Van N.B., Chien N.Q., Dung N.Q. Study on chemical composition of essential oil of Baeckea frutescens L. from Vietnam. Pharm. J. 1998;12:7–8. [Google Scholar]
- 17.Tam N.T., Thuam D.T., Bighelli A., Castola V., Muselli A., Richomme P., Casanova J. Baeckea frutescens leaf oil from Vietnam: Composition and chemical variability. Flavour Fragr. J. 2004;19:217–220. doi: 10.1002/ffj.1281. [DOI] [Google Scholar]
- 18.Dai D., Thang T., Olayiwola T., Ogunwande I. Chemical composition of essential oil of Baeckea frutescens L. Int. Res. J. Pure Appl. Chem. 2015;8:26–32. doi: 10.9734/IRJPAC/2015/17199. [DOI] [Google Scholar]
- 19.Ji X., Zhao G., Pu Q., Cai Q., Jiang D. GC/MS Analysis of the essential oil of Backea frutrescens Linn. Acta Pharm. Sin. 1980;15:766–768. [PubMed] [Google Scholar]
- 20.Jantan I., Ahmad A.S., Bakar S.A.A., Ahmad A.R., Trockenbrodt M., Chak C.V. Constituents of the essential oil of Baeckea frutescens L. From Malaysia. Flavour Fragr. J. 1998;13:245–247. doi: 10.1002/(SICI)1099-1026(1998070)13:4<245::AID-FFJ736>3.0.CO;2-J. [DOI] [Google Scholar]
- 21.NIST17. National Institute of Standards and Technology; Gaithersburg, MD, USA: 2017. [Google Scholar]
- 22.Mondello L. FFNSC 3. Shimadzu Scientific Instruments; Columbia, MD, USA: 2016. [Google Scholar]
- 23.Satyal P. Ph.D. Thesis. University of Alabama in Huntsville; Huntsville, AL, USA: 2015. Development of GC-MS Database of Essential Oil Components by the Analysis of Natural Essential Oils and Synthetic Compounds and Discovery of Biologically Active Novel Chemotypes in Essential Oils. [Google Scholar]
- 24.Riaz M., Chaudhary F.M. The chemical composition of Pakistani Callistemon citrinus oils. J. Essent. Oil Res. 1990;2:327–328. doi: 10.1080/10412905.1990.9697891. [DOI] [Google Scholar]
- 25.Brophy J.J., Goldsack R.J., Forster P.I., Craven L.A., Lepschi B.J. The leaf essential oils of the Australian members of the genus Callistemon (Myrtaceae) J. Essent. Oil Res. 1998;10:595–606. doi: 10.1080/10412905.1998.9700986. [DOI] [Google Scholar]
- 26.Andola H.C., Haider S.Z., Negi P.S., Arunachalam K. Composition of the essential oil of Callistemon citrinus (Curtis) Skeels from Uttarakhand (India) Natl. Acad. Sci. Lett. 2017;40:389–392. doi: 10.1007/s40009-017-0594-x. [DOI] [Google Scholar]
- 27.Chane-Ming J., Vera R.R., Fraisse D.J. Chemical composition of essential oil of Callistemon citrinus (Curtis) Skeel from Reunion. J. Essent. Oil Res. 1998;10:429–431. doi: 10.1080/10412905.1998.9700935. [DOI] [Google Scholar]
- 28.Srivastava S.K., Ahmad A., Jain N., Aggarwal K.K., Syamasundar K.V. Essential oil composition of Callistemon citrinus leaves from the lower region of Himalayas. J. Essent. Oil Res. 2001;13:359–361. doi: 10.1080/10412905.2001.9712233. [DOI] [Google Scholar]
- 29.Sharma R.K., Kotoky R., Bhattacharyya P.R. Volatile oil from the leaves of Callistemon lanceolatus D.C. grown in north-eastern India. Flavour Fragr. J. 2006;21:239–240. doi: 10.1002/ffj.1564. [DOI] [Google Scholar]
- 30.Oyedeji O.O., Lawal O.A., Shode F.O., Oyedeji A.O. Chemical composition and antibacterial activity of the essential oils of Callistemon citrinus and Callistemon viminalis from South Africa. Molecules. 2009;14:1990–1998. doi: 10.3390/molecules14061990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silva C.J., Barbosa L.C.A., Demuner A.J., Montanari R.M., Pinheiro A.L., Dias I., Andrade N.J. Chemical composition and antibacterial activities from the essential oils of Myrtaceae species planted in Brazil. Quim. Nova. 2010;33:104–108. doi: 10.1590/S0100-40422010000100019. [DOI] [Google Scholar]
- 32.Zandi-Sohani N., Hojjati M., Carbonell-Barrachina Á.A. Insecticidal and repellent activities of the essential oil of Callistemon citrinus (Myrtaceae) against Callosobruchus maculatus (F.) (Coleoptera: Bruchidae) Neotrop. Entomol. 2013;42:89–94. doi: 10.1007/s13744-012-0087-z. [DOI] [PubMed] [Google Scholar]
- 33.Shrestha S., Poudel A., Satyal P., Dosoky N.S., Chhetri B.K., Setzer W.N. Chemical composition and biological activity of the leaf essential oil of Callistemon citrinus from Nepal. Am. J. Essent. Oils Nat. Prod. 2015;2:29–33. [Google Scholar]
- 34.Kumar D., Sukapaka M., Babu G.D.K., Padwad Y. Chemical composition and in vitro cytotoxicity of essential oils from leaves and flowers of Callistemon citrinus from western Himalayas. PLoS ONE. 2015;10:e0133823. doi: 10.1371/journal.pone.0133823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silva C.J., Barbosa L.C.A., Maltha C.R.A., Pinheiro A.L., Ismail F.M.D. Comparative study of the essential oils of seven Melaleuca (Myrtaceae) species grown in Brazil. Flavour Fragr. J. 2007;22:474–478. doi: 10.1002/ffj.1823. [DOI] [Google Scholar]
- 36.Siddique S., Parveen Z., Firdaus-e-Bareen, Mazhar S. Chemical composition, antibacterial and antioxidant activities of essential oils from leaves of three Melaleuca species of Pakistani flora. Arab. J. Chem. 2020;13:67–74. doi: 10.1016/j.arabjc.2017.01.018. [DOI] [Google Scholar]
- 37.Padalia R.C., Verma R.S., Chauhan A., Chanotiya C.S. The essential oil composition of Melaleuca leucadendra L. grown in India: A novel source of (E)-nerolidol. Ind. Crops Prod. 2015;69:224–227. doi: 10.1016/j.indcrop.2015.02.019. [DOI] [Google Scholar]
- 38.Da Silva M.M.C., da Camara C.A.G., de Moraes M.M., de Melo J.P.R., dos Santos R.B., Neves R.C.S. Insecticidal and acaricidal activity of essential oils rich in (E)-nerolidol from Melaleuca leucadendra occurring in the state of Pernambuco (Brazil) and effects on two important agricultural pests. J. Braz. Chem. Soc. 2020;31:813–820. doi: 10.21577/0103-5053.20190245. [DOI] [Google Scholar]
- 39.Pino J., Bello A., Urquiola A., Aguero J., Marbot R. Chemical composition of cajuput oil (Melaleuca leucadendra L.) from Cuba. J. Essent. Oil Res. 2002;14:10–11. doi: 10.1080/10412905.2002.9699744. [DOI] [Google Scholar]
- 40.Siani A.C., Nakamura M.J., das Neves G.P., Monteiro S.D.S., Ramos M.F.S. Leaf essential oil from three exotic Mytaceae species growing in the botanical garden of Rio de Janeiro, Brazil. Am. J. Plant Sci. 2016;7:834–840. doi: 10.4236/ajps.2016.76079. [DOI] [Google Scholar]
- 41.Kumar A., Tandon S., Yadav A. Chemical composition of the essential oil from fresh leaves of Melaleuca leucadendron L. from north India. J. Essent. Oil Bear. Plants. 2005;8:19–22. doi: 10.1080/0972060X.2005.10643415. [DOI] [Google Scholar]
- 42.Pino J.A., Regalado E.L., Rodríguez J.L., Fernández M.D. Phytochemical analysis and in vitro free-radical-scavenging activities of the essential oils from leaf and fruit of Melaleuca leucadendra L. Chem. Biodivers. 2010;7:2281–2288. doi: 10.1002/cbdv.200900162. [DOI] [PubMed] [Google Scholar]
- 43.Rini P., Ohtani Y., Ichiura H. Antioxidant, anti-hyaluronidase and antifungal activities of Melaleuca leucadendron Linn. leaf oils. J. Wood Sci. 2012;58:429–436. doi: 10.1007/s10086-012-1270-x. [DOI] [Google Scholar]
- 44.Adjalian E., Sessou P., Yehouenou B., Bothon F.T.D., Noudogbessi J.-P., Kossou D., Menut C., Sohounhloue D. Anti-oviposition and repellent activity of essential oil from Melaleuca leucadendron leaf acclimated in Bénin against the Angoumois grain moth. Int. J. Biol. Pharm. Allied Sci. 2015;4:797–806. [Google Scholar]
- 45.Tia E.V., Lozano P., Menut C., Lozano Y.F., Martin T., Niamké S., Adima A.A. Potentialité des huiles essentielles dans la lutte biologique contre la mouche blanche Bemisia tabaci Genn. Phytotherapie. 2013;11:31–38. doi: 10.1007/s10298-012-0736-8. [DOI] [Google Scholar]
- 46.Muchtaridi M., Tjiraresmi A., Febriyanti R. Analysis of active compounds in blood plasma of mice after inhalation of cajuput essential oil (Melaleuca leucadendron L.) Indones. J. Pharm. 2016;26:219–227. doi: 10.14499/indonesianjpharm26iss4pp219. [DOI] [Google Scholar]
- 47.Brophy J.J., Lassak E.V. Melaleuca leucadendra L. leaf oil: Two phenylpropanoid chemotypes. Flavour Fragr. J. 1988;3:43–46. doi: 10.1002/ffj.2730030109. [DOI] [Google Scholar]
- 48.Liu B., Peng W. Component analysis of essential oil from Melaleuca leucadendron L. J. Instrum. Anal. 1999;18:70–72. [Google Scholar]
- 49.González de Colmenares N., Ojeda de Rodríguez G., Prieto A., Crescente O., Cabrera L. Phytoconstituents and antimicrobial activity of Melaleuca leucadendron leaf essential oil from Venezuela. Ciencia. 1998;6:123–128. [Google Scholar]
- 50.Farag R.S., Shalaby A.S., El-Baroty G.A., Ibrahim N.A., Ali M.A., Hassan E.M. Chemical and biological evaluation of the essential oils of different Melaleuca species. Phyther. Res. 2004;18:30–35. doi: 10.1002/ptr.1348. [DOI] [PubMed] [Google Scholar]
- 51.Dung N.X., Van Luu H., Khoi T.T., Leclercq P.A. GC and GC/MS analysis of the leaf oil of Cleistocalyx operculatus Roxb. Merr. et Perry (syn. Eugenia operculata Roxb.; Syzygicum mervosum DC.) J. Essent. Oil Res. 1994;6:661–662. doi: 10.1080/10412905.1994.9699366. [DOI] [Google Scholar]
- 52.Dosoky N.S., Pokharel S.K., Setzer W.N. Leaf essential oil composition, antimicrobial and cytotoxic activities of Cleistocalyx operculatus from Hetauda, Nepal. Am. J. Essent. Oils Nat. Prod. 2015;2:34–37. [Google Scholar]
- 53.Perumalsamy H., Kim N.-J., Ahn Y.-J. Larvicidal activity of compounds isolated from Asarum heterotropoides against Culex pipiens pallens, Aedes aegypti, and Ochlerotatus togoi (Diptera: Culicidae) J. Med. Entomol. 2009;46:1420–1423. doi: 10.1603/033.046.0624. [DOI] [PubMed] [Google Scholar]
- 54.Sakhanokho H.F., Sampson B.J., Tabanca N., Wedge D.E., Demirci B., Baser K.H.C., Bernier U.R., Tsikolia M., Agramonte N.M., Becnel J.J., et al. Chemical composition, antifungal and insecticidal activities of Hedychium essential oils. Molecules. 2013;18:4308–4327. doi: 10.3390/molecules18044308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pavela R. Acute toxicity and synergistic and antagonistic effects of the aromatic compounds of some essential oils against Culex quinquefasciatus Say larvae. Parasitol. Res. 2015;114:3835–3853. doi: 10.1007/s00436-015-4614-9. [DOI] [PubMed] [Google Scholar]
- 56.Scalerandi E., Flores G.A., Palacio M., Defagó M.T., Carpinella M.C., Valladares G., Bertoni A., Palacios S.M. Understanding synergistic toxicity of terpenes as insecticides: Contribution of metabolic detoxification in Musca domestica. Front. Plant Sci. 2018;9:1579. doi: 10.3389/fpls.2018.01579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee D.C., Ahn Y.J. Laboratory and simulated field bioassays to evaluate larvicidal activity of Pinus densiflora hydrodistillate, its constituents and structurally related compounds against Aedes albopictus, Aedes aegypti and Culex pipiens pallens in relation to their inhibitory effects on acetylcholinesterase activity. Insects. 2013;4:217–229. doi: 10.3390/insects4020217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cavalcanti E.S.B., de Morais S.M., Lima M.A.A., Santana E.W.P. Larvicidal activity of essential oils from Brazilian plants against Aedes aegypti L. Mem. Inst. Oswaldo Cruz. 2004;99:541–544. doi: 10.1590/S0074-02762004000500015. [DOI] [PubMed] [Google Scholar]
- 59.Van Zyl R.L., Seatlholo S.T., van Vuuren S.F., Viljoen A.M. The biological activities of 20 nature identical essential oil constituents. J. Essent. Oil Res. 2006;18:129–133. doi: 10.1080/10412905.2006.12067134. [DOI] [Google Scholar]
- 60.Schmidt J.M., Noletto J.A., Vogler B., Setzer W.N. Abaco bush medicine: Chemical composition of the essential oils of four aromatic medicinal plants from Abaco Island, Bahamas. J. Herbs Spices Med. Plants. 2006;12:43–65. doi: 10.1300/J044v12n03_04. [DOI] [Google Scholar]
- 61.Hammer K.A., Carson C.F., Riley T.V. Antifungal activity of the components of Melaleuca alternifolia (tea tree) oil. J. Appl. Microbiol. 2003;95:853–860. doi: 10.1046/j.1365-2672.2003.02059.x. [DOI] [PubMed] [Google Scholar]
- 62.Ojeda-Sana A.M., van Baren C.M., Elechosa M.A., Juárez M.A., Moreno S. New insights into antibacterial and antioxidant activities of rosemary essential oils and their main components. Food Control. 2013;31:189–195. doi: 10.1016/j.foodcont.2012.09.022. [DOI] [Google Scholar]
- 63.Crevelin E.J., Caixeta S.C., Dias H.J., Groppo M., Cunha W.R., Martins C.H.G., Crotti A.E.M. Antimicrobial activity of the essential oil of Plectranthus neochilus against cariogenic bacteria. Evid. Based Complement. Altern. Med. 2015;2015:102317. doi: 10.1155/2015/102317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Salehi P., Sonboli A., Asghari B. Chemical composition of the essential oil of Stachys acerosa and its antibacterial and antioxidant activities. Chem. Nat. Compd. 2007;43:339–341. doi: 10.1007/s10600-007-0126-x. [DOI] [Google Scholar]
- 65.Pichette A., Larouche P.-L., Lebrun M., Legault J. Composition and antibacterial activity of Abies balsamea essential oil. Phyther. Res. 2006;20:371–373. doi: 10.1002/ptr.1863. [DOI] [PubMed] [Google Scholar]
- 66.Rather M.A., Dar B.A., Dar M.Y., Wani B.A., Shah W.A., Bhat B.A., Ganai B.A., Bhat K.A., Anand R., Qurishi M.A. Chemical composition, antioxidant and antibacterial activities of the leaf essential oil of Juglans regia L. and its constituents. Phytomedicine. 2012;19:1185–1190. doi: 10.1016/j.phymed.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 67.Filipowicz N., Kamiński M., Kurlenda J., Asztemborska M., Ochocka J.R. Antibacterial and antifungal activity of juniper berry oil and its selected components. Phyther. Res. 2003;17:227–231. doi: 10.1002/ptr.1110. [DOI] [PubMed] [Google Scholar]
- 68.Reichling J., Suschke U., Schneele J., Geiss H.K. Antibacterial activity and irritation potential of selected essential oil components—Structure-activity relationship. Nat. Prod. Commun. 2006;1:1003–1012. doi: 10.1177/1934578X0600101116. [DOI] [Google Scholar]
- 69.Tampieri M.P., Galuppi R., MacChioni F., Carelle M.S., Falcioni L., Cioni P.L., Morelli I. The inhibition of Candida albicans by selected essential oils and their major components. Mycopathologia. 2005;159:339–345. doi: 10.1007/s11046-003-4790-5. [DOI] [PubMed] [Google Scholar]
- 70.Jirovetz L., Bail S., Buchbauer G., Denkova Z., Slavchev A., Stoyanova A., Schmidt E., Geissler M. Antimicrobial testings, gas chromatographic analysis and olfactory evaluation of an essential oil of hop cones (Humulus lupulus L.) from Bavaria and some of its main compounds. Sci. Pharm. 2006;74:189–201. doi: 10.3797/scipharm.2006.74.189. [DOI] [Google Scholar]
- 71.Maggi F., Cecchini C., Cresci A., Coman M.M., Tirillini B., Sagratini G., Papa F. Chemical composition and antimicrobial activity of the essential oil from Ferula glauca L. (F. communis L. subsp. glauca) growing in Marche (central Italy) Fitoterapia. 2009;80:68–72. doi: 10.1016/j.fitote.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 72.Höferl M., Buchbauer G., Jirovetz L., Schmidt E., Stoyanova A., Denkova Z., Slavchev A., Geissler M. Correlation of antimicrobial activities of various essential oils and their main aromatic volatile constituents. J. Essent. Oil Res. 2009;21:459–463. doi: 10.1080/10412905.2009.9700218. [DOI] [Google Scholar]
- 73.Vardar-Ünlü G., Ünlü M., Dönmez E., Vural N. Chemical composition and in vitro antimicrobial activity of the essential oil of Origanum minutiflorum O Schwarz & H Davis. J. Sci. Food Agric. 2007;87:255–259. [Google Scholar]
- 74.Carson C.F., Riley T.V. Antimicrobial activity of the major components of the essential oil of Melaleuca alternifolia. J. Appl. Bacteriol. 1995;78:264–269. doi: 10.1111/j.1365-2672.1995.tb05025.x. [DOI] [PubMed] [Google Scholar]
- 75.Van Vuuren S.F., Viljoen A.M. Antimicrobial activity of limonene enantiomers and 1,8-cineole alone and in combination. Flavour Fragr. J. 2007;22:540–544. doi: 10.1002/ffj.1843. [DOI] [Google Scholar]
- 76.Mulyaningsih S., Sporer F., Zimmermann S., Reichling J., Wink M. Synergistic properties of the terpenoids aromadendrene and 1,8-cineole from the essential oil of Eucalyptus globulus against antibiotic-susceptible and antibiotic-resistant pathogens. Phytomedicine. 2010;17:1061–1066. doi: 10.1016/j.phymed.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 77.Chang S.-T., Chen P.-F., Chang S.-C. Antibacterial activity of leaf essential oils and their constituents from Cinnamomum osmophloeum. J. Ethnopharmacol. 2001;77:123–127. doi: 10.1016/S0378-8741(01)00273-2. [DOI] [PubMed] [Google Scholar]
- 78.Schmidt E., Bail S., Friedl S.M., Jirovetz L., Buchbauer G., Wanner J., Denkova Z., Slavchev A., Stoyanova A., Geissler M. Antimicrobial activities of single aroma compounds. Nat. Prod. Commun. 2010;5:1365–1368. doi: 10.1177/1934578X1000500906. [DOI] [PubMed] [Google Scholar]
- 79.Juliani H.R., Biurrun F., Koroch A.R., Oliva M.M., Demo M.S., Trippi V.S., Zygadlo J.A. Chemical constituents and antimicrobial activity of the essential oil of Lantana xenica. Planta Med. 2002;68:762–764. doi: 10.1055/s-2002-33803. [DOI] [PubMed] [Google Scholar]
- 80.Duarte Moreira R.R., Zimmermann Martins G., Teixeira Botelho V., dos Santos L.E., Cavaleiro C., Salgueiro L., Andrade G., Gomes Martins C.H. Composition and activity against oral pathogens of the essential oil of Melampodium divaricatum (Rich.) DC. Chem. Biodivers. 2014;11:438–444. doi: 10.1002/cbdv.201300322. [DOI] [PubMed] [Google Scholar]
- 81.Hung N.H., Satyal P., Hieu H.V., Chuong N.T.H., Dai D.N., Huong L.T., Tai T.A., Setzer W.N. Mosquito larvicidal activity of the essential oils of Erechtites species growing wild in Vietnam. Insects. 2019;10:47. doi: 10.3390/insects10020047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dai D.N., Chung N.T., Huong L.T., Hung N.H., Chau D.T.M., Yen N.T., Setzer W.N. Chemical compositions, mosquito larvicidal and antimicrobial activities of essential oils from five species of Cinnamomum growing wild in north central Vietnam. Molecules. 2020;25:1303. doi: 10.3390/molecules25061303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu Z.L., He Q., Chu S.S., Wang C.F., Du S.S., Deng Z.W. Essential oil composition and larvicidal activity of Saussurea lappa roots against the mosquito Aedes albopictus (Diptera: Culicidae) Parasitol. Res. 2012;110:2125–2130. doi: 10.1007/s00436-011-2738-0. [DOI] [PubMed] [Google Scholar]
- 84.Finney D. Probit Analysis. Cambridge University Press; Cambridge, UK: 2009. Reissue Edition. [Google Scholar]