SUMMARY
Lung squamous cell carcinoma (LSCC) is a prevalent form of lung cancer exhibiting distinctive histological and genetic characteristics. Chromosome 3q26 copy number gain (CNG) is a genetic hallmark of LSCC present in >90% of tumors. We report that 3q26 CNGs occur early in LSCC tumorigenesis, persist during tumor progression, and drive coordinate overexpression of PRKCI, SOX2, and ECT2. Overexpression of PRKCI, SOX2, and ECT2 in the context of Trp53 loss is sufficient to transform mouse lung basal stem cells into tumors with histological and genomic features of LSCC. Functionally, PRKCI and SOX2 collaborate to activate an extensive transcriptional program that enforces a lineage-restricted LSCC phenotype, whereas PRKCI and ECT2 collaborate to promote oncogenic growth. Gene signatures indicative of PKCi-SOX2 and PKCi-ECT2 signaling activity are enriched in the classical subtype of human LSCC and predict distinct therapeutic vulnerabilities. Thus, the PRKCI, SOX2, and ECT2 oncogenes represent a multigenic driver of LSCC.
In Brief
Liu et al. report that three oncogenes, PRKCI, SOX2, and ECT2, which are coordinately amplified and overexpressed in lung squamous cell carcinoma (LSCC), can transform Trp53−/− mouse lung basal stem cells into tumors with histological and genomic features of LSCC and drive oncogenic signaling necessary to maintain a LSCC phenotype.
Graphical Abstract

INTRODUCTION
Lung squamous cell carcinoma (LSCC) is a major form of non- small-cell lung cancer (NSCLC) accounting for 30% of lung cancer diagnoses, and exhibiting histological, biochemical, and genetic characteristics distinct from other forms of NSCLC including lung adenocarcinoma (LADC). Copy number gains (CNGs) at chromosome 3q26 and mutation of the tumor sup- pressor gene TP53 are the most prevalent genetic alterations in LSCC, occurring concomitantly in >90% of LSCC cases. Although multiple 3q26 genes have been implicated in LSCC (Fields et al., 2016), it is not clear whether 3q26 CNG is an oncogenic driver of LSCC, and if so, which 3q26 genes are necessary and sufficient to drive LSCC tumorigenesis.
We have previously reported extensive genetic, biological, and biochemical cooperativity between three 3q26 genes, PRKCI, SOX2, and ECT2, that contribute to the transformed phenotype of LSCC cells. PRKCI (encoding for PKCi) directly phosphorylates SOX2, an event that regulates SOX2 binding to the promoter region of the Hedgehog (Hh) acyl transferase (HHAT) gene and promotes human LSCC tumor-initiating cell (TIC) growth (Justilien et al., 2014). In addition, PKCi-mediated phosphorylation of ECT2 regulates its guanine nucleotide exchange (GEF) activity toward RAC1, thereby activating proliferative MEK-ERK signaling, and stimulating ribosomal DNA transcription (Justilien et al., 2011, 2017, 2019; Justilien and Fields, 2009), two pathways required for the transformed growth of LSCC cells.
Here, we assessed whether PRKCI, SOX2, and ECT2 CNGs and overexpression are early and persistent events in LSCC tumorigenesis, identified and functionally characterized a transcriptional program that is activated by the PKCi-SOX2 signaling axis in LSCC cells and tumors, and assessed the effect of overexpression of PRKCI, SOX2, and ECT2 on transformation of mouse lung basal stem cells (LBSCs), a major cell of origin for LSCC. Our results demonstrate that PRKCI, SOX2, and ECT2 are cooperative 3q26 oncogenes that are necessary and sufficient in the context of TP53 loss to drive LSCC tumorigenesis.
RESULTS
Coordinate PRKCI, SOX2, and ECT2 CNGs and Overexpression Are Frequent Early Events in LSCC
Interrogation of The Cancer Genome Atlas (TCGA) LSCC dataset revealed that PRKCI, SOX2, and ECT2 CNGs occur concomitantly in ~91% of LSCC tumors (Figure 1A). Low CNG (genomic identification of significant targets in cancer [GISTIC] score of +1) and high CNG/gene amplification (GISTIC score of +2) lead to a stepwise increase in PRKCI, SOX2, and ECT2 expression when compared to tumors without CNG (GISTIC scores 0 or −1) (Figures 1B–1D). Interestingly, PRKCI, SOX2, and ECT2 CNG and overexpression are equally prevalent in early stage I LSCC tumors and later stage tumors (Figures 1E–1H), demonstrating that these are early and persistent events in LSCC tumor initiation and progression.
Figure 1. Analysis of PRKCI, SOX2, and ECT2 CNGs and Expression in Primary LSCC Tumors.
(A) Oncoprint showing amplification (red, GISTIC score +2) and significant copy number gain (CNG) (pink, GISTIC score +1) of PRKCI, SOX2, and ECT2 in LSCC tumors (n = 478). Blue, shallow copy number deletion (GISTIC score of −1); gray, no alterations in copy number (GISTIC score of 0).
(B–D) Expression of PRKCI (B), SOX2 (C), and ECT2 (D) in LSCC tumors. Results plotted for all tumor samples (T/All; n = 478), normal lung tissues (N; n = 49), and according to PRKCI (B) GISTIC score (0,−1, n = 47; +1, n = 223; +2, n = 208), SOX2 (C) GISTIC score (0,−1, n = 44; +1, n = 202; +2, n = 232), and ECT2 (D) GISTIC score (0,−1, n = 47; +1, n = 220; +2, n = 211). Data represent median, boxes indicate 25% and 75% confidence intervals; error bars indicate 95% confidence interval. Dots indicate outliers. Comparison was by two-tailed Student’s t test; *p < 1.2 × 10−9 and **p = 0.003 versus indicated comparator. NS, not significant.
(E) Prevalence of 3q26 CNGs in LSCC by clinical stage. Data represent the percentage of tumors at each clinical stage harboring 3q26 CNG (stage I, n = 230; stage II, n = 155; and stage III+IV, n = 88). No significant difference in 3q26 CNG prevalence was observed across clinical stages as assessed by chi-square analysis. NS, not significant.
(F–H) Expression of PRKCI (F), SOX2 (G), and ECT2 (H) in LSCC tumors by clinical stages. Data represent median, boxes indicate 25% and 75% confidence intervals, and error bars indicate 95% confidence interval. Dots indicate outliers. Comparison was by unpaired two-tailed Student’s t test; *p < 1.2 × 10−9 compared to normal (n = 49).
PRKCI Regulates an Extensive SOX2 Transcriptional Program in LSCC Cells
PKCi regulates SOX2-dependent transcription of Hedgehog (Hh) acyltransferase (HHAT), leading to activation of Hh signaling in LSCC cells (Justilien et al., 2014). To assess whether PKCi regulates SOX2 transcriptional activity more broadly, we conducted RNA sequencing of H1299 cells in which either PRKCI or SOX2 was silenced by validated lentiviral short hairpin RNA (shRNA) constructs (Justilien et al., 2014). H1299 lung carcinoma cells were chosen for analysis because they harbor PRKCI and SOX2 CNG and homozygous TP53 loss, express elevated PKCi and SOX2 mRNA and protein, exhibit PKCi-, SOX2-dependent transformed growth, and show activated PKCi-SOX2-HHAT signaling (Justilien et al., 2014). qPCR confirmed efficient knockdown (KD) of PKCi and SOX2 in PRKCI and SOX2 KD cells, respectively, and decreased expression of the PKCi-dependent SOX2 transcriptional target HHAT (Justilien et al., 2014; Figure S1A).
Gene set enrichment analysis (GSEA) revealed significant regulation (false discovery rate [FDR] q value < 0.25) of 16 oncogenic hallmarks after PRKCI KD and 19 after SOX2 KD; strikingly, 13 of these hallmarks were commonly regulated by PRKCI and SOX2, suggesting a highly coordinated effect on gene expression (Figure 2A). Among the prominent common hallmarks are Hh, Wnt, and Notch, three oncogenic pathways implicated in cancer cell stemness and LSCC tumorigenesis (Figure 2B; see Table S1). Thus, PKCi regulates a broad SOX2 transcriptional program driving stemness in LSCC cells.
Figure 2. Identification of Direct PKCi-SOX2 Target Genes in LSCC Cells and Tumors.
(A) Venn diagram showing overlap (green) between oncogenic hallmarks regulated by PRKCI (yellow) and SOX2 (blue). Genes set enrichment analysis (GSEA) was conducted on RNA-seq gene expression data from PRKCI KD, SOX2 KD, and NT H1299 LSCC cells. Significantly altered GSEA hallmarks are given in Table S1.
(B) Major cancer stem cells related pathways, Hedgehog, Wnt, and Notch, are significantly enriched in LSCC cells by PRKCI and SOX2 (FDR < 0.25).
(C) Ingenuity Pathway Analysis (IPA) reveals five major oncogenic signaling pathways coordinately activated in PRKCI(H).SOX2(H) versus PRKCI(L).SOX2(L) primary LSCC tumors (lane 1), NT versus PRKCI KD H1299 cells (lane 2), and NT versus SOX2 KD H1299 cells (lane 3). Differentially expressed genes that drive pathway activation are listed (gene targets). p values and z scores for pathways are given in Table S2.
(D) Heatmap showing expression of PRKCI, SOX2, ECT2, and identified PKCi-SOX2 gene targets in stage 1 primary LSCC tumors. Tumors exhibiting high PRKCI (PRKCI(H); n = 42) or low PKCi expression (PRKCI(L); n = 42) were compared. Expression of indicated genes is significantly associated with PRKCI expression. NS, not significant. p values for individual gene associations are given in Table S3.
(E) Heatmap showing expression of PRKCI, SOX2, ECT2, and the identified PKCi-SOX2 gene targets in stage 1 primary LADC tumors. Tumors exhibiting high PRKCI (PRKCI(H); n = 43) or low PRKCI expression (PRKCI(L); n = 43) were compared. The asterisk (*) indicates a statistically significant association with PRKCI. p values for individual gene associations are given in Table S3. NS, not significant.
(F–I) Chromatin immunoprecipitation (ChIP) and ChIP-qPCR to assess binding of SOX2 to the promoter region of the indicated genes in H1299 (F), H520 (G), H2170 (H), and A549 (I) cells expressing either non-target (NT) or PRKCI (PRKCI KD) shRNA. Results are expressed as percentage input ± SEM and are representative of three independent experiments. n = 3. *p < 0.05 compared to NT cells. ND, not detected. NS, not significant. (J–M) qPCR analysis for expression of the indicated genes in H1299 (J), H520 (K), H2170 (L), and A549 (M) cells expressing non-target (NT), PRKCI (PRKCI KD), or SOX2 (SOX2 KD) shRNA. RNA abundance is expressed as fold of NT cells ± SD and are representative of three independent experiments. n = 3. *p < 0.05 compared to NT cells. ND, not detected. NS, not significant. Significance was assessed by unpaired two-tailed Student’s t test.
See also Figures S1 and S2, and Tables S1, S2, and S3.
To identify pathways, and associated genes, that correlate with PRKCI and SOX2 expression in LSCC cells and primary LSCC tumors, we first identified genes differentially expressed in LSCC tumors expressing high PRKCI and SOX2 versus low PRKCI and SOX2 (top versus bottom 20%; n = 44/group) within the TCGA LSCC dataset (Hammerman et al., 2012). We then used RNA sequencing (RNA-seq) to identify genes differentially expressed in NT versus PRKCI KD and SOX2 KD H1299 cells. Ingenuity Pathway Analysis (IPA) revealed significant enrichment of five oncogenic signaling pathways in primary LSCC tumors expressing high PRKCI and SOX2 (p < 0.05, z score > 1.3): Planar Cell Polarity, Basal Cell Carcinoma, Glioblastoma Multiforme, Notch, and Colorectal Cancer Metastasis signaling pathways (Figure 2C, lane 1). Strikingly, these same five pathways were also significantly regulated by PRKCI and SOX2 in H1299 cells (Figure 2C, lanes 2 and 3). Nineteen PKCi-SOX2 target genes were identified whose expression is significantly elevated, and drive pathway enrichment, in all three datasets (Figure 2C; see also Table S2). IPA of gene expression datasets from mouse Sox2-Pten-Cdkn2ab (SOX2-PC) LSCC and KP LADC tumor models (Ferone et al., 2016), revealed enrichment of the same five oncogenic pathways, and elevated expression of 15/19 potential PKCi-SOX2 target genes, in SOX2-PC tumors, suggesting the relevance of these genes and pathways in this LSCC model (Figure S1B).
Interestingly, expression of 13/19 PKCi-SOX2 target genes were significantly elevated in stage 1 LSCC expressing high PRKCI versus low PRKCI (Figure 2D), but these genes showed little or no association with PRKCI expression in stage 1 LADC (Figure 2E), indicating these genes are LSCC-selective PRKCI targets (see Table S3). Expression of PKCi-SOX2 target genes was not inhibited by ECT2 KD in H1299 cells, or H520 LSCC cells harboring 3q26 CNG, demonstrating that the PKCi-ECT2 signaling axis is not involved in their regulation (Figures S1C and S1D). Thus, PKCi-SOX2 signaling activates an extensive oncogenic transcriptional program in LSCC tumors, but not LADC tumors.
Identification of Direct PKCi-Dependent SOX2 Transcriptional Targets
Many of the identified PKCi-SOX2 target genes contain SOX2 binding motifs near their proximal promoters (Figure S2A), suggesting PKCi-SOX2 dependent transcriptional activation. SOX2 chromatin immunoprecipitation (ChIP)-qPCR revealed that SOX2 promoter occupancy is significantly reduced in PRKCI KD cells compared to NT cells in the majority (9/13) of these genes (Figure 2F). Two genes (NOTCH3 and ADCY3) exhibited SOX2 binding that was unaffected by PRKCI KD; two other genes (PARD6G and HEY1) exhibited no appreciable SOX2 binding in the presence or absence of PKCi (data not shown). PKCi-dependent SOX2 binding was validated by ChIP-qPCR using a second primer-probe set to each gene (Figure S2B). PKCi-dependent SOX2 promoter occupancy was also observed in H520 LSCC cells harboring PRKCI and SOX2 CNG (Figure 2G) but not in H2170 LSSC cells without PRKCI and SOX2 CNG (Figure 2H), or in A549 LADC cells (Figure 2I). PRKCI and SOX2 KD significantly inhibited expression of target genes in H1299 and H520 cells (Figures 2J and 2K) but had little or no effect on gene expression in H2170 or A549 cells (Figures 2L and 2M). Thus, PKCi preferentially regulates SOX2-dependent promoter binding and expression of 9 genes in cells harboring PRKCI and SOX2 CNG.
PKCi-SOX2 Regulated Genes Contribute to Transformed Growth of LSCC Cells
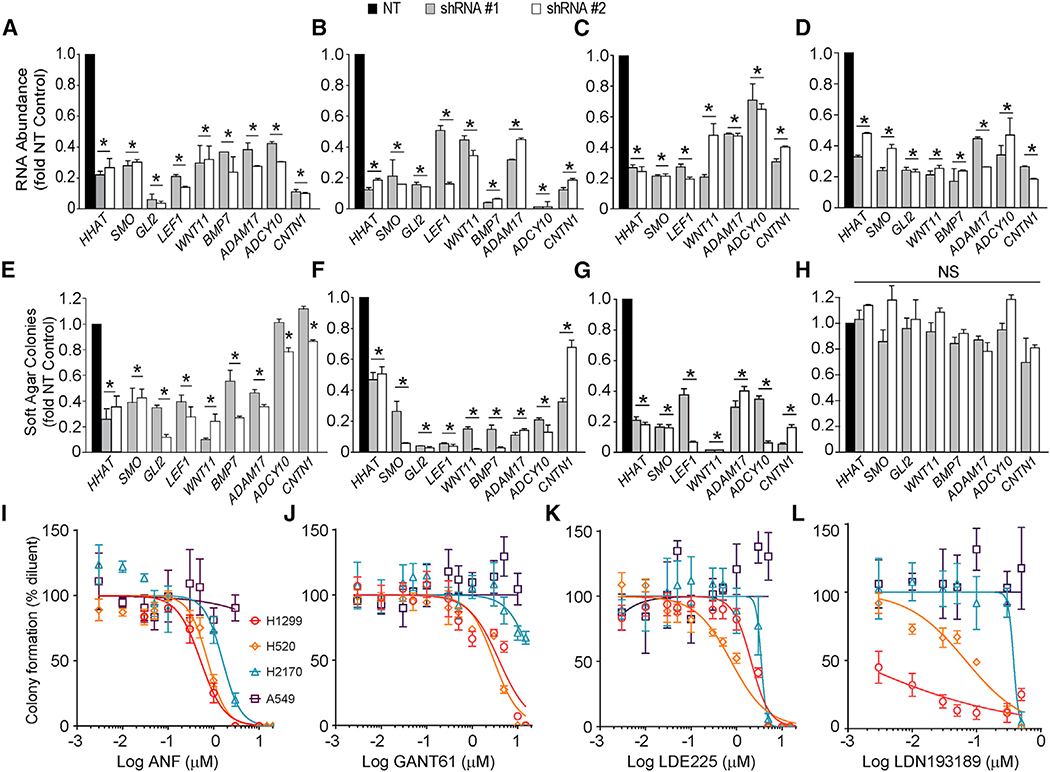
We next assessed the effect of shRNA-mediated knockdown of the 9 direct PKCi-SOX2 regulated genes (Figures 3A–3D) on transformed growth in H1299, H520, H2170, and A549 cells (Figures 3E–3H). In each case, shRNA-mediated KD significantly inhibited transformed growth of H1299, H520, and H2170 cells, but had little or no effect on A549 LADC cells. Interestingly, treatment of H1299 and H520 cells with the PKCi inhibitor Auranofin (ANF), the GLI inhibitor GANT61, the Smoothened inhibitor LDE225, or the BMP inhibitor LDN-193189 led to dose-dependent inhibition of transformed growth with IC50 values consistent with target inhibition (Figures 3I–3L; see also Table S4), whereas H2170 and A549 cells exhibited little or no response. Thus, these genes, and the signaling pathways within which they reside, are selectively involved in transformed growth of cells harboring 3q26 CNG.
Figure 3. Effect of Silencing PKCi-SOX2 Gene Targets on Transformed Growth of LSCC Cells.
(A–D) shRNA-mediated knockdown of the indicated genes in H1299 (A), H520 (B), H2170 (C), and A549 (D) cells using two independent shRNA constructs. Results are presented as fold of NT cells ± SD and are representative of three independent experiments. n = 3. *p < 0.05 compared to NT cells.
(E–H) Effect of shRNA-mediated knockdown of the indicated genes on soft agar growth of H1299 (E), H520 (F), H2170 (G), and A549 (H) cells. Results are presented as fold of NT cells ± SD and are representative of three independent experiments. n = 3. *p < 0.05 compared to NT cells. NS, not significant. (I–L) Effect of ANF (I), GANT61 (J), LDE225 (K), and LDN-193189 (L) on soft agar growth in H1299, H520, H2170, and A549 cells. Results are normalized to diluent and represent mean ± SD; n = 5. Significance was assessed by unpaired two-tailed Student’s t test.
See also Table S4.
PKCi-SOX2 and PKCi-ECT2 Signaling Axes Are Activated in the Classical LSCC Subtype
To assess whether PKCi-SOX2 and PKCi-ECT2 signaling is active in primary LSCC tumors, we generated Eigengenes reflecting PKCi-SOX2 and PKCi-ECT2 signaling activity, respectively. A PKCi-SOX2 Eigengene consisted of the 9 direct PKCi- SOX2 transcriptional targets identified and characterized above. Since PKCi-ECT2 signaling drives rDNA transcription, and ECT2 expression correlates strongly with expression of ribosomal RNA processing genes (Justilien et al., 2017, 2019), our PKCi-ECT2 Eigengene consisted of the 12 ribosomal RNA processing genes that most highly correlate with PRKCI and ECT2 expression the TCGA LSCC dataset (top 10%; indicated in bold in Table S5). Analysis of the TCGA LSCC dataset (n = 478) revealed that PKCi-SOX2 Eigengene scores distribute non-randomly across the four distinct LSCC subtypes (Primitive, Classical, Secretory, and Basal) as defined by molecular profiling (Hammerman et al., 2012; Figure 4A), with Classical LSCC subtype tumors exhibiting significantly higher PKCi-SOX2 Eigengene scores than other subtypes (Figure 4B). PKCi-ECT2 Eigengene scores also distribute non-randomly (Figure 4C), with Classical and Primitive LSCC subtypes exhibiting higher PKCi-ECT2 Eigengene scores than other subtypes (Figure 4D). Interestingly, PRKCI, SOX2, and ECT2 expression is significantly higher in the Classical LSCC subtype, consistent with the activation of the PKCi-SOX2 and PKCi-ECT2 signaling axes in these tumors (Figures S3A–S3C). Analysis of an independent LSCC gene expression dataset (Wilkerson et al., 2010) revealed a similar enrichment of PKCi-SOX2 and PKCi-ECT2 Eigengene scores in Classical and Primitive LSCC subtype tumors, respectively, providing independent validation (Figures S3D and S3E). Heatmap analysis revealed that the 9 PKCi-SOX2 Eigengene genes are significantly and specifically overexpressed in Classical LSCC tumors (162/478 [34%] of LSCC tumors), whereas the 12 PKCi-ECT2 Eigengene genes are overexpressed in both Primitive and Classical LSCC subtypes (Figure 4E). Analysis of a gene expression dataset of bronchial premalignant lesions (PMLs) (n = 431) that were classified into four molecular subtypes: Secretory, Normal-like, Inflammatory, and Proliferative (Beane et al., 2019), revealed significantly higher PKCi-SOX2 Eigengene scores in Proliferative PMLs compared to other subtypes (Figure 4F), whereas PKCi-ECT2 Eigengene scores did not differ significantly across PML subtypes (Figure 4G). Thus, PKCi-SOX2 signaling is preferentially activated in Proliferative PMLs, which exhibit a strong association with smoking behavior, increased histological progression to dysplasia, an elevated propensity to progress to LSSC, and genomic features consistent with the Classical subtype of LSCC tumors (Hammerman et al., 2012).
Figure 4. PKCi, SOX2, and ECT2 Expression Profiling of Human LSCC Tumors.
(A) Distribution of PKCi-SOX2 Eigengene scores across LSCC molecular subtypes. Data presented as percentage of tumors of each indicated subtype within the indicated Eigengene score range. n, number of tumors in the indicated Eigengene score range.
(B) Violin plot showing PKCi-SOX2 Eigengene scores across LSCC molecular subtypes (Primitive, n = 69; Classical, n = 162; Secretory, n = 115; Basal, n = 132). Middle vertical bar indicates range; box shows the median and 75% and 25% confidence intervals. Width indicates frequency. The asterisk (*) indicates a significant difference between Classical subtype and other subtypes. Significance was assessed by ANOVA test. *p < 4.8 × 10−18.
(C) Distribution of PKCi-ECT2 Eigengene scores across molecular subtypes of LSCC tumors. Data presented as percentage of tumors of each indicate subtype within the indicated Eigengene score range. n, number of tumors in the indicated Eigengene score range.
(D) Violin plot showing the distribution of PKCi-ECT2 Eigengene scores across LSCC molecular subtypes (Primitive, n = 69; Classical, n = 162; Secretory, n = 115; Basal, n = 132). Middle vertical bar indicates range; box shows the median and 75% and 25% confidence intervals. Width indicates frequency. The asterisk (*) indicates statistically significant difference between Primitive and Classical subtype with other subtypes. Significance was assessed by ANOVA test. *p < 1.6 × 10−18.
(E) Heatmap showing 3q26 CNG and expression of the 9 PKCi-SOX2 target genes and the 12 genes in the PKCi-ECT2 Eigengene across molecular LSCC subtypes.
(F) Violin plot showing PKCi-SOX2 Eigengene scores across four molecular subtypes of bronchial premalignant lesions (PMLs; Secretory, n = 136; Normal-like, n = 106; Inflammatory, n = 83; Proliferative, n = 106). Middle vertical bar indicates range; box shows the median and 75% and 25% confidence intervals. Width indicates frequency. The asterisk (*) indicates statistically significant difference between Proliferative subtype and other subtypes. Significance was assessed by ANOVA test. *p < 0.05.
(G) Violin plot showing PKCi-ECT2 Eigengene scores across molecular subtypes of bronchial premalignant lesions (PMLs; Secretory, n = 136; Normal-like, n = 106; Inflammatory, n = 83; Proliferative, n = 106). Middle vertical bar indicates range; box shows the median and 75% and 25% confidence intervals. Width indicates frequency. Significance was assessed by ANOVA test. NS, no significant differences between subtypes.
Coordinate Overexpression of PRKCI, SOX2, and ECT2 Is Necessary and Sufficient to Drive LSCC Tumorigenesis
Teixeira et al. (2019) performed genomic profiling of 39 human pulmonary carcinoma in situ (CIS) lesions and classified them as either progressive or regressive based on whether they subsequently progressed to LSCC. Interrogation of these data revealed that 3q26 CNG (Figure 5A), and elevated expression of PRKCI, SOX2, and ECT2 (Figures 5B–5D), are significantly associated with progressive CIS lesions. Interestingly, progressive CIS lesions also exhibited a significantly higher incidence of TP53 mutations (28/29) than regressive lesions (5/10), whereas mutations in other tumor suppressors were detected with much lower prevalence and did not significantly associate with progressive versus regressive lesions (Figure 5A). Interestingly, three of the regressive CIS lesions harboring a TP53 mutation and 3q26 CNG ultimately progressed to LSCC after the preset clinical endpoint, making the correlation between these genetic alterations and oncogenic progression even stronger. Analysis of the TCGA LSCC dataset revealed that TP53 mutation is by far the most frequently mutated tumor suppressor gene in LSCC (~86% of tumors), rivaling in frequency, and occurring concomitantly with, PRKCI, SOX2, and ECT2 CNGs (Figure S4A). Like 3q26 CNG (Figure 1E), TP53 mutations are detected at equally high frequency in early- and late-stage LSCC tumors (Figure S4B), consistent with the early acquisition of TP53 mutation in progressive premalignant CIS lesions (Figure 5A).
Figure 5. Effect of PRKCI and SOX2 Overexpression on LBSC Transformation.
(A) Heatmap showing the distribution of chromosome 3q26 CNGs and mutations in tumor suppressor genes TP53, CDKN2A/CDKN2B, PTEN, KEAP1, and STK11 in progressive and regressive CIS lesions. Blue bars, CIS lesions with 3q26 copy number gain. Red bars, CIS lesions with mutation. White bars, CIS lesions without alterations. Significance was assessed by chi-square analysis. *p < 0.05. NS, not significant.
(B–D) Expression of PRKCI (B), SOX2 (C), and ECT2 (D) mRNA in regressive (Reg.) and progressive (Prog.) CIS lesions. *p < 0.05 compared to regressive CIS lesions.
(E) qPCR analysis for expression of Trp63, Ngfr, Krt14, Aqp3, Krt5, Sox2, Prkci, and Ect2 in isolated mouse lung basal stem cells (LBSCs). RNA abundance expressed as fold of total lung epithelial cells (TLECs). Values represent mean ± SEM and are representative of three independent experiments. n = 3.
(F) Effect of Ad-Cre on expression of Trp53 in LBSCs. qPCR of mRNA (lower panel) and genomic DNA (upper panel) for Trp53 RNA and DNA, respectively, in LBSCs treated with Ad null (Null) or Ad-Cre (Cre) virus. Trp53 mRNA abundance normalized to mouse ubiquitin C. Values represent mean ± SEM and are representative of three independent experiments. n = 3. unrec., unrecombined; rec., recombined; ND, not detected.(G) Overexpression of SOX2 and PRKCI in LBSCs. Immunoblot analysis of LBSCs transduced with the indicated lentivirus for PKCi and SOX2. Actin is included as a loading control. Lysate from an equivalent number of H520 cells served as a comparator.
(H–J) Effect of Trp53 loss and overexpression of SOX2 and PRKCI on LBSC morphology (H), sphere number (I), and sphere size (J) in three-dimensional Matrigel culture. Results in (H) are representative bright-field and immunofluorescence images stained for E-cadherin (red) and DAPI (blue). Scale bars, 100 μm. Results in (I) are normalized to Trp53+/+ and represent mean ± SEM; n = 6. Results in (J) represent mean ± SEM; n > 50; *p < 0.05 comparing to Trp53+/+ cells; **p < 0.05, comparing to Trp53−/− control vector cells; ***p < 0.05 comparing S/Trp53−/− LBSCs. V, control vector.
(K) Immunoblot analysis of LBSCs expressing SOX2 (S) or SOX2 and PRKCI (S, P) for total SOX2 and phospho-T118 SOX2.
(L) qPCR analysis of Trp53−/− and S/P/Trp53−/− LBSCs for expression of PKCi-SOX2 target genes. Results represent mRNA abundance relative to Trp53−/− LBSCs ± SEM and are representative of three independent experiments. n = 3. *p < 0.05 compared to Trp53−/− LBSCs. S, SOX2; P, PRKCI. Significance was assessed by unpaired two-tailed Student’s t test.
See also Figure S4.
We next established an ex vivo model to directly assess whether combined Trp53 loss, and PRKCI, SOX2, and ECT2 overexpression, is sufficient to drive transformation of mouse lung basal stem cells (LBSCs), a prominent cell of origin for LSCC (Ferone et al., 2016; Hong et al., 2004; Rock et al., 2009; Shaykhiev et al., 2013). As expected, LBSCs from Trp53fl/fl mice (Hegab et al., 2012) express elevated basal cell markers Tumor Protein 63 (Tp63), Nerve Growth Factor Receptor (Ngfr), Keratin 14 (Krt14), Aquaporin 3 (Aqp3), and Keratin 5 (Krt5) compared to total lung epithelial cells (TLECs) (Figure 5E). LBSCs also express higher Sox2, and similar Prkci and Ect2, levels compared to TLECs.
Adenovirus-Cre recombinase (Ad-Cre) induced loss of Trp53fl/fl alleles and Trp53 expression, mimicking the TP53 inactivation observed in progressive CIS lesions and LSCC tumors (Figure 5F). Transduction of Trp53−/− LBSCs with SOX2 alone, or together with PRKCI, achieved SOX2 and PKCi protein levels comparable to H520 cells harboring 3q26 CNG (Figure 5G). Interestingly, Trp53−/− and Ad Null-treated Trp53fl/fl LBSCs form spheres of similar morphology in three-dimensional Matrigel culture (Figure 5H). However, Trp53−/− LBSCs cultures exhibit an increase in sphere number and size compared to Ad Null-treated Trp53fl/fl LBSC cultures (Figures 5I and 5J), consistent with the established role of Trp53 loss in LBSC proliferation ex vivo (Jeong et al., 2017). Indeed, we find that Ad Null-treated Trp53 fl/fl LBSCs fail to grow after serial passaging, whereas Trp53−/− LBSCs continue to grow as non-transformed spheres upon serially passaging (data not shown).
Overexpression of SOX2 in Trp53−/− LBSCs (S/Trp53−/−LBSCs) leads to a small but significant increase in sphere number and size (Figures 5I and 5J) while retaining morphology similar to Ad-Null and Trp53−/− LBSCs (Figure 5H). SOX2/PRKCI/Trp53−/− (S/P/Trp53−/−) LBSCs exhibit a further increase in sphere number and size (Figures 5I and 5J), accompanied by a profound change in morphology from a single layer of highly polarized epithelial cells, to disorganized cellular masses exhibiting loss of cell polarity (Figure 5H). S/P/Trp53−/− LBSCs express elevated phospho-Thr118-SOX2 (Figure 5K) and increased expression of the 9 validated direct PKCi-SOX2 transcriptional targets (Figure 5L). Thus, S/P/Trp53−/− LBSCs exhibit enhanced proliferation, morphological transformation, and activated PKCi-SOX2 signaling consistent with that observed in human Proliferative PMLs, progressive CIS lesions, and Classical LSCC tumors.
ECT2 Enhances Proliferation of PRKCI-, SOX2- Transformed LBSCs
Expression of ECT2 in S/P/Trp53−/− LBSCs (S/P/E/Trp53−/−LBSCs) to levels comparable to H520 cells (Figure 6A), caused an increase in sphere number and size compared to S/P/Trp53−/− LBSCs, while retaining morphology induced by PRKCI expression (Figures 6B–6D). S/P/E/Trp53−/− LBSCs exhibit increased abundance of Mmp10 and 45S ribosomal RNA (Figure 6E) and elevated levels of PKCi-induced phospho-Thr328-ECT2 (Figure 6F), biochemical changes consistent with activation of oncogenic PKCi-ECT2 signaling (Justilien et al., 2011, 2017, 2019). Interestingly, overexpression of ECT2 in S/Trp53−/− LBSCs (Figure S5A) led to a small but significant increase in sphere size, but no change in sphere number or morphology (Figures S5B–S5D), indicating that full morphological transformation and enhanced transformed growth of LBSCs occurs only when all three cooperating 3q26 oncogenes are coordinately overexpressed.
Figure 6. Effect of Overexpressing ECT2 on SOX2/PRKCI/Trp53−/− LBSCs In Vitro.
(A) Expression of ECT2 in S/P/Trp53−/− LBSCs. Immunoblot analysis for ECT2, PKCi and SOX2 in the indicated LBSCs. Actin is included as a loading control. Lysate from an equivalent number of H520 cells served as a comparator.
(B) Effect of overexpressing ECT2 on the growth and morphology of LBSCs expressing SOX2 and PKCi grown in three-dimensional Matrigel culture. Representative bright-field and immunofluorescence images stained for E-cadherin (red) and DAPI (blue) are shown. Scale bars, 100 μm.
(C and D) Effect of overexpressing ECT2 on sphere number (C) and size (D). Results in (C) are normalized to S/P/Trp53−/− LBSCs and represent mean ± SEM; n = 6; *p < 0.05. Results in (D) represent mean ± SEM; n < 50; *p < 0.05.
(E) Effect of overexpressing ECT2 on expression of Mmp10 and ribosomal 45S RNA in LBSCs. Results represent RNA abundance relative to Trp53−/− LBSCs ± SEM and are representative of three independent experiments. n = 3. *p < 0.05; **p < 1.5 3 10−5 compared to Trp53−/− LBSCs.
(F) Immunoblot analysis showing expression of total ECT2 and phospho-Thr328 ECT2 in the indicated LBSCs.
(G–I) Effect of expressing SOX2 and ECT2 phosphorylation site mutants on the morphology (G), number (H), and size (I) of LBSCs. Results in (H) were normalized to Vector/Trp53−/− LBSCs and represent mean ± SEM; n = 5; *p < 0.002, **p < 0.001. Results in (I) represent the mean ± SEM; n > 85; *p < 0.0001 compared to Vector control; **p < 0.0001 compared to SOX2, PRKCI, and ECT2. Scale bars, 100 μm.
(J) Effect of silencing PKCi-SOX2 gene targets on S/P/E/Trp53−/− LBSC growth. Two independent shRNA constructs were used to knock down the indicated genes in S/P/E/Trp53−/− LBSC. Results are presented as fold NT control cells ± SD and are representative of three independent experiments. n = 3. *p < 0.05 compared to NT cells.
(K) Effect of ANF on sphere formation in S/P/E/Trp53—/— LBSCs. Results were normalized to DMSO diluent treatment and represent mean ± SD; n = 4.
(L) Effect of GANT61, LDN193189, and LDE225 on sphere formation in S/P/E/Trp53−/− LBSCs. S/P/E/Trp53−/− LBSCs were treated with 10 μM GANT61, 300 nM LDN193189, and 3 μM LDE225, respectively. Results were normalized to diluent treatment and represent mean ± SD; n = 3. *p < 0.05. S, SOX2; P, PRKCI; E, ECT2; ST118A, SOX2 T118A phospho mutant; ET328A, ECT2 T328A phospho mutant. Significance was assessed by unpaired two-tailed Student’s t test.
See also Figure S5.
To assess the role of PKCi-mediated SOX2 and ECT2 phosphorylation in LBSC transformation, we utilized lentiviruses expressing previously characterized SOX2 and ECT2 mutants, SOX2T118A (ST118A) and ECT2T328A (ET328A), that cannot be phosphorylated by PKCi (Justilien et al., 2011, 2014; Figure 6G). As expected, S/P/E/Trp53−/− LBSCs produced large, proliferative cell masses exhibiting loss of cell polarity, whereas ST118A/P/E/Trp53−/− LBSCs produce polarized cysts resembling S/Trp53−/− LBSCs, but of increased size comparable to S/P/E/Trp53−/− LBSCs (Figures 6G–6I). In contrast, S/P/ET328A/Trp53−/− LBSCs produced spheres with morphology consistent with S/P/ E/Trp53−/− LBSCs but of a smaller size consistent with S/P/ Trp53−/− LBSCs. Thus, PKCi and PKCi-phosphorylatable SOX2 are required for increased sphere formation and loss of polarity characteristic of S/P/E/Trp53−/− LBSCs, whereas ECT2 is largely dispensable for these phenotypes. Conversely, PKCi and PKCi-phosphorylatable ECT2 are required for the enhanced growth exhibited by S/P/E/Trp53−/− LBSCs, a phenotype that is less dependent upon wild-type SOX2. Thus, the PKCi-SOX2 and PKCi-ECT2 signaling axes play critical but distinct roles in the transformed phenotype of S/P/E/Trp53−/− LBSCs.
We next used two independent lentiviral shRNA constructs to knock down of each of the nine PKCi-SOX2 target genes (Figure S5E) and assessed the effect on transformed growth of S/P/E/Trp53−/− LBSCs (Figure 6J). In each case, target gene knockdown induced a significant decrease in the number of S/P/E/Trp53−/− LBSC spheres, indicating that each of these PKCi-SOX2 target genes contributes to mouse LBSC transformation. Treatment of S/P/E/Trp53−/− LBSCs with ANF revealed that these cells are extremely sensitive to PKCi inhibition, exhibiting an IC50 of 10 nM (Figure 6K). Furthermore, treatment of S/P/E/Trp53−/− LBSCs with GANT61, LDE225, and LDN- 193189 at concentrations that inhibited transformed growth of LSCC cells exhibiting 3q26 CNG (Figures 3J–3L), significantly inhibited sphere growth (Figure 6L).
PRKCI, SOX2, and ECT2 Drive LSCC Tumor Formation In Vivo
We next assessed whether expression of PRKCI, SOX2, and ECT2 is sufficient to drive LSCC tumor formation in vivo. For this purpose, S/P/E/Trp53−/− and S/Trp53−/− LBSCs expressing a Cre-activated luciferase allele (LSL-lucRosa26) were in- jected orthotopically into the lungs of syngeneic mice using established protocols (Ali et al., 2016; Justilien et al., 2014, 2017, 2019). IVIS imaging revealed that S/Trp53−/− LBSCs form small, slow-growing lesions whose growth plateaus after approximately 4 weeks (Figure 7A). In contrast, S/P/E/Trp53−/− LBSCs generate larger, exponentially growing tumors (Figure 7A) that are clearly detectable by both IVIS and micro-computed tomography (mCT) as distinct intrapulmonary masses (Figure 7B). Histological analysis revealed that S/Trp53−/− LBSCs form small benign cysts consisting of a single epithelial cell layer surrounding largely empty lumens reminiscent of S/Trp53—/— LBSC cultures ex vivo (Figure 7C, upper panel, left). In contrast, S/P/E/ Trp53—/— LBSC tumors exhibited classical LSCC pathology characterized by nuclear atypia, loss of cyst-like glandular architecture, prominent intercellular bridges, and prominent extracellular keratin pearls (Figure 7C, middle panel, left). All S/P/E/Trp53—/— LBSC tumors (14 tumors from three independent experiments) exhibited similar LSCC pathology that was clearly distinct from that of mouse KP LADC tumors, which consisted of solid masses of polygonal cells with frequent nuclear atypia, abnormal mitoses, and multinucleated giant cells characteristic of classical, solid LADC (Figure 7C, lower panel, left). S/Trp53−/− and S/P/E/Trp53−/− lesions retain expression of SOX2, and the basal cell markers TP63 and KRT5, confirming their squamous nature (Figure 7C, upper and middle panels). Interestingly, S/Trp53−/− lesions exhibit low KI67 staining consistent with limited proliferative potential, whereas S/P/E/ Trp53−/− tumors show elevated KI67 staining indicative of aggressive growth (Figure 7C, upper and middle panels, right).
Figure 7. Effect of PRKCI, SOX2, and ECT2 Expression on LBSC Transformation In Vivo.
(A) Growth of S/Trp53−/− and S/P/E/Trp53−/− LBSCs as lung orthotopic tumors in syngeneic mice. Results represent mean bioluminescence flux ± SEM. n = 5. Significance was assessed by unpaired two-tailed Student’s t test. *p < 0.05 compared to S/Trp53−/− LBSCs.
(B) Representative S/P/E/Trp53−/− lung orthotopic LBSC tumor sequentially imaged by IVIS and μCT at 8 weeks post-inoculation. The bioluminescence and μCT datasets were co-registered in 3D using the Living Image 4.1 soft ware.
(C) Histological and immunohistochemical (IHC) analysis of S/Trp53—/— and S/P/E/Trp53—/— LBSC tumors. Sections from S/Trp53−/− and S/P/E/Trp53−/− LBSC tumors stained with hematoxylin/eosin (H&E), and by IHC for SOX2, TP63, KRT5, and KI67. Analysis of LSL-KrasG12D/Trp53—/— (KP) LADC tumors is included for comparison. Scale bars, 50 μm.
(D) IHC for PKCi-SOX2 target genes. H&E and IHC staining for WNT11, GLI2, LEF1, and PKCi, in S/Trp53−/− LBSC, S/P/E/Trp53—/— LBSC and KP tumors. S, SOX2; P, PRKCI; E, ECT2. Scale bars, 50 μm.
In contrast, KP LADC tumors stain negative for the squamous markers SOX2, TP63, or KRT5, but stain positively for KI67, consistent with their highly proliferative nature (Figure 7C, lower panels).
We next characterized S/Trp53−/−, S/P/E/Trp53−/− and KP lesions for expression of direct PKCi-SOX2 transcriptional targets. Immunohistochemistry (IHC) revealed that S/Trp53−/− lesions express low but detectable levels of WNT11, GLI2, and LEF1, and moderate levels of PKCi (Figure 7D, upper panels). S/P/E/Trp53−/− LSCC tumors express elevated levels of each of these markers, consistent with activation of the PKCi-SOX2 transcriptional program (Figure 7D, middle panels). In contrast, KP LADC tumors expressed low levels of WNT11, GLI2, and LEF1, despite expressing elevated PKCi (Figure 7D, lower panels). These data support the conclusion that the PKCi-SOX2 transcriptional program identified here is selectively activated in LSCC tumors but not in LADC tumors.
DISCUSSION
We have characterized extensive biochemical and functional links between three 3q26 candidate driver genes, PRKCI, SOX2, and ECT2, in LSCC biology (Fields et al., 2016; Justilien et al., 2011, 2014, 2019). Here, we report that CNG and coordinate overexpression of PRKCI, ECT2, and SOX2 are common and early events in LSCC tumor development. Importantly, we found that PRKCI, SOX2, and ECT2 overexpression in the context of Trp53 loss is sufficient to transform LBSCs, a major cell of origin for LSCC, and drive formation of tumors with pathological and biochemical characteristics of LSCC. Our data provide compelling evidence that PRKCI, SOX2, and ECT2 are cooperating oncogenic drivers of LSCC tumorigenesis, and that PRKCI-, SOX2-, and ECT2-driven LSCC tumor formation is dependent upon PKCi-SOX2 and PKCi-ECT2 signaling pathways that are activated by direct PKCi-mediated SOX2 and ECT2 phosphorylation.
SOX2 is a well-established lineage-restricting oncogene (Bass et al., 2009; Hussenet et al., 2010) that can induce squamous tumors in transgenic mouse models (Ferone et al., 2016; Fukazawa et al., 2016; Mollaoglu et al., 2018; Tata et al., 2018; Watanabe et al., 2014). SOX2 transcriptional targets have been identified through ChIP-sequencing (ChIP-seq) analyses, and it is clear that SOX2 transcriptional programming is critical for its oncogenic activity. However, while a few SOX2 target genes have been functionally validated, including EVT4, CDKN1A, and CXCL5 (Fukazawa et al., 2016; Mollaoglu et al., 2018; Watanabe et al., 2014), the role of many other SOX2 targets in SOX2-dependent squamous transformation has not been verified. We have identified 9 direct PKCi-dependent SOX2 gene targets and functionally characterized their role in LSCC trans- formation. Interestingly, many of these PKCi-SOX2 target genes were also identified as potential SOX2 targets in the studies cited above, suggesting that they may be major drivers of SOX2 oncogenesis in multiple contexts. The direct PKCi- SOX2 transcriptional targets identified herein reside in three oncogenic pathways, Hh, Wnt, and Notch, each of which can be pharmacologically targeted with small molecular inhibitors. In this regard, LSCC tumor cells exhibiting PKCi-SOX2-driven activation of these pathways are highly responsive to the growth-inhibitory effects of PKCi, SMO, GLI, and BMP inhibitors. Finally, genomic signatures reflective of PKCi-SOX2 and PKCi-ECT2 signaling activity are enriched in the Classical LSCC subtype, suggesting that Classical LSCC tumors may be responsive to these pathway inhibitors. Our current results significantly advance our molecular understanding of oncogenic SOX2 transcriptional programming, and identify potential pharmacologic vulnerabilities associated with SOX2-driven LSCC tumors that could improve therapeutic treatment of these aggressive tumors.
Genetically engineered mouse models of SOX2-dependent squamous tumorigenesis demonstrate that SOX2 can collaborate with loss of multiple tumor suppressor genes, including Pten, Cdkn1a, Cdkn2a/b, Lkb1, Keap1, Trp53, and Nkx2–1 (Ferone et al., 2016; Fukazawa et al., 2016; Jeong et al., 2017; Mollaoglu et al., 2018; Mukhopadhyay et al., 2014; Tata et al., 2018; Watanabe et al., 2014; Xu et al., 2014), and can induce tumors with squamous histology in multiple cells of origin through trans differentiation. These data attest to the powerful lineage-restricted oncogenic potential of Sox2 and may provide critical insights into the genetic and biology of LADC with squamous or adenosquamous characteristics. The proportion of early human squamous lesions with PTEN and CDKN2A/B alterations is relatively small, and their loss is not significantly associated with progressive versus regressive squamous CIS lesions. Thus, mutation of these tumor suppressors, while clearly relevant to LSCC, may contribute preferentially to LSCC progression rather than initiation.
TP53 is the most frequently mutated tumor suppressor in LSCC and is significantly associated with 3q26 CNG both in LSCC tumors and progressive CIS lesions, suggesting that TP53 loss plays a critical role in LSCC initiation. Interestingly, Trp53 has been shown to control proliferation and differentiation of progenitor cells including regional airway stem cells (McConnell et al., 2016) and inactivation of Trp53 promotes LBSC self-renewal in vivo (Jeong et al., 2017). Our newly described model of LSCC tumor initiation, driven solely by collaboration of three key 3q26 oncogenes and loss of Trp53, provides a valuable complement to previously described LSCC models, and represents a genetically tractable model of the Classical subtype of LSCC, the most prevalent LSCC molecular subtype.
The recurrent 3q26 amplicon contains a number of additional potential drivers or modifier genes that may be of biologic and therapeutic relevance to LSCC tumorigenesis (Fields et al., 2016). Further studies will be necessary to determine the contributory role of other 3q26 genes in LSCC tumorigenesis (Fields et al., 2016). Our ex vivo LSCC model should prove useful in identifying and characterizing the involvement of additional 3q26 genes in LSCC tumorigenesis. A major advantage of our model is that it allows precise, temporally controlled targeting of multiple gene manipulations exclusively to a defined LSCC cell of origin, LBSCs. Our model also facilitates biochemical and genetic dissection of the roles of specific genetic alterations, and their associated signaling pathways, in LSCC initiation and progression ex vivo, and in tumor initiation and maintenance in syngeneic mice in vivo. Finally, our genetically tractable model of 3q26-driven LSCC provides a platform for pre-clinical evaluation of new therapeutic strategies targeting specific molecular signaling mechanisms involved in LSCC tumorigenesis.
STAR⋆METHODS
LEAD CONTACT AND MATERIALS AVAILABILITY
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Dr. Alan P. Fields (Fields.Alan@mayo.edu). All unique/stable reagents generated in this study are available from the Lead Contact with a completed Materials Transfer Agreement.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Human Cell lines
Human lung carcinoma cell lines (H1299 male, H520 male, H2170 male and A549 male) were obtained from the American Type Culture Collection and maintained in low passage culture as recommended. Cells were grown as oncospheres in serum-free DMEM-F12 (1:1) medium (Life Technologies) and maintained in ultra-low attachment flasks (Corning, NY). Growth media was modi- fied by adding 50 mg/ml insulin and 0.4% Albumin Bovine Fraction V from (Sigma-Aldrich St. Louis, MO), N2 Plus Media Supplement and B-27 Supplement (Life Technologies), 20 mg/ml EGF and 10 mg/ml basic FGF purchased from (PeproTech, Rocky Hill, NJ). All cultures were maintained in a humidified tissue culture incubator at 37°C in 5% CO2.
Establishment of ex vivo LBSC cultures
Trp53 conditional null mice (Trp53fl/fl) (8~10 weeks old; both male and female) were anesthetized with ketamine/xylazine by intraperitoneal injection. The mice were exsanguinated and the trachea was isolated in cold DMEM:F12 2X P/S/F media and opened to expose the lumen using dissecting scope. The trachea was incubated in Dispase (16U/ml) for 40 min followed by DNase I (0.5mg/ml) for 30 min at room temperature. The digested tracheas were transferred to a dish containing fresh media. Using 2 pair of forceps, sheets of epithelial cells were stripped off from digested trachea and subsequently trypsinized to obtain single cell suspension. Cell suspensions were filtered through 40 micron cell strainers (Falcon, BD). The Fc interactions were blocked with CD16/CD32 antibody for 15 min at room temperature and stained with TROP2 and CD49f antibodies and sorted for TROP2+/ CD49f+ LBSCs. The LBSCs were treated with Adenovirus-Cre recombinase to remove Trp53f/f alleles and grown embedded in 50% Matrigel for approximately 21 days. After establishing Trp53—/— LBSC cells, the cells were dissociated from Matrigel, treated with polybrene (2–5 mg/ml) and incubated with appropriate volume of recombinant lentivirus expressing SOX2, PRKCI or ECT2 cDNAs in serum free DMEM:F12 media for 6–8 hours on Matrigel in a humidified tissue culture incubator at 37°C in 5% CO2. The cells were re-embedded in Matrigel and maintained in 3D Matrigel culture for approximately 21 days. For sphere formation assays, cells were released from Matrigel (BD Biosciences), and dissociated into single cells by trypsinization. Cells were counted and resus- pended in 50% Matrigel and plated in Matrigel coated plates at a density of 1000 cells per well.
Orthotopic mouse LBSC tumor studies
S/Trp53—/—, and S/P/E/Trp53—/— LBSCs were injected orthotopically into the left lungs (final volume 50 ml) of 8~10 weeks old wild-type C57BL/6 mice (both male and female) using a 30-gauge needle. For IVIS and μCT imaging, mice were anaesthetized by isoflurane inhalation, administered 150 mg kg−1 d-luciferin by intraperitoneal injection and placed in a mouse imaging shuttle device that maintains the sedated mouse in a stationary position. At 8 minutes after luciferin injection, bioluminescence was measured using a Perkin Elmer IVIS imaging system (Caliper Life Sciences-Xenogen, Hopkinton, MA). For μCT imaging, mice were scanned for 4 min under isoflurane anesthesia using a small animal Quantum FX μCT (PerkinElmer) at 45 μm resolution, 90 kV, with 160 μA current. Images were acquired using PerkinElmer Quantum GX software. The bioluminescence optical and CT datasets were co-registered in 3-D using the Living Image 4.5 software. Mouse lung tissues were prepared for histology and hematoxylin/eosin staining was per- formed as previously described (Regala et al., 2009). All animal experiments were performed under an approved IACUC protocol of Mayo Clinic.
METHOD DETAILS
Total Lung Epithelial Cell (TLEC) Cultures
Mice were anesthetized with ketamine/xylazine by intraperitoneal injection. The trachea was isolated, cannulated and the lungs perfused with 10ml 0.9% saline. Dispase (3 ml) was injected into the lung through the trachea followed by 0.5 mL agarose (45°C). Lungs were immediately covered with ice for 2 min, removed from the mice, and incubated in 3 mL dispase for 45 min at room tem- perature. Lungs were subsequently transferred to tissue culture dishes with HEPES-buffered DMEM (100 U/ml DNase I per 7 ml), and lung tissue was gently teased into small pieces. Cell suspensions were filtered through cell strainers and red blood cells were lysed by Ammonium chloride solution. Cells were further purified through CD45/CD31 negative selection as described previously (Regala et al., 2009). Total lung epithelial cells were resuspended and cultured in Matrigel-coated plates.
Histology, Immunohistochemistry and Immunofluorescence
Mice were sacrificed, exsanguinated, and lungs processed for histologic and immunohistochemical analysis as previously described (Regala et al., 2009). Briefly, the tissue was deparaffinized by placing slides into three changes of xylene and rehydrated in a graded ethanol series. The rehydrated tissue samples were rinsed in water and subjected to antigen retrieval in citrate buffer (pH 6.0). Slides were treated with 3% H2O2 for 5 minutes to reduce endogenous peroxidase activity and washed with PBS containing 0.5% (w/v) Tween 20 and incubated with the indicated antibodies: SOX2, TP63, KRT5, KI67, WNT11, GLI2, LEF1 and PKCi. Slide images were captured and analyzed using a ScanScope scanner and ImageScope software (Aperio Technologies).
For immunofluorescence staining, spheres were released from Matrigel, fixed in 4% paraformaldehyde and embedded in paraffin. Paraffin embedded spheres were sectioned, mounted on slides and deparaffinized by placing slides into three changes of xylene and rehydrated in a graded ethanol series. The rehydrated sections were rinsed in water and subjected to antigen retrieval in citrate buffer (pH 6.0) followed by permeabilization with 0.1% Triton X-100 in PBS, blocked with 1% BSA, glycine and 0.1% Tween 20 in PBS, and incubated with E-cadherin (1:500) diluted in 1% BSA and 0.1% Tween 20 in PBS at 4C overnight. Sections were washed 3 times with PBS and incubated with Alexa Fluor 594 secondary antibody (1:500) diluted in 1% BSA and 0.1% Tween 20 for 1hour at room temperature. Sections were then washed 3 times in PBS and coverslipped Prolong Gold anti-fade mounting medium containing 4, 6-diamidino-2-phenylindole (DAPI). Images were captured using an Olympus IX71 inverted microscope (Olympus) with a 10X objective).
Immunoblot protein expression assay
For protein expression analysis, total cell lysates were resolved using gradient SDS-PAGE (Novex, Life Technologies, Carlsbad, CA) and transferred to polyvinylidene difluoride membranes (Immobilon-P; Millipore). Membranes were incubated with the appropriate primary antibodies followed by incubation with HRP-conjugated secondary antibodies purchased from Santa Cruz Biotechology (Santa Cruz, CA). Proteins bands were visualized by chemiluminescence detection (Perkin-Elmer Life Sciences) using KODAK BioMax MR Film (Carestream Health, Rochester, NY).
Lentiviral RNAi Constructs and Transfections
Lentiviral vectors containing human and mouse NT, PKCi, SOX2, ECT2 and potential targets shRNA were obtained from Sigma, packaged into recombinant lentivirus, and used to establish stable cell transfectants as described previously (Frederick et al., 2008). LSCC and LADC cells, as well as SOX2/PRKCI/ECT2/Trp53−/− LBSCs, were transduced with recombinant lentivirus and stable transfectants were selected for puromycin resistance. Knock down efficiency of PKCi, SOX2, ECT2 and potential targets was assessed by measuring the mRNA levels by QPCR. An NT control vector that does not recognize any mouse or human genes was used as a negative control. Sequences of all lentiviral shRNA constructs are listed in Key Resources Table.
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| β-Actin Antibody | Cell Signaling | Cat#4967; RRID: AB_330288 |
| Mouse Anti-PKC iota Monoclonal Antibody | BD Biosciences | Cat#610176; RRID: AB_397575 |
| Sox2 (D6D9) XP® Rabbit mAb (ChIP Formulated) | Cell Signaling | Cat#5024; RRID: AB_1904142 |
| Sox2 Antibody | Cell Signaling | Cat#2748; RRID: AB_823640 |
| Anti-ECT2 Antibody | Millipore Sigma | Cat#07–1364; RRID: AB_10805932 |
| Donkey anti-Goat IgG (H+L) Cross-Adsorbed Secondary Antibody, Alexa Fluor 568 | Thermo Fisher Scientific | Cat#A-11057; RRID: AB_2534104 |
| Donkey anti-Rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 488 | Thermo Fisher Scientific | Cat#A-21206; RRID: AB_2535792 |
| Goat Anti-P53 Polyclonal antibody | Santa Cruz Biotechnology | Cat#sc-6243-G; RRID: AB_653754 |
| p-SOX2 (T118) | 21st centry biochemicals | PMID: 24525231 |
| p-ECT2 (T328) | 21st centry biochemicals | PMID: 21189248 |
| Human p63/TP73L Antibody | R&D systems | Cat#AF1916; RRID:AB_2207174 |
| Cytokeratin 5 antibody | Abcam | Cat# ab52635; RRID:AB_869890 |
| Ki67 antibody | Abcam | Cat# ab15580; RRID:AB_443209 |
| normal rabbit IgG-B antibody | Santa Cruz Biotechnology | Cat#sc-2763; RRID: AB_737189 |
| Anti-Gli2 antibody | Abcam | Cat#ab26056; RRID:AB_2111901 |
| Anti-Wnt11 antibody | Abcam | Cat#ab31962; RRID:AB_883537 |
| Recombinant Anti-LEF1 antibody | Abcam | Cat#ab137872 |
| TROP2 / TACSTD2 Antibody (Extracellular Domain, CF) | LifeSpan | Cat#LS-C126415; RRID:AB_10833496 |
| Alexa Fluor® 647 anti-human/mouse CD49f Antibody | BioLegend | Cat#313610; RRID:AB_493637 |
| Purified Rat Anti-Mouse CD16/CD32 (Mouse BD Fc Block) | BD Biosciences | Cat#553142; RRID: AB_394657 |
| Biotin Rat Anti-Mouse CD31 | BD Biosciences | Cat#553371; RRID: AB_394817 |
| Biotin Mouse Anti-Mouse CD45.1 | BD Biosciences | Cat#553774; RRID: AB_395042 |
| Bacterial and Virus Strains | ||
| E. coli DH5α | Thermo Fisher Scientific | Cat#18265017 |
| Ad-CMV-iCre | Vector biolabs | Cat#1045 |
| Ad-CMV-Null | Vector biolabs | Cat#1300 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| TaqMan Fast Universal PCR Master Mix | Thermo Fisher Scientific | Cat#4352042 |
| PowerUp SYBR Green Master Mix | Applied Biosystems | Cat#A25778 |
| Protein G Mag Sepharose® Xtra | GE Healthcare | Cat#GE28–9670-70 |
| Pierce 16% Formaldehyde (w/v), Methanol-free | Thermo Scientific | Cat#28906 |
| Halt Protease Inhibitor Cocktail, EDTA-Free | Thermo Scientific | Cat#78437 |
| Pierce DTT (Dithiothreitol), No-Weigh Format | Thermo Scientific | Cat#A39255 |
| Micrococcal Nuclease | New England Biolabs | Cat#M0247S |
| RNase A, DNase and protease-free (10 mg/mL) | Thermo Scientific | Cat#EN0531 |
| Proteinase K Solution (20 mg/mL) | Thermo Scientific | Cat#AM2546 |
| SeaPlaque GTGTM Agarose | Lonza | Cat#50111 |
| Auranofin (ANF) | Santa Cruz Biotechnology | Cat#sc-202476A |
| GANT61 | Selleckchem | Cat#S8075 |
| LDE225 (Sonidegib, Erismodegib) | Selleckchem | Cat#S2151 |
| LDN-193189 | Selleckchem | Cat#S2618 |
| Dispase (1 U/mL) | STEMCELL Technologies | Cat#07923 |
| DNase I, RNase-free (1 U/μL) | Thermo Fisher Scientific | Cat#EN0521 |
| Bronchial Epithelial Cell Growth Medium (BEGM) | Lonza | Cat#CC-3170 |
| Matrigel Matrix | CORNING | Cat#354234 |
| Critical Commercial Assays | ||
| High-Capacity cDNA Reverse Transcription Kit | Thermo Fisher Scientific | Cat#4368813 |
| TURBO DNA-free Kit | Thermo Fisher Scientific | Cat#AM1907 |
| RNAqueous Total RNA Isolation Kit | Thermo Fisher Scientific | Cat#AM1912 |
| ViraPower lentiviral packaging mix | Invitrogen | Cat#K4975–00 |
| NucleoSpin® RNA Virus Kit | Silica-membrane technology | Cat#740956 |
| QIAprep Spin Miniprep Kit | QIAGEN | Cat#27106 |
| MinElute PCR Purification Kit | QIAGEN | Cat#28006 |
| QIAprep Spin Mininprep Kit | QIAGEN | Cat#27104 |
| Agilent RNA 6000 Nano Kit | Agilent | Cat#5067–1511 |
| Deposited Data | ||
| RNaseq of H1299 NT RNAi, H1299 PKCi RNAi and H1299 SOX2 RNAi cells | This paper | GSE141666 |
| Experimental Models: Cell Lines | ||
| NCI-H1299 | ATCC (the American Type Culture Collection) | CRL-5803; RRID: CVCL_0060 |
| NCI-H520 | ATCC (the American Type Culture Collection) | HTB-182; RRID: CVCL_1566 |
| NCI-H2170 | ATCC (the American Type Culture Collection) | CRL-5928; RRID: CVCL_1535 |
| A549 | ATCC (the American Type Culture Collection) | CCL-185; RRID: CVCL_0023 |
| Experimental Models: Organisms/Strains | ||
| Trp53fl/fl mice | The Jackson Laboratory | 008462 |
| KP mice | Alan Fields Laboratory | N/A |
| Oligonucleotides | ||
| See Table S6 | N/A | |
| Recombinant DNA | ||
| See Table S7 | N/A | |
| Software and Algorithms | ||
| cBioportal for Cancer Genomics | Memorial Sloan Kettering Cancer Center | http://www.cbioportal.org/ |
| SigmaPlot | Systat Software Inc. | https://systatsoftware.com/products/sigmaplot/ |
| Image-Pro Plus 7.0 | MEDIA CYBERNETICS | https://www.mediacy.com/imageproplus |
| Living Image software | PerkinElmer | https://www.perkinelmer.com/lab-products-and-services/resources/in-vivo-imaging-software-downloads.html#LivingImage |
| Gene Set Enrichment Analysis (GSEA), hallmark analysis (version h.all.v6.2) | Broad Institute | http://software.broadinstitute.org/gsea/index.jsp |
| Ingenuity Pathway Analysis (IPA) | QIAGEN Bioinformatics | https://www.qiagenbioinformatics.com/products/ingenuity-pathway-analysis/ |
| Transcription Factor Target Gene Database | Institute for Systems Bioloy | http://tfbsdb.systemsbiology.net/ |
| The MEME Suite: Motif-based sequence analysis tools | National Institutes of Health | http://meme-suite.org/ |
| GraphPad Prism 7 | GraphPad | https://www.graphpad.com/scientific-software/prism/ |
| Aperio ImageScope | Leica Biosystems | https://www.leicabiosystems.com/digital-pathology/manage/aperio-imagescope/ |
RNA sample preparation
Total RNA was extracted from three independent spheroid cultured H1299 NT RNAi, PKCi RNAi and SOX2 RNAi cells using the RNAqueous phenol-free total RNA isolation protocols (Ambion, Life Technologies, Grand Island, NY). Extracted RNA was subjected to DNase treatment to eliminate DNA contamination according to provider’s instructions using TURBO DNA-free™ (Ambion, Life Technologies, CA). Quality of RNA samples was determined using an Agilent Bioanalyzer and only samples with RINs > 9.0 were used for genomic sequencing analysis. Sequencing was carried out at Mayo Clinic Advanced Genomic Technology Center at Rochester, MN. RPKM-normalized gene counts were generated and used to perform Gene Set Enrichment Analysis (GSEA) and Ingenuity Pathway Analysis (IPA).
Chromatin Immunoprecipitation (ChIP) assay
Cells were harvested by centrifugation and crosslinked to final 1% formaldehyde (Thermo Scientific) for 10 min at room temperature. The protein-DNA cross-linking reaction was quenched using 250 mM glycine for 5 min. Cells were washed with 1X-PBS and re-suspended in cell lysis buffer (1% Nonidet P-40, 150 mM NaCl, 50 mM Tris$HCl, (pH 8.0), 25 μM MG-132, and 1X Complete protease inhibitor (Roche) mixture). After 10 min of incubation on ice, cells were digested with MNase (NEB) and incubated in the Isotemp (Fisher Scientific) at 37C for 10 min. Then samples are sonicated in the Diagenode Bioruptor (15 cycles in Stop/ChIP buffer: 30 s On, 30 Sec Off). In this condition, at least 80%–90% of chromatins are digested into mono- and di-nucleosomes. After sonication, cells were centrifuged at 15,000 x rpm for 10 min to remove debris, the clarified supernatant was transferred to fresh tubes and one percent of the supernatant was saved as input to compare and analyze antibody pull-down. The remainder of each supernatant was incubated overnight at 4C with specific SOX2 or non-specific rabbit IgG antibody as indicated in the figures. After addition of protein G-magnetic beads (Invitrogen), samples were incubated at 4C on a tube rocker for 4 hr. Protein G-magnetic beads were sequentially washed with the following buffers: a) low salt wash [20 mM Tris•Cl, (pH 8.1), 150 mM NaCl, 1% Triton X-100, 2 mM, EDTA, and 1 3 Complete protease inhibitor mixture (Roche)], b) high salt wash [20 mM Tris•Cl, (pH 8.1), 500 mM NaCl, 1% Triton X-100, 2 mM EDTA], c) LiCl wash [10 mM Tris$Cl, (pH 8.1), 250 mM LiCl, 1% deoxycholate, 1% Nonidet P-40, 1 mM EDTA], and d) T.E wash (twice) [10 mM Tris•Cl, (pH 8.1), 1 mM EDTA]. All the washes were performed at 4C for 10 min followed by centrifuge at 9000 RPM (1 min) to remove the supernatant. After washing, bound DNA was eluted in 1% SDS, 100 mM NaHCO3. Cross-linking was reversed by incubating at 65○C overnight in elution buffer after addition of 200 mM NaCl. DNA was purified and eluted using MinElute PCR Purification Kit (QIAGEN).
QPCR mRNA expression and ChIP-QPCR
Gene expression was assessed using TaqMan Fast Universal PCR Master Mix. QPCR amplification analysis was performed using Applied Biosystems ViiA7 thermal cycler (Foster City, CA). QPCR reagents were purchased from Applied Biosystems or custom designed from Invitrogen. Relative mRNA expression values were determined by using human GAPDH or mouse Ubc as internal controls. Fold change (FC) of expression was calculated by FC = 2-DDCt. See Key Resources Table for QPCR Primers used.
For enrichment analysis, QPCR was performed using primer sets designed by Primer 3 (http://bioinfo.ut.ee/primer3-0.4.0/) to amplify the promoter regions of the potential genes (ChIP-QPCR primers are listed in Key Resources Table) using SYBR Green (Life Technologies, Grand Island, NY) dye detection on an Applied Biosystems ViiA7 thermal cycler. The comparative Ct method was used for quantitation, and ChIP enrichment was determined as % input.
Anchorage-independent growth and Clonal expansion assay
Anchorage-independent growth was assessed by the ability of cells suspended in agarose to form colonies (SeaPlaque GTG Agarose from Lonza, Rockland, ME). Complete 2X media was mixed with 0.8% agarose at 1:1 ratio to achieve a final agar concentration of 0.4% in growth media plated into 35 mm tissue culture dishes to create bottom layer of soft agar. Single cell suspensions containing 5,000–10,000 cells per plate were mixed in soft agar and dispensed over the solidified bottom layer of soft agar. For drug dose response studies, inhibitors to PKCi (Auranofin), GLI2 (GANT61), BMP7 (LDN193189), and SMO (LDE 225) were added to the soft agar at plating at the concentrations detailed in figures. Plates were incubated at 37C in 5% CO2 and colony growth assessed after 4–5 weeks. For drug studies on S/P/E/Trp53−/− LBSCs, inhibitors were added to both layers of growth factor reduced Matrigel at the concentrations indicated in the figure legend. Plates were incubated at 37C in 5% CO2 and sphere number assessed after 10 days. Plates were stained with Giemsa (EMD Millipore, Darmstadt, Germany) for quantification. Briefly, plates were fixed in meth- anol for 20 min followed by two 1X-PBS wash. Giemsa stain was diluted (1:20) in 1X-PBS and fixed colonies were stained at room temperature for 1–2 hr. Stained plates were washed with 1X-PBS and plates were imaged using BioSpectrum (UVP, Upland, CA). Colony number on soft agar was determined using ImagePro Plus version 7 software. The spheroids on Matrigel were quantified on day 10 under microscope (4X magnification).
Computational Analysis
We downloaded the data of primary LSCC and LADC tumors from the Cancer Genome Atlas (TCGA) data portal (https://www.cbioportal.org/). We collected RNA-seq data from 527 LSCC samples which include 478 patients diagnosed with LSCC and 49 normal epithelial tissues adjacent to primary LSCC tumors. The scaled expression data was used for data analysis (Li and Dewey, 2011). To identify chromosome 3q26 amplification is a prominent feature of LSCC, we classified tumors with different copy number alterations using GISTIC (Genomic Identification of Significant Targets in Cancer) software. To investigate PKCi-SOX2 dual mediated target genes, we further divided the 478 samples into two groups based on their PKCi and SOX2 expression values; the PKCiH.SOX2H group contained 44 LSCC samples with 20% of tumors expressing the highest levels of PKCi and SOX2; and 44 LSCC samples with 20% of tumors expressing the lowest levels of PKCi and SOX2 comprised the PKCiL.SOX2L group. To investigate 3q26 upregulation is an early clinical event in LSCC but not in LADC, 230 clinical stage I LSCC tumors and 214 clinical stage I LADC tumors were analyzed ranking by PKCi expression. Statistical differences between samples were assessed with two way unpaired t test or Chi-square test.
Independent dataset GSE78948 was downloaded from the NCBI Gene Expression Omnibus (GEO) database to validate PKCi-SOX2 transcriptional pathways/targets. GSE108124 was downloaded to analyze TP53 mutation, 3q26 CN and PKCi, SOX2, and ECT2 expression in regressive and progressive LSCC CIS lesions. GSE109743 was downloaded to analyze PKCi-SOX2 and PKCi-ECT2 Eigengene scores across 4 LSCC pre-malignant lesion sub-types. GSE17710 was downloaded to validate PKCi- SOX2 and PKCi-ECT2 Eigengene scores across 4 LSCC sub-types.
QUANTIFICATION AND STATISTICAL ANALYSIS
Gene Set Enrichment Analysis (GSEA)
The Gene Set Enrichment Analysis (GSEA) software is publicly available from the Broad Institute of MIT and Harvard University (http://software.broadinstitute.org/gsea/index.jsp). RPKM-normalized gene counts from H1299 RNA-seq were downloaded to GSEA software for hallmark analysis (version h.all.v6.2). Analysis of differentially enriched hallmarks (NT RNAi versus PKCi RNAi, or SOX2 RNAi) in H1299 cells was performed separately. GSEA was performed using the gene permutation option and gene sets smaller than 15 or larger than 500 were excluded. The false discovery rate (FDR) of hallmark with less than 0.25 (FDR < 0.25) was considered as significant enriched.
Ingenuity Pathway Analysis (IPA)
Genes that were up (or down) regulated in PKCiH.SOX2H group and that responded to PKCiL.SOX2L group with significance (p < 0.05) and folder change (F > 1.5) were considered to be differential expressed genes (DEGs) in TCGA LSCC primary tumors. Genes that were up (or down) regulated in oncosphere NT cells and that responded to PKCi (or SOX2) knock down with significance (p < 0.05) and fold change (F > 1.5) were considered to be DEGs in H1299 LSCC cells. Three independent lists of DEGs from TCGA
LSCC primary tumors and LSCC cells were generated and used to perform Ingenuity Pathway Analysis (IPA). The core analysis was performed using default settings: direct and indirect relationships between molecules supported by experimentally observed data were considered, networks did not exceed 35 molecules, and all sources of data from human, mouse, and rat studies in the Ingenuity Knowledge Base were considered. This generated priority lists for canonical pathways. Score values were calculated from hypergeometric distribution and right-tailed Fisher’s exact test. Canonical pathways were further filtered by log(p value) > 1.3 and Z-score > 1.3.
Eigengene score analysis of TCGA LSCC
The Eigengene score represents the principal component analysis (PCA) of the expression matrix of PKCi-SOX2 and PKCi-ECT2 genes and positively correlates with mean expression of those genes. The Eigengene score can be applied using the built-in R prcomp and princomp functions.
Statistical Analysis for Experimental Data
The details of statistical analysis of experiments can be found in the figure legends. Statistical analysis of differences between samples was performed using two-tailed Student’s t tests, and p < 0.05 was defined as significant. The analysis of percentile 3q26 CNGs and TP53 mutations across clinical stages was performed using the chi-square test. When comparing various groups one-way ANOVA statistical test was used applying the Dunnett’s method or Tukey’s method to correct for multiple comparisons. Statistical analysis was performed using the computing environment R.
DATA AND CODE AVAILABILITY
All RNA-Seq data has been deposited in Gene Expression Omnibus under the accession Number GSE141666 (GEO: GSE141666).
Supplementary Material
Highlights.
3q26 CNG and TP53 mutation associate with progression of carcinoma in situ to LSCC
PRKCI, SOX2, and ECT2 overexpression transforms Trp53−/− mouse lung basal stem cells
Active PKCi-SOX2 and PKCi-ECT2 signaling is required for LBSC transformation
A PKCi-SOX2 molecular signature suggests unique therapeutic vulnerabilities in LSCC
ACKNOWLEDGMENTS
We thank Ms. Kayla Lewis, Ms. Capella Weems, and Mr. Jorge Lombardi for technical assistance; Dr. Laura Lewis-Tuffin and the Mayo Clinic Cellular Imaging and Flow Cytometry Facility for assistance with cell sorting; Ms. Brandy Edenfield and the Mayo Clinic Cancer Biology Histology Facility for processing tumor tissues for analysis; and the Mayo Clinic Sequencing Facility for RNA-seq runs. We also acknowledge members of the Fields laboratory for critical feedback on the manuscript. This work was supported by grants from the National Institutes of Health/National Cancer Institute (R01 CA081436–22 and R01 CA206267–04 to A.P.F.; R01 CA140290–05 to N.R.M.; and R03 CA235189 to V.J.). A.P.F. is the Monica Flynn Jacoby Professor of Cancer Research, an endowment fund that provides partial support for the investigator’s research program. V.J. was supported in part by a Mayo Clinic Center for Biomedical Discovery Career Development Award and an American Cancer Society Research Scholar Award (RSG-18–201-01). Y.L. and N.Y. are recipients of the Edward C. Kendall Fellowship in Biochemistry from the Mayo Clinic Graduate School.
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
SUPPLEMENTAL INFORMATION
Supplemental Information can be found online at https://doi.org/10.1016/j.celrep.2019.12.071.
REFERENCES
- Ali SA, Justilien V, Jamieson L, Murray NR, and Fields AP (2016). Protein kinase Ci drives a NOTCH3-dependent stem-like phenotype in mutant KRAS lung adenocarcinoma. Cancer Cell 29, 367–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass AJ, Watanabe H, Mermel CH, Yu S, Perner S, Verhaak RG, Kim SY, Wardwell L, Tamayo P, Gat-Viks I, et al. (2009). SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nat. Genet. 41, 1238–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beane JE, Mazzilli SA, Campbell JD, Duclos G, Krysan K, Moy C, Perdomo C, Schaffer M, Liu G, Zhang S, et al. (2019). Molecular subtyping reveals immune alterations associated with progression of bronchial pre-malignant lesions. Nat. Commun. 10, 1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferone G, Song JY, Sutherland KD, Bhaskaran R, Monkhorst K, Lambooij JP, Proost N, Gargiulo G, and Berns A (2016). SOX2 is the determining oncogenic switch in promoting lung squamous cell carcinoma from different cells of origin. Cancer Cell 30, 519–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields AP, Justilien V, and Murray NR (2016). The chromosome 3q26 OncCassette: a multigenic driver of human cancer. Adv. Biol. Regul. 60, 47–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick LA, Matthews JA, Jamieson L, Justilien V, Thompson EA, Radisky DC, and Fields AP (2008). Matrix metalloproteinase-10 is a critical effector of protein kinase Ciota-Par6alpha-mediated lung cancer. Oncogene 27, 4841–4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukazawa T, Guo M, Ishida N, Yamatsuji T, Takaoka M, Yokota E, Haisa M, Miyake N, Ikeda T, Okui T, et al. (2016). SOX2 suppresses CDKN1A to sustain growth of lung squamous cell carcinoma. Sci. Rep. 6, 20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerman PS, Lawrence MS, Voet D, Jing R, Cibulskis K, Sivachenko A, Stojanov P, McKenna A, Lander ES, Gabriel S, et al. ; Cancer Genome Atlas Research Network (2012). Comprehensive genomic characterization of squamous cell lung cancers. Nature 489, 519–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegab AE, Ha VL, Attiga YS, Nickerson DW, and Gomperts BN (2012). Isolation of basal cells and submucosal gland duct cells from mouse trachea. J. Vis. Exp. 2012, e3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong KU, Reynolds SD, Watkins S, Fuchs E, and Stripp BR (2004). Basal cells are a multipotent progenitor capable of renewing the bronchial epithelium. Am. J. Pathol. 164, 577–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussenet T, Dali S, Exinger J, Monga B, Jost B, Dembeleé D,,Martinet N, Thibault C, Huelsken J, Brambilla E, and du Manoir S (2010). SOX2 is an oncogene activated by recurrent 3q26.3 amplifications in human lung squamous cell carcinomas. PLoS ONE 5, e8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong Y, Hoang NT, Lovejoy A, Stehr H, Newman AM, Gentles AJ, Kong W, Truong D, Martin S, Chaudhuri A, et al. (2017). Role of KEAP1/ NRF2 and TP53 mutations in lung squamous cell carcinoma development and radiation resistance. Cancer Discov. 7, 86–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justilien V, and Fields AP (2009). Ect2 links the PKCiota-Par6alpha com plex to Rac1 activation and cellular transformation. Oncogene 28, 3597–3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justilien V, Jameison L, Der CJ, Rossman KL, and Fields AP (2011). Oncogenic activity of Ect2 is regulated through protein kinase C iota-mediated phosphorylation. J. Biol. Chem. 286, 8149–8157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justilien V, Walsh MP, Ali SA, Thompson EA, Murray NR, and Fields AP (2014). The PRKCI and SOX2 oncogenes are coamplified and cooperate to activate Hedgehog signaling in lung squamous cell carcinoma. Cancer Cell 25, 139–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justilien V, Ali SA, Jamieson L, Yin N, Cox AD, Der CJ, Murray NR, and Fields AP (2017). Ect2-dependent rRNA synthesis is required for KRAS- TRP53-driven lung adenocarcinoma. Cancer Cell 31, 256–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justilien V, Lewis KC, Murray NR, and Fields AP (2019). Oncogenic Ect2 signaling regulates rRNA synthesis in NSCLC. Small GTPases 10, 388–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, and Dewey CN (2011). RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 12, 323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell AM, Yao C, Yeckes AR, Wang Y, Selvaggio AS, Tang J, Kirsch DG, and Stripp BR (2016). p53 regulates progenitor cell quiescence and differentiation in the airway. Cell Rep. 17, 2173–2182. [DOI] [PubMed] [Google Scholar]
- Mollaoglu G, Jones A, Wait SJ, Mukhopadhyay A, Jeong S, Arya R, Camolotto SA, Mosbruger TL, Stubben CJ, Conley CJ, et al. (2018). The lineage-defining transcription factors SOX2 and NKX2–1 determine lung cancer cell fate and shape the tumor immune microenvironment. Immunity 49, 764–779.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay A, Berrett KC, Kc U, Clair PM, Pop SM, Carr SR, Witt BL, and Oliver TG (2014). Sox2 cooperates with Lkb1 loss in a mouse model of squamous cell lung cancer. Cell Rep. 8, 40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regala RP, Davis RK, Kunz A, Khoor A, Leitges M, and Fields AP (2009). Atypical protein kinase Ciota is required for bronchioalveolar stem cell expansion and lung tumorigenesis. Cancer Res. 69, 7603–7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, Randell SH, and Hogan BL (2009). Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc. Natl. Acad. Sci. USA 106, 12771–12775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaykhiev R, Wang R, Zwick RK, Hackett NR, Leung R, Moore MA, Sima CS, Chao IW, Downey RJ, Strulovici-Barel Y, et al. (2013). Airway basal cells of healthy smokers express an embryonic stem cell signature relevant to lung cancer. Stem Cells 31, 1992–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata PR, Chow RD, Saladi SV, Tata A, Konkimalla A, Bara A, Montoro D, Hariri LP, Shih AR, Mino-Kenudson M, et al. (2018). Developmental history provides a roadmap for the emergence of tumor plasticity. Dev. Cell 44, 679–693.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira VH, Pipinikas CP, Pennycuick A, Lee-Six H, Chandrasekharan D, Beane J, Morris TJ, Karpathakis A, Feber A, Breeze CE, et al. (2019). Deciphering the genomic, epigenomic, and transcriptomic landscapes of pre-invasive lung cancer lesions. Nat. Med. 25, 517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H, Ma Q, Peng S, Adelmant G, Swain D, Song W, Fox C, Francis JM, Pedamallu CS, DeLuca DS, et al. (2014). SOX2 and p63 colocalize at genetic loci in squamous cell carcinomas. J. Clin. Invest. 124, 1636–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkerson MD, Yin X, Hoadley KA, Liu Y, Hayward MC, Cabanski CR, Muldrew K, Miller CR, Randell SH, Socinski MA, et al. (2010). Lung squamous cell carcinoma mRNA expression subtypes are reproducible, clinically important, and correspond to normal cell types. Clin. Cancer Res. 16, 4864–4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Fillmore CM, Koyama S, Wu H, Zhao Y, Chen Z, Herter-Sprie GS, Akbay EA, Tchaicha JH, Altabef A, et al. (2014). Loss of Lkb1 and Pten leads to lung squamous cell carcinoma with elevated PD-L1 expression. Cancer Cell 25, 590–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All RNA-Seq data has been deposited in Gene Expression Omnibus under the accession Number GSE141666 (GEO: GSE141666).