Abstract
Accumulation of a positive fluid balance is common in critically ill patients, and is associated with adverse outcomes, including mortality. However, there are few randomised clinical trials to guide clinicians as to the most appropriate fluid strategy following initial resuscitation and on the use of deresuscitation (removal of accumulated fluid using diuretics and/or renal replacement therapy). To inform the design of randomised trials, we surveyed critical care physicians with regard to perceptions of fluid overload in critical care, self-reported practice, acceptability of a variety of approaches to deresuscitation, appropriate safety parameters, and overall acceptability of a randomised trial of deresuscitation. Of 524 critical care specialists completing the survey, the majority practiced in mixed medical/surgical intensive care units in the United Kingdom. Most (309 of 363 respondents, 85%) believed fluid overload to be a modifiable source of morbidity; there was strong support (395 of 457, 86%) for a randomised trial of deresuscitation in critical illness. Marked practice variability was evident among respondents. In a given clinical scenario, self-reported practice ranged from the administration of fluid (N = 59, 14%) to the administration of a diuretic (N = 285, 67%). The majority (95%) considered it appropriate to administer diuretics for fluid overload in the setting of noradrenaline infusion and to continue to administer diuretics despite mild dysnatraemias, hypotension, metabolic alkalosis, and hypokalaemia. The majority of critical care physicians view fluid overload as a common and modifiable source of morbidity; deresuscitation is widely practiced, and there is widespread support for randomised trials of deresuscitation in critical illness.
Keywords: Critical illness, diuretics, fluid therapy, deresuscitation, water–electrolyte balance, critical care
Background
The accumulation of a positive fluid balance is a frequent occurrence in critically ill patients. Contributory factors include the administration of intravenous fluid for volume expansion, maintenance fluids administered to provide estimated daily requirements of water and electrolytes, and fluid given as drug diluents and as nutrition, blood product transfusion, and others.1 The effect is compounded by fluid retention caused by the endocrine stress response to critical illness and by acute kidney injury.
Numerous studies have demonstrated a strong and consistent association between the accumulation of a positive fluid balance and adverse outcomes, particularly mortality.2–5 Several trials have investigated restrictive approaches to fluid administration or the use of diuretics to remove accumulated fluid, an approach described as ‘deresuscitation’.6–8 In a recent systematic review and meta-analysis including 2051 patients in 11 randomised trials, we found that a conservative or deresuscitative fluid strategy resulted in increased ventilator-free days and a shorter length of ICU stay, but no difference in mortality.9 Considerable heterogeneity was evident in the therapeutic approaches tested in these trials, highlighting the challenges involved in designing clinical trials on this topic. Areas of uncertainty include the relative efficacy of intermittent bolus dosing versus infusion of loop diuretics7,10,11 with loop diuretics.
To inform the design of randomised trials in this area of practice, we surveyed practicing critical care physicians. Our objectives were to: (a) ascertain views on the issue of fluid overload in critical care, (b) ascertain the acceptability and importance of randomised trials of deresuscitation to the critical care community, (c) explore self-reported practice in deresuscitation, (d) determine the acceptability of different approaches to deresuscitation and important safety parameters, and (e) compare responses between UK-based specialists and those practicing in other countries.
Methods
Survey design
Using an online survey tool (www.surveymonkey.com), we designed a brief survey consisting of demographic, attitudinal, and practice-based questions, together with case vignettes and associated therapeutic options. We utilised a combination of multiple-choice questions, Likert scales, and free text responses (full survey in supplementary material). Survey questions were piloted among colleagues from the Canadian Critical Care Trials Group and the Belfast Health and Social Care Trust, and were revised prior to distribution.
Survey distribution
A link to the survey was distributed electronically to members of the United Kingdom Intensive Care Society (ICS) and the European Society of Intensive Care Medicine (ESICM), and to subscribers to an electronic mailing list (criticalcarereviews.com). Subsequent follow-up requests were sent. Only consultants (specialists) in Intensive Care Medicine were asked to complete the survey and respondents were asked to confirm their status before being able to proceed to complete any further questions. Participation was voluntary, and consent for participation was implied by completion of the survey. The survey was anonymous, although participants had the option to leave comments and personal information on a voluntary basis.
Statistical analysis
Statistical analysis was performed using Stata v14.2 (StataCorp, Texas, USA). Variables are reported as mean (standard deviation) where normally distributed and as median (interquartile range) where not. Due to relatively low numbers of responses from countries other than the UK, geographic comparisons were limited to UK versus non-UK respondents. Data from Likert scales were enumerated as ordinal data ranging from 1 to 5, with 1 = strongly agree and 5 = strongly disagree). Comparisons of ordinal data between groups were made using the Mann–Whitney U-test. For categorical data, the chi-squared test was used. Statistical significance was defined by a P value < 0.05.
Results
Respondents
The survey was distributed to 1550 consultants on the ICS electronic mailing list, ≈11,500 specialists and non-specialists on the ESICM electronic mailing list, and 6288 individuals on the criticalcarereviews.com mailing list. A total of 524 responses were received from critical care specialists, although not all respondents answered all questions. The majority (N = 440, 87%) of respondents worked in mixed medical–surgical ICUs, with the remainder from specialist cardiac, neurological, medical, or surgical units. Most respondents practiced in the United Kingdom (N = 309, 61%), with smaller proportions practicing in other European countries (N = 99, 20%) and non-European countries (N = 96, 19%).
Attitudinal questions
We asked a number of questions to elucidate physicians' perceptions of fluid overload (defined as a positive fluid balance with oedema) in clinical practice (Table 1). While the majority (270, 74%) of the 367 respondents believed that fluid overload was inevitable as the result of appropriate fluid resuscitation, and many viewed this as a manifestation of endocrine factors and acute kidney injury (246 of 364, 67.6%), there was nevertheless a strong perception that fluid overload represents a modifiable source of morbidity (309 of 363, 85.1%). These perceptions were expressed more strongly by non-UK respondents (Supplementary material 2). Very few respondents believed fluid overload to be benign (N = 12, 3%).
Table 1.
Broadly speaking, how do you perceive the issue of fluid overload (positive fluid balance with oedema) in ICU patients? (N = 367).
| Strongly agree |
Agree |
Uncertain/neither agree nor disagree |
Disagree |
Strongly disagree |
Total |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | N | |
| An inevitable consequence of appropriate fluid resuscitation in the presence of capillary leak | 84 | (23.1%) | 186 | (51.2%) | 45 | (12.4%) | 43 | (11.9%) | 5 | (1.4%) | 363 |
| A modifiable consequence of fluid administration from multiple sources | 75 | (20.6%) | 234 | (64.3%) | 42 | (11.5%) | 12 | (3.3%) | 1 | (0.3%) | 364 |
| A manifestation of sodium and water retention due to endocrine factors and acute kidney injury | 29 | (8.0%) | 217 | (59.6%) | 78 | (21.4%) | 36 | (9.9%) | 4 | (1.1%) | 364 |
| An issue which will resolve spontaneously with resolution of the underlying illness | 30 | (8.3%) | 136 | (37.8%) | 91 | (25.3%) | 90 | (25.0%) | 13 | (3.6%) | 360 |
| A finding without clinical consequence | 3 | (0.8%) | 9 | (2.5%) | 24 | (6.7%) | 139 | (38.5%) | 186 | (51.5%) | 361 |
| A modifiable source of morbidity | 129 | (35.5%) | 180 | (49.6%) | 38 | (10.5%) | 11 | (3.0%) | 5 | (1.4%) | 363 |
ICU: intensive care unit.
We sought views from respondents as to the importance of the research question ‘does deresuscitation of critically ill patients with fluid overload improve patient outcomes’, and willingness or otherwise to enrol patients to a clinical trial designed to answer this question. The majority of respondents (N = 399 of 457, 87% overall) believed this to be an important or very important research question, with UK respondents attaching less importance to the question than those from outside the UK (very important: 114 of 278 (41%) versus 97 of 179 (54%), P<0.01). Willingness to enrol patients to a clinical trial of deresuscitation was similarly strong for both UK and non-UK respondents (236 of 278 (85%) versus 159 of 179 (88%), P = 0.23).
Case vignettes
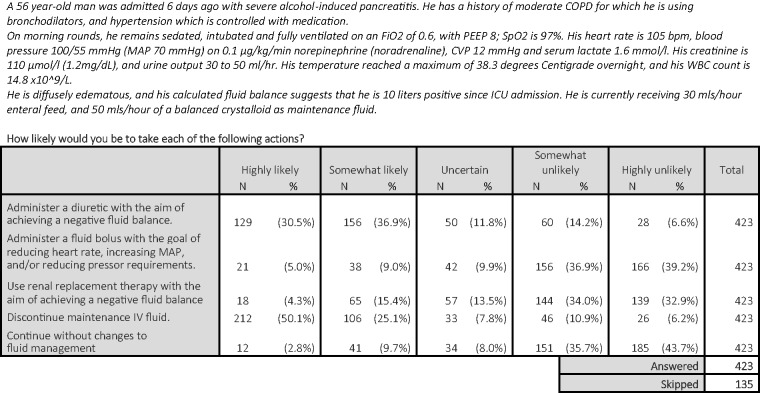
We presented respondents with clinical scenarios designed to reflect clinical practice, seeking to ascertain therapeutic approaches with regard to fluid management. In a given scenario designed to reflect a typical patient with evidence of fluid overload and abnormal haemodynamics, there was marked variability in suggested therapeutic approach (Figure 1). While 285 respondents (67%) were likely or highly likely to administer a diuretic, a minority (N = 59, 14%) were likely or highly likely to administer a fluid bolus in the same scenario, and 72 (17%) were likely to continue maintenance fluid.
Figure 1.
Case vignette 1. COPD: chronic obstructive pulmonary disease; CVP: central venous pressure; ICU: intensive care unit; MAP: mean arterial pressure; PEEP: positive end-expiratory pressure; WBC: white blood cells.
Presented with the same patient after a poor response to an initial diuretic bolus and ongoing positive fluid balance, there was no clear consensus on the preferred therapeutic approach. Of the 335 respondents who were uncertain or likely to administer a diuretic initially, 122 (36%) were likely or highly likely to repeat the same dose, while 216 (65%) were likely or highly likely to administer a higher dose.
Respondents were then asked to consider the same patient in the presence of acute kidney injury. In this context, respondents were less likely to favour diuretic administration (N = 164, 39% likely or highly likely versus N = 210, 50% at least somewhat unlikely).
In a second scenario (Figure 2), designed to reflect a hypothetical patient with clear evidence of organ dysfunction associated with fluid overload and oliguric acute kidney injury, respondents were asked to consider the use of renal replacement therapy to remove fluid in the absence of classic indications for RRT. The majority of respondents (N = 248, 62%) would initiate RRT in this scenario. A further 72 (18%) would do so if additional criteria were met, the most common of which was failure to respond to diuretics. The majority of respondents (N = 187, 72%) would target a negative fluid balance of between 500 and 1500 ml, and faced with mild isolated hypotension would continue fluid removal, if necessary administering vasopressors and/or an albumin solution to treat hypotension.
Figure 2.
Case vignette 2. CVP: central venous pressures.
Self-reported practice
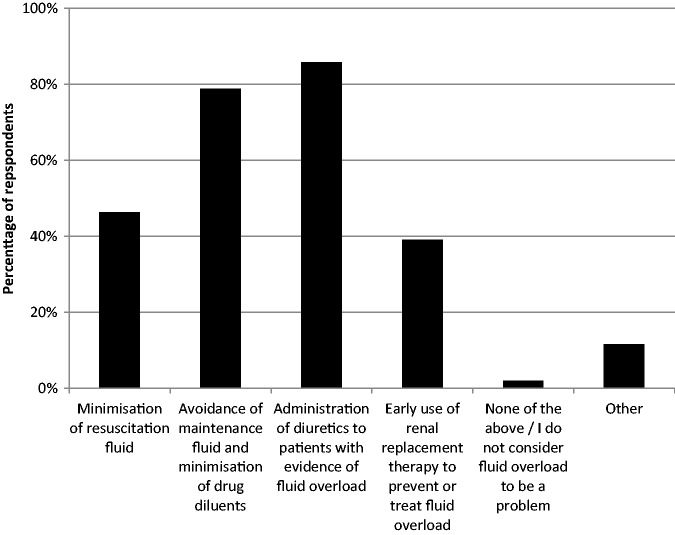
A large majority of respondents (N = 373, 94%) reported fluid overload as a common occurrence in practice and reported the use of several preventative and treatment strategies (Figure 3), with diuretic administration being the most frequently used. The majority of respondents (N = 263, 66%) reported using diuretics to treat fluid overload on at least 50% of days working in ICU, with greater reported use from non-UK specialists (Supplementary material 2). The most commonly reported approach was intermittent bolus doses of loop diuretics (N = 211, 54%) with similar proportions of respondents reporting the use of thiazides, potassium-sparing diuretics, and carbonic anhydrase inhibitors, typically on an infrequent basis (Table 2).
Figure 3.
Strategies for prevention or treatment of fluid overload. CVP: central venous pressure; ICU: intensive care unit.
Table 2.
Use of adjunctive diuretics alongside loop diuretics.
| Of the occasions on which you administer loop diuretics to achieve a negative fluid balance, how often do you use the following agents (either as adjuncts or alternatives)? | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Always or nearly all of the time |
Frequently (>50% of the time) |
Sometimes (20–50% of the time) |
Rarely (<20% of the time) |
Never |
Total |
||||||
| N | % | N | % | N | % | N | % | N | % | N | |
| Thiazides (e.g. bendroflumethiazide) | 23 | (6.0%) | 32 | (8.4%) | 47 | (12.3%) | 121 | (31.6%) | 160 | (41.8%) | 383 |
| Potassium-sparing (e.g. spironolactone) | 10 | (2.6%) | 48 | (12.5%) | 115 | (29.9%) | 165 | (42.9%) | 47 | (12.2%) | 385 |
| Carbonic anhydrase inhibitors (e.g. acetazolamide) | 5 | (1.3%) | 10 | (2.6%) | 56 | (14.7%) | 163 | (42.8%) | 147 | (38.6%) | 381 |
| Answered | 393 | ||||||||||
| Skipped | 165 | ||||||||||
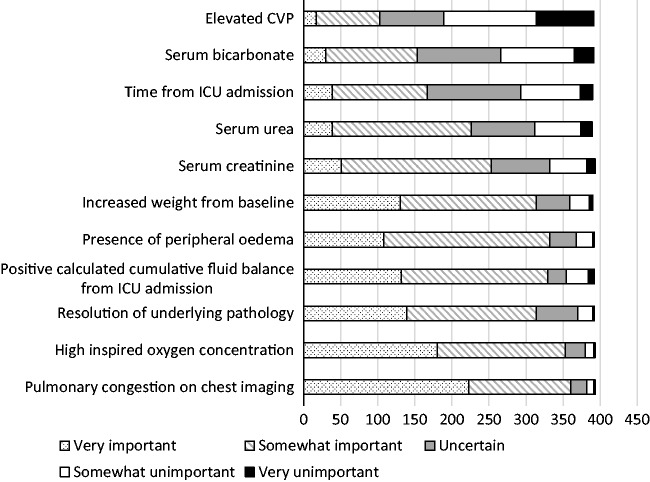
A broad range of factors were considered to be important in the decision to attempt deresuscitation in practice (Figure 4).
Figure 4.
Reponses to the question ‘Recognising that the decision to initiate a deresuscitation strategy (using diuretics and/or dialysis to target a negative fluid balance) is complex and patient-dependent, how important do you consider each of the following indications for deresuscitation?’ N = 393. IV: intravenous; MAP: mean arterial pressure.
Safety factors
Respondents were asked their willingness to administer diuretics to patients receiving vasopressors and their response to several common side effects of diuretic administration. A small minority of respondents reported unwillingness to administer diuretics to patients on a norepinephrine infusion (N = 20, 5%), while most (N = 197, 50%) did not have a fixed dose ceiling for norepinephrine above which they would not administer diuretics. Of those respondents who did report a fixed dose ceiling, the most frequent was 0.1 mcg/kg/min (N = 59, 34%).
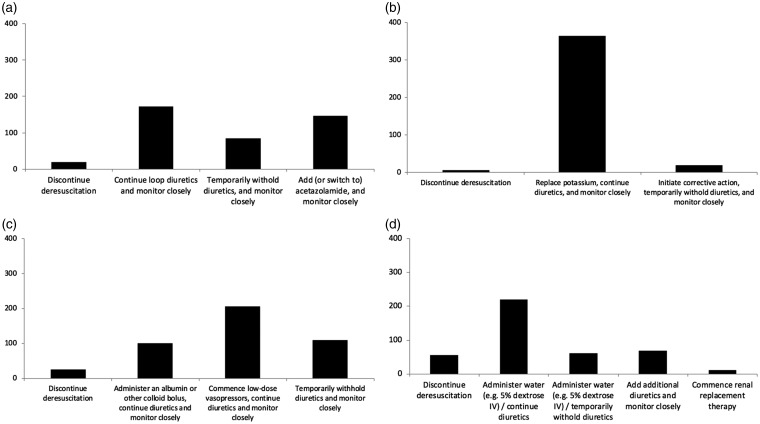
Likely therapeutic responses to possible side effects of diuretic therapy are shown in Figure 5. The majority of respondents expressed willingness to continue with diuretics with close monitoring even in the context of mild side effects (e.g. metabolic alkalosis, mild hypernatraemia).
Figure 5.
Response to common side effects of diuretics. (a) Metabolic alkalosis (bicarbonate > 30 mmol/l), (b) mild hypokalaemia (K + 3.0–3.5 mmol/l), (c) mild hypotension (MAP 55–65 mmHg), and (d) mild hypernatraemia (sodium 145–150 mmol/l).
Discussion
Our survey demonstrates that the majority of critical care physicians view fluid overload as a major modifiable source of morbidity in critically ill patients. Considerable variability in practice was evident. While the majority of respondents reported the use of deresuscitation in practice, the indications, techniques, and threshold for doing so were highly variable. This was highlighted in the responses to case vignettes, in which clinicians faced with the same scenario might either administer fluid or administer a diuretic. Despite widespread use of deresuscitative measures, fluid overload remains a common problem.1
Fluid management remains a controversial topic in critical care, with increasing discussion being focused on the concept of treating fluids with the same care and attention as drugs: with regard to dose, indications and side effects, timing, and of balancing benefits and harms.12 This approach was reflected in responses to this survey, in which the overwhelming majority of respondents perceived this as an important topic for research, and expressed support for enrolment of patients to a randomised controlled trial to address the research question: ‘does deresuscitation of critically ill patients with fluid overload improve patient outcomes?’ Several previous surveys have investigated clinician attitudes and practice with regard to early fluid resuscitation in septic shock,13 the use of fluid boluses in the ICU,14 and definitions of fluid overload and the use of continuous renal replacement therapy to treat fluid overload.15 The common theme through these surveys is the marked degree of uncertainty which persists in the area of fluid management in critical illness, despite fluid administration being one of the most common interventions in critical care.
Designing interventions to treat and/or prevent fluid overload in a critically ill population is necessarily complex and not without risk. For this reason, the views of clinicians were sought as to the acceptability of deresuscitation in the presence of mild physiological and metabolic derangement. That the majority of respondents were willing to continue to administer diuretics despite mild dysnatraemias, hypotension, metabolic alkalosis, and hypokalaemia is informative in designing protocols for intervention which will inevitably need to address these potential complications.
This survey has a number of limitations. Most obviously, the study population is poorly defined, with distribution to some respondents through more than one source. It is a theoretical possibility that the same individual could have completed the survey on more than one occasion, although this appears unlikely in practice. Furthermore, the response rate is low and differed between distribution channels. This may be the result of ‘survey fatigue’: the ease with which opinions can be sought from a large number of potential respondents on a range of topics through platforms such as surveymonkey may predispose to potential participants being selective as to which surveys they respond.
It is likely that respondents are those with greater than average levels of interest in the topic and potentially more polarised views. Responses may therefore be poorly representative of the views of the critical care community as a whole, and comparisons between geographic regions must be treated with caution. Nevertheless, the practice variability demonstrated in this study illustrates clearly a state of equipoise with regard to appropriate fluid strategy in the post-resuscitation phase of critical illness and highlights the need for randomised trials to address this fundamental question of widespread interest to critical care physicians and others who care for the critically ill.
Conclusions
This survey illustrates wide variation in decision-making with regard to fluid management in critical illness and highlights the complex nature of these decisions. While deresuscitative measures are widely used, there is no consensus as to the appropriate indications, timing, and techniques used, and there is widespread support for randomised controlled trials of deresuscitation in the critically ill.
Supplemental Material
Supplemental material, Supplemental Material1 for Fluid management and deresuscitation practices: A survey of critical care physicians by Jonathan A Silversides, Daniel F McAuley, Bronagh Blackwood, Eddy Fan, Andrew J Ferguson and John C Marshall in Journal of the Intensive Care Society
Supplemental material, Supplemental Material2 for Fluid management and deresuscitation practices: A survey of critical care physicians by Jonathan A Silversides, Daniel F McAuley, Bronagh Blackwood, Eddy Fan, Andrew J Ferguson and John C Marshall in Journal of the Intensive Care Society
Acknowledgments
The authors wish to acknowledge colleagues from the Canadian Critical Care Trials Group and from Belfast Health and Social Care Trust for providing feedback on draft versions of the survey. The ICS and ESICM circulated the survey to their members, and Dr Rob MacSweeney kindly distributed the survey through the www.criticalcarereviews.com mailing list.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Silversides JA, Fitzgerald E, Manickavasagam US, et al. Deresuscitation of patients with iatrogenic fluid overload is associated with reduced mortality in critical illness. Crit Care Med 2018; 46: 1600–1607. [DOI] [PubMed] [Google Scholar]
- 2.Murphy CV, Schramm GE, Doherty JA, et al. The importance of fluid management in acute lung injury secondary to septic shock. Chest 2009; 136: 102–109. [DOI] [PubMed] [Google Scholar]
- 3.Payen D, de Pont A-CJM, Sakr Y, et al. A positive fluid balance is associated with a worse outcome in patients with acute renal failure. Crit Care 2008; 12: R74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Acheampong A, Vincent JL. A positive fluid balance is an independent prognostic factor in patients with sepsis. Crit Care 2015; 19: 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaara ST, Korhonen A-M, Kaukonen K-M, et al. Fluid overload is associated with an increased risk for 90-day mortality in critically ill patients with renal replacement therapy: data from the prospective FINNAKI study. Crit Care 2012; 16: R197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network, Wiedemann HP, Wheeler AP, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006; 354: 2564–2575. [DOI] [PubMed] [Google Scholar]
- 7.Martin GS, Moss M, Wheeler AP, et al. A randomized, controlled trial of furosemide with or without albumin in hypoproteinemic patients with acute lung injury. Crit Care Med 2005; 33: 1681–1687. [DOI] [PubMed] [Google Scholar]
- 8.Chen C, Kollef MH. Targeted fluid minimization following initial resuscitation in septic shock: a pilot study. Chest 2015; 148: 1462–1469. [DOI] [PubMed] [Google Scholar]
- 9.Silversides JA, Major E, Ferguson AJ, et al. Conservative fluid management or deresuscitation for patients with sepsis or acute respiratory distress syndrome following the resuscitation phase of critical illness: a systematic review and meta-analysis. Intensive Care Med 2017; 43: 155–170. [DOI] [PubMed] [Google Scholar]
- 10.Ng KT, Velayit A, Khoo DKY, et al. Continuous Infusion versus Intermittent Bolus Injection of Furosemide in Critically Ill Patients: A Systematic Review and Meta-analysis. J Cardiothorac Vasc Anesth 2018; 32: 2303–2310. [DOI] [PubMed]
- 11.Bihari S, Holt AW, Prakash S, et al.. Addition of indapamide to frusemide increases natriuresis and creatinine clearance, but not diuresis, in fluid overloaded ICU patients. J Crit Care 2016; 33: 200–206. [DOI] [PubMed]
- 12.Hoste EA, Maitland K, Brudney CS, et al. Four phases of intravenous fluid therapy: a conceptual model. Br J Anaesth 2014; 113: 740–747. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McIntyre L, Rowe BH, Walsh TS, et al. Multicountry survey of emergency and critical care medicine physiciansã fluid resuscitation practices for adult patients with early septic shock. BMJ Open 2016; 6: e010041. [DOI] [PMC free article] [PubMed]
- 14.Glassford NJ, Mårtensson J, Eastwood G, et al. Defining the characteristics and expectations of fluid bolus therapy: A worldwide perspective. J Crit Care 2016; 35: 126–132. [DOI] [PubMed]
- 15.O'Connor ME, Jones SL, Glassford NJ, et al. Defining fluid removal in the intensive care unit: A national and international survey of critical care practice. J Intensive Care Soc 2017; 18: 282–288. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental Material1 for Fluid management and deresuscitation practices: A survey of critical care physicians by Jonathan A Silversides, Daniel F McAuley, Bronagh Blackwood, Eddy Fan, Andrew J Ferguson and John C Marshall in Journal of the Intensive Care Society
Supplemental material, Supplemental Material2 for Fluid management and deresuscitation practices: A survey of critical care physicians by Jonathan A Silversides, Daniel F McAuley, Bronagh Blackwood, Eddy Fan, Andrew J Ferguson and John C Marshall in Journal of the Intensive Care Society