Abstract
Carnitine palmitoyltransferase 2 deficiency is an inherited metabolic disorder involving a deficiency in a mitochondrial enzyme necessary for long chain fatty acid oxidation, and therefore decreased utilisation of fatty acids. The adult form of this condition leads to recurrent rhabdomyolysis triggered by exercise, fasting and infection. It is a very rare condition with only a few hundred reported cases worldwide. Here we present a case of severe rhabdomyolysis in the context of carnitine palmitoyltransferase 2 deficiency in which major organ involvement was avoided, and organ support was not needed. This prompted us to perform a systematic review of the existing case reports in the literature to ascertain the most frequent patterns of organ involvement and assess the outcomes that are seen in these patients. Our findings suggest that these patients most frequently develop isolated renal failure, often requiring renal replacement therapy; however, the outcomes following this are very good, supporting the early involvement of intensive care teams.
Keywords: Rhabdomyolysis, carnitine palmitoyltransferase, renal failure, myoglobinuria, long chain fatty acids
Introduction
Carnitine palmitoyltransferase (CPT) 2 is a crucial enzyme for long chain fatty acid (LCFA) oxidation within the mitochondria. LCFAs cannot readily diffuse across the mitochondrial membrane. After conjugation to coenzyme-A (CoA) by long chain fatty acyl-CoA synthetase they are transferred across the mitochondrial membrane by the carnitine shuttle: this comprises of CPT1 at the outer membrane, a carnitine acyl carnitine translocase spanning the inner mitochondrial membrane and CPT2 on the inner membrane.1 CPT2 acts to transfer the conjugated fatty acid from carnitine within the mitochondrial membrane back to CoA within the mitochondrial matrix.1,2 This, therefore, allows for beta-oxidation of LCFAs within mitochondria – one of the mainstays of energy homeostasis within the body. There are three variant forms of CPT2 deficiency: lethal neonatal, severe infantile hepatocardiomuscular and myopathic.3 The adult myopathic form of CPT2 deficiency is inherited in an autosomal recessive manner.3 Globally there is heterogeneity within the genotypic variation. For example, a novel mutation, S373P, was recently discovered in a south American family.4 Additionally, Japanese patients appear to demonstrate a different profile of mutation within CPT2 – the most common mutation being F383Y.5 Roughly 90% of cases within Caucasians are accounted for by the S113L missense mutation,6,7 although there has been much debate about the exact mechanism of reduced function. This particular variant is multifactorial: it seems to be expressed to the same extent as wild type CPT2,8 and the recombinant human mutated CPT2 shows increased thermolability compared to control CPT2.9,10 In contrast to the wild type CPT2 the mutated variant appears to demonstrate a lack of activation by cardiolipins11 as well as abnormal inhibition by malonyl-CoA.12 Malonyl-CoA is an intermediate of fatty acid synthesis,13 and as such it acts as a metabolic brake on CPT1 activity – preventing concomitant de novo lipogenesis and beta-oxidation. CPT2 is crucially characterised by being insensitive to malonyl-CoA inhibition, a feature that is altered in the majority of CPT2 deficient patients.12 A recent genotype-phenotype analysis confirmed that homozygotes for the S113L missense mutation all demonstrated similar extents of CPT2 activity inhibition with malonyl CoA.14
These factors underpin why CPT2 deficiency results in an energy crisis within the fatty acid metabolic pathways and a deficit of ATP production under specific trigger conditions, such as fasting, exercise, or infection. This crisis occurring within the skeletal muscle causes rhabdomyolysis, typically presenting with myalgia, accompanied by darkening of the urine due to myoglobinuria. Clinically, patients often display diffuse muscular tenderness, and may display signs of the infection provoking the attack. These attacks can be complicated by renal failure, and severe electrolyte disturbances requiring renal replacement therapy (RRT). Crucially, between attacks patients are asymptomatic and have no clinical or biochemical evidence of myopathy.
Before genetic testing, a new diagnosis of myopathic CPT2 deficiency may be suspected in those with recurrent myalgia, or those presenting with attacks of myalgia with myoglobinuria and occasionally muscle weakness following episodes of exercise or infection.15 Supportive laboratory findings may be elevated creatine kinase (CK) during attacks more than five times the upper limit of normal. Further to this analysis of plasma acyl-carnitine profiles via high-performance liquid chromatography tandem mass spectrometry demonstrating increased levels of C16 and C18:1 species specifically with an overall increase of C12 to C18 acylcarnitines will support a diagnosis.16 Specifically the (C16 + C18:1)/C2 ratio being most indicative of CPT2 being a possible diagnosis.16 However, for definitive diagnosis, genetic testing is required for affected individuals. This is either via single gene or multipanel testing to rule out other differential diagnoses including: carnitine-acylcarnitine translocase deficiency, glutaricacidaemia II, TANGO2-related metabolic encephalopathy and arrhythmias, as well as other defects in oxidative phosphorylation or lipid metabolism and recurrent bouts of rhabdomyolysis from environmental causes.3
Long-term management for CPT2 deficiency is aimed at trigger avoidance and dietary modification to restrict lipid intake (<20%) and increase carbohydrate intake (70%) to provide fuel for glycolysis.17–19 The medium-chain fatty acid triheptanoin appears to be an effective therapy for adult-onset CPT II deficiency.18
Management of acute rhabdomyolysis in the context of CPT2 deficiency comprises of:
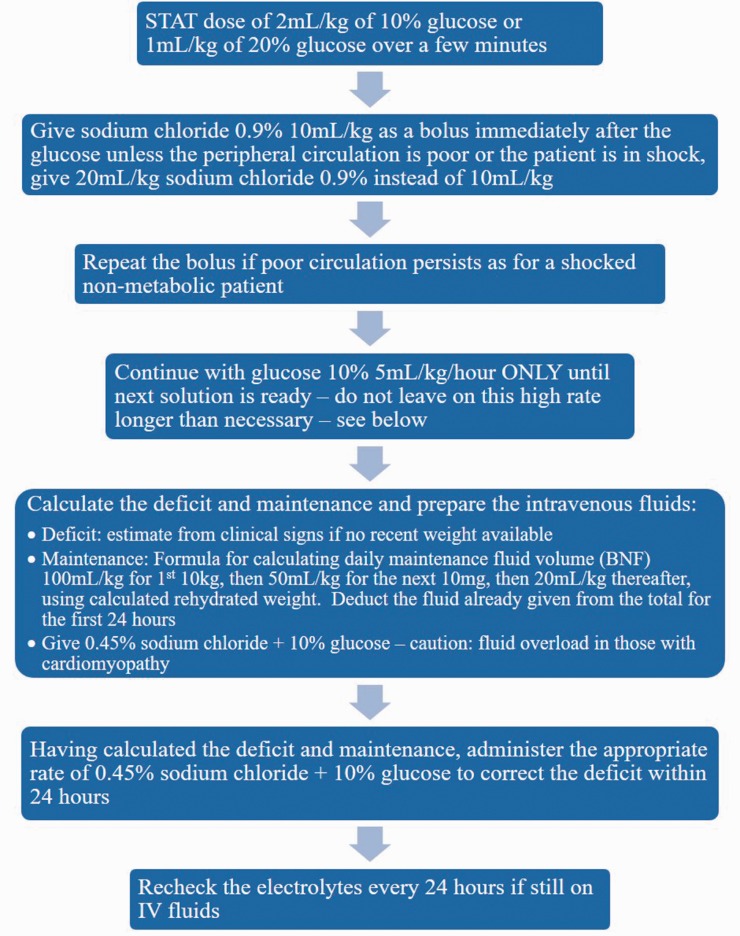
IV glucose administration: The primary acute treatment is to supply adequate glucose to prevent adipose tissue lipolysis3,19–21 (see Figure 1). Hypoglycaemia only occurs at relatively late stages; bedside testing for glucose should not be relied on, and treatment with IV glucose should not be delayed due to a normal blood glucose.3
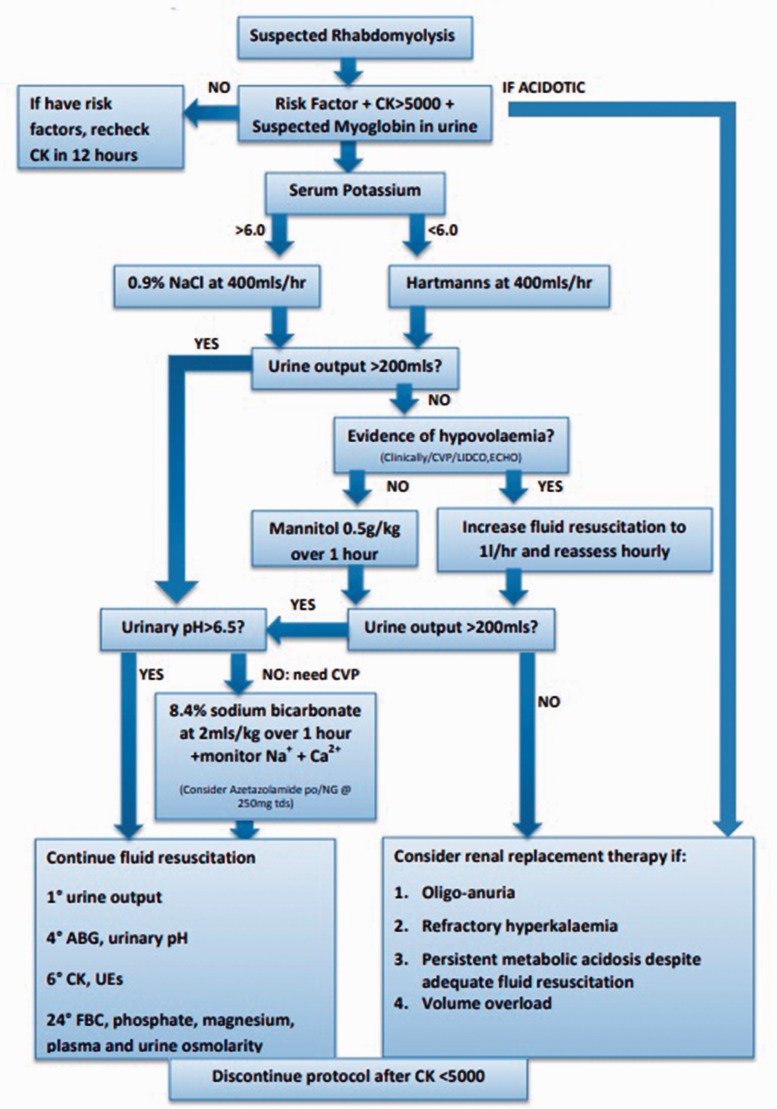
Management of rhabdomyolysis: as with rhabdomyolysis of any aetiology, the preventive measure for renal injury is aggressive fluid resuscitation (see Figure 2). If acute kidney injury is associated with acidosis, early consideration for RRT is indicated. Volaemic state can be assessed clinically or through invasive monitoring, e.g. bed side echocardiography, cardiac output monitoring or central venous pressure.22–26 The need for potassium should be considered; this should be added to the regime once urine flow is confirmed to be normal and the plasma potassium concentration is known.
Identify and stop any medications contributing towards/associated with a high risk of rhabdomyolysis: a full medicine reconciliation should be considered, including prescribed medications from their GP, secondary care consultant(s), over-the-counter medication or herbal medicines.
Figure 1.
Intravenous glucose management in acute rhabdomyolysis acutely decompensated LCFA disorders,18 from the British Inherited Metabolic Disease Group. LCFA: long chain fatty acid.
Figure 2.
Protocol for the management of acute rhabdomyolysis in critical care.22
Other agents which may offer benefit in the acute scenario include carnitine (100–200 mg/kg/24 h in 4 divided doses)18 which will convert potentially toxic long-chain acyl-CoAs to acylcarnitines. Sodium bicarbonate can be used for urinary alkalinisation by decreasing deposition of myoglobin in the renal tubules; however, if pH is not >6.5 after 4 to 6 h, if sodium >155 mmol/L or if the patient develops symptomatic hypocalcaemia then it should be discontinued.22–24,26 Acetazolamide can be considered when urinary alkalinisation is inadequate with the above measures.27 Mannitol is an osmotic diuretic agent that can be used if the patient is normotensive; it causes vasodilation of renal vasculature and acts as a free radical scavenger.
Important in both the acute and non-acute settings is the avoidance of medications which can trigger or exacerbate episodes of muscle injury; important medications include statins (due to their risk of rhabdomyolysis in the general population), ibuprofen (mechanism unknown) and valproic acid.28
The condition has been described extensively from genetic and metabolic perspectives; however, there is not a large amount of literature describing the management of these patients acutely.
Case report
A 34-year-old man presented to emergency department with suprapubic abdominal pain, and aching pains across his groin and thighs. The only associated symptoms were darkening of his urine and fevers. He had no shortness of breath or cough, no palpitations or chest pain, no dysuria or frequency, and no nausea or vomiting or change in bowel habit. There was no history of trauma, he denied coryzal or flu-like symptoms and he had not undertaken any strenuous physical exercise. He admitted to a poor oral intake once his abdominal pain had started. He had no significant past medical or surgical history apart from known CPT2 deficiency, and resulting rhabdomyolysis for which he had previously been admitted to hospital. Previous episodes were triggered by exercise, presenting with muscle aches in his arms and stomach, followed by darkening of his urine. At the time of presentation, he was unemployed, was a non-smoker and rarely drank alcohol. His sister had the same condition.
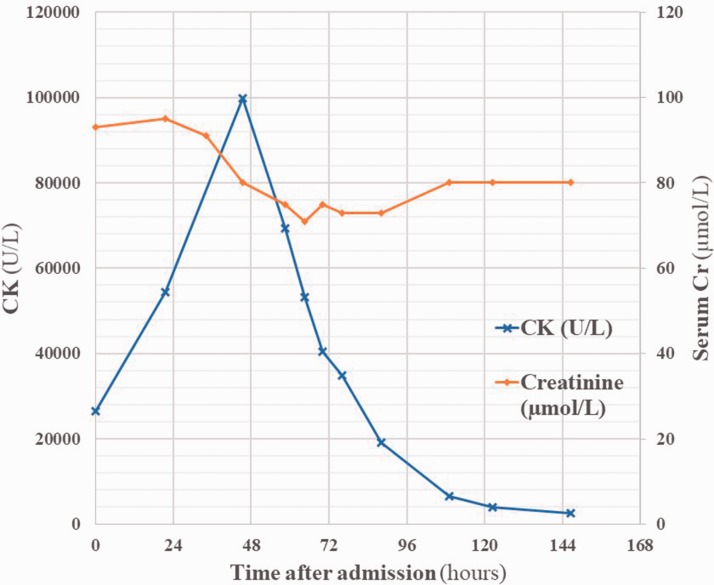
On admission his temperature was 38.2℃, heart rate 60 bpm sinus rhythm, respiratory rate 16 per minute, blood pressure 141/87 mmHg, and O2 saturations 97% on room air. He appeared unwell and sweaty. Cardiovascular and respiratory examinations were normal. He had diffuse mild abdominal tenderness, and his urine was visibly dark. Neurological examination was normal; however, he had diffuse muscular tenderness over his lower limbs. Urinalysis was strongly positive for blood. Biochemical investigations showed white cell count 9.5 × 109/L, haemoglobin 148 g/L, CK 26488 U/L, Cr 93 µmol/L, and alanine aminotransferase (ALT) 148 U/L (see Figure 3).
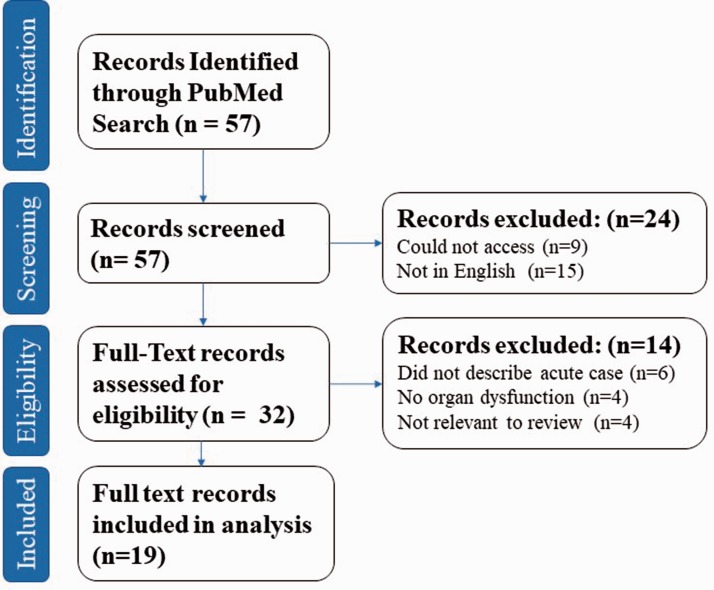
Figure 4.
PRISMA flowsheet outlining numbers in each group of inclusion and exclusion. Full details of the search terms can be found in Supplementary Figure 5. PRISMA: Preferred Reporting Items for Systematic Review and Meta-Analysis.
Figure 3.
Serum creatine kinase and creatinine levels over the course of the admission. 10% glucose IV infusion 2 mg/kg/h was started within 4 h of admission. Hartman’s solution was commenced at a slower rate of 83 mL/h after 34 h. Hartman’s solution at the full rate of 400 mL/h was commenced at 54 h.
Initial treatment comprised of 10% dextrose infusion at a rate of 2 mg/kg/h. His pain settled; however, he then became tachycardic and hyperpyrexial (>40℃). An infusion of Hartman’s solution at a rate of 400 mL/h was added. CK values continued to increase, and the patient was transferred to ICU.
CK values peaked at 99,874 U/L and then fell towards normal values over 48 h. Serum creatinine was stable throughout and his urine output was appropriate for his rate of fluid input. Respiratory viral swabs yielded a positive result for influenza A, and the patient was isolated and started on oseltamivir 75 mg twice daily. Abdominal ultrasound scanning showed that his liver was diffusely hyperechoic, in keeping with fatty infiltration. Echocardiography showed eccentric left ventricular hypertrophy (LVH) with dynamic systolic function. Biochemical investigations before discharge including CK and Cr were normal apart from ALT which returned to normal post discharge from hospital. He was discharged with advice regarding diet and exercise.
Aims
The following systematic review was performed with the aims of answering the following questions:
What are the patterns or combinations of organ involvement that occur with rhabdomyolysis in the context of CPT2 deficiency?
What modalities of organ support are required for these patients? (i.e. what is the role of ICU/HDU teams in the management of these patients?)
What are the patient outcomes that can be expected in the acute setting in terms of in-hospital mortality and renal function?
Methods
This systematic review was performed in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) statement recommendations.29 The study protocol was registered to PROSPERO (registration ID: CRD42019123686).
The PubMed database was searched (search terms can be found within Supplementary Figure 5) with no restrictions on publication date. Inclusion criteria were case reports or case series, describing patients diagnosed with the adult form of CPTII deficiency, experiencing acute rhabdomyolysis (here defined as symptoms or clinical signs consistent with rhabdomyolysis and a value of CK > 1000 U/L), with resulting organ involvement (here defined as biochemical evidence of organ dysfunction, for example PaO2 < 10 kPa for respiratory dysfunction, serum creatinine (Cr) > range predicted for age and sex for renal dysfunction) in the acute setting.
Articles were screened by a single researcher (NI) and if any doubt regarding eligibility criteria fulfilment arose then a second independent investigator was involved (VDT). Articles not in English or that could not be accessed were excluded. After identification of eligible articles, data regarding patient demographics, suspected triggers, biochemical investigations, medical management of the patient, and patient outcomes in terms of in-hospital mortality were extracted. Risk of bias was assessed at the study level with the completeness of the reporting of the case and outcomes. Omitted data were not included in the descriptive analysis.
A formal meta-analysis was not appropriate due to the heterogeneity of the studies and their methodologies. At the study level there was the risk of publication bias. The patterns of organ involvement and organ support required were described categorically – such that a patient with multiple organ failure/requiring multiple organ support would be placed into a new category of this combination, rather than the comprising groups for each organ affected/supported. Patient demographics, serum creatinine and CK values on admission were described, and the rate of in hospital mortality was calculated, and these were compared between the groups that did and did not require organ support. Amongst the cases in which renal function at discharge was described, the rate of residual renal dysfunction at discharge was described.
Results
Of all the articles identified in searches, 19 were included in the final analysis28,30–48 (see Figure 4 and Supplementary Table 1). These comprised of 18 case reports and one case series; the case series included one patient who met the inclusion criteria. Full details of the included cases can be found in Table 1 (online supplement). Of the 19 patients, the mean age was 25.3 years (standard deviation 13.6, range 3–47 years). Sex was stated in 18 cases, 15 of which (83.3%) were male. Mean serum creatinine on admission was 512 µmol/L, and mean CK on admission was 88,413 U/L. The most frequent suspected triggers (stated in 17 cases) for rhabdomyolysis were infection (7 patients, 41.2%), exercise (6 patients, 35.3%), drugs (2 patients, 11.76%), and fasting and extremes of temperature (1 patient each, 5.9% each).
The most common patterns of organ involvement in the acute setting was single organ renal involvement in 16 patients (84.2%), followed by renal failure with respiratory failure (2 patients, 10.6%), followed by isolated respiratory failure (1 patient, 5.3%). This corresponded to the requirements for organ support (stated in 18 cases); most frequently patients required RRT (11 patients, 61.1%), followed by no support (4 patients, 22.2%), followed by RRT and ventilation (2 patients, 11.1%), followed by ventilation alone (1 patient, 5.6%).
In terms of patient outcomes, whether the patient survived was stated in 18 cases, of which 1 patient (5.9%) died, who showed isolated renal involvement requiring haemodialysis.45 Renal function at the point of discharge was stated in nine cases, one of which (11.1%) still had evidence of renal dysfunction at this time point.34 This patient had isolated renal involvement treated with intravenous hydration, intravenous bicarbonate, and diuretics, requiring RRT. Renal function after discharge was only stated in four cases; however, three of these were in reference to a further attack of rhabdomyolysis.
When comparing the group of patients who required no organ support and those that did, mean age was similar (21.5 versus 24 years respectively) and serum creatinine on admission was similar (551 versus 500 µmol/L respectively); however, mean CK on admission was strikingly different (10,544 U/L in the group requiring no support versus 110,660 U/L in the group requiring support).
Discussion
For this patient, intravenous glucose 10% was given at a rate of 2 mL/kg/h to maintain high to normal level of plasma glucose. This patient also received 13.6 L total of IV Hartmann’s solution at 400 mL/h during their admission, stopped when CK values fell sufficiently. This patient also received potassium replacement at a maximum rate of 10 mmol/h (120 mmol over total admission). With this patient the trigger for rhabdomyolysis was less clear early in the admission than in other cases; initially the myalgia and hyperpyrexia were attributed to the rhabdomyolysis itself rather than related to a potential cause. The overlap in symptomology of influenza (and viral in general) infection and rhabdomyolysis in these patients means that in the absence of a clear trigger other than viral infection, care teams should have a low threshold for isolation, respiratory contact precautions, respiratory viral swabs, and empirical antiviral administration.
With the management as outlined above, the patient reported here avoided all organ involvement during this episode of rhabdomyolysis, and as such required no organ support. Despite this, he benefitted in this instance from early admission to ICU from the environment which allowed close monitoring of fluid balance, his haemodynamic status, his electrolytes, and his CK values. This may be even more important in individuals who at greater risk of fluid overload or cardiac failure – although cardiac involvement in the phenotype of the disorder is not a universally recognised element, echocardiography in this otherwise healthy man patient displayed LVH.
The findings of our systematic review suggest that he was most at risk of developing isolated renal failure – early ICU admission also confers the benefit of prompt initiation of RRT in the case of an indication arising. Our results suggest that despite patients frequently requiring RRT, outcomes in terms of patient survival and renal function are favourable, suggesting that all patients in this cohort should be offered RRT if indicated. Our results also generate the interesting hypothesis that the CK levels on admission may be of predictive value in terms of the eventual need for organ support, and so high CK values in the first instance may further support the case for early ICU admission. The outcomes of this patient taken with his CK of 26,884 U/L on admission are consistent with this hypothesis; however, this requires further directed study to support this.
Limitations of our study at the review level were the small number of cases, incomplete retrieval of identified research, and the potential for publication bias. Limitations at the study level were heterogeneity between the patients themselves, the healthcare systems and teams in which they were cared for, and the reporting of the cases.
Supplemental Material
Supplemental material, sj-pdf-1-inc-10.1177_1751143719889766 for Rhabdomyolysis caused by carnitine palmitoyltransferase 2 deficiency: A case report and systematic review of the literature by Nicholas Ivin, Valentina Della Torre, Francis Sanders and Matthew Youngman in Journal of the Intensive Care Society
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent
Written informed consent was given by the patient for information and images to be published.
ORCID iD
Nicholas Ivin https://orcid.org/0000-0003-2974-2916
Matthew Youngman https://orcid.org/0000-0001-8965-1691
Supplemental material
Supplemental material for this article is available online.
References
- 1.McGarry JD, Brown NF. The mitochondrial carnitine palmitoyltransferase system. From concept to molecular analysis. Eur J Biochem 1997; 244: 1–14. [DOI] [PubMed] [Google Scholar]
- 2.Bonnefont J-P, Djouadi F, Prip-Buus C, et al. Carnitine palmitoyltransferases 1 and 2: biochemical, molecular and medical aspects. Mol Aspects Med 2004; 25: 495–520. [DOI] [PubMed] [Google Scholar]
- 3.Wieser T. Carnitine palmitoyltransferase II deficiency. In: Adam MP, et al. (ed). GeneReviews®, Seattle: University of Washington, 2004. [PubMed] [Google Scholar]
- 4.Avila-Smirnow D, Boutron A, Beytia-Reyes M, et al. Carnitine palmitoyltransferase type 2 deficiency: novel mutation in a Native South American family with whole-body muscle magnetic resonance imaging findings: two case reports. J Med Case Rep 2018; 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yasuno T, Kaneoka H, Tokuyasu T, et al. Mutations of carnitine palmitoyltransferase II (CPT II) in Japanese patients with CPT II deficiency. Clin Genet 2008; 73: 496–501. [DOI] [PubMed] [Google Scholar]
- 6.Isackson PJ, Bennett MJ, Vladutiu GD. Identification of 16 new disease-causing mutations in the CPT2 gene resulting in carnitine palmitoyltransferase II deficiency. Mol Genet Metab 2006; 89: 323–331. [DOI] [PubMed] [Google Scholar]
- 7.Deschauer M, Wieser T, Zierz S. Muscle carnitine palmitoyltransferase II deficiency: clinical and molecular genetic features and diagnostic aspects. Arch Neurol 2005; 62: 37–41. [DOI] [PubMed] [Google Scholar]
- 8.Lehmann D, Zierz S. Normal protein content but abnormally inhibited enzyme activity in muscle carnitine palmitoyltransferase II deficiency. J Neurol Sci 2014; 339: 183–188. [DOI] [PubMed] [Google Scholar]
- 9.Yao M, Cai M, Dengfu Y, et al. Abbreviated half-lives and impaired fuel utilization in carnitine palmitoyltransferase II variant fibroblasts. PLoS ONE 2015; 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Motlagh L, Golbik R, Sippl W, et al. Stabilization of the thermolabile variant S113L of carnitine palmitoyltransferase II. Neurol Genet 2016; 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Motlagh Scholle L, Thaele A, Beckers M, et al. Lack of activation of the S113L variant of carnitine palmitoyltransfersase II by cardiolipin. J Bioenerg Biomembr 2018; 50: 461–466. [DOI] [PubMed] [Google Scholar]
- 12.Motlagh L, Golbik R, Sippl W, et al. Malony-CoA inhibits the S113L variant of carnitine-palmitoyltransferase II. Biochim Biophys Acta 2016; 1861: 34–40. [DOI] [PubMed] [Google Scholar]
- 13.Sanders FWB, Griffin JL. De novo lipogenesis in the liver in health and disease: more than just a shunting yard for glucose. Biol Rev Camb Philos Soc 2016; 91: 452–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joshi PR, Deschauer M, Zierz S. Carnitine palmitoyltransferase II (CPT II) deficiency: genotype-phenotype analysis of 50 patients. J Neurol Sci 2014; 338: 107–111. [DOI] [PubMed] [Google Scholar]
- 15.Joshi PR, Deschauer M, Zierz S. Carnitine palmitoyltransferase II (CPT II) deficiency: genotype–phenotype analysis of 50 patients. J Neurol Sci 2014; 338: 107–111. [DOI] [PubMed] [Google Scholar]
- 16.de Sain-van der Velden MGM, Diekman EF, Jans JJ, et al. Differences between acylcarnitine profiles in plasma and bloodspots. Mol Genet Metab 2013; 110: 116–121. [DOI] [PubMed] [Google Scholar]
- 17.Wieser T. Carnitine palmitoyltransferase II deficiency. In: Adam MP, et al. (ed). GeneReviews®, Seattle: University of Washington, 1993. [PubMed] [Google Scholar]
- 18.BIMDG: British Inherited Metabolic Disease Group, http://www.bimdg.org.uk/guidelines/guidelines-adult.asp?s=az&i=l (accessed 29 March 2019).
- 19.RESERVED IU-AR. Orphanet: carnitine palmitoyltransferase II deficiency, https://www.orpha.net/consor/cgi-bin/OC_Exp.php?Lng=EN&Expert=157 (accessed 29 March 2019).
- 20.Reference GH. CPT II deficiency. Genetics home reference, https://ghr.nlm.nih.gov/condition/carnitine-palmitoyltransferase-ii-deficiency (accessed 29 March 2019).
- 21.Mochel F, DeLonlay P, Touati G, et al. Pyruvate carboxylase deficiency: clinical and biochemical response to anaplerotic diet therapy. Mol Genet Metab 2005; 84: 305–312. [DOI] [PubMed] [Google Scholar]
- 22.Rhabdomyolysis, Critical Care Guidelines 2016 (updated 2019), Critical Care Unit, West Suffolk Hospital NHS Foundation Trust, Bury St Edmunds, UK (accessed February 2019).
- 23.Bosch X, Poch E, Grau JM. Rhabdomyolysis and acute kidney injury. N Engl J Med 2009; 361: 62–72. [DOI] [PubMed] [Google Scholar]
- 24.Zimmerman JL, Shen MC. Rhabdomyolysis. Chest 2013; 144: 1058–1065. [DOI] [PubMed] [Google Scholar]
- 25.Cho YS, Lim H, Kim SH. Comparison of lactated Ringer’s solution and 0.9% saline in the treatment of rhabdomyolysis induced by doxylamine intoxication. Emerg Med J 2007; 24: 276–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams J, Thorpe C. Rhabdomyolysis. Contin Educ Anaesth Crit Care Pain 2014; 14: 163–166. [Google Scholar]
- 27.Thondebhavi Subbaramaiah M, Sapsford D, Banham-Hall E. Acetazolamide as an adjunct to sodium bicarbonate in the treatment of rhabdomyolysis. Anaesth Intensive Care 2010; 38: 398. [PubMed] [Google Scholar]
- 28.Kottlors M, Jaksch M, Ketelsen U-P, et al. Valproic acid triggers acute rhabdomyolysis in a patient with carnitine palmitoyltransferase type II deficiency. Neuromuscul Disord 2001; 11: 757–759. [DOI] [PubMed] [Google Scholar]
- 29.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alaygut D, Torun Bayram M, Kasap B, et al. Rhabdomyolysis with different etiologies in childhood. World J Clin Pediatr 2017; 6: 161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alečković-Halilović M, Mešić E, Sinanović O, et al. Carnitine palmitoyl transferase deficiency – unrecognized cause of recurrent acute kidney injury. Ren Fail 2013; 35: 732–734. [DOI] [PubMed] [Google Scholar]
- 32.Blah N, Sudrie-Arnaud B, Torre S, et al. Acute respiratory infection unveiling CPT II deficiency. Int J Mol Sci 2018; 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brownell AK, Severson DL, Thompson CD, et al. Cold induced rhabdomyolysis in carnitine palmyityl transferase deficiency. Can J Neurol Sci 1979; 6: 367–370. [DOI] [PubMed] [Google Scholar]
- 34.Cucchiari D, Colombo I, Amato O, et al. Exertional rhabdomyolysis leading to acute kidney injury: when genetic defects are diagnosed in adult life. CEN Case Rep 2017; 7: 62–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deutsch M, Vassilopoulos D, Sevastos N, et al. Severe rhabdomyolysis with hypoglycemia in an adult patient with carnitine palmitoyltransferase II deficiency. Eur J Intern Med 2008; 19: 289–291. [DOI] [PubMed] [Google Scholar]
- 36.Donato SD, Castiglione A, Rimoldi M, et al. Heterogeneity of carnitine-palmitoyltransferase deficiency. J Neurol Sci 1981; 50: 207–215. [DOI] [PubMed] [Google Scholar]
- 37.Ferrara AM, Sciacco M, Zovato S, et al. Coexistence of VHL disease and CPT2 deficiency: a case report. Cancer Res Treat Off J Korean Cancer Assoc 2016; 48: 1438–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gentili A, Iannella E, Masciopinto F, et al. Rhabdomyolysis and respiratory failure: rare presentation of carnitine palmityl-transferase II deficiency. Minerva Anestesiol 2008; 74: 205–208. [PubMed] [Google Scholar]
- 39.Gjorgjievski N, Dzekova-Vidimliski P, Petronijevic Z, et al. Carnitine palmitoyltransferase II deficiency (CPT II) followed by rhabdomyolysis and acute kidney injury. Open Access Maced J Med Sci 2018; 6: 666–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hargreaves IP, Heales SJ, Olpin SE, et al. The diagnosis of carnitine palmitoyltransferase II deficiency is now possible in small skeletal muscle biopsies. J Inherit Metab Dis 2000; 23: 352–354. [DOI] [PubMed] [Google Scholar]
- 41.Joussain C, Lamireau D, Espil-Taris C, et al. A 10-year-old boy with dark urine and acute kidney injury: question. Pediatr Nephrol 2011; 26: 1229–1230. [DOI] [PubMed] [Google Scholar]
- 42.Kaneoka H, Uesugi N, Moriguchi A, et al. Carnitine palmitoyltransferase II deficiency due to a novel gene variant in a patient with rhabdomyolysis and ARF. Am J Kidney Dis 2005; 45: 596–602. [DOI] [PubMed] [Google Scholar]
- 43.Ross NS, Hoppel CL. Partial muscle carnitine palmitoyltransferase – a deficiency: rhabdomyolysis associated with transiently decreased muscle carnitine content after ibuprofen therapy. JAMA 1987; 257: 62–65. [DOI] [PubMed] [Google Scholar]
- 44.Sciacco M, Prelle A, Fagiolari G, et al. A case of CPT deficiency, homoplasmic mtDNA mutation and ragged red fibers at muscle biopsy. J Neurol Sci 2005; 239: 21–24. [DOI] [PubMed] [Google Scholar]
- 45.Shintani S, Shiigai T, Sugiyama N. Atypical presentation of carnitine palmitoyltransferase (CPT) deficiency as status epilepticus. J Neurol Sci 1995; 129: 69–73. [DOI] [PubMed] [Google Scholar]
- 46.Smolle KH, Kaufmann P, Gasser R. Recurrent rhabdomyolysis and acute respiratory failure due to carnitine palmityltransferase deficiency. Intensive Care Med 2001; 27: 1235. [DOI] [PubMed] [Google Scholar]
- 47.Uzel B, Altiparmak MR, Ataman R, et al. Acute renal failure due to carnitine palmitoyltransferase II deficiency. Neth J Med 2003; 61: 417–420. [PubMed] [Google Scholar]
- 48.Villard J, Fischer A, Mandon G, et al. Recurrent myoglobinuria due to carnitine palmitoyltransferase II deficiency: expression of the molecular phenotype in cultured muscle cells. J Neurol Sci 1996; 136: 178–181. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-inc-10.1177_1751143719889766 for Rhabdomyolysis caused by carnitine palmitoyltransferase 2 deficiency: A case report and systematic review of the literature by Nicholas Ivin, Valentina Della Torre, Francis Sanders and Matthew Youngman in Journal of the Intensive Care Society