Abstract
Variation in the time interval between consecutive R wave peaks of the QRS complex has long been recognised. Measurement of this RR interval is used to derive heart rate variability. Heart rate variability is thought to reflect modulation of automaticity of the sinus node by the sympathetic and parasympathetic components of the autonomic nervous system. The clinical application of heart rate variability in determining prognosis post myocardial infarction and the risk of sudden cardiac death is well recognised. More recently, analysis of heart rate variability has found utility in predicting foetal deterioration, deterioration due to sepsis and impending multiorgan dysfunction syndrome in critically unwell adults. Moreover, reductions in heart rate variability have been associated with increased mortality in patients admitted to the intensive care unit. It is hypothesised that heart rate variability reflects and quantifies the neural regulation of organ systems such as the cardiovascular and respiratory systems. In disease states, it is thought that there is an ‘uncoupling’ of organ systems, leading to alterations in ‘inter-organ communication’ and a clinically detectable reduction in heart rate variability. Despite the increasing evidence of the utility of measuring heart rate variability, there remains debate as to the methodology that best represents clinically relevant outcomes. With continuing advances in technology, our understanding of the physiology responsible for heart rate variability evolves. In this article, we review the current understanding of the physiological basis of heart rate variability and the methods available for its measurement. Finally, we review the emerging use of heart rate variability analysis in intensive care medicine and conditions in which heart rate variability has shown promise as a potential physiomarker of disease.
Keywords: Heart rate variability, autonomic nervous system, critical care medicine, electrocardiography, physiology, variability analysis
Introduction
Stephen Hales in 1733 was the first to report that the time interval between individual arterial pulsations varied in horses.1 Since then, the introduction of ambulatory ECG has led to the recognition that the time period between successive R waves on the ECG varies in mammals.1,2 This variability between heartbeats or R-R interval (RRi) (Figure 1) is a feature of the healthy cardiovascular system and is more commonly known as the heart rate variability (HRV).2,3
Hon and Lee first recognised the clinical potential of HRV when they noted that acute alterations in the HRV were a marker of foetal distress and predicted foetal hypoxia.4 Today, monitoring the variability of foetal heart rate has become a standard of care and has been responsible for significant reductions in foetal morbidity and mortality.4,5 Similar alterations in HRV have been recognised post myocardial infarction and are associated with a five-fold increase in mortality.6,7 More recently, reduced HRV parameters have been reported as an independent predictor of 30-day mortality and provided additional predictive value over APACHE II scores in critically unwell patients.8
The increased appreciation of the clinical potential of HRV analysis has led to its use in various clinical situations common to intensive care medicine including multiorgan dysfunction syndrome (MODS), sepsis and trauma.9–11 With this in mind, the following review aims to discuss the physiological basis of HRV, the measurement of HRV and the emerging clinical role of HRV analysis in intensive care medicine.
Physiological basis of HRV
Automaticity is common to cardiac pacemaker tissue; however, heart rate and rhythm are continuously altered and regulated by the autonomic nervous system (ANS).2,12
The parasympathetic nervous system (PNS) innervates the sinoatrial node, the atrioventricular node and the atrial myocardium via the vagus nerve.1,13 Parasympathetic activation leads to release of acetylcholine (ACh), which slows the heart rate and lengthens the R-R interval.1,13 Parasympathetic activation leads to an almost immediate reduction in heart rate due to the very short latency of effect of ACh, and the rate at which ACh is rapidly metabolised and cleared.1,2 Therefore, the PNS regulates heart rate on a near beat by beat basis.1 In contrast, sympathetic nervous system (SNS) activation initiates the synaptic release of catecholamines that increase cardiac contractility and heart rate.1,2 The action of catecholamines is slow compared to that of ACh and results in a delay between the onset of sympathetic stimulation and changes in heart rate of approximately 5 s.1,14 Despite the slower onset, sympathetic stimulation has a longer duration of action, affecting heart rate for 5–10 s following the cessation of a sympathetic stimulus.1,14 The differences in neurotransmitters between the PNS and SNS has led to the recognition that the effects of each arm of the ANS are not opposite and symmetrical but confer overlapping and different time frequencies of action.1
In healthy individuals' cyclical changes in HRV occur with respiration and fluctuations in blood pressure.15,16 Frequency domain and power spectral density (PSD) analysis utilises fast Fourier transform (FFT) analysis to describe oscillations in the RRi and transform them into discrete frequencies that help to conceptualise our understanding of the physiological mechanisms responsible for HRV.17,18
Since cyclical changes in HRV are associated with respiration and occur at a high frequency (HF) of 0.25 Hz, they are thought to dominate a number of cardiorespiratory and neural interactions.2,16 These interactions are responsible for the observation of respiratory sinus arrhythmia, characterised by shortening of the RRi with inspiration and lengthening with expiration.1 Abolition of these HF oscillations can be achieved by parasympathetic blockade with atropine, suggesting that they are parasympathetically mediated.15
Cyclical changes associated with fluctuations in arterial blood pressure (ABP) occur at a low frequency (LF) of 0.10 Hz and are thought to be mediated by the SNS.2 These oscillations occur in synchrony with arterial Mayer waves.1 Mayer waves are spontaneous oscillations in ABP whose amplitude is thought to measure sympathetic vasomotor tone.16 Mayer wave oscillations are thought to parallel oscillations in HRV and in particular the LF oscillations recognised in HRV.1 These are attenuated and completely abolished by alpha adrenergic antagonist drugs, suggesting that sympathetic activity is important in the generation of these oscillations.1 There remains debate as to the precise physiological origin of Mayer waves in the generation of heart rate frequencies at 0.10 Hz and controversy exists in attributing all LF HRV oscillations to sympathetic modulation.19 Research has demonstrated that parasympathetic blockade also produces modulation of LF oscillations in HRV.1 Despite this, measurement of HF and LF oscillations calculated as a ratio of LF/HF has been suggested as a measure of sympathovagal balance with relative changes in the magnitude of each frequency reflecting the dominance of a particular arm of the ANS.2,15
HF and LF components of HRV account for only 5% of the total power of HRV recordings measured by PSD analysis. The remaining 95% is accounted for by two other frequencies called the very low frequency (VLF) band and ultra-low frequency (ULF) band.13 Historically, these frequency components have not been well characterised. However, recent research suggests that the VLF band is associated with thermoregulatory mechanisms, changes in peripheral chemoreceptor activity and fluctuations in the renin-angiotensin system (RAAS), whilst the ULF band is thought to reflect oscillations due to circadian rhythm.17,20 Despite relatively less being known about the VLF and ULF frequencies, they appear to be clinically important as reduced variability in the VLF band is associated with arrhythmias, high inflammation levels and increased mortality.21
Measuring HRV
In 1996, The European Society of Cardiology and the North American Society of Pacing and Electrophysiology published guidelines aimed at standardising the terminology and methodology used in the measurement of HRV.12 These guidelines describe a number of methods for measuring HRV including linear measures such as time domain and frequency domain measures and non-linear measures such as the Poincare plot.12 Recent advances in biological systems theory, HRV analysis and complexity analysis have resulted in updated guidance for non-linear techniques such as entropy and fractal analysis that focus on similarities in the RRi over a given time period.20,22
Time domain measures
Time domain measures derive HRV using either statistical or geometric analysis.12 Statistical analyses (e.g. standard deviation) are applied to the RRi to measure variation over a specified period of time from <1 min to 24 h.12,20 Geometric derivation of HRV requires that a series of RRi are converted into a geometric pattern, such as a sample density distribution of RRi and analysed using statistical methods (Table 1).12,23
Table 1.
Most common linear and non-linear HRV measures.
| Variability analysis | Parameters | Units | Description |
|---|---|---|---|
| Time domain | |||
| Statistical Geometric | SDNN SDANN RMSSD SDNN index SDSD NN50 pNN50 HRV triangular index TINN Differential index Logarithmic index | ms ms ms ms ms % ms ms ms−1 | Standard deviation of the NN intervala Standard deviation of average of NN intervals in 5 min epochs of entire recording Square of the root of the mean of the sum of square differences between adjacent NN intervals Mean of the standard deviations of all NN intervals for all 5 min segments Standard deviation of differences between adjacent NN intervals Number of pairs of adjacent NN intervals differing by more than 50 ms NN50 count divided by the total number of all NN intervals Total NN intervals divided by height of histogram of all NN intervals Baseline width of the minimum square difference triangular interpolation of the highest peak of the histogram of all NN intervals Difference between widths of the histograms of differences between adjacent NN intervals measured at selected heights |
| Frequency domain | |||
| Short-term recording (5 min) Long-term recording (24 h) | Total power VLF LF LF norm HF HF norm LF/HF α | ms2 ms2 ms2 n.u ms2 n.u | Variance of the NN intervals over the temporal segment Power of very low frequency range (0.0033 Hz–0.04 Hz) Power of the low frequency range (0.04 Hz–0.15 Hz) LF in normalised units Ratio of the LF to HF Power of the high frequency HF in normalised units (0.15 Hz–0.40 Hz) Slope of the linear interpolation of the spectrum in a log-log scale |
| Non-linear measures | |||
| Poincare analysis Entropy analysis Detrended fluctuation analysis | S SD1 SD2 SD1/SD2 ApEn SampEn DFAα1 DFAα2 D2 | ms ms ms % | Area of the ellipse, representing total variability Standard deviation perpendicular to the line of identity Standard deviation along the line of identity Ratio of SD1 to SD2 Approximate entropy, measures the regularity and complexity associated with a time series Sample entropy, measures the regularity and complexity associated with a time series Detrended fluctuation analysis that describes short term fluctuations (<10–11 beat) Detrended fluctuation analysis that describes long term fluctuations (>10 beat) Correlation dimension (estimates the minimum number of variables required to construct a model of system dynamics |
Note: Adapted from: Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, Standards of measurement of HRV guidelines.12
NN interval refers to RRi for normal R wave peaks, i.e. those that are not abnormal due to arrhythmia/interference/artefact. In practice, RRi and NN interval are used synonymously.
Time domain measures are easy to calculate and simple to derive.12,24 However, they are sensitive to artefact particularly supraventricular and ventricular extrasystolic beats.24 Therefore, ECG recordings need careful pre-processing to ensure removal of extrasystolic beats and interference. Similarly, they require stationarity in the time series (i.e. the mean heart rate does not change significantly), which is a property not often met in biological systems.24 For these reasons, time domain measures cannot discriminate between alterations in SNS or PNS output. Despite this, they can be used to assess overall ANS activity and provide useful clinical information.1,24
Frequency domain measures
Frequency domain measures describe variation in the RRi following transformation into different frequency components. Frequency domain measures are derived using FFT analysis to provide information on the frequency components of HRV over a time series (Figure 2).2,12,24 In analysis of 2 to 5 min ECG recordings, three characteristic frequencies are recognised, LF, HF and VLF (Table 1).24 In 24 h recordings, the ULF band is recognised with the VLF band.12 In general, to accurately determine the power of a LF banding, a recording greater or equal to approximately 5/f is required. Frequency domain, like time domain analysis, is sensitive to artefact, ectopic beats and require stationarity in the data series.24 Physiological mechanisms such as changes in posture, levels of stress and movement are thought to alter LF and HF readings; therefore, factors that are known to modulate the ANS should be controlled during HRV measurement.12,24,25
Figure 1.
Electrocardiogram demonstrating the interval between R waves. Heart rate variability is derived by measuring the variability in consecutive time intervals between each R wave.
Non-linear measures of HRV
Non-linear measures overcome the requirement of stationarity in data unlike the linear measures.20,26 They include techniques such as the Poincare plot, detrended fluctuation analysis (DFA) and approximate and sample entropy analysis (ApEN and SampEN).26 Non-linear measures model dynamic systems using variables that cannot be plotted on a straight line.22 Physiological systems are dynamic due to complex interactions between cardiovascular, endocrine and autonomic systems and do not ordinarily display stationarity. Therefore, non-linear measures may offer a number of advantages over linear HRV measures when stationarity cannot be guaranteed.26 The non-linear methods implicitly assume that the factors that create HRV occur as oscillatory inputs with associated random variation.27 Non-linear methods borrow techniques from fractal mathematics and produce variables that describe the pattern of variability by analysing temporal similarities in the signals.27 Typically, parameters are derived that separately describe the scaling of short-term variability (e.g. < 10 beats) and longer term trends. Whilst, as yet, these parameters do not offer a great deal of mechanistic insight, they are robust and can distinguish between patient groups.2
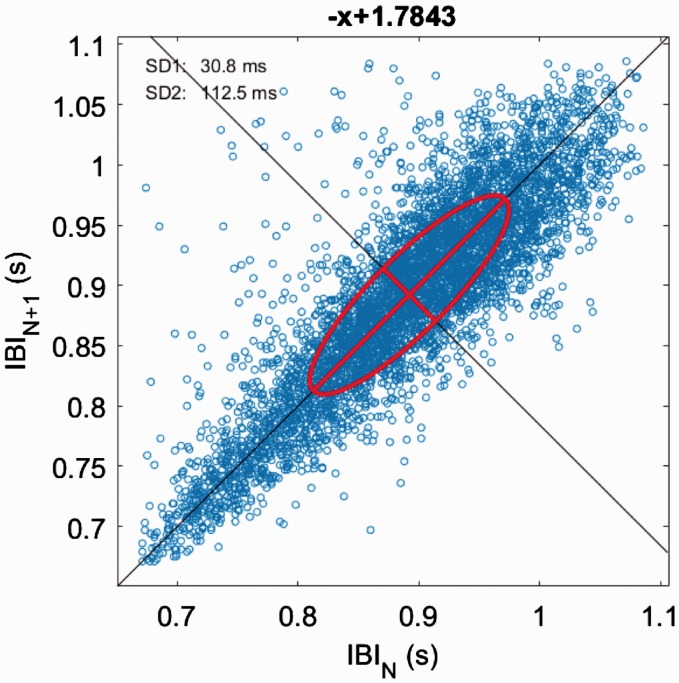
Poincare plot
Poincare plots are a graphical representation (scatter plot) of HRV generated by plotting each RRi against the prior RRi (Figure 3).20
Figure 2.
Power spectral density analysis showing frequency domain measures of heart rate variability. Three characteristic peaks are seen corresponding to the low frequency (LF) domain 0.03–0.15 Hz, high frequency domain (HF) 0.15–0.40 Hz and very low frequency (VLF) 0.003–0.04 Hz domain of heart rate variability recordings. Five-minute recording taken from healthy male and analysed using Kubios V3.1 software.
Figure 3.
Poincare plot of healthy individual taken from 5-min ECG recording and analysed in Kubios V3.1. A Poincare plot is a non-linear heart rate variability technique that plots each RR interval as a function of the previous RR interval. The plot provides summary information such as total variability represented as a central ellipse. The plot is analysed by calculating the standard deviations SD1 relating to fast beat-to-beat variability and SD2 describing longer term variability.
Poincare plots are analysed by fitting an ellipse to the data series. Three non-linear measures are typically derived, SD, SD1 and SD2 (Table 1).20 Total variability (S) in the sample is represented by the entire area of the ellipse.20
DFA
DFA correlates the fluctuations between RRi over different time scales and analyses temporal self-similarities in the RRi.27 Short-term fluctuations are represented by DFAα1, whilst long-term fluctuations are represented by DFAα2.20 The calculation of DFA involves several steps, and during the calculation, non-stationarity in the signal is addressed by subtraction of extrinsic fluctuations; this has been extensively reviewed elsewhere.28 The primary advantage of DFA is removal of confounding due to non-stationarity during DFA calculation.29 However, it requires large data sets and whether it offers further information compared to other techniques requires further investigation.24,28
Entropy
Entropy analysis can be applied to a series of RRi and provides a measure of the degree of irregularity or ‘randomness’ within the series.24 The technique essentially calculates the probability that any given sequence of intervals within the RRi series will be repeated.27 The more likely to be repeated, the lower the calculated entropy. Measures of such entropy include the ApEN and SampEN, respectively. Clinically, lower entropy values correlate to a state of illness.24,30 SampEN was introduced to address the sensitivity of ApEN to sample size and the inaccuracy of ApEN when the number of data points are low in a time series.24
Factors affecting HRV measurement
Despite the promising ability of HRV to provide information on biological systems, there remains a number of physiological and technical issues that need to be considered when interpreting HRV clinically. The context of HRV recording is crucial, as numerous factors including age (increased age leads to reduced HRV), gender (higher HRV in females), resting heart rate and recent physical activity, are thought to alter HRV.20 Factors such as posture and movement also need to be considered as it has been shown that HRV is markedly altered between standing and supine positioning.12 HRV is also affected by a number of technical factors such as ECG sampling frequency, length of ECG recording and the presence of artefact or interference.12,20 To detect the R wave fiducial point on the ECG, a sampling frequency minimum of 500 Hz is recommended.12 However, as HRV decreases with illness, it may be necessary to sample at a much higher frequency to ensure adequate resolution and accuracy.20 A recent systematic review of HRV use in critical care highlighted that a significant number of studies used sampling frequencies as low as 250 Hz and these results should be considered with caution.11 Similarly, the length of recording is crucial and can significantly affect time and frequency domain HRV measures. Recommendations have been made regarding acceptable ECG recording lengths for each HRV measure; however, the existing literature often fails to accurately report the duration of ECG recordings used in studies, potentially introducing an element of uncertainty to their results.11,12 Artefacts can significantly distort time and frequency domain HRV measures and the bias of a single artefact can distort the entire HRV recording. Manual inspection of ECG is recommended to ensure that HRV analysis is conducted on ECG segments that are free of artefact, ectopic beats, missed beats and interference.12 Artefacts such as missed and ectopic beats can be resolved by artefact removal and interpolation of an R wave based on previous QRS intervals.20 However, with increasing interpolation of R waves, a significant amount of noise to signal ratio can be introduced in the data series and lead to errors in HRV measures. Similarly, arrhythmias such as atrial fibrillation (AF) can introduce significant distortion in HRV and therefore should not be considered accurate in patients with AF. These factors need to be considered when interpreting HRV in the clinical context.
HRV in intensive care medicine
HRV is frequently used to describe the activity of the SNS and PNS. However, this relies on the assumption that the ANS is in balance, with low PNS activity associated with a correspondingly high SNS activity and vice versa.31 Many authors have refuted this, and it is generally accepted that the relative balance of the ANS is more complex.
Similarly, mechanisms responsible for RRi and HRV are complex and reflect inputs from multiple physiological systems, including the SNS, PNS, RAAS, thermoregulatory systems, as well as mechanical inputs from respiration and alterations in ABP.32
Despite debate regarding the association between HRV and the ANS, the previous two decades have witnessed a significant expansion in the use of HRV analysis and increasing evidence supporting its use in intensive care.11
Autonomic dysfunction is common to a number of disorders seen in critical care patients, such as MODS, sepsis, myocardial infarction, decompensated heart failure and severe brain injury (SBI).11,33,34 The ability to assess autonomic function may provide valuable information regarding the pathophysiology, severity and prognosis of these disorders.33 However, the reader is reminded that whilst an association between HRV and the ANS certainly exists, HRV does not directly measure autonomic activity and any association is likely a combination of complex physiological inputs.31 With this in mind, the remainder of this review will focus on areas in which HRV has found utility in intensive care medicine.
Multiorgan dysfunction syndrome and Sepsis
As early as 1995, it was recognised that SDNN, LF, LF/HF are reduced in sepsis.11,35–37 Godin and Buchman suggested that organ systems are connected to each other via neural, hormonal and cytokine networks and that they each behave as biological oscillators.38 They hypothesised that sepsis resulted in an uncoupling of organ systems and leads to a reduction in HRV parameters.30,38 They proposed that HRV was a method for the quantification of ‘inter-organ communication’ and yielded valuable information in the pathophysiology of sepsis and prognosis of patients admitted to the intensive care unit (ICU).38 Recently, Bishop et al. reported that reduction in the VLF domain was predictive of 30 day all-cause mortality in patients admitted to ICU.39 Similar findings have been reported by Schmidt et al. who found that a reduced VLF was predictive of 28-day mortality in patients with MODS.40 HRV analysis may be able to predict mortality early in a patient's presentation, with Chen et al. reporting that a reduced SDNN was predictive of in-hospital mortality in septic patients admitted to the accident and emergency department.9 Interestingly, Chen et al. also reported that an increased HF was predictive of hospital survival, suggesting that health is associated with a high degree of variability.9 This was confirmed by Papaioannou et al. in a novel study that tracked changing HRV in response to a patient's pathophysiological state.41 SOFA scores were longitudinally tracked with a number of HRV measures over time and revealed that entropy was reduced in non-survivors, and the long term non-linear HRV parameter DFAα2 correlated with length of ICU stay.41 Moreover, patients who were more clinically unstable had a reduced LF/HF ratio, and a reduction in overall variance.41 This recovered as patients improved and were finally discharged from ICU, suggesting that HRV analysis may be valuable as a method of monitoring physiological deterioration and offer real-time prognostication in critically unwell patients.41
HRV may also serve to predict those patients at risk of deterioration and those who may benefit from early ICU admission. In a recent observational study in septic emergency department patients, Samsudin et al. report a scoring system utilising two vital signs (respiratory rate and systolic blood pressure), age and two HRV measures (mean RRi and DFAα2).42 They revealed that the use of HRV not only outperformed SOFA, NEWS and MEWS scoring at prediction of 30-day mortality but was also able to accurately predict those patients requiring ICU admission and intubation.42 Similar scoring systems utilising HRV have already shown promise in neonatal patients. In the landmark HeRO Trial, Moorman et al. revealed that monitoring heart rate characteristics including reduced variability and transient heart rate decelerations, led to a 22% relative reduction in mortality in very low birthweight neonates.43 The HeRO trial provided clinicians with a score based on a composite measure utilising SD RRi, sample asymmetry (a measure of transient accelerations and deceleration of the heart rate) and SampEN.44 Using multivariable logistic regression and mathematical algorithms, the HeRO score provides continuous non-invasive monitoring that estimates the fold-increase in the probability of sepsis.43,44 The HeRO trial and scoring systems developed by Samsudin et al. hint at the possibility of a new generation of physiomarkers for the earlier detection of deterioration and sepsis.42,44
HRV and inflammation
Inflammation is associated with a number of conditions that present to ICU such as myocardial infarction, sepsis, systemic inflammatory response syndrome, MODS and severe trauma.45 Factors that trigger inflammation also enhance anti-inflammatory pathways that counterbalance the initial pro-inflammatory signal.21,45 An inflammatory reflex has been described, in which cytokines induce neuroendocrine modulatory mechanisms that signal via the ANS.21,45 In response to inflammation, vagal outflow increases systemically and more specifically to organs such as the spleen that are thought to be responsible for the upregulation of anti-inflammatory cytokine levels.45,46 It is thought that this counter-regulatory mechanism confers protection against unregulated tissue damage in inflammatory conditions and poly-microbial infection and is known as the ‘cholinergic anti-inflammatory pathway’.45 HRV analysis has helped elucidate the role the ANS plays in the inflammatory reflex, and a depressed parasympathetic activity has been implicated in the pathogenesis of diseases associated with an exaggerated inflammatory response.45 A number of authors have correlated HRV with inflammatory markers.21,47,48 Tateishi et al. investigated the relationship between IL-6 and HRV in patients admitted to critical care with sepsis and found that IL-6 was negatively correlated with the LF component of HRV analysis.47 Papaioannou et al. tracked patients from admission to critical care and reported an inverse correlation between LF and LF/HF and C-reactive protein (CRP) levels.21 HF HRV was correlated with IL-10 levels, suggesting that LF/HF ratio and reduced LF HRV are related to both pro-inflammatory and anti-inflammatory responses.21 Furthermore, those patients who developed shock had increased biomarkers (CRP, IL-6, IL-10) and decreased HRV, reaching statistical significance in patients with a SOFA score >10.21 This suggests that HRV is related to both anti-inflammatory and pro-inflammatory signals with a stronger association being present in patients who are more unwell.21
There is strong evidence that the ANS influences the physiological response to inflammation and recent research suggests that the anticholinergic anti-inflammatory pathway may hold promise as a therapeutic target.21,49 HRV measurement may therefore prove to be a novel physiomarker that characterises the cardiorespiratory responses to inflammation and may have prognostic value in any future anti-inflammatory treatments.50
Cardiovascular disorders, arrhythmias and cardiac arrest
It is generally accepted that HRV is a powerful predictor of cardiac mortality, arrhythmia and sudden cardiac death, and is independent of other risk factors (left ventricular ejection fraction, ventricular extra-systoles and episodes of non-sustained ventricular tachycardia) after myocardial infarction.5,7,12,51 A substudy of the large ATRAMI trial found that decreased SDNN and impaired heart rate response to an increase in blood pressure (baroreceptor sensitivity) were predictors of cardiac mortality.52 In patients with a reduced ejection fraction, the presence of a reduced SDNN or low baroreceptor sensitivity carried a relative risk of mortality of 6.7 and 8.7, respectively.52 Reduced HRV may also provide an early warning of deterioration as Passariello et al. have shown that patients who suffer sudden cardiac death secondary to fatal arrhythmia have a marked decrease in SDNN in the 5 min preceding its onset.53 Similar findings are reported in patients who suffer from paroxysmal AF, where ApEN was decreased up to 100 min prior to the onset of arrhythmia.22 That HRV analysis appears to be able to predict patients at risk of cardiac mortality and arrhythmias may prove useful for risk stratification, particularly in patients at increased cardiovascular risk such as in the peri-operative period.33
HRV has also been used to monitor the responses to drug treatment in patients with cardiovascular disease and hypertension. Beta-antagonists such as metoprolol and atenolol tend to augment HF whilst reducing LF in patients with hypertension.54 Similar findings have been reported post myocardial infarction, where the addition of metoprolol leads to a reduction in LF output.54 However, cardiovascular drugs such as, statins and calcium channel antagonists have been found to have a variable effect on HRV.11 Interestingly, drugs that would be expected to have profound effects on the ANS such as catecholamines have also been shown to have variable effects on HRV. A recent systematic review reported three studies that did not show any association between HRV parameters and vasopressor requirement or administration of exogenous catecholamines.11 Despite no finding of an association, the authors highlighted that the majority of studies failed to report the administration of cardiovascular drugs, vasopressors or catecholamines and had limited ability to draw any conclusions regarding the potential effects on HRV.11,54
HRV may also offer important information regarding neurological recovery post cardiac arrest.33,55 Tiainen et al. in a randomised trial reported significantly higher HRV measures in those patients who underwent therapeutic hypothermia (TH) compared to normothermia post cardiac arrest.55 Higher SDNN, SDANN, HF and LF measures were recorded in the first 48 h of TH.55 The authors suggest that higher HRV measures may represent a beneficial effect on myocardial function and preservation of ANS function or the neuroprotective effects of cooling.55 However, they acknowledge that this finding may be due to confounding and secondary to the relative bradycardia that TH induces in patients.55 The exact mechanism underlying altered HRV with TH remains uncertain, despite this the potential of HRV to predict outcome post cardiac arrest should be confirmed with larger trials.33,55
Neurological disorders
Lowensohn et al. were amongst the first authors to investigate the links between HRV and neurological disorders.56 In brain-damaged adults, Lowensohn et al. revealed that HRV decreased and rapidly diminished in line with increases in intracranial pressure (ICP).56 A more recent study in 145 trauma patients confirmed that an increase in ICP, as measured by invasive ICP monitoring, is preceded by a reduction in HRV.57 Reduction in HRV has been shown to be proportional to the increase in ICP, with more marked alterations in HRV occurring when ICP was >30 mmHg or cerebral perfusion pressure <40 mmHg.33 Moreover, reductions in HRV preceded changes in ICP by approximately 24 h.57 These findings suggest that HRV may function as a non-invasive method of monitoring early changes in ICP and may identify those patients who would benefit from invasive monitoring.57
Complications following subarachnoid haemorrhage (SAH) can include severe vasospasm, neurogenic stress cardiomyopathy and cardiac arrhythmias.58 Reduction in RMSSD has been shown to be associated with neurogenic stress cardiomyopathy following SAH.58 Similar alterations in HRV have been recognised in extradural, subdural and intracerebral haematomas.33 Schmidt et al. have investigated VLF reductions and delayed cerebral ischaemia secondary to cerebral vasospasm in SAH patients.59 It is thought that VLF may partly represent parasympathetic outflow and reductions in VLF are associated with states of high inflammation.20 Both RMSSD and VLF have been shown to predict complications following SAH, and it has been suggested that this may be related to the pro-inflammatory response contributing to the development of cerebral ischaemia after SAH.59
Changes in HRV have also been shown to be an early indication of the occurrence of brain death.60 Conci et al. reported a reduction in the total power of frequency domain analysis and suggested that these changes likely mirror a cessation of the activity of cardiorespiratory brainstem centres.60 These findings have been confirmed by others who measured continuous HRV and found that the loss of spectral power occurred during the transition to brain death.61 Taken together, these findings may be useful as a complementary method in the diagnosis of brain stem death and help inform when more formal brain stem death testing should occur.60,61
Conclusion
HRV analysis offers a unique monitoring modality that provides information regarding variability in complex biological signals. Unlike existing monitoring, HRV can potentially detect and track the state of the whole physiological system over time and during the development of illness, potentially even before it is clinically apparent. Goldberger et al. described illness as the de-complexification of complex biological systems and suggested that health is characterised by ‘organised variability’ whilst reduced variability is associated with disease states, such as MODS and sepsis.62 The inclusion of HRV measures into current early warning scoring systems such as NEWS could potentially lead to a new generation of physiomarkers that can predict deterioration earlier and help target those patients at greatest risk of mortality.42 The HeRO trial and HeRO monitoring system have shown that incorporation of HRV measures can potentially lead to earlier investigation and treatment and significantly improved clinical outcomes.44
Despite the potential of HRV measurement, it is still largely a research technique and has not become part of routine monitoring in critical care.63 There are a number of potential reasons for this. First, despite the large number of experimental studies, the majority are cohort or case-control studies of low methodological quality.11 Many studies also failed to fully account for confounding factors such as commonly used drugs in ICU, including anti-arrhythmic medications and the impact that interventions in ICU such as mechanical ventilation have on HRV parameters.11 Second, there is a lack of standardised methodology for the recording, processing and derivation of HRV from ECG. Despite guidelines from The European Society of Cardiology and the North American Society of Pacing and Electrophysiology, obtaining clinically useful HRV parameters still requires clinicians to pre-process ECG and RRi data using standard ECG monitoring equipment before using standalone software to derive HRV parameters.12 A number of open source software packages written in Matlab mathematical language are available as well as a number of paid software packages such as Kubios and ARTiiFACT.64–66 To date, the authors are not aware of any monitoring systems that derive HRV in real time at the bedside and this likely limits its widespread use in ICU. Third, despite evidence to suggest that HRV can predict deterioration, arrhythmias and MODS, the exact pathophysiological mechanisms underlying these associations remain unclear. Throughout this review, we have discussed HRV as a measure of autonomic function. In reality, individual HRV parameters are more complex and multiple physiological factors impact upon them.31 Until the exact mechanisms responsible for measured HRV parameters are uncovered, it is difficult to fully define a mechanistic basis for HRV.31
Measurement of HRV, along with advances in biomedical engineering and computational methods, has increased our understanding of the role the ANS plays in the pathophysiology of disease and illness. But for HRV analysis to become a standard of monitoring in critical care, prospective studies are needed to address the technical considerations, determine what factors confound HRV analysis and develop consensus standards for HRV monitoring in ICU. In conclusion, if these challenges are addressed, HRV analysis has the potential to revolutionise critical care monitoring and introduce an era of monitoring based on individualised variability analysis.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Draghici AE, Taylor JA. The physiological basis and measurement of heart rate variability in humans. J Physiol Anthropol 2016; 35: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pumprla J, Howorka K, Groves D, et al. Functional assessment of heart rate variability: physiological basis and practical applications. Int J Cardiol 2002; 84: 1–14. [DOI] [PubMed] [Google Scholar]
- 3.Lombardi F, Stein PK. Origin of heart rate variability and turbulence: an appraisal of autonomic modulation of cardiovascular function. Front Physiol 2011; 2: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hon EH, Lee ST. Electronic evaluation of the fetal heart rate. Viii. patterns preceding fetal death, further observations. Am J Obstet Gynecol 1963; 87: 814–826. [PubMed] [Google Scholar]
- 5.Billman GE. Heart rate variability – a historical perspective. Front Physiol 2011; 2: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolf MM, Varigos GA, Hunt D, et al. Sinus arrhythmia in acute myocardial infarction. Med J Aust 1978; 2: 52–53. [DOI] [PubMed] [Google Scholar]
- 7.Kleiger RE, Miller JP, Bigger JT, et al. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol 1987; 59: 258–282. [DOI] [PubMed] [Google Scholar]
- 8.Bishop DG, Wise RD, Lee C, et al. Heart rate variability predicts 30-day all-cause mortality in intensive care units. South Afr J Anaesth Analg 2016; 22: 125–128. [Google Scholar]
- 9.Chen WL, Chen JH, Huang CC, et al. Heart rate variability measures as predictors of in-hospital mortality in ED patients with sepsis. Am J Emerg Med 2008; 26: 395–401. [DOI] [PubMed] [Google Scholar]
- 10.Ahmad S, Ramsay T, Huebsch L, et al. Continuous multi-parameter heart rate variability analysis heralds onset of sepsis in adults. PLoS One 2009; 4: e6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karmali SN, Sciusco A, May SM, et al. Heart rate variability in critical care medicine: a systematic review. Intensive Care Med Exp 2017; 5: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malik M, Bigger JT, Camm AJ, et al. Heart rate variability Standards of measurement, physiological interpretation, and clinical use. Eur Heart J 1996; 17: 354–381. . [PubMed] [Google Scholar]
- 13.Shaffer F, McCraty R, Zerr CL. A healthy heart is not a metronome: an integrative review of the heart's anatomy and heart rate variability. Front Psychol 2014; 5: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hainsworth R, Malik M, Camm A. The control and physiological importance of heart rate: heart rate variability, Armonk, NY: Futura Publishing Company, 1995. [Google Scholar]
- 15.Keselbrener L, Akselrod S. Autonomic responses to blockades and provocations. In: M Malik. (ed). Clinical guide to cardiac autonomic tests, Netherlands: Springer, 1998, pp. 101–148. [Google Scholar]
- 16.Pagani M, Lombardi F, Guzzetti S, et al. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ Res 1986; 59: 178–193. [DOI] [PubMed] [Google Scholar]
- 17.Akselrod S, Gordon D, Ubel FA, et al. Power spectrum analysis of heart rate fluctuation: a quantitative. Science 1981; 213: 220–222. [DOI] [PubMed] [Google Scholar]
- 18.Perini R, Veicsteinas A. Heart rate variability and autonomic activity at rest and during exercise in various physiological conditions. Eur J Appl Physiol 2003; 90: 317–325. [DOI] [PubMed] [Google Scholar]
- 19.Julien C. The enigma of Mayer waves: facts and models. Cardiovasc Res 2006; 70: 12–21. [DOI] [PubMed] [Google Scholar]
- 20.Shaffer F, Ginsberg JP. An overview of heart rate variability metrics and norms. Front Public Heal 2017; 5: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papaioannou VE, Dragoumanis C, Theodorou V, et al. Relation of heart rate variability to serum levels of C-reactive protein, interleukin 6, and 10 in patients with sepsis and septic shock. J Crit Care 2009; 24: 625e1–625.e7. [DOI] [PubMed] [Google Scholar]
- 22.Sassi R, et al. Advances in heart rate variability signal analysis: joint position statement by the e-Cardiology ESC Working Group and the European Heart Rhythm Association co-endorsed by the Asia Pacific Heart Rhythm Society. Europace 2015; 17: 1341–1353. [DOI] [PubMed] [Google Scholar]
- 23.Hnatkova K, Copie X, Staunton A, et al. Numeric processing of Lorenz plots of R-R intervals from long-term ECGs. Comparison with time-domain measures of heart rate variability for risk stratification after myocardial infarction. J Electrocardiol 1995; 28: 74–80. [DOI] [PubMed] [Google Scholar]
- 24.Seely AJE, Macklem PT. Complex systems and the technology of variability analysis. Crit Care 2004; 8: R367–R384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merri M, Farden DC, Mottley JG, et al. Sampling frequency of the electrocardiogram for spectral analysis of the heart rate variability. IEEE Trans Biomed Eng 1990; 37: 99–106. [DOI] [PubMed] [Google Scholar]
- 26.Buccelletti F, Bocci MG, Gilardi E, et al. Linear and nonlinear heart rate variability indexes in clinical practice. Comput Math Methods Med 2012. 1: 1–5. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ernst G. Hidden signals – the history and methods of heart rate variability. Front Public Health 2017; 5: 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peng CK, Havlin S, Stanley HE, et al. Quantification of scaling exponents and crossover phenomena in nonstationary heartbeat time series. Chaos 1995; 5: 82–87. [DOI] [PubMed] [Google Scholar]
- 29.Goldberger AL, et al. PhysioBank, PhysioToolkit, and PhysioNet: components of a new research resource for complex physiologic signals. Circulation 2002; 101: E215–E220. [DOI] [PubMed] [Google Scholar]
- 30.Pincus SM. Assessing serial irregularity and its implications for health. Ann N Y Acad Sci 2001; 954: 245–267. [DOI] [PubMed] [Google Scholar]
- 31.Ernst G. Heart-rate variability – more than heart beats?. Front Public Health 2017; 5: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fleisher L. Heart rate variability as an assessment of cardiovascular status. J. Cardiothorac Vasc Anesth 1996; 10: 659–671. [DOI] [PubMed] [Google Scholar]
- 33.Mazzeo AT, La Monaca E, Di Leo R, et al. Heart rate variability: a diagnostic and prognostic tool in anesthesia and intensive care. Acta Anaesthesiol Scand 2011; 55: 797–811. [DOI] [PubMed] [Google Scholar]
- 34.Shaffer F, et al. Association of heart rate variability and inflammatory response in patients with cardiovascular diseases: current strengths and limitations. Front Physiol 2013; 5: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Annane D, et al. Inappropriate sympathetic activation at onset of septic shock: a spectral analysis approach. Am J Respir Crit Care Med 1999; 160: 458–465. [DOI] [PubMed] [Google Scholar]
- 36.Buchan CA, Bravi A, Seely AJE. Variability analysis and the diagnosis, management, and treatment of sepsis. Curr Infect Dis Rep 2012; 14: 512–521. [DOI] [PubMed] [Google Scholar]
- 37.Piepoli M, Garrard CS, Kontoyannis DA, et al. Autonomic control of the heart and peripheral vessels in human septic shock. Intensive Care Med 1995; 21: 112–119. [DOI] [PubMed] [Google Scholar]
- 38.Godin PJ, Buchman TG. Uncoupling of biological oscillators: a complementary hypothesis concerning the pathogenesis of multiple organ dysfunction syndrome. Crit Care Med 1996; 24: 1107–1116. [DOI] [PubMed] [Google Scholar]
- 39.Bishop DG, Wise RD, Lee C, et al. Heart rate variability predicts 30-day all-cause mortality in intensive care units. South Afr J Anaesth Analg 2016; 22: 125–128. [Google Scholar]
- 40.Schmidt H, Hoyer D, Hennen R, et al. Autonomic dysfunction predicts both 1- and 2-month mortality in middle-aged patients with multiple organ dysfunction syndrome. Crit Care Med 2008; 36: 967–970. [DOI] [PubMed] [Google Scholar]
- 41.Papaioannou VE, Maglaveras N, Houvarda I, et al. Investigation of altered heart rate variability, nonlinear properties of heart rate signals, and organ dysfunction longitudinally over time in intensive care unit patients. J Crit Care 2006; 21: 95–103. [DOI] [PubMed] [Google Scholar]
- 42.Samsudin MI, Liu N, Prabhakar SM, et al. A novel heart rate variability based risk prediction model for septic patients presenting to the emergency department. Medicine 2018; 97: e10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moorman JR, Carlo WA, Kattwinkel J, et al. Mortality reduction by heart rate characteristic monitoring in very low birth weight neonates: a randomized trial. J Pediatr 2011; 159: 900–906.e1. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fairchild KD, Schelonka RL, Kaufman DA, et al. Septicemia mortality reduction in neonates in a heart rate characteristics monitoring trial. Pediatr Res 2013; 74: 570–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huston JM, Tracey KJ. The pulse of inflammation: heart rate variability, the cholinergic anti-inflammatory pathway, and implications for therapy. J Intern Med 2011; 269: 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Borovikova LV, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000; 405: 458–462. [DOI] [PubMed] [Google Scholar]
- 47.Tateishi Y, Oda S, Nakamura M, et al. Depressed heart rate variability is associated with high Il-6 blood level and decline in the blood pressure in septic patients. Shock 2007; 28: 549–553. [DOI] [PubMed] [Google Scholar]
- 48.Jan BU, Coyle SM, Macor MA, et al. Relationship of basal heart rate variability to in vivo cytokine responses after endotoxin exposure. Shock 2010; 33: 363–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kanashiro A, Sônego F, Ferreira RG, et al. Therapeutic potential and limitations of cholinergic anti-inflammatory pathway in sepsis. Pharmacol Res 2017; 117: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pontet J, Contreras P, Curbelo A, et al. Heart rate variability as early marker of multiple organ dysfunction syndrome in septic patients. J Crit Care 2003; 18: 156–163. [DOI] [PubMed] [Google Scholar]
- 51.Billman GE, Huikuri HV, Sacha J, et al. An introduction to heart rate variability: methodological considerations and clinical applications. Front Physiol 2015; 6: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.La Rovere MT, Bigger JT, Marcus FI, et al. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet 1998; 351: 478–484. [DOI] [PubMed] [Google Scholar]
- 53.Passariello G, Peluso A, Moniello G, et al. Effect of autonomic nervous system dysfunction on sudden death in ischemic patients with anginal syndrome died during electrocardiographic monitoring in Intensive Care Unit. Minerva Anestesiol 2007; 73: 207–212. [PubMed] [Google Scholar]
- 54.Rajendra Acharya U, Paul Joseph K, et al. Heart rate variability: a review. Med Biol Eng Comput 2006; 44: 1031–1051. [DOI] [PubMed] [Google Scholar]
- 55.Tiainen M, Parikka HJ, Mäkijärvi MA, et al. Arrhythmias and heart rate variability during and after therapeutic hypothermia for cardiac arrest. Crit Care Med 2009; 37: 403–409. [DOI] [PubMed] [Google Scholar]
- 56.Lowensohn RI, Weiss M, Hon EH. Heart-rate variability in brain-damaged adults. Lancet 1977; 1: 626–628. [DOI] [PubMed] [Google Scholar]
- 57.Mowery NT, Norris PR, Riordan W, et al. Cardiac uncoupling and heart rate variability are associated with intracranial hypertension and mortality: a study of 145 trauma patients with continuous monitoring. J Trauma Inj Infect Crit Care 2008; 65: 621–627. [DOI] [PubMed] [Google Scholar]
- 58.Park S, Kaffashi F, Loparo KA, et al. The use of heart rate variability for the early detection of treatable complications after aneurysmal subarachnoid hemorrhage. J Clin Monit Comput 2013; 27: 385–393. [DOI] [PubMed] [Google Scholar]
- 59.Schmidt JM, Sow D, Crimmins M, et al. Heart rate variability for preclinical detection of secondary complications after subarachnoid hemorrhage. Neurocrit Care 2014; 20: 382–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Conci F, Rienzo D, Castiglioni P. Blood pressure and heart rate variability and baroreflex sensitivity before and after brain death. J Neurol Neurosurg Psychiatry 2001; 71: 621–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baillard C, Vivien B, Mansier P, et al. Brain death assessment using instant spectral analysis of heart rate variability. Crit Care Med 2002; 30: 306–310. [DOI] [PubMed] [Google Scholar]
- 62.Stanley HE, Amaral LAN, Goldberger AL, et al. Statistical physics and physiology: monofractal and multifractal approaches. Phys A Stat Mech Appl 1999; 270: 309–324. [DOI] [PubMed] [Google Scholar]
- 63.Sztajzel J. Heart rate variability: a noninvasive electrocardiographic method to measure the autonomic nervous system. Swiss Med Wkly 2004; 134: 514–522. [DOI] [PubMed] [Google Scholar]
- 64.Niskanen J-P, Tarvainen MP, Ranta-aho PO, et al. Software for advanced HRV analysis. Comput Methods Programs Biomed 2004; 76: 73–81. [DOI] [PubMed] [Google Scholar]
- 65.Tarvainen MP, Niskanen J-P, Lipponen JA, et al. Heart rate variability analysis software. Comput Methods Programs Biomed 2014; 113: 210–220. [DOI] [PubMed] [Google Scholar]
- 66.Kaufmann T, Sütterlin S, Schulz SM, et al. ARTiiFACT: a tool for heart rate artifact processing and heart rate variability analysis. Behav Res Methods 2011; 43: 1161–1170. [DOI] [PubMed] [Google Scholar]