Abstract
Rationale
Acute hypoxemic respiratory failure is a condition that comprises a wide array of entities. Obtaining a histological lung sample might help reach a diagnosis and direct an appropriate treatment in a select group of patients.
Objective
To describe our experience in the use of cryobiopsy for the diagnosis of acute hypoxemic respiratory failure of undetermined origin.
Methods
Retrospective analysis of case series of patients with acute hypoxemic respiratory failure who underwent lung cryobiopsy at the Intensive Care Unit of the Hospital Italiano de Buenos Aires, Argentina.
Results
Cryobiopsy yielded a histological diagnosis in all patients (n = 10, 100%). This led to either a change in therapy or continuation of a specific treatment in eight of these patients. Cryobiopsy was found to be contributive in all the patients who did not meet Berlin criteria for acute respiratory distress syndrome. No major complications were associated with the procedure.
Conclusions
Cryobiopsy is a safe procedure with a high diagnostic yield in a selected group of patients.
Keywords: Acute lung injury, respiratory distress syndrome, adult, cryoprobe biopsy
Introduction
Acute hypoxemic respiratory failure (AHRF) is a condition that comprises a wide array of entities. The acute respiratory distress syndrome (ARDS) is a life-threatening form of respiratory failure.1 As a syndrome, it has different causes, pathophysiological mechanisms and histologic changes,2 and its diagnosis is based on clinical and radiologic findings.3 Several entities may mimic ARDS, and reaching a definite diagnosis – and consequently providing an appropriate treatment – can sometimes be a major challenge.2 When diagnosis of the underlying cause is not clear after performing a minimally invasive evaluation with bronchoscopy with bronchoalveolar lavage (BAL), many physicians opt for empirical treatments such as broad-spectrum antibiotics, diuretics, or immunosuppressive agents. Such strategies might sometimes be ineffective or even potentially injurious. In patients who do not improve after initial therapy, or those at risk of having serious complications with aggressive empirical treatments, having a histological sample might help reach a diagnosis and guide therapy.
Up to date, open lung biopsy (OLB) is the most common method for obtaining a pulmonary sample for most lung diseases. However, it is rarely performed in patients with AHRF because of potential severe complications.4 A recent meta-analysis of 14 case series including 512 mechanically ventilated patients who underwent OLB showed that therapeutic changes occurred in 78% of the patients. However, 29% of them presented procedure-related complications and overall mortality after the procedure was 54%.5 Transbronchial lung (TBLB) biopsy on the other hand, although a less invasive procedure, has a low diagnostic yield and high incidence of complications in ARDS6 even when taking multiple samples.7 Transbronchial lung cryobiopsy is a procedure that has proven to have a high diagnostic yield in diffuse lung diseases, quite similar to OLB in some series, with an acceptable safety profile and lower morbidity and mortality than surgical procedures.8 Moreover, it provides larger samples than TBLB and more preserved tissue architecture for histologic interpretation.9,10
Our purpose is to report our experience on cryoprobe lung biopsy in mechanically ventilated patients with hypoxemic respiratory failure and pulmonary infiltrates of unclear etiology.
Methods
Patients
We performed a retrospective review of hospital charts of all mechanically ventilated patients who underwent bronchoscopic cryobiopsy for the diagnosis of hypoxemic respiratory failure with pulmonary infiltrates of unclear etiology at the Intensive Care Unit (ICU) of the Hospital Italiano de Buenos Aires from January 2016 to March 2018. The study was approved by the ethics committee of the Hospital Italiano de Buenos Aires (Comité de Ética en Protocolos de Investigación). Demographic data and underlying conditions were recorded. Acute Physiology and Chronic Health Evaluation II Score (APACHE II) was calculated on ICU admission. Laboratory data were recorded on the day of lung biopsy.
Subjects were included if they met the following criteria: (1) age >18 years old, (2) receiving mechanical ventilation on the day of the biopsy, (3) PaO2/FiO2 ratio <300 mmHg, and (4) undiagnosed pulmonary infiltrate on computed tomography (CT) scan. We included patients with and without ARDS. Cryobiopsy was performed after infection was ruled out (via BAL) or when an alternative diagnosis was suspected.
Cryoprobe biopsy was performed by a trained respirologist at the bedside in the ICU under general anesthesia with a Pentax flexible bronchoscope using the ERBECRYO 2 probe (Tübingen, Germany). Briefly, the cryoprobe was advanced through the bronchoscope's working channel until a resistance point was met. The probe was then withdrawn 2 cm and a freezing time of four seconds was applied. Both the bronchoscope and probe were then fully withdrawn, and the cryoprobe was then placed in sterile saline 0.9% solution to accelerate defrosting and facilitate the specimen's extraction. Finally, a second bronchoscopy was performed, and the area where the biopsy was taken from was mechanically occluded with the bronchoscope for two minutes. Chest X-ray and lung ultrasound were performed after the procedure to rule out pneumothorax.
All samples were examined by a pathologist specialized in lung pathology.
Definitions
ARDS was defined according to the Berlin criteria.3 ARDS mimickers were defined as patients that met the Berlin criteria but had no exposure to a known insult.2 Patients who met neither criteria were labeled as hypoxemic respiratory failure of unclear origin.
Cryobiopsy was considered to be contributive when the pathological findings either resulted in a change in therapy or management or when a specific treatment was continued based on the results.11
Procedure-related complications were defined as: bleeding (Type 1: resolved without endoscopic maneuvers; Type 2: resolved with endoscopic maneuvers) local epinephrine, bronchial occlusion balloon, endoscopic occlusion); Type 3: required angiography or surgery), hypoxemia (PaO2/FiO2 ratio drop of 20 mmHg), pneumothorax, persistent air leak, hemodynamic instability (mean arterial pressure drop of 20 mmHg not related to bleeding), and biopsy-related death (death occurring within 24h of the procedure).
Statistical analysis
Continuous data are expressed as mean and standard deviation or median and interquartile range (IQR) depending on their distribution. Categorical data are expressed as counts and percentages.
Results
From January 2016 to March 2018, 90 patients were admitted to the ICU for AHRF. Among them, 10 patients (11.1%) underwent bronchoscopic cryobiopsy. The basal characteristics of the population are given in Table 1. Four (40%) patients met the Berlin criteria for ARDS and 1 (10%) patient was defined as an ARDS mimicker. Eight patients had a BAL performed prior to the cryobiopsy, and in the remaining two cases, the BAL was done at the same time. Only one BAL had a positive culture for Pseudomonas aeruginosa. All patients had a previous CT scan. The following CT patterns were seen: diffuse ground-glass opacities (N = 10), consolidation (N = 8), pleural effusion (N = 7), septal thickening (N = 1), crazy paving (N = 1), multiple nodules (N = 1), and mediastinal adenopathy (N = 4). It was the first lung biopsy for nine of the patients. The only patient who had a repeat biopsy had previously undergone a lung transplant. The average number of biopsies was four, and the mean duration of the procedure was 37 min.
Table 1.
Patients characteristics.
| Characteristics | All patients (N = 10) |
|---|---|
| Age, years – median (IQR) | 58 (34–78) |
| Female sex – no. (%) | 6 (60%) |
| Previous lung disease – no. (%) | |
| COPD | 1 (10%) |
| Interstitial lung disease | 1 (10%) |
| Lung transplant | 1 (10%) |
| Comorbidities – no. (%) | |
| Hypertension | 4 (40%) |
| Diabetes | 2 (20%) |
| Cancer | 1 (10%) |
| Immunocompromised – no. (%) | 6 (60%) |
| APACHE II score – median (IQR) | 20 (13–22) |
| PaO2/FiO2 ratio – median (IQR) | 203 (181–240) |
| Days from admission to biopsy – median (IQR) | 3 (1–4) |
| Bilateral infiltrate on CT scan – no. (%) | 10 (100%) |
| ARDSa – no. (%) | 4 (40%) |
| Contributive biopsy – no. (%) | 8 (80%) |
According to the Berlin definition.3
All biopsies yielded a pathological diagnosis. Results are summarized in Table 2. Diffuse alveolar damage (DAD) was observed in four (40%) samples, one of them being organizing DAD (Figure 1). Other diagnoses were: histological findings compatible with drug toxicity (amiodarone and bleomycin) (N = 2 (20%)), tuberculosis (N = 1 (10%)), eosinophilic pneumonia (N = 1 (10%)), acute fibrinous organizing pneumonia (AFOP) (N = 1 (10%)), and bacterial pneumonia (N = 1 (10%)). One patient had a combination of DAD and cancer. Of the four patients who met ARDS criteria, three had DAD (75%) and one patient with DAD did not meet Berlin criteria (did not meet the timeframe criteria). Five of six patients (83.3%) who did not meet ARDS criteria had a pathological finding different to DAD.
Table 2.
Histological findings.
| Result of biopsy | n (%) |
|---|---|
| Diffuse alveolar damage | 4 (40%) |
| Acute eosinophilic pneumonia | 1 (10%) |
| Findings compatible with drug toxicitya | 2 (20%) |
| Cancer | 1 (10%) |
| AFOP | 1 (10%) |
| Infectious disease | |
| Tuberculosis | 1 (10%) |
| Pseudomonas pneumonia | 1 (10%) |
Amiodarone and bleomycin toxicity.
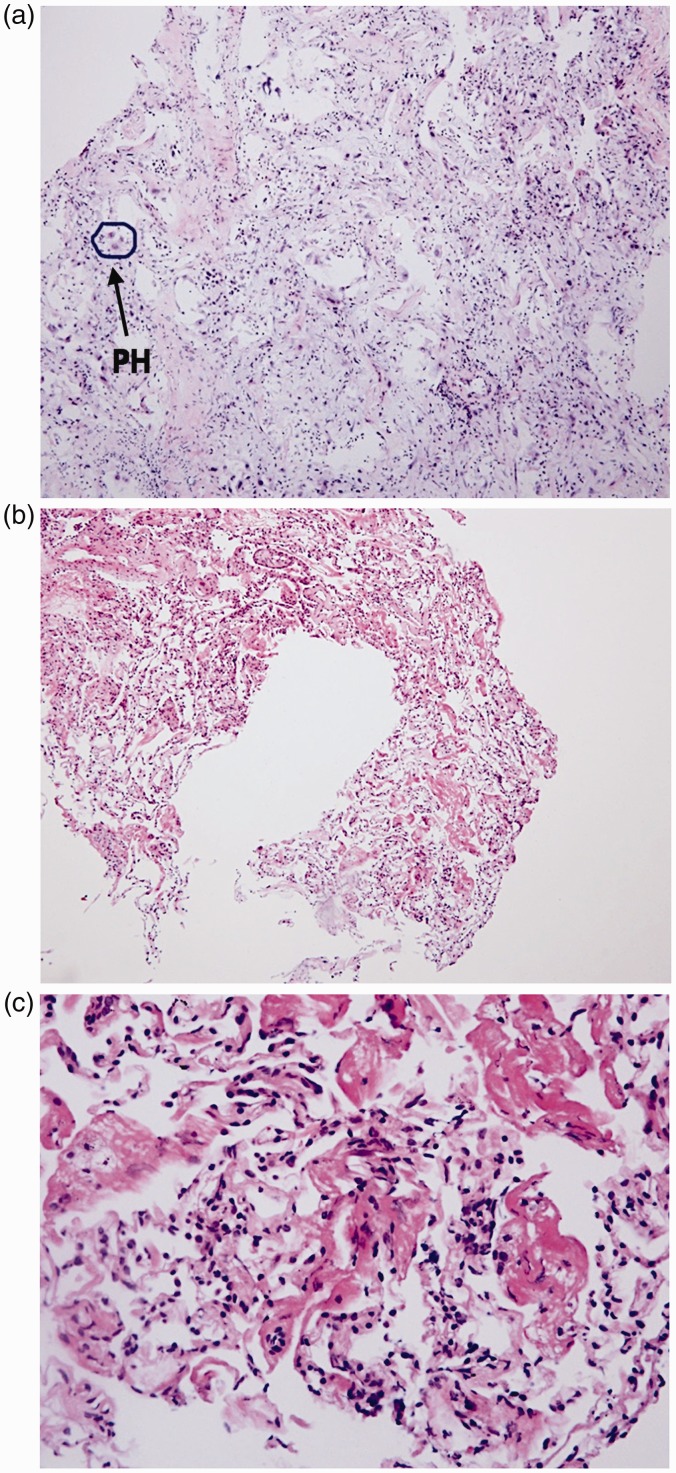
Figure 1.
Cryoprobe biopsy showing different stages of DAD. Representative hematoxylin and eosin stained (HES) cryobiopsies showing different stages of DAD. (a) Diffuse alveolar damage with pneumocyte hyperplasia (HES × 20), (b) exudative diffuse alveolar damage (HES × 20), and (c) diffuse alveolar damage, exudative phase (HES × 60).
Cryobiopsy was found to be contributive in 8 of the 10 patients (80%). High-dose steroids were started or continued in three cases and discontinued in two cases. Immunosuppression was discontinued in one case. Antimicrobials were changed to treat specific microorganisms in one case and discontinued in two cases. Amiodarone was discontinued in one of the patients with findings compatible with drug toxicity (bleomycin had already been discontinued in the other drug toxicity patient). When analyzing the subgroup of patients who did not meet Berlin criteria for ARDS, biopsy was found to be contributive in all of them.
Seven patients had type 1 bleeding (70%) and one patient had type 2 bleeding (10%). No patients presented hemodynamic instability related to a bleeding event. Three patients developed transient hypoxemia after the procedure which resolved within the following 60 min without the need for medical interventions. No patients developed pneumothorax or persistent air leak after the procedure. No biopsy-related death was recorded.
Discussion
In our small case series of highly selected patients with AHRF of unclear origin, we found a high diagnostic yield and contribution to guide management with cryoprobe biopsy. No major complications were associated with the procedure.
DAD was seen in 40% of the samples, in keeping with the findings of Philipponnet et al.11 Most of the patients (75%) with DAD met the Berlin criteria. In their cohort, Thille et al.12 found DAD in 45% of the autopsies of patients with ARDS. This difference might be explained by the fact that our sample is small. Moreover, although DAD is considered the pathological hallmark of ARDS,13 both cannot be considered the same entity.14 Interestingly, in the subgroup of patients who did not have ARDS according to the Berlin definition, biopsy showed a diagnosis different to DAD in 83% of the patients and led to a change in therapy in all the subjects in this group. This suggests that in the subgroup of patients where Berlin criteria is not met, it is more probable to obtain a different diagnosis than DAD. OLB has shown to be contributive in 60–80% in previous studies.4,11,15–19 Our findings are therefore consistent with these results. It has to be noted that in many studies, the therapeutic changes reported included the decision to initiate steroids if the diagnosis was organizing DAD. This is not a common practice in our center as there is no robust evidence to support this treatment.20–22
We observed no major complications associated with the procedure. Bleeding was the most common complication, and it resolved on its own in most of the patients. Only one patient required topical administration of epinephrine. OLB, as previously described, carries a higher risk of complications.15,23,24 The mean number of biopsies was four, and this is both usual practice in our center and the recommended number of specimens suggested in the literature.25
This study has limitations. First, it is a retrospective study in a single center with a small sample size. Moreover, the procedure has to be performed by a trained interventional bronchoscopist. This makes generalization of our findings difficult. Second, this is a highly selected population of patients with AHRF, so we cannot exclude the possibility that both the diagnostic yield and the changes in therapy might be overestimated due to selection bias. However, both the indication for biopsy and the changes in therapy were documented in the medical charts. Third, although based on previously published criteria,2,15 the definition of contributive biopsy might be considered arbitrary and broad. Nevertheless, we still found a significative contribution of cryobiopsy, even when steroids were not initiated for the treatment of organizing DAD.
To the best of our knowledge, this is the largest case series of cryoprobe biopsy in patients with hypoxemic respiratory failure of unclear origin. Ours is the first report of cryobiopsy findings in a population of hypoxemic respiratory failure with and without ARDS.
Our report of case-series shows that cryobiopsy is a safe procedure with a high diagnostic yield and can lead to changes in management in a highly selected group of patients in which diagnosis is not clear despite an adequate and extensive diagnostic process. Larger studies are needed to prove our findings and better identify those patients who are likely to benefit from this intervention.
Authors' contributions
MJLH and JMD conceived the study; MJLH, JMD, GS, SEG and ESR designed the study; MJLH, JMD and MT acquired and analyzed the study measurements; and JMD conducted the statistical analysis. All authors contributed to the interpretation of the findings. MJLH and JMD prepared the first draft of the manuscript and all authors critically revised the manuscript. All authors gave final approval for the publication of the work and accepted responsibility for the integrity of the work.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Fan E, Brodie D, Slutsky AS. Acute respiratory distress syndrome. Advances in diagnosis and treatment. JAMA 2018; 319: 698–710. [DOI] [PubMed] [Google Scholar]
- 2.Aublanc M, Perinel S, Guérin C. Acute respiratory distress syndrome mimics: the role of lung biopsy. Curr Opin Crit Care 2017; 23: 24–29. [DOI] [PubMed] [Google Scholar]
- 3.Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA 2012; 307: 2526–2533. [DOI] [PubMed] [Google Scholar]
- 4.Patel SR, Karmpaliotis D, Ayas NT, et al. The role of open-lung biopsy in ARDS. Chest 2004; 125: 197–202. [DOI] [PubMed] [Google Scholar]
- 5.Wong AK, Walkey AJ. Open lung biopsy among critically ill, mechanically ventilated patients: a metaanalysis. Ann Am Thorac Soc 2015; 12: 1226–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bulpa PA, Dive AM, Mertens L, et al. Combined bronchoalveolar lavage and transbronchial lung biopsy: safety and yield in ventilated patients. Eur Respir J 2003; 21: 489–494. [DOI] [PubMed] [Google Scholar]
- 7.Fruchter O, Fridel L, Rosengarten D, et al. Transbronchial cryobiopsy in immunocompromised patients with pulmonary infiltrates: a pilot study. Lung 2013; 191: 619–624. [DOI] [PubMed] [Google Scholar]
- 8.Pajares V, Puzo C, Castillo D, et al. Diagnostic yield of transbronchial cryobiopsy in interstitial lung disease: a randomized trial. Respirology 2014; 19: 900–906. [DOI] [PubMed] [Google Scholar]
- 9.Tomic R, Podgaetz E, Andrade RS, et al. Cryotechnology in diagnosing and treating lung diseases. J Bronchol Interv Pulmonol 2015; 22: 76–84. [DOI] [PubMed] [Google Scholar]
- 10.Kropski JA, Pritchett JM, Mason WR, et al. Bronchoscopic cryobiopsy for the diagnosis of diffuse parenchymal lung disease. PLoS One 2013; 8: 6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Philipponnet C, Cassagnes L, Pereira B, et al. Diagnostic yield and therapeutic impact of open lung biopsy in the critically ill patient. PLoS One 2018; 13: e0196795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thille AW, Esteban A, Fernández-Segoviano P, et al. Comparison of the berlin definition for acute respiratory distress syndrome with autopsy. Am J Respir Crit Care Med 2013; 187: 761–767. [DOI] [PubMed] [Google Scholar]
- 13.Katzenstein AL, Bloor CM, Leibow AA. Diffuse alveolar damage – the role of oxygen, shock, and related factors. A review. Am J Pathol 1976; 85: 209–228. [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson BT, Guérin C, Esteban A. Should ARDS be renamed diffuse alveolar damage?. Intens Care Med 2016; 42: 653–655. [DOI] [PubMed] [Google Scholar]
- 15.Papazian L, Doddoli C, Chetaille B, et al. A contributive result of open-lung biopsy improves survival in acute respiratory distress syndrome patients. Crit Care Med 2007; 35: 755–762. [DOI] [PubMed] [Google Scholar]
- 16.Baumann HJ, Kluge S, Balke L, et al. Yield and safety of bedside open lung biopsy in mechanically ventilated patients with acute lung injury or acute respiratory distress syndrome. Surgery 2008; 143: 426–433. [DOI] [PubMed] [Google Scholar]
- 17.Libby LJ, Gelbman BD, Altorki NK, et al. Surgical lung biopsy in adult respiratory distress syndrome: a meta-analysis. Ann Thorac Surg 2014; 98: 1254–1260. [DOI] [PubMed] [Google Scholar]
- 18.Almotairi A, Biswas S, Shahin J. The role of open lung biopsy in critically ill patients with hypoxic respiratory failure: a retrospective cohort study. Can Respir J. Epub ahead of print 4 May 2016. DOI: 10.1155%2F2016%2F8715024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerard L, Bidoul T, Castanares-Zapatero D, et al. Open lung biopsy in nonresolving acute respiratory distress syndrome commonly identifies corticosteroid-sensitive pathologies, associated with better outcome. Crit Care Med 2018; 46: 907–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steinberg K, Hudson LD, Goodman R, et al. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med 2006; 354: 1671–1684. [DOI] [PubMed] [Google Scholar]
- 21.Lamontagne F, Briel M, Guyatt GH, et al. Corticosteroid therapy for acute lung injury, acute respiratory distress syndrome, and severe pneumonia: a meta-analysis of randomized controlled trials. J Crit Care 2010; 25: 420–435. [DOI] [PubMed] [Google Scholar]
- 22.Tonelli A, Zein J, Adams J, et al. Effects of interventions on survival in acute respiratory distress syndrome: an umbrella review of 159 published randomized trials and 29 meta-analyses. Intens Care Med 2014; 40: 769–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donati SY, Papazian L. Role of open-lung biopsy in acute respiratory distress syndrome. Curr Opin Crit Care 2008; 14: 75–79. [DOI] [PubMed] [Google Scholar]
- 24.Guerin C, Bayle F, Leray V, et al. Open lung biopsy in nonresolving ARDS frequently identifies diffuse alveolar damage regardless of the severity stage and may have implications for patient management. Intens Care Med 2014; 41: 222–230. [DOI] [PubMed] [Google Scholar]
- 25.Poletti V, Casoni GL, Gurioli C, et al. Lung cryobiopsies: a paradigm shift in diagnostic bronchoscopy?. Respirology 2014; 19: 645–654. [DOI] [PubMed] [Google Scholar]